Abstract
Objective
The presence of macroscopic residual disease after primary cytoreductive surgery (PCS) is an important factor influencing survival for patients with high-grade serous ovarian cancer (HGSC). More research is needed to identify factors associated with having macroscopic residual disease. We analyzed 12 lifestyle and personal exposures known to be related to ovarian cancer risk or inflammation to identify those associated with having residual disease after surgery.
Methods
This analysis used data on 2,054 patients with advanced stage HGSC from the Ovarian Cancer Association Consortium. The exposures were body mass index, breastfeeding, oral contraceptive use, depot-medroxyprogesterone acetate use, endometriosis, first-degree family history of ovarian cancer, incomplete pregnancy, menopausal hormone therapy use, menopausal status, parity, smoking, and tubal ligation. Logistic regression models were fit to assess the association between these exposures and having residual disease following PCS.
Results
Menopausal estrogen-only therapy (ET) use was associated with 33% lower odds of having macroscopic residual disease compared to never use (OR=0.67, 95%CI 0.46–0.97, p=0.033). Compared to nulliparous women, parous women who did not breastfeed had 36% lower odds of having residual disease (OR=0.64, 95%CI 0.43–0.94, p=0.022), while there was no association among parous women who breastfed (OR=0.90, 95%CI 0.65–1.25, p=0.53).
Conclusions
The association between ET and having no macroscopic residual disease is plausible given a strong underlying biologic hypothesis between this exposure and diagnosis with HGSC. If this or the parity finding is replicated, these factors could be included in risk stratification models to determine whether HGSC patients should receive PCS or neoadjuvant chemotherapy.
Keywords: ovarian cancer, residual disease, primary cytoreductive surgery, lifestyle
Introduction
Ovarian carcinoma is the deadliest gynecologic cancer with about 20,000 new cases and more than 13,000 deaths in the US in 20221. High-grade serous cancer (HGSC) is the most common histotype, comprising ~70% of all epithelial ovarian cancers2. About 80% of HGSCs are diagnosed at an advanced stage3 and the five-year survival rate is very low (~32%)4. An important factor influencing survival for HGSC patients is whether no macroscopic residual disease is achieved during primary cytoreductive surgery (PCS)5, 6.
Factors known to be associated with residual disease following ovarian cancer PCS include disease stage and age7–9. Significant efforts have been made to identify additional factors, including personal and lifestyle exposures. We have previously reported that use of menopausal hormone therapy for five or more years before diagnosis was associated with 29% lower odds of having residual disease compared to never use (odds ratio OR=0.71, 95% confidence interval CI 0.54–0.93)10. Other studies have suggested that having a family history of cancer11, a personal history of endometriosis11, or use of combined oral contraceptives (COCs)12 are associated with a higher likelihood of achieving no macroscopic residual disease or cytoreduction to residual disease ≤1 cm. Conversely, post-menopausal status11–13, higher parity12, 14, higher body mass index (BMI)12, 13, 15, and ever smoking12 have been associated with a lower likelihood of achieving no macroscopic residual disease or cytoreduction to residual disease ≤1 cm.
However, previous studies have significant limitations. Most findings of associations with the presence of residual disease are difficult to interpret because there was no adjustment for potential confounders, small sample sizes, and heterogeneity in outcome definitions. Less rigorous definitions of residual disease following PCS has been used, e.g., ‘optimal’ cytoreduction (residual disease ≤1 cm) versus ‘suboptimal’ cytoreduction (residual disease >1 cm)8, 11. There is evidence that factors associated with achieving no macroscopic residual disease after PCS may not mirror factors associated with cytoreduction to ≤1 cm residual disease15.
To address these limitations, we comprehensively examined the association between 12 lifestyle and personal factors and the likelihood of having macroscopic residual disease after PCS in 2,054 patients with advanced-stage HGSC. Data from seven studies from the international Ovarian Cancer Association Consortium (OCAC; https://ocac.ccge.medschl.cam.ac.uk/) were used. We were able to adjust for important confounders and to consider temporal changes in clinical practice.
Materials and Methods
Study population
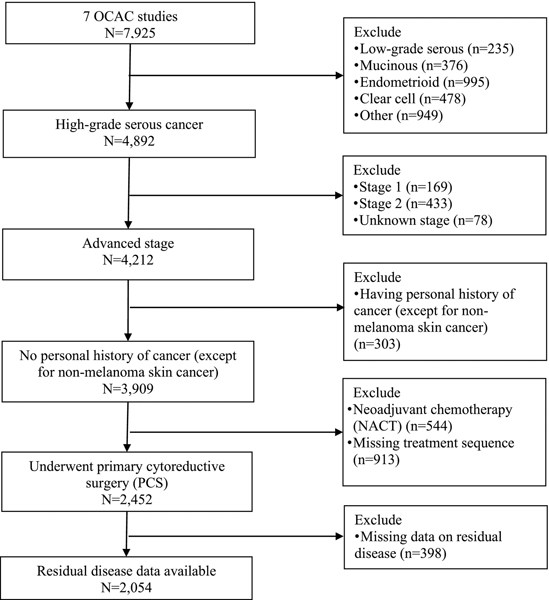
All OCAC studies obtained institutional review board approval and all participants provided written consent. Studies that had information on residual disease following PCS and data on at least eight of the 12 exposures of interest (see below) were included. Two studies from Australia and five from the United States (US) met these criteria (Table 1). Figure 1 presents the flow chart of patients considered for this analysis. People who were diagnosed with advanced stage (i.e., distant stage) invasive HGSC (tubal, peritoneal or ovarian), had no prior cancer (except non-melanoma skin cancer), underwent PCS, and had information on residual disease following PCS were included in the analysis. Women undergoing neoadjuvant chemotherapy (NACT; N=544), those who were missing information on whether they had PCS or NACT (N=913), and those without residual disease information (N=398) were excluded. This left 2,054 participants available for this analysis (Figure 1).
Table 1:
Characteristics of studies included in the analysis
| Study abbreviation | Study full name | Study location | Year of diagnosis | Total of participants included in the main analysis | Diagnosed before 2007 N=1,359 (66.2%) |
Diagnosed in 2007 or later N=695 (33.8%) |
||
|---|---|---|---|---|---|---|---|---|
| Macrosco pic residual disease n (%) | No macroscopic residual disease n (%) | Macroscopic residual disease n (%) | No macroscopic residual disease n (%) | |||||
|
|
|
|||||||
| AUS29 | Australian Ovarian Cancer Study |
Australia | 2001-2006 | 544 | 424 (77.9%) | 120 (22.1%) | 0 | 0 |
| OPL30 | Ovarian Cancer Prognosis and Lifestyle Study |
Australia | 2012-2015 | 245 | 0 | 0 | 148 (60.4%) | 97 (39.6%) |
| HAW31 | Hawaii Ovarian Cancer Case-Control Study |
Hawai’i, US | 1994-2006 | 65 | 47 (72.3%) | 18 (27.7%) | 0 | 0 |
| HOP32 | Hormones and Ovarian Cancer Prediction |
Western Pennsylvani a, Northeast Ohio, Western New York, US |
2003-2008 | 289 | 148 (76.7%) | 45 (23.3%) | 64 (66.7%) | 32 (33.3%) |
| LAX | Women’s Cancer Program at the Samuel Oschin Comprehensive Cancer Institute |
California, US |
1986-2008 | 134 | 51 (45.9%) | 60 (54.1%) | 13 (56.5%) | 10 (43.5%) |
| MAYO33, 34 | Mayo Clinic Ovarian Cancer Study |
Minnesota, US | 1993-2014 | 569 | 177 (70.0%) | 76 (30.0%) | 144 (45.6%) | 172 (54.4%) |
| NEC35 | New England Case Control Study |
New Hampshire and Eastern Massachuse tts, US |
1992-2008 | 208 | 141 (73.1%) | 52 (26.9%) | 11 (73.3%) | 4 (26.7%) |
|
|
|
|||||||
| 2,054 | 988 (72.7%) | 371 (27.3%) | 380 (54.7%) | 315 (45.3%) | ||||
Figure 1:
Flowchart of participants included in the analysis
Outcome and Exposure Variables
The outcome of interest was macroscopic residual disease after ovarian cancer PCS. The exposures we considered for this analysis were those that are known to be associated with ovarian cancer risk or inflammation. The 12 self-reported pre-diagnosis exposures of interest are: BMI (<18.5, 18.5–24.99, 25–29.99, 30+ kg/m2); combined oral contraceptive (COC) duration of use (<1 year, 1–4.99, 5–9.99, 10+ years); depot-medroxyprogesterone acetate (DMPA) use for contraception (yes, no); personal history of endometriosis (yes, no); first-degree family history of ovarian cancer (yes, no); incomplete pregnancy (yes, no); menopausal hormone therapy use (never use, estrogen-only therapy [ET] use, combined estrogen-progestin therapy [EPT] use, other [use of both ET and EPT or type unknown]); menopausal status (pre-, post-menopausal); parity/breastfeeding (nulliparous, parous/never breastfed, parous/breastfed); smoking (never, former, current); and tubal ligation (yes, no). Results were similar when conducting analyses on finer categories of parity (0, 1, 2, 3+), incomplete pregnancy (0, 1, 2+), breastfeeding duration (never breastfed, breastfed <12, 12–23, 24+ months), and menopausal hormone therapy duration of use (never use, use for <5 years and 5+ years) separately for ET and EPT use. We considered other exposures but did not include them in the final analysis due to a high proportion of missing values, i.e., >50% (history of polycystic ovary syndrome and pelvic inflammatory disease, alcohol consumption, exposure to environmental smoking, use of talcum powder, and use of non-steroidal anti-inflammatory drugs, aspirin, or acetaminophen).
Multiple imputation
The proportion of missingness for the 12 exposures ranged from 5% for menopausal status to 33% for parity/breastfeeding among the 2,054 participants included in this analysis. Multiple imputation was carried out using the mice package in R to generate 20 imputed datasets. All variables were imputed, except for the outcome (residual disease). All variables were included in the imputation models, except for those with 70% of missingness or higher. All studies were imputed together; OCAC study site (n=7) and country (Australia and US) were included as predictors in the imputation models. Results were pooled from 20 imputed datasets using Rubin’s rule16.
Statistical analyses
Patients included in this analysis were diagnosed between 1986 and 2015, a time period over which surgical techniques and treatment approach changed. The value of achieving no macroscopic residual disease became more evident, thus over time patients have generally undergone longer and more extensive and aggressive surgeries17. We adjusted for this by including a linear term for year of surgery in the model. However, the frequency of NACT followed by interval debulking surgery increased over time beginning around 2007; prior to 2007 about 10% of patients in the US received NACT whereas by 2018 that number was around 40%, according to the National Cancer Database18. Notably, following the clinical trials19–22 showing non-inferiority of NACT compared to PCS, the number of patients receiving NACT increased across most regions. PCS has increasingly been used in patients most likely to be able to be cytoreduced to no macroscopic residual disease, a decision which is institution-/surgeon-specific. Since our analysis was restricted to patients who did not receive NACT, the proportion of patients cytoreduced to no macroscopic residual disease would therefore be anticipated to be higher in the later calendar periods compared to the earlier periods. We studied the effect of this by estimating the associations separately for year of diagnosis before 2007 versus 2007 and later and carrying out a meta-analysis for the exposures for the two time periods. I2 are provided for each exposure across the two time periods.
Logistic regression models in the two time periods were fit regressing the presence of macroscopic residual disease on the 12 exposures of interest, adjusted for age at diagnosis (per five years); race/ethnicity (non-Hispanic White, Black, Asian, other); education level (<high school, high school, some college, college or above); year of diagnosis (continuous); Federation of Gynecology and Obstetrics (FIGO) stage (IIIA, IIIB, IIIC, III NOS, and IV); grade (moderately differentiated and poorly differentiated/undifferentiated); CA125 within one month of PCS; and OCAC study site.
Sensitivity analyses
We conducted a sensitivity analysis to assess the appropriateness of pooling the data from the OCAC studies. Meta-analysis results among patients diagnosed before 2007 showed little evidence of heterogeneity in the associations between the exposures and having macroscopic residual disease across the OCAC studies: I2=0.0% for 16 of the total 19 comparisons (i.e., the number of categories of all exposures excluding the reference groups), except for COC use for 14.99 years (I2=26%) and 10+ years (I2=61%), and tubal ligation (I2=28%). Heterogeneity across OCAC study sites among patients diagnosed in 2007 or later could not be assessed due to the smaller sample size. Thus, given the limited evidence of heterogeneity, the OCAC studies were pooled and analyzed as described above.
Statistical significance was defined as p≤0.05 using a 2-sided test. Analyses were conducted using R version 4.2.0.
Data availability
The data generated in this study are not publicly available due to limitations imposed by the original studies in which these data were collected. The corresponding author will facilitate access through existing data request processes for the OCAC.
Results
Of the total 2,054 advanced stage HGSC patients included in the analysis, 1,359 (66.2%) were diagnosed before 2007 and 695 (33.8%) in 2007 or later (Table 1). The proportion of participants with macroscopic residual disease following PCS was higher among patients before 2007 compared to those who were diagnosed in 2007 or later (72.7% and 54.7%, respectively; Table 1). Participant characteristics are shown in Table 2. In both calendar periods, patients with FIGO Stage IIIC and IV disease were more likely to have residual disease compared with those with FIGO stage IIIA/B (Table 3); stage IIIA and IIIB accounted for only 13% of the study population.
Table 2:
Characteristics of participants included in the main analysis based on the unimputed dataset
| Diagnosed before 2007 | Diagnosed in 2007 or later | |||
|---|---|---|---|---|
|
| ||||
| Macroscopic residual disease n=988 | No macroscopic residual disease n=371 | Macroscopic residual disease n=380 | No macroscopic residual disease n=315 | |
|
| ||||
| Age at diagnosis | ||||
| Mean [SD] | 60.7 [10.3] | 59.3 [11.2] | 62.0 [10.6] | 60.6 [11.1] |
| Median [IQR] | 61.0 [14.0] | 60.0 [16.5] | 62.5 [15.0] | 60.0 [15.5] |
| FIGO stage | ||||
| IIIA and IIIB | 39 (3.9%) | 60 (16.2%) | 13 (3.4%) | 41 (13.0%) |
| III (NOS) | 133 (13.5%) | 52 (14.0%) | 11 (2.9%) | 4 (1.3%) |
| IIIC | 657 (66.5%) | 229 (61.7%) | 289 (76.1%) | 241 (76.5%) |
| IV | 159 (16.1%) | 30 (8.1%) | 67 (17.6%) | 29 (9.2%) |
| Grade | ||||
| Moderately differentiated | 147 (14.9%) | 50 (13.5%) | 23 (6.1%) | 21 (6.7%) |
| Poorly differentiated or undifferentiated | 841 (85.1%) | 321 (86.5%) | 357 (93.9%) | 294 (93.3%) |
| CA125 | ||||
| Mean [SD] | 2680 [7,370] | 1080 [1,960] | 1,990 [3,160] | 1480 [8,900] |
| Median [IQR] | 904 [1,740] | 433 [915] | 920 [1,970] | 386 [962] |
| Missing | 337 (34.1%) | 131 (35.3%) | 98 (25.8%) | 84 (26.7%) |
| Education | ||||
| < high school | 159 (16.1%) | 50 (13.5%) | 68 (17.9%) | 37 (11.7%) |
| High school | 247 (25.0%) | 82 (22.1%) | 90 (23.7%) | 80 (25.4%) |
| Some college | 238 (24.1%) | 73 (19.7%) | 84 (22.1%) | 85 (27.0%) |
| College or above | 202 (20.4%) | 80 (21.6%) | 112 (29.5%) | 92 (29.2%) |
| Missing | 142 (14.4%) | 86 (23.2%) | 26 (6.8%) | 21 (6.7%) |
| Race/ethnicity | ||||
| Non-Hispanic White | 904 (91.5%) | 327 (88.1%) | 352 (92.6%) | 285 (90.5%) |
| Black | 12 (1.2%) | 3 (0.8%) | 1 (0.3%) | 2 (0.6%) |
| Asian | 20 (2.0%) | 18 (4.9%) | 13 (3.4%) | 13 (4.1%) |
| Other | 23 (2.3%) | 14 (3.8%) | 12 (3.2%) | 12 (3.8%) |
| Missing | 29 (2.9%) | 9 (2.4%) | 2 (0.5%) | 3 (1.0%) |
| BMI (kg/m2) | ||||
| <18.5 | 20 (2.0%) | 5 (1.3%) | 3 (0.8%) | 4 (1.3%) |
| 18.5–24.99 | 364 (36.8%) | 137 (36.9%) | 159 (41.8%) | 123 (39.0%) |
| 25–29.99 | 293 (29.7%) | 108 (29.1%) | 115 (30.3%) | 85 (27.0%) |
| 30+ | 225 (22.8%) | 79 (21.3%) | 84 (22.1%) | 83 (26.3%) |
| Missing | 86 (8.7%) | 42 (11.3%) | 19 (5.0%) | 20 (6.3%) |
| COC use (years) | ||||
| <1 | 475 (48.1%) | 160 (43.1%) | 136 (35.8%) | 99 (31.4%) |
| 1–4.99 | 190 (19.2%) | 63 (17.0%) | 82 (21.6%) | 81 (25.7%) |
| 5–9.99 | 109 (11.0%) | 42 (11.3%) | 69 (18.2%) | 51 (16.2%) |
| 10+ | 115 (11.6%) | 31 (8.4%) | 62 (16.3%) | 58 (18.4%) |
| Missing | 99 (10.0%) | 75 (20.2%) | 31 (8.2%) | 26 (8.3%) |
| DMPA use | ||||
| Never | 709 (71.8%) | 220 (59.3%) | 218 (57.4%) | 133 (42.2%) |
| Ever | 5 (0.5%) | 3 (0.8%) | 88 (23.2%) | 117 (37.1%) |
| Missing | 274 (27.7%) | 148 (39.9%) | 74 (19.5%) | 65 (20.6%) |
| Endometriosis | ||||
| No | 769 (77.8%) | 253 (68.2%) | 336 (88.4%) | 264 (83.8%) |
| Yes | 51 (5.2%) | 19 (5.1%) | 18 (4.7%) | 25 (7.9%) |
| Missing | 168 (17.0%) | 99 (26.7%) | 26 (6.8%) | 26 (8.3%) |
| Family history of ovarian cancer | ||||
| No | 785 (79.5%) | 276 (74.4%) | 326 (85.8%) | 271 (86.0%) |
| Yes | 37 (3.7%) | 30 (8.1%) | 24 (6.3%) | 20 (6.3%) |
| Missing | 166 (16.8%) | 65 (17.5%) | 30 (7.9%) | 24 (7.6%) |
| Incomplete pregnancy | ||||
| No | 605 (61.2%) | 231 (62.3%) | 246 (64.7%) | 200 (63.5%) |
| Yes | 320 (32.4%) | 110 (29.6%) | 118 (31.1%) | 100 (31.7%) |
| Missing | 63 (6.4%) | 30 (8.1%) | 16 (4.2%) | 15 (4.8%) |
| Menopausal status | ||||
| Pre-menopause | 184 (18.6%) | 82 (22.1%) | 65 (17.1%) | 65 (20.6%) |
| Post-menopause | 757 (76.6%) | 262 (70.6%) | 303 (79.7%) | 236 (74.9%) |
| Missing | 47 (4.8%) | 27 (7.3%) | 12 (3.2%) | 14 (4.4%) |
| Menopausal hormone use | ||||
| Never use | 486 (49.2%) | 159 (42.9%) | 225 (59.2%) | 187 (59.4%) |
| ET use | 93 (9.4%) | 42 (11.3%) | 29 (7.6%) | 25 (7.9%) |
| EPT use | 155 (15.7%) | 51 (13.7%) | 67 (17.6%) | 55 (17.5%) |
| Other | 38 (3.8%) | 13 (3.5%) | 27 (7.1%) | 16 (5.1%) |
| Missing | 216 (21.9%) | 106 (28.6%) | 32 (8.4%) | 32 (10.2%) |
| Parity/Breastfeeding | ||||
| Nulliparous | 145 (14.7%) | 47 (12.7%) | 56 (14.7%) | 46 (14.6%) |
| Parous/never breastfed | 189 (19.1%) | 86 (23.2%) | 57 (15.0%) | 35 (11.1%) |
| Parous/breastfed | 405 (41.0%) | 108 (29.1%) | 124 (32.6%) | 74 (23.5%) |
| Missing | 249 (25.2%) | 130 (35.0%) | 143 (37.6%) | 160 (50.8%) |
| Smoking | ||||
| Never | 499 (50.5%) | 168 (45.3%) | 179 (47.1%) | 154 (48.9%) |
| Current | 134 (13.6%) | 30 (8.1%) | 44 (11.6%) | 26 (8.3%) |
| Former | 247 (25.0%) | 106 (28.6%) | 118 (31.1%) | 111 (35.2%) |
| Missing | 108 (10.9%) | 67 (18.1%) | 39 (10.3%) | 24 (7.6%) |
| Tubal ligation | ||||
| No | 629 (63.7%) | 234 (63.1%) | 208 (54.7%) | 156 (49.5%) |
| Yes | 192 (19.4%) | 71 (19.1%) | 70 (18.4%) | 52 (16.5%) |
| Missing | 167 (16.9%) | 66 (17.8%) | 102 (26.8%) | 107 (34.0%) |
Abbreviations: BMI: body mass index; COC: combined oral contraceptive; DMPA: depot medroxyprogesterone acetate; EPT: estrogen plus progestin therapy; ET: estrogen therapy; FIGO: International Federation of Gynecology and Obstetrics; NOS, not otherwise specified; SD: standard deviation
Table 3:
Association between clinical factors and year at diagnosis and having macroscopic residual disease after ovarian cancer primary cytoreductive surgery by calendar period of diagnosis (before 2007 vs 2007 or later)
| Diagnosed before 2007 N=1,359 |
Diagnosed in 2007 or later N=695 |
|||
|---|---|---|---|---|
| OR (95% CI)* | p-value | OR (95% CI)* | p-value | |
|
| ||||
| Age at diagnosis | ||||
| Every 5 years | 1.05 (0.96–1.16) | 0.30 | 1.06 (0.94–1.18) | 0.34 |
| Year at diagnosis | ||||
| Every calendar year | 1.00 (0.94–1.05) | 0.92 | 0.93 (0.83–1.04) | 0.18 |
| CA125 | ||||
| Every 200 units | 1.02 (1.00–1.03) | 0.063 | 1.00 (0.99–1.01) | 0.72 |
| FIGO stage | ||||
| IIIA+IIIB | 1.0 | 1.0 | ||
| III (NOS) | 5.75 (1.94–16.99) | 0.002 | 10.24 (0.89–117.77) | 0.062 |
| IIIC | 5.52 (3.45–8.84) | <0.001 | 4.96 (2.46–9.97) | <0.001 |
| IV | 10.50 (5.63–19.59) | <0.001 | 11.40 (4.94–26.32) | <0.001 |
| Grade | ||||
| Moderately differentiated | 1.0 | 1.0 | ||
| Poorly differentiated or undifferentiated | 0.94 (0.63–1.39) | 0.74 | 1.38 (0.69–2.77) | 0.36 |
Pooled results using the 20 imputed datasets.
Abbreviations: CI: confidence interval; FIGO: International Federation of Gynecology and Obstetrics; NOS, not otherwise specified; OR: odds ratio
Meta-analysis across the two calendar periods (patients diagnosed in 2007 or later compared to those diagnosed before 2007) showed no evidence of heterogeneity, with the exception of first-degree family history of ovarian cancer. First-degree family history of ovarian cancer was statistically significantly inversely associated with macroscopic residual disease in patients diagnosed before 2007 (OR=0.47, 95% CI 0.27–0.80; p=0.005), however in patients diagnosed in 2007 or later, the association was positive, but not statistically significant (OR=1.28, 95% CI 0.64–2.56, p=0.48; Table 4).
Table 4:
Association between the exposures of interest and having macroscopic residual disease after ovarian cancer primary cytoreductive surgery by calendar period of diagnosis (before 2007 vs 2007 or later)
| Diagnosed before 2007 N=1,359 | Diagnosed in 2007 or later N=695 | Meta-analysis** | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| OR (95% CI)* | p-value | OR (95% CI)* | p-value | OR (95% CI) | p-value | I 2 | |
|
| |||||||
| BMI (kg/m 2 ) | |||||||
| 18.5–24.99 | 1.0 | 1.0 | 1.0 | ||||
| 25–29.99 | 0.99 (0.71-1.38) | 0.95 | 1.04 (0.69-1.55) | 0.86 | 1.01 (0.78-1.30) | 0.95 | 0% |
| 30+ | 0.98 (0.68-1.41) | 0.91 | 0.78 (0.51-1.18) | 0.24 | 0.89 (0.67-1.17) | 0.39 | 0% |
| COC use (years) | |||||||
| <1 | 1.0 | 1.0 | 1.0 | ||||
| 1–4.99 | 1.06 (0.73-1.55) | 0.75 | 0.97 (0.61-1.54) | 0.89 | 1.02 (0.76-1.37) | 0.88 | 0% |
| 5–9.99 | 0.91 (0.58-1.44) | 0.70 | 1.02 (0.62-1.67) | 0.95 | 0.96 (0.69-1.34) | 0.81 | 0% |
| 10+ | 1.14 (0.69-1.89) | 0.60 | 0.89 (0.54-1.46) | 0.64 | 1.01 (0.71-1.43) | 0.97 | 0% |
| DMPA use | |||||||
| No | 1.0 | 1.0 | 1.0 | ||||
| Yes | 0.65 (0.14-2.92) | 0.57 | 0.92 (0.29-2.94) | 0.89 | 0.81 (0.33-2.00) | 0.64 | 0% |
| Endometriosis | |||||||
| No | 1.0 | 1.0 | 1.0 | ||||
| Yes | 1.03 (0.55-1.95) | 0.92 | 0.73 (0.36-1.47) | 0.38 | 0.88 (0.55-1.41) | 0.61 | 0% |
| First-degree family history of ovarian cancer | |||||||
| No | 1.0 | 1.0 | 1.0 | ||||
| Yes | 0.47 (0.27-0.80) | 0.005 | 1.28 (0.64-2.56) | 0.48 | 0.68 (0.45-1.04) | 0.075 | 80.5 % |
| Incomplete pregnancy | |||||||
| No | 1.0 | 1.0 | 1.0 | ||||
| Yes | 1.12 (0.84-1.50) | 0.44 | 1.00 (0.69-1.44) | 0.99 | 1.07 (0.85-1.35) | 0.55 | 0% |
| Menopausal status | |||||||
| Pre-menopausal | 0.84 (0.52-1.33) | 0.45 | 0.87 (0.48-1.56) | 0.64 | 0.85 (0.59-1.22) | 0.38 | 0% |
| Post-menopausal | 1.0 | 1.0 | 1.0 | ||||
| Menopausal hormone therapy use | |||||||
| Never | 1.0 | 1.0 | 1.0 | ||||
| EPT use only | 0.62 (0.39-0.98) | 0.42 | 0.77 (0.40-1.46) | 0.42 | 0.67 (0.46-0.97) | 0.033 | 0% |
| EPT use only | 0.92 (0.61-1.37) | 0.67 | 1.10 (0.69-1.76) | 0.69 | 0.99 (0.73-1.34) | 0.95 | 0% |
| Other | 0.99 (0.48-2.03) | 0.97 | 1.07 (0.51-2.24) | 0.86 | 1.02 (0.61-1.72) | 0.93 | 0% |
| Parity/Breastfeeding | |||||||
| Nulliparous | 1.0 | 1.0 | 1.0 | ||||
| Parous/never breastfed | 0.53 (0.33-0.87) | 0.013 | 0.85 (0.45-1.59) | 0.60 | 0.64 (0.43-0.94) | 0.022 | 21.9% |
| Parous/breastfed | 0.84 (0.55-1.31) | 0.45 | 0.98 (0.59-1.64) | 0.94 | 0.90 (0.65-1.25) | 0.53 | 0% |
| Smoking | |||||||
| Never | 1.0 | 1.0 | 1.0 | ||||
| Current | 1.50 (0.93-2.41) | 0.094 | 1.36 (0.74-2.48) | 0.32 | 1.44 (0.99-2.09) | 0.053 | 0% |
| Former | 0.78 (0.57-1.08) | 0.13 | 0.86 (0.59-1.26) | 0.44 | 0.81 (0.64-1.04) | 0.100 | 0% |
| Tubal ligation | |||||||
| No | 1.0 | 1.0 | 1.0 | ||||
| Yes | 0.93 (0.65-1.32) | 0.67 | 0.98 (0.61-1.59) | 0.94 | 0.95 (0.71-1.26) | 0.70 | 0% |
Models adjusted for age at diagnosis, race/ethnicity, education level, year of diagnosis, FIGO stage, grade, CA125, and OCAC study site.
Fixed odds ratios, 95% confidence intervals and p-values from meta-analyses across calendar period of diagnosis (before 2007 vs 2007 or later). ND=not done.
Abbreviations: BMI: body mass index; CI: confidence interval; COC: combined oral contraceptive; DMPA: depot medroxyprogesterone acetate; EPT: estrogen plus progestin therapy; ET: estrogen therapy; FIGO: International Federation of Gynecology and Obstetrics; OCAC: Ovarian Cancer Association Consortium; OR: odds ratio
ET use was statistically significantly associated with 33% lower odds of having macroscopic residual disease compared to never use (OR=0.67, 95% CI 0.46–0.97, p-value=0.033; Table 4). Compared to nulliparous women, parous women who did not breastfeed had 36% lower odds of having residual disease (OR=0.64, 95% CI 0.43–0.94, p=0.022), while there was no association among parous women who breastfed (OR=0.90, 95% CI 0.65–1.25, p=0.53). There was some suggestion that smoking was associated with having residual disease, but this relationship was only of borderline statistical significance. Compared to never smoking, current smoking was associated with increased odds of having macroscopic residual disease whereas former smoking was associated with reduced odds (OR=1.44, p=0.053, and OR=0.81, p=0.100; Table 4). None of the other exposures was associated with macroscopic residual disease following PCS (Table 4).
Discussion
We comprehensively examined the association between 12 lifestyle and personal factors and the presence of macroscopic residual disease after PCS for advanced stage HGSC. We found evidence for a role of ET and parity in residual disease after PCS. Previously, using data from OCAC we found that people who used menopausal hormone therapy for 5+ years were more likely to have no macroscopic residual disease after ovarian cancer surgery10, but we did not evaluate ET and EPT use separately. In the current analysis, we did not find an association between EPT use and having macroscopic residual disease.
The mechanism for the association between ET use and having macroscopic residual disease after PCS is unknown. This is unlikely due to access to care, since the majority of our patients were diagnosed at stage IIIC or IV (85.7%; Table 2). We have previously hypothesized that exposure to estrogen makes the tumor less adhesive to their neighbor tissues and thus easier to resect10. Estrogen promotes the epithelial-mesenchymal transition process, through which the tumor detaches from nearby tissues. Estrogen also promotes tumor mobility by regulating estrogen responsive genes that are related to survivin, cyclin D1, cyclin E and cathepsin D23. Additionally, estrogen may alter the anatomic distribution of disease within a given stage and thus improve resectability. Another possibility is ET might be related to resectability through inflammation. Inflammation may be associated with resectability because the cytokines secreted by immune cells during inflammatory reactions such as IL-6, TNF-α and CXCR2 promote angiogenesis and tumor growth24. Estrogen at high concentrations promotes an anti-inflammatory environment25, 26 and this milieu may make it easier to achieve no macroscopic residual disease. However, further studies are warranted to elucidate the precise mechanisms through which menopausal estrogen use is associated with resectability.
We found that parity was inversely associated with having macroscopic residual disease among people who did not breastfeed. Contrary to our results, two previous studies found that ovarian cancer patients who had residual disease (>1cm) after PCS had more births than women who were optimally debulked (residual disease ≤1cm)12, 14. However, the results from these studies were not adjusted for potential confounders, had a different outcome definition, and did not take breastfeeding into account. Parity is associated with high exposure to both estrogen and progesterone and it is possible that exposure of the pelvis and upper abdomen to these hormones creates an environment that ultimately makes HGSC easier to resect. It is also possible that the association between parity and the presence of residual disease after PCS among women who did not breastfeed was due to chance, given the lack of a clear underlying biologic mechanism and the number of hypotheses we tested. More studies are needed to explore the role of hormones in risk of residual disease after ovarian cancer PCS.
The observation that current smokers have higher odds of having macroscopic residual disease after PCS compared to never smokers could also be related to inflammation given that smoking leads to a pro-inflammatory environment. Former smokers had lower odds of having macroscopic residual disease after PCS compared to never smokers. This may be because people who quit smoking adopt healthier lifestyles, including diets27, which are associated with less inflammation. However, the associations between smoking and having residual disease following PCS were only of borderline statistical significance.
We found that a first-degree family history of ovarian cancer was statistically significantly associated with lower odds of having residual disease among patients diagnosed before 2007, but was associated with a higher odds among patients diagnosed in 2007 or later although this result was not statistically significant. This could be due to the smaller sample size among patients diagnosed in the later period. It is also possible that the proportion of BRCA mutation carriers is different among patients in the two calendar periods in our study. There is suggestive evidence that ovarian cancer patients with BRCA mutations are more likely to have cytoreduction to ≤1 cm residual disease compared to noncarriers28. However, we did not have information on who carried a pathogenic BRCA variant in our study population.
Strengths of the current study include the large sample size and the ability to adjust for confounders. However, we did not have information on surgical expertise, effort, complexity, or comorbidities which may impact surgical outcome. Most of the data in the OCAC studies came from patients who received care at large treatment centers where surgical expertise would be expected to be high. In addition, we adjusted for tumor stage in the analysis which could serve as a proxy for surgical complexity. However, we acknowledge that OCAC study site and tumor stage may not fully capture surgical expertise, effort, and complexity. Surgical expertise and effort could be effect modifiers. Our analysis included seven study sites, some of which are made up of multiple hospitals and surgeons. Further, the proportion of patients undergoing PCS as well as the proportion of patients with no macroscopic residual disease varies somewhat across study site (Table 1). However, our meta-analysis found no heterogeneity in the effect of menopausal hormone therapy, parity/breastfeeding, or smoking on the presence of macroscopic residual disease across study site. This suggests that the associations observed in this study are independent of surgical expertise or effort, primary cytoreductive surgery rate, and debulking rate, thus enhancing the generalizability of our findings. Also, because of the increase in the use of NACT over time, the participants who were recruited in early calendar years were likely materially different from those enrolled in later years. To address this issue, we stratified our analyses on calendar period and meta-analyzed the results across the two calendar periods. Lastly, a quarter of participants were excluded due to missing information on treatment sequence (PCS vs NACT). We did not impute this information because there was heterogeneity across the OCAC study sites in year, which could affect the treatment sequence selection.
In conclusion, our results suggest that parity among women who did not breastfeed and ET use are associated with lower odds of having macroscopic residual disease after ovarian cancer PCS. The association between ET use and having no macroscopic residual disease is plausible given a strong underlying biologic hypothesis between this exposure and diagnosis with ovarian cancer. If this finding or the parity association is replicated, these factors may be able to be included in prospective risk stratification models to determine whether HGSC patients should be managed with PCS or NACT. Future studies on the mechanisms of these associations are warranted.
Highlights.
Menopausal estrogen-only therapy use was associated with 33% lower odds of having macroscopic residual disease following PCS.
Being a current smoker may be associated with higher odds of having macroscopic residual disease following PCS.
These factors could be included in models to determine if patients should receive PCS or neoadjuvant chemotherapy.
Acknowledgements
We are grateful to the family and friends of Kathryn Sladek Smith for their generous support of the Ovarian Cancer Association Consortium through their donations to the Ovarian Cancer Research Fund. We thank the study participants, doctors, nurses, clinical and scientific collaborators, health care providers and health information sources who have contributed to the many studies contributing to this manuscript.
Acknowledgements for individual studies: AUS: The AOCS also acknowledges the cooperation of the participating institutions in Australia, and the contribution of the study nurses, research assistants and all clinical and scientific collaborators. The complete AOCS Study Group can be found at www.aocstudy.org. We would like to thank all of the women who participated in this research program; OPL: Members of the OPAL Study Group (http://opalstudy.qimrberghofer.edu.au/);
Funding
The Ovarian Cancer Association Consortium is supported by a grant from the Ovarian Cancer Research Fund thanks to donations by the family and friends of Kathryn Sladek Smith (PPD/RPCI.07). The scientific development and funding for this project were in part supported by the US National Cancer Institute GAME-ON Post-GWAS Initiative (U19-CA148112). This study made use of data generated by the Wellcome Trust Case Control consortium that was funded by the Wellcome Trust under award 076113. The results published here are in part based upon data generated by The Cancer Genome Atlas Pilot Project established by the National Cancer Institute and National Human Genome Research Institute (dbGap accession number phs000178.v8.p7).
Funding for individual studies: AUS: The Australian Ovarian Cancer Study (AOCS) was supported by the U.S. Army Medical Research and Materiel Command (DAMD17–01-1–0729), National Health & Medical Research Council of Australia (199600, 400413 and 400281), Cancer Councils of New South Wales, Victoria, Queensland, South Australia and Tasmania and Cancer Foundation of Western Australia (Multi-State Applications 191, 211 and 182). AOCS gratefully acknowledges additional support from Ovarian Cancer Australia and the Peter MacCallum Foundation; HAW: U.S. National Institutes of Health (R01-CA58598, N01-CN-55424 and N01-PC-67001); HOP: University of Pittsburgh School of Medicine Dean’s Faculty Advancement Award (F. Modugno), Department of Defense (DAMD17–02-1–0669) and NCI (K07-CA080668, R01-CA95023, P50-CA159981 MO1-RR000056 R01-CA126841); LAX: American Cancer Society Early Detection Professorship (SIOP-06–258-01-COUN) and the National Center for Advancing Translational Sciences (NCATS), Grant UL1TR000124; MAYO: National Institutes of Health (R01-CA122443, P30-CA15083, P50-CA136393); Mayo Foundation; Minnesota Ovarian Cancer Alliance; Fred C. and Katherine B. Andersen Foundation; Fraternal Order of Eagles; NEC: National Institutes of Health R01-CA54419 and P50-CA105009 and Department of Defense W81XWH-10–1-02802; OPL: National Health and Medical Research Council (NHMRC) of Australia (GNT1025142, GNT1120431).
TVG is a Senior Clinical Investigator of the Fund for Scientific Research-Flanders (FWO Vlaanderen 18B2921N). SJR is supported by National Health and Medical Research Council of Australia (NHMRC) grant APP2009840. PMW is supported by NHMRC GNT1173346. The contents of the published material are solely the responsibility of the authors and do not reflect the views of NHMRC, NIH and other funders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin 2022;72: 7–33. [DOI] [PubMed] [Google Scholar]
- 2.Peres LC, Cushing-Haugen KL, Anglesio M, Wicklund K, Bentley R, Berchuck A, Kelemen LE, Nazeran TM, Gilks CB, Harris HR, Huntsman DG, Schildkraut JM, et al. Histotype classification of ovarian carcinoma: A comparison of approaches. Gynecol Oncol 2018;151: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer Facts & Figures 2022ed. Atlanta: American Cancer Society, 2022. [Google Scholar]
- 4.Peres LC, Cushing-Haugen KL, Köbel M, Harris HR, Berchuck A, Rossing MA, Schildkraut JM, Doherty JA. Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage. J Natl Cancer Inst 2019;111: 60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elattar A, Bryant A, Winter‐Roach BA, Hatem M, Naik R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database Syst Rev 2011;2011: CD007565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang SJ, Hodeib M, Chang J, Bristow RE. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: a meta-analysis. Gynecol Oncol 2013;130: 493–8. [DOI] [PubMed] [Google Scholar]
- 7.Horowitz NS, Maxwell GL, Miller A, Hamilton CA, Rungruang B, Rodriguez N, Richard SD, Krivak TC, Fowler JM, Mutch DG, Van Le L, Lee RB, et al. Predictive Modeling for Determination of Microscopic Residual Disease at Primary Cytoreduction: An NRG Oncology/Gynecologic Oncology Group 182 Study. Gynecol Oncol 2018;148: 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eo W, Kim HB, Lee YJ, Suh DS, Kim KH, Kim H. Preoperative Lymphocyte-Monocyte Ratio Is a Predictor of Suboptimal Cytoreduction in Stage III-IV Epithelial Ovarian Cancer. J Cancer 2016;7: 1772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suidan RS, Ramirez PT, Sarasohn DM, Teitcher JB, Iyer RB, Zhou Q, Iasonos A, Denesopolis J, Zivanovic O, Long Roche KC, Sonoda Y, Coleman RL, et al. A multicenter assessment of the ability of preoperative computed tomography scan and CA-125 to predict gross residual disease at primary debulking for advanced epithelial ovarian cancer. Gynecol Oncol 2017;145: 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brieger KK, Peterson S, Lee AW, Mukherjee B, Bakulski KM, Alimujiang A, Anton-Culver H, Anglesio MS, Bandera EV, Berchuck A, Bowtell DDL, Chenevix-Trench G, et al. Menopausal hormone therapy prior to the diagnosis of ovarian cancer is associated with improved survival. Gynecol Oncol 2020;158: 702–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sananpanichkul P, Muangtan S, Suknikhom W, Bhamarapravatana K, Suwannarurk K. Does Endometriosis Hinder Successful Ovarian Debulking Surgery? Asian Pac J Cancer Prev 2018;19: 509–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin F, Lin K, Xie X, Zhou C. Increased ERCC1 protein expression is associated with suboptimal debulking in advanced epithelial ovarian cancer. Anticancer Res 2010;30: 2447–52. [PubMed] [Google Scholar]
- 13.Chesnais M, Lecuru F, Mimouni M, Ngo C, Fauconnier A, Huchon C. A pre-operative predictive score to evaluate the feasibility of complete cytoreductive surgery in patients with epithelial ovarian cancer. PLoS One 2017;12: e0187245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arab M, Jamdar F, Hosseini MS, Ghodssi-Ghasemabadi R, Farzaneh F, Ashrafganjoei T . Model for Prediction of Optimal Debulking of Epithelial Ovarian Cancer. Asian Pac J Cancer Prev 2018;19: 1319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janco JM, Glaser G, Kim B, McGree ME, Weaver AL, Cliby WA, Dowdy SC, Bakkum-Gamez JN. Development of a prediction model for residual disease in newly diagnosed advanced ovarian cancer. Gynecol Oncol 2015;138: 70–7. [DOI] [PubMed] [Google Scholar]
- 16.Collins LM, Schafer JL, Kam CM. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol Methods 2001;6: 330–51. [PubMed] [Google Scholar]
- 17.Jones NL, Chen L, Chatterjee S, Tergas AI, Burke WM, Hou JY, Ananth CV, Neugut AI, Hershman DL, Wright JD. National Trends in Extended Procedures for Ovarian Cancer Debulking Surgery. Int J Gynecol Cancer 2018;28: 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knisely AT, St Clair CM, Hou JY, Collado FK, Hershman DL, Wright JD, Melamed A. Trends in Primary Treatment and Median Survival Among Women With Advanced-Stage Epithelial Ovarian Cancer in the US From 2004 to 2016. JAMA Netw Open 2020;3: e2017517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, Verheijen RH, van der Burg ME, Lacave AJ, Panici PB, Kenter GG, Casado A, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010;363: 943–53. [DOI] [PubMed] [Google Scholar]
- 20.Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, Luesley D, Perren T, Bannoo S, Mascarenhas M, Dobbs S, Essapen S, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet 2015;386: 249–57. [DOI] [PubMed] [Google Scholar]
- 21.Onda T, Satoh T, Ogawa G, Saito T, Kasamatsu T, Nakanishi T, Mizutani T, Takehara K, Okamoto A, Ushijima K, Kobayashi H, Kawana K, et al. Comparison of survival between primary debulking surgery and neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in phase III randomised trial. Eur J Cancer 2020;130: 114–25. [DOI] [PubMed] [Google Scholar]
- 22.Fagotti A, Vizzielli G, Ferrandina G, Fanfani F, Gallotta V, Chiantera V, Costantini B, Margariti PA, Gueli Alletti S, Cosentino F. Survival analyses from a randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer with high tumor load (SCORPION trial), vol. 36: American Society of Clinical Oncology, 2018:5516. [Google Scholar]
- 23.Jeon SY, Hwang KA, Choi KC. Effect of steroid hormones, estrogen and progesterone, on epithelial mesenchymal transition in ovarian cancer development. J Steroid Biochem Mol Biol 2016;158: 1–8. [DOI] [PubMed] [Google Scholar]
- 24.Ono M. Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci 2008;99: 1501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martín-Millán M, Castañeda S. Estrogens, osteoarthritis and inflammation. Joint Bone Spine 2013;80: 368–73. [DOI] [PubMed] [Google Scholar]
- 26.Straub RH. The complex role of estrogens in inflammation. Endocr Rev 2007;28: 521–74. [DOI] [PubMed] [Google Scholar]
- 27.Alkerwi A, Baydarlioglu B, Sauvageot N, Stranges S, Lemmens P, Shivappa N, Hébert JR. Smoking status is inversely associated with overall diet quality: Findings from the ORISCAV-LUX study. Clin Nutr 2017;36: 1275–82. [DOI] [PubMed] [Google Scholar]
- 28.Hyman DM, Long KC, Tanner EJ, Grisham RN, Arnold AG, Bhatia J, Phillips MF, Spriggs DR, Soslow RA, Kauff ND, Levine DA. Outcomes of Primary Surgical Cytoreduction in Patients with BRCA-associated High-grade Serous Ovarian Carcinoma. Gynecol Oncol 2012;126: 224–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merritt MA, Green AC, Nagle CM, Webb PM, Australian Cancer Study (Ovarian Cancer), Australian Ovarian Cancer Study Group. Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. Int J Cancer 2008;122: 170–6. [DOI] [PubMed] [Google Scholar]
- 30.Beesley VL, Smith DD, Nagle CM, Friedlander M, Grant P, DeFazio A, Webb PM, OPAL Study Group. Coping strategies, trajectories, and their associations with patient-reported outcomes among women with ovarian cancer. Support Care Cancer 2018;26: 4133–42. [DOI] [PubMed] [Google Scholar]
- 31.Goodman MT, Lurie G, Thompson PJ, McDuffie KE, Carney ME. Association of two common single-nucleotide polymorphisms in the CYP19A1 locus and ovarian cancer risk. Endocr Relat Cancer 2008;15: 1055–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo-Ciganic WH, Zgibor JC, Bunker CH, Moysich KB, Edwards RP, Ness RB. Aspirin, nonaspirin nonsteroidal anti-inflammatory drugs, or acetaminophen and risk of ovarian cancer. Epidemiology 2012;23: 311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goode EL, Maurer MJ, Sellers TA, Phelan CM, Kalli KR, Fridley BL, Vierkant RA, Armasu SM, White KL, Keeney GL, Cliby WA, Rider DN, et al. Inherited determinants of ovarian cancer survival. Clin Cancer Res 2010;16: 995–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelemen LE, Sellers TA, Schildkraut JM, Cunningham JM, Vierkant RA, Pankratz VS, Fredericksen ZS, Gadre MK, Rider DN, Liebow M, Goode EL. Genetic variation in the onecarbon transfer pathway and ovarian cancer risk. Cancer Res 2008;68: 2498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terry KL, De Vivo I, Titus-Ernstoff L, Shih MC, Cramer DW. Androgen receptor cytosine, adenine, guanine repeats, and haplotypes in relation to ovarian cancer risk. Cancer Res 2005;65: 5974–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in this study are not publicly available due to limitations imposed by the original studies in which these data were collected. The corresponding author will facilitate access through existing data request processes for the OCAC.