Abstract
Protein secretion is essential for epithelial tissue homoeostasis and therefore has to be tightly regulated. However, while the mechanisms regulating polarized protein sorting and trafficking have been widely studied in the past decade, those governing polarized secretion remain elusive. The calcium manganese pump SPCA1 and the calcium-binding protein Cab45 were recently shown to regulate the secretion of a subset of soluble cargoes in nonpolarized HeLa cells. Interestingly, we demonstrated that in polarized epithelial cells calcium levels in the trans-Golgi network (TGN), controlled by SPCA1, and Cab45 are critical for the apical sorting of glycosylphosphatidylinositol-anchored proteins (GPI-APs), a class of integral membrane proteins containing a soluble protein attached to the membrane by the GPI anchor, prompting us to investigate the mechanism regulating the polarized secretion of soluble cargoes. By reducing Cab45 expression level or overexpressing an inactive mutant of SPCA1, we found that Cab45 and calcium levels in the TGN drive the polarized apical secretion of a secretory form of placental alkaline phosphatase, exogenously expressed, and the endogenous soluble protein clusterin/Gp80 in Madin–Darby canine kidney (MDCK) cells. These data highlight the critical role of a calcium-dependent Cab45 mechanism regulating apical exocytosis in polarized MDCK cells.
INTRODUCTION
Polarity of epithelial cells is essential for proper cellular functions and is often challenged in human diseases (Wilson, 1997; Mellman and Nelson, 2008). Polarized epithelial cells constitute a protective barrier but also serve as exchange interfaces. This paradigm results from their asymmetrical plasma membrane, which is physically separated by the apical junctional complexes into apical and basolateral domains that differ in protein and lipid composition and in functions (Yeaman et al., 1999; Mostov et al., 2003; Mostov, 2003; Rodriguez-Boulan et al., 2005; Rodriguez-Boulan and Macara, 2014; Mellman and Nelson, 2008; Eaton and Martin-Belmonte, 2014). Therefore, with respect to other cell types, epithelial cells need to properly sort proteins and lipids to the two surface domains in order to exert their functions. Epithelial cell polarity relies on a tight regulated polarized intracellular protein trafficking, secretion, recycling, and endocytosis (Mostov et al., 2003; Rodriguez-Boulan et al., 2005; Mellman and Nelson, 2008; Cao et al., 2012; Lebreton et al., 2018). Roughly one-third of all human proteins are following the conventional secretory pathway and are sorted at the level of the Golgi apparatus, the main sorting hub in cells, before reaching their final destination. The Golgi complex exhibits the capacity to handle membrane fluxes from both the secretory and the endocytic pathways but also to sort cargoes diverse in topology and size such as transmembrane proteins, glycosylphosphatidylinositol-anchored proteins (GPI-APs), and soluble proteins (Cao et al., 2012; Zurzolo and Simons, 2015; Ortega and Welling, 2018; Lebreton et al., 2019).
Transmembrane protein sorting relies on the specific recognition of signals decrypted by the cellular machinery. These signals either can be found in the primary sequence and/or can be a posttranslational modification such as glycosylation for instance (Ortega and Welling, 2018). Regarding GPI-APs, it was shown that clustering in Golgi relying on cholesterol levels regulates their apical sorting, and this in turn affects their organization at the apical surface and their biological activities (Paladino et al., 2014; Lebreton et al., 2018). More recently, we demonstrated that calcium ions, the transmembrane calcium/manganese pump, secretory pathway Ca2+-ATPase pump type 1 (SPCA1), and a Golgi resident calcium-binding protein, Cab45, are key players of the cellular machinery regulating the apical sorting of GPI-APs (Lebreton et al., 2021) without affecting trafficking of basolateral GPI-APs and of transmembrane proteins.
However, our knowledge regarding the mechanisms regulating the apical sorting of soluble cargoes in polarized epithelial cells is limited and more fragmented.
Secretion of soluble protein, depending on cell types, occurs through the constitutive or regulated secretory pathways. In the first case, proteins are secreted as fast as they are synthesized: after Golgi sorting they are incorporated into secretory vesicles that through fusion with the plasma membrane release their contents by exocytosis.
In secretory cells such as neurons and endocrine cells, protein exocytosis is governed by the storage of newly synthesized proteins in intracellular vesicles, named secretory storage granules, that release their content into the extracellular space only upon specific extracellular stimuli (Kelly, 1985; Nickel and Rabouille, 2009; Pompa et al., 2017). Several studies showed that a high calcium concentration and acidification are required for TGN protein aggregation, thereby favoring the incorporation of proteins in secretory granules (Kelly, 1985; Tooze and Huttner, 1990; Nickel and Rabouille, 2009; Pompa et al., 2017). More recently, studies in HeLa cells pointed out an interesting interplay between actin, calcium uptake, and sphingomyelin as the driving force for protein secretion at the TGN. Specifically, cofilin has been shown to rearrange the actin cytoskeleton that binds and favors the activation of SPCA1, allowing calcium ion uptake in the TGN. This in turn leads to oligomerization of the Golgi luminal calcium-binding protein Cab45, which favors the exocytosis of a subset of soluble cargoes such as the cartilage oligomeric matrix protein (COMP) or lysozyme C (LyzC) through direct interaction with them (Scherer et al., 1996; von Blume et al., 2011; Crevenna et al., 2016; Blank and von Blume, 2017; Pakdel and von Blume, 2018). It was further proposed that in addition to actin, sphingomyelin synthesis regulates SPCA1 activity into the lumen of the TGN required for the export of some soluble cargoes (Deng et al., 2018).
Interestingly, we recently demonstrated that calcium ions, SPCA1, and Cab45 have a major role in the apical sorting of GPI-APs in polarized Madin–Darby canine kidney (MDCK) cells, prompting us to assess here whether a calcium-dependent mechanism involving Cab45 could regulate the apical secretion of soluble cargoes in polarized epithelial cells.
For this purpose, we used as a model protein a truncated form of the native GPI-AP placental alkaline phosphatase (PLAP) devoid of its GPI-attachment signal, PLAP-sec, a physiologically relevant cargo whose altered secretion is the main phenotypic hallmark in the hyperphosphatasia mental retardation syndrome (HPMR) (Murakami et al., 2012), which was previously demonstrated to be apically secreted in thyroid polarized cells (Lipardi et al., 2000, 2002).
We demonstrate that Cab45 and SPCA1-regulated calcium levels in the trans-Golgi network (TGN) drive the polarized apical secretion of exogenously expressed PLAP-sec and of the endogenous soluble clusterin/Gp80 in MDCK cells.
RESULTS AND DISCUSSION
PLAP-sec is apically secreted in polarized MDCK cells
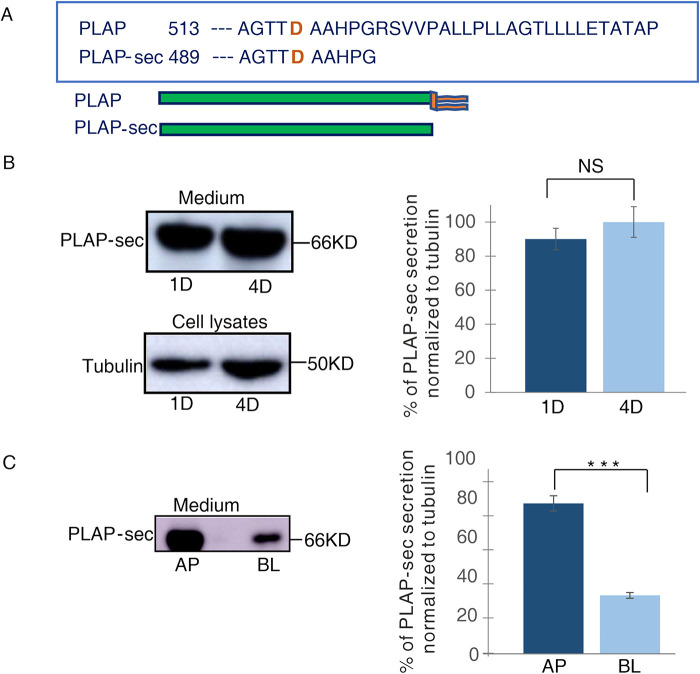
To decipher whether a calcium-dependent Cab45 mechanism regulates the apical secretion of soluble cargoes in epithelial cells, we monitored the secretion of the secretory form of the native GPI-AP PLAP, PLAP-sec (Figure 1A), in polarized MDCK cells. We generated MDCK clones stably expressing high PLAP-sec levels (Materials and Methods and Figure 1B) and analyzed by Western blot the secreted pool of PLAP-sec in the cellular medium (Figure 1B). As expected for constitutive secretory proteins, intracellular PLAP-sec is mainly enriched in the endoplasmic reticulum (ER) as evidenced by the colocalization with an ER marker (Pearson coefficient of 0.39 ± 0.1; Supplemental Figure 1A) by immunofluorescence.
FIGURE 1:
PLAP-sec is apically secreted in polarized MDCK cells. (A) Schematic representation of secretory PLAP-sec. (B) MDCK cells stably expressing PLAP-sec (MDCK PLAP-sec) cultured for 1 d (1D) or 4 d (4D) were left to secrete for 4 h before cellular media were collected to monitor PLAP-sec secretion. In parallel, intracellular tubulin levels were monitored. On the right quantification of PLAP-sec secretion in 1D and 4D normalized to intracellular tubulin in four different experiments is shown. No statistical differences were revealed. (C) MDCK PLAP-sec cultured for 4 d on filters were left to secrete for 4 h before the cellular media were collected to further monitor apical (AP) or basolateral (BL) PLAP-sec secretion. On the right, quantification from three different experiments, ***p < 0.001.
Next, we decided to monitor the rate of PLAP-sec secretion during epithelial polarization. We analyzed the amount of PLAP-sec in media collected after 1 or 4 d of culture in MDCK cells, conditions that mimic the unpolarized or polarized state, respectively (Paladino et al., 2014). Comparable levels of PLAP-sec secretion were observed at 1 or 4 d (Figure 1B), indicating that the rate of PLAP-sec secretion is independent of the acquisition of polarized phenotype. Next, we analyzed the polarity of secretion of PLAP-sec in MDCK cells grown for 4 d in filters and found that PLAP-sec protein is largely secreted (73% ± 5) in the apical medium (Figure 1C) as previously reported in thyroid FRT cells (Lipardi et al., 2000, 2002). These data suggest a mechanism common to epithelia notwithdstanding their organ origin, ensuring the apical secretion of PLAP-sec.
Apical secretion of PLAP-sec and clusterin/Gp80 is dependent on Cab45
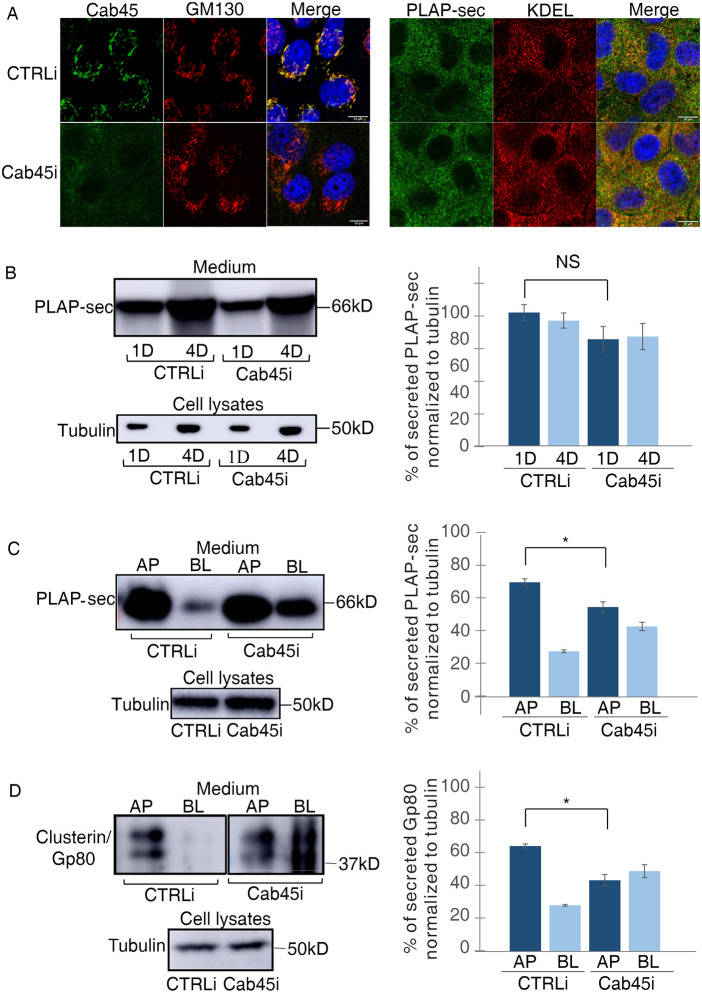
To evaluate whether Cab45 plays a role in the apical secretion of PLAP-sec, we generated stable MDCK PLAP-sec cells knocked down for Cab45 (MDCK PLAP-sec Cab45i) (Figure 2A, left panels). Quantification of the colocalization between PLAP-sec and an ER marker revealed that the intracellular PLAP-sec localization is unchanged in Cab45i cells compared with CTRLi MDCK cells (Figure 2A, right panels), suggesting that the reduction of Cab45 levels does not modify PLAP-sec intracellular localization (Pearson coefficient 0.37 ± 0.08% in CTRLi vs. 0.33 ± 0.09% in Cab45i) along the secretory pathway. We also found that the levels of PLAP-sec collected in the media of CTRLi and Cab45i cells at 1 and 4 d in culture are similar (Figure 2B), thus indicating that Cab45 knockdown does not affect the PLAP-sec secretion level.
FIGURE 2:
Cab45 regulates the apical secretion of exogenous PLAP-sec and endogenous clusterin/Gp80. (A) MDCK PLAP-sec knockdown for Cab45 (Cab45i) or not (CTRLi) plated on coverslip was used to reveal intracellular Cab45 and Golgi (GM130) (left panels) or PLAP-sec and ER (KDEL) (right panels). Scale bars, 10 μm. (B) Cellular media of 1 d (1D) and 4 d (4D) MDCK PLAP-sec CTRLi or Cab45i were collected as previously to monitor PLAP-sec secretion. On the right, quantification of PLAP-sec secretion normalized to intracellular tubulin levels. Experiments were performed three times. No statistical significance was observed. (C, D) Apical (AP) or basolateral (BL) media of MDCK PLAP-sec CTRLi or Cab45i plated on filters for 4 d was considered to monitor PLAP-sec secretion (C) or clusterin/Gp80 (D). On the right, quantification of AP and BL PLAP-sec (C) or clusterin/Gp80 (D) secretion normalized to intracellular tubulin levels. Experiments were performed six times; in both cases statistical significance in AP/BL secretion of PLAP-sec or clusterin/Gp80 was evaluated *, p < 0.05.
Next, we have investigated the effect of Cab45 knockdown on the polarity of the secretion. Importantly, we have previously shown that the polarized distribution of apical and basolateral proteins, GP114 and E-cadherin, respectively, were unaffected in Cab45 MDCK knockdown cells (Lebreton et al., 2021), indicating that Cab45 knockdown does not cause either polarization defects or alteration in the integrity of the epithelial monolayer. The levels of secreted PLAP-sec in the apical and basolateral media of MDCK cells grown on filters for 4 d were evaluated.
Similar to wild-type (WT) MDCK cells (Figure 1D), PLAP-sec was apically secreted (75% of secretion) in CTRLi cells, while it resulted secreted in an unpolarized manner in Cab45i cells, as it was largely found in the basolateral medium (46% in Cab45i cells compared with 25% in CTRLi cells; *, p < 0.05) (Figure 2C), thus highlighting the critical role of Cab45 in apical PLAP-sec polarized secretion.
To understand whether Cab45-dependent sorting may be a general mechanism driving the polarized trafficking of soluble cargoes, we monitored the secretion of the endogenous soluble clusterin/Gp80 protein in both CTRLi and Cab45i cells. While in CTRLi cells clusterin was mostly apically secreted as previously shown (Urban et al., 1987; Scheiffele et al., 1995), it became secreted in an unpolarized manner in Cab45i cells (70% apical in control cells vs. 47% in Cab45i cells; *, p < 0.05) (Figure 2D).
Next, to test whether Cab45 had a specific role in apical secretion, we analyzed the secretion of Wnt3a, a basolaterally secreted protein (Yamamoto et al., 2013). Therefore, we generated MDCK Wnt3a CTRLi and Cab45i and found that Wnt3a is still basolaterally secreted in Cab45i cells (Supplemental Figure S1, B–D), further supporting the specific role of Cab45 in the apical secretion of soluble cargoes.
Cab45 regulates the apical secretion of PLAP-sec without modifying its secreted and intracellular clustering state
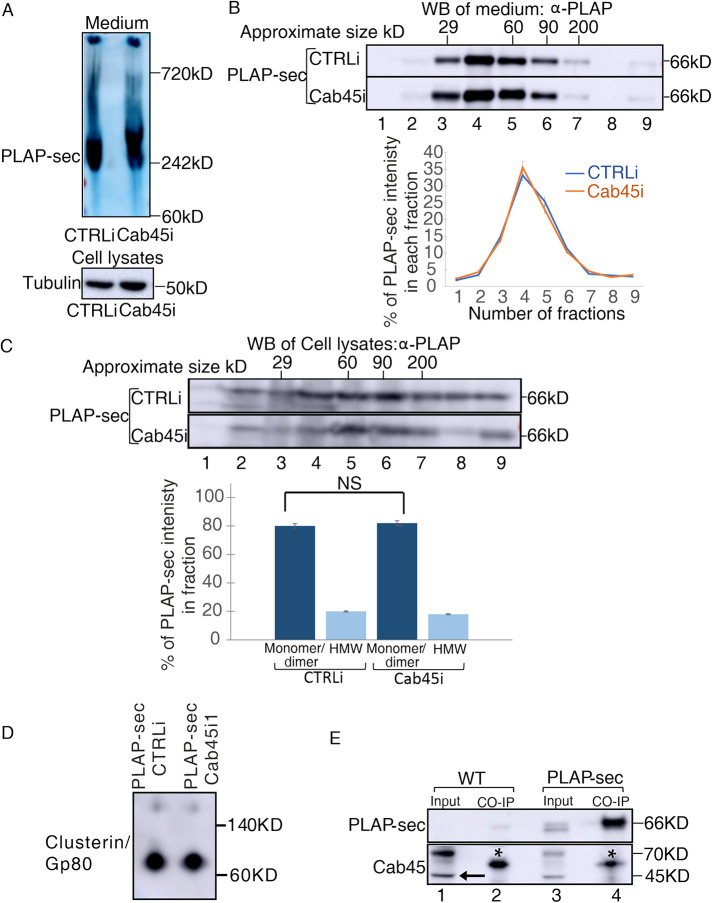
Cab45 is essential for clustering of GPI-APs in the Golgi of polarized MDCK cells to ensure their apical sorting (Lebreton et al., 2021) but also favors exocytosis of a subset of soluble cargoes in HeLa cells. However, in the case of secretory proteins in nonpolarized cells, it was shown that Cab45 directly binds to selective soluble cargoes; whether this leads to oligomerization and whether oligomerization is necessary for their secretion were not assessed. We analyzed the clustering state of secreted and intracellular PLAP-sec. Media of MDCK PLAP-sec CTRLi and Cab45i cells grown to confluence for 4 d on dishes were collected, and the aggregation state of PLAP-sec was evaluated by Native PAGE. We found that secreted PLAP-sec migrates around 240 kDa in both CTRLi and Cab45i cells (Figure 3A), indicating that it is mostly organized as trimers. Alternatively, we purified the collected media of CTRLi and Cab45i cells on velocity sedimentation gradients (Figure 3B), a technique that allows separating the proteins according to their molecular weight. Consistent with the Native-PAGE data, PLAP-sec was highly enriched in CTRLi in fractions 4, 5, and 6, corresponding to the molecular weight of the monomer/dimer (Figure 3B). A comparable PLAP-sec pattern on gradient was observed in Cab45i cells (Figure 3B, compare the distribution curves), thus indicating that Cab45 silencing did not affect the clustering state of secreted PLAP-sec.
FIGURE 3:
The loss of Cab45 does not affect secreted and intracellular PLAP-sec clustering. (A, B) Cellular media of MDCK PLAP-sec CTRLi and Cab45i cells plated for 4 d were analyzed by (A) Native PAGE or (B) velocity gradient where fractions collected from top (fraction 1) to bottom (fraction 9) were loaded on SDS–PAGE gel to monitor clustering of secreted PLAP-sec. The representative molecular weight markers are indicated. Native-PAGE experiments were performed three times and velocity gradient five times with the quantification shown on the right. (C) Cell lysates of MDCK PLAP-sec CTRLi or Cab45i cells seeded for 4 d were collected and run on velocity gradient (method). Fractions were collected and then trichloroacetic acid (TCA) precipitated and analyzed by SDS–PAGE with PLAP antibody. The experiment was repeated four times, and the quantification is shown. (D) Cell lysates of MDCK PLAP-sec CTRLi or Cab45i seeded for 4 d were run on Native PAGE to monitor the Gp80/clusterin. (E) Cell lysates of MDCK PLAP-sec or MDCK WT cells seeded for 4 d were immunoprecipitated with PLAP antibody together with the protein A Sepharose beads and revealed with either anti-PLAP (top) or anti-Cab45 (bottom) antibodies. Aliquots of cell lysates (1: input; 2: pull down) were loaded. *, most probably heavy chain of immunoglobulin G. Note that no interaction could be monitored between PLAP-sec and Cab45.
Next, we investigated whether intracellular PLAP-sec could cluster and whether Cab45 could play a role in this organization. Lysates of MDCK PLAP-sec CTRLi and Cab45i cells plated for 4 d were analyzed by velocity gradients. In both cases, about 20% of intracellular PLAP-sec was found in high-molecular-weight complexes, indicating that intracellular PLAP-sec clustering is independent of Cab45. Similarly, the state of clusterin/Gp80 was unaffected upon Cab45 knockdown (Figure 3D). Indeed, as previously reported (Urban et al., 1987), it migrates as an 80 kDa band in a Native PAGE in both CTRLi and Cab45i cells (Figure 3D), thus excluding an effect of Cab45 on its clustering state.
Overall, these data also reinforce that Cab45 regulates the apical sorting of GPI proteins and the apical secretion of soluble cargoes by two distinct molecular mechanisms, dependent on and independent of clustering, respectively.
We next wonder whether Cab45 would directly bind to PLAP-sec as it does in HeLa Cells (von Blume et al., 2012). To this end we performed coimmunoprecipitation assays in MDCK PLAP-sec or MDCK WT cell extracts using PLAP antibody as previously performed (Paladino et al., 2014). Then, PLAP-sec immunoprecipitates were revealed by Western blot using a specific anti-Cab45 antibody. Nonetheless, PLAP-sec is enriched in the eluted immunoprecipitates (Figure 3E, top gel, compare lane 3, input, with lane 4, pull down); we could not detect coimmunoprecipitation between PLAP-sec and endogenous Cab45 (Figure 3E, bottom gel). These data may imply that 1) Cab45 does not interact with PLAP-sec or, alternatively, 2) their interaction is transient or indirect.
Altogether our data indicate that Cab45 is essential for the apical secretion of soluble cargoes in polarized MDCK cells but its action is not through a clustering mechanism of the cargo.
Overexpression of SPCA1 mutant impairing calcium uptake in the Golgi leads to unpolarized secretion of PLAP-sec and clusterin in polarized MDCK cells
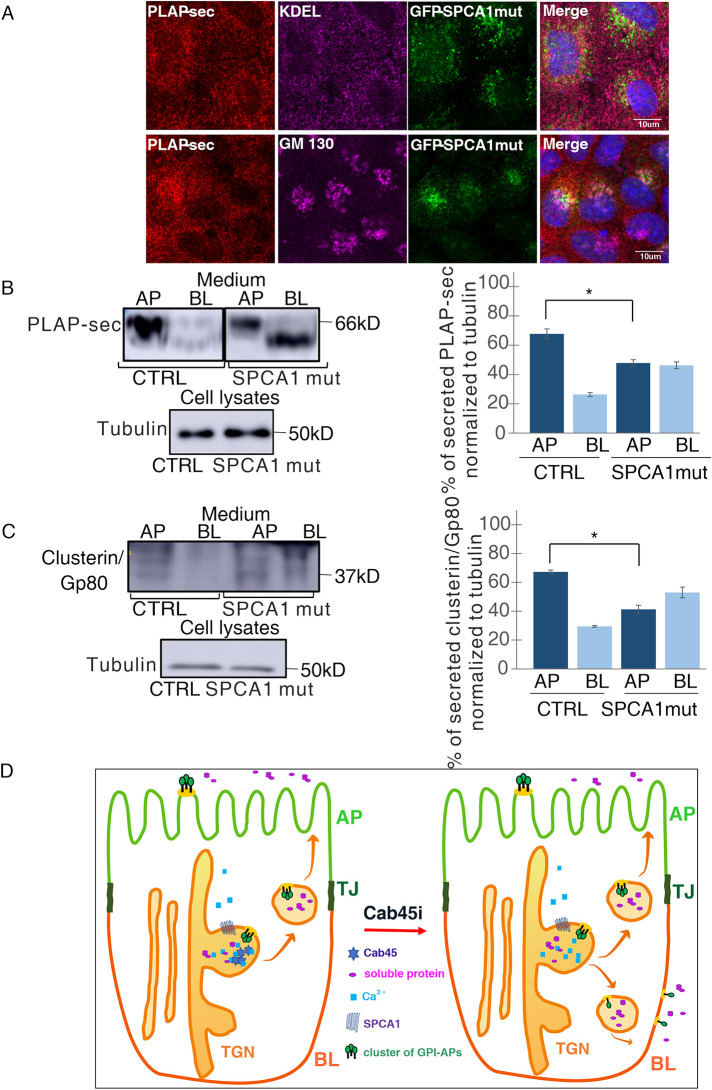
Because Cab45 is a calcium-binding protein, we next asked whether calcium levels in the Golgi would regulate the apical secretion of PLAP-sec in polarized MDCK cells as it has been reported earlier to be essential for the exocytosis of some soluble cargoes in HeLa cells (von Blume et al., 2012; Crevenna et al., 2016). In cells stably overexpressing a GFP-SPCA1 mutant (SPCA1 mut), which impairs calcium uptake in the Golgi (Kienzle and von Blume, 2014), intracellular localization of PLAP-sec remained unchanged as evidenced by similar Pearson coefficients between PLAP-sec and ER marker or PLAP-sec and GM130 (Figure 4A) (as, respectively, 0.39 ± 1 and 0.18 ± 0.06 vs. 0.31 ± 0.07 and 0.2 ± 0.07). The same cells were grown on filters for 4 d, and then the medium from apical and basolateral chambers were collected after 4 h in serum-free medium as aforementioned. Interestingly, we found that the overexpression of the SPCA1 mutant leads to unpolarized secretion of PLAP-sec and clusterin/Gp80 (Figure 4, B and C), further highlighting the critical role of functional SPCA1 and therefore of calcium in the secretion of apical soluble cargoes in polarized MDCK cells. Of interest, basolaterally secreted PLAP-sec in SPCA1 mut cells migrates at a lower molecular weight compared to control cells, suggesting a difference of N-glycosylation state of PLAP-sec (Catino et al., 2008).
FIGURE 4:
Overexpression of SPCA1 mutant impairing calcium uptakes leads to unpolarized secretion of PLAP-sec and clusterin/Gp80. (A) MDCK PLAP-sec stably expressing the GFP-SPCA1 mutant seeded on coverslip was used to reveal intracellular PLAP-sec localization and ER marker (top) or Golgi marker (bottom). (B, C) AP and BL media of MDCK PLAP-sec CTRL or the overexpressing GFP-SPCA1 mutant plated on filters for 4 d were collected to monitor PLAP-sec secretion (B) or clusterin/Gp80 (C). On the right, quantification of AP and BL PLAP-sec (B) or clusterin/Gp80 (C) secretion normalized to intracellular tubulin levels. Experiments were performed three times; in both cases the statistical significance in AP/BL secretion of PLAP-sec or clusterin/Gp80 was evaluated *, p < 0.05. (D) Model of calcium-dependent Cab45 mechanisms regulating apical secretion of soluble cargoes and apical sorting of GPI-APs. Cab45 plays a crucial role in the apical sorting of GPI-APs (in green) and in the apical secretion of soluble cargoes (in pink) through clustering-dependent and -independent mechanisms, respectively.
GPI-APs are integral membrane proteins exhibiting peculiarity in their sorting and trafficking because they contain beside the protein portion, the glycolipid anchor that confers high affinity for cholesterol and sphingolipid membrane microdomains. Thus, although the same molecular factors govern apical sorting of GPI-APs and secretion of soluble cargoes (Figure 4D), the underlying mechanisms are not superimposable as evidenced by the regulation of clustering mechanisms. It is intriguing that the same molecular machinery involving SPCA1 and Cab45 regulates the apical sorting of both GPI-APs and soluble cargoes, but with different downstream effects on the clustering state of the protein.
In HeLa cells, SPCA1 or Cab45 knockdown leads to the retention of soluble cargoes in the Golgi apparatus (von Blume et al., 2012), but this is not the case in polarized MDCK cells, highlighting a possible different mechanism in the two cells lines of different tissue origins (Paladino et al., 2014). Interestingly, an increased expression of Cab45 in a cancer cell line exhibiting higher metastatic potential was reported (Luo et al., 2016). In addition, in cervical and esophageal human cancer samples the expression of Cab45 correlates with the expression of matrix metalloproteinase 2, MMP2, essential to remodel the extracellular matrix during cancer cell migration (Luo et al., 2016). Finally, Cab45 was reported to be a positive regulator of epithelial–mesenchymal transition and cancer migration (Luo et al., 2016), thus suggesting a potential critical role of Cab45 in tumorigensis. Furthermore, secretory proteins in tumor tissues are important components of the tumor microenvironment (Patel et al., 2018; Baghban et al., 2020) as cancer cells display their own secretome (Karagiannis et al., 2010; Baghban et al., 2020). Hence, it will be very interesting to evaluate the link between Cab45 and cancer and in particular how secretion of soluble cargoes may change when the epithelial polarity is lost through epithelial-mesenchymal transition in MDCK cells as previously described (Sampaio et al., 2011; Zuccarini et al., 2017; Pastula and Lundmark, 2021).
MATERIALS AND METHODS
Request a protocol through Bio-protocol.
Cell cultures, transfections, and antibodies
MDCK cells were cultured in DMEM (Sigma-Aldrich; D6429) containing 5% fetal calf serum.
The cDNA encoding PLAP-sec was a gift of Andre Le Bivic (Evolution and Morphogenesis of Epithelia UMR 7288 Case 907 - Parc Scientifique de Luminy 13288 Marseille Cedex 9 – France) and was previously described (Berger et al., 1989; Lipardi et al., 2000).
MDCK cells stably expressing PLAP-sec were generated by transfecting the cDNA encoding PLAP-sec using lipofectamine 2000. After 48 h transfection, a serial dilution of the cells in 96-well plates (0.5–1 cell/well) was performed and then the cells were selected with G418 (800 µg/ml; Gibco/Fisher; 10131-019). Several clones have been produced, and because we obtained exactly the same unpolarized secretions of PLAP-sec and clusterin/Gp80 in Cab45I and SPCA1mut cells, we decided to show results coming from one clone only.
To silence Cab45 expression, MDCK PLAP-sec cells were infected using the lentiviral vector pRFP-CB-sh sequence TGTGAATACTGACCGGAAGATAAGCGCCA (Origene) or with a noneffective 29-mer scrambled short hairpin RNA cassette in the same p-RFP-CB-sh lentiviral vector (ref. TR30033) as negative control. After infection for 24 h, stable clones were selected by using blasticidin (10 µg/ml). Screening of positive clones was carried out by analyzing RFP fluorescence (Lebreton et al., 2021). The SPCA1 mutant is a kind gift of Julia von Blume (Yale University, New Haven, CT; Kienzle and von Blume, 2014). The stable MDCK PLAP-sec–overexpressing SPCA1 mutant was produced by retroviral infection as previously described (Morita et al., 2000). The antibodies used for biochemistry were as follows: polyclonal anti-PLAP 1:1000 (from Rockland), polyclonal Wnt3a 1:1000 (Cell Signaling), polyclonal apoliprotein J 1:1000 (Thermo Fisher), and polyclonal Cab45 1:1000 from the J. V. Blume laboratory. The secondary antibodies considered were rabbit (GE Healthcare UK; NA934V) and mouse (Cytiva; NXA931). Regarding immunostaining, polyclonal KDEL to reveal ER 1:500 (from ABR Affinity/Ozyme; SPA-827), polyclonal GM130 to reveal cis/medial Golgi 1:500 (from DB Transduction; 610823), and the same PLAP 1:500 and Cab45 1:500 antibody. The secondary antibodies Alexa Fluor 488 rabbit (A11034), Alexa Fluor 633 rabbit (A21070), Alexa Fluor 546 rabbit (A11035), Alexa Fluor 546 mouse (A11030), Alexa Fluor 633 mouse (A21050), and Alexa Fluor 488 mouse (A11029) were purchased from Thermo Fisher Scientific. Monoclonal anti-PLAP 1:1000 (Sigma-Aldrich; A2951) was used in the immunoprecipitation experiment.
Immunofluorescence
MDCK cells grown on coverslips for 1 or 4 d were washed with phosphate-buffered saline (PBS) containing CaCl2 and MgCl2, fixed with 4% paraformaldehyde for 20 min, and quenched with 50 mM NH4Cl for 10 min. After saturation and permeabilization of the cells for 30 min in PBS containing CaCl2/MgCl2, 0.2% gelatin, 0.075% saponin, cells were incubated for 30 min with specific antibodies in permeabilized conditions. Primary antibodies were detected with Alexa 488 or 546 (Thermo Fisher Scientific). The images were acquired using a laser scanning confocal microscope (LSM 700; Carl Zeiss MicroImaging) equipped with a Plan Apo 63× oil immersion (NA1.4) objective lens.
Medium collection
MDCK cells stably expressing PLAP-sec were seeded on polyester filters (2 million) with 0.4 μm pores (Corning; 3450) for 4 d or on 10 cm culture dishes (1 million) for 1 or 4 d. Afterward, cells were washed twice with serum-free medium and then incubated in serum-free medium (Sigma-Aldrich; D1145) for 4 h at 37°C. Then, the medium from each condition was collected and concentrated by 10 KD Vivaspin6 (Dutscher; 28-9322-96), which allows the passage of proteins above 10 kDa. In parallel, the corresponding cells were scraped in lysis buffer (NaCl 150 mM, Tris 20 mM, EDTA 5 mM, Triton-100 1%). The same volume of cell lysates was taken to detect the intracellular PLAP-sec and tubulin as loading control.
Western blot
Cell lysates or concentrated cellular medium with protein inhibitor (Roche; 11836170001) were reduced in Laemmli buffer containing 5% β-mercaptoethanol and boiled at 100°C for 5 min before being run on different concentration of SDS–polyacrylamide gels: 1) 4–12% Bis-Tris gels (BioRad; 3450124) or 2) 8% acrylamide/bisacrylamide gel. The gel was run using MOPS buffer (BioRad; 161-0788) or TGS buffer (BioRad; 1610732) at 90 V constant. Proteins were transferred on polyvinylidene difluoride membranes (Dutscher; 10600023). Next, the membrane was incubated in blocking solution containing powdered milk and then with primary and secondary antibodies. Finally, membranes were revealed using the ECL prime reagent (Dutscher; RPN2236). To determine the apparent molecular weights of the protein bands, a PageRuler plus prestained protein ladder (Thermo Fisher Scientific; 26619) was used.
Native PAGE
Cellular medium was collected and concentrated using Vivaspin before supplementation with 5% G-250 native sample buffer additive (Thermo Fisher Scientific; BN2004), protein inhibitor and native sample buffer (Thermo Fisher Scientific; BN2003). Then proteins were loaded under nondenaturing conditions in Native Bis-Tris PAGE, 3–12% (Thermo Fisher Scientific; BN1001) and run at 200 V. The Native-PAGE protein ladder (Thermo Fisher Scientific; Invitrogen; LC0725) was loaded and stained using Coomassie blue. Proteins were transferred onto a polyvinylidene fluoride membrane for 90 min at 90 V and then the membrane was immersed in 6% acetic acid for 5 min, air dried, and washed with 100% methanol. Finally, membranes were blocked in 4% bovine serum albumin (BSA) (Sigma-Aldrich; A9647) in Tris-Buffered Saline (TBS) overnight at 4°C and incubated with the primary PLAP antibody overnight at 4°C before being incubated with secondary antibodies and revealed as mentioned earlier.
Velocity gradients
Velocity gradients, a biochemical method that allows purifying proteins according to their molecular weight, were performed as previously published (Tivodar et al., 2006). Briefly, cells were lysed in 20 mM Tris, pH 7.4, 100 mM NaCl, 0. 2% TX-100, with 0.4% SDS. Cells were scraped and cell lysates were sheared through a 26-gauge needle or the concentrated medium were layered on top of a discontinuous glycerol gradient (20–40%) in the same buffer containing 0.2% TX-100. After centrifugation at 45,000 rpm for 16 h in a Beckman SW50 ultracentrifuge, fractions of 500 μl were harvested from the top of the gradient and trichloroacetic acid–precipitated. The proteins were revealed by Western blot using specific antibodies.
Immunoprecipitation and coimmunoprecipitation assays
Cells were lysed in JS buffer (NaCl 150 mM, Tris 20 mM, EDTA 5 mM, Triton-100 0.5%, SDS 0.1%, BSA 0.2%) containing protease inhibitor cocktail for 20 min and scraped from the dishes. After nucleus pelleting, lysates were precleared with protein A Sepharose beads (5 mg/sample; Cytiva; 17-0780-01) for 1 h at 4°C and then incubated with PLAP antibody (Sigma-Aldrich; A2951) 1:1000 overnight at 4°C. Afterward, protein A Sepharose beads were incubated with cell lysate for 2 h at 4°C. The pellets were washed three times with cold buffer (washing: twice with complete JS, twice with buffer 3, and twice with buffer 4), centrifuged for 2 min at 14,000 rpm for each wash, then boiled with Laemmli buffer, run on SDS–PAGE, and revealed with either PLAP or Cab45 antibodies. MDCK WT cells were used as negative control.
Statistical analysis
Image analyses were performed by using ImageJ, and then a t test was used to address the statistical significance.
Supplementary Material
Acknowledgments
We thank Julia Von Blume for sharing the SPCA1 mutant DNA and Akira Kikuchi for sharing with us MDCK Wnt3a cells. We also warmly thank Christel Brou for her critical reading of the manuscript. Our work was supported by the Agence Nationale de la Recherche (ANR-20-CE13-0032), the China Scholarship Council (PhD grant to D. L.), and the program Explore de l’Institut Pasteur.
Abbreviations used:
- ADF/cofilin
actin-filament severing protein
- Ca 2+
calcium
- Cab45
45kDa Calcium binding protein
- Cab45i
Cab45 interfered cells
- cDNA
complementary DNA
- COMP
cartilage oligomeric matrix protein
- CTRL
control
- CTRLi
scrambled interfered cells
- 1D
1d (day)
- 4D
4 d (day)
- DMEM
Dulbecco’s modified eagle medium
- ECL
enchanced chemiluminescence
- EMT
epithelial-to-mesenchymal Transition
- ER
endoplasmic reticulum
- FCS
fetal bovine serum
- GFP
green fluorescent protein
- GM130
130 kDa cis Golgi matrix protein
- Gp80
glycoprotein 80/clusterin
- GPI-APs
glycosylphosphatidylinositolanchored
- GPI
glycosyl phosphatidyl inositol
- HPMR
hyperphosphatasia mental retardation syndrome
- IF
immunofluorescence
- IP
immunoprecipitation proteins
- KDEL
Lys-Asp-Glu-Leu
- KRB
modified Krebs-Ringer buffer
- LyzC
Lysozyme C
- MDCK
Madin-Darby canine kidney
- mRNA
messenger RNA
- PLAP-sec
secretory form of PLAP
- PLAP
placental alkaline phosphatase
- SDS–PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SDS
sodium dodecyl sulfate
- ShRNA
short hairpin RNA
- SPCA1
secretory pathway Ca 2+ -ATPase pump type 1
- TGN
trans Golgi network
- WB
western blot
- Wnt
wingless/int conserved family of secreted proteins
- Wnt11
Wnt family member 11
- WT
wild-type
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E22-12-0549) on May 10, 2023.
REFERENCES
- Baghban R, Roshangar L, Jahanban-Esfahlan R, Seidi K, Ebrahimi-Kalan A, Jaymand M, Kolahian S, Javaheri T, Zare P (2020). Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal 18, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J, Micanovic R, Greenspan RJ, Udenfriend S (1989). Conversion of placental alkaline phosphatase from a phosphatidylinositol-glycan-anchored protein to an integral transmembrane protein. Proc Natl Acad Sci USA 86, 1457–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank B, von Blume J (2017). Cab45—unraveling key features of a novel secretory cargo sorter at the trans-Golgi network. Eur J Cell Biol 96, 383–390. [DOI] [PubMed] [Google Scholar]
- Cao X, Surma MA, Simons K (2012). Polarized sorting and trafficking in epithelial cells. Cell Res 22, 793–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catino MA, Paladino S, Tivodar S, Pocard T, Zurzolo C (2008). N- and O-glycans are not directly involved in the oligomerization and apical sorting of GPI proteins. Traffic 9, 2141–2150. [DOI] [PubMed] [Google Scholar]
- Crevenna AH, Blank B, Maiser A, Emin D, Prescher J, Beck G, Kienzle C, Bartnik K, Habermann B, Pakdel M, et al. (2016). Secretory cargo sorting by Ca2+-dependent Cab45 oligomerization at the trans-Golgi network. J Cell Biol 213, 305–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Pakdel M, Blank B, Sundberg EL, Burd CG, von Blume J (2018). Activity of the SPCA1 calcium pump couples sphingomyelin synthesis to sorting of secretory proteins in the trans-Golgi network. Dev Cell 47, 464–478.e468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton S, Martin-Belmonte F (2014). Cargo sorting in the endocytic pathway: a key regulator of cell polarity and tissue dynamics. Cold Spring Harb Perspect Biol 6, a016899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis GS, Pavlou MP, Diamandis EP (2010). Cancer secretomics reveal pathophysiological pathways in cancer molecular oncology. Mol Oncol 4, 496–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RB (1985). Pathways of protein secretion in eukaryotes. Science 230, 25–32. [DOI] [PubMed] [Google Scholar]
- Kienzle C, von Blume J (2014). Secretory cargo sorting at the trans-Golgi network. Trends Cell Biol 24, 584–593. [DOI] [PubMed] [Google Scholar]
- Lebreton S, Paladino S, Liu D, Nitti M, von Blume J, Pinton P, Zurzolo C (2021). Calcium levels in the Golgi complex regulate clustering and apical sorting of GPI-APs in polarized epithelial cells. Proc Natl Acad Sci USA 118, e2014709118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebreton S, Paladino S, Zurzolo C (2019). Clustering in the Golgi apparatus governs sorting and function of GPI-APs in polarized epithelial cells. FEBS Lett 593, 2351–2365. [DOI] [PubMed] [Google Scholar]
- Lebreton S, Zurzolo C, Paladino S (2018). Organization of GPI-anchored proteins at the cell surface and its physiopathological relevance. Crit Rev Biochem Mol Biol 53, 403–419. [DOI] [PubMed] [Google Scholar]
- Lipardi C, Nitsch L, Zurzolo C (2000). Detergent-insoluble GPI-anchored proteins are apically sorted in fischer rat thyroid cells, but interference with cholesterol or sphingolipids differentially affects detergent insolubility and apical sorting. Mol Biol Cell 11, 531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipardi C, Ruggiano G, Perrone L, Paladino S, Monlauzeur L, Nitsch L, Le Bivic A, Zurzolo C (2002). Differential recognition of a tyrosine-dependent signal in the basolateral and endocytic pathways of thyroid epithelial cells. Endocrinology 143, 1291–1301. [DOI] [PubMed] [Google Scholar]
- Luo J, Li Z, Zhu H, Wang C, Zheng W, He Y, Song J, Wang W, Zhou X, Lu X, et al. (2016). A novel role of Cab45-G in mediating cell migration in cancer cells. Int J Biol Sci 12, 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Nelson WJ (2008). Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol 9, 833–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita S, Kojima T, Kitamura T (2000). Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther 7, 1063–1066. [DOI] [PubMed] [Google Scholar]
- Mostov K, Su T, ter Beest M (2003). Polarized epithelial membrane traffic: conservation and plasticity. Nat Cell Biol 5, 287–293. [DOI] [PubMed] [Google Scholar]
- Mostov KE (2003). Epithelial polarity and morphogenesis. Methods 30, 189–190. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Kanzawa N, Saito K, Krawitz PM, Mundlos S, Robinson PN, Karadimitris A, Maeda Y, Kinoshita T (2012). Mechanism for release of alkaline phosphatase caused by glycosylphosphatidylinositol deficiency in patients with hyperphosphatasia mental retardation syndrome. J Biol Chem 287, 6318–6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W, Rabouille C (2009). Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol 10, 148–155. [DOI] [PubMed] [Google Scholar]
- Ortega B, Welling PA (2018). Chapter 45—molecular mechanisms of polarized protein trafficking in epithelial cells. In: Physiology of the Gastrointestinal Tract, 6th ed., ed. Said H.M., Irvine, CA: Academic Press, 1027–1050. [Google Scholar]
- Pakdel M, von Blume J (2018). Exploring new routes for secretory protein export from the trans-Golgi network. Mol Biol Cell 29, 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladino S, Lebreton S, Tivodar S, Formiggini F, Ossato G, Gratton E, Tramier M, Coppey-Moisan M, Zurzolo C (2014). Golgi sorting regulates organization and activity of GPI proteins at apical membranes. Nat Chem Biol 10, 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastula A, Lundmark R (2021). Induction of epithelial-mesenchymal transition in MDCK II cells. Bio Protoc 11, e3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel H, Nilendu P, Jahagirdar D, Pal JK, Sharma NK (2018). Modulating secreted components of tumor microenvironment: a masterstroke in tumor therapeutics. Cancer Biol Ther 19, 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompa A, De Marchis F, Pallotta MT, Benitez-Alfonso Y, Jones A, Schipper K, Moreau K, Zarsky V, Di Sansebastiano GP, Bellucci M (2017). Unconventional transport routes of soluble and membrane proteins and their role in developmental biology. Int J Mol Sci 18, 703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Kreitzer G, Musch A (2005). Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol 6, 233–247. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Macara IG (2014). Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol 15, 225–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio JL, Gerl MJ, Klose C, Ejsing CS, Beug H, Simons K, Shevchenko A (2011). Membrane lipidome of an epithelial cell line. Proc Natl Acad Sci USA 108, 1903–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Peranen J, Simons K (1995). N-glycans as apical sorting signals in epithelial cells. Nature 378, 96–98. [DOI] [PubMed] [Google Scholar]
- Scherer PE, Lederkremer GZ, Williams S, Fogliano M, Baldini G, Lodish HF (1996). Cab45, a novel (Ca2+)-binding protein localized to the Golgi lumen. J Cell Biol 133, 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tivodar S, Paladino S, Pillich R, Prinetti A, Chigorno V, van Meer G, Sonnino S, Zurzolo C (2006). Analysis of detergent-resistant membranes associated with apical and basolateral GPI-anchored proteins in polarized epithelial cells. FEBS Lett 580, 5705–5712. [DOI] [PubMed] [Google Scholar]
- Tooze SA, Huttner WB (1990). Cell-free protein sorting to the regulated and constitutive secretory pathways. Cell 60, 837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J, Parczyk K, Leutz A, Kayne M, Kondor-Koch C (1987). Constitutive apical secretion of an 80-kD sulfated glycoprotein complex in the polarized epithelial Madin-Darby canine kidney cell line. J Cell Biol 105, 2735–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Blume J, Alleaume AM, Cantero-Recasens G, Curwin A, Carreras-Sureda A, Zimmermann T, van Galen J, Wakana Y, Valverde MA, Malhotra V (2011). ADF/cofilin regulates secretory cargo sorting at the TGN via the Ca2+ ATPase SPCA1. Dev Cell 20, 652–662. [DOI] [PubMed] [Google Scholar]
- von Blume J, Alleaume AM, Kienzle C, Carreras-Sureda A, Valverde M, Malhotra V (2012). Cab45 is required for Ca(2+)-dependent secretory cargo sorting at the trans-Golgi network. J Cell Biol 199, 1057–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PD (1997). Epithelial cell polarity and disease. Am J Physiol 272, F434–F442. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Awada C, Hanaki H, Sakane H, Tsujimoto I, Takahashi Y, Takao T, Kikuchi A (2013). The apical and basolateral secretion of Wnt11 and Wnt3a in polarized epithelial cells is regulated by different mechanisms. J Cell Sci 126, 2931–2943. [DOI] [PubMed] [Google Scholar]
- Yeaman C, Grindstaff KK, Nelson WJ (1999). New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev 79, 73–98. [DOI] [PubMed] [Google Scholar]
- Zuccarini M, Giuliani P, Buccella S, Di Liberto V, Mudo G, Belluardo N, Carluccio M, Rossini M, Condorelli DF, Rathbone MP, et al. (2017). Modulation of the TGF-beta1-induced epithelial to mesenchymal transition (EMT) mediated by P1 and P2 purine receptors in MDCK cells. Purinergic Signal 13, 429–442. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zurzolo C, Simons K (2015). Glycosylphosphatidylinositol-anchored proteins: membrane organization and transport. Biochim Biophys Acta 1858, 632–639. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.