Abstract
Pulmonary fibrosis is a chronic progressive interstitial lung disease caused by a variety of etiologies. The disease can eventually lead to irreversible damage to the lung tissue structure, severely affecting respiratory function and posing a serious threat to human health. Currently, glucocorticoids and immunosuppressants are the main drugs used in the clinical treatment of pulmonary fibrosis, but their efficacy is limited and they can cause serious adverse effects. Traditional Chinese medicines have important research value and potential for clinical application in anti-pulmonary fibrosis. In recent years, more and more scientific researches have been conducted on the use of traditional Chinese medicine to improve or reduce pulmonary fibrosis, and some important breakthroughs have been made. This review paper systematically summarized the research progress of pharmacological mechanism of traditional Chinese medicines and their active compounds in improving or reducing pulmonary fibrosis. We conducted a systematic search in several main scientific databases, including PubMed, Web of Science, and Google Scholar, using keywords such as idiopathic pulmonary fibrosis, pulmonary fibrosis, interstitial pneumonia, natural products, herbal medicine, and therapeutic methods. Ultimately, 252 articles were included and systematically evaluated in this analysis. The anti-fibrotic mechanisms of these traditional Chinese medicine studies can be roughly categorized into 5 main aspects, including inhibition of epithelial-mesenchymal transition, anti-inflammatory and antioxidant effects, improvement of extracellular matrix deposition, mediation of apoptosis and autophagy, and inhibition of endoplasmic reticulum stress. The purpose of this article is to provide pharmaceutical researchers with information on the progress of scientific research on improving or reducing Pulmonary fibrosis with traditional Chinese medicine, and to provide reference for further pharmacological research.
Keywords: Pulmonary fibrosis, Traditional Chinese medicine, Anti-inflammation, Anti-oxidation, Endoplasmic reticulum stress
Introduction
Pulmonary fibrosis (abbreviated as PF) is a chronic, progressive, irreversible interstitial lung disease commonly caused by multiple etiologies and characterized by the accumulation of inflammatory cells such as macrophages, neutrophils and lymphocytes in the alveoli, the proliferation and differentiation of fibroblasts, and the development of fibrous connective tissue. Ultimately, it leads to structural changes in the patient's normal lung tissue [1, 2]. In the late stages of many pulmonary diseases, PF is a common pathological manifestation. In modern medicine, interstitial lung disease is divided into two types: secondary interstitial lung disease and idiopathic interstitial lung disease. The former has a relatively clear etiology, including silicosis, pneumoconiosis, asbestosis, radiation-induced PF, and drug-induced interstitial lung disease. In contrast, the etiology of the latter is unknown, including idiopathic pulmonary fibrosis (abbreviated as IPF), cystic fibrosis, and interstitial pneumonia with autoimmune features, among which IPF is the most important [1, 3]. Due to IPF's wide involvement, long course, high mortality rate, and other characteristics, it is also known as a tumor-like disease [2]. Unfortunately, the prognosis for patients with IPF is often unfavorable, as they typically suffer from significant lung function impairment and a reduced quality of life during recovery [4, 5]. Epidemiological surveys have shown that the prevalence and incidence of IPF is increasing year by year and is more prevalent in the elderly. The mortality rate of IPF is high, and more than half of patients with IPF who were hospitalized for acute exacerbations will die during their hospitalization, and their average survival period after diagnosis is 2 to 4 years [6–8]. Notably, following the outbreak of COVID-19 in 2019, clinical observations have identified fibrosis in the lungs of patients with COVID-19 [9–11]. If PF is not controlled promptly and effectively, it will lead to a decline in lung function and seriously affect the quality of life and life expectancy of patients [12]. At present, glucocorticoids and Immunosuppressive drug, such as pirfenidone and nifanyl, are the main clinical treatments for PF, but their clinical efficacy is not satisfactory, and these drugs are also expensive and have many side effects [13–15]. In recent years, traditional Chinese medicines (abbreviated as TCM) has made great progress in improving or reducing of PF, and has become one of the alternative therapies for clinical treatment of IPF due to its significant efficacy and few side effects [16–18]. Of particular interest is that in the battle against COVID-19, TCM has shown the advantages of high efficiency and low toxicity in lung injury caused by the novel coronavirus and in the prognosis of rehabilitation, playing an important role in blocking the progression of PF and promoting the recovery of patients [19–21]. In this review article, we focus on the research progress of TCM in improving PF from a pharmacological perspective. Firstly, we summarized the research progress on the pharmacological mechanisms related to PF. Secondly, we systematically summarized the potential active compounds in TCM that can be used to improve PF, and classified the targets of these compounds. Finally, future research directions were envisioned. The following search terms were used: IPF, PF, interstitial pneumonia, natural product, herbs. The time limit is from June 2017 to June 2023. We did a systematic search of several major scientific databases, including PubMed, Web of Science, and Google Scholar. A total of 252 articles were retrieved. At that time, the focus was on screening original experimental articles that matched the theme, totaling 184 articles. We evaluated these literature and systematically reviewed the research progress of TCM in improving PF in the past five years.
Pathogenesis of pulmonary fibrosis
The pathogenesis of PF is not yet fully elucidated, but it is known to be caused by a variety of factors: for example, PF due to silica inhalation [22], PF induced by chemicals, such as bleomycin, paraquat [23, 24], and induced by different primary diseases [25].
Modern medicine generally agrees that fibrosis can be described as an irrational form of injury repair [26, 27]. Repeated microdamage to the alveolar epithelium is the first driving factor that induces an abnormal repair process in which persistent alveolar epithelial cell damage and repair abnormalities, proliferation of fibroblasts, and accumulation of extracellular matrix (abbreviated as ECM) lead to structural disorders in the lung and the formation of fibrosis [28–30]. In the early stages of PF, there are different influencing factors, but in the later stages of fibrosis, there are the same mechanisms of action [31, 32]. The pathogenesis of PF can be roughly divided into three stages: injury, inflammation, repair. The first stage: the lung is damaged or otherwise noxiously stimulated and fibroblasts are activated and begin to secrete ECM. This phase is disease-specific and it consists mainly of lymphocyte activation and differentiation, autoimmune and immune-mediated conditions of excessive immune response, and chronic granulomatous inflammation. This is due to the persistence of identified or unidentified antigens, or other exposures. These multiple environmental risk factors, such as smoking, occupational exposure, air pollution, toxic compounds, viral infections, can repeatedly damage alveolar cells [2, 33, 34]. The second stage: mitogen-activated protein kinase (abbreviated as MAPK) and nuclear factor kappa B (abbreviated as NF-κB) pathways are activated to promote the production of a large number of cytokines [35]. Activated fibroblasts undergo structural and phenotypic changes and produce a large amount of ECM [36]. Through paracrine, inflammatory cells, including macrophages, move to the stimulated site. T cells are activated to secrete fibrogenic growth factors, such as interleukin and tumor necrosis factor alpha (abbreviated as TNF-α) [37]. Macrophages promote the proliferation and differentiation of fibroblasts and secrete a variety of cytokines, including transforming growth factor beta (abbreviated as TGF-β) and Interleukins-1 (abbreviated as IL-1) [38]. The third phase: the injury factors persisted, resulting in repeating damage of alveolar epithelial cells. Fibroblasts continue to be activated to produce more ECM [39–41]. Cytokines continue to cause tissue inflammation and collagen overexpression, ECM deposition, the beginning of a vicious circle, and finally lead to the gradual formation of PF, the loss of lung function at the idiopathic site [42–44].
Pulmonary fibrosis-related signaling pathways
TGF-β/Smad
Transforming growth factor-β/Smad (TGF-β/Smad) is a pleiotropic signaling pathway that plays a key role in inflammation, wound healing, fibrosis processes such as epithelial injury, myofibroblast fibroblast proliferation and differentiation and ECM production [45, 46]. TGF-β exerts its biological activity through activation of Smad-dependent and non-dependent pathways. The Smad protein family can be divided into three categories: receptor-activated (R-Smads, including Smads 1, 2, 3, 5, and 8), general-purpose (Co-Smad, including Smad 4), and inhibitory (I-Smads, including Smad 6 and 7) [47]. The TGF-β receptor is a receptor that belongs to the group with endogenous Ser/Thr kinase activity and binds to its type I and type II receptors to form a complex that leads to phosphorylation of Smad2 and Smad3 [48]. The phosphorylated Smad2 and Smad3 then further form a complex with Smad4, which undergoes nuclear translocation, activating the expression of transcription factors downstream of Epithelial-mesenchymal transition (EMT) and promoting EMT [49]. TGF-β1 also activates MAPK, phosphoinositide 3-kinase/protein kinase B pathway, and Rho pathways, induces EMT, increases the expression of collagen, fibronectin, and tissue inhibitor of matrix metalloproteinases (TIMPs), and promotes PF. Recent studies have shown that numerous active substances of natural products can improve PF through the TGF-β/Smad signaling pathway [50].
Nrf2/ARE
When the organism is damaged by external oxidative and chemical stimuli and other stresses, it generates corresponding self-defense responses and induces a series of protective proteins. The Nuclear Factor erythroid 2-Related Factor 2/antioxidant response element (Nrf2/ARE) pathway is a classical defensive transduction pathway that can reduce the oxidative stress damage occurring in cells [51]. Nrf2 is a key factor in the cellular oxidative stress response, with antioxidant, anti-inflammatory response and cytoprotective effects [52]. Nrf2 is a key transcription factor that is essentially expressed in oxidative stress and is present in multiple organs throughout the body, and its deletion or impaired activation directly causes changes in cellular sensitivity to stressors changes in the sensitivity of cells to stressors [53, 54]. The Nrf2/ARE pathway in the lung mainly regulates the expression of antioxidant genes, thus providing protection to lung tissue [55]. When cells are attacked by reactive oxygen species or other electrophile reagents in a state of oxidative stress, Nrf2 is uncoupled from keap1 and translocated across the membrane into the nucleus. By regulating ARE activity, it further initiates the transcription of downstream regulatory antioxidant proteins and phase II detoxification enzymes, and regulates the expression of various antioxidant genes, thereby increasing the production of antioxidant substances, reducing cellular oxidative damage and maintaining intracellular redox homeostasis, thus playing an antioxidant and anti-fibrotic role [55]. By activating the Nrf2/ARE signaling pathway, the synthesis of antioxidant proteins can be increased, and thus the body’s enhanced antioxidant capacity can be achieved to delay the progression of PF [56]. The Nrf2/ARE pathway can also mediate a variety of antioxidant enzymes and phase II detoxification enzymes to protect tissues.
PI3K/AKT
The phosphoinositide 3-kinase/protein kinase B pathway (PI3K/AKT) is one of the central intracellular signaling pathways regulating cell growth, proliferation, motility, metabolism and survival [57, 58]. PI3K is a signal transduction enzyme that phosphorylates PI (4,5) P2 to form PI (3,4,5) P3, which is activated by tyrosine kinase receptors, G protein-coupled receptors/cytokine receptors and Ras protein-associated GD Pase receptors to promote cell proliferation, survival, adhesion, differentiation, cytoskeleton organization, etc. [59, 60]. Protein kinase B (AKT) is a serine/threonine kinase downstream of PI3k, and AKT binds to PI(3,4,5) P3 near the cell membrane to form a complex. The binding of the complex to 3-phosphoinositide-dependent protein kinase 1 promotes the phosphorylation of the PH domain at the amino acid terminus of AKT, which activates downstream factors such as hypoxia-inducible factor-1 and mammalian target of rapamycin (mTOR) to participate in cell proliferation and differentiation [59, 60]. AKT is a direct target protein downstream of PI3K, which can participate in regulating cell proliferation and metabolism, promoting fibrosis-related gene transcription and protein synthesis, and activated AKT can participate in PF by activating mTOR [61]. AKT2-deficient mice can counteract bleomycin (BLM)-induced PF and inflammation, suggesting that PI3K/AKT signaling plays an important role in IPF development [62]. In addition, activation of PI3K/AKT can be involved in the development of PF by regulating its downstream genes such as mTOR, hypoxia-inducible factor-1 and Fox family [63]. It is due to the important role of PI3K/AKT in regulating receptor-mediated signaling that targeting PI3K/AKT has become a promising strategy for the treatment of IPF.
Wnt/β-catenin
Wnt signaling pathway can be divided into classical Wnt signaling pathway (i.e. Wnt/β-catenin signaling pathway) and non-classical Wnt signaling pathway. Among them, β-catenin is the key molecule that mediates classical Wnt signaling. Wnt proteins are a group of secreted glycoproteins expressed in a variety of tissue cells, involved in a variety of signaling pathways, and play a key role in cell differentiation, cell migration, organogenesis, stem cell self-renewal and maintenance of tissue homeostasis. β-catenin is a cytoskeletal protein that, together with E-cadherin and α-catenin, is involved in the construction of cell junctions and intercellular adhesion mechanisms, and plays an important role in maintaining the stability of the intracellular environment and signaling into the nucleus [64, 65].
The Wnt/β-catenin signaling pathway plays an important role in many pathological processes in the lung and is one of the major regulatory pathways in PF [66]. In pulmonary endothelial cells Wnt/β-catenin signaling causes a shift from perivascular fibroblasts to myofibroblast-like cells, leading to ECM accumulation and increased tissue stiffness, further promoting PF [67]. Downregulation of the Wnt signaling pathway also inhibited myofibroblast differentiation, thereby ameliorating PF lesions [68]. The Wnt/β-catenin signaling pathway was significantly activated in the IPF animal model [69], and blockade of the Wnt/β-catenin pathway was also effective in attenuating lung fibrosis in mice [70].
It has been shown that Wnt/β-catenin signaling is involved in the induction of EMT during the development of fibrosis [71]. Wnt1, Wnt3A, Wnt7B, Wnt10B, Fzd2, Fzd3 and β-catenin expression were significantly increased in lung tissues of IPF patients [72]. Wnt5A and Wnt5B ligands have been reported to exert effects on pulmonary fibroblast differentiation via TGF-β [73]. The Wnt/β-catenin pathway also interacts with the TGF-β/Smad, PI3K/AKT signaling pathway and plays an important role in the pathogenesis of IPF, and inhibition of this pathway can reduce or reverse PF [74].
NF-κB
NF-κB is one of the major nuclear transcription factors that regulate inflammatory and immune responses, as well as a signaling pathway that is present in many cell types and closely associated with intracellular biological functions and inflammatory responses [75, 76]. In addition, NF-κB binds to fixed nucleotide sequences in the promoter regions of many genes to initiate gene transcription, which plays a crucial role in the inflammatory response, the regulation of the immune system, and cell growth [77, 78]. Five transcription factors comprise the NF-κB family: NF-κB1 (p50), NF-κB2 (p52), Rel A (p65), Rel B, and c-Rel [79]. NF-κB proteins function as dimers that bind to the κB site and affect the transcription of target genes [80]. The phosphorylation of NF-κB is responsible for activating the NF-κB signaling pathway [81]. Inflammation is now believed to be one of the factors contributing to fibrosis [82–84], and during PF, NF-κB is activated, which promotes the release of large amounts of inflammatory factors such as TNF-α, IL-1β, IL-8 and TGF-β1 [85], stimulating the proliferation of fibroblasts and the deposition of collagen fibers, thereby promoting the development of organ fibrosis [86]. Several studies [87, 88] have elucidated the crucial role of the NF-κB signaling pathway in regulating acute lung injury-induced PF. NF-κB also plays a key role in the secretion of pro-fibrotic cytokines during the progression of PF [89]. In addition to inflammation, cellular senescence is an important factor leading to fibrosis that can promote the development of IPF through a variety of mechanisms, such as senescence associated secretory phenotype (SASP), telomere dysfunction, etc. [90, 91]. The NF-κB signaling pathway is a key regulator of SASP, according to [92]. SASP has been shown to be inhibited by the inhibition or knockdown of multiple components that regulate NF-κB signaling [93].
Cytokines related to pulmonary fibrosis
Transforming growth factor β
Transforming Growth Factor β (TGF-β) has been implicated as a central factor in the development of PF [94–96]. TGF-β has several biological roles, such as promoting wound repair by increasing ECM deposition, inflammatory cell recruitment and fibroblast differentiation [97, 98]. TGF-β1 is currently recognized as the most critical fibrogenic factor and the most potent promoter of ECM deposition ever identified. It has been shown in the literatures that the TGF-β1/Smad signaling pathway is activated during PF [99, 100], prompting the conversion of fibroblasts to myofibroblasts and alveolar epithelial and endothelial cells to mesenchymal cells. In addition, activation of the TGF-β1/Smad signaling pathway reduces the secretion and inhibits the activity of matrix protein metallases, while increasing the synthesis and secretion of matrix metalloproteinase tissue inhibitory factor, which inhibits myofibroblast apoptosis and leads to the production of large amounts of ECM and its failure to degrade properly, allowing it to accumulate in the lung and cause PF [36, 101].
Platelet-derived growth factor
Platelet-derived growth factor (PDGF) is a peptide-like regulatory factor stored mainly in platelets, and when PF occurs, epithelial cells, alveolar macrophages and activated platelets will secrete large amounts of PDGF [102]. PDGF is closely related to the proliferation and differentiation of lung fibroblasts [103]. PDGF promotes the formation and development of PF by promoting the proliferation, migration and aggregation of lung fibroblasts, as well as regulating the synthesis and deposition of ECM. Therefore, PDGF is known as an energizing factor for fibroblast proliferation [104, 105]. In the process of PF, PDGF is mainly produced at the site of lung tissue injury, and TGF-β1 and TNF can regulate the expression of PDGF. On the one hand, PDGF can cross the cell membrane barrier through the damaged lung epithelial cells into the alveolar mesenchyme and chemotactic mesenchymal cells; On the other hand, similar to the function of TGF-β1, it can also induce fibroblast proliferation and differentiation and stimulate fibroblasts to secrete collagen, but the mechanism of action may be different from that of TGF-β1 [106]. It has been found that increased release of PDGF is observed in the lungs of IPF patients and that blocking PDGF receptor signaling in animal models of IPF attenuates the extent of PF [107].
Interleukins
Interleukins (IL) are a class of cytokines produced mainly by lymphocytes, monocytes or macrophages and act on a variety of cells. They are complex in structure and function and play an important role in a range of processes including immune regulation and inflammation in lung tissue [108, 109]. Thirty-eight species have been named, of which approximately one third are involved in the development of PF. During PF, IL-1, IL-4, IL-6, IL-11, and IL-13 play important roles in promoting proliferation and aggregation of pulmonary fibroblasts, ECM deposition, collagen synthesis, and lung tissue remodeling [110, 111]. Among them, IL-13 has a significant effect on tissue fibrosis. It has been shown that IL-4 and IL-13 can synergistically exert activation effects on M2 type macrophages, and the activated M2 type macrophages secrete pro-fibroblastic cytokines thereby promoting the development of fibrosis [112]. IL-7, IL-10, IL-12, and IL-18 reduce PF by inhibiting inflammatory factors and modulating immunity [113–117]. Among them, IL-10 may activate macrophages through the CCL2/CCR2 axis, causing fibroblast accumulation and eventually causing fibrous degeneration [118].
Tumor necrosis factor-α
Tumor necrosis factor-α (TNF-α) is a multi-temporal, cellular immune defense factor produced by mononuclear macrophages. TNF-α is highly expressed in the pathological process of lung injury and can participate in the local injury and inflammatory response, leading to the aggregation of inflammatory cells, which in turn stimulates massive proliferation of lung fibroblasts and secretion of collagen. Therefore, TNF-α is one of the important indicators for clinical testing of PF. On the one hand, TNF-α is involved in the process of acute inflammatory response and inhibits the repair of lung injury by promoting apoptosis of type II alveolar cells [119]. On the other hand, TNF-α promotes the differentiation of lung resident mesenchymal stem cells into myofibroblasts [120]. In the early stages of PF, macrophages aggregate, synthesize and release large amounts of TNF-α. TNF-α stimulates a massive increase in chemotactic and adhesion molecules, creating an inflammatory cell infiltrate. Therefore, TNF-α is also known as an early response factor [121]. In addition, TNF-α can act synergistically with IL-1 to promote neutrophil activation and aggregation and regulate the inflammatory response in the early stages of PF [122]. TNF-α acts mainly through the NF-κB pathway, and its mechanism may be related to the Wnt/β-catenin signaling pathway. There are NF-κB binding sites on the transcriptional promoter region or enhancer of the TNF-α gene, and the two promote each other and jointly regulate the development of PF [123].
Pharmacological mechanism of TCM in improving pulmonary fibrosis
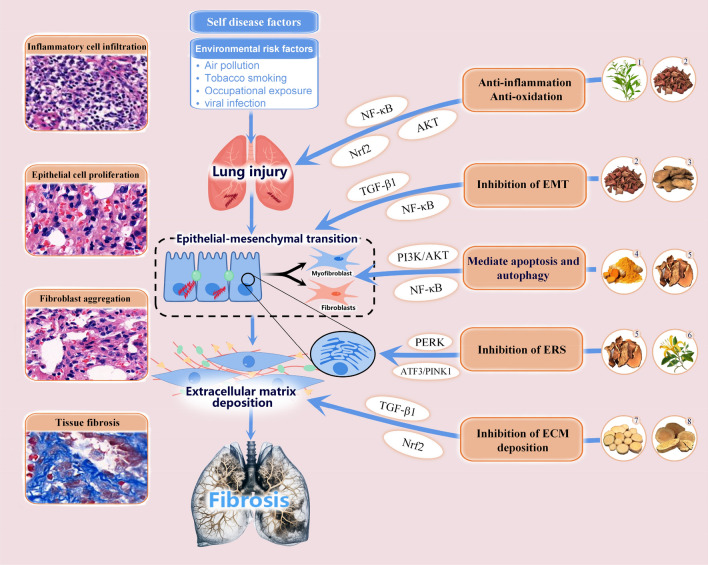
TCM has demonstrated significant clinical efficacy and unique advantages in improving IPF. PF is closely related with TCM terminologies such as ‘lung obstruction’, ‘lung atrophy’, and ‘lung abscess'. TCM mainly employs a dialectical treatment approach from the perspectives of tonifying the lung and kidney, invigorating the spleen and lung, and promoting blood circulation and removing blood stasis. In recent years, research on TCM therapy for PF has been increasing, and significant progress has been made in some aspects. In this section, we reviewed recent literatures and summarized that the mechanisms by which TCM improving PF can be roughly divided into five categories: inhibiting EMT, anti-inflammatory and anti-oxidative stress, improving ECM deposition, mediating cell apoptosis and autophagy, and inhibiting endoplasmic reticulum stress (ERS), a simple classification information was shown in Fig. 1.
Fig. 1.
Schematic diagram of the main intervention targets of traditional Chinese medicine against pulmonary fibrosis
Inhibition of epithelial cell-mesenchymal transformation
EMT is the process by which epithelial cells lose cellular activity through a specific procedure and are then transformed into mesenchymal cells. EMT is the main source of fibroblasts and myofibroblasts in IPF [124, 125]. It has been shown that approximately 33% of myofibroblasts in the lung can be traced to cells undergoing EMT in a bleomycin-induced lung fibrosis model [126]. EMT is mainly divided into 3 types: type I EMT is mainly related to normal physiological activities of cells; type II EMT is mainly related to injury repair, tissue regeneration and organ fibrosis; type III EMT is related to tumor metastasis [127, 128]. Among them, type II EMT is mainly caused by persistent inflammation and injury, regulated by a variety of signaling factors and signaling pathways, and is an important mechanism for the occurrence and development of PF, which can be treated by inhibiting EMT progression. There is growing evidence that EMT is closely associated with fibroblast activation and PF, characterized by increased expression of α-SMA and vimentin and decreased expression of the intercellular adhesion molecule E-cadherin. Therefore, inhibition of EMT is an important way to treat PF. At present, the signaling pathways involved in EMT inhibition by TCM mainly include TGF-β/Smad, NF-κB, PI3K-Akt, and so on.
Andrographolide, a diterpenoid compound isolated from andrographis paniculata, it has pharmacological effects such as anti-inflammatory, antioxidant, and participation in regulating EMT [129, 130]. Sachin Karkale's research team found that Andrographolide can effectively reduce the expression of mesenchymal markers in PF mice and increase the expression of epithelial markers [131]. This study revealed for the first time that andrographolide could shown good anti-fibrosis activity by inhibiting inflammation and targeting EMT, which provided a new idea for the study of TCM to improve silicon induced PF. In addition to silicon induced occupational PF, andrographolide also has a certain inhibitory effect on bleomycin induced IPF. The research results of Li Jingpei's team shown that andrographolide could improve BLM induced PF in rats, and explore its mechanism from different perspectives. First, andrographolide could improve PF by inhibiting the proliferation of fibroblasts and the differentiation of myofibroblasts. This process is affected by TGF-β1 mediated regulation of Smad dependent and non dependent pathways [132]. Secondly, andrographide could inhibit TGF β1 in alveolar epithelial cells (AEC) by regulating the Smad2/3 and Erk1/2 signaling pathways [132]. Then, andrographolide could inhibit EMT in lung epithelial cells through AKT/mTOR signaling pathway, thereby reducing BLM induced PF [134]. These results shown that andrographis paniculata could improve PF through multiple ways and targets, which has great development potential and is worth our in-depth study.
Emodin is an anthraquinone compound with multiple biological activities isolated and purified from rhubarb. Zhou's team research shown that emodin could inhibit NE induced EMT in RLE-6TN and A549 cells, and its mechanism of action was related to NE induced Notch1 lysis [135]. This study preliminarily revealed the mechanism of emodin inhibiting EMT at the cellular level, which has certain reference value for further understanding the pharmacological effect of emodin in improving PF. Emodin can also regulated NF-κB and TGF- β1/Smad3 signaling pathway inhibits EMT and improves silica induced PF [136]. Rhapontin is another important compound in rhubarb, and Tao's team found in vitro experiments that rhapontin could activate AMPK and inhibit TGF- β/Smad pathway reversal of ECM [137]. This experiment confirmed the anti-PF activity of rhapontin for the first time. On the whole, rhubarb has important medicinal value in improving PF, but its pharmacology and toxicology still need further experimental and clinical research.
Salvia miltiorrhiza is a commonly used herbal medicine to treat cardiovascular and pulmonary diseases. Salvianolic acid B is a bioactive component extracted from Salvia miltiorrhiza, which has strong anti-inflammatory and antioxidant effects. Liu's team first confirmed the anti fibrotic activity of salvianolic acid B through a cell model, and further found in animal models that salvianolic acid B inhibits the transdifferentiation of lung fibroblasts by activating Nrf2 signaling [138]. Cryptotanshinone is a diterpenoid compound with antioxidant, anti-inflammatory, and antibacterial activities. Research has shown that CPT exhibits good anti-fibrotic effects in both in vitro and in vivo, inhibiting various cell proliferation and TGF-β induced EMT [139]. Although these research results suggest that Salvia miltiorrhiza may be a potential drug for improving IPF, these studies have only been conducted on animal or cell models and further clinical research is still needed.
In addition to the above-mentioned TCM that can improve PF, the detailed information of other TCM studies in the past five years that can improve PF by inhibiting EMT were summarized and presented in Table 1. It should be noted that the traditional efficacy of most Chinese medicines in the table is related to clearing away heat and detoxification, promoting blood circulation to remove blood stasis, relieving cough and resolving phlegm, which may suggest that the traditional efficacy is a reference factor that cannot be ignored when we are looking for natural drugs with anti PF activity.
Table 1.
Details about some traditional Chinese medicines improving pulmonary fibrosis by inhibiting epithelial cell-mesenchymal transformation
| No | Source | Genus information | Traditional efficacy | Active compound | Experiment model | Administration dose | Therapeutic target | Cytokine | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Scutellariae radix | Scutellaria | Clearing away heat and dampness, purging fire and detoxifying | Astragalus polysaccharides | BLM-induced PF mice and A549 cells | Mice (200 mg/kg) | Inhibit NF-κB signaling | TGF-β1, NF-κB, α-SMA, Collagen I, CHOP | [140] |
| 2 | Baicalin | BLM-induced PF rats and RPF | Rats (50 mg/kg) | Regulation of CaMKII and PI3K/AKT signaling | PI3K, AKT, Bax, Bcl-2 | [141] | |||
| Cell (20, 40, 60, 80 μg/mL) | |||||||||
| 3 | Radiation-induced EMT model | Cell (2, 10, 50 μmol/L) | Suppress EMT | Smad2/3, ERK, GSK3β | [142] | ||||
| 4 | Baicalein | MRC-5 cells | Cell (1–80 μmol/L) | Inhibit miR-21 | STAT3, TGF-β1, COL1 A1, α-SMA, | [143] | |||
| 5 | Calycosin, CA | BLM-induced PF mice and MLE-12 cells | Mice (7, 14 mg/kg) | Inhibit AKT/GSK3β/β-catenin signaling | AKT, GSK3β, β-catenin, E-cadherin | [144] | |||
| Cell (0–80 μmol/L) | |||||||||
| 6 | Curcumae longae rhizoma | Zingiberaceae | Promoting blood circulation and removing blood stasis, Regulating menstruation and relieving pain | Curcumin | HUVEC cells | Cell (5, 10 μmol/L) | Regulation of NRF2-DDAH-ADMA-NO signaling | TGF-β1, Nrf2, DDAH, PRMT, ERK1/2 | [145] |
| 7 | BLM-induced PF mice and A549 cells | Mice (75 mg/kg) | Suppress EMT | EGFR, Ki67 | [146] | ||||
| Cell (20 μmol/L) | |||||||||
| 8 | A549 cells | Cell (20 μmol/L) | Inhibit TGF-β1/Smad/non Smad signaling | TGF-β1, Smad | [147] | ||||
| 9 | A549 cells | Cell (0–1000 μmol/L) | Suppress EMT | ROS, α-SMA, TGF-β1 | [148] | ||||
| 10 | NHLF cells | Cell (10 μmol/L) | Down-regulation of hsa-miR-6724-5p expression | KLF10 | [149] | ||||
| 11 | CCD-19Lu cells | Cell (0–50 μmol/L) | activate PPARγ | TGF-β1, α-SMA, PPARγ | [150] | ||||
| 12 | BLM-induced PF mice | Mice (75, 150 mg/kg) | Activates PPARγ and CREB signaling | PPARγ, CREB | [151] | ||||
| 13 | Andrographis herba | Acanthaceae | Clearing heat and detoxification, cooling blood | Andrographolide | NIH 3T3, PLF cells | Rats (10 mg/kg) | Inhibits TGF-β1/Smad2/3 and Erk1/2 signaling | TGF-β1, Smad2/3 | [132] |
| Cell (2, 5, 10 μmol/L) | |||||||||
| 14 | BLM-induced PF rats and A549 cells | Rats (10 mg/kg) | Regulation of AKT/mTOR signaling | AKT, mTOR | [134] | ||||
| 15 | Silica-induced PF mice | Mice (3, 10 mg/kg) | Inhibition of ECM precipitate formation | GSH, NF-κB, CTGF | [131] | ||||
| 16 | A549 cells | Cell (0–20 μmol/L) | Inhibits A and B signaling | TGF-β1, Smad2/3, MMP-9 | [133] | ||||
| 17 | Salviae miltiorrhizae | Lamiaceae | Promoting blood circulation and removing blood stasis, reducing swelling and relieving pain | Tanshinone IIA | BLM-induced PF mice and PLFs, NIH-3T3 cells | Mice (5, 10, 20 mg/kg) | Inhibition of TGF-β1-Smad3 signaling | Nrf2, TGF-β1 | [152] |
| Cell (0.01–500 μmol/L) | |||||||||
| 18 | Salvianolic acid B | BLM-induced PF rats and MRC-5 cells | Rats (20 mg/kg) | Regulates Nrf2 signaling | Nrf2, α-SMA, TGF-β1 | [138] | |||
| Cell (40 μmol/L) | |||||||||
| 19 | Salvianolic acid B/sodium tanshinone IIA sulfonate | THP-1 cells | Cell (0–600 μg/mL) | Inhibit TGF-β1 signaling | TGF-β1, α-SMA | [153] | |||
| 20 | Cryptotanshinone | BLM-induced PF mice and A549, NIH/3T3, HPF cells | Mice (30, 60 mg/kg) | Suppress EMT | [139] | ||||
| Cell (0–20 μmol/L) | |||||||||
| 21 | Rhei radix et rhizoma | Polygonaceae | Purging and defecating, clearing heat and detoxification | Emodin | Silica-induced PF mice and A549 cells | Mice (25, 50, 100 mg/kg) | Regulation of NF-κB and TGF-β1/Smad3 signaling | NF-κB, TGF-β1 | [136] |
| 22 | RLE-6TN, A549 cells | Cell (50, 100, 200 nmol/L) | Suppress EMT | Notch1, C-MYC | [135] | ||||
| 23 | rhapontin | BLM-induced PF rats and THP-1 cells | Rats (25, 50, 100 mg/kg) | Regulation of TGF-β/Smad signaling | TGF-β, Smad | [137] | |||
| Cell (50 μmol/L) | |||||||||
| 24 | Various plant sources | quercetin | RLE/Abca3 cells | Cell (20 μmol/L) | Regulation of Smad and b-catenin signaling | Smad, b-catenin | [154] | ||
| 25 | BLM-induced PF mice and HELF cells | Mice (25, 50, 100 mg/kg) | Inhibits SphK1/S1P signaling | TGF-β, SphK1, S1P | [155] | ||||
| Cell (10, 20, 40 μmol/L) | |||||||||
| 26 | Polygoni cuspidati rhizoma et radix | Polygonaceae | Clearing heat and detoxification, promoting blood circulation and removing blood stasis | Polydatin | BLM-induced PF rats and A549 cells | Rats (10, 40, 160 mg/kg) | Inhibit TGF-β1/Smad signaling | TGF-β1, Smad2/3, Erk1/2 | [156] |
| Cell (0–120 μmol/L) | |||||||||
| 27 | BLM-induced PF rats and HFL-1 cells | Rats (100 mg/kg) | Regulation of TGF-β/Smad signaling | TGF-β, Smad2/3, TNF-α, IL-1β | [157] | ||||
| Cell (50, 150 mmol/L) | |||||||||
| 28 | Astragali radix | Fabaceae | Replenishing qi and solidifying the surface, strengthening the upright and dispelling evil | astragaloside IV | A549 cells | Cell (20 mg/mL) | Inhibit the expression of NLRP3 | NLRP3, TGF-β1, Smad2/3, α-SMA | [158] |
| 29 | BLM-induced PF rats and A549 cells | Rats (20 mg/kg) | Inhibit TGF-β1/PI3K/Akt signaling | TGF-β1, PI3K, Akt | [159] | ||||
| Cell (100 μg/mL) | |||||||||
| 30 | Tripterygium wilfordii Hook. f | Ranunculaceae | Clearing heat and detoxification, promoting blood circulation and removing blood stasis | Triptolide | HFL-1 cells | Cell (5, 10, 15, 20 nmol/L) | Regulation of FAK/calin signaling | FAK, calain | [160] |
| 31 | paraquat-induced PF mice and BEAS-2B cells | Mice (0.25 mg/kg) | Suppress EMT | TGF-β1, α-SMA, Smad3, E-cadherin | [161] | ||||
| 32 | Schisandrae chinensis fructus | Magnoliaceae | Astringent and astringent, replenishing qi and invigorating fluid | schisantherin A | BLM-induced PF mice and A549 cells | Mice (1, 2, 4 mg/kg) | Inhibition of ERK signaling | ERK, α-SMA, IL-1β, IL-6, TNF-α | [162] |
| Cell (0.625, 10 μmol/L) | |||||||||
| 33 | schisandrin B | BLM-induced PF mice | Mice (5, 10, 20 mg/kg) | Inhibits Wnt signaling | MMP7, SOD, TGF-β1 | [163] | |||
| 34 | Inulae flos | Asteraceae | Clearing heat and detoxification, eliminating phlegm and relieving cough | Inula japonica Thunb.extract | Mlg, CAGA-NIH3T3 cells | Cell (0–80 μmol/L) | Inhibit TGF-β1/Smad3 signaling | TGF-β1, Smad3 | [164] |
| 35 | BLM-induced PF mice | Mice (100, 200, 400 mg/kg) | Regulates GSK3β signaling | GSK3β, COX-2, GSK3β, p65 | [165] | ||||
| 36 | Ferulae resina | Apiaceae | Activating qi to relieve pain, warming menstruation and dispelling cold | ferulic acid | Silica-induced PF mice | Mice (100, 300 mg/kg) | Inhibit TGF-β1 signaling | TGF-β1, Smad2/3, CTGF, Slug | [166] |
| 37 | Psoraleae fructus | Fabaceae | Warm the kidney and consolidate the essence, strengthen the muscles and bones | Psoralen | BLM-induced PF mice and NIH3T3 cells | Mice (5 mg/kg) | Inhibition of ECM precipitate formation | TNF-α, IL‐1β | [167] |
| Cell (5, 10, 20, 40 μmol/L) | |||||||||
| 38 | Atractylodis rhizoma | Asteraceae | Invigorating spleen and stomach, tonifying qi and blood | atractylon | OVA-induced asthma mice and TC-1 cells | Mice (25 mg/kg) | Regulate the mmu_circ_0000981/miR-211-5p/ TGFBR2 axis | TFGBR2, Vimentin, α-SMA, collagen | [168] |
| 39 | Angelicae sinensis radix | Apiaceae | Tonifying blood and activating blood, regulating menstruation and relieving pain | Angelica Sinensis Polysaccharide | BLM-induced PF rats and RLE-6TN cells | Rats (20 mg/kg) | Suppress EMT | DANCR, AUF1 | [169] |
| Cell (μmol/L) | |||||||||
| 40 | Pyrethrum parthenium (L.) Sm | Asteraceae | Dispelling wind and relieving pain and headache | Parthenolide | BLM-induced PF mice and A549 cells | Mice (12.5, 25, 50 mg/kg) | Inhibits NF-κB/Snail signaling | NF-κB, Snail | [170] |
| Cell (5, 10 μmol/L) | |||||||||
| 41 | Erigeron breviscapus | Asteraceae | Promoting blood circulation and removing blood stasis, clearing heat and detoxification | Scutellarin | BLM-induced PF mice and A549 cells | Mice (30, 60, 90 mg/kg) | Regulation of NF-κB/NLRP3 signaling | NF-κB, NLRP3 | [171] |
| Cell (0.1, 0.2, 0.4 mmol/L) | |||||||||
| 42 | Scutellarein | BLM-induced PF mice | Mice (10 mg/kg) | Inhibits PI3K/Akt signaling | PI3K, Akt, Smad2/3, α-SMA | [172] | |||
| 43 | Spirulina platensis | phycocyanin | BLM-induced PF mice | Mice (50 mg/kg) | Regulation of TLR2-MyD88-NF-κB signaling | TLR2, NF-κB | [173] | ||
| 44 | Curcuma aromatica Salisb | Zingiberaceae | Promoting blood circulation and removing blood stasis, reducing swelling and relieving pain | Curdione | BLM-induced PF mice and HPFS cells | Mice (100 mg/kg) | Inhibition of TGF-β/Smad3 signaling | TGF-β1, α-SMA, Collagen 1, Erk1/2 | [174] |
| Cell (100–500 μmol/L) | |||||||||
| 45 | Citrus aurantium L | Rutaceae | Soothing the liver and regulating qi, resolving phlegm and relieving cough | Hesperidin | A549 cells | Cell (40–200 μmol/L) | Inhibit TGF-β/Smad2/3 signaling | TGF-β1, Smad2/3, Smad4, Smad7 | [175] |
| 46 | Alpiniae officinarum rhizoma | Zingiberaceae | Regulating qi and relieving pain, removing dampness and resolving phlegm | Galangin | BLM-induced PF mice and A549 cells | Mice (25, 50 mg/kg) | Suppress EMT | TGF-β1, E-cadherin | [176] |
| Cell (0–100 μmol/L) | |||||||||
| 47 | Aronia melanocarpa | Rosaceae | Cyanidin-3-galactoside | Silica-induced PF mice | Mice (100, 200, 400 mg/kg) | Inhibition of TGF-β/mTOR signaling through the NRF2/HO-1 pathway | TGF-β1, p-mTOR, NRF2 | [177] | |
| 48 | Carthami flos | Asteraceae | Promoting blood circulation and removing blood stasis, regulating menstruation and relieving pain | safflower yellow | paraquat-induced PF rats | Rats (50 mg/kg) | Regulate Hippo signaling | Hippo, Smad2/3, TGF-β1 | [178] |
| 49 | Juglans mandshurica | Juglandaceae | Moistening the intestines and relieving defecation, tonifying the kidney and strengthening yang | Juglanin | BLM-induced PF mice and MRC-5, MLE-12 cells | mice (80 mg/kg) | Suppress Sting | Sting, MMP-9, α-SMA, TGF-β1 | [179] |
| Cell (0–160 μmol/L) | |||||||||
| 50 | Trigonellae semen | Fabaceae | Warming the middle and dispelling cold, regulating qi and relieving pain | Diosgenin | BLM-induced PF rats | Rats (100 mg/kg) | Regulation of TGF-β/Smad signaling | TGF-β1, snail, NF-κB, COX-2, IL-1β | [180] |
| Cell (10–30 μmol/L) | |||||||||
| 51 | Gynostemma pentaphyllum (Thunb.) Makino | Cucurbitaceae | Clearing heat and detoxification, lowering blood pressure | Gypenoside, Gyps | BLM-induced PF mice | Mice (200 mg/kg) | Inhibits AKT/mTOR/c-Myc signaling | AKT, mTOR | [181] |
| 52 | Sophorae fla vescentis radix | Fabaceae | Clearing heat and detoxification, diuresis and purging gonorrhea | Matrine | MRC-5 cells | Cell (10 μmol/L) | Inhibit TGF-β/Smad2/3 signaling | TGF-β, Smad2/3 | [182] |
| 53 | Vaccinium spp. | Ericaceae | Pterostilbene | A549 cells | Cell (0–100 μmol/L) | Inhibit TGF-β1 signaling | TGF-β1, Bcl-2, BAX, P62, P-21 | [183] | |
| 54 | Cyanobacteria | C-Phycocyanin | Oleic acid-induced PF mice and A549, HFL-1 cells | Mice (1, 3, 9 mg/kg) | Regulation of TGF-β/Smad and MAPK signaling | TGF-β1, MAPK | [184] | ||
| Cell (10, 30 μmol/L) | |||||||||
| 55 | Eclipta prostrata (L.) L | Saururaceae | Clearing heat and detoxification, stopping bleeding and generating hair | wedelolactone | BLM-induced PF mice and PLFs cells | Mice (2, 10 mg/kg) | Inhibition of RAF1-MAPKs signaling | Col I, α-SAM, AMPK | [185] |
| Cell (10 μmol/L) | |||||||||
| 56 | Nelumbinis semen | Nelumbonaceae | Clear the heart and calm the mind, moisturize the lungs and relieve cough | Lotus Plumule Extract | BLM-induced PF mice | Mice (80, 160, 240 mg/kg) | Inhibit TGF-β1/Smad3 signaling | TGF-β, α-SMA | [186] |
| 57 | Gentianae radix et rhizoma | Gentianaceae | Clearing heat, dryness and dampness, purging liver and gallbladder fire | Gentiopicroside | BLM-induced PF mice | Mice (2.5, 10 mg/kg) | Anti-inflammatory | TNF-α, IL‐1β, TGF-β1, CTGF | [187] |
| 58 | Siratia grosvenorii | Cucurbitaceae | Clear heat and moisturize the lungs and open pharynx | Mogrol | BLM-induced PF mice and NIH3T3 cells | Mice (1, 5, 10 mg/kg) | Regulation of TGF-β1 and AMPK signaling | TGF-β1, AMPK | [188] |
| Cell (1, 5, 10 μmol/L) | |||||||||
| 59 | Mangifera indica L. | Anacardiaceae | Clearing heat and detoxification, invigorating the stomach and eliminating food | Mangiferin | BLM-induced PF rats and A549 cells | Rats (40 mg/kg) | Inhibit TGF-β1/Smad2/3 signaling | TLR4, TGF-β1 | [189] |
| Cell (10 μg/mL) | |||||||||
| 60 | Atractylodis rhizoma | Asteraceae | Invigorate the spleen and appetizer, remove dampness and dissipate phlegm | Atractylodin | BLM-induced PF mice and A549 cells | Mice (50, 100 mg/kg) | Inhibit TGF-β1/Smad signaling | TGF-β1, Snail | [190] |
| Cell (0–100 μmol/L) | |||||||||
| 61 | Indigo naturalis | Brassicaceae | Clearing heat and detoxification, reducing swelling and relieving pain | Indirubin | BLM-induced PF mice and PMLFs HPFs cells | Mice (12.5, 25 mg/kg) | Inhibit TGF-β1/Smad signaling | TGF-β1, Collagen I, α-SMA, Smad2/3 | [191] |
| Cell (2.5–60 μmol/L) | |||||||||
| 62 | Ginseng radix et rhizoma | Araliaceae | Replenish qi and nourish blood, invigorate body and quench thirst | ginsenoside Rg3 | BLM-induced PF mice and LL 29 cells | Mice (5 mg/kg) | Inhibits nuclear localization of HIF-1α | HIF-1α, TGF-β1 | [192] |
| 63 | Inonotus sanghuang | Clearing heat and detoxification, promoting blood circulation and removing blood stasis | Inonotus sanghuang extract (ISE) | BLM-induced PF mice and A549 cells | Mice (0.6% w/w) | Inhibit TGF-β1/Smad signaling | TGF-β1, Smad2/3 | [193] | |
| Cell (2, 4 μg/mL) | |||||||||
| 64 | Hippophae fructus | Elaeagnaceae | Invigorate the stomach and eliminate food, relieve cough and expectoration | Isorhamnetin | BLM-induced PF mice | Mice (10, 30 mg/kg) | Suppress EMT | PERK, α-SMA, Collagen I , TGF-β1 | [194] |
| Cell (25, 50, 100 μmol/L) | |||||||||
| 65 | Zingiberis rhizoma recens | Zingiberaceae | Warm the middle to dissipate the cold, solve the surface and dissipate the cold | 6 gingerol | BLM-induced PF mice and | Mice (100, 250 mg/kg) | Activates SIRT1 signaling signaling | SIRT1, α-SMA, TNF-α, IL-6, IL-1β | [195] |
| human embryonic lung fibroblasts MRC-5 | Cell (10, 20 μmol/L) | ||||||||
| 66 | Silybi fructus | Asteraceae | Clearing heat and detoxification, soothing the liver and promoting gallbladder | Silibinin | Silica-induced PF mice | Mice (100, 300 mg/kg) | Anti-inflammatory Inhibits EMT | IL-1β, smad 2/3, α-SMA, TGF-β1 | [196] |
| 67 | Ampelopsis grossedentata (Hand.-Mazz.) W. T. Wang (Vitaceae) | Lamiaceae | Promoting blood circulation and regulating menstruation, diuresis and detumescence | Dihydromyricetin | BLM-induced PF mice and MLG cells | Mice (50, 100, 200 mg/kg) | Inhibit TGF-β1/Smad signaling | TGF-β1, α-SMA | [197] |
| 68 | PMLFs, PHLFs cells | Cell (100, 200, 300 μmol/L) | Regulation of STAT3/p-STAT3/GLUT1 signaling | STAT3,p-STAT3,GLUT1 | [198] | ||||
| 69 | Dendrobii officinalis caulis | Orchidaceae | Lung heat coughing, deficiency heat dispelling thirst | Polysaccharides from Dendrobium officinale | BLM-induced PF rats | Rats (200 mg/kg) | Inhibition of TGF-β1-Smad2/3 signaling | TGF-β, Smad2/3, α-SMA, Collagen I I | [199] |
| 70 | Galla chinensis | Anacardiaceae | Restrain the lungs and reduce the fire, astringent intestines and stop diarrhea | Tannic acid | BLM-induced PF mice | Mice (10 mg/kg) | Inhibit TGF-β receptor signaling | TGF-β, Smad2/3 | [200] |
| Cell (1, 3 μmol/L) | |||||||||
| 71 | Camptotheca acuminata Decne | Nyssaceae | Relieve cough and resolve phlegm | Hyperoside | BLM-induced PF mice | Mice (50 mg/kg) | Regulation of AKT/GSK3b signaling | AKT, GSK3b | [201] |
| 72 | armeniacae semen amarum | Rosaceae | Moistening the bowels, relieving cough and resolving phlegm | amygdalin | smoking combined with LPS-induced COPD mice and BEAS-2B cells | mice (5, 10, 20 mg/kg) | Inhibit TGF-β/Smad2/3 signaling | TGF-β, Smad2/3 | [202] |
| Cell (0–2000 μg/mL) | |||||||||
| 73 | Arenaria kansuensis Maxim | Caryophyllaceae | Clearing heat and detoxification, diuresis and purging gonorrhea | A. kansuensis ethanol extract | paraquat-induced PF rats | Rats (170, 350, 700 mg/kg) | Inhibit NF-κB/TGF-β1/Smad2/3 signal transduction | TGF-β1, Smad2/3 | [203] |
| 74 | Myrica rubra Sieb | Myricaceae | Moistening the lung and relieving cough, invigorating body and relieving thirst | Myricetin | BLM-induced PF mice and A549, HFL1 cells | Mice (25, 50, 100 mg/kg) | Regulation of TGF-β/Smad and non-Smad signaling | TGF-β, Smad | [204] |
| cell (μmol/L) | |||||||||
| 75 | Epimedii folium | Berberidaceae | Kidney deficiency and impotence, sore waist and knees | Icariin | BLM-induced PF mice and NIH3T3, HLF-1 cells | Mice (0.04, 0.02, 1 mg/kg) | Inhibit TGF-β1 signaling | TGF-β1, α-SMA | [205] |
| Cell (3 μmol/L) | |||||||||
| 76 | Houttuyniae herba | Saururaceae | Heat-clearing and detoxification, sore waist and knees | Sodium Houttuyfonate | BLM-induced PF mice | Mice (45, 90 mg/kg) | Anti-inflammatory | IL-1β, TNF-α | [206] |
| 77 | Hypericum longistylum | Hypericaceae | Promoting blood circulation and removing blood stasis, reducing swelling and relieving pain | Hypericum longistylum | MLFC cells | Cell (0–80 μmol/L) | Inhibition of TGF-β/Smad3 signaling | TGF-β | [207] |
| 78 | Aurantii fructus immaturus | Rutaceae | Regulating qi stagnation, resolving phlegm and relieving cough | Neohesperidin | BLM-induced PF mice and NIH3T3, MLg, A549 cells | Mice (20 mg/kg) | Inhibition of TGF-β/Smad3 signaling | TGF-β1, Smad2/3, Erk, p-38, JNK | [208] |
| cell (0–200 μmol/L) | |||||||||
| 79 | Multiple plant sources | Hederagenin | BLM-induced PF rats | Rats (10, 20, 50 mg/kg) | Adjust Ras/JNK/NFAT4 axis | JNK, NFAT4 | [209] | ||
| 80 | Polyporus | Polyporaceae | Diuresis, detumescence and phlegm | Polyporus Polysaccharide | BLM-induced PF mice and HLF cells | Mice (50, 100 mg/kg) | Inhibit TGF-β1/Smad2/3 signaling | TGF-β1, Smad2/3 | [210] |
| Cell (1 mg/mL) | |||||||||
| 81 | Podocarpus nagi | Podocarpaceae | Clearing heat and detoxification, dispelling wind and promoting dampness | Nagilactone D | BLM-induced PF mice and WI-38, VA-13, HFL-1 cells | Mice (1, 3 mg/kg) | Inhibition of TGF-β/Smad3 signaling | TGF-β1, Collagen I, α-SMA | [211] |
| Cell (1, 2 μmol/L) |
Anti-inflammation and anti-oxidation
Oxidative stress is a pathological state in which the body undergoes some kind of stimulation resulting in excessive production of reactive nitrogen radicals and reactive oxygen radicals, leading to an oxidative/antioxidative imbalance. Oxidative stress is a major cellular stressor that can act directly or indirectly on cells, leading to structural necrosis, apoptosis and tissue inflammation [212]. The imbalance between oxidants and antioxidants plays a role in the pathophysiology of IPF, and NADPH oxidase (NOX), which generates reactive oxygen species (ROS), is the primary cause of IPF [213]. Excessive ROS and free radical production can cause lung damage [214]. The level of systemic oxidative stress and disease severity in IPF patients are significantly correlated with dyspnea, as shown by numerous studies [215]. Therefore, anti-oxidative stress is essential for the successful treatment of PF [216]. The inflammatory response is a defense mechanism of the body, and the inflammatory response of the body to different degrees of injury is one of the important factors against lung injury [217, 218]. The pathogenesis of PF may be due to damage to lung epithelial cells by fibrotic stimuli. Therefore, lung inflammation plays an important role in the development of PF. And inflammation is controlled by a variety of cells and cytokines [212, 219] Pro-inflammatory cytokines control tissue differentiation and morphogenesis through adhesion molecules and promote fibrotic responses in lung tissue [220]. Currently, many anti-inflammatory and antioxidant agents have shown effective antifibrotic effects in BLM-induced PF models. Therefore, the imbalance between oxidative stress, oxidants and antioxidants, and inflammation in the development of PF deserve further attention [221, 222].
Emodin is an anthraquinone compound extracted from rhubarb, which has antiviral, anti-cancer, anti-inflammatory and other pharmacological effects [223, 224]. Tian's team found that emodin can significantly reduce the increase of proinflammatory cytokines and oxidative damage caused by BLM [225]. Further experiments found that the anti-inflammatory and antioxidant activities of emodin may be through regulating NF-κB and Nrf2 signal pathways. This study preliminarily revealed the anti-inflammatory and antioxidant activities of emodin in improving PF, and preliminarily explored the possible signal pathways involved. However, the mechanism by which emodin exerts its biological activity still needs to be further explored in order to better play its potential in the treatment of PF. Qi's team found the protective effect and potential mechanism of chrysophanol in IPF through research [226]. The research results shown that chrysophanol can effectively reduce ECM deposition and inflammatory cytokine levels in PF model mice, and chrysophanol can also inhibit Wnt/β-catenin signaling pathway and inhibition of lung fibroblast proliferation to alleviate BLM induced mouse PF. This study demonstrates that chrysophanol has anti-inflammatory biological activity, but further experimental verification is needed. In addition to emodin and chrysophanol, rhubarb also contains many active ingredients with anti-inflammatory and antioxidant effects. The improvement of PF by these active ingredients deserves further research.
Studies have shown that ethyl acetate extract of Salvia miltiorrhiza can reduce the degree of active oxygen-related PF by targeting Nrf2-NOX4 REDOX equilibrium [227, 238]. Tanshinone IIA is a bioactive ingredient extracted from Salvia miltiorrhiza with anti-inflammatory, antioxidant and anti-fibrotic properties. Feng's research team studied the protective effect of Tanshinone IIA on silica-induced PF and its potential mechanism [229], and the results showed that tanshinone IIA could down-regulate the level of oxidative stress markers in silicosis rats and attenuate pulmonary inflammatory response. In addition, Tanshinone IIA may protect the lung from silica damage by inhibiting TGF-β1/Smad signaling, inhibiting NOX4 expression, and activating the Nrf2/ARE pathway. An’s team found a similar phenomenon in mice with bleomycin-induced PF treated with tanshinone IIA [152]. The study found that tanshinone IIA can inhibit PF by activating Nrf2, regulating REDOX homeostasis and glutamine breakdown. Liu's study showed that salvianolic acid B can reduce experimental lung inflammation by protecting endothelial cells from oxidative stress [230], further demonstrating that the anti-inflammatory effects of Salvianolic acid B may be mediated through MAPK and NF-κB signaling pathways. Notably, Salvianolic acid B and Tanshinone IIA sulfonates reduce PF by affecting the inflammatory system and controlling the TGF-β1 pathway, which may be the result of a synergistic effect between the two drugs [153]. In summary, many active compounds in salvia miltiorrhiza have pharmacological effects on improving PF, and the synergistic effect between these compounds is worthy of further study. In addition to the above-mentioned TCM that can improve PF, the detailed information of other TCM studies in the past five years that can improve PF through anti-inflammation and anti-oxidation were summarized and presented in Table 2.
Table 2.
Details about some traditional Chinese medicines improving pulmonary fibrosis by anti-inflammation and anti-oxidation effects
| No | Source | Genus information | Traditional efficacy | Active compound | Experiment model | Administration dose | Therapeutic target | Cytokine | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Salviae miltiorrhizae radix et rhizoma | Lamiaceae | Promoting blood circulation and removing blood stasis, reducing swelling and relieving pain | Salvia miltiorrhiza | BLM-induced PF mice and NIH3T3 cells | Mice (21, 40, 80 mg/kg) | Anti-oxidation | Nrf2, NOX4 | [228] |
| Cell (0.1, 1, 3 μmol/L) | |||||||||
| 2 | Tanshinone IIA | Silica-induced PF rats | Rats (25 mg/kg) | Regulation of TGF-β1/Smad and Nrf2/ARE signaling | TGF-β1, Nrf2 | [229] | |||
| 3 | BLM-induced PF mice and PLFs, NIH-3T3 cells | Mice (5, 10, 20 mg/kg) | Inhibition of TGF-β1-Smad3 signaling | Nrf2, TGF-β1 | [152] | ||||
| Cell (0.01–500 μmol/L) | |||||||||
| 4 | Sodium Tanshinone IIA sulfonate | Silica-induced PF rats and A549, RLE-6TN, MRC-5, NIH-3T3 cells | Rats (25 mg/kg) | Up-regulation of Nrf2 nuclear expression | Nrf2, Trx, TrxR | [231] | |||
| Cell (μmol/L) | |||||||||
| 5 | Salvianolic acid B | BLM-induced PF mice and hy926 cells | Mice (10 mg/kg) | Anti-inflammatory and antioxidant | IL-1β, TNF-α, NF-κB | [230] | |||
| Cell (50 μg/mL) | |||||||||
| 6 | BLM-induced PF rats and MRC-5 cells | Rats (20 mg/kg) | Regulates Nrf2 signaling | Nrf2, α-SMA, TGF-β1 | [138] | ||||
| Cell (40 μmol/L) | |||||||||
| 7 | Salvianolic acid B/sodium tanshinone IIA sulfonate | THP-1 cells | Cell (0–600 μg/mL) | Inhibit TGF-β1 signaling | TGF-β1, α-SMA | [153] | |||
| 8 | Curcumae longae rhizoma | Zingiberaceae | Promoting blood circulation and removing blood stasis, Regulating menstruation and relieving pain | Curcumin | BLM-induced PF mice | Mice (75 mg/kg) | Inhibit NF-κB signaling | AMPK, COX-2 | [232] |
| 9 | paraquat-induced PF rats | Rats (200 mg/kg) | Improve pulmonary fibrosis | Smad 4, Smurf 2 | [233] | ||||
| 10 | LMSCs cells | Cell (2.5, 5, 10, 20 μmol/L) | Regulation of Akt/Nrf2/HO-1 signaling | Akt, Nrf2, HO-1 | [234] | ||||
| 11 | BLM-induced PF rats | Rats (300 mg/kg) | Inhibition of ECM precipitate formation | / | [235] | ||||
| 12 | A549 cells | Cell (20 μmol/L) | Inhibit TGF-β1/Smad/non Smad signaling | TGF-β1, Smad | [147] | ||||
| 13 | Rhei radix et rhizoma | Polygonaceae | Purging and defecating, clearing heat and detoxification | Chrysophanol | BLM-induced PF mice | Mice (10 mg/kg) | Inhibits Wnt/β-catenin signaling | β-catenin, Bax, TNF-α, IL-1β | [226] |
| 14 | Emodin | BLM-induced PF rats and A549 cells | Rats (20 mg/kg) | Anti-inflammatory and antioxidant | IL-1β, IL-6, TNF-α, NF-κB | [225] | |||
| Cell (60 μmol/L) | |||||||||
| 15 | Rhapontin | BLM-induced PF rats and THP-1 cells | Rats (25, 50, 100 mg/kg) | Regulation of TGF-β/Smad signaling | TGF-β1, Smad | [137] | |||
| Cell (50 μmol/L) | |||||||||
| 16 | Polygoni cuspidati rhizoma et radix | Polygonaceae | Clearing heat and detoxification, promoting blood circulation and removing blood stasis | Polydatin | MTX‐induced PF rats | Rats (25, 50, 100 mg/kg) | Inhibit TGF-β1 signaling | TGF-β1, HYP, α-SMA, TNF-α | [236] |
| 17 | BLM-induced PF rats and A549 cells | Rats (10, 40, 160 mg/kg) | Inhibit TGF-β1/Smad signaling | TGF-β1, Smad2/3, Erk1/2 | [156] | ||||
| Cell (0–120 μmol/L) | |||||||||
| 18 | BLM-induced PF rats and HFL-1 cells | Rats (100 mg/kg) | Regulation of TGF-β/Smad signaling | TGF-β, Smad2/3, TNF-α, IL-1β | [157] | ||||
| Cell (50, 150 mmol/L) | |||||||||
| 19 | Astragali radix | Fabaceae | Replenishing qi and solidifying the surface, strengthening the upright and dispelling evil | Baicalin | BLM-induced PF rats | Rats (25, 100 mg/kg) | Activate SOD | / | [237] |
| 20 | Astragaloside IV | Silica-induced PF rats | Rats (20 mg/kg) | Inhibit TGF-β1/Smad signaling | TGF-β1, α-SMA | [238] | |||
| 21 | A549 cells | Cell (20 mg/mL) | Inhibit the expression of NLRP3 | NLRP3, TGF-β1, Smad2/3, α-SMA | [158] | ||||
| 22 | Tripterygium wilfordii Hook. f | Ranunculaceae | Clearing heat and detoxification, promoting blood circulation and removing blood stasis | Triptolide | radiation-induced PF mice and NIH3T3 cells | Mice (0.25 mg/kg) | Inhibit NF-κB signaling | NF-κB, LOX, IκBα | [239] |
| cell (5 ng/mL) | |||||||||
| 23 | HFL-1 cells | Cell (5, 10, 15, 20 nmol/L) | Regulation of FAK/calin signaling | FAK, calain | [160] | ||||
| 24 | Isorhynchophylline | Silica-induced PF mice | Mice (20 mg/kg) | Anti-inflammatory | / | [240] | |||
| 25 | Andrographis herba | Acanthaceae | Clearing heat and detoxification, cooling blood | Andrographolide | BLM-induced PF rats and A549 cells | Rats (10 mg/kg) | Regulation of AKT/mTOR signaling | AKT, mTOR | [134] |
| 26 | A549 cells | Cell (0–20 μmol/L) | Inhibits A and B signaling | TGF-β1, Smad2/3, MMP-9 | [133] | ||||
| 27 | Schisandrae chinensis fructus | Magnoliaceae | Astringent and astringent, replenishing qi and invigorating fluid | Schisantherin A | BLM-induced PF mice and A549 cells | Mice (1, 2, 4 mg/kg) | Inhibition of ERK signaling | ERK, α-SMA, IL-1β, IL-6, TNF-α | [162] |
| Cell (0.625, 10 μmol/L) | |||||||||
| 28 | Schisandrin B | BLM-induced PF mice | Mice (5, 10, 20 mg/kg) | Inhibits Wnt signaling | MMP7, SOD, TGF-β1 | [163] | |||
| 29 | glycyrrhizae radix et rhizoma | Fabaceae | Nourishes qi and nourishes yin, clears away heat and detoxifies | Licorice extract | paraquat-induced PF mice and A549, HepG2 cells | Mice (20, 40, 60 mg/kg) | Regulates Nrf2 signaling | Nrf2, TGF-β1, CYP3A4, MDA | [241] |
| Cell (0–100 μmol/L) | |||||||||
| 30 | Deglycyrrhizinated licorice | BLM-induced PF rats | Rats (75, 150, 300 mg/kg) | Anti-inflammatory and antioxidant | / | [242] | |||
| 31 | Ferulae resina | Apiaceae | Activating qi to relieve pain, warming menstruation and dispelling cold | Ferulic acid | Silica-induced PF mice | Mice (100, 300 mg/kg) | Inhibit TGF-β1 signaling | TGF-β1, Smad2/3, CTGF, Slug | [166] |
| 32 | Birch bark | Betulaceae | Clear away heat and dampness, detoxify | Betulinic acid | BLM-induced PF mice and MLG, PPF cells | Mice (20, 40, 80 mg/kg) | Inhibit Wnt/β-catenin signaling | β-catenin, Col 1, α-SMA | [69] |
| Cell (5, 10, 20 μmol/L) | |||||||||
| 33 | Bletillae rhizoma | Orchidaceae | Convergence to stop bleeding, reduce swelling and promote muscle growth | Bletilla striata | RAW264.7 cells | Cell (2.5 μg/mL) | Anti-inflammatory | / | [243] |
| 34 | Chelidonii herba | Papaveraceae | Clearing heat and detoxification, reducing swelling and relieving pain | Chelerythrine | BLM-induced PF mice | Mice (0.375, 0.75 mg/kg) | activate Nrf2/ARE signal transduction | Nrf2, ARE | [244] |
| 35 | Stemonae radix | Stemonaceae | Expelling phlegm and relieving cough, killing insects and expelling Ascaris | Stemona alkaloids | BLM-induced PF mice and PFB cells | Mice (30, 60 mg/kg) | Inhibits JAK2/STAT3 and CXCR4/PI3K/AKT1 signaling | STAT3, PI3K, AKT1 | [245] |
| Cell (1, 10, 100 μg/mL) | |||||||||
| 36 | Atractylodis rhizoma | Asteraceae | Invigorate the spleen and appetizer, remove dampness and dissipate phlegm | Atractylenolide III | BLM-induced PF rats | Rats (0.6, 1.2, 2.4 mg/kg) | activate Nrf2/NQO1/HO-1 signal transduction | Nrf2, NQO1, HO-1 | [246] |
| 37 | Rehmanniae radix | Oleaceae | Nourishing yin and tonifying blood, clearing heat and cooling blood | catalpol | BLM-induced PF rats | Rats (10, 20, 40 mg/kg) | Antioxidant inhibits EMT | TGF-β, Smad3 | [247] |
| 38 | Erigeron breviscapus | Asteraceae | Promoting blood circulation and removing blood stasis, clearing heat and detoxification | Scutellarin | BLM-induced PF mice and A549 cells | Mice (30, 60, 90 mg/kg) | Regulation of NF-κB/NLRP3 signaling | NF-κB, NLRP3 | [171] |
| cell (0.1, 0.2, 0.4 mmol/L) | |||||||||
| 39 | Spirulina | Phycocyanin | BLM-induced PF mice | Mice (50 mg/kg) | Anti-inflammatory | IL-6, TNF-α | [248] | ||
| 40 | Various plant sources | Epicatechin | BLM-induced PF mice | Mice (20, 50, 100 mg/kg) | Anti-inflammatory and antioxidant | / | [249] | ||
| 41 | Sinapic acid | BLM-induced PF rats | Rats (10, 20 mg/kg) | Inhibit NF-κB/NRF2/HO-1 signaling | NF-κB, NRF2, HO-1 | [250] | |||
| 42 | Pinocembrin | BLM-induced PF mice | Mice (5, 25, 50 mg/kg) | Inhibit TLR4/NF-κB/NLRP3 signaling | NF-κB, NLRP3 | [251] | |||
| cell (100, 200, 300 μmol/L) | |||||||||
| 43 | Cymbopogon winterianus | Cymbopogon | Essential oil of Cymbopogon winterianus | BLM-induced PF rats | Rats (50, 100, 200 mg/kg) | Inhibit TGF-β1 signaling | TGF-β1, SOD. MDA | [252] | |
| 44 | Citrus fruits | Rutaceae | Invigorate the spleen and replenish qi, moisturize the lungs and relieve cough | Hesperidin | BLM-induced PF rats | Rats (25, 50, 100 mg/kg) | Inhibits TGF-β1/Smad3/AMPK and I-κBα/NF-κB signaling | TGF-β1, NF-κB | [253] |
| 45 | Alpiniae officinarum rhizoma | Zingiberaceae | Regulating qi and relieving pain, removing dampness and resolving phlegm | Galangin | BLM-induced PF mice and A549 cells | Mice (25, 50 mg/kg) | Suppress EMT | E-cadherin, vimentin, α-SMA, MMP-9 | [176] |
| Cell (0–100 μmol/L) | |||||||||
| 46 | Puerariae lobatae radix | Fabaceae | Relieving cold, sweating and detoxification | Puerarin | HLF1 cells | Cell (200, 400, 600 μg/mL) | Inhibition of TGF-β/Smad3 signaling | TGF-β1, Caspase3, Bcl-2, Smad3 | [254] |
| 47 | Glycyrrhizae radix et rhizoma | Fabaceae | Clearing heat and detoxification, moistening the lungs and relieving cough | Glycyrrhiza glabra | BLM-induced PF rats | Rats (500 mg/kg) | Anti-inflammatory and antioxidant | HYP, LPO | [255] |
| 48 | Laminaria japonica | Phaeophyta | Clearing heat and detoxification, softening and dispersing knots | Low molecular weight fucoidan | BLM-induced PF mice and A549 cells | Mice (25, 50, 100 mg/kg) | Antioxidant inhibits fibrosis | NRF-2, HO-1, NQO1 | [256] |
| Cell (50, 100, 200 μg/mL) | |||||||||
| 49 | Carthami flos | Asteraceae | Promoting blood circulation and removing blood stasis, regulating menstruation and relieving pain | Hydroxysafflor Yellow A | MRC-5 cells | Cell (5, 15, 45 μmol/L) | Inhibit NF-κB/AP-1 signaling | NF-κB, AP-1 | [257] |
| 50 | BLM-induced PF rats | Rats (35.6, 53.3, 80 mg/kg) | Anti-inflammatory | α-SMA, IL-1β, IL-6, TNF-α, TGF-β | [258] | ||||
| 51 | Rhodiolae crenulatae radix et rhizoma | Crassulaceae | Replenishing qi and activating blood circulation, dredging pulse and relieving asthma | Rutin | BLM-induced PF rats | Rats (50, 100 mg/kg) | Regulation of TGF-β1/α-SMA/Col I/III signaling | TGF-β1, α-SMA | [259] |
| 52 | Juglans | Juglandaceae | Moistening the bowels, relieving cough and resolving phlegm | Juglanin | BLM-induced PF mice and MRC-5, MLE-12 cells | Mice (80 mg/kg) | Suppress sting | Sting, MMP-9, α-SMA, TGF-β1 | [179] |
| Cell (0–160 μmol/L) | |||||||||
| 53 | Trigonellae semen | Fabaceae | Warming the middle and dispelling cold, regulating qi and relieving pain | Diosgenin | BLM-induced PF rats | Rats (100 mg/kg) | Regulation of TGF-β/Smad signaling | TGF-β1, snail, NF-κB, COX-2, IL-1β | [180] |
| Cell (10–30 μmol/L) | |||||||||
| 54 | Coptidis rhizoma | Ranunculaceae | Clearing heat and dryness, purging fire and detoxification | Berberine | BLM-induced PF mice | Mice (50, 100, 200 mg/kg) | Activate PPAR-γ | HGF, PPAR-γ | [260] |
| 55 | Astragali radix/ferulae resina | Fabaceae/Apiaceae | Replenishing qi and solidifying the surface, strengthening the upright and dispelling evil | Astragaloside IV/ferulic acid | BLM-induced PF mice | Mice (24 + 40.8 mg/kg) | Inhibit TGF-β1/Smad3 signaling | TGF-β1, Nrf2 | [261] |
| 56 | Scutellariae radix | Scutellaria | Clearing away heat and dampness, purging fire and detoxifying | Baicalin | BLM-induced PF rats and RPF | Rats (50 mg/kg) | Regulation of CaMKII and PI3K/AKT signaling | PI3K, AKT | [141] |
| Cell (20, 40, 60, 80 μg/mL) | |||||||||
| 57 | Calycosin, CA | BLM-induced PF mice and MLE-12 cells | Mice (7, 14 mg/kg) | Inhibit AKT/GSK3β/β-catenin signaling | AKT, GSK3β | [144] | |||
| cell (0–80 μmol/L) | |||||||||
| 58 | Centellae herba | Apiaceae | Clearing heat and detoxification, promoting diuresis and detumescence | Asiaticoside | BLM-induced PF mice | Mice (50 mg/kg) | Activates cAMP and RAP1 signaling | A2AR, RAP1 | [262] |
| 59 | BLM-induced PF mice | Mice (50 mg/kg) | Up-regulation of BMP7/Smad1/5 signaling | BMP7, Smad1/5 | [263] | ||||
| 60 | Tribuli fructus | Cucurbitaceae | Soothing the liver and relieving depression, promoting diuresis and reducing swelling | Terrestrosin D | BLM-induced PF mice | Mice (10 mg/kg) | Anti-inflammatory | HYP, IL-6, IL-8, TGF-β, PDGF-AB | [264] |
| 61 | Zingiberis rhizoma recens | Zingiberaceae | Warm the middle to dissipate the cold, solve the surface and dissipate the cold | Zingerone | BLM-induced PF rats | Rats (50, 100 mg/kg) | Affects TGF-β1 and iNOS signaling | TGF-β1, MDA, SOD, TNF-α, IL-1β | [265] |
| 62 | Lonicerae japonicae caulis | Caprifoliaceae | Clearing heat and detoxification, relieving surface and dissipating heat | Blue honeysuckle extract | Silica-induced PF mice and HLN cells | Mice (100, 200, 400 mg/kg) | Regulation of NRF2/HO-1 MAPK signaling | NRF2, HO-1, MAPK | [266] |
| 63 | Cyanidin-3-glucoside | Silica-induced PF mice | Mice (100, 200, 400 mg/kg) | Inhibits STAT1/3 signaling | STAT1, STAT3 | [267] | |||
| 64 | Blueberry | Ericaceae | Pterostilbene | LPS-induced PF mice | Mice (12.5, 25, 50 mg/kg) | Activation of Keap-1/Nrf2 inhibits A20/NF-κB and NLRP3 signaling | NF-κB, NLRP3 | [268] | |
| 65 | Cyanobacteria | C-Phycocyanin | Oleic acid-induced PF mice and A549, HFL-1 cells | Mice (1, 3, 9 mg/kg) | Regulation of TGF-β/Smad and MAPK signaling | TGF-β1, MAPK | [184] | ||
| Cell (10, 30 μmol/L) | |||||||||
| 66 | Eclipta prostrata L | Asteraceae | Heat-clearing and detoxification, black hair | Wedelolactone | BLM-induced PF mice and PLFs cells | Mice (2, 10 mg/kg) | Inhibition of RAF1-MAPKs signaling | Col I, α-SAM, AMPK | [185] |
| cell (10 μmol/L) | |||||||||
| 67 | Nelumbinis semen | Nelumbonaceae | Clear the heart and calm the mind, moisturize the lungs and relieve cough | Lotus plumule extract | BLM-induced PF mice | mice (80, 160, 240 mg/kg) | Inhibit TGF-β1/Smad3 signaling | TGF-β1, α-SMA | [186] |
| 68 | Oxytropis falcata Bunge | Fabaceae | Clearing heat and detoxification, dispelling wind and dispersing blood stasis | Flavonoids of Oxytropis falcata | BLM-induced PF rats | Rats (100, 200, 400 mg/kg) | Regulates JAK/STAT1 signaling | SOCS, p-JAK1 | [269] |
| 69 | gentianae radix et rhizoma | Gentianaceae | Clearing heat, dryness and dampness, purging liver and gallbladder fire | Gentiopicroside | BLM-induced PF mice | Mice (2.5, 10 mg/kg) | Anti-inflammatory | TNF-α, IL‐1β, TGF-β1, CTGF | [187] |
| 70 | Cervi cornu pantotrichum | Tonifying the kidney and tonifying essence, strengthening muscles and bones | Pilose antler peptide | BLM-induced PF mice and A549 cells | Mice (50, 100 mg/kg) | Regulation of ROCK/NF-κB signaling | NF-κB, MPO, SOD, IL-1β, IL-6, TNF-α, IκBα | [270] | |
| 71 | Siratia grosvenorii | Cucurbitaceae | Clear heat and moisturize the lungs, invigorate body and quench thirst | Mogrol | BLM-induced PF mice and NIH3T3 cells | Mice (1, 5, 10 mg/kg) | Regulation of TGF-β1 and AMPK signaling | TGF-β1, AMPK | [188] |
| Cell (1, 5, 10 μmol/L) | |||||||||
| 72 | Gallnut | Anacardiaceae | Stop diarrhea, converge, astringent intestines | Gallic acid derivative | BLM-induced PF mice | Mice (75, 150, 300 mg/kg) | Anti-inflammatory and antioxidant | NOX4, Nrf2 | [271] |
| 73 | Ophiopogonis radix | Liliaceae | Nourish yin and moisturize dryness, clear the heart and calm the mind | Ophiopogonin C | radiation-induced PF mice | Mice (3 mg/kg) | Inhibit TGF-β1 signaling | TGF-β1, IL-6, HYP, SOD, MDA, MMP-2 | [272] |
| 74 | Mangifera indica | Anacardiaceae | Clearing heat and detoxification, invigorating the stomach and eliminating food | Mangiferin | BLM-induced PF rats and A549 cells | Rats (40 mg/kg) | Inhibit TGF-β1/Smad2/3 signaling | TLR4, TGF-β1 | [189] |
| Cell (10 μg/mL) | |||||||||
| 75 | Rosmarinus officinalis | Lamiaceae | Rosmarinic Acid | radiation-induced lung damage rats | Rats (30, 60, 120 mg/kg) | Inhibits RhoA/Rock signaling | RhoA, Rock | [273] | |
| 76 | Carnosol | BLM-induced PF rats | Rats (10, 20, 40 mg/kg) | Anti-inflammatory and antioxidant | HYP, MDA, PC, NO, GSH, SOD, TNF-α, IL-6 | [274] | |||
| 77 | Oroxyli semen | Palmaceae | Detoxify and remove phlegm, relieve cough and asthma | Chrysin | BLM-induced PF rats | Rats (50 mg/kg) | Inhibit TGF-β1 signaling | TGF-β1, TXNIP | [275] |
| 78 | Aucklandiae radix | Asteraceae | Regulate spleen and stomach, remove dampness and eliminate phlegm | Costunolide | BLM-induced PF mice and HELF cells | Mice (10, 20 mg/kg) | Regulation of NF-kB and TGF-β1/Smad2/Nrf2-NOX4 signaling | NF-kB, TGF-β1 | [276] |
| Cell (10, 20 μmol/L) | |||||||||
| 79 | Grape | Resveratrol | BLM-induced PF rats and BEAS-2B cells | Rats (25, 50, 100 mg/kg) | Inhibits HIF-1α and NF-κB signaling | HIF-1α, NF-κB | [277] | ||
| Cell (2.5, 5, 10 mg/mL) | |||||||||
| 80 | PM-induced PF mice and BEAS-2B cells | Mice (100 mg/kg) | Inhibit the expression of NLRP3 | NLRP3, TGF-β1, IL-1β, IL-6, TNF-α, α-SMA | [278] | ||||
| Cell (1, 5 μmol/L) | |||||||||
| 81 | FCA-induced arthritis rats | Rats (10 mg/kg) | Regulation of JAK/STAT/RANKL signaling | JAK, STAT | [279] | ||||
| 82 | Grape seed proanthocyanidin extract (GSPE), | BLM-induced PF mice and A549 cells | Mice (30, 60, 90; 50, 100, 150 mg/kg) | Inhibition of oxidative stress inhibits epithelial cell apoptosis | HYP, TNF-α, IL-1β, IL-6, PARP, Bak | [280] | |||
| Cell (1 μg/mL) | |||||||||
| 83 | Indigo naturalis | Brassicaceae | Clearing heat and detoxification, reducing swelling and relieving pain | Indirubin | BLM-induced PF mice and PMLFs HPFs cells | Mice (12.5, 25 mg/kg) | Inhibit TGF-β1/Smad signaling | TGF-β1, ALT, CR, HYP, Collagen I, α-SMA | [191] |
| Cell (2.5–60 μmol/L) | |||||||||
| 84 | Artemisia annua L. | Asteraceae | Clear deficiency heat and remove bone steaming | Dihydroartemisinin | BLM-induced PF mice | Mice (25, 50, 100 mg/kg) | Reduced expression of TNF-α, IL-6 and TGF-β1 via TGF-β/JAK2/STAT3 signaling | TGF-β1, JAK2, STAT3 | [281] |
| 85 | BLM-induced PF rats | Rats (50 mg/kg) | anti-oxidation | SOD, GSH, MDA, HO-1, Nrf2 | [282] | ||||
| 86 | Nervilia fordii | Orchidaceae | Clearing heat and moistening the lungs, relieving cough and resolving phlegm | The extract of Nervilia fordii | BLM-induced PF rats and CT6 cells | Rats (100, 200, 400 mg/kg) | Inhibit TGF-β1/Smad signaling | TGF-β1, HYP, MPO, T-AOC, GSH, SOD, TNF-α | [283] |
| Cell (100, 200, 500 μg/mL) | |||||||||
| 87 | Cinnamomi cortex | Lauraceae | Warming the meridians and dispelling cold, dredging yang and reaching the camp | Trans-cinnamaldehyde | V79-4 cells | Cell (0–50 μmol/L) | Activates Nrf2/HO-1 signaling | Nrf2, HO-1, ROS, MMP | [284] |
| 88 | Notoginseng radix et rhizoma | Araliaceae | Promoting blood circulation and removing blood stasis, clearing heat and detoxification | Panax notoginseng saponin | ferric trichloride-induced PF Japanese rabbits | Rabbit(50 mg/kg) | Alleviate lung damage | IL-6, NF-κB | [285] |
| 89 | Inonotus sanghuang | Clearing heat and detoxification, promoting blood circulation and removing blood stasis | Inonotus sanghuang extract (ISE) | BLM-induced PF mice and A549 cells | Mice (0.6% w/w) | Inhibit TGF-β1/Smad signaling | TGF-β1, Smad2/3 | [193] | |
| Cell (2, 4 μg/mL) | |||||||||
| 90 | Kaempferiae rhizoma | Zingiberaceae | Promoting qi to relieve pain and regulating qi in a broad way | Alpha-Mangostin | BLM-induced PF mice | Mice (10 mg/kg) | Regulates AMPK signaling | AMPK, α-SMA, Smad2/3, Col I, MMP-9 | [286] |
| 91 | Cnidii fructus | Loranthaceae | Warming the kidney to help yang, tonifying essence and astringent | Osthole | BLM-induced PF mice | Mice (40 mg/kg) | Downregulation of TGF-β1/nox4 signaling | TGF-β1, nox4 | [287] |
| 92 | Zingiberis rhizoma recens | Zingiberaceae | Warm the middle to dissipate the cold, solve the surface and dissipate the cold | 6 gingerol | BLM-induced PF mice and | Mice (100, 250 mg/kg) | Activates SIRT1 signaling signaling | SIRT1, α-SMA, TNF-α, IL-6, IL-1β | [195] |
| human embryonic lung fibroblasts MRC-5 | Cell (10, 20 μmol/L) | ||||||||
| 93 | Amaryllidaceae | Amaryllidaceae | Dispel wind and detoxification, kill insects and stop itching | Lycorine | BLM-induced PF mice and BMDMs cells | Mice (5, 10, 20 mg/kg) | Inhibit the expression of NLRP3 | NLRP3, MPO, IL-6, IL-1β, α-SMA | [288] |
| Cell (0–40 mmol/L) | |||||||||
| 94 | Salvia officinalis | Lamiaceae | Clearing heat and detoxification, dispelling wind and relieving pain | Salvia officinalis | BLM-induced PF rats | Rats (50, 100, 150 mg/kg) | Anti-oxidation | CAT, MDA, SOD | [289] |
| 95 | Silybi fructus | Asteraceae | Clearing heat and detoxification, soothing the liver and promoting gallbladder | Silibinin | Silica-induced PF mice | Mice (100, 300 mg/kg) | Anti-inflammatory Inhibits EMT | MDA, GSH, HYP, IL-6, IL-1β, IL-17, TGF-β1 | [196] |
| 96 | Ampelopsis grossedentata (Hand.-Mazz.) W. T. Wang (Vitaceae) | Lamiaceae | Promoting blood circulation and regulating menstruation, diuresis and detumescence | Dihydromyricetin | BLM-induced PF mice and MLG cells | Mice (50, 100, 200 mg/kg) | Inhibit TGF-β1/Smad signaling | TGF-β1, α-SMA | [197] |
| 97 | Schisandrae chinensis fructus/glycyrrhizae radix et rhizoma | Magnoliaceae/Fabaceae | Astringent and astringent, replenishing qi and invigorating fluid | Schizandrin B + Glycyrrhizic acid | BLM-induced PF mice | Mice (100, 75, 100 + 75 mg/kg) | Regulation of TGF-β/Smad2 signaling | NOX1, Smad2 | [290] |
| 98 | Croci stigma | Iridaceae | Promoting blood circulation and removing blood stasis, regulating menstruation and relieving pain | Crocin | BLM-induced PF rats | Rats (25 mg/kg) | Anti-inflammatory and antioxidant | HYP, GSH, CAT, SOD, TNF-α, MDA | [291] |
| 99 | BLM-induced PF rats | Rats (20 mg/kg) | Regulation of NRF2 and HO-1 signaling | NRF2, HO-1 | [292] | ||||
| 100 | Rhododendron brachycarpum G. Don | Ericaceae | Clearing heat and detoxification, reducing swelling and relieving pain | Hyperoside | BLM-induced PF mice | Mice (50 mg/kg) | Regulation of AKT/GSK3b signaling | AKT, GSK3b | [203] |
| 101 | Arenaria kansuensis | Caryophyllaceae | Clearing heat and detoxification, diuresis and purging gonorrhea | A. kansuensis ethanol extract | paraquat-induced PF rats | Rats (170, 350, 700 mg/kg) | Inhibit NF-κB/TGF-β1/Smad2/3 signal transduction | NF-κB, TGF-β1, Smad2/3 | [203] |
| 102 | Morchella esculenta | Morchellaceae | Tonifying the kidney, tonifying qi, nourishing blood and calming the mind | FMP-1 | A549 cells | Cell (50–300 μg/mL) | Regulation of PI3K/AKT-Nrf2/HO-1 signaling | ROS, PI3K | [293] |
| 103 | Leonuri herba | Lamiaceae | Promoting blood circulation and regulating menstruation, reducing swelling and relieving pain | Leonurine | BLM-induced PF mice | Mice (50, 100 mg/kg) | Up-regulation of AKT signaling | AKT, ECAD, TGF-β, BAX, ACTA2 | [294] |
| 104 | Epimedii folium | Berberidaceae | Kidney deficiency and impotence, sore waist and knees | Icariin | BLM-induced PF rats | Rats (60 mg/kg) | Inhibit hippo signaling | YAP, IL-1β, IL-6, TGF-β1, TNF-α | [295] |
| 105 | Houttuyniae herba | Saururaceae | Heat-clearing and detoxification, sore waist and knees | Sodium Houttuyfonate | BLM-induced PF mice | Mice (45, 90 mg/kg) | Anti-inflammatory | IL-1β, TNF-α | [206] |
| 106 | Anemarrhenae rhizoma | Liliaceae | Clearing heat and moistening dryness, promoting fluid to quench thirst | total extract of Anemarrhenae Rhizoma (TEAR) | BLM-induced PF rats | Rats (1.33, 4, 12 g/kg) | Inhibit TGF-β1/Smad signaling | TGF-β1, HYP, COlI, ColIII, MPO, NO | [296] |
| 107 | Hedara nepalensis var.sinensis | Araliaceae | Clearing heat and detoxification, dispelling wind and promoting dampness | Hederagenin | BLM-induced PF rats | Rats (10, 20, 50 mg/kg) | Adjust Ras/JNK/NFAT4 axis | JNK, NFAT4 | [209] |
Improve extracellular matrix deposition
ECM is a three-dimensional network of non-cellular macromolecules, made primarily of collagen, non-collagenous proteins, and glycoproteins, among others [297]. A pathological characteristic of PF is the massive buildup of ECM in the interstitium. Excessive ECM deposition promotes alveolar structural loss, which generates or aggravates PF. Initial damage to alveolar epithelial cells stimulates the production of multiple pro-fibrotic cytokines that induce fibroblast proliferation, aggregation, and transdifferentiation, generating myofibroblasts that secrete stronger ECM, releasing high levels of ECM, and promoting the development of PF [298]. Under pathological situations, excessive deposition of ECM in the interstitial lung can stimulate ECM synthesis and alter the regulatory function of matrix metalloproteinases (MMPs)/TIMPs [299, 300]. Under pathological situations, excessive deposition of ECM in the interstitial lung can stimulate ECM synthesis and alter the regulatory function of matrix metalloproteinases (MMPs)/TIMPs [301]. The majority of animal investigations have concentrated on the independent detection of TGF-β1, Smads, and MMPs/TIMPs, and the precise mechanism of action is still unknown. Furthermore, Smads, MMPs, and TIMPs comprise several family members whose activities and mutual effects have yet to be thoroughly investigated [45].
In addition to its anti-inflammatory and antioxidant properties, Salvia miltiorrhiza can also play an anti PF role by inhibiting ECM deposition. Feng’s research suggests that tanshinone IIA inhibits TGF-β1/Smad signaling pathway to reduce silica induced PF [302]. Further research found that tanshinone IIA may improve silicosis fibrosis by inhibiting the EMT phase. Cryptotanshinone is a lipophilicity compound derived from salvia miltiorrhiza, which has antioxidant, anti-inflammatory and anti-angiogenesis properties [303]. Zhang research team found that cryptotanshinone improved the lung function of the rat model of bleomycin induced PF, alleviated pathological changes and inhibited ECM precipitation [304]. This experiment found that cryptotanshinone may prevent PF by inhibiting Smad and STAT3 signaling pathways through cell experiments. In conclusion, many active compounds in Salvia miltiorrhiza have good anti PF effects in terms of anti inflammation, anti-oxidation, inhibition of EMT and inhibition of ECM precipitation.
Astragalus membranaceus is a TCM with various pharmacological effects such as enhancing immune function, protecting lung function, and reducing oxidative stress. Astragaloside IV is one of the most important active ingredients in astragalus membranaceus. Some studies have shown that astragaloside IV not only improves the secretion of collagen induced by bleomycin, but also reduces the level of type III collagen, serum laminin and hyaluronic acid in lung homogenate [305]. These findings indicated that astragaloside IV can effectively inhibit ECM deposition in PF mice, providing experimental data support for its use as a candidate compound for the treatment of PF. Li’s team also studied the anti PF effect of astragaloside IV [306]. The difference is that this experiment uses the silicon induced PF model. The experiment shown that astragaloside IV can effectively inhibit the formation of silicosis fibrosis. Further cell experiments have found that astragaloside IV can inhibit ECM precipitation in fibroblasts, and its mechanism of action may be related to TGF-β1/Smad signaling pathway is involved. These results suggest that astragalus may have the potential to improve PF by inhibiting ECM precipitation in a variety of disease induced PF. In addition to the above-mentioned TCM that can improve PF, the detailed information of other TCM studies in the past five years that can improve PF through inhibition of ECM deposition were summarized and presented in Table 3.
Table 3.
Details about some traditional Chinese medicines improving pulmonary fibrosis by inhibiting extracellular matrix deposition
| No | Source | Genus information | Traditional efficacy | Active compound | Experiment model | Administration dose | Therapeutic target | Cytokine | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Andrographis herba | Acanthaceae | Clearing heat and detoxification, cooling blood | Andrographolide | NIH 3T3, PLF cells | Rats (10 mg/kg) | Inhibits TGF-β1/Smad2/3 and Erk1/2 signaling | TGF-β1, Smad2/3 | [132] |
| cell (2, 5, 10 μmol/L) | |||||||||
| 2 | Silica-induced PF mice | Mice (3, 10 mg/kg) | Inhibition of ECM precipitate formation | GSH, HYP, MDA, IL-1β, IL-6, TNF-α, TGF-β1 | [131] | ||||
| 3 | Rhei radix et rhizoma | Polygonaceae | Purging and defecating, clearing heat and detoxification | Chrysophanol | BLM-induced PF mice | Mice (10 mg/kg) | Inhibits Wnt/β-catenin signaling | β-catenin, HYP, TNF-α, IL-1β, IL-6, IFN-γ | [226] |
| 4 | Rhapontin | BLM-induced PF rats and THP-1 cells | Rats (25, 50, 100 mg/kg) | Regulation of TGF-β/Smad signaling | TGF-β, Smad | [137] | |||
| cell (50 μmol/L) | |||||||||
| 5 | Salviae miltiorrhizae radix et rhizoma | Lamiaceae | Promoting blood circulation and removing blood stasis, reducing swelling and relieving pain | Salvia miltiorrhiza | BLM-induced PF mice and NIH3T3 cells | Mice (21, 40, 80 mg/kg) | anti-oxidation | Nrf2, NOX4 | [228] |
| Cell (0.1, 1, 3 μmol/L) | |||||||||
| 6 | Tanshinone IIA | Silica-induced PF mice | Mice (25 mg/kg) | Inhibit TGF-β1/Smad signaling | TGF-β1, α-SMA, Smad2/3/4/7 | [307] | |||
| 7 | Cryptotanshinone | BLM-induced PF rats and HLFs cells | Rats (7.5, 15, 30, 60 mg/kg) | Inhibition of TGF-β/Smad signaling | TGF-β1, HYP, TNF-α, IL-6, ColI, ColIII, α-SMA | [304] | |||
| Cell (0.75–18 mg/mL) | |||||||||
| 8 | Astragalus membranaceus | Fabaceae | Replenishing qi and solidifying the surface, strengthening the upright and dispelling evil | Astragaloside IV | Silica-induced PF rats | Rats (20 mg/kg) | Inhibition of TGF-β/Smad3 signaling | TGF-β1, α-SMA, Collagen III, Smad2/3 | [306] |
| Cell (7, 20, 60 μg/mL) | |||||||||
| 9 | BLM-induced PF rats | Rats (10 mg/kg) | Inhibition of ECM precipitate formation | HMGB1, HYP, Col-III, LN, HA | [305] | ||||
| 10 | Scutellaria baicalensis Georgi | Scutellaria | Clearing away heat and dampness, purging fire and detoxifying | Baicalein | MRC-5 cells | Cell (1, 10 μmol/L) | Downregulation of CTGF expression | CTGF, TGF-β1, Smad2/3 | [308] |
| 11 | Curcumae longae rhizoma | Zingiberaceae | Promoting blood circulation and removing blood stasis, Regulating menstruation and relieving pain | Curcumin | BLM-induced PF rats | Rats (300 mg/kg) | Inhibit TGF-β1 signaling | TGF-β1, HYP, Collagen III | [309] |
| 12 | BLM-induced PF rats | Rats (300 mg/kg) | Inhibition of ECM precipitate formation | fibronectin, lung glycoproteins | [235] | ||||
| 13 | Tripterygium wilfordii Hook. f | Ranunculaceae | Clearing heat and detoxification, promoting blood circulation and removing blood stasis | Triptolide | radiation-induced PF mice and NIH3T3 cells | Mice (0.25 mg/kg) | Inhibit NF-κB signaling | NF-κB, LOX, IκBα | [239] |
| Cell (5 ng/mL) | |||||||||
| 14 | Isorhynchophylline | Silica-induced PF mice | Mice (20 mg/kg) | Anti-inflammatory | / | [240] | |||
| 15 | Rhodiolae crenulatae radix et rhizoma | Crassulaceae | Replenishing qi and activating blood circulation, dredging pulse and relieving asthma | Rutin | BLM-induced PF rats | Rats (50, 100 mg/kg) | Regulation of TGF-β1/α-SMA/Col I/III signaling | TGF-β1, α-SMA | [259] |
| 16 | Ginseng radix et rhizoma | Araliaceae | Replenish qi and nourish blood, invigorate body and quench thirst | Total ginsenoside | BLM-induced PF mice | Mice (40, 80, 160 mg/kg) | Regulation of TGF-β1/Smad signaling | TGF-β1, α-SMA, Smad2/3/7, MMP-2, MMP-9 | [310] |
| 17 | Dioscorea polystachya Turczaninow | Dioscoreaceae | Tonifying spleen and lung, nourishing yin and moistening dryness | Dioscin | Silica-induced PF mice and AM MH-S cells | Mice (mg/kg) | Promotes autophagy in alveolar macrophages | LC3, p62, AKT, mTOR, BECN1 | [311] |
| Cell (200, 400, 800 nmol/L) | |||||||||
| 18 | Artemisia annua L | Asteraceae | Clear deficiency heat and remove bone steaming | Dihydromyricetin | BLM-induced PF mice and MLG cells | Mice (50, 100, 200 mg/kg) | Inhibit TGF-β1/Smad signaling | TGF-β1, α-SMA | [197] |
| 19 | Epimedii folium | Berberidaceae | Kidney deficiency and impotence, sore waist and knees | Icariin | BLM-induced PF rats | Rats (60 mg/kg) | Inhibit Hippo signaling | YAP, IL-1β, IL-6, TGF-β1, TNF-α | [295] |
| 20 | Chelidonii herba | Papaveraceae | Clearing heat and detoxification, reducing swelling and relieving pain | Chelerythrine | BLM-induced PF mice | Mice (0.375, 0.75 mg/kg) | activate Nrf2/ARE signal transduction | Nrf2, ARE | [244] |
| 21 | Psoraleae fructus | Fabaceae | Warm the kidney and consolidate the essence, strengthen the muscles and bones | Psoralen | BLM-induced PF mice and NIH3T3 cells | Mice (5 mg/kg) | Inhibition of ECM precipitate formation | TNF-α, IL‐1β | [167] |
| Cell (5, 10, 20, 40 μmol/L) | |||||||||
| 22 | Citri reticulatae pericarpium | Rutaceae | Regulating qi and eliminating food, resolving phlegm and relieving cough | citrus alkaline extracts (CAEs) | BLM-induced PF mice | Mice (16, 32, 64 mg/kg) | Inhibit TGF-β1/Smad3 signaling | TGF-β1, LOX, HYP | [312] |
| 23 | Salviae miltiorrhizae radix et rhizoma/chuanxiong rhizoma | Lamiaceae/Apiaceae | Promoting blood circulation and removing blood stasis, Clearing heat and detoxification | Salvia miltiorrhiza and ligustrazine | BLM-induced PF rats | Rats (125 + 43.75, 250 + 87.5, 500 + 175 mg/kg) | Regulation of TGF-β/Smad signaling | TNF-α, TGF-β1 | [313] |
| 24 | Rabdosiae rubescentis herba | Lamiaceae | Clearing heat and detoxification, reducing swelling and relieving pain | Oridonin | BLM-induced PF mice and MRC-5 cells | Mice (10, 20 mg/kg) | Regulation of TGF-β/Smad signaling | TGF-β, Smad | [314] |
| Cell (5, 10 μmol/L) | |||||||||
| 25 | Curcumae longae rhizoma | Zingiberaceae | Promoting blood circulation and removing blood stasis, Regulating menstruation and relieving pain | curcumin/Curcumol | HFL cells | Cell (8, 16, 32 μg/mL) | Inhibition of ECM precipitate formation | Col-I, Col-III, TGF-β1, α-SMA | [315] |
| 26 | Citrus fruits | Rutaceae | Invigorate the spleen and replenish qi, moisturize the lungs and relieve cough | Hesperidin | BLM-induced PF rats | Rats (25, 50, 100 mg/kg) | Inhibits TGF-β1/Smad3/AMPK and I-κBα/NF-κB signaling | TGF-β1, NF-κB | [253] |
| 27 | Polygoni cuspidati rhizoma et radix | Polygonaceae | Clearing heat and detoxification, promoting blood circulation and removing blood stasis | Polydatin | BLM-induced PF rats and A549 cells | Rats (10, 40, 160 mg/kg) | Inhibit TGF-β1/Smad signaling | TGF-β1, Col-1, α-SMA, TNF-α, IL-6, IL-13 | [156] |
| Cell (0–120 μmol/L) | |||||||||
| 28 | Coptidis rhizoma | Ranunculaceae | Clearing heat and dryness, purging fire and detoxification | berberine | BLM-induced PF mice | Mice (50, 100, 200 mg/kg) | activate PPAR-γ | HGF, PPAR-γ | [260] |
| 29 | Astragali radix/ferulae resina | Fabaceae/Apiaceae | Replenishing qi and solidifying the surface, strengthening the upright and dispelling evil | astragaloside IV/ferulic acid | BLM-induced PF mice | Mice (24 + 40.8 mg/kg) | Inhibit TGF-β1/Smad3 signaling | TGF-β1, Nrf2 | [261] |
| 30 | Centellae herba | Apiaceae | Clearing heat and detoxification, promoting diuresis and detumescence | Asiaticoside | BLM-induced PF mice | Mice (50 mg/kg) | Up-regulation of BMP7/Smad1/5 signaling | BMP7, Smad1/5 | [263] |
| 31 | sophorae fla vescentis radix | Fabaceae | Clearing heat and detoxification, diuresis and purging gonorrhea | Matrine | MRC-5 cells | Cell (10 μmol/L) | Inhibit TGF-β/Smad2/3 signaling | TGF-β, Smad2/3 | [182] |
| 32 | Blueberry | Ericaceae | Pterostilbene | A549 cells | Cell (0–100 μmol/L) | Inhibit TGF-β1 signaling | TGF-β1, Bcl-2, Bax, LC3, p62, p21 | [183] | |
| 33 | Siratia grosvenorii | Cucurbitaceae | Clear heat and moisturize the lungs and open pharynx | Mogrol | BLM-induced PF mice and NIH3T3 cells | Mice (1, 5, 10 mg/kg) | Regulation of TGF-β1 and AMPK signaling | TGF-β1, AMPK | [188] |
| Cell (1, 5, 10 μmol/L) | |||||||||
| 34 | Rosmarinus officinalis | Lamiaceae | Rosmarinic Acid | radiation-induced lung damage rats | Rats (30, 60, 120 mg/kg) | Inhibits RhoA/Rock signaling | RhoA, Rock | [273] | |
| 35 | Grape | Resveratrol | BLM-induced PF rats and MRC-5 cells | Rats (60 mg/kg) | Regulates MAPK/AP-1 signaling | MAPK/AP-1 | [316] | ||
| Cell (10 μmol/L) | |||||||||
| 36 | Kaempferiae rhizoma | Zingiberaceae | Promoting qi to relieve pain and regulating qi in a broad way | Alpha-Mangostin | BLM-induced PF mice | Mice (10 mg/kg) | Regulates AMPK signaling | AMPK, α-SMA, Smad2/3, Col I, MMP-9 | [286] |
| 37 | Silybi fructus | Asteraceae | Clearing heat and detoxification, soothing the liver and promoting gallbladder | Silibinin | Silica-induced PF mice | Mice (100, 300 mg/kg) | Anti-inflammatory Inhibits EMT | MDA, GSH, HYP, IL-6, IL-1β, IL-17, TGF-β1 | [196] |
| 38 | Pseudostellariae radix | Caryophyllaceae | Yiqi Jianpi, Shengjin fluid | Heterophyllin B | BLM-induced PF mice and MLE-12 cells | Mice (5, 20 mg/kg) | Activates AMPK, inhibits TGF-β1-Smad2/3 signaling, and downregulates STING | TGF-β1, CoL-1, α-SMA | [317] |
| Cell (1–100 μmol/L) | |||||||||
| 39 | Arenaria kansuensis | Caryophyllaceae | Clearing heat and detoxification, diuresis and purging gonorrhea | A. kansuensis ethanol extract | paraquat-induced PF rats | Rats (170, 350, 700 mg/kg) | Inhibit NF-κB/TGF-β1/Smad2/3 signal transduction | NF-κB, TGF-β1, Smad2/3 | [203] |
| 40 | Aurantii fructus immaturus | Rutaceae | Regulating qi stagnation, resolving phlegm and relieving cough | Neohesperidin | BLM-induced PF mice and NIH3T3, MLg, A549 cells | Mice (20 mg/kg) | Inhibition of TGF-β/Smad3 signaling | TGF-β1, Smad2/3, Erk, p-38, JNK | [208] |
| Cell (0–200 μmol/L) | |||||||||
| 41 | Polyporus | Polyporaceae | Diuresis, detumescence and phlegm | Polyporus Polysaccharide | BLM-induced PF mice and HLF cells | Mice (50, 100 mg/kg) | Inhibit TGF-β1/Smad2/3 signaling | TGF-β1, Smad2/3 | [210] |
| Cell (1 mg/mL) | |||||||||
| 42 | Arnebiae radix | Boraginaceae | Clearing heat and detoxification, moistening dryness and invigorating muscle | Shikonin | MLFC cells | Cell (0.1, 0.3, 1, 3, 10 μmol/L) | Inhibit Akt signaling | MAPK, akt | [318] |
Mediate apoptosis and autophagy
Autophagy is the degradation of intracytoplasmic foreign bodies, damaged, and senescent cells by the cell's own structures via lysosomes, which helps maintain a homeostatic equilibrium between degradation and recirculation [319]. Cellular autophagy can promote PF by promoting fibroblast activation, myofibroblast differentiation, and ECM deposition, indicating that autophagy may be one of the key mechanisms in the pathogenesis of PF [320, 321]. The expression of cellular autophagy is typically deficient in all IPF lung tissues, which appears to be one of the risk factors for IPF, as suggested by the current study [322]. When autophagy is inhibited, lung fibroblast epithelial senescence and myofibroblast differentiation can be accelerated. Numerous studies have demonstrated that herbal medicine regulates cellular autophagy primarily via mTOR-dependent and -independent pathways. Apoptosis is a form of programmed cell death characterized primarily by cell shrinkage and the formation of apoptotic vesicles [323]. Excessive apoptosis of alveolar epithelial cells induces aberrant secretion of numerous cytokines, including TGF-β1, and accelerates the progression of PF [324]. Inadequate apoptosis of lung fibroblasts results in their massive transformation into myofibroblasts, which promotes ECM deposition and lung fibrosis [325]. There are multiple signaling pathways involved in the role of apoptosis in lung fibrosis. It has been hypothesized that the MAPK/NF-κB signaling pathway plays a crucial role in this.Autophagy and apoptosis are complex mechanisms that control cell growth and death under physiological and pathological conditions. It has been suggested that the two processes, reduced autophagic activity and apoptotic resistance, may be interrelated in IPF fibroblasts [326]. When mTOR activity is inhibited, autophagy increases, apoptosis increases, and resistance to apoptosis decreases. Enhanced autophagy is accompanied by increased apoptosis. Consequently, insufficient autophagy can result in insufficient apoptosis in fibroblastic cells and excessive apoptosis in alveolar epithelial cells, which, through a cascade of biological responses, leads to IPF.
Various components in the peel of citrus reticulata blanco have anti-inflammatory and antioxidant activities [327]. Wu’s experimental team extracted primary lung fibroblasts from normal mice and bleomycin induced PF mice for experiments. The results indicate that citrus alkaloid extract can effectively induce apoptosis in mouse lung fibroblasts, and its mechanism of action may be related to the p38/COX-2/Fas signaling pathway regulated by oxidative stress [328]. In addition, the experimental team explored the intervention effect of citrus alkaline extract on bleomycin induced PF in mice and its mechanism [329]. The experiment shows that citrine extract can effectively delay the degree of PF mice, and its mechanism may be through inhibiting NF-κB/p38-mediated signaling pathway inhibits cell apoptosis. In addition, studies have shown that citrus alkaloid extracts can activate COX-2 and β-Catenin/P53 pathway inhibits cellular senescence and reduces PF [330, 331]. To sum up, citrus extract can mediate cell apoptosis through multiple signaling pathways, but the specific pharmacodynamic substances in the extract that play a role in improving PF are still unclear, and further experimental research is needed.
Quercetin is a flavonol compound in TCM [332], which has anti-inflammatory, antioxidant, immunomodulatory and anti-tumor activities [333]. Literature studies have shown that quercetin alone cannot promote apoptosis, but quercetin can eliminate fibroblast resistance to apoptosis [334]. Further studies suggest that quercetin may enhance the susceptibility of aging fibroblasts to apoptosis by regulating the expression of caveolin-1 and Fas and activating AKT. In addition, Xiao found that quercetin not only promotes autophagy activity, but also improves fibrosis by inhibiting pro-fibrotic factors and AKT/mTOR signaling pathway [335]. These studies demonstrate quercetin's potential to combat PF by mediating apoptosis, adding to therapeutic strategies for IPF and other fibrotic diseases. However, these studies have only been conducted in vitro or in animals, and further clinical studies are still needed. In addition to the above-mentioned TCM that can improve PF, the detailed information of other TCM studies in the past five years that can improve PF through mediate apoptosis and autophagy were summarized and presented in Table 4.
Table 4.
Details about some traditional Chinese medicine improving pulmonary fibrosis by mediating apoptosis and autophagy
| No | Source | Genus information | Traditional efficacy | Active compound | Experiment model | Administration dose | Therapeutic target | cytokine | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Citri reticulatae pericarpium | Rutaceae | Regulating qi and eliminating food, resolving phlegm and relieving cough | citrus alkaline extracts (CAEs) | A549 cells | Cell (0.01 μmol/L) | Inhibit b-catenin/p53 signaling | PDGF, TNF-α, p21, p53, MMP-7, CTGF | [331] |
| 2 | BLM-induced PF mice and PMLF, MRC-5 cells | Mice (32, 64, 96 mg/kg) | prevent cellular senescence | COX-2, α-SMA, Fibronectin, p21, p16 | [330] | ||||
| Cell (50, 100, 200 μmol/L) | |||||||||
| 3 | BLM-induced PF mice | Mice (16, 32, 64 mg/kg) | Inhibit p38/NF-κB signaling | p38, NF-κB | [329] | ||||
| 4 | HPFS cells | Cell (50, 250, 500 μmol/L) | Induces apoptosis of lung fibroblasts | COX-2, Fas | [328] | ||||
| 5 | BLM-induced PF mice and A549 cells | Mice (32, 64, 96 mg/kg) | Regulates PERK and ATF3/PINK1 signaling | PERK, ATF3, PINK1 | [336] | ||||
| Cell (50, 100, 200 μg/mL) | |||||||||
| 6 | Curcumae longae rhizoma | Zingiberaceae | Promoting blood circulation and removing blood stasis, Regulating menstruation and relieving pain | Curcumin | LMSCs cells | Cell (2.5, 5, 10, 20 μmol/L) | Regulation of Akt/Nrf2/HO-1 signaling | Akt, Nrf2, HO-1 | [234] |
| 7 | BLM-induced PF rats | Rats (300 mg/kg) | Inhibit TGF-β1 signaling | TGF-β1, HYP, Collagen III | [309] | ||||
| 8 | Erigeron breviscapus | Asteraceae | Promoting blood circulation and removing blood stasis, clearing heat and detoxification | Scutellarein | BLM-induced PF mice | Mice (10 mg/kg) | Inhibits PI3K/Akt signaling | PI3K, Akt, Smad2/3, α-SMA | [172] |
| 9 | Amaryllidaceae | Amaryllidaceae | Dispel wind and detoxification, kill insects and stop itching | Lycorine | BLM-induced PF mice and BMDMs cells | Mice (5, 10, 20 mg/kg) | Inhibit the expression of NLRP3 | NLRP3, MPO, IL-6, IL-1β, α-SMA | [288] |
| Cell (0–40 mmol/L) | |||||||||
| 10 | Dioscorea polystachya Turczaninow | Dioscoreaceae | Tonifying spleen and lung, nourishing yin and moistening dryness | Dioscin | Silica-induced PF mice and AM MH-S cells | Mice (mg/kg) | Promotes autophagy in alveolar macrophages | MMP9,mtROS | [311] |
| Cell (200, 400, 800 nmol/L) | |||||||||
| 11 | Blueberry | Ericaceae | Pterostilbene | LPS-induced PF mice | Mice (12.5, 25, 50 mg/kg) | Activation of Keap-1/Nrf2 inhibits A20/NF-κB and NLRP3 signaling | NF-κB, NLRP3 | [268] | |
| 12 | Various plant sources | Quercetin | Tracheal Trauma Rabbit and WI-38 cells | Cell (5–200 μmol/L) | Inhibits AKT/mTOR signaling | AKT, mTOR | [335] | ||
| 13 | BLM-induced PF mice | Mice (30 mg/kg) | Enhance fibroblast apoptosis | Fas, DR4/5, CAveolin-1, AKT | [334] | ||||
| 14 | Sinapic acid | BLM-induced PF rats | Rats (10, 20 mg/kg) | Inhibit NF-κB/NRF2/HO-1 signaling | NF-κB, NRF2, HO-1 | [250] | |||
| 15 | Bletillae rhizoma | Orchidaceae | Convergence to stop bleeding, reduce swelling and promote muscle growth | Bletilla striata | Silica-induced PF mice and A549 cells | Mice (20, 40 mg/kg) | Inhibit Bax/Bcl-2 signaling | HO-1, Nrf2, γGCSc, Bax, Bcl-2 | [337] |
| Cell (10, 25, 50 μmol/L) | |||||||||
| 16 | Atractylodis rhizoma | Asteraceae | Invigorating spleen and stomach, tonifying qi and blood | Atractylenolide III | BLM-induced PF rats | Rats (0.6, 1.2, 2.4 mg/kg) | activate Nrf2/NQO1/HO-1 signal transduction | Nrf2, NQO1, HO-1 | [246] |
| 17 | Chuanxiong rhizoma | Apiaceae | Activating blood circulation and promoting qi, expelling wind and relieving pain | Ligustrazin | paraquat-induced PF mice and A549 cells | Mice (30 mg/kg) | Inhibit mTOR/Akt signaling | mTOR, Akt | [338] |
| 18 | Rhei radix et rhizoma | Polygonaceae | Purging and defecating, clearing heat and detoxification | Emodin | Silica-induced PF mice and A549 cells | Mice (25, 50, 100 mg/kg) | Regulation of NF-κB and TGF-β1/Smad3 signaling | NF-κB, TGF-β1 | [136] |
| 19 | Salviae miltiorrhizae radix et rhizoma/puerariae lobatae radix | Lamiaceae/Fabaceae | Promoting blood circulation and removing blood stasis, reducing swelling and relieving pain | Tanshinone IIA/puerarin | BLM-induced PF mice and MRC-5 cells | Mice (5 + 14 mg/kg) | Inhibition of IL 6-JAK2-STAT3/STAT1 signaling | IL6, STAT1 | [339] |
| 20 | Glycyrrhizae radix et rhizoma | Fabaceae | Nourishes qi and nourishes yin, clears away heat and detoxifies | Isoliquiritigenin | MRC-5 cells | Cell (0–40 mg/L) | Inhibit PI3K/AKT/mTOR signaling | PI3K, AKT, mTOR | [340] |
| 21 | Scutellariae radix | Scutellaria | Clearing away heat and dampness, purging fire and detoxifying | Baicalin | BLM-induced PF rats and RPF | Rats (50 mg/kg) | Regulation of CaMKII and PI3K/AKT signaling | PI3K, AKT | [141] |
| Cell (20, 40, 60, 80 μg/mL) | |||||||||
| 22 | Rosmarinus officinalis | Lamiaceae/Fabaceae | Rosmarinic Acid + carnosic acid | BLM-induced PF rats and HLFs cells | Rats (5 + 3 mg/kg) | Induces apoptosis of lung fibroblasts | p21, AKT, p38 | [341] | |
| Cell (50 + 10, 100 + 20 μmol/L) | |||||||||
| 23 | Cinnamomi cortex | Lauraceae | Warming the meridians and dispelling cold, dredging yang and reaching the camp | Trans-cinnamaldehyde | V79-4 cells | Cell (0–50 μmol/L) | Activates Nrf2/HO-1 signaling | Nrf2, HO-1 | [284] |
| 24 | Rhizoma Kaempferiae | Zingiberaceae | Relieving pain by activating qi and dispelling cold in the middle of warming | Kaempferol | Silica-induced PF mice | Mice (150 mg/kg) | Anti-inflammatory | Beclin-1, p62, LC3, MMP-9, MMP-2 | [342] |
| 25 | Schisandrae chinensis fructus | Magnoliaceae | Astringent and astringent, replenishing qi and invigorating fluid | Schisandra | BLM-induced PF rats | Rats (5 mg/kg) | Inhibit TGF-β1/Smad signaling | TGF-β1, Smad3/4/7 | [343] |
| 26 | Leonuri herba | Lamiaceae | Promoting blood circulation and regulating menstruation, reducing swelling and relieving pain | Leonurine | BLM-induced PF mice | Mice (50, 100 mg/kg) | Up-regulation of AKT signaling | AKT, ECAD, TGF-β1, ACTA2, BAX | [294] |
| 27 | Ginkgo folium | Ginkgoaceae | Calming the liver and eyesight, moistening the lungs and relieving cough | Ginkgo biloba Extract EGb761 | BLM-induced PF mice | Mice (25, 50, 100 mg/kg) | Regulation of NF-κB signaling | NF-κB, TNF-β, IL-1β, IL-6, α-SMA, TGF-β1 | [344] |
Inhibition of endoplasmic reticulum stress
The endoplasmic reticulum (ER) is an organelle responsible for maintaining protein homeostasis in cells. The accumulation of misfolded proteins in the ER is referred to as ERS. Existing literature suggests that ERS is a key mechanism mediating PF in AEC [345, 346]. For example, ER stress promotes inflammation, induces EMT, and activates pro-apoptotic pathways, leading to the generation of PF [345]. In order to maintain homeostasis, cells rely on protective mechanisms to help them cope with ER stress, collectively known as the unfolded protein response (UPR). When the UPR mechanism fails or is over-activated, it can lead to cell apoptosis [347].
ERS can be triggered by various factors, including genetics, environmental exposure, viral infections, and cellular aging. Studies have demonstrated that ERS induced by genetic mutations, such as those found in surfactant protein C (SFTPC), plays a significant role in the development of PF [348, 349]. Additionally, environmental factors can also induce ERS, which is a critical factor in the pathogenesis of PF. For example, research has shown that environmental factors like silica and cigarette smoke can trigger ERS, and airborne particulate matter can activate signaling pathways like PERK and ATF, inducing ERS in cells [350]. Cigarette smoke can cause ERS in bronchial epithelial cells, leading to cell apoptosis in both in vitro and in vivo settings [351, 352]. Viral infections may contribute to fibrosis development by inducing ERS and activating UPR. Research has demonstrated that aging mice infected with herpes virus may develop PF through mechanisms related to ERS [353]. Additionally, aging can impair normal ER function in organisms [354, 355], which is in line with clinical observations indicating that PF is more prevalent in older individuals. In conclusion, enhancing protein processing and mitigating downstream effects of ERS may be an effective strategy to treat fibrosis resulting from ERS.
Citrus reticulata Blanco peel is used to treat lung diseases in Chinese Traditional medicine. Recently, Wang's team carried out a study on citrus extract to improve PF [336]. The results shown that citrus extract could reduce collagen deposition in PF mice and inhibit the increase of endoplasmic reticulum stress biomarkers. Further cell experiments shown that citrus extract regulated ERS through PERK and ATF3/PINK1 pathways. However, it is unclear whether citrus alkaline extract can improve PF by regulating ERS, which still needs further experiments. This study provides an important reference for the mechanism of citrus extract in improving PF.
Chlorogenic acid is the main active ingredient of many TCMs, which has antibacterial, antiviral, and free radical scavenging pharmacological effects. Wang's team evaluated the effect of chlorogenic acid on improving PF related markers through mice and cell models [356]. The results shown that chlorogenic acid can effectively regulate the expression of PF related pathological markers, and reduce the degree of PF by inhibiting the ERS pathway. At the same time, chlorogenic acid plays a certain role in regulating apoptosis involved in PF. Although this study did not deeply explore the signal pathway involved in chlorogenic acid inhibiting ERS, it provided a new experimental reference for chlorogenic acid to improve PF.
Isorhamnetin is a flavonoid active compound in hippophae fructus, which has pharmacological effects such as antioxidant, anti-inflammatory, and anti-tumor. Recent research literature suggests that isorhamnetin can effectively inhibit bleomycin induced collagen deposition and reduce type I collagen and α-SMA [194]. In addition, they further demonstrated that isorhamnetin can improve the degree of PF by inhibiting EMT and ERS. Although more studies are needed to clarify the mechanism of Isorhamnetin in improving PF, this study shown for the first time the potential important value of isorhamnetin in improving PF.
In recent years, the research of citrus alkaline extracts, isorhamnetin and chlorogenic acid to improve PF has made great progress. These studies have explored the activity and pharmacological mechanism of these compounds to improve PF in animal or cell experiments, making these compounds possible candidates for new drugs to treat PF. However, it should be pointed out that these studies have failed to delve into the association between ERS and inflammatory response, EMT, and cell apoptosis, and also lack in-depth research on signaling pathways and molecular mechanisms. Therefore, future research should pay more attention to exploring the molecular mechanism and signal pathway of TCM in improving PF, so as to better understand the pharmacology and toxicology of these candidate compounds. In addition to the above-mentioned TCM that can improve PF, the detailed information of other TCM studies in the past five years that can improve PF through inhibition of endoplasmic reticulum stress were summarized and presented in Table 5.
Table 5.
Details about some traditional Chinese medicines improving pulmonary fibrosis by inhibiting endoplasmic reticulum stress
| No | Source | Genus information | Traditional efficacy | Active compound | Experiment model | Administration dose | Therapeutic target | cytokine | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Citri reticulatae pericarpium | Rutaceae | Regulating qi and eliminating food, resolving phlegm and relieving cough | citrus alkaline extracts (CAEs) | BLM-induced PF mice and A549 cells | Mice (32, 64, 96 mg/kg) | Regulates PERK and ATF3/PINK1 signaling | PERK, ATF3, PINK1 | [336] |
| cell (50, 100, 200 μg/mL) | |||||||||
| 2 | Hippophae fructus | Elaeagnaceae | Invigorate the stomach and eliminate food, relieve cough and expectoration | Isorhamnetin | BLM-induced PF mice | Mice (10, 30 mg/kg) | Suppress EMT | PERK, α-SMA, Col III, CHOP, GRP78, TGF-β1 | [194] |
| Cell (25, 50, 100 μmol/L) | |||||||||
| 3 | Lonicerae japonicae flos | Caprifoliaceae | Clearing heat and detoxification, dispelling wind and detoxification | Chlorogenic acid | BLM-induced PF mice | Mice (15, 30, 60 mg/kg) | Inhibits endoplasmic reticulum stress | α-SMA, Col I, CHOP, PERK, IRE-1, GRP78 | [356] |
Conclusion
PF is a prevalent lung disease, the prevalence and incidence of IPF increasing annually [6, 7]. If PF is not promptly and effectively treated, it will lead to a decline in lung function, which will have a negative impact on patients' quality of life and life expectancy [12]. In the last decade, glucocorticoids and immunosuppressants are commonly used in the treatment of PF, such as pirfenidone and nintedanib, but their efficacy is not ideal, they have many side effects, and they are expensive [14, 15]. Finding some reliable drugs from natural plants is becoming a research hotspot. TCM has been widely concerned because of its remarkable efficacy in treating or improving PF, mild side effects and low price. In the last five years, the scientific research on the improvement of PF by TCMs are increasing and has made great progress. Systematic and comprehensive summary of these research advances will help pharmaceutical researchers to understand the current research progress more quickly [16–18].
This article systematically reviews the progress in improving or reversing PF using traditional Chinese medicine in the last 5 years, and analyzes the major signaling pathways involved from a pharmacological perspective. Overall, the mechanism of improving PF mainly includes inhibiting EMT, anti-inflammatory and antioxidant, improving ECM deposition, mediating apoptosis and inhibiting ERS. The involved signaling pathways include TGF-β1/Smad, Nrf2/ARE, PI3K/AKT, NF-κB, etc. It is worth noting that the same Chinese medicine often involves multiple signaling pathways to improve pulmonary fibrosis, suggesting that these Chinese medicines have multi-target effects.
Although the experimental research of improving or treating PF with TCM has made some important progress, there are still some shortcomings. First, these experiments are mostly based on animal or cellular models and lack clinical trial validation. Secondly, most experiments often study only one or a few signaling pathways, lacking overall and comprehensive research. Third, because the pathogenesis of PF has not been fully elucidated, it also limits the depth of corresponding research in TCM, especially the toxicology research needs to be strengthened. What is most worth thinking about is how current pharmacological research can support the transformation of these TCM into new drugs.
On the basis of the existing known mechanisms of drug action, research methods based on artificial intelligence and big data computing are becoming the mainstream of drug development. The combination of computer aided drug design, drug molecular-target interaction, signaling pathway and pharmacological network, these new technologies will provide more than traditional research approaches to decrypt TCM treatment of PF, which will become a research hotspot in the near future.
Abbreviations
- PF
Pulmonary fibrosis
- IPF
Idiopathic pulmonary fibrosis
- ECM
Extracellular matrix
- MAPK
Mitogen-activated protein kinase
- IL
Interleukin
- TNF-α
Tumor necrosis factor alpha
- TGF-β
Transforming growth factor Beta
- TIMPs
Tissue inhibitor of matrix metalloproteinases
- ARE
Antioxidant response element
- PI3K
Phosphoinositide 3-kinase
- AKT
Protein kinase B
- mTOR
Mammalian target of rapamycin
- SASP
Senescence associated secretory phenotype
- PDGF
Platelet-derived growth factor
- TCM
Traditional Chinese medicine
- EMT
Epithelial-mesenchymal transition
- SAB
Salvianolic acid B
- Tan-IIA
Tanshinone IIA
- CPT
Cryptotanshinone
- ROS
Reactive oxygen species
- AG
Andrographolide
- NOX
NADPH oxidase
- TanIIA
Tanshinone IIA
- ASV
Astragalus methyloside
- Pts
Pterostilbene
- ERS
Endoplasmic reticulum stress
- AEC
Alveolar epithelial cells
- ER
Endoplasmic reticulum
Author contributions
TP and ZJN conceived and designed the review; QSB and ZYY drafted the manuscript and designed the table; QSB designed the fgure and edited the graphic abstract; ZYY modifed the language and checked the text; QSB and ZYY checked the fgure and table, confrmed information. All authors read and approved the fnal manuscript.
Funding
This work was supported by Sichuan Science and Technology Program (Grant numbers 2022YFS0429, 2022ZYD0102, 2022JDKY0036).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shanbo Qin, Email: 1193023297@qq.com.
Peng Tan, Email: 2013tanpeng@sina.com.
Junjie Xie, Email: 552344522@qq.com.
Yongfeng Zhou, Email: 852064509@qq.com.
Junning Zhao, Email: zarmy@189.cn.
References
- 1.Richeldi L, Collard HR, Jones MG. Idiopathic pulmonary fibrosis. Lancet. 2017;389(10082):1941–1952. doi: 10.1016/S0140-6736(17)30866-8. [DOI] [PubMed] [Google Scholar]
- 2.Wijsenbeek M, Cottin V. Spectrum of fibrotic lung diseases. N Engl J Med. 2020;383(10):958–968. doi: 10.1056/NEJMra2005230. [DOI] [PubMed] [Google Scholar]
- 3.Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. doi: 10.1038/nrdp.2017.74. [DOI] [PubMed] [Google Scholar]
- 4.Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, et al. Diagnosis of idiopathic pulmonary fibrosis an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198(5):E44–68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 5.Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis an international working group report. Am J Respir Crit Care Med. 2016;194(3):265–275. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 6.Hutchinson J, Fogarty A, Hubbard R, McKeever T. Global incidence and mortality of idiopathic pulmonary fibrosis: a systematic review. Eur Respir J. 2015;46(3):795–806. doi: 10.1183/09031936.00185114. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins RB, Burke N, Fell C, Dion G, Kolb M. Epidemiology and survival of idiopathic pulmonary fibrosis from national data in Canada. Eur Respir J. 2016;48(1):187–195. doi: 10.1183/13993003.01504-2015. [DOI] [PubMed] [Google Scholar]
- 8.Lee HE, Myong JP, Kim HR, Rhee CK, Yoon HK, Koo JW. Incidence and prevalence of idiopathic interstitial pneumonia and idiopathic pulmonary fibrosis in Korea. Int J Tuberc Lung Dis. 2016;20(7):978–984. doi: 10.5588/ijtld.16.0003. [DOI] [PubMed] [Google Scholar]
- 9.George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and covid-19: the potential role for antifibrotic therapy. Lancet Respir Med. 2020;8(8):807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu YH, Dong JH, An WM, Lv XY, Yin XP, Zhang JZ, et al. Clinical and computed tomographic imaging features of novel coronavirus pneumonia caused by SARS-cov-2. J Infect. 2020;80(4):394–400. doi: 10.1016/j.jinf.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spagnolo P, Balestro E, Aliberti S, Cocconcelli E, Biondini D, Della Casa G, et al. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med. 2020;8(8):750–752. doi: 10.1016/S2213-2600(20)30222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378(19):1811–1823. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
- 13.Somogyi V, Chaudhuri N, Torrisi SE, Kahn N, Muller V, Kreuter M. The therapy of idiopathic pulmonary fibrosis: what is next? Eur Respir Rev. 2019;28(153):190021. doi: 10.1183/16000617.0021-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 15.Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh S, Inoue Y, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718–1727. doi: 10.1056/NEJMoa1908681. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Zhao XT, Feng WW, Li YX, Peng C. Inhibiting TGF-beta 1-mediated cellular processes as an effective strategy for the treatment of pulmonary fibrosis with chinese herbal medicines. Am J Chin Med. 2021;49(08):1965–1999. doi: 10.1142/S0192415X21500932. [DOI] [PubMed] [Google Scholar]
- 17.Chen DQ, Feng YL, Cao G, Zhao YY. Natural products as a source for antifibrosis therapy. Trends Pharmacol Sci. 2018;39(11):937–952. doi: 10.1016/j.tips.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Hosseini S, Imenshahidi M, Hosseinzadeh H, Karimi G. Effects of plant extracts and bioactive compounds on attenuation of bleomycin-induced pulmonary fibrosis. Biomed Pharmacother. 2018;107:1454–1465. doi: 10.1016/j.biopha.2018.08.111. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Islam MS, Wang J, Li Y, Chen X. Traditional chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-cov-2): a review and perspective. Int J Biol Sci. 2020;16(10):1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang K, Zhang P, Zhang ZH, Youn JY, Wang C, Zhang HC, et al. Traditional chinese medicine (TCM) in the treatment of COVID-19 and other viral infections: efficacies and mechanisms. Pharmacol Ther. 2021;225:107843. doi: 10.1016/j.pharmthera.2021.107843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang FF, Li Y, Leung E, Liu XH, Liu KF, Wang Q, et al. A review of therapeutic agents and chinese herbal medicines against SARS-cov-2 (covid-19) Pharmacol Res. 2020;158:104929. doi: 10.1016/j.phrs.2020.104929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoy RF, Baird T, Hammerschlag G, Hart D, Johnson AR, King P, et al. Artificial stone-associated silicosis: a rapidly emerging occupational lung disease. Occup Environ Med. 2018;75(1):3–5. doi: 10.1136/oemed-2017-104428. [DOI] [PubMed] [Google Scholar]
- 23.Della Latta V, Cecchettini A, Del Ry S, Morales MA. Bleomycin in the setting of lung fibrosis induction: from biological mechanisms to counteractions. Pharmacol Res. 2015;97:122–130. doi: 10.1016/j.phrs.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Xu LJ, Xu J, Wang Z. Molecular mechanisms of paraquat-induced acute lung injury: a current review. Drug Chem Toxicol. 2014;37(2):130–134. doi: 10.3109/01480545.2013.834361. [DOI] [PubMed] [Google Scholar]
- 25.Crouser ED, Maier LA, Wilson KC, Bonham CA, Morgenthau AS, Patterson KC, et al. Diagnosis and detection of sarcoidosis an official american thoracic society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(8):E26–51. doi: 10.1164/rccm.202002-0251ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pardo A, Selman M. Lung fibroblasts, aging, and idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2016;13:S417–S421. doi: 10.1513/AnnalsATS.201605-341AW. [DOI] [PubMed] [Google Scholar]
- 27.Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol. 2014;9:157–79. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mack M. Inflammation and fibrosis. Matrix Biol. 2018;68–69:106–121. doi: 10.1016/j.matbio.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Heukels P, Moor CC, von der Thusen JH, Wijsenbeek MS, Kool M. Inflammation and immunity in IPF pathogenesis and treatment. Respir Med. 2019;147:79–91. doi: 10.1016/j.rmed.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Spagnolo P, Distler O, Ryerson CJ, Tzouvelekis A, Lee JS, Bonella F, et al. Mechanisms of progressive fibrosis in connective tissue disease (CTD)-associated interstitial lung diseases (ILDs) Ann Rheum Dis. 2021;80(2):143–150. doi: 10.1136/annrheumdis-2020-217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiskirchen R, Weiskirchen S, Tacke F. Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol Aspects Med. 2019;65:2–15. doi: 10.1016/j.mam.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Distler J, Gyorfi AH, Ramanujam M, Whitfield ML, Konigshoff M, Lafyatis R. Shared and distinct mechanisms of fibrosis. Nat Rev Rheumatol. 2019;15(12):705–730. doi: 10.1038/s41584-019-0322-7. [DOI] [PubMed] [Google Scholar]
- 33.Sgalla G, Iovene B, Calvello M, Ori M, Varone F, Richeldi L. Idiopathic pulmonary fibrosis: pathogenesis and management. Respir Res. 2018;19(1):32. doi: 10.1186/s12931-018-0730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cottin V, Hirani NA, Hotchkin DL, Nambiar AM, Ogura T, Otaola M, et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev. 2018;27(150):180076. doi: 10.1183/16000617.0076-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phan T, Paliogiannis P, Nasrallah GK, Giordo R, Eid AH, Fois AG, et al. Emerging cellular and molecular determinants of idiopathic pulmonary fibrosis. Cell Mol Life Sci. 2021;78(5):2031–2057. doi: 10.1007/s00018-020-03693-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herrera J, Henke CA, Bitterman PB. Extracellular matrix as a driver of progressive fibrosis. J Clin Invest. 2018;128(1):45–53. doi: 10.1172/JCI93557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gieseck RL, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18(1):62–76. doi: 10.1038/nri.2017.90. [DOI] [PubMed] [Google Scholar]
- 38.Morse C, Tabib T, Sembrat J, Buschur KL, Bittar HT, Valenzi E, et al. Proliferating SPP1/MERTK-expressing macrophages in idiopathic pulmonary fibrosis. Eur Respir J. 2019;54(2):1802441. doi: 10.1183/13993003.02441-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adams TS, Schupp JC, Poli S, Ayaub EA, Neumark N, Ahangari F, et al. Single-cell rna-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv. 2020;6(28):eaba1983. doi: 10.1126/sciadv.aba1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinz B, Lagares D. Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases. Nat Rev Rheumatol. 2020;16(1):11–31. doi: 10.1038/s41584-019-0324-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chanda D, Otoupalova E, Smith SR, Volckaert T, De Langhe SP, Thannickal VJ. Developmental pathways in the pathogenesis of lung fibrosis. Mol Aspects Med. 2019;65:56–69. doi: 10.1016/j.mam.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Upagupta C, Shimbori C, Alsilmi R, Kolb M. Matrix abnormalities in pulmonary fibrosis. Eur Respir Rev. 2018;27(148):180033. doi: 10.1183/16000617.0033-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noble PW, Barkauskas CE, Jiang DH. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest. 2012;122(8):2756–2762. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luppi F, Kalluri M, Faverio P, Kreuter M, Ferrara G. Idiopathic pulmonary fibrosis beyond the lung: understanding disease mechanisms to improve diagnosis and management. Respir Res. 2021;22(1):109. doi: 10.1186/s12931-021-01711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan Z, Kui Z, Ping Z. Reviews and prospectives of signaling pathway analysis in idiopathic pulmonary fibrosis. Autoimmun Rev. 2014;13(10):1020–1025. doi: 10.1016/j.autrev.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 46.Li XH, Ma L, Huang K, Wei YL, Long SD, Liu QY, et al. Regorafenib-attenuated, bleomycin-induced pulmonary fibrosis by inhibiting the TGF-beta 1 signaling pathway. Int J Mol Sci. 2021;22(4):1985. doi: 10.3390/ijms22041985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macias MJ, Martin-Malpartida P, Massague J. Structural determinants of smad function in TGF-beta signaling. Trends Biochem Sci. 2015;40(6):296–308. doi: 10.1016/j.tibs.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wrighton KH, Lin X, Feng XH. Phospho-control of TGF-beta superfamily signaling. Cell Res. 2009;19(1):8–20. doi: 10.1038/cr.2008.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyazono K. Transforming growth factor-beta signaling in epithelial-mesenchymal transition and progression of cancer. Proc Jpn Acad Ser B Phys Biol Sci. 2009;85(8):314–323. doi: 10.2183/pjab.85.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi KY, Jiang JZ, Ma TL, Xie J, Duan LR, Chen RH, et al. Pathogenesis pathways of idiopathic pulmonary fibrosis in bleomycin-induced lung injury model in mice. Respir Physiol Neurobiol. 2014;190(1):113–117. doi: 10.1016/j.resp.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Zhao YW, Tang HC, Zeng X, Ye DC, Liu JJ. Resveratrol inhibits proliferation, migration and invasion via Akt and ERK1/2 signaling pathways in renal cell carcinoma cells. Biomed Pharmacother. 2018;98:36–44. doi: 10.1016/j.biopha.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 52.Ramkumar KM, Sekar TV, Foygel K, Elango B, Paulmurugan R. Reporter protein complementation imaging assay to screen and study Nrf2 activators in cells and living animals. Anal Chem. 2013;85(15):7542–7549. doi: 10.1021/ac401569j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dodson M, de la Vega MR, Cholanians AB, Schmidlin CJ, Chapman E, Zhang DD. Modulating NRF2 in disease: timing is everything. Annu Rev Pharmacol Toxicol. 2019;59:555–575. doi: 10.1146/annurev-pharmtox-010818-021856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iddir M, Brito A, Dingeo G, Del Campo S, Samouda H, La Frano MR, et al. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the covid-19 crisis. Nutrients. 2020;12(6):1562. doi: 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, et al. Reversal of persistent fibrosis in aging by targeting NOX4-NRF2 redox imbalance. Sci Transl Med. 2014;6(231):231–247. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buendia I, Michalska P, Navarro E, Gameiro I, Egea J, Leon R. Nrf2-ARE pathway: an emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol Ther. 2016;157:84–104. doi: 10.1016/j.pharmthera.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Wang JC, Hu KL, Cai XY, Yang B, He QJ, Wang JJ, et al. Targeting PI3K/AKT signaling for treatment of idiopathic pulmonary fibrosis. Acta Pharm Sin B. 2022;12(1):18–32. doi: 10.1016/j.apsb.2021.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barnes PJ, Baker J, Donnelly LE. Cellular senescence as a mechanism and target in chronic lung diseases. Am J Respir Crit Care Med. 2019;200(5):556–564. doi: 10.1164/rccm.201810-1975TR. [DOI] [PubMed] [Google Scholar]
- 59.Spangle JM, Roberts TM, Zhao JJ. The emerging role of PI3K/AKT-mediated epigenetic regulation in cancer. Biochim Biophys Acta Rev Cancer. 2017;1868(1):123–131. doi: 10.1016/j.bbcan.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017;170(4):605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Zhang L, Wu GR, Zhou Q, Yue HH, Rao LZ, et al. MBD2 serves as a viable target against pulmonary fibrosis by inhibiting macrophage M2 program. Sci Adv. 2021;7(1):eabb6075. doi: 10.1126/sciadv.abb6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qiu T, Tian YQ, Gao YJ, Ma M, Li H, Liu XQ, et al. Pten loss regulates alveolar epithelial cell senescence in pulmonary fibrosis depending on akt activation. Aging. 2019;11(18):7492–7509. doi: 10.18632/aging.102262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lawrence J, Nho R. The role of the mammalian target of rapamycin (mTOR) in pulmonary fibrosis. Int J Mol Sci. 2018;19(3):778. doi: 10.3390/ijms19030778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bastakoty D, Young PP. Wnt/β-catenin pathway in tissue injury: roles in pathology and therapeutic opportunities for regeneration. FASEB J. 2016;30(10):3271–84. doi: 10.1096/fj.201600502R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tzouvelekis A, Gomatou G, Bouros E, Trigidou R, Tzilas V, Bouros D. Common pathogenic mechanisms between idiopathic pulmonary fibrosis and lung cancer. Chest. 2019;156(2):383–391. doi: 10.1016/j.chest.2019.04.114. [DOI] [PubMed] [Google Scholar]
- 66.Okazaki H, Sato S, Koyama K, Morizumi S, Abe S, Azuma M, et al. The novel inhibitor PRI-724 for Wnt/β-catenin/CBP signaling ameliorates bleomycin-induced pulmonary fibrosis in mice. Exp Lung Res. 2019;45(7):188–199. doi: 10.1080/01902148.2019.1638466. [DOI] [PubMed] [Google Scholar]
- 67.Andersson-Sjöland A, Karlsson JC, Rydell-Törmänen K. ROS-induced endothelial stress contributes to pulmonary fibrosis through pericytes and Wnt signaling. Lab Invest. 2016;96(2):206–217. doi: 10.1038/labinvest.2015.100. [DOI] [PubMed] [Google Scholar]
- 68.Cao H, Chen X, Hou J, Wang C, Xiang Z, Shen Y, et al. The Shh/Gli signaling cascade regulates myofibroblastic activation of lung-resident mesenchymal stem cells via the modulation of Wnt10a expression during pulmonary fibrogenesis. Lab Invest. 2020;100(3):363–377. doi: 10.1038/s41374-019-0316-8. [DOI] [PubMed] [Google Scholar]
- 69.Li X, Liu X, Deng R, Gao S, Jiang Q, Liu R, et al. Betulinic acid attenuated bleomycin-induced pulmonary fibrosis by effectively intervening Wnt/β-catenin signaling. Phytomedicine. 2021;81:153428. doi: 10.1016/j.phymed.2020.153428. [DOI] [PubMed] [Google Scholar]
- 70.Ji J, Hou J, Xia Y, Xiang Z, Han X. NLRP3 inflammasome activation in alveolar epithelial cells promotes myofibroblast differentiation of lung-resident mesenchymal stem cells during pulmonary fibrogenesis. Biochim Biophys Acta Mol Basis Dis. 2021;1867(5):166077. doi: 10.1016/j.bbadis.2021.166077. [DOI] [PubMed] [Google Scholar]
- 71.Froidure A, Marchal-Duval E, Homps-Legrand M, Ghanem M, Justet A, Crestani B, et al. Chaotic activation of developmental signalling pathways drives idiopathic pulmonary fibrosis. Eur Respir Rev. 2020;29(158):190140. doi: 10.1183/16000617.0140-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vukmirovic M, Kaminski N. Impact of transcriptomics on our understanding of pulmonary fibrosis. Front Med. 2018;5:87. doi: 10.3389/fmed.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Newman DR, Sills WS, Hanrahan K, Ziegler A, Tidd KM, Cook E, et al. Expression of WNT5A in idiopathic pulmonary fibrosis and its control by TGF-β and WNT7B in human lung fibroblasts. J Histochem Cytochem. 2016;64(2):99–111. doi: 10.1369/0022155415617988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li X, Liu X, Deng R, Gao S, Yu H, Huang K, et al. Nintedanib inhibits Wnt3a-induced myofibroblast activation by suppressing the Src/β-catenin pathway. Front Pharmacol. 2020;11:310. doi: 10.3389/fphar.2020.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu T, Zhang L, Joo D, Sun S. NF-kappa B signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu H, Lin L, Zhang Z, Zhang H, Hu H. Targeting NF-kappa B pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct Target Ther. 2020;5(1):209. doi: 10.1038/s41392-020-00312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhong Z, Umemura A, Sanchez-Lopez E, Liang S, Shalapour S, Wong J, et al. NF-kappa B restricts inflammasome activation via elimination of damaged mitochondria. Cell. 2016;164(5):896–910. doi: 10.1016/j.cell.2015.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, et al. MicroRNA-181B regulates NF-kappa B-mediated vascular inflammation. J Clin Invest. 2012;122(6):1973–1990. doi: 10.1172/JCI61495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ghosh S, May M, Kopp E. NF-kappa B and REL proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 80.Hayden MS, Ghosh S. Nf-kappa b, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26(3):203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Christian F, Smith EL, Carmody RJ. The regulation of NF-kappa B subunits by phosphorylation. Cells. 2016;5(1):12. doi: 10.3390/cells5010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang WG, Ablin RJ. Cancer metastasis, challenges, progress and the opportunities. Front Biosci. 2011;3(1):391–394. doi: 10.2741/e254. [DOI] [PubMed] [Google Scholar]
- 83.Afonina IS, Zhong Z, Karin M, Beyaert R. Limiting inflammation-the negative regulation of NF-kappa B and the NLRP3 inflammasome. Nat Immunol. 2017;18(8):861–869. doi: 10.1038/ni.3772. [DOI] [PubMed] [Google Scholar]
- 84.Mitchell JP, Carmody RJ. NF-kappa B and the transcriptional control of inflammation. Int Rev Cell Mol Biol. 2018;335:41–84. doi: 10.1016/bs.ircmb.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 85.Li C, Xia W, Huo L, Lim S, Wu Y, Hsu JL, et al. Epithelial-mesenchymal transition induced by TNF-alpha requires NF-kappa B-mediated transcriptional upregulation of Twist1. Cancer Res. 2012;72(5):1290–1300. doi: 10.1158/0008-5472.CAN-11-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sigal LH. Basic science for the clinician 39-NF-kappa B—function, activation, control, and consequences. J Clin Rheumatol. 2006;12(4):207–211. doi: 10.1097/01.rhu.0000231385.94784.e4. [DOI] [PubMed] [Google Scholar]
- 87.Gross CM, Kellner M, Wang T, Lu Q, Sun X, Zemskov EA, et al. LPS-induced acute lung injury involves NF-kappa B-mediated downregulation of SOX18. Am J Respir Cell Mol Biol. 2018;58(5):614–624. doi: 10.1165/rcmb.2016-0390OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ju M, Liu B, He H, Gu Z, Liu Y, Su Y, et al. MicroRNA-27A alleviates lps-induced acute lung injury in mice via inhibiting inflammation and apoptosis through modulating TLR4/MyD88/NF-kappa B pathway. Cell Cycle. 2018;17(16):2001–2018. doi: 10.1080/15384101.2018.1509635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu H, Dong F, Li G, Niu M, Zhang C, Han Y, et al. Liuweiwuling tablets attenuate BDL-induced hepatic fibrosis via modulation of TGF-beta/smad and NF-kappa B signaling pathways. J Ethnopharmacol. 2018;210:232–241. doi: 10.1016/j.jep.2017.08.029. [DOI] [PubMed] [Google Scholar]
- 90.Blokland KEC, Waters DW, Schuliga M, Read J, Pouwels SD, Grainge CL, et al. Senescence of IPF lung fibroblasts disrupt alveolar epithelial cell proliferation and promote migration in wound healing. Pharmaceutics. 2020;12(4):389. doi: 10.3390/pharmaceutics12040389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee J, La J, Aziz S, Brownell R, Jones K, Green G, et al. Molecular markers of telomere dysfunction and senescence are common findings in the usual interstitial pneumonia pattern of lung fibrosis. Eur Respir J. 2018;52:67–76. doi: 10.1111/his.14334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lopes-Paciencia S, Saint-Germain E, Rowell M, Ruiz AF, Kalegari P, Ferbeyre G. The senescence-associated secretory phenotype and its regulation. Cytokine. 2019;117:15–22. doi: 10.1016/j.cyto.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 93.Fafian-Labora JA, O'Loghlen A. Classical and nonclassical intercellular communication in senescence and ageing. Trends Cell Biol. 2020;30(8):628–639. doi: 10.1016/j.tcb.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 94.Lodyga M, Hinz B. TGF-beta 1-a truly transforming growth factor in fibrosis and immunity. Semin Cell Dev Biol. 2020;101:123–139. doi: 10.1016/j.semcdb.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 95.Liu G, Philp AM, Corte T, Travis MA, Schilter H, Hansbro NG, et al. Therapeutic targets in lung tissue remodelling and fibrosis. Pharmacol Ther. 2021;225:107839. doi: 10.1016/j.pharmthera.2021.107839. [DOI] [PubMed] [Google Scholar]
- 96.Caja L, Dituri F, Mancarella S, Caballero-Diaz D, Moustakas A, Giannelli G, et al. TGF-beta and the tissue microenvironment: relevance in fibrosis and cancer. Int J Mol Sci. 2018;19(5):1294. doi: 10.3390/ijms19051294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim KK, Sheppard D, Chapman HA. TGF-beta 1 signaling and tissue fibrosis. Cold Spring Harb Perspect Biol. 2018;10(4):a022293. doi: 10.1101/cshperspect.a022293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hinz B. The extracellular matrix and transforming growth factor-beta 1: tale of a strained relationship. Matrix Biol. 2015;47:54–65. doi: 10.1016/j.matbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 99.Saito A, Horie M, Nagase T. TGF-beta signaling in lung health and disease. Int J Mol Sci. 2018;19(8):2460. doi: 10.3390/ijms19082460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Walton KL, Johnson KE, Harrison CA. Targeting TGF-beta mediated smad signaling for the prevention of fibrosis. Front Pharmacol. 2017;8:461. doi: 10.3389/fphar.2017.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hu HH, Chen DQ, Wang YN, Feng YL, Cao G, Vaziri ND, et al. New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem Biol Interact. 2018;292:76–83. doi: 10.1016/j.cbi.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 102.Nagaoka I, Trapnell BC, Crystal RG. Upregulation of platelet-derived growth factor-B and -B gene expression in alveolar macrophages of individuals with idiopathic pulmonary fibrosis. J Clin Invest. 1990;85(6):2023–2027. doi: 10.1172/JCI114669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sasaki M, Kashima M, Ito T, Watanabe A, Izumiyama N, Sano M, et al. Differential regulation of metalloproteinase production, proliferation and chemotaxis of human lung fibroblasts by PDGF, interleukin-1β and TNF-α. Mediators Inflamm. 2000;9(3–4):155–160. doi: 10.1080/09629350020002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Q, Niu YM, Diao HJ, Wang LT, Chen XP, Wang YT, et al. In situ sequestration of endogenous PDGF-BB with an ECM-mimetic sponge for accelerated wound healing. Biomaterials. 2017;148:54–68. doi: 10.1016/j.biomaterials.2017.09.028. [DOI] [PubMed] [Google Scholar]
- 105.Sun QZ, Liu L, Mandal J, Molino A, Stolz D, Tamm M, et al. PDGF-BB induces PRMT1 expression through ERK1/2 dependent STAT1 activation and regulates remodeling in primary human lung fibroblasts. Cell Signal. 2016;28(4):307–315. doi: 10.1016/j.cellsig.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 106.Sato S, Shinohara S, Hayashi S, Morizumi S, Abe S, Okazaki H, et al. Anti-fibrotic efficacy of nintedanib in pulmonary fibrosis via the inhibition of fibrocyte activity. Respir Res. 2017;18(1):172. doi: 10.1186/s12931-017-0654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Grimminger F, Gunther A, Vancheri C. The role of tyrosine kinases in the pathogenesis of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(5):1426–1433. doi: 10.1183/09031936.00149614. [DOI] [PubMed] [Google Scholar]
- 108.Ruaro B, Salton F, Braga L, Wade B, Confalonieri P, Volpe MC, et al. The history and mystery of alveolar epithelial type II cells: focus on their physiologic and pathologic role in lung. Int J Mol Sci. 2021;22(5):2566. doi: 10.3390/ijms22052566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Geffen C, Deissler A, Quante M, Renz H, Hartl D, Kolahian S. Regulatory immune cells in idiopathic pulmonary fibrosis: friends or foes? Front Immunol. 2021;12:663203. doi: 10.3389/fimmu.2021.663203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Klee S, Lehmann M, Wagner DE, Baarsma HA, Konigshoff M. WISP1 mediates IL-6-dependent proliferation in primary human lung fibroblasts. Sci Rep. 2016;6:20547. doi: 10.1038/srep20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Passalacqua G, Mincarini M, Colombo D, Troisi G, Ferrari M, Bagnasco D, et al. IL-13 and idiopathic pulmonary fibrosis: possible links and new therapeutic strategies. Pulm Pharmacol Ther. 2017;45:95–100. doi: 10.1016/j.pupt.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 112.Su SC, Zhao QY, He CH, Huang D, Liu J, Chen F, et al. miR-142-5p and miR-130a-3p are regulated by IL-4 and IL-13 and control profibrogenic macrophage program. Nat Commun. 2015;6:8523. doi: 10.1038/ncomms9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Song C, He LJ, Zhang J, Ma H, Yuan XN, Hu GY, et al. Fluorofenidone attenuates pulmonary inflammation and fibrosis via inhibiting the activation of NALP3 inflammasome and IL-1/IL-1R1/MyD88/NF-B pathway. J Cell Mol Med. 2016;20(11):2064–2077. doi: 10.1111/jcmm.12898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sziksz E, Pap D, Lippai R, Beres NJ, Fekete A, Szabo AJ, et al. Fibrosis related inflammatory mediators: role of the IL-10 cytokine family. Mediators Inflamm. 2015;2015:764641. doi: 10.1155/2015/764641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huang M, Sharma S, Zhu LX, Keane MP, Luo J, Zhang L, et al. IL-7 inhibits fibroblast TGF-beta production and signaling in pulmonary fibrosis. J Clin Invest. 2002;109(7):931–937. doi: 10.1172/JCI0214685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Groves AM, Johnston CJ, Misra RS, Williams JP, Finkelstein JN. Effects of IL-4 on pulmonary fibrosis and the accumulation and phenotype of macrophage subpopulations following thoracic irradiation. Int J Radiat Biol. 2016;92(12):754–765. doi: 10.1080/09553002.2016.1222094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu Y, Lu F, Kang LR, Wang ZH, Wang YF. Pirfenidone attenuates bleomycin-induced pulmonary fibrosis in mice by regulating Nrf2/Bach1 equilibrium. BMC Pulm Med. 2017;17(1):63. doi: 10.1186/s12890-017-0405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun L, Louie MC, Vannella KM, Wilke CA, LeVine AM, Moore BB, et al. New concepts of IL-10-induced lung fibrosis: fibrocyte recruitment and M2 activation in a CCL2/CCR2 axis. Am J Physiol Lung Cell Mol Physiol. 2011;300(3):L341–L353. doi: 10.1152/ajplung.00122.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kuroki M, Noguchi Y, Shimono M, Tomono K, Tashiro T, Obata Y, et al. Repression of bleomycin-induced pneumopathy by TNF. J Immunol. 2003;170(1):567–574. doi: 10.4049/jimmunol.170.1.567. [DOI] [PubMed] [Google Scholar]
- 120.Sinclair K, Yerkovich ST, Chambers DC. Mesenchymal stem cells and the lung. Respirology. 2013;18(3):397–411. doi: 10.1111/resp.12050. [DOI] [PubMed] [Google Scholar]
- 121.Huang XP, Wang X, Xie XL, Zeng SL, Li ZF, Xu XX, et al. Kallistatin protects against bleomycin-induced idiopathic pulmonary fibrosis by inhibiting angiogenesis and inflammation. Am J Transl Res. 2017;9(3):999–1011. [PMC free article] [PubMed] [Google Scholar]
- 122.Dinarello CA. Role of pro- and anti-inflammatory cytokines during inflammation: experimental and clinical findings. J Biol Regul Homeost Agents. 1997;11(3):91–103. [PubMed] [Google Scholar]
- 123.Hou J, Ma T, Cao H, Chen Y, Wang C, Chen X, et al. Tnf-α-induced nf-κb activation promotes myofibroblast differentiation of lr-mscs and exacerbates bleomycin-induced pulmonary fibrosis. J Cell Physiol. 2018;233(3):2409–2419. doi: 10.1002/jcp.26112. [DOI] [PubMed] [Google Scholar]
- 124.King TEJ, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378(9807):1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 125.Ikegami T, Zhang Y, Matsuzaki Y. Liver fibrosis: possible involvement of EMT. Cells Tissues Organs. 2007;185(1–3):213–221. doi: 10.1159/000101322. [DOI] [PubMed] [Google Scholar]
- 126.Tanjore H, Xu XC, Polosukhin VV, Degryse AL, Li B, Han W, et al. Contribution of epithelial-derived fibroblasts to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2009;180(7):657–665. doi: 10.1164/rccm.200903-0322OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim KK, Sisson TH, Horowitz JC. Fibroblast growth factors and pulmonary fibrosis: it’s more complex than it sounds. J Pathol. 2017;241(1):6–09. doi: 10.1002/path.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Desai P, Yang J, Tian B, Sun H, Kalita M, Ju H, et al. Mixed-effects model of epithelial-mesenchymal transition reveals rewiring of signaling networks. Cell Signal. 2015;27(7):1413–1425. doi: 10.1016/j.cellsig.2015.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ji X, Li C, Ou Y, Li N, Yuan K, Yang G, et al. Andrographolide ameliorates diabetic nephropathy by attenuating hyperglycemia-mediated renal oxidative stress and inflammation via Akt/NF-κB pathway. Mol Cell Endocrinol. 2016;437:268–279. doi: 10.1016/j.mce.2016.06.029. [DOI] [PubMed] [Google Scholar]
- 130.Kayastha F, Johar K, Gajjar D, Arora A, Madhu H, Ganatra D, et al. Andrographolide suppresses epithelial mesenchymal transition by inhibition of MAPK signalling pathway in lens epithelial cells. J Biosci. 2015;40(2):313–324. doi: 10.1007/s12038-015-9513-9. [DOI] [PubMed] [Google Scholar]
- 131.Karkale S, Khurana A, Saifi MA, Godugu C, Talla V. Andrographolide ameliorates silica induced pulmonary fibrosis. Int Immunopharmacol. 2018;62:191–202. doi: 10.1016/j.intimp.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 132.Li J, Feng M, Sun R, Li Z, Hu L, Peng G, et al. Andrographolide ameliorates bleomycin-induced pulmonary fibrosis by suppressing cell proliferation and myofibroblast differentiation of fibroblasts via the TGF-β1-mediated smad-dependent and -independent pathways. Toxicol Lett. 2020;321:103–113. doi: 10.1016/j.toxlet.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 133.Li J, Liu J, Yue W, Xu K, Cai W, Cui F, et al. Andrographolide attenuates epithelial-mesenchymal transition induced by TGF-β1 in alveolar epithelial cells. J Cell Mol Med. 2020;24(18):10501–10511. doi: 10.1111/jcmm.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li J, Yang X, Yang P, Xu K, Peng X, Cai W, et al. Andrographolide alleviates bleomycin-induced NLRP3 inflammasome activation and epithelial-mesenchymal transition in lung epithelial cells by suppressing AKT/mTOR signaling pathway. Ann Transl Med. 2021;9(9):764. doi: 10.21037/atm-20-7973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhou L, Gao R, Hong H, Li X, Yang J, Shen W, et al. Emodin inhibiting neutrophil elastase-induced epithelial-mesenchymal transition through Notch1 signalling in alveolar epithelial cells. J Cell Mol Med. 2020;24(20):11998–12007. doi: 10.1111/jcmm.15827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pang X, Shao L, Nie X, Yan H, Li C, Yeo AJ, et al. Emodin attenuates silica-induced lung injury by inhibition of inflammation, apoptosis and epithelial-mesenchymal transition. Int Immunopharmacol. 2021;91:107277. doi: 10.1016/j.intimp.2020.107277. [DOI] [PubMed] [Google Scholar]
- 137.Tao L, Cao J, Wei W, Xie H, Zhang M, Zhang C. Protective role of rhapontin in experimental pulmonary fibrosis in vitro and in vivo. Int Immunopharmacol. 2017;47:38–46. doi: 10.1016/j.intimp.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 138.Liu M, Xu H, Zhang L, Zhang C, Yang L, Ma E, et al. Salvianolic acid B inhibits myofibroblast transdifferentiation in experimental pulmonary fibrosis via the up-regulation of Nrf2. Biochem Biophys Res Commun. 2018;495(1):325–331. doi: 10.1016/j.bbrc.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 139.Zhang Q, Gan C, Liu H, Wang L, Li Y, Tan Z, et al. Cryptotanshinone reverses the epithelial-mesenchymal transformation process and attenuates bleomycin-induced pulmonary fibrosis. Phytother Res PTR. 2020;34(10):2685–2696. doi: 10.1002/ptr.6699. [DOI] [PubMed] [Google Scholar]
- 140.Zhang R, Xu L, An X, Sui X, Lin S. Astragalus polysaccharides attenuate pulmonary fibrosis by inhibiting the epithelial-mesenchymal transition and NF-κB pathway activation. Int J Mol Med. 2020;46(1):331–339. doi: 10.3892/ijmm.2020.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhao H, Li C, Li L, Liu J, Gao Y, Mu K, et al. Baicalin alleviates bleomycin-induced pulmonary fibrosis and fibroblast proliferation in rats via the PI3K/AKT signaling pathway. Mol Med Rep. 2020;21(6):2321–2334. doi: 10.3892/mmr.2020.11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lu J, Zhong Y, Lin Z, Lin X, Chen Z, Wu X, et al. Baicalin alleviates radiation-induced epithelial-mesenchymal transition of primary type II alveolar epithelial cells via TGF-β and ERK/GSK3Β signaling pathways. Biomed Pharmacother. 2017;95:1219–1224. doi: 10.1016/j.biopha.2017.09.037. [DOI] [PubMed] [Google Scholar]
- 143.Cui X, Sun X, Lu F, Jiang X. Baicalein represses TGF-β1-induced fibroblast differentiation through the inhibition of miR-21. Toxicol Appl Pharmacol. 2018;358:35–42. doi: 10.1016/j.taap.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 144.Liu X, Shao Y, Zhang X, Ji X, Xie M, Liu H. Calycosin attenuates pulmonary fibrosis by the epithelial-mesenchymal transition repression upon inhibiting the AKT/GSK3Β/β-catenin signaling pathway. Acta Histochem. 2021;123(5):151746. doi: 10.1016/j.acthis.2021.151746. [DOI] [PubMed] [Google Scholar]
- 145.Chen X, Chen X, Shi X, Gao Z, Guo Z. Curcumin attenuates endothelial cell fibrosis through inhibiting endothelial-interstitial transformation. Clin Exp Pharmacol Physiol. 2020;47(7):1182–1192. doi: 10.1111/1440-1681.13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shaikh SB, Prabhu A, Bhandary YP. Curcumin suppresses epithelial growth factor receptor (EGFR) and proliferative protein (Ki 67) in acute lung injury and lung fibrosis in vitro and in vivo. Endocr Metab Immune Disord Drug Targets. 2020;20(4):558–563. doi: 10.2174/1871530319666190823160230. [DOI] [PubMed] [Google Scholar]
- 147.Shaikh SB, Prabhu A, Bhandary YP. Curcumin targets p53-fibrinolytic system in TGF-β1 mediated alveolar epithelial mesenchymal transition in alveolar epithelial cells. Endocr Metab Immune Disord Drug Targets. 2021;21(8):1441–1452. doi: 10.2174/1871530320666200929142503. [DOI] [PubMed] [Google Scholar]
- 148.Tyagi N, Singh DK, Dash D, Singh R. Curcumin modulates paraquat-induced epithelial to mesenchymal transition by regulating transforming growth factor-β (TGF-β) in A549 cells. Inflammation. 2019;42(4):1441–1455. doi: 10.1007/s10753-019-01006-0. [DOI] [PubMed] [Google Scholar]
- 149.Chang W, Chen C, Sheu C, Liao S, Hsu Y, Tsai M, et al. The potential effects of curcumin on pulmonary fibroblasts of idiopathic pulmonary fibrosis (IPF)-approaching with next-generation sequencing and bioinformatics. Molecules. 2020;25(22):5458. doi: 10.3390/molecules25225458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Saidi A, Kasabova M, Vanderlynden L, Wartenberg M, Kara-Ali GH, Marc D, et al. Curcumin inhibits the TGF-β1-dependent differentiation of lung fibroblasts via PPARγ-driven upregulation of cathepsins B and L. Sci Rep. 2019;9(1):491. doi: 10.1038/s41598-018-36858-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Miao Y, Zhang Y, Qiao S, Xia Y, Wei Z, Dai Y. Oral administration of curcumin ameliorates pulmonary fibrosis in mice through 15d-PGJ2-mediated induction of hepatocyte growth factor in the colon. Acta Pharmacol Sin. 2021;42(3):422–435. doi: 10.1038/s41401-020-0469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.An L, Peng L, Sun N, Yang Y, Zhang X, Li B, et al. Tanshinone iia activates nuclear factor-erythroid 2-related factor 2 to restrain pulmonary fibrosis via regulation of redox homeostasis and glutaminolysis. Antioxid Redox Signal. 2019;30(15):1831–1848. doi: 10.1089/ars.2018.7569. [DOI] [PubMed] [Google Scholar]
- 153.Jiang L, Wang J, Ju J, Dai J. Salvianolic acid b and sodium tanshinone II A sulfonate prevent pulmonary fibrosis through anti-inflammatory and anti-fibrotic process. Eur J Pharmacol. 2020;883:173352. doi: 10.1016/j.ejphar.2020.173352. [DOI] [PubMed] [Google Scholar]
- 154.Takano M, Deguchi J, Senoo S, Izumi M, Kawami M, Yumoto R. Suppressive effect of quercetin against bleomycin-induced epithelial-mesenchymal transition in alveolar epithelial cells. Drug Metab Pharmacokinet. 2020;35(6):522–526. doi: 10.1016/j.dmpk.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 155.Zhang X, Cai Y, Zhang W, Chen X. Quercetin ameliorates pulmonary fibrosis by inhibiting Sphk1/S1P signaling. Biochem Cell Biol. 2018;96(6):742–751. doi: 10.1139/bcb-2017-0302. [DOI] [PubMed] [Google Scholar]
- 156.Liu Y, Chen B, Nie J, Zhao G, Zhuo J, Yuan J, et al. Polydatin prevents bleomycin-induced pulmonary fibrosis by inhibiting the TGF-β/Smad/ERK signaling pathway. Exp Ther Med. 2020;20(5):62. doi: 10.3892/etm.2020.9190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Qiu Y, Pan X, Hu Y. Polydatin ameliorates pulmonary fibrosis by suppressing inflammation and the epithelial mesenchymal transition via inhibiting the TGF-β/Smad signaling pathway. Rsc Adv. 2019;9(14):8104–8112. doi: 10.1039/C8RA08659A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hou Y, Zhen Y, Xue Q, Wang W. Astragaloside IV attenuates TGF-β-mediated epithelial-mesenchymal transition of pulmonary fibrosis via suppressing NLRP3 expression in vitro. Pharmazie. 2021;76(2):97–102. doi: 10.1691/ph.2021.0933. [DOI] [PubMed] [Google Scholar]
- 159.Qian W, Cai X, Qian Q, Zhang W, Wang D. Astragaloside IV modulates TGF-β1-dependent epithelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. J Cell Mol Med. 2018;22(9):4354–4365. doi: 10.1111/jcmm.13725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang P, Liu J, Zong R. Triptolide protects against TGF-β1-induced pulmonary fibrosis by regulating FAK/calpain signaling. Exp Ther Med. 2019;18(6):4781–4789. doi: 10.3892/etm.2019.8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen H, Chen Q, Jiang C, Shi G, Sui B, Zhang W, et al. Triptolide suppresses paraquat induced idiopathic pulmonary fibrosis by inhibiting TGFB1-dependent epithelial mesenchymal transition. Toxicol Lett. 2018;284:1–9. doi: 10.1016/j.toxlet.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 162.Zhuang WY, Zhao N, Li D, Su XM, Wang YY, Chen JG, et al. Schisantherin a inhibits pulmonary fibrosis via regulating ERK signaling pathway. Nat Prod Commun. 2020;15(8):1934578X2094835. [Google Scholar]
- 163.Wang Y, Dong X, Zhao N, Su X, Wang Y, Li Y, et al. Schisandrin b attenuates bleomycin-induced pulmonary fibrosis in mice through the wingless/integrase-1 signaling pathway. Exp Lung Res. 2020;46(6):185–194. doi: 10.1080/01902148.2020.1760964. [DOI] [PubMed] [Google Scholar]
- 164.Li XH, Huang MY, Liu F, Gao SY, Bai JK, Liu SS, et al. Analysis of chemical composition of inula japonica thumb. Extract and in vitro screening for anti-pulmonary fibrosis active components. Phytochem Lett. 2020;36:144–49. doi: 10.1016/j.phytol.2020.02.003. [DOI] [Google Scholar]
- 165.Zhao W, Luan Z, Liu T, Ming W, Huo X, Huang H, et al. Inula japonica ameliorated bleomycin-induced pulmonary fibrosis via inhibiting soluble epoxide hydrolase. Bioorg Chem. 2020;102:104065. doi: 10.1016/j.bioorg.2020.104065. [DOI] [PubMed] [Google Scholar]
- 166.Ali SA, Saifi MA, Pulivendala G, Godugu C, Talla V. Ferulic acid ameliorates the progression of pulmonary fibrosis via inhibition of TGF-β/Smad signalling. Food Chem Toxicol. 2021;149:111980. doi: 10.1016/j.fct.2021.111980. [DOI] [PubMed] [Google Scholar]
- 167.Du M, Duan J, Zhang C, Yang H, Guan X, Zhong W, et al. Psoralen attenuates bleomycin-induced pulmonary fibrosis in mice through inhibiting myofibroblast activation and collagen deposition. Cell Biol Int. 2019;44(1):98–107. doi: 10.1002/cbin.11205. [DOI] [PubMed] [Google Scholar]
- 168.Zeng H, Gao H, Zhang M, Wang J, Gu Y, Wang Y, et al. Atractylon treatment attenuates pulmonary fibrosis via regulation of the mmu_circ_0000981/miR-211-5p/TGFBR2 axis in an ovalbumin-induced asthma mouse model. Inflammation. 2021;44(5):1856–1864. doi: 10.1007/s10753-021-01463-6. [DOI] [PubMed] [Google Scholar]
- 169.Qian W, Cai X, Qian Q, Wang D, Zhang L. Angelica sinensis polysaccharide suppresses epithelial-mesenchymal transition and pulmonary fibrosis via a DANCR/AUF-1/FOXO3 regulatory axis. Aging Dis. 2020;11(1):17–30. doi: 10.14336/AD.2019.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li X, Xiao T, Yang J, Qin Y, Gao J, Liu H, et al. Parthenolide attenuated bleomycin-induced pulmonary fibrosis via the NF-κB/snail signaling pathway. Respir Res. 2018;19(1):111. doi: 10.1186/s12931-018-0806-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Peng L, Wen L, Shi Q, Gao F, Huang B, Meng J, et al. Scutellarin ameliorates pulmonary fibrosis through inhibiting NF-κB/NLRP3-mediated epithelial-mesenchymal transition and inflammation. Cell Death Dis. 2020;11(11):978. doi: 10.1038/s41419-020-03178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Miao K, Pan T, Mou Y, Zhang L, Xiong W, Xu Y, et al. Scutellarein inhibits blm-mediated pulmonary fibrosis by affecting fibroblast differentiation, proliferation, and apoptosis. Ther Adv Chronic Dis. 2020;11:1754231961. doi: 10.1177/2040622320940185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Li C, Yu Y, Li W, Liu B, Jiao X, Song X, et al. Phycocyanin attenuates pulmonary fibrosis via the TLR2-Myd88-NF-κB signaling pathway. Sci Rep. 2017;7(1):5843. doi: 10.1038/s41598-017-06021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu P, Miao K, Zhang L, Mou Y, Xu Y, Xiong W, et al. Curdione ameliorates bleomycin-induced pulmonary fibrosis by repressing TGF-β-induced fibroblast to myofibroblast differentiation. Respir Res. 2020;21(1):58. doi: 10.1186/s12931-020-1300-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ren ZX, Shen JL, Mei XF, Dong HR, Li JS, Yu HB. Hesperidin inhibits the epithelial to mesenchymal transition induced by transforming growth factor-beta 1 in A549 cells through smad signaling in the cytoplasm. Braz J Pharm Sci. 2019;55:e18172. doi: 10.1590/s2175-97902019000218172. [DOI] [Google Scholar]
- 176.Wang L, Liu H, He Q, Gan C, Li Y, Zhang Q, et al. Galangin ameliorated pulmonary fibrosis in vivo and in vitro by regulating epithelial-mesenchymal transition. Bioorg Med Chem. 2020;28(19):115663. doi: 10.1016/j.bmc.2020.115663. [DOI] [PubMed] [Google Scholar]
- 177.Cui Y, Zhao J, Chen J, Kong Y, Wang M, Ma Y, et al. Cyanidin-3-galactoside from aronia melanocarpa ameliorates silica-induced pulmonary fibrosis by modulating the TGF-β/mTOR and NRF2/HO-1 pathways. Food Sci Nutr. 2022;10(8):2558–2567. doi: 10.1002/fsn3.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li H, Kan B, Song L, Liu Y, Jian X. Role of the hippo signaling pathway in safflower yellow pigment treatment of paraquat-induced pulmonary fibrosis. J Int Med Res. 2020;48(9):300060520905425. doi: 10.1177/0300060520905425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sun S, Han R, Hou S, Yi H, Chi S, Zhang A. Juglanin alleviates bleomycin-induced lung injury by suppressing inflammation and fibrosis via targeting sting signaling. Biomed Pharmacother. 2020;127:110119. doi: 10.1016/j.biopha.2020.110119. [DOI] [PubMed] [Google Scholar]
- 180.Dinesh Babu V, Suresh Kumar A, Sudhandiran G. Diosgenin inhibits TGF-β1/Smad signaling and regulates epithelial mesenchymal transition in experimental pulmonary fibrosis. Drug Chem Toxicol. 2022;45(3):1264–1275. doi: 10.1080/01480545.2020.1814803. [DOI] [PubMed] [Google Scholar]
- 181.Liu S, Yang Q, Dong B, Qi C, Yang T, Li M, et al. Gypenosides attenuate pulmonary fibrosis by inhibiting the AKT/mTOR/c-Myc pathway. Front Pharmacol. 2021;12:806312. doi: 10.3389/fphar.2021.806312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li L, Ma L, Wang D, Jia H, Yu M, Gu Y, et al. Design and synthesis of matrine derivatives as novel anti-pulmonary fibrotic agents via repression of the TGFΒ/Smad pathway. Molecules. 2019;24(6):1108. doi: 10.3390/molecules24061108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Peng Y, Zhang Y, Zhang Y, Wang X, Xia Y. Pterostilbene alleviates pulmonary fibrosis by regulating ASIC2. Chin Med. 2021;16(1):66. doi: 10.1186/s13020-021-00474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li Q, Peng W, Zhang Z, Pei X, Sun Z, Ou Y. A phycocyanin derived eicosapeptide attenuates lung fibrosis development. Eur J Pharmacol. 2021;908:174356. doi: 10.1016/j.ejphar.2021.174356. [DOI] [PubMed] [Google Scholar]
- 185.Yang J, Tao L, Liu B, You X, Zhang C, Xie H, et al. Wedelolactone attenuates pulmonary fibrosis partly through activating AMPK and regulating Raf-MAPKs signaling pathway. Front Pharmacol. 2019;10:151. doi: 10.3389/fphar.2019.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Guo P, Li B, Liu M, Li Y, Weng G, Gao Y. Protective effects of lotus plumule ethanol extracts on bleomycin-induced pulmonary fibrosis in mice. Drug Chem Toxicol. 2022;45(3):1432–1441. doi: 10.1080/01480545.2021.1993670. [DOI] [PubMed] [Google Scholar]
- 187.Chen C, Wang Y, Wang Y, Cheng M, Yin J, Zhang X, et al. Gentiopicroside ameliorates bleomycin-induced pulmonary fibrosis in mice via inhibiting inflammatory and fibrotic process. Biochem Biophys Res Commun. 2018;495(4):2396–2403. doi: 10.1016/j.bbrc.2017.12.112. [DOI] [PubMed] [Google Scholar]
- 188.Liu B, Yang JY, Hao JT, Xie HF, Shimizu K, Li RS, et al. Natural product mogrol attenuates bleomycin-induced pulmonary fibrosis development through promoting AMPK activation. J Funct Foods. 2021;77(8):104280. doi: 10.1016/j.jff.2020.104280. [DOI] [Google Scholar]
- 189.Jia L, Sun P, Gao H, Shen J, Gao Y, Meng C, et al. Mangiferin attenuates bleomycin-induced pulmonary fibrosis in mice through inhibiting TLR4/p65 and TGF-β1/Smad2/3 pathway. J Pharm Pharmacol. 2019;71(6):1017–1028. doi: 10.1111/jphp.13077. [DOI] [PubMed] [Google Scholar]
- 190.Chang K, Zhang X, Lin S, Lin Y, Li C, Akhrymuk I, et al. Atractylodin suppresses TGF-β-mediated epithelial-mesenchymal transition in alveolar epithelial cells and attenuates bleomycin-induced pulmonary fibrosis in mice. Int J Mol Sci. 2021;22(20):11152. doi: 10.3390/ijms222011152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wang Q, Yu J, Hu Y, Chen X, Zhang L, Pan T, et al. Indirubin alleviates bleomycin-induced pulmonary fibrosis in mice by suppressing fibroblast to myofibroblast differentiation. Biomed Pharmacother. 2020;131:110715. doi: 10.1016/j.biopha.2020.110715. [DOI] [PubMed] [Google Scholar]
- 192.Fu Z, Xu Y, Cai C. Ginsenoside Rg3 inhibits pulmonary fibrosis by preventing HIF-1α nuclear localisation. Bmc Pulm Med. 2021;21(1):70. doi: 10.1186/s12890-021-01426-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Su X, Liu K, Xie Y, Zhang M, Wu X, Zhang Y, et al. Mushroom inonotus sanghuang alleviates experimental pulmonary fibrosis: implications for therapy of pulmonary fibrosis. Biomed Pharmacother. 2021;133:110919. doi: 10.1016/j.biopha.2020.110919. [DOI] [PubMed] [Google Scholar]
- 194.Zheng Q, Tong M, Ou B, Liu C, Hu C, Yang Y. Isorhamnetin protects against bleomycin-induced pulmonary fibrosis by inhibiting endoplasmic reticulum stress and epithelial-mesenchymal transition. Int J Mol Med. 2019;43(1):117–126. doi: 10.3892/ijmm.2018.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Liu L, Yu N, Leng W, Lu Y, Xia X, Yuan H. 6-Gingerol, a functional polyphenol of ginger, reduces pulmonary fibrosis by activating Sirtuin1. Allergol Immunopathol. 2022;50(2):104–114. doi: 10.15586/aei.v50i2.533. [DOI] [PubMed] [Google Scholar]
- 196.Ali SA, Saifi MA, Godugu C, Talla V. Silibinin alleviates silica-induced pulmonary fibrosis: potential role in modulating inflammation and epithelial-mesenchymal transition. Phytother Res. 2021;35(9):5290–5304. doi: 10.1002/ptr.7210. [DOI] [PubMed] [Google Scholar]
- 197.Xiao T, Wei Y, Cui M, Li X, Ruan H, Zhang L, et al. Effect of dihydromyricetin on SARS-cov-2 viral replication and pulmonary inflammation and fibrosis. Phytomedicine. 2021;91:153704. doi: 10.1016/j.phymed.2021.153704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li Z, Geng J, Xie B, He J, Wang J, Peng L, et al. Dihydromyricetin alleviates pulmonary fibrosis by regulating abnormal fibroblasts through the Stat3/p-Stat3/Glut1 signaling pathway. Front Pharmacol. 2022;13:834604. doi: 10.3389/fphar.2022.834604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chen J, Lu J, Wang B, Zhang X, Huang Q, Yuan J, et al. Polysaccharides from dendrobium officinale inhibit bleomycin-induced pulmonary fibrosis via the TGFΒ1-Smad2/3 axis. Int J Biol Macromol. 2018;118(Pt B):2163–2175. doi: 10.1016/j.ijbiomac.2018.07.056. [DOI] [PubMed] [Google Scholar]
- 200.Reed EB, Ard S, La J, Park CY, Culligan L, Fredberg JJ, et al. Anti-fibrotic effects of tannic acid through regulation of a sustained TGF-beta receptor signaling. Respir Res. 2019;20(1):168. doi: 10.1186/s12931-019-1141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Huang J, Tong X, Zhang L, Zhang Y, Wang L, Wang D, et al. Hyperoside attenuates bleomycin-induced pulmonary fibrosis development in mice. Front Pharmacol. 2020;11:550955. doi: 10.3389/fphar.2020.550955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang Z, Fang K, Wang G, Guan X, Pang Z, Guo Y, et al. Protective effect of amygdalin on epithelial-mesenchymal transformation in experimental chronic obstructive pulmonary disease mice. Phytother Res PTR. 2019;33(3):808–817. doi: 10.1002/ptr.6274. [DOI] [PubMed] [Google Scholar]
- 203.Cui Y, Xin H, Tao Y, Mei L, Wang Z. Arenaria kansuensis attenuates pulmonary fibrosis in mice via the activation of Nrf2 pathway and the inhibition of NF-kB/TGF-beta1/Smad2/3 pathway. Phytother Res. 2021;35(2):974–986. doi: 10.1002/ptr.6857. [DOI] [PubMed] [Google Scholar]
- 204.Li X, Yu H, Liang L, Bi Z, Wang Y, Gao S, et al. Myricetin ameliorates bleomycin-induced pulmonary fibrosis in mice by inhibiting TGF-β signaling via targeting HSP90β. Biochem Pharmacol. 2020;178:114097. doi: 10.1016/j.bcp.2020.114097. [DOI] [PubMed] [Google Scholar]
- 205.Hua Q, Huang X, Xie W, Zhao F, Cheng H, Luo Z, et al. Pparγ mediates the anti-pulmonary fibrosis effect of icaritin. Toxicol Lett. 2021;350:81–90. doi: 10.1016/j.toxlet.2021.06.014. [DOI] [PubMed] [Google Scholar]
- 206.Shen Y, Cheng M, Liu X, Zhu D, Gao J. Sodium houttuyfonate inhibits bleomycin induced pulmonary fibrosis in mice. Front Pharmacol. 2021;12:596492. doi: 10.3389/fphar.2021.596492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Li X, Liu S, Zhai Y, Cao X, Gao S, Huang M, et al. In vitro screening for compounds from hypericum longistylum with anti-pulmonary fibrosis activity. Bioorg Med Chem Lett. 2019;29(22):126695. doi: 10.1016/j.bmcl.2019.126695. [DOI] [PubMed] [Google Scholar]
- 208.Guo J, Fang Y, Jiang F, Li L, Zhou H, Xu X, et al. Neohesperidin inhibits TGF-β1/Smad3 signaling and alleviates bleomycin-induced pulmonary fibrosis in mice. Eur J Pharmacol. 2019;864:172712. doi: 10.1016/j.ejphar.2019.172712. [DOI] [PubMed] [Google Scholar]
- 209.Ma W, Huang Q, Xiong G, Deng L, He Y. The protective effect of hederagenin on pulmonary fibrosis by regulating the Ras/JNK/NFAT4 axis in rats. Biosci Biotechnol Biochem. 2020;84(6):1131–1138. doi: 10.1080/09168451.2020.1721263. [DOI] [PubMed] [Google Scholar]
- 210.Jiang J, Wang F, Luo A, Lin S, Feng X, Yan W, et al. Polyporus polysaccharide ameliorates bleomycin-induced pulmonary fibrosis by suppressing myofibroblast differentiation via TGF-β/Smad2/3 pathway. Front Pharmacol. 2020;11:767. doi: 10.3389/fphar.2020.00767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Li A, Xiao X, Feng Z, Chen X, Liu L, Lin L, et al. Nagilactone d ameliorates experimental pulmonary fibrosis in vitro and in vivo via modulating TGF-β/Smad signaling pathway. Toxicol Appl Pharmacol. 2020;389:114882. doi: 10.1016/j.taap.2020.114882. [DOI] [PubMed] [Google Scholar]
- 212.Cheresh P, Kim S, Tulasiram S, Kamp DW. Oxidative stress and pulmonary fibrosis. Biochim Biophys Acta. 2013;1832(7):1028–1040. doi: 10.1016/j.bbadis.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Masamune A, Watanabe T, Kikuta K, Satoh K, Shimosegawa T. Nadph oxidase plays a crucial role in the activation of pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2008;294(1):G99–108. doi: 10.1152/ajpgi.00272.2007. [DOI] [PubMed] [Google Scholar]
- 214.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Daniil ZD, Papageorgiou E, Koutsokera A, Kostikas K, Kiropoulos T, Papaioannou AI, et al. Serum levels of oxidative stress as a marker of disease severity in idiopathic pulmonary fibrosis. Pulm Pharmacol Ther. 2008;21(1):26–31. doi: 10.1016/j.pupt.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 216.Rodriguez LR, Bui SN, Beuschel RT, Ellis E, Liberti EM, Chhina MK, et al. Curcumin induced oxidative stress attenuation by N-acetylcysteine co-treatment: a fibroblast and epithelial cell in-vitro study in idiopathic pulmonary fibrosis. Mol Med. 2019;25(1):27. doi: 10.1186/s10020-019-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nathan C. Points of control in inflammation. Nature. 2002;420(6917):846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 218.Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140(6):871–882. doi: 10.1016/j.cell.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 219.Wuyts WA, Agostini C, Antoniou KM, Bouros D, Chambers RC, Cottin V, et al. The pathogenesis of pulmonary fibrosis: a moving target. Eur Respir J. 2013;41(5):1207–1218. doi: 10.1183/09031936.00073012. [DOI] [PubMed] [Google Scholar]
- 220.Gorowiec MR, Borthwick LA, Parker SM, Kirby JA, Saretzki GC, Fisher AJ. Free radical generation induces epithelial-to-mesenchymal transition in lung epithelium via a TGF-β1-dependent mechanism. Free Radic Biol Med. 2012;52(6):1024–1032. doi: 10.1016/j.freeradbiomed.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 221.Cameli P, Carleo A, Bergantini L, Landi C, Prasse A, Bargagli E. Oxidant/antioxidant disequilibrium in idiopathic pulmonary fibrosis pathogenesis. Inflammation. 2020;43(1):1–7. doi: 10.1007/s10753-019-01059-1. [DOI] [PubMed] [Google Scholar]
- 222.Otoupalova E, Smith S, Cheng G, Thannickal VJ. Oxidative stress in pulmonary fibrosis. Compr Physiol. 2020;10(2):509–547. doi: 10.1002/cphy.c190017. [DOI] [PubMed] [Google Scholar]
- 223.Rim H, Moon P, Choi I, Lee E, Kim H, Jeong H. Sososo or its active ingredient chrysophanol regulates production of inflammatory cytokines & adipokine in both macrophages & adipocytes. Indian J Med Res. 2013;137(1):142–150. [PMC free article] [PubMed] [Google Scholar]
- 224.Chen J, Ma M, Lu Y, Wang L, Wu C, Duan H. Rhaponticin from rhubarb rhizomes alleviates liver steatosis and improves blood glucose and lipid profiles in KK/Ay diabetic mice. Planta Med. 2009;75(5):472–477. doi: 10.1055/s-0029-1185304. [DOI] [PubMed] [Google Scholar]
- 225.Tian S, Yang Y, Liu X, Xu Q. Emodin attenuates bleomycin-induced pulmonary fibrosis via anti-inflammatory and anti-oxidative activities in rats. Med Sci Monit. 2018;24:1–10. doi: 10.12659/MSM.905496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Qi XW, Ou YM, Xie Y, Cai KR, Gan HL, Wan QQ, et al. Chrysophanol administration alleviates bleomycin-induced pulmonary fibrosis by inhibiting lung fibroblast proliferation and wnt/beta-catenin signaling. Trop J Pharm Res. 2020;19(5):971–976. doi: 10.4314/tjpr.v19i5.9. [DOI] [Google Scholar]
- 227.Zhou L, Zuo Z, Chow MSS. Danshen: an overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J Clin Pharmacol. 2005;45(12):1345–1359. doi: 10.1177/0091270005282630. [DOI] [PubMed] [Google Scholar]
- 228.Peng L, An L, Sun N, Ma Y, Zhang X, Liu W, et al. Salvia miltiorrhiza restrains reactive oxygen species-associated pulmonary fibrosis via targeting Nrf2-Nox4 redox balance. Am J Chin Med. 2019;47(5):1113–1131. doi: 10.1142/S0192415X19500575. [DOI] [PubMed] [Google Scholar]
- 229.Feng F, Cheng P, Zhang H, Li N, Qi Y, Wang H, et al. The protective role of tanshinone iia in silicosis rat model via TGF-β1/Smad signaling suppression, NOX4 inhibition and Nrf2/ARE signaling activation. Drug Des Devel Ther. 2019;13:4275–4290. doi: 10.2147/DDDT.S230572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Liu Q, Shi X, Tang L, Xu W, Jiang S, Ding W, et al. Salvianolic acid b attenuates experimental pulmonary inflammation by protecting endothelial cells against oxidative stress injury. Eur J Pharmacol. 2018;840:9–19. doi: 10.1016/j.ejphar.2018.09.030. [DOI] [PubMed] [Google Scholar]
- 231.Zhu Z, Li Q, Xu C, Zhao J, Li S, Wang Y, et al. Sodium tanshinone iia sulfonate attenuates silica-induced pulmonary fibrosis in rats via activation of the Nrf2 and thioredoxin system. Environ Toxicol Pharmacol. 2020;80:103461. doi: 10.1016/j.etap.2020.103461. [DOI] [PubMed] [Google Scholar]
- 232.Shaikh SB, Prabhakar BY. Effect of curcumin on il-17a mediated pulmonary AMPK kinase/cyclooxygenase-2 expressions via activation of NFkB in bleomycin-induced acute lung injury in vivo. Int Immunopharmacol. 2020;85:106676. doi: 10.1016/j.intimp.2020.106676. [DOI] [PubMed] [Google Scholar]
- 233.Chen H, Yang R, Tang Y, Fu X. Effects of curcumin on artery blood gas index of rats with pulmonary fibrosis caused by paraquat poisoning and the expression of Smad 4, Smurf 2, interleukin-4 and interferon-γ. Exp Ther Med. 2019;17(5):3664–3670. doi: 10.3892/etm.2019.7341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ke S, Zhang Y, Lan Z, Li S, Zhu W, Liu L. Curcumin protects murine lung mesenchymal stem cells from H(2)O(2) by modulating the Akt/Nrf2/HO-1 pathway. J Int Med Res. 2020;48(4):300060520910665. doi: 10.1177/0300060520910665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Durairaj P, Venkatesan S, Narayanan V, Babu M. Protective effects of curcumin on bleomycin-induced changes in lung glycoproteins. Mol Cell Biochem. 2020;469(1–2):159–167. doi: 10.1007/s11010-020-03737-3. [DOI] [PubMed] [Google Scholar]
- 236.Ali YA, Ahmed AAE, Abd El-Raouf OM, Elkhoely A, Gad AM. Polydatin combats methotrexate-induced pulmonary fibrosis in rats: involvement of biochemical and histopathological assessment. J Biochem Mol Toxicol. 2022;36(5):e23019. doi: 10.1002/jbt.23019. [DOI] [PubMed] [Google Scholar]
- 237.Chang H, Meng H, Bai W, Meng Q. A metabolomic approach to elucidate the inhibitory effects of baicalin in pulmonary fibrosis. Pharm Biol. 2021;59(1):1016–1025. doi: 10.1080/13880209.2021.1950192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Li N, Wu K, Feng F, Wang L, Zhou X, Wang W. Astragaloside iv alleviates silica-induced pulmonary fibrosis via inactivation of the TGF-β1/Smad2/3 signaling pathway. Int J Mol Med. 2021;47(3):16. doi: 10.3892/ijmm.2021.4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Guo K, Chen J, Chen Z, Luo G, Yang S, Zhang M, et al. Triptolide alleviates radiation-induced pulmonary fibrosis via inhibiting IKKΒ stimulated LOX production. Biochem Biophys Res Commun. 2020;527(1):283–288. doi: 10.1016/j.bbrc.2020.04.023. [DOI] [PubMed] [Google Scholar]
- 240.Qiu M, Yang Z, Bian M, Liu C, Zhao Y, Liu Q. Protective effects of isorhynchophylline against silicon-dioxide-induced lung injury in mice. Artif Cells Nanomed Biotechnol. 2020;48(1):1125–1134. doi: 10.1080/21691401.2020.1814315. [DOI] [PubMed] [Google Scholar]
- 241.Liu Z, Zhong J, Zhang M, Chen Z, Wang J, Chen H, et al. The alexipharmic mechanisms of five licorice ingredients involved in CYP450 and Nrf2 pathways in paraquat-induced mice acute lung injury. Oxid Med Cell Longev. 2019;2019:7283104. doi: 10.1155/2019/7283104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Gad El-Hak HN, Mohamed OE, Nabil ZI. Evaluating the protective role of deglycyrrhizinated licorice root supplement on bleomycin induced pulmonary oxidative damage. Toxicol Mech Methods. 2022;32(3):180–193. doi: 10.1080/15376516.2021.1977881. [DOI] [PubMed] [Google Scholar]
- 243.Jiang F, Li M, Wang H, Ding B, Zhang C, Ding Z, et al. Coelonin, an anti-inflammation active component of Bletilla striata and its potential mechanism. Int J Mol Sci. 2019;20(18):4422. doi: 10.3390/ijms20184422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Peng L, Wen L, Shi Q, Gao F, Huang B, Wang C. Chelerythrine ameliorates pulmonary fibrosis via activating the Nrf2/ARE signaling pathway. Cell Biochem Biophys. 2021;79(2):337–347. doi: 10.1007/s12013-021-00967-0. [DOI] [PubMed] [Google Scholar]
- 245.Ding Q, Sun J, Xie W, Zhang M, Zhang C, Xu X. Stemona alkaloids suppress the positive feedback loop between M2 polarization and fibroblast differentiation by inhibiting JAK2/Stat3 pathway in fibroblasts and CXCR4/PI(3)K/AKT1 pathway in macrophages. Int Immunopharmacol. 2019;72:385–394. doi: 10.1016/j.intimp.2019.04.030. [DOI] [PubMed] [Google Scholar]
- 246.Huai B, Ding J. Atractylenolide III attenuates bleomycin-induced experimental pulmonary fibrosis and oxidative stress in rat model via Nrf2/NQO1/Ho-1 pathway activation. Immunopharmacol Immunotoxicol. 2020;42(5):436–444. doi: 10.1080/08923973.2020.1806871. [DOI] [PubMed] [Google Scholar]
- 247.Yang F, Hou Z, Zhu H, Chen X, Li W, Cao R, et al. Catalpol protects against pulmonary fibrosis through inhibiting TGF-β1/Smad3 and Wnt/β-catenin signaling pathways. Front Pharmacol. 2020;11:594139. doi: 10.3389/fphar.2020.594139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Xie Y, Li W, Lu C, Zhu L, Qin S, Du Z. The effects of phycocyanin on bleomycin-induced pulmonary fibrosis and the intestinal microbiota in C57BL/6 mice. Appl Microbiol Biotechnol. 2019;103(20):8559–8569. doi: 10.1007/s00253-019-10018-7. [DOI] [PubMed] [Google Scholar]
- 249.Shariati S, Kalantar H, Pashmforoosh M, Mansouri E, Khodayar MJ. Epicatechin protective effects on bleomycin-induced pulmonary oxidative stress and fibrosis in mice. Biomed Pharmacother. 2019;114:108776. doi: 10.1016/j.biopha.2019.108776. [DOI] [PubMed] [Google Scholar]
- 250.Raish M, Ahmad A, Ahmad Ansari M, Ahad A, Al-Jenoobi FI, Al-Mohizea AM, et al. Sinapic acid ameliorates bleomycin-induced lung fibrosis in rats. Biomed Pharmacother. 2018;108:224–231. doi: 10.1016/j.biopha.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 251.Gan W, Li X, Cui Y, Xiao T, Liu R, Wang M, et al. Pinocembrin relieves lipopolysaccharide and bleomycin induced lung inflammation via inhibiting TLR4-NF-κB-NLRP3 inflammasome signaling pathway. Int Immunopharmacol. 2021;90:107230. doi: 10.1016/j.intimp.2020.107230. [DOI] [PubMed] [Google Scholar]
- 252.Tavares LA, Rezende AA, Santos JL, Estevam CS, Silva AMO, Schneider JK, et al. Cymbopogon winterianus essential oil attenuates bleomycin-induced pulmonary fibrosis in a murine model. Pharmaceutics. 2021;13(5):679. doi: 10.3390/pharmaceutics13050679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zhou Z, Kandhare AD, Kandhare AA, Bodhankar SL. Hesperidin ameliorates bleomycin-induced experimental pulmonary fibrosis via inhibition of TGF-b1/Smad3/AMPK and IkBa/NF-kB pathways. Excli J. 2019;2019(18):723–745. doi: 10.17179/excli2019-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Hu X, Huang X. Alleviation of inflammatory response of pulmonary fibrosis in acute respiratory distress syndrome by puerarin via transforming growth factor (TGF-β1) Med Sci Monit. 2019;25:6523–6531. doi: 10.12659/MSM.915570. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 255.Samareh Fekri M, Poursalehi HR, Sharififar F, Mandegary A, Rostamzadeh F, Mahmoodi R. The effects of methanolic extract of glycyrrhiza glabra on the prevention and treatment of bleomycin-induced pulmonary fibrosis in rat: experimental study. Comparative Study Drug Chem Toxicol. 2021;44(4):365–371. doi: 10.1080/01480545.2019.1606232. [DOI] [PubMed] [Google Scholar]
- 256.Dong H, Xue T, Liu Y, He S, Yi Y, Zhang B, et al. Low molecular weight fucoidan inhibits pulmonary fibrosis in vivo and in vitro via antioxidant activity. Oxid Med Cell Longev. 2022;2022:7038834. doi: 10.1155/2022/7038834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Liu S, Wang Y, Wen H, Sun X, Wang Y. Hydroxysafflor yellow a inhibits TNF-α-induced inflammation of human fetal lung fibroblasts via Nf-κB signaling pathway. Evid Based Complement Alternat Med. 2019;2019:4050327. doi: 10.1155/2019/4050327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Jin M, Wang L, Wu Y, Zang B, Tan L. Protective effect of hydroxysafflor yellow a on bleomycin- induced pulmonary inflammation and fibrosis in rats. Chin J Integr Med. 2018;24(1):32–39. doi: 10.1007/s11655-017-2094-z. [DOI] [PubMed] [Google Scholar]
- 259.Bai L, Li A, Gong C, Ning X, Wang Z. Protective effect of rutin against bleomycin induced lung fibrosis: involvement of TGF-β1/α-SMA/Col I and III pathway. BioFactors. 2020;46(4):637–644. doi: 10.1002/biof.1629. [DOI] [PubMed] [Google Scholar]
- 260.Guan C, Qiao S, Lv Q, Cao N, Wang K, Dai Y, et al. Orally administered berberine ameliorates bleomycin-induced pulmonary fibrosis in mice through promoting activation of PPAR-γ and subsequent expression of HGF in colons. Toxicol Appl Pharmacol. 2018;343:1–15. doi: 10.1016/j.taap.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 261.Tong J, Wu Z, Wang Y, Hao Q, Liu H, Cao F, et al. Astragaloside IV synergizing with ferulic acid ameliorates pulmonary fibrosis by TGF-β1/Smad3 signaling. Evid Based Complement Alternat Med. 2021;2021:8845798. doi: 10.1155/2021/8845798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Luo J, Zhang T, Zhu C, Sun J, Zhu W, Ai W, et al. Asiaticoside might attenuate bleomycin-induced pulmonary fibrosis by activating cAMP and Rap1 signalling pathway assisted by A2AR. J Cell Mol Med. 2020;24(14):8248–8261. doi: 10.1111/jcmm.15505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zhang T, Dai J, Ye W, Cai L, Wei J, Chen M, et al. Asiaticoside attenuates bleomycin-induced pulmonary fibrosis in A2aR(-/-) mice by promoting the BMP7/Smad1/5 signaling pathway. Biochem Biophys Res Commun. 2020;527(3):662–667. doi: 10.1016/j.bbrc.2020.04.156. [DOI] [PubMed] [Google Scholar]
- 264.Qiu M, An M, Bian M, Yu S, Liu C, Liu Q. Terrestrosin d from tribulus terrestris attenuates bleomycin-induced inflammation and suppresses fibrotic changes in the lungs of mice. Pharm Biol. 2019;57(1):694–700. doi: 10.1080/13880209.2019.1672754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Gungor H, Ekici M, Onder Karayigit M, Turgut NH, Kara H, Arslanbas E. Zingerone ameliorates oxidative stress and inflammation in bleomycin-induced pulmonary fibrosis: modulation of the expression of TGF-β1 and iNOS. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(9):1659–1670. doi: 10.1007/s00210-020-01881-7. [DOI] [PubMed] [Google Scholar]
- 266.Zhao J, Zang J, Lin Y, Wang YH, Li DN, Meng XJ. Polyphenol-rich blue honeysuckle extract alleviates silica-induced lung fibrosis by modulating th immune response and NRF2/Ho-1 mapk signaling. J Funct Foods. 2019;53:176–186. doi: 10.1016/j.jff.2018.12.030. [DOI] [Google Scholar]
- 267.Zhao J, Ma JX, Zhang Q, Tian JL, Wang YH, Meng XJ. Cyanidin-3-glucoside attenuates silica-induced pulmonary inflammatory responses by modulating t cell immune responses and STAT1/STAT3 signaling. J Funct Foods. 2020;68:103911. doi: 10.1016/j.jff.2020.103911. [DOI] [Google Scholar]
- 268.Yang H, Hua C, Yang X, Fan X, Song H, Peng L, et al. Pterostilbene prevents lps-induced early pulmonary fibrosis by suppressing oxidative stress, inflammation and apoptosis in vivo. Food Funct. 2020;11(5):4471–4484. doi: 10.1039/C9FO02521A. [DOI] [PubMed] [Google Scholar]
- 269.Wang Y, Li Y, Wang X, Li X, Chen Y, Yang L, et al. Effect of total flavonoids of oxytropis falcata bunge on the expression of p-JAK1-and p-STAT1-related proteins in idiopathic pulmonary fibrosis. Evid Based Complement Alternat Med. 2020;2020:2407239. doi: 10.1155/2020/2407239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zhao S, Zuo W, Chen H, Bao T, Liu X, Sun T, et al. Effects of pilose antler peptide on bleomycin-induced pulmonary fibrosis in mice. Biomed Pharmacother. 2019;109:2078–2083. doi: 10.1016/j.biopha.2018.08.114. [DOI] [PubMed] [Google Scholar]
- 271.Rong Y, Cao B, Liu B, Li W, Chen Y, Chen H, et al. A novel gallic acid derivative attenuates blm-induced pulmonary fibrosis in mice. Int Immunopharmacol. 2018;64:183–191. doi: 10.1016/j.intimp.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 272.Fu X, Li T, Yao Q. The effect of ophiopogonin c in ameliorating radiation-induced pulmonary fibrosis in C57BL/6 mice: an update study. Front Oncol. 2022;12:811183. doi: 10.3389/fonc.2022.811183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zhang T, Ma S, Liu C, Hu K, Xu M, Wang R. Rosmarinic acid prevents radiation-induced pulmonary fibrosis through attenuation of ROS/MYPT1/TGFΒ1 signaling via miR-19b-3p. Dose Response. 2020;18(4):1559325820968413. doi: 10.1177/1559325820968413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Kalantar H, Sadeghi E, Abolnezhadian F, Goudarzi M, Hemmati AA, Basir Z, et al. Carnosol attenuates bleomycin-induced lung damage via suppressing fibrosis, oxidative stress and inflammation in rats. Life Sci. 2021;287:120059. doi: 10.1016/j.lfs.2021.120059. [DOI] [PubMed] [Google Scholar]
- 275.Kseibati MO, Sharawy MH, Salem HA. Chrysin mitigates bleomycin-induced pulmonary fibrosis in rats through regulating inflammation, oxidative stress, and hypoxia. Int Immunopharmacol. 2020;89(Pt A):107011. doi: 10.1016/j.intimp.2020.107011. [DOI] [PubMed] [Google Scholar]
- 276.Liu B, Rong Y, Sun D, Li W, Chen H, Cao B, et al. Costunolide inhibits pulmonary fibrosis via regulating NF-kB and TGF-β(1)/Smad(2)/Nrf(2)-NOX(4) signaling pathways. Biochem Biophys Res Commun. 2019;510(2):329–333. doi: 10.1016/j.bbrc.2019.01.104. [DOI] [PubMed] [Google Scholar]
- 277.Wang Z, Li X, Chen H, Han L, Ji X, Wang Q, et al. Resveratrol alleviates bleomycin-induced pulmonary fibrosis via suppressing HIF-1α and Nf-κB expression. Aging. 2021;13(3):4605–4616. doi: 10.18632/aging.202420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Ding S, Wang H, Wang M, Bai L, Yu P, Wu W. Resveratrol alleviates chronic “real-world” ambient particulate matter-induced lung inflammation and fibrosis by inhibiting nlrp3 inflammasome activation in mice. Ecotoxicol Environ Saf. 2019;182:109425. doi: 10.1016/j.ecoenv.2019.109425. [DOI] [PubMed] [Google Scholar]
- 279.Yang G, Lyu L, Wang X, Bao L, Lyu B, Lin Z. Systemic treatment with resveratrol alleviates adjuvant arthritis-interstitial lung disease in rats via modulation of JAK/STAT/RANKL signaling pathway. Pulm Pharmacol Ther. 2019;56:69–74. doi: 10.1016/j.pupt.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 280.Sul OJ, Kim JH, Lee T, Seo KW, Cha HJ, Kwon B, et al. Gspe protects against bleomycin-induced pulmonary fibrosis in mice via ameliorating epithelial apoptosis through inhibition of oxidative stress. Oxid Med Cell Longev. 2022;2022:8200189. doi: 10.1155/2022/8200189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.You X, Jiang X, Zhang C, Jiang K, Zhao X, Guo T, et al. Dihydroartemisinin attenuates pulmonary inflammation and fibrosis in rats by suppressing JAK2/STAT3 signaling. Aging. 2022;14(3):1110–1127. doi: 10.18632/aging.203874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Yang D, Qiu J, Zhou H, Yu Y, Zhou D, Xu Y, et al. Dihydroartemisinin alleviates oxidative stress in bleomycin-induced pulmonary fibrosis. Life Sci. 2018;205:176–183. doi: 10.1016/j.lfs.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 283.Yao Y, Yuan Y, Lu Z, Ma Y, Xie Y, Wang M, et al. Effects of Nervilia fordii extract on pulmonary fibrosis through TGF-β/smad signaling pathway. Front Pharmacol. 2021;12:659627. doi: 10.3389/fphar.2021.659627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Choi YH. Trans-cinnamaldehyde prevents oxidative stress-induced apoptosis in V79-4 Chinese hamster lung fibroblasts through the Nrf2-mediated HO-1 activation. Biol Pharm Bull. 2020;43(11):1707–1714. doi: 10.1248/bpb.b20-00407. [DOI] [PubMed] [Google Scholar]
- 285.Zhang J, Li Q, Shao Q, Song J, Zhou B, Shu P. Effects of panax notoginseng saponin on the pathological ultrastructure and serum il-6 and il-8 in pulmonary fibrosis in rabbits. J Cell Biochem. 2018;119(10):8410–8418. doi: 10.1002/jcb.27045. [DOI] [PubMed] [Google Scholar]
- 286.Li R, Xu G, Cao J, Liu B, Xie H, Ishii Y, et al. Alpha-mangostin ameliorates bleomycin-induced pulmonary fibrosis in mice partly through activating adenosine 5'-monophosphate-activated protein kinase. Front Pharmacol. 2019;10:1305. doi: 10.3389/fphar.2019.01305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Fang L, Wang W, Chen J, Zuo A, Gao H, Yan T, et al. Osthole attenuates bleomycin-induced pulmonary fibrosis by modulating nadph oxidase 4-derived oxidative stress in mice. Oxid Med Cell Longev. 2021;2021:3309944. doi: 10.1155/2021/3309944. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 288.Liang Q, Cai W, Zhao Y, Xu H, Tang H, Chen D, et al. Lycorine ameliorates bleomycin-induced pulmonary fibrosis via inhibiting NLRP3 inflammasome activation and pyroptosis. Pharmacol Res. 2020;158:104884. doi: 10.1016/j.phrs.2020.104884. [DOI] [PubMed] [Google Scholar]
- 289.Bahri S, Ben Ali R, Nahdi A, Mlika M, Abdennabi R, Jameleddine S. Salvia officinalis attenuates bleomycin-induced oxidative stress and lung fibrosis in rats. Nutr Cancer. 2020;72(7):1135–1145. doi: 10.1080/01635581.2019.1675724. [DOI] [PubMed] [Google Scholar]
- 290.Zhang D, Liu B, Cao B, Wei F, Yu X, Li G, et al. Synergistic protection of schizandrin b and glycyrrhizic acid against bleomycin-induced pulmonary fibrosis by inhibiting TGF-β1/Smad2 pathways and overexpression of NOX4. Int Immunopharmacol. 2017;48:67–75. doi: 10.1016/j.intimp.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 291.Mehrabani M, Goudarzi M, Mehrzadi S, Siahpoosh A, Mohammadi M, Khalili H, et al. Crocin: a protective natural antioxidant against pulmonary fibrosis induced by bleomycin. Pharmacol Rep. 2020;72(4):992–1001. doi: 10.1007/s43440-019-00023-y. [DOI] [PubMed] [Google Scholar]
- 292.Zaghloul MS, Said E, Suddek GM, Salem HA. Crocin attenuates lung inflammation and pulmonary vascular dysfunction in a rat model of bleomycin-induced pulmonary fibrosis. Life Sci. 2019;235:116794. doi: 10.1016/j.lfs.2019.116794. [DOI] [PubMed] [Google Scholar]
- 293.Li W, Cai Z, Mehmood S, Wang Y, Pan W, Zhang W, et al. Polysaccharide FMP-1 from Morchella esculenta attenuates cellular oxidative damage in human alveolar epithelial A549 cells through PI3K/AKT/Nrf2/HO-1 pathway. Int J Biol Macromol. 2018;120(Pt A):865–875. doi: 10.1016/j.ijbiomac.2018.08.148. [DOI] [PubMed] [Google Scholar]
- 294.Zhu YP, Lin BX, Ding FD, Ma FF, Zhou XH, Zong HY, et al. Leonurine negatively modulates T cells activity by suppressing recombination activation gene protein 2 in pulmonary fibrosis. Eur J Inflamm. 2021;19:205873922110359. doi: 10.1177/20587392211035907. [DOI] [Google Scholar]
- 295.Du W, Tang Z, Yang F, Liu X, Dong J. Icariin attenuates bleomycin-induced pulmonary fibrosis by targeting hippo/yap pathway. Biomed Pharmacother. 2021;143:112152. doi: 10.1016/j.biopha.2021.112152. [DOI] [PubMed] [Google Scholar]
- 296.Shen X, Ding D, Yu L, Ni J, Liu Y, Wang W, et al. Total extract of anemarrhenae rhizoma attenuates bleomycin-induced pulmonary fibrosis in rats. Bioorg Chem. 2022;119:105546. doi: 10.1016/j.bioorg.2021.105546. [DOI] [PubMed] [Google Scholar]
- 297.Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 298.Li J, Yao W, Zhang L, Bao L, Chen H, Wang D, et al. Genome-wide dna methylation analysis in lung fibroblasts co-cultured with silica-exposed alveolar macrophages. Respir Res. 2017;18(1):91. doi: 10.1186/s12931-017-0576-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Robert S, Gicquel T, Victoni T, Valença S, Barreto E, Bailly-Maître B, et al. Involvement of matrix metalloproteinases (MMPs) and inflammasome pathway in molecular mechanisms of fibrosis. Biosci Rep. 2016;36(4):e00360. doi: 10.1042/BSR20160107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Giannandrea M, Parks WC. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech. 2014;7(2):193–203. doi: 10.1242/dmm.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Cutroneo KR, White SL, Phan SH, Ehrlich HP. Therapies for bleomycin induced lung fibrosis through regulation of TGF-beta1 induced collagen gene expression. J Cell Physiol. 2007;211(3):585–589. doi: 10.1002/jcp.20972. [DOI] [PubMed] [Google Scholar]
- 302.Feng F, Cheng P, Xu S, Li N, Wang H, Zhang Y, et al. Tanshinone iia attenuates silica-induced pulmonary fibrosis via nrf2-mediated inhibition of EMT and TGF-β1/Smad signaling. Chem Biol Interact. 2020;319:109024. doi: 10.1016/j.cbi.2020.109024. [DOI] [PubMed] [Google Scholar]
- 303.Tang Y, Chen Y, Chu Z, Yan B, Xu L. Protective effect of cryptotanshinone on lipopolysaccharide-induced acute lung injury in mice. Eur J Pharmacol. 2014;723:494–500. doi: 10.1016/j.ejphar.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 304.Zhang Y, Lu W, Zhang X, Lu J, Xu S, Chen S, et al. Cryptotanshinone protects against pulmonary fibrosis through inhibiting Smad and STAT3 signaling pathways. Pharmacol Res. 2019;147:104307. doi: 10.1016/j.phrs.2019.104307. [DOI] [PubMed] [Google Scholar]
- 305.Li L, Xu L, Hu Y, Cui W, Cui W, Zhou W, et al. Astragaloside iv improves bleomycin-induced pulmonary fibrosis in rats by attenuating extracellular matrix deposition. Front Pharmacol. 2017;8:513. doi: 10.3389/fphar.2017.00513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Li N, Feng F, Wu K, Zhang H, Zhang W, Wang W. Inhibitory effects of astragaloside IV on silica-induced pulmonary fibrosis via inactivating TGF-β1/Smad3 signaling. Biomed Pharmacother. 2019;119:109387. doi: 10.1016/j.biopha.2019.109387. [DOI] [PubMed] [Google Scholar]
- 307.Feng F, Li N, Cheng P, Zhang H, Wang H, Wang Y, et al. Tanshinone IIA attenuates silica-induced pulmonary fibrosis via inhibition of TGF-β1-smad signaling pathway. Biomed Pharmacother. 2020;121:109586. doi: 10.1016/j.biopha.2019.109586. [DOI] [PubMed] [Google Scholar]
- 308.Sun X, Cui X, Chen X, Jiang X. Baicalein alleviated tgf β1-induced type I collagen production in lung fibroblasts via downregulation of connective tissue growth factor. Biomed Pharmacother. 2020;131:110744. doi: 10.1016/j.biopha.2020.110744. [DOI] [PubMed] [Google Scholar]
- 309.Durairaj P, Venkatesan S, Narayanan V, Babu M. Curcumin inhibition of bleomycin-induced changes in lung collagen synthesis, deposition and assembly. Mol Biol Rep. 2021;48(12):7775–7785. doi: 10.1007/s11033-021-06790-3. [DOI] [PubMed] [Google Scholar]
- 310.Yang L, Chen P, Luo M, Shi W, Hou D, Gao Y, et al. Inhibitory effects of total ginsenoside on bleomycin-induced pulmonary fibrosis in mice. Biomed Pharmacother. 2019;114:108851. doi: 10.1016/j.biopha.2019.108851. [DOI] [PubMed] [Google Scholar]
- 311.Du S, Li C, Lu Y, Lei X, Zhang Y, Li S, et al. Dioscin alleviates crystalline silica-induced pulmonary inflammation and fibrosis through promoting alveolar macrophage autophagy. Theranostics. 2019;9(7):1878–1892. doi: 10.7150/thno.29682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Li R, Chen X, Xu Y, Feng F, He H, Zhou X. Inhibitory effects of alkaline extract from the pericarp of citrus reticulata blanco on collagen behavior in bleomycin-induced pulmonary fibrosis. J Ethnopharmacol. 2021;269:113761. doi: 10.1016/j.jep.2020.113761. [DOI] [PubMed] [Google Scholar]
- 313.Huang C, Wu X, Wang S, Wang W, Guo F, Chen Y, et al. Combination of salvia miltiorrhiza and ligustrazine attenuates bleomycin-induced pulmonary fibrosis in rats via modulating tnf-α and tgf-β. Chin Med. 2018;13:36. doi: 10.1186/s13020-018-0194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Fu Y, Zhao P, Xie Z, Wang L, Chen S. Oridonin inhibits myofibroblast differentiation and bleomycin-induced pulmonary fibrosis by regulating transforming growth factor β (TGFβ)/Smad pathway. Med Sci Monit. 2018;24:7548–7555. doi: 10.12659/MSM.912740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Chun-Bin S, Yi Y, Qin-Yi W, Yang L, Jing-Ze Y, Hai-Jing X, et al. The main active components of curcuma zedoaria reduces collagen deposition in human lung fibroblast via autophagy. Mol Immunol. 2020;124:109–116. doi: 10.1016/j.molimm.2020.05.017. [DOI] [PubMed] [Google Scholar]
- 316.Wang J, He F, Chen L, Li Q, Jin S, Zheng H, et al. Resveratrol inhibits pulmonary fibrosis by regulating mIR-21 through MAPK/AP-1 pathways. Biomed Pharmacother. 2018;105:37–44. doi: 10.1016/j.biopha.2018.05.104. [DOI] [PubMed] [Google Scholar]
- 317.Shi W, Hao J, Wu Y, Liu C, Shimizu K, Li R, et al. Protective effects of heterophyllin B against bleomycin-induced pulmonary fibrosis in mice via AMPK activation. Eur J Pharmacol. 2022;921:174825. doi: 10.1016/j.ejphar.2022.174825. [DOI] [PubMed] [Google Scholar]
- 318.Nie Y, Yang Y, Zhang J, Cai G, Chang Y, Chai G, et al. Shikonin suppresses pulmonary fibroblasts proliferation and activation by regulating AKT and P38 MAPK signaling pathways. Biomed Pharmacother. 2017;95:1119–1128. doi: 10.1016/j.biopha.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 319.Doherty J, Baehrecke EH. Life, death and autophagy. Nat Cell Biol. 2018;20(10):1110–1117. doi: 10.1038/s41556-018-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Araya J, Kojima J, Takasaka N, Ito S, Fujii S, Hara H, et al. Insufficient autophagy in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304(1):L56–69. doi: 10.1152/ajplung.00213.2012. [DOI] [PubMed] [Google Scholar]
- 321.Mizumura K, Cloonan S, Choi ME, Hashimoto S, Nakahira K, Ryter SW, et al. Autophagy: friend or foe in lung disease? Ann Am Thorac Soc. 2016;13(Suppl 1):S40–47. doi: 10.1513/AnnalsATS.201507-450MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Haspel JA, Choi AMK. Autophagy: a core cellular process with emerging links to pulmonary disease. Am J Respir Crit Care Med. 2011;184(11):1237–1246. doi: 10.1164/rccm.201106-0966CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Wang RF. Progress in imaging agents of cell apoptosis. Anticancer Agents Med Chem. 2009;9(9):996–1002. doi: 10.2174/187152009789377781. [DOI] [PubMed] [Google Scholar]
- 324.Jin Y, Peng L, Zhao A. Hyperoxia induces the apoptosis of alveolar epithelial cells and changes of pulmonary surfactant proteins. Eur Rev Med Pharmacol Sci. 2018;22(2):492–497. doi: 10.26355/eurrev_201801_14200. [DOI] [PubMed] [Google Scholar]
- 325.Im J, Kim K, Hergert P, Nho RS. Idiopathic pulmonary fibrosis fibroblasts become resistant to FAS ligand-dependent apoptosis via the alteration of decoy receptor 3. J Pathol. 2016;240(1):25–37. doi: 10.1002/path.4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Romero Y, Bueno M, Ramirez R, Álvarez D, Sembrat JC, Goncharova EA, et al. Mtorc1 activation decreases autophagy in aging and idiopathic pulmonary fibrosis and contributes to apoptosis resistance in IPF fibroblasts. Aging Cell. 2016;15(6):1103–1112. doi: 10.1111/acel.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Testai L, Calderone V. Nutraceutical value of citrus flavanones and their implications in cardiovascular disease. Nutrients. 2017;9(5):502. doi: 10.3390/nu9050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Wu Q, Zhou Y, Feng F, Jin Y, Wang Z, Zhou X. Probing into the mechanism of alkaline citrus extract promoted apoptosis in pulmonary fibroblasts of bleomycin-induced pulmonary fibrosis mice. Evid Based Complement Alternat Med: eCAM. 2018;2018:9658950. doi: 10.1155/2018/9658950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Wu Q, Zhou Y, Zhou X. Citrus alkaline extract delayed the progression of pulmonary fibrosis by inhibiting P38/Nf-κB signaling pathway-induced cell apoptosis. Evid Based Complement Alternat Med eCAM. 2019;2019:1528586. doi: 10.1155/2019/1528586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Feng F, Wang Z, Li R, Wu Q, Gu C, Xu Y, et al. Citrus alkaline extracts prevent fibroblast senescence to ameliorate pulmonary fibrosis via activation of cox-2. Biomed Pharmacother. 2019;112:108669. doi: 10.1016/j.biopha.2019.108669. [DOI] [PubMed] [Google Scholar]
- 331.Han D, Xu Y, Peng W, Feng F, Wang Z, Gu C, et al. Citrus alkaline extracts inhibit senescence of A549 cells to alleviate pulmonary fibrosis via the β-catenin/P53 pathway. Med Sci Monit. 2021;27:e928547. doi: 10.12659/MSM.928547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Häkkinen SH, Kärenlampi SO, Heinonen IM, Mykkänen HM, Törrönen AR. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J Agric Food Chem. 1999;47(6):2274–2279. doi: 10.1021/jf9811065. [DOI] [PubMed] [Google Scholar]
- 333.Lee M, Yun S, Lee H, Yang J. Quercetin mitigates inflammatory responses induced by vascular endothelial growth factor in mouse retinal photoreceptor cells through suppression of nuclear factor kappa B. Int J Mol Sci. 2017;18(11):2497. doi: 10.3390/ijms18112497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Hohmann MS, Habiel DM, Coelho AL, Verri WAJ, Hogaboam CM. Quercetin enhances ligand-induced apoptosis in senescent idiopathic pulmonary fibrosis fibroblasts and reduces lung fibrosis in vivo. Am J Respir Cell Mol Biol. 2019;60(1):28–40. doi: 10.1165/rcmb.2017-0289OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Xiao Y, Zhou L, Zhang T, Qin C, Wei P, Luo L, et al. Anti-fibrosis activity of quercetin attenuates rabbit tracheal stenosis via the TGF-β/AKT/mTOR signaling pathway. Life Sci. 2020;250:117552. doi: 10.1016/j.lfs.2020.117552. [DOI] [PubMed] [Google Scholar]
- 336.Wang Z, Feng F, He H, Wu Q, Gu C, Hrovat J, et al. Citrus alkaline extracts prevent endoplasmic reticulum stress in type II alveolar epithelial cells to ameliorate pulmonary fibrosis via the ATF3/PINK1 pathway. Phytomedicine. 2021;89:153599. doi: 10.1016/j.phymed.2021.153599. [DOI] [PubMed] [Google Scholar]
- 337.Chen G, Chang W, Li X, Han L, Zhou D, Feng Y, et al. N-buoh extract of Bletilla striata exerts chemopreventive effects on lung against SiO(2) nanoparticles through activation of Nrf2 pathway. Phytomedicine. 2021;82:153445. doi: 10.1016/j.phymed.2020.153445. [DOI] [PubMed] [Google Scholar]
- 338.Liu M, Su M, Tang D, Hao L, Xun X, Huang Y. Ligustrazin increases lung cell autophagy and ameliorates paraquat-induced pulmonary fibrosis by inhibiting PI3K/Akt/mTOR and hedgehog signalling via increasing mir-193a expression. BMC Pulm Med. 2019;19(1):35. doi: 10.1186/s12890-019-0799-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Xue Z, Zhao F, Sang X, Qiao Y, Shao R, Wang Y, et al. Combination therapy of tanshinone IIA and puerarin for pulmonary fibrosis via targeting IL6-JAK2-STAT3/STAT1 signaling pathways. Phytother Res PTR. 2021;35(10):5883–5898. doi: 10.1002/ptr.7253. [DOI] [PubMed] [Google Scholar]
- 340.He J, Peng H, Wang M, Liu Y, Guo X, Wang B, et al. Isoliquiritigenin inhibits TGF-β1-induced fibrogenesis through activating autophagy via PI3K/AKT/mTOR pathway in MRC-5 cells. Acta Biochim Biophys Sin. 2020;52(8):810–820. doi: 10.1093/abbs/gmaa067. [DOI] [PubMed] [Google Scholar]
- 341.Bahri S, Mies F, Ben Ali R, Mlika M, Jameleddine S, Mc Entee K, et al. Rosmarinic acid potentiates carnosic acid induced apoptosis in lung fibroblasts. PLoS ONE. 2017;12(9):e184368. doi: 10.1371/journal.pone.0184368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Liu H, Yu H, Cao Z, Gu J, Pei L, Jia M, et al. Kaempferol modulates autophagy and alleviates silica-induced pulmonary fibrosis. Dna Cell Biol. 2019;38(12):1418–1426. doi: 10.1089/dna.2019.4941. [DOI] [PubMed] [Google Scholar]
- 343.Guo Z, Li S, Zhang N, Kang Q, Zhai H. Schisandra inhibit bleomycin-induced idiopathic pulmonary fibrosis in rats via suppressing M2 macrophage polarization. Biomed Res Int. 2020;2020:5137349. doi: 10.1155/2020/5137349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Pan L, Lu Y, Li Z, Tan Y, Yang H, Ruan P, et al. Ginkgo biloba extract EGb761 attenuates bleomycin-induced experimental pulmonary fibrosis in mice by regulating the balance of M1/M2 macrophages and nuclear factor kappa b (nf-κb)-mediated cellular apoptosis. Med Sci Monit. 2020;26:e922634. doi: 10.12659/MSM.922634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Tanjore H, Lawson WE, Blackwell TS. Endoplasmic reticulum stress as a pro-fibrotic stimulus. Biochem Biophys Acta. 2013;1832(7):940–947. doi: 10.1016/j.bbadis.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Tanjore H, Blackwell TS, Lawson WE. Emerging evidence for endoplasmic reticulum stress in the pathogenesis of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2012;302(8):L721–L729. doi: 10.1152/ajplung.00410.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Lin JH, Walter P, Yen TSB. Endoplasmic reticulum stress in disease pathogenesis. Annu Rev Pathol. 2008;3:399–425. doi: 10.1146/annurev.pathmechdis.3.121806.151434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Nogee LM, Dunbar AER, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein c gene associated with familial interstitial lung disease. N Engl J Med. 2001;334(8):573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 349.Thomas AQ, Lane K, Phillips JR, Prince M, Markin C, Speer M, et al. Heterozygosity for a surfactant protein c gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165(9):1322–1328. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 350.Jorgensen E, Stinson A, Shan L, Yang J, Gietl D, Albino AP. Cigarette smoke induces endoplasmic reticulum stress and the unfolded protein response in normal and malignant human lung cells. BMC Cancer. 2008;8:229. doi: 10.1186/1471-2407-8-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Laing S, Wang G, Briazova T, Zhang C, Wang A, Zheng Z, et al. Airborne particulate matter selectively activates endoplasmic reticulum stress response in the lung and liver tissues. Am J Physiol Cell Physiol. 2010;299(4):C736–C749. doi: 10.1152/ajpcell.00529.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Tagawa Y, Hiramatsu N, Kasai A, Hayakawa K, Okamura M, Yao J, et al. Induction of apoptosis by cigarette smoke via ROS-dependent endoplasmic reticulum stress and CCAAT/enhancer-binding protein-homologous protein (CHOP) Free Radic Biol Med. 2008;45(1):50–59. doi: 10.1016/j.freeradbiomed.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 353.Torres-González E, Bueno M, Tanaka A, Krug LT, Cheng D, Polosukhin VV, et al. Role of endoplasmic reticulum stress in age-related susceptibility to lung fibrosis. Am J Respir Cell Mol Biol. 2012;46(6):748–756. doi: 10.1165/rcmb.2011-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Castriotta RJ, Eldadah BA, Foster WM, Halter JB, Hazzard WR, Kiley JP, et al. Workshop on idiopathic pulmonary fibrosis in older adults. Chest. 2010;138(3):693–703. doi: 10.1378/chest.09-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Selman M, Rojas M, Mora AL, Pardo A. Aging and interstitial lung diseases: unraveling an old forgotten player in the pathogenesis of lung fibrosis. Semin Respir Crit Care Med. 2010;31(5):607–617. doi: 10.1055/s-0030-1265901. [DOI] [PubMed] [Google Scholar]
- 356.Wang Y, Dong J, Nie J, Zhu J, Wang H, Chen Q, et al. Amelioration of bleomycin-induced pulmonary fibrosis by chlorogenic acid through endoplasmic reticulum stress inhibition. Apoptosis. 2017;22(9):1147–1156. doi: 10.1007/s10495-017-1393-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.