Abstract
This longitudinal cohort study examines if 1) cognitive decline varies by birth cohort, adjusting for covariates, and 2) edentulism and nonuse of dental care predict 10-y cognitive decline (2008–2018). The Health and Retirement Study (HRS) features a representative sample of US adults over age 50. Eligibility criteria included having cognitive interview data available and responding to the question, “Have you lost all of your upper and lower natural permanent teeth?” at 2+ time points between 2006 and 2018. Use of dental care in the past 2 y was assessed. Linear mixed models for repeated measures estimated the trajectories of mean cognition over time for the birth cohorts, adjusted for baseline cognition, dentition status, dental care use, and covariates (demographic characteristics, health behaviors, and medical conditions). Cohort-by-time interaction terms were included to assess if cognitive decline varied by birth cohort. Ten-year change in cognition status (measured by HRS Cogtot27)—categorized as dementia (<7); cognitive impairment, not demented (7-11) 7≤Cogtot27<12; and normal (≥12)—was also investigated according to birth cohort, dentition status, and dental care use. Mean (SD) baseline age was 63.4 (10.1) y (n = 22,728). Older birth cohorts had greater cognitive decline than younger cohorts. Linear mixed-model estimates and 95% confidence intervals for protective factors for cognitive decline included higher baseline cognition (HRS Cogtot27) (0.49; 0.48–0.50), use of dental care in the past 2 y (0.17; 0.10–0.23), and covariates such as greater household wealth and being married. Risk increased with being edentulous (−0.42; −0.56 to −0.28), history of stroke or diabetes, less education, Medicaid recipient, current smoker, loneliness, and poor/fair self-rated health. Edentulism and irregular dental care are among important predictors of cognitive decline. Tooth retention and regular dental care throughout life appear to be important for maintaining oral and cognitive health.
Keywords: tooth loss, dental care use, epidemiology, longitudinal, dementia, aging
Introduction
Global increases in cognitive impairment result in high costs to society and families. In the United States (US), Alzheimer’s disease (AD) and other dementias occur in 8.8% of older adults (Kemle and Ackermann 2018). AD incidence is expected to double by 2050 (Alzheimer’s Association 2022). In 2022, the total cost of care for Americans with dementia was estimated at $321 billion, or $41,757/person (Alzheimer’s Association 2022). Thus, the importance of understanding the predictors and causes of cognitive decline cannot be overstated.
Correlates of cognitive impairment include oral conditions, notably periodontal diseases and tooth loss. Theoretical mechanisms for a relationship between periodontal disease (PD) and neurocognitive disorders have been examined (Singhrao et al. 2015). Elwishhahy et al. (2021) found the data were insufficient to evaluate the association between Porphyromonas gingivalis and AD in a systematic review (n = 6 studies). Yet they suggest that P. gingivalis may play a role through its effect on systemic inflammation. A separate systematic review and meta-analysis of case-control studies (n = 9) concluded that AD patients experienced edentulism and more tooth loss than non-AD patients (Dioguardi et al. 2019).
Recent prospective studies suggest that tooth loss may increase the risk of dementia (Fang et al. 2018; Saito et al. 2018; Han et al. 2020). Han et al. (2020) analyzed this relationship for edentulism and dental care use using the US Health and Retirement Study (HRS) data through 2014, finding both edentulism and infrequent dental visits associated with cognitive decline. A cohort study among South Korean older adults reported an association of early-stage cognitive impairment with increases in tooth loss and decreases with periodontal treatment (Yoo et al. 2019). Cerutti-Kopplin et al. (2016), in a systematic review (n = 10 studies), showed that adults with <20 teeth had a higher risk for cognitive decline than persons with >20 teeth. Chen et al.’s (2018) meta-analysis of 8 cohort studies reported that tooth loss conferred a 1.34 times greater risk of developing dementia; further, increasing the number of teeth lost increased relative risk. In a systematic review and meta-analysis of 11 cohort studies, Oh et al. (2018) suggest that having more teeth is associated with an almost 50% lower risk of dementia. However, the quality of the evidence was rated as very low. More recent meta-analyses by Fang et al. (2018) and Qi et al. (2021) studied the longitudinal relationship between tooth loss and cognitive impairment. However, not all studies controlled for education, income, smoking, or self-reported health.
Thomson and Barak (2021) proposed a model of the effects of tooth loss on cognitive function highlighting the importance of life course experiences on cognitive development in childhood. Wu et al. (2016) suggested why findings from different studies and systematic reviews are mixed. More recently, a systematic review and meta-analysis (Asher et al. 2022) showed that poor periodontal health and tooth loss may increase the risks of cognitive loss and dementia. Again, however, they cite the low quality of evidence as a concern, as well as concerns regarding “reverse causality.” Thus, there remains a need for clear evidence on this topic.
This longitudinal cohort study focused on 2 research questions. First, does cognitive decline vary by birth cohort, adjusting for other covariates? Second, do dental care use and dentition status at baseline predict cognition decline? We hypothesized the following: 1) birth cohort predicts rate of decline of cognitive function, and 2) dentition status (edentulism) and less use of dental care are associated with declines in cognitive function.
Methods
Design
This was a longitudinal cohort study of whether tooth loss and dental care use predict cognitive decline in participants of the US HRS.
Institutional Review Board
The project (study 20-2429) was reviewed by the University of North Carolina at Chapel Hill Office of Human Research Ethics, which determined that it was not human subjects research as defined under federal regulations and did not require institutional review board approval. All data were deidentified and obtained online for public use. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for cohort studies (von Elm et al. 2007).
Conceptual Model
Guided by a variation on Thomson and Barak’s (2021) conceptual model, we estimated trajectories of cognition decline over time in the HRS birth cohorts and determined the extent to which total tooth loss (edentulism) and less frequent dental care use are predictors of cognitive decline in the HRS.
Sample Data Source
The HRS is an ongoing, longitudinal study that began in 1992, sponsored by the National Institute on Aging (U01AG009740) and conducted by the University of Michigan. The HRS is a nationally representative sample of US adults over age 50 y (Sonnega et al. 2014; Health and Retirement Study [HRS] 2022). Approximately 20,000 people participate over time; additional cohorts are periodically recruited (HRS 2008a, 2008b), most recently in 2012 and 2018.
The present study uses rich, longitudinal data in the HRS, including extensive information about potential confounding factors, covariates, and social determinants associated with cognition and oral health. Analyses used biennial CORE data from 2006 to 2018 that were collected using face-to-face and telephone interviews.
Eligibility Criteria
The study included adults >50 y who participated in 2 or more CORE HRS waves from 2006 to 2018 (even years), with known dentition status (including some values that were imputed as described below) and observed cognition for at least 2 time points to assess change.
Outcome
The primary outcome of interest in the study is cognition, as measured by the HRS variable Cogtot27. HRS Cogtot27 is a multidimensional measure of cognitive tests of orientation, memory, numeracy, and word recognition (Brandt et al. 1988), ranging from 0 to 27, modeled on the Mini-Mental State Exam (Folstein et al. 1975), and in the biennial CORE questionnaire in the HRS. Besides the Cogtot27 scale, cognitive status is categorized as <7 (dementia), 7 to 7≤Cogtot27<12 (cognitive impairment, not demented [CIND]), and ≥12 (normal).
Time
This study used time as a linear variable in 2-y increments ranging from 0 to 5. Time 0 was the first exam chronologically after the baseline exam. Time at baseline varied for each person depending on the first exam where cognitive tests were administered between 2006 and 2018. Duration of time also varied for each person. For example, if someone had all 7 exam cycles with complete data, the 2006 exam would be the baseline exam. The analysis used Cogtot27 from 2008 to 2018 as the outcome, adjusting for baseline Cogtot27.
Predictors
This study focused on time-varying exposure variables of edentulism and dental care use as predictors of cognition over time. The longitudinal HRS data allowed for comparison of birth cohorts over time with adjustment for baseline cognitive status and covariates including demographic characteristics, health behaviors, and medical conditions as described in detail below.
Birth Cohorts
HRS birth cohorts include the Asset and Health Dynamics Among the Oldest Old (AHEAD), born before 1924; Children of the Depression Age (CODA), born between 1924 and 1930; the original HRS cohort born between 1931 and 1941; War Babies, born between 1942 and 1947; and Early, Middle, and Late Baby Boomers, born between 1948 and 1953, 1954 and 1959, and 1960 and 1965, respectively. The above birth cohorts used by the HRS were collapsed due to smaller sample sizes for the oldest birth cohorts and relative similarities among the Baby Boomer cohorts. The resultant 4 cohorts by the range of birth years for each were as follows: ≤1930, AHEAD and CODA; 1931–1941, HRS; 1942–1947, War Babies; and 1948–1965, Baby Boomers.
Tooth Loss
Data on complete tooth loss (edentulism) are present in the 2006, 2012, and 2018 HRS CORE questionnaires with the question, “Have you lost all of your upper and lower permanent teeth?” In other years (2008, 2010, 2014, and 2016), dentition status (dentate versus edentulous) was imputed only when it could be logically determined under the premise “once edentulous, always edentulous.” That is, once there was complete tooth loss, we carried it forward to subsequent time points. Similarly, when a participant was recorded as dentate at 2 time points, the status of dentate was also assigned at intervening time points.
Retirement/Employment
Retirement transitions are often not linear, and retirement patterns differ by age, cohort, gender, race, and ethnicity (Gustman and Steinmeier 2000; Denton and Spencer 2009; Manski et al. 2010; Carr et al. 2020). We used self-described retirement status from HRS CORE current work status questions as a time-varying covariate (Gustman and Steinmeier 2000).
Demographics and Lifestyle Covariables
Demographic characteristics and lifestyle covariables relevant in this study include gender, race/ethnicity, education, urban/rural location, wealth estimation (net value of all wealth components less all debt: HRS-created variable), and other time-varying covariates, namely, marital status (Liu et al. 2020), smoking, alcohol use, Medicaid status, and felt lonely. Health-related covariates were obtained from CORE data: baseline and incident medical conditions including diabetes and stroke, self-reported health, and functional limitations (activities of daily living or ADLs), as used in prior analysis (Weintraub et al. 2019).
Analyses
Linear mixed models for repeated measures with adjustment for baseline cognition were used to estimate 1) the trajectories of mean cognition over time for the 4 birth cohorts and 2) the effects of dentition status and dental care use on mean cognition. These dual assessments were made without adjustment (model 1) and with adjustment (model 2) for the covariates described. Baseline cognition (HRS Cogtot27) was determined at the first available visit with observations included in the analytic data set for subsequent visits. Both models included the main effects of birth cohort, time (linear in 2-y units, ranging from 0 to 5), their interaction (to allow for the comparison of the cohorts’ slopes), and the time-varying exposures of dental care use (“In the last 2 y, have you seen a dentist for dental care, including dentures?”) and dentition status and their interaction. To account for repeated measures, correlated subject-specific random intercepts and slopes were included. In model 2, all available visits with complete covariate data were used for each participant. Trajectories of unadjusted and covariate-adjusted mean cognition scores for the birth cohorts were plotted with the latter using model-based predictions fixing all covariates at their mean values.
Secondary analyses evaluated population-level changes in the distribution of cognitive status over time as well as within-person 10-y change in cognitive status (for those participants with a 10-y follow-up assessment), stratified separately by birth cohorts, dentition status, and dental care use. Spearman rank correlations were used to summarize the association of number of years since baseline and cognitive status, with larger correlations representing greater cognitive decline.
Results
Characteristics of the study sample by their baseline year and sample sizes, 2008 to 2018, are shown in Table 1 (and Appendix Table 1), as participants had their first visit during this time period at different 2-y cycles. Note that new participants were recruited by the HRS in 2012 and 2018. Thus, the baseline sample size ranges from 12,158 in 2016 to 18,606 in 2012. Over time, the HRS made attempts to increase the race/ethnic diversity of the overall sample to reflect the US population, thus the decline in the percent Caucasian with time. With time, the proportion of participants in the oldest cohort declined to 4.2%, and the Baby Boomers become the prominent group, 63%. There were less dramatic changes in the proportions of people with other characteristics at baseline by study year. Overall, there were 22,728 participants in our analysis with a mean (SD) baseline age of 63.4 (10.1) years and 42% male. Figure 1 depicts the flow diagram of cohort participation.
Table 1.
Study Variable Distribution at Each Participant’s Baseline, a HRS, 2008–2018.
| Variable | Baseline Percent |
|---|---|
| Sample size (n) | 22,321 |
| Age, mean (SD), y | 63.4 (10.1) |
| Caucasian | 62.3 |
| African American | 19.8 |
| Hispanic | 13.8 |
| Other | 4.1 |
| Male | 41.8 |
| Birth cohort | |
| ≤1930 AHEAD and CODA | 11.5 |
| 1931–1941 HRS | 24.2 |
| 1942–1947 War Babies | 11.0 |
| 1948–1965 Baby Boomers | 53.3 |
| Consider self-retired | 47.0 |
| Education | |
| No degree | 18.4 |
| High school or equivalent | 58.4 |
| College or more | 23.2 |
| Married | 59.1 |
| Household net wealth | |
| Negative–$50,000 | 33.8 |
| $50,000–$200,000 | 24.2 |
| $200,000–$500,000 | 19.9 |
| $500,000+ | 22.2 |
| Medicaid | 11.6 |
| Urban | 53.9 |
| Suburban | 21.8 |
| Ex-urban | 24.4 |
| Felt lonely much of past week | 16.9 |
| Self-rated general health: fair, poor | 27.7 |
| Current smoker | 16.7 |
| Ever drink alcohol | 56.0 |
| Edentulous | 15.5 |
| Not seen dentist within 2 y | 38.3 |
| Diabetes | 21.7 |
| Heart condition | 20.3 |
| Stroke | 5.6 |
| ADL | 29.0 |
ADL, activity of daily living; AHEAD, Asset and Health Dynamics Among the Oldest Old; CODA, Children of the Depression; HRS, Health and Retirement Study.
At the first appearance of each participant at baseline.
Figure 1.
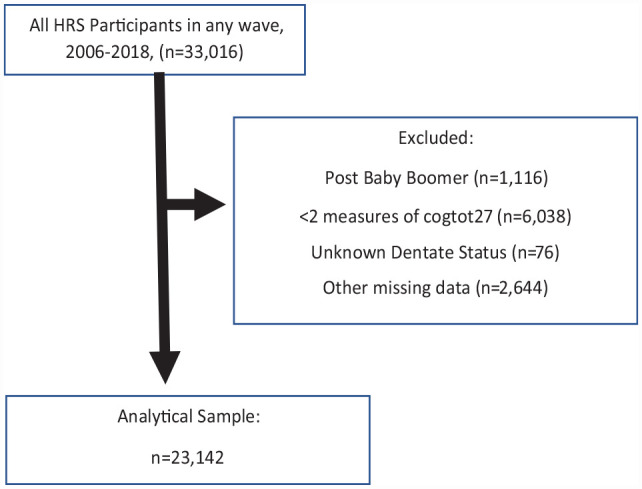
Flow diagram of participant inclusion.
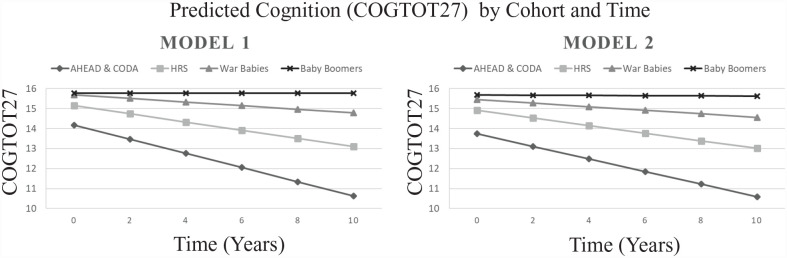
Estimates and 95% confidence intervals (CIs) for cognition trajectories (slopes) by birth cohort and the effects of the 2 exposures on cognitive scores from the linear repeated-measures mixed model are shown in Table 2. Estimated slopes for cognitive decline for the 4 birth cohorts vary (birth cohort × time interaction effect, P < .0001) for unadjusted and covariate-adjusted models, while the slope for each birth cohort is similar between models, suggesting that confounding is only mildly present (Fig. 2). While cognition for Baby Boomers did not significantly change over time (slope = −0.01; 95% CI, −0.03 to 0.02, which contains the null value of zero in model 2), the oldest old (AHEAD and CODA) had the highest rate of decline, HRS Cogtot27 units over 10 y, that is, 5*(−0.01, −0.62) = −3.15. Overall, the negative interaction estimates indicated increasing amounts of cognitive decline with increasing age of birth cohort.
Table 2.
HRS Estimated Regression Coefficients (95% Confidence Intervals) for Cognition from Linear Mixed Models, 2008–2018.
| Variable | Model 1 (n = 23,142) | Model 2 a (n = 22,728) |
|---|---|---|
| Intercept | 6.41 (6.26, 6.57) | 9.88 (9.66, 10.1) |
| Baseline cognition | 0.61 (0.61, 0.62) | 0.48 (0.48, 0.50) |
| Time (per 2-y cycle) | 0.00 (−0.02, 0.02) | −0.01 (−0.03, 0.02) |
| Birth cohort ≤1930 AHEAD and CODA 1931–1941 HRS 1942–1947 War Babies 1948–1965 Baby Boomers Interaction with time (P < 0.0001) ≤1930 AHEAD and CODA 1931–1941 HRS 1942–1947 War Babies 1948–1965 Baby Boomers |
−1.59 (−1.72, −1.46) −0.62 (−0.72, −0.53) −0.08 (−0.21, 0.04) Reference −0.71 (−0.76, −0.66) −0.41 (−0.44, −0.38) −0.18 (−0.22, −0.15) Reference |
−1.94 (−2.08, −1.81) −0.77 (−0.87, −0.68) −0.22 (−0.34, −0.09) Reference −0.62 (−0.67, −0.57) −0.37 (−0.40, −0.34) −0.17 (−0.21, −0.13) Reference |
| Not seen dentist within 2 y | −0.48 (−0.54, −0.42) | −0.17 (−0.23, −0.10) |
| Edentulous a | −1.01 (−1.15, −0.87) | −0.42 (−0.56, −0.28) |
| Not seen dentist × edentulous | 0.27 (0.13, 0.42) (P = 0.0002) |
0.07 (−0.08, 0.22) (P = 0.22) |
| Education Less than high school High school of equivalent College or more |
— |
−2.03 (−2.15, −1.91) −0.75 (−0.84, −0.67) Reference |
| Race Caucasian African American Hispanic Other |
— |
Reference −0.90 (−0.99, −0.80) −0.38 (−0.50, −0.27) −0.36, (−0.54, −0.17) |
| Male | — | −0.40 (−0.47, −0.33) |
AHEAD, Asset and Health Dynamics Among the Oldest Old; CODA, Children of the Depression; HRS, Health and Retirement Study.
Model 2 includes covariates married, household net wealth, Medicaid, urban, felt lonely much of past week, self-rate general health, current smoker, ever drink alcohol, diabetes, stroke, and ADL, activity of daily living with full results shown in Appendix Table 3.
Figure 2.
Predicted cognition by birth cohort and time.
Compared to dentate participants, persons who were or became edentulous exhibited greater cognitive decline (−0.42; 95% CI, −0.56 to −0.28, for those who had seen a dentist; model 2); given the interaction with use of dental care, the effect was similar (−0.42 + 0.07 = −0.35) for those who had not seen a dentist. The impacts of edentulous and not seeing a dentist were greater when covariates were not adjusted (model 1), implying a confounding role of covariates for these effects. In model 2, less education, non-White race, Hispanic/other ethnicity, and lower household wealth were associated with greater cognitive decline, as were stroke, loneliness, fair or poor self-rated health, and smoking (see Appendix Table 3).
In the analysis of cognitive status over time, there is little if any change in cognitive status over time for Baby Boomers. The AHEAD and CODA group had the largest population shift in the percent of CIND/dementia over time from 33% at baseline to 45.7% 12 y later over time (Table 3) and the greatest within-subject change among birth cohorts, with 35% experiencing worsening cognitive status in 10 y ((170 + 41 + 41)/728 × 100%; Appendix Table 4). Individuals who were edentulous at baseline experienced greater decline in cognitive status than individuals who were dentate on both a population level (Appendix Table 5) and with respect to within-subject change (Appendix Table 6). On the other hand, individuals with dental care use (correlation with time of 0.11) had similar population-averaged decline over time in cognitive status than individuals without dental care use (correlation with time of 0.13; Appendix Table 7), while the latter group had a greater level of dementia at baseline. With respect to within-person change over 10 y, 24.2% of individuals without dental care use at baseline experienced cognitive decline versus 12.3% of individuals who had seen a dentist (Appendix Table 8).
Table 3.
Association of Time since Baseline and Cognitive Status for Birth Cohorts, n = 25,660.
| Characteristic | Years since Baseline | Normal, n (%) | CIND, n (%) | Dementia, n (%) | Total N | Rank Correlation |
|---|---|---|---|---|---|---|
| AHEAD and CODA | 0 | 2,365 (66.7) | 927 (26.1) | 254 (7.2) | 3,546 | 0.32 |
| 2 | 1,638 (64.0) | 686 (26.8) | 236 (9.2) | 2,560 | ||
| 4 | 1,249 (58.1) | 676 (31.5) | 224 (10.4) | 2,149 | ||
| 6 | 1,111 (54.6) | 634 (31.1) | 291 (14.3) | 2,036 | ||
| 8 | 531 (57.8) | 263 (28.6) | 125 (13.6) | 919 | ||
| 10 | 395 (54.3) | 237 (32.6) | 96 (13.2) | 728 | ||
| 12 | 346 (54.3) | 210 (33.0) | 81 (12.7) | 637 | ||
| HRS | 0 | 5,215 (80.8) | 1,024 (15.9) | 217 (3.4) | 6,456 | 0.20 |
| 2 | 4,350 (80.6) | 852 (15.8) | 196 (3.6) | 5,398 | ||
| 4 | 3,948 (76.8) | 963 (18.7) | 231 (4.5) | 5,142 | ||
| 6 | 3,787 (73.4) | 1,091 (21.1) | 282 (5.5) | 5,160 | ||
| 8 | 2,564 (73.9) | 713 (20.5) | 195 (5.6) | 3,472 | ||
| 10 | 265 (71.1) | 715 (20.5) | 205 (6.4) | 3,185 | ||
| 12 | 2,131 (70.7) | 677 (22.5) | 208 (6.9) | 3,016 | ||
| War Babies | 0 | 2,484 (87.7) | 295 (10.4) | 52 (1.8) | 2,831 | 0.10 |
| 2 | 2,153 (87.3) | 276 (11.2) | 36 (1.5) | 2,465 | ||
| 4 | 2,040 (86.7) | 274 (11.6) | 40 (1.7) | 2,354 | ||
| 6 | 2,009 (84.4) | 329 (13.8) | 43 (1.8) | 2,381 | ||
| 8 | 1,539 (85.4) | 216 (12.0) | 48 (2.7) | 1,803 | ||
| 10 | 1,373 (84.6) | 215 (13.3) | 35 (2.2) | 1,623 | ||
| 12 | 1,385 (86.7) | 173 (10.8) | 40 (2.5) | 1,598 | ||
| Baby Boomers | 0 | 10,883 (84.8) | 1,707 (13.3) | 237 (1.9) | 12,827 | 0.02 |
| 2 | 10,048 (84.5) | 1,598 (13.4) | 252 (2.1) | 11,898 | ||
| 4 | 6,155 (85.6) | 897 (12.5) | 138 (1.9) | 7,190 | ||
| 6 | 6,060 (85.0) | 906 (12.7) | 166 (2.3) | 7,132 | ||
| 8 | 5,414 (85.4) | 778 (12.3) | 150 (2.4) | 6,342 | ||
| 10 | 2,110 (85.9) | 301 (12.3) | 46 (1.9) | 2,457 | ||
| 12 | 2,126 (87.2) | 265 (10.9) | 48 (2.0) | 2,439 |
AHEAD, Asset and Health Dynamics Among the Oldest Old; CIND, cognitive impairment, not demented; CODA, Children of the Depression; HRS, Health and Retirement Study.
Discussion
This longitudinal cohort study examined if 1) cognitive decline varies by birth cohort, 2) mean cognition varies by dentition status and dental care use, and 3) edentulism predicts 10-y cognitive decline (2008–2018). We found that cognition declines over time for the 3 oldest birth cohorts and that the rate of decline increases with age of the birth cohorts. The older 2 cohorts had lower initial cognitive scores, and the 3 older cohorts had near-parallel trajectories, with slightly steeper trajectories among progressively older cohorts.
With respect to the dental variables, edentulous persons with irregular dental care had the highest percentage who, over time, develop CIND and dementia, followed by edentulous persons who use dental care regularly, then dentate persons who use dental care irregularly. Possible reasons for these findings could be nutritional declines with edentulism and suboptimal health behaviors. A review (Azuma et al. 2017) suggested that mastication and stimulation from chewing, which can be impaired with tooth loss, can affect hippocampal function, leading to cognitive deficits. However, the exact mechanisms cannot be ascertained from HRS data. What can be said is that even after controlling for education, race and ethnicity, health status, functional status, wealth, behaviors (smoking, alcohol use), loneliness, and birth cohort, the longitudinal effects of dentition status on declines in cognition remain. Thus, the lowest rates of development of CIND/dementia are among dentate persons who use dental care regularly. Disaggregating the data by birth cohort showed that among the older cohorts, the patterns become less strong as the prevalence of CIND/dementia increased among the oldest old.
Another question is that of directionality. Our previous work examined predictors of edentulism and found that cognitive decline predicts 12-y edentulism (Preisser et al. 2022). The present work examines if dental care use and edentulism predict cognitive decline, even after adjusting for known confounders. Data from the fully adjusted model in Table 2 and Appendix Table 3 clearly show that edentulism and, to a lesser extent, nonuse of dental care predict cognitive decline. Furthermore, while the magnitude of the effect is less than the impact of birth cohort, education, and race and ethnicity, it is on the order of magnitude of baseline cognition (although in the opposite direction). Thus, it may be that the relation between tooth loss and cognition is bidirectional.
We expected that the relationship between tooth loss and cognition would be affected by environmental and dental care factors that have changed over time and have affected different birth cohorts differently. Ettinger and Marchini (2020) have written about how key historical, economic, and dental events have affected the health behaviors and dental care use of different cohorts of older adults. The oldest cohort in their analysis lived through the Depression and, subsequent groups, World War II, later wars, and different periods of economic prosperity and recession. Medicaid began in 1965, the World Wide Web in 1990. Over time, dental care developments included licensing of dentists (1921); lidocaine (1943); public water fluoridation (1945); high-speed handpieces (1950s); acid-etch technique, sealants, and composites (1970s); dental implants (1980s); and, more recently, people maintaining more of their natural teeth.
Strengths of this study are its longitudinal analysis, large sample size, and availability of cognitive, dental, demographic characteristics, health, and behavioral data at multiple time points. Recent additions of participants to the younger cohorts have included more Black, Latino, and Asian Americans to better represent society. The results are generalizable to the US population of older adults who are willing and able to participate in the HRS study over an extended period of time.
A major limitation of this study was that clinically determined oral health information was not available. Furthermore, many variables were self-reported, and some questions, such as dentition status, were not asked at every biennial cycle. Another limitation is that the cohorts are continuously added to, resulting in a changing baseline over time, making data analysis and interpretation less straightforward. However, this limitation is balanced by the increase in Black, Latino, and Asian Americans. In this study, generally <1% of data were missing for any baseline variable; overall, 5.7% of participants were missing any data (Appendix Table 2). Furthermore, people with dementia could be less likely to be in the data set, affecting results. A final limitation was that the HRS core data do not include questions regarding dental implants, which would make dentures fit more securely.
Conclusions
A national, longitudinal study of 22,728 participants in the Health and Retirement Study during 2008 to 2018 found that cognitive decline varied by birth cohort, with the steepest declines among the oldest old. Birth cohorts, education, race and ethnicity, edentulism, and use of dental care, after adjustment for other demographic characteristics, health status, and health behavior variables, were associated with cognitive decline.
Author Contributions
J.A. Jones, T.L. Finlayson, J.S. Preisser, J.A. Weintraub, contributed to conception and design, data analysis and interpretation, drafted and critically revised the manuscript; K. Moss, contributed to conception and design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave their final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-docx-1-jdr-10.1177_00220345231167805 for Edentulism Predicts Cognitive Decline in the US Health and Retirement Cohort Study by J.A. Jones, K. Moss, T.L. Finlayson, J.S. Preisser and J.A. Weintraub in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Health and Retirement Study. Produced and distributed by the University of Michigan with funding from the National Institute on Aging (grant number U01AG009740), Ann Arbor, MI.
Analyses were supported by the National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research, grant number 1 R03DE030161-01. Publicly available data are online at the Health and Retirement Study website: https://hrs.isr.umich.edu/data-products.
ORCID iDs: J.A. Jones  https://orcid.org/0000-0002-0126-0790
https://orcid.org/0000-0002-0126-0790
T.L. Finlayson  https://orcid.org/0000-0003-2457-4183
https://orcid.org/0000-0003-2457-4183
J.S. Preisser  https://orcid.org/0000-0002-7869-2057
https://orcid.org/0000-0002-7869-2057
J.A. Weintraub  https://orcid.org/0000-0001-7178-2028
https://orcid.org/0000-0001-7178-2028
References
- Alzheimer’s Association. 2022. Alzheimer’s facts and figures [accessed 2022 Sep 13]. https://www.Alz.Org/.
- Asher S, Stephen R, Mäntylä P, Suominen AL, Solomon A. 2022. Periodontal health, cognitive decline, and dementia: a systematic review and meta-analysis of longitudinal studies. J Am Geriatr Soc. 70(9):2695–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma K, Zhou Q, Niwa M, Kubo KY. 2017. Association between mastication, the hippocampus, and the HPA axis: a comprehensive review. Int J Mol Sci. 18(8):1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Spencer M, Folstein M. 1988. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1(2):111–117. [Google Scholar]
- Carr DC, Willis R, Kail BL, Carstensen LL. 2020. Alternative retirement paths and cognitive performance: exploring the role of preretirement job complexity. Gerontologist. 60(3):460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti-Kopplin D, Feine J, Padilha DM, de Souza RF, Ahmadi M, Rompré P, Booij L, Emami E. 2016. Tooth loss increases the risk of diminished cognitive function: a systematic review and meta-analysis. JDR Clin Trans Res. 1(1):10–19. [DOI] [PubMed] [Google Scholar]
- Chen JR, Ren C-J, Wu L, Xia L-Y, Shao J, Leng W-D, Zeng X-T. 2018. Tooth loss is associated with increased risk of dementia and with a dose-response relationship. Front Aging Neurosci. 10:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton FT, Spencer BG. 2009. What is retirement? A review and assessment of alternative concepts and measures. Can J Aging. 28(1):63–76. [DOI] [PubMed] [Google Scholar]
- Dioguardi M, Gioia GD, Caloro GA, Capocasale G, Zhurakivska K, Troiano G, Russo LL, Muzio LL. 2019. The association between tooth loss and alzheimer’s disease: a systematic review with meta-analysis of case control studies. Dent J (Basel). 7(2):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwishahy A, Antia K, Bhusari S, Ilechukwu NC, Horstick O, Winkler V. 2021. Porphyromonas gingivalis as a risk factor to alzheimer’s disease: a systematic review. J Alzheimers Dis Rep. 5(1):721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger RL, Marchini L. 2020. Cohort differences among aging populations: an update. J Am Dent Assoc. 151(7):519–526. [DOI] [PubMed] [Google Scholar]
- Fang WL, Jiang MJ, Gu BB, Wei YM, Fan SN, Liao W, Zheng YQ, Liao SW, Xiong Y, Li Y, et al. 2018. Tooth loss as a risk factor for dementia: systematic review and meta-analysis of 21 observational studies. BMC Psychiatry. 18(1):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. 1975. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 12(3):189–198. [DOI] [PubMed] [Google Scholar]
- Gustman A, Steinmeier T. 2000. Retirement outcomes in the health and retirement study. Working paper 7588. Soc Secur Bull. 63(4):57–71. [DOI] [PubMed] [Google Scholar]
- Han SH, Wu B, Burr JA. 2020. Edentulism and trajectories of cognitive functioning among older adults: the role of dental care service utilization. J Aging Health. 32(7–8):744–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health and Retirement Study (HRS). 2008. a. Design history. Ann Arbor: Survey Research Center, Institute for Social Research, University of Michigan; [accessed 2023 Mar 20]. https://hrs.isr.umich.edu/sites/default/files/biblio/DesignHistory.pdf. [Google Scholar]
- Health and Retirement Study (HRS). 2008. b. Sample evolution: 1992–1998. Ann Arbor: Survey Research Center, Institute for Social Research, University of Michigan; [accessed 2023 Mar 20]. https://hrs.isr.umich.edu/sites/default/files/biblio/surveydesign.pdf. [Google Scholar]
- Health and Retirement Study (HRS). 2022. The Health and Retirement Study. Institute for Social Research, University of Michigan; [accessed 2022 June 27]. https://hrs.isr.umich.edu/about. [Google Scholar]
- Kemle K, Ackermann RJ. 2018. Issues in geriatric care: Alzheimer disease. FP Essent. 468:26–34. [PubMed] [Google Scholar]
- Liu H, Zhang Z, Choi SW, Langa KM. 2020. Marital status and dementia: evidence from the health and retirement study. J Gerontol B Psychol Sci Soc Sci. 75(8):1783–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manski RJ, Moeller J, Schimmel J, St Clair PA, Chen H, Magder L, Pepper JV. 2010. Dental care coverage and retirement. J Public Health Dent. 70(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh B, Han D-H, Han K-T, Liu X, Ukken J, Chang C, Dounis K, Yoo JW. 2018. Association between residual teeth number in later life and incidence of dementia: a systematic review and meta-analysis. BMC Geriatr. 18(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisser JS, Moss K, Finlayson TL, Jones JA, Weintraub JA. 2022. Prediction model development and validation of 12-year incident edentulism of older adults in the United States [epub ahead of print 9 Aug 2022]. doi: 10.1177/23800844221112062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Zhu Z, Plassman BL, Wu B. 2021. Dose-response meta-analysis on tooth loss with the risk of cognitive impairment and dementia. J Am Med Dir Assoc. 22(10):2039–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Ohi T, Murakami T, Komiyama T, Miyoshi Y, Endo K, Satoh M, Asayama K, Inoue R, Kikuya M, et al. 2018. Association between tooth loss and cognitive impairment in community-dwelling older Japanese adults: a 4-year prospective cohort study from the Ohasama study. BMC Oral Health. 18(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhrao SK, Harding A, Poole S, Kesavalu L, Crean S. 2015. Porphyromonas gingivalis periodontal infection and its putative links with Alzheimer’s disease. Mediators Inflamm. 2015:137357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. 2014. Cohort profile: the Health and Retirement Study (HRS). Int J Epidemiol. 43(2):576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson WM, Barak Y. 2021. Tooth loss and dementia: a critical examination. J Dent Res. 100(3):226–231. [DOI] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. 2007. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 147(8):573–577. [DOI] [PubMed] [Google Scholar]
- Weintraub JA, Orleans B, Fontana M, Phillips C, Jones JA. 2019. Factors associated with becoming edentulous in the US health and retirement study. J Am Geriatr Soc. 67(11):2318–2324. [DOI] [PubMed] [Google Scholar]
- Wu B, Fillenbaum GG, Plassman BL, Guo L. 2016. Association between oral health and cognitive status: a systematic review. J Am Geriatr Soc. 64(4):739–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JJ, Yoon JH, Kang MJ, Kim M, Oh N. 2019. The effect of missing teeth on dementia in older people: a nationwide population-based cohort study in South Korea. BMC Oral Health. 19(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jdr-10.1177_00220345231167805 for Edentulism Predicts Cognitive Decline in the US Health and Retirement Cohort Study by J.A. Jones, K. Moss, T.L. Finlayson, J.S. Preisser and J.A. Weintraub in Journal of Dental Research