Abstract
The pathogenicities of the murine AIDS (MAIDS) virus complex (LP-BM5) and ecotropic helper virus (BM5eco) isolated from the complex to BALB/c nude mice were studied to elucidate the possible role of replication-competent helper virus in inducing the monoclonal outgrowth of lymphoid cells. Neither LP-BM5 nor BM5eco was pathogenic in adult BALB/c nude mice. However, B-cell lymphoma developed with a very high frequency when either virus was inoculated into newborn BALB/c nude (nu/nu) mice. The cells from the B-cell lymphoma were easily transplanted into nude mice. These results suggested that ecotropic helper virus in the MAIDS virus complex plays an important role in inducing the monoclonal outgrowth of lymphoid cells under immunodeficient conditions caused by defective virus.
The current understanding of the murine AIDS (MAIDS) virus-induced pathogenesis is that the virus complex causes the development of immunodeficiency concomitant primarily with polyclonal and ultimately oligo- or monoclonal outgrowth of lymphoid cells (12). Although the MAIDS virus causes MAIDS in C57BL/6 and C57BL/10 mice and not in BALB/c mice when inoculated into adult mice (6), MAIDS develops in BALB/c mice when the virus is inoculated neonatally (17). However, although adult nu/nu mice with a C57BL/10 background (16) were resistant to the lethal effects of MAIDS induced by MAIDS virus infection, the pathogenicity of the virus to nude mice with a BALB/c background has not been examined. An experiment using C57BL/10 nude mice suggested that the presence of functional T lymphocytes is required for the development of MAIDS.
The MAIDS virus complex consists of a replication-competent helper virus and a replication-defective virus which can induce immunodeficiency (1, 4). However, the pathogenicity of the helper virus has not been well characterized. The main component of the helper virus is a B-tropic, ecotropic murine leukemia virus (MuLV) which was molecularly cloned and reported to be nonpathogenic in adult C57BL/6 mice (5, 22). Mink cell focus-forming virus was also isolated as a helper for the defective virus (4, 5).
In this study, the pathogenicity of MAIDS virus infection in BALB/c nude mice was examined to elucidate the role of the helper ecotropic virus in the MAIDS virus complex in inducing the outgrowth of lymphoid cells and their progression to malignant transplantable lymphoid cells (Table 1).
TABLE 1.
Susceptibility of BALB/c nude mice to MAIDS virus
| Mouse group
|
Virusb | No. of mice with splenomegalyc/total no. of mice inoculated | No. of mo. after inoculation (avg) when splenomegaly was detectedd | Transplantability of the cells into nu/nu micee | Virus detected in spleenf | |
|---|---|---|---|---|---|---|
| Agea | Genotype | |||||
| NB | nu/nu | LP-BM5 | 14/16 | 4–9 (7.2) | Easy | M |
| NB | nu/+ | LP-BM5 | 12/13 | 3–8 (5.2) | Difficult | M |
| NB | +/+ | LP-BM5 | 15/15 | 3–6 (5.1) | Difficult | M |
| NB | nu/nu | BM5eco | 11/12 | 7–12 (9.7) | Easy | Eco |
| NB | nu/+ | BM5eco | 1/14 | 10 | Easy | Eco |
| NB | +/+ | BM5eco | 0/9 | |||
| Adult | nu/nu | LP-BM5 | 0/10 | |||
| Adult | nu/nu | BM5eco | 0/10 | |||
| Adult | +/+ | LP-BM5 | 0/10 | |||
| Adult | +/+ | BM5eco | 0/10 | |||
NB, newborn (less than 36 h old). Adult, 5 weeks old.
A 0.5-ml volume of the virus given to newborn mice was inoculated intraperitoneally into adult mice.
Mice were observed for more than 12 months or until the moribund stage. In our colony (nu/nu, nu/+, and +/+), many female BALB/c mice older than 12 months died due to spontaneous mammary tumors.
Period when the mice were nearing the moribund stage of the disease.
Easy, transplantation was successful in almost all cases. Difficult, transplantation was rarely successful.
M, virus showing the same pathogenicity as LP-BM5 when inoculated into adult C57BL/6 mice. Eco, virus without pathogenicity to adult C57BL/6 mice (but B-tropic ecotropic MuLV was detected by the UV-XC test).
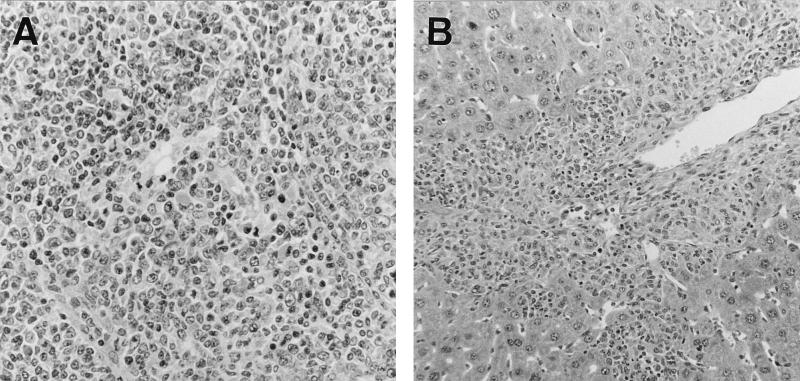
Aliquots of 0.1 ml of MAIDS virus (LP-BM5), harvested from the G6 line of SC-1 cells containing defective virus and helper ecotropic virus (obtained from J. Hartley), were inoculated into 16 newborn BALB/c nude (nu/nu) mice and 13 newborn BALB/c (nu/+) mice (13). Significant hepatosplenomegaly and lymphadenopathy appeared around 3 or 4 months after inoculation in nu/+ mice. Although 1 of the inoculated nu/+ mice survived for an extended period (8 months), 11 of 13 inoculated nu/+ mice reached the moribund stage within 6 months (an average of 5.2 months). Some of the animals were killed for further analyses at this stage. The pathogenicities of MAIDS virus to BALB/c (nu/+) and normal BALB/c mice were indistinguishable histopathologically. Although cells from the enlarged spleens of BALB/c mice infected with MAIDS virus were reported to be transplantable (22), it was always very difficult to transplant the BALB/c cells into nude mice, unlike the cells from C57BL/6 mice (13). In 14 of the 16 neonatally infected nude (nu/nu) mice, significant hepatosplenomegaly with lymphadenopathy appeared from 4 to 9 months after inoculation (an average of 7.2 months), which was a little later than in nu/+ mice. Cells from the enlarged spleens of all 14 nu/nu mice were easily transplantable into BALB/c nude mice, which suggested that malignant lymphomas had developed in the nu/nu mice (Fig. 1). All cell-free supernatants prepared from the serially transplanted lymphoma cell lines from nu/nu and nu/+ mice caused the development of MAIDS when they were inoculated into C57BL/6 mice, which suggested that both the defective-pathogenic component and the replication-competent helper component of MAIDS virus still proliferated in the transplanted cell lines.
FIG. 1.
(A) Nodular proliferation of atypical lymphoblastoid cells in the spleen of a BALB/c nude mouse 5 months after neonatal infection with LP-BM5 virus. Magnification, ×194. (B) Infiltration of lymphoma cells into the hepatic portal area. Magnification, ×97.
To determine the role of the helper virus component in the MAIDS virus complex, newborn BALB/c (nu/nu) and BALB/c (nu/+) mice were inoculated with the B-tropic ecotropic helper virus, BM5eco, which was harvested from SC-1 cells transfected with molecularly cloned DNA (obtained from S. Chattopadhyay) (5). Aliquots of 0.1 ml of BM5eco virus (103 PFU on SC-1 cells assayed by the UV-XC test), which contained the same titer of the ecotropic helper virus component as LP-BM5 virus inoculum, were inoculated into 12 newborn BALB/c (nu/nu) and 14 newborn BALB/c (nu/+) mice. Surprisingly, lymphomas developed in 11 of the 12 nu/nu mice between 7 and 12 months (an average of 9.7 months) after infection. Severe infiltration of the liver with lymphoid cells, showing colonial growth of malignant cells in the liver, was observed in many nu/nu mice infected with BM5eco virus. Cells from the spleens of nude mice were also serially transplanted into nude mice as easily as those from nude mice infected with LP-BM5. The histopathologies of the spleen and liver were almost the same as those of nude mice infected with LP-BM5. B-tropic ecotropic MuLV at very high titers was detected by the UV-XC test (18) in the cell extracts from all lymphomas developed in nude mice infected with BM5eco virus, which suggested that lymphomas were developed by B-tropic ecotropic BM5eco virus infection, since the isolation of B-tropic ecotropic MuLV from spontaneously developed primary tumor cells is not a frequent event, as implied by the results of this experiment. Inoculation of cell extracts prepared from helper virus-induced BALB/c nude mouse tumor cells into adult C57BL/6 mice did not cause MAIDS, which suggested neither contamination with a defective component of MAIDS virus nor induction of a virus such as the MAIDS-developing component in the helper virus-infected mice. Interestingly, a lymphoma (765-7) developed in 1 of the 14 inoculated nu/+ mice 9.5 months after infection.
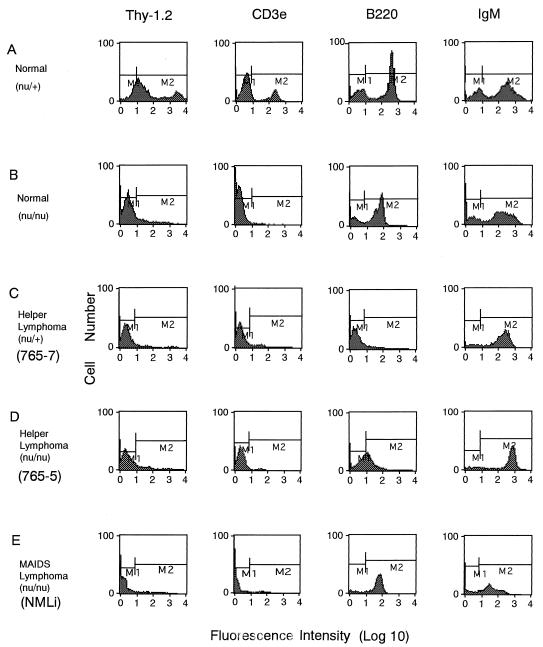
The transplantable cells were characterized by fluorocytometry with a FACScan and tested for markers specific for T cells (CD3e and Thy-1.2), B cells (B220, CD19, and immunoglobulin M [IgM] μ chain), early B cells (BP-1), macrophages (Mac-1), and granulocytes (Gr-1), and most of the cell lines were shown to belong to the B-cell lineage and not the early-B-cell lineage. Although the presence of IgM was detected on most of the transplantable cell lines, such as NMLi (Fig. 2E), another B-cell-specific marker, B220, was not detected on many cell lines, such as lymphoma 765-7 (Fig. 2C), or was present at low levels on some cell lines, such as lymphoma 765-5 (Fig. 2D). To confirm the origins of such transplantable cells, rearrangements of the immunoglobulin heavy-chain gene and T-cell-receptor gene were examined by Southern blot hybridization with a JH4 probe for the immunoglobulin heavy-chain gene and a Cβ2 probe for the T-cell-receptor gene obtained from A. Shimizu and T. Honjo (Fig. 3).
FIG. 2.
Expression of cell surface markers on normal spleen cells from BALB/c nu/+ (A) and BALB/c nu/nu (B) mice, on transplantable lymphoma cells developed with BM5eco in nu/+ (765-7) (C) and nu/nu (765-5) (D) mice, and on transplantable lymphoma cells developed with LP-BM5 in nu/nu (NMLi) (E) mice. Transplantable cells for FACScan analysis were prepared from the enlarged spleens of recipient BALB/c nude mice. After the Fc receptor was blocked with rat anti-mouse CD16-CD32 monoclonal antibody, lymphoid cells were treated with fluorescein isothiocyanate-conjugated hamster anti-mouse CD3 antibody (CD3e), rat anti-mouse Thy-1.2 antibody, rat anti-mouse B220 monoclonal antibody, or goat anti-mouse IgM(μ) antibody. M1, negative range; M2, positive range.
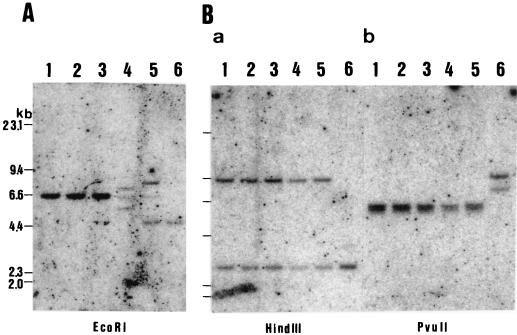
FIG. 3.
DNA was extracted from nude BALB/c (nu/nu) mouse livers (lanes 1), BALB/c (nu/+) livers (lanes 2), BALB/c (+/+) livers (lanes 3), BALB/c (nu/nu) tumor cells (765-5) (lanes 4), BALB/c (nu/+) tumor cells (765-7) (lanes 5), and a BALB/c (nu/nu) B-cell line (lane 6 in panel A contains a positive control for immunoglobulin heavy-chain gene rearrangement) or mouse T-cell line L8313 (lanes 6 in panel B contain a positive control for T-cell-receptor gene rearrangement) (19). In panel A, lanes 4 and 5, two rearranged bands were detected in addition to a germ line band of 6.6 kb, indicating a clonal B-cell population in both lymphomas. In panel B, lanes 4 and 5, no rearranged T-cell-receptor genes were detected, indicating that neither lymphoma was of T-cell origin.
DNA isolated from lymphomas developed in a BALB/c nude (nu/nu) mouse (765-5) and a BALB/c ((nu/+) mouse (765-7) with helper virus infection was analyzed. High-molecular-weight DNA was isolated from the livers of normal BALB/c mice with a nu/nu, nu/+, or +/+ genotype and from the spleens with transplantable tumor cells (765-5) of BALB/c nude (nu/nu) mouse origin and tumor cells (765-7) of BALB/c (nu/+) origin. Each DNA preparation (4 μg) was digested with EcoRI (Fig. 3A), HindIII (Fig. 3B, panel a), or PvuII (Fig. 3B, panel b) and electrophoresed in 0.6% agarose gels in Boyer’s buffer. The size-fractionated DNA was blotted onto nitrocellulose filter paper, hybridized with a 32P-labeled molecular probe at 65°C, washed with 0.3 × SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at 65°C, and analyzed with a BAS 2000 image analyzer (Fuji Film Co. Ltd.). To detect the B-lymphocyte clonal populations, a JH4 probe (a 1.2-kbp EcoRI-HindIII fragment) was used to determine the profile of immunoglobulin heavy-chain gene rearrangement (Fig. 3A) (20). T-lymphocyte clonality was examined with a mouse Cβ2 probe (a 730-bp EcoRI fragment) to determine the profile of T-cell-receptor gene rearrangement (Fig. 3B) (10).
Data obtained by fluorocytometry and Southern blot hybridization showed that all of the transplantable cell lines examined belonged to the B-cell lineage. However, Southern blotting analysis with a helper-specific probe (14) showed uncountable bands of ecotropic helper virus in the DNA prepared from transplantable cells induced with LP-BM5 or BM5eco virus. This suggested that the transplantable cells still consisted of oligoclonal cells and that there were multiple integrations of the viruses. The discrepancy of monoclonality of the transplantable cells detected by Southern blot hybridization with the virus-specific probe and the JH4 probe for the immunoglobulin heavy-chain gene does not exclude the possibility that the lymphomas acquired ecotropic virus insertions in a clonal somatic manner consistent with a contribution of insertional mutagenesis to the induction of lymphoma. One possible explanation for this discrepancy is that the B cells were transformed after rearrangement of the immunoglobulin heavy-chain gene, since the cell surface markers detected on the transplantable cells were those for mature B cells.
The effects of ecotropic helper virus infection on the immune systems of adult C57BL/6 mice were not remarkable compared with those of whole MAIDS virus infection (15). Although infection of adult C57BL/6 mice with the ecotropic virus component of the LP-BM5 MuLV mixture was not sufficient to induce the major manifestations of MAIDS (4, 5), the frequent integration of ecotropic virus into a transplantable cell line established from MAIDS virus complex-infected C57BL/6 mice suggested that the ecotropic virus is an important factor in making cells transplantable (22).
The age-dependent susceptibility of BALB/c nu/nu and +/+ mice to MAIDS virus-induced lymphoma has not yet been examined in detail. Adult BALB/c nu/nu mice are resistant to BM5eco infection, but neonates of this strain are susceptible. Although the difference has been attributed mainly to the immunological immaturity of neonates, further analysis of the development of resistance in adult nu/nu mice is required. It is also possible that this difference may be explained by different susceptibilities of the target B cells in nu/nu neonates to virus infection.
Although the MAIDS virus-induced immunodeficiency in normal mice is not the same as that in nude mice, the severe oncogenicity of the ecotropic helper virus in nude mice suggests that this virus in the LP-BM5 virus complex causes opportunistic leukemia under immunodeficient conditions in C57BL/6 or BALB/c mice similar to the leukemia observed in human AIDS patients. To study the opportunistic oncogenesis caused by these viruses, one useful approach would be to infect nude mice. It has been reported that the Friend spleen focus-forming virus resistance gene, Fv-2rr, was not effective in C57BL/6 nude mice (11) and that another Friend helper virus resistance gene, Fv-4, caused a phenotype in BALB/c nude mice different from that in normal BALB/c mice: resistance was dominant in normal BALB/c mice and recessive in BALB/c nude mice (7).
Although the experiment using a helper-free stock of defective MAIDS virus showed that the helper virus is not necessary to induce early nonmalignant B-cell proliferation, it is unclear whether the presence of ecotropic virus is necessary to induce the malignant proliferation of lymphoid cells in MAIDS virus-infected mice. Induction of clonal growth of lymphoid cells in C57BL/6 mice upon infection with a helper-free stock of defective MAIDS virus (8) does not exclude the possibility that the ecotropic helper virus plays an important role in inducing the malignant outgrowth of lymphoid cells. The frequent appearance of replication-competent helper virus in transplantable T-cell lines developed in mice infected with a helper-free stock (21) also suggested a possible role for it in leukemogenesis, such as insertional mutagenesis by the helper virus (9). The defective virus appears to cause the development of the early nonmalignant proliferation of B cells, while the helper virus develops the malignant B- or T-cell lymphoma much later in MAIDS; the transplantable B cells are different from the B cells proliferating in early MAIDS. The mechanism responsible for the abnormal proliferation of B cells induced by defective virus infection is also unknown. The mechanism of leukemogenesis caused by the MAIDS virus may be explained by analogy to the previous reports that spleen focus-forming virus in the Friend MuLV complex transformed erythroid cells by the insertional mutagenesis of proto-oncogenes such as p53 and spi-1 collaborating with the replication-competent helper virus (2, 3).
Acknowledgments
Lamin Tayar and Kyoko Higo contributed equally to this study.
We thank Toshio Hattori, Hiroshi Hiai, and Kagemasa Kuribayashi for useful technical advice. We thank Akira Shimizu and Tasuku Honjo for helpful discussions and providing materials. We also thank Yuki Sato and Chiemi Tajima for taking care of the mice.
This work was partly supported by a Sasakawa Scientific Research Grant from the Japan Science Society to Lamin Tayar and by Grants-in-Aid for Science Research and Cancer Research from the Ministry of Education, Science and Culture of Japan to Akinori Ishimoto and Hiroyuki Sakai.
REFERENCES
- 1.Aziz D C, Hanna Z, Jolicoeur P. Severe immunodeficiency disease induced by a defective murine leukaemia virus. Nature. 1989;338:505–508. doi: 10.1038/338505a0. [DOI] [PubMed] [Google Scholar]
- 2.Ben-David Y, Giddens E B, Bernstein A. Identification and mapping of a common proviral integration site Fli-1 in erythroleukemia cells induced by Friend murine leukemia virus. Proc Natl Acad Sci USA. 1990;87:1332–1336. doi: 10.1073/pnas.87.4.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-David Y, Lavigueur A, Cheong G Y, Bernstein A. Insertional inactivation of the p53 gene during Friend leukemia: a new strategy for identifying tumor suppressor genes. New Biol. 1990;2:1015–1023. [PubMed] [Google Scholar]
- 4.Chattopadhyay S K, Morse III H C, Makino M, Ruscetti S K, Hartley J W. Defective virus is associated with induction of murine retrovirus-induced immunodeficiency syndrome. Proc Natl Acad Sci USA. 1989;86:3862–3866. doi: 10.1073/pnas.86.10.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chattopadhyay S K, Sengupta D N, Fredrickson T N, Morse III H C, Hartley J W. Characteristics and contributions of defective, ecotropic, and mink cell focus-inducing viruses involved in a retrovirus-induced immunodeficiency syndrome of mice. J Virol. 1991;65:4232–4241. doi: 10.1128/jvi.65.8.4232-4241.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartley J W, Fredrickson T N, Yetter R A, Makino M, Morse H C., III Retrovirus-induced murine acquired immunodeficiency syndrome: natural history of infection and differing susceptibility of inbred mouse strains. J Virol. 1989;63:1223–1231. doi: 10.1128/jvi.63.3.1223-1231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higo K, Kubo Y, Iwatani Y, Ono T, Maeda M, Hiai H, Masuda T, Kuribayashi K, Zhang F, Lamin T Y, Adachi A, Ishimoto A. Susceptibility of nude mice carrying the Fv-4 gene to Friend murine leukemia virus infection. J Virol. 1997;71:750–754. doi: 10.1128/jvi.71.1.750-754.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang M, Simard C, Jolicoeur P. Immunodeficiency and clonal growth of target cells induced by helper-free defective retrovirus. Science. 1989;246:1614–1617. doi: 10.1126/science.2480643. [DOI] [PubMed] [Google Scholar]
- 9.Huang M, Takac M, Kozak C A, Jolicoeur P. The murine AIDS defective provirus acts as an insertional mutagen in its infected target B cells. J Virol. 1995;69:4069–4078. doi: 10.1128/jvi.69.7.4069-4078.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikuta K, Hattori M, Wake K, Kano S, Honjo T, Yodoi J, Minato N. Expression and rearrangement of the α, β, and γ chain genes of the T cell receptor in cloned murine large granular lymphocyte lines. No correlation with the cytotoxic spectrum. J Exp Med. 1986;164:428–442. doi: 10.1084/jem.164.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitagawa M, Matsubara O, Kasuga T. Dynamics of lymphocytic subpopulations in Friend leukemia virus-induced leukemia. Cancer Res. 1986;46:3034–3039. [PubMed] [Google Scholar]
- 12.Klinken S P, Fredrickson T N, Hartley J W, Yetter R A, Morse H C., III Evolution of B cell lineage lymphomas in mice with a retrovirus-induced immunodeficiency syndrome, MAIDS. J Immunol. 1988;140:1123–1131. [PubMed] [Google Scholar]
- 13.Kubo Y, Nakagawa Y, Kakimi K, Matsui H, Iwashiro M, Kuribayashi K, Masuda T, Hiai H, Hirama T, Yanagawa S-I, Ishimoto A. Presence of transplantable T-lymphoid cells in C57BL/6 mice infected with murine AIDS virus. J Virol. 1992;66:5691–5695. doi: 10.1128/jvi.66.9.5691-5695.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubo Y, Nakagawa Y, Kakimi K, Matsui H, Higo K, Ling W, Kobayashi H, Hirama T, Ishimoto A. Molecular cloning and characterization of a murine AIDS virus-related endogenous transcript expressed in C57BL/6 mice. J Gen Virol. 1994;75:881–888. doi: 10.1099/0022-1317-75-4-881. [DOI] [PubMed] [Google Scholar]
- 15.Lee J S, Giese N A, Elkins K L, Yetter R A, Holmes K L, Hartley J W, Morse H C., III Effects of exogenous, nonleukemogenic, ecotropic murine leukemia virus infections on the immune systems of adult C57BL/6 mice. J Virol. 1995;69:4182–4188. doi: 10.1128/jvi.69.7.4182-4188.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mosier D E, Yetter R A, Morse H C., III Functional T lymphocytes are required for a murine retrovirus-induced immunodeficiency disease (MAIDS) J Exp Med. 1987;165:1737–1742. doi: 10.1084/jem.165.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakagawa Y, Kakimi K, Ling W, Kubo Y, Higo K, Masuda T, Kuribayashi K, Iwashiro M, Komatz Y, Hirama T, Adachi A, Ishimoto A. Inhibition of murine AIDS (MAIDS) development by the transplantation of bone marrow cells carrying the Fv-4 resistance gene to MAIDS virus-infected mice. J Virol. 1994;68:1438–1441. doi: 10.1128/jvi.68.3.1438-1441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowe W P, Pugh W E, Hartley J W. Plaque assay techniques for murine leukemia viruses. Virology. 1970;42:1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- 19.Sawada H, Itoh K, Kirikae T, Sakoda H, Tezuka H, Kuribayashi K, Maeda M, Yoshida Y, Uchino H, Hanaoka M, Mori K. Establishment of a hemopoietic stimulating factor producing murine leukemia cell lines: pathogenesis of granulocytosis in L8313 bearing mice. Leuk Res. 1988;12:763–771. doi: 10.1016/0145-2126(88)90010-0. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu A, Takahashi N, Yaoita Y, Honjo T. Organization of the constant-region gene family of the mouse immunoglobulin heavy chain. Cell. 1982;28:499–506. doi: 10.1016/0092-8674(82)90204-5. [DOI] [PubMed] [Google Scholar]
- 21.Simard C, Huang M, Jolicoeur P. Establishment of leukemic, T-cell lines from mice inoculated with the MAIDS defective virus. Virology. 1995;206:555–563. doi: 10.1016/s0042-6822(95)80072-7. [DOI] [PubMed] [Google Scholar]
- 22.Tang Y, Chattopadhyay S K, Hartley J W, Fredrickson T N, Morse H C., III Clonal outgrowths of T and B cells in SCID mice reconstituted with cells from mice with MAIDS. In Vivo. 1994;8:953–960. [PubMed] [Google Scholar]