Abstract
Lungs are constantly exposed to environmental perturbations and therefore have remarkable capacity to regenerate in response to injury. Sustained lung injuries, aging, and increased genomic instability, however, make lungs particularly susceptible to disrepair and fibrosis. Pulmonary fibrosis constitutes a major cause of morbidity and is often relentlessly progressive, leading to death from respiratory failure. The pulmonary vasculature, which is critical for gas exchanges and plays a key role during lung development, repair, and regeneration, becomes aberrantly remodeled in patients with progressive pulmonary fibrosis. Although capillary rarefaction and increased vascular permeability are recognized as distinctive features of fibrotic lungs, the role of vasculature dysfunction in the pathogenesis of pulmonary fibrosis has only recently emerged as an important contributor to the progression of this disease. This review summarizes current findings related to lung vascular repair and regeneration and provides recent insights into the vascular abnormalities associated with the development of persistent lung fibrosis.
Keywords: pulmonary fibrosis, pulmonary vasculature, lung repair, lung regeneration, endothelial heterogeneity
The lung is a highly vascularized organ with a vital role in respiration and gas exchange. The pulmonary vasculature consists of arterial and venous trees connected by an extensive network of capillaries lining the alveoli, the gas-exchanging units of the lung (1). In addition to regulating oxygen exchange and vascular permeability, pulmonary endothelial cells (ECs) deploy specific paracrine signaling molecules (i.e., angiocrine factors) to orchestrate lung homeostasis, regeneration, and repair after injury (2). Disruption of endothelial homeostasis due to stressful stimuli or during aging results in endothelial dysfunction that facilitates the progression of numerous chronic lung diseases, including pulmonary fibrosis (3).
Pulmonary fibrosis is a disease characterized by pathologic lung scarring leading to progressive respiratory failure and death (4). Pulmonary fibrosis belongs to a group of chronic lung disorders characterized by abnormalities of the lung interstitium known as interstitial lung diseases (ILDs). Idiopathic pulmonary fibrosis (IPF) and systemic sclerosis–associated pulmonary fibrosis are the most common and severe forms of ILD, with estimated prevalences of 8.2 and 12.1 cases per 100,000, respectively. IPF is an aging-associated disease, with 90–100% of patients exhibiting progressive fibrosis (5) and a median survival duration of 3–5 years after diagnosis (6). Therapeutic options are limited and mainly rely on drugs that slow the progression of the disease rather than block it (6). IPF is characterized by the progressive accumulation of activated fibroblasts (myofibroblasts), which are responsible for the excessive extracellular matrix (ECM) deposition and increased stiffening of the lung parenchyma (7–10). Myofibroblasts communicate with other resident lung cells and receive pathogenic signals during the fibrogenic process, which ultimately contributes to the pathogenesis of IPF.
Although the majority of studies have focused on epithelial cells, fibroblasts, and immune cells as critical players in the pathogenesis of IPF, the role of the pulmonary vasculature in the progression of this disease has only recently begun to emerge. In this review, we provide a comprehensive summary of the current knowledge of the physiopathology of the pulmonary vasculature in the context of lung injury, repair, and fibrosis.
Anatomy of the Pulmonary Vasculature
The lung is supplied by two blood circulations, the bronchial circulation and the pulmonary circulation (11). The bronchial circulation, which is part of the systemic circulation, is a high-pressure and low-capacitance system responsible for delivering oxygen to the bronchial tree and disposing of its metabolic waste products. The bronchial circulation is therefore not involved in blood oxygenation (11). The pulmonary circulation, on the contrary, is a low-pressure and high-capacitance system that provides a large surface area for gas exchange in the alveolar air spaces (11). It originates from the pulmonary artery, which transports the entire cardiac output from the right ventricle to the lungs for oxygenation of circulating blood and removal of carbon dioxide. The main pulmonary artery bifurcates into two main branches, right and left, which further branch into medium and small arteries, arterioles, and finally an extensive network of alveolar capillaries. The capillaries are drained by postcapillary venules and veins of increasingly larger size, which finally form the pulmonary veins that carry oxygenated blood to the left atrium of the heart (12). A healthy, intact network of alveolar capillaries is critical to provide the maximum surface required for optimal gas exchange.
The process of gas exchange takes place in the alveoli, which are surrounded by a dense network of capillaries (13). The alveolar walls are composed of an epithelial layer, facing the alveolar air space, and an endothelial layer, which lines the capillary lumen (13). A thin basement membrane composed of extracellular proteins, mainly type IV collagen and laminin, separates these two layers and brings them close through adhesive mechanisms to facilitate efficient gas exchange (14). Even though most studies have focused on the alveolar epithelium and its contribution to lung homeostasis, repair, and fibrosis (15–17), recent investigations have shed new light on the crucial role played by the alveolar capillaries in response to lung injury (18–20), highlighting the importance of the capillary ECs as orchestrators of wound healing responses.
Lung Endothelial Heterogeneity
Although the vascular endothelium has long been considered a homogeneous cell layer, there is now increasing appreciation that it is instead very heterogeneous in structure and function (21). Vascular heterogeneity exists between ECs in different organs, as well as in ECs located in different vascular segments of the same organ (21). ECs along the arterial–capillary–venous axis exhibit remarkable differences and can differently adapt to homeostatic and pathogenic stimuli (22, 23). Although anatomical distinctions have been recognized between different lung vascular beds, specific endothelial transcriptional signatures that comprehensively define the heterogeneity of the pulmonary vasculature have been largely unknown.
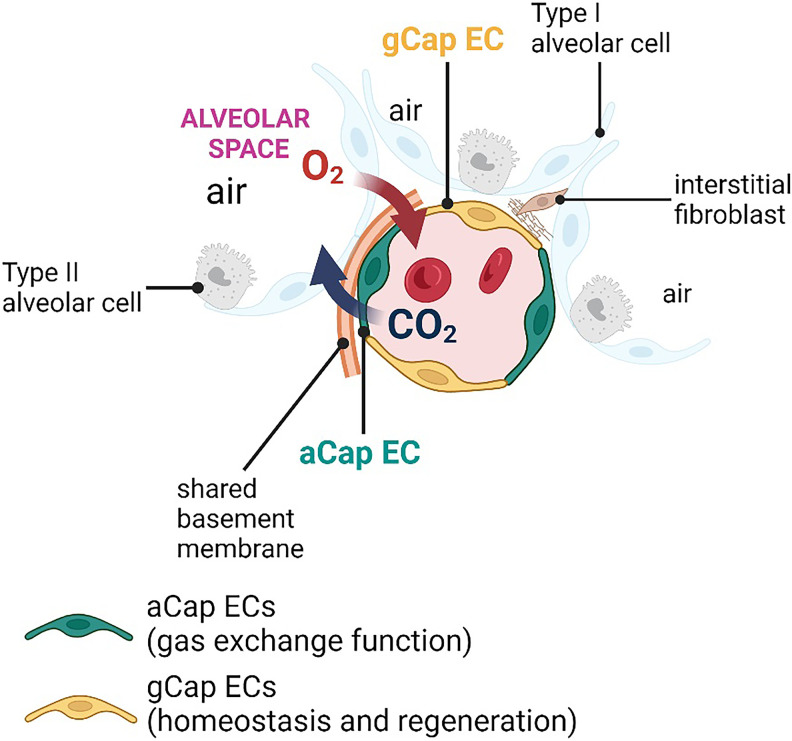
In the context of the lung, the use of single-cell RNA sequencing (scRNA-seq) technology combined with imaging and lineage-tracing studies in mice has revealed that the pulmonary vasculature is characterized by a remarkable transcriptional heterogeneity (18, 20, 24). For example, recent studies have identified two different subsets of pulmonary capillary ECs with distinct morphological and transcriptional features to serve distinct lung functions. Gillich and colleagues named these newly identified pulmonary capillary ECs “aCap ECs” (i.e., aerocytes) and “gCap ECs” (i.e., general capillaries) (18) (Figure 1). The two EC subtypes were also found within human alveolar capillaries (18), and these findings were recently confirmed by Schupp and colleagues (25), suggesting that this endothelial specialization is conserved across mouse and human lungs.
Figure 1.
Lung endothelial heterogeneity. Two distinct subsets of pulmonary capillary endothelial cells (ECs), named aCap ECs (aerocytes) and gCap ECs (general capillary), characterize the pulmonary vasculature. The aCap ECs are unique to the lung and are localized in the thin regions of the gas-exchange surface in close contact with type 1 alveolar cells, through which oxygen diffusion occurs. The gCap ECs, localized in the thick regions of the alveoli, serve as specialized stem/progenitor cells that give rise to gCap ECs and aCap ECs in response to injury and during normal homeostasis.
The aCap ECs, which are unique to the lung, are specialized in gas exchange and leukocyte trafficking. They are large cells (spanning >100 μm) with extensive cellular projections that establish contact with multiple alveoli. aCap ECs are localized in the thin regions of the gas-exchange surface in close contact with alveolar type 1 (AT1) cells, through which oxygen diffusion occurs. Transcriptionally, aCap ECs express a distinct set of genes encoding for proteins implicated in gas exchange, such as Car4 (Carbonic Anhydrase 4) (18, 24), and leukocyte trafficking, such as Icam1 (Intercellular Adhesion Molecule 1) (18). In addition, aCap ECs express genes important in angiogenesis, including Apln (Apelin receptor ligand), Kitl (kit ligand), and Kdr (Kinase Insert Domain Receptor; encoding for VEGFR2, vascular endothelial growth factor receptor 2) (18).
The gCap ECs share numerous similarities with ECs from capillary beds of other organs. gCap ECs regulate vascular tone and serve as specialized stem/progenitor cells that give rise to gCap ECs and aCap ECs in response to injury and during normal homeostasis (18). gCap ECs are smaller than aCap ECs, spanning less than 40 μm, with few cytoplasmic extensions that form intimate connections with cells of the interstitial space, including interstitial fibroblasts and pericytes. gCap ECs also express genes related to lipid metabolism, such as Gpihbp1 (Glycosylphosphatidylinositol Anchored High Density Lipoprotein Binding Protein 1), and vasomotor control, including Edn1 (endothelin 1) and endothelial Nos3 (Nitric Oxide Synthase 3). Genes encoding for membrane receptors implicated in angiogenesis, including Aplnr (apelin receptor), Kit, and Tek (Tek receptor kinase, also known as Tie2 or angiopoietin-1 receptor) are also highly enriched in gCap ECs (18).
Based on their transcriptomes, aCap and gCap ECs are predicted to cross-talk and also signal with nonendothelial cells of the alveolar wall. Indeed, aCap ECs produce Apln and Kitl ligands recognized by the Aplnr and Kit receptors on gCap ECs, whereas gCap ECs produce Edn1, which can be recognized by endothelin receptor type B (Ednrb) on aCap ECs and endothelin receptor type A (Ednra) on pericytes (18). Other studies demonstrated that Car4-high ECs respond to VEGF (vascular endothelial growth factor) signaling derived from neighboring AT1 cells during formation and postnatal maintenance (24) and in the setting of acute lung injury (20).
To understand whether the different transcriptional profiles of the identified EC subpopulations result in different functions, Gillich and colleagues combined a series of elegant in vivo experiments performed using a mouse model of lung emphysema and demonstrated that gCap ECs proliferate and are essential to orchestrate capillary repair following elastase-induced lung injury (18). In another work, Niethamer and colleagues found increased Car4-high ECs in areas of alveolar damage after influenza or bleomycin injury, suggesting that this EC subtype may be responsible for regeneration of injured lung (20).
Although these studies are not conclusive, they provide the foundation for future investigations aimed at elucidating the contribution of different EC subpopulations to lung wound healing and the development of new therapies for the treatment of lung disease caused by a defective response to injury.
Role of the Pulmonary Vasculature in Lung Repair
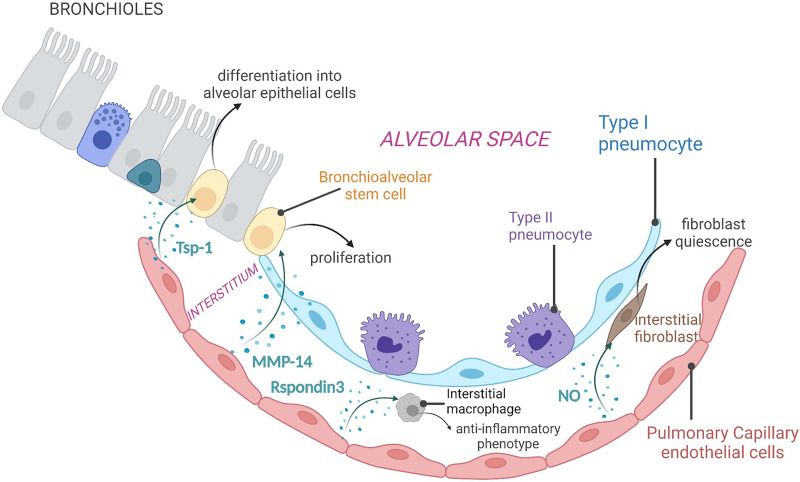
It is well recognized that capillary ECs do not just form the lining of small vascular tubes, but they also influence adjacent cells through the secretion of paracrine factors, known as angiocrine factors (2, 26, 27). These endothelial-derived secreted molecules actively participate in organ homeostasis, repair, and regeneration after injury in multiple organs (28–30), including the lung (2, 27) (Figure 2).
Figure 2.
Angiocrine factors in lung regeneration and repair. Pulmonary capillary ECs secrete a plethora of endothelial-derived soluble factors, known as angiocrine factors, in the extracellular environment. These endothelial-derived soluble molecules, such as Tsp-1 (thrombospondin-1), MMP-14 (matrix metalloproteinase-14), Rspondin3, and nitric oxide, target neighboring bronchioalveolar stem cells, interstitial macrophages, and fibroblasts to promote lung repair and regeneration in response to injury. NO = nitric oxide
Studies in mice have shown that pulmonary ECs promote lung repair in response to injury by supporting the differentiation of resident stem cells of the lung. For example, an elegant study by Lee and colleagues showed that lung ECs promote differentiation of the bronchioalveolar stem cells (BASCs), a population of epithelial stem cells of the distal lung involved in lung repair and homeostasis (31–33). The authors showed that lung injury activates the BMP4 (Bone Morphogenic Protein 4) pathway in lung ECs, which induces the activation of the transcription factor NFATC1 (Nuclear Factor of Activated T Cells 1), resulting in the upregulation and secretion of endothelial-derived Tsp1 (thrombospondin-1). Tsp1, in turn, signals to BASCs to promote their differentiation into epithelial cells of alveolar lineage. The authors then sought to investigate whether this effect was organ-specific, and therefore unique to the pulmonary vasculature, or could be broadly elicited by ECs from other vascular beds. Interestingly, they demonstrated that BASC differentiation was dependent on pulmonary ECs, as ECs from the liver were not able to support this process, thus reinforcing the concept that different organ ECs produce angiocrine factors to perform organ-specific activities (33).
To assess the contribution of the pulmonary vasculature in the context of alveolar regeneration, Ding and colleagues employed the mouse model of unilateral pneumonectomy (PNX), which is widely used to study mechanisms of compensatory lung growth (2, 34). The authors demonstrated that the activation of VEGFR2 (vascular endothelial growth factor receptor 2) and FGFR2 (Fibroblast Growth Factor Receptor 2) in pulmonary capillary ECs leads to the secretion of MT1-MMP/MMP14 (membrane-type 1 matrix metalloproteinase) (2). Endothelial-derived MMP14 cleaves the EGF-like ectodomain on EGFR (Epidermal Growth Factor Receptor) on alveolar progenitor cells, leading to its autoactivation and stimulation of epithelial cell proliferation and alveolarization (2). In an effort to find additional mechanisms underlying activation of pulmonary capillary ECs and secretion of MMP14, Ding and colleagues also discovered that, after PNX, platelets become activated and secrete SDF1 (stromal cell–derived factor 1). SDF1 in turn stimulates CXCR4 (C-X-C Motif Chemokine Receptor 4) and CXCR7 (C-X-C Chemokine Receptor Type 7) on pulmonary capillary ECs, leading to secretion of MMP14 and alveolar epithelial cell proliferation (35).
In addition to interacting with lung cells of the epithelial lineage, lung ECs also establish intimate contact with lung-resident macrophages and can regulate their functions (36, 37). Although functional interactions between ECs and macrophages have been reported in other organs (38, 39), the contribution of this cellular cross-talk in lung homeostasis and disease remains largely unknown. In a recent study, Zhou and colleagues shed some light on the interaction of lung ECs with macrophages in the setting of inflammatory injury. With a series of elegant experiments, they demonstrated that pulmonary ECs secrete the Wnt signaling activator Rspondin3, which in turn activates β-catenin in interstitial macrophages, resulting in their reprogramming toward an antiinflammatory phenotype that promotes resolution of inflammation and lung repair (40). These findings laid the foundation for future work aimed at identifying endothelial signals that play a role in immune cell polarization and inflammation with the goal of further expanding our knowledge of EC-mediated paracrine communication in the context of lung fibrosis resolution.
Besides the secretion of endothelial-derived proreparative factors active on the lung microenvironment, the process of vascular regeneration itself is also critical for overall lung regeneration (41). In this respect, Mammoto and colleagues investigated the contribution of the mechanical environment to lung vascular regeneration. They demonstrated that the activation of the endothelial YAP1 (Yes Associated Protein), a transcriptional cofactor and mechanoregulator downstream of the Hippo pathway, modulates the compensatory growth after PNX in mice through the activation of the Tie2 signaling pathway in lung ECs (42). Loss of endothelial YAP1 inhibited vascular and alveolar morphogenesis, thereby affecting compensatory lung growth, further demonstrating the role of mechano-derived endothelial signaling in vascular remodeling during lung regeneration (42). Intriguingly, the function of YAP and its coactivator TAZ have been largely studied in lung fibroblasts, in which the YAP/TAZ signaling pathway contributes to their conversion to synthetic and contractile myofibroblasts in response to fibrogenic stimuli (8, 43, 44). Given the divergent outcomes of activated YAP signaling in different lung cell types, further investigations aimed at understanding the cross-talk of YAP with other signaling pathways in lung ECs would be critical to better define the regenerative and pathogenic functions of this transcriptional regulator.
Altogether, these studies shed light on the important role of pulmonary ECs in driving lung repair and regeneration. This effect can be mediated by endothelial-derived paracrine factors with proreparative effects on neighboring cells, or it can be dependent on the vascular regeneration itself, which raises the possibility of harnessing these endothelial mechanisms to develop therapeutics to promote lung-reparative responses to injury.
The Pulmonary Vasculature in Lung Fibrosis
IPF is characterized by the histologic pattern of usual interstitial pneumonia, a spatially heterogeneous mix of normal lung tissue, patchy fibrosis, and aggregates of collagen-producing fibroblasts (i.e., fibroblastic foci) (45). Vascular abnormalities, including vessel dilation and capillary rarefaction, have been observed in IPF lungs (3,46–50). Although these vascular abnormalities have been well documented, whether they are important drivers of disease progression still remains debated.
The first study reporting vascular alterations in lungs of patients with IPF was reported in 1963 by Turner-Warwick, who used a microradiographic technique to study vascular anastomosis between the bronchial and the pulmonary systemic circulation in fibrotic human lungs. This study revealed that, in the large majority of fibrotic lungs, the bronchial vasculature expanded to form an extensive network of vessels throughout the lungs, forming anastomoses (i.e., connections between vascular structures that are originally separated) with the pulmonary systemic microvasculature (51). Subsequent studies reporting vascular abnormalities in IPF have led to controversial theories regarding the potential contribution of the lung endothelium to the pathogenesis of IPF, with some studies supporting an increased number of vessels (52, 53) and others reporting reduced vascularization in IPF lungs (49, 50). More recent evidence suggested that these seemingly divergent pathologic features may be due to the histological heterogeneity of this disease. In fact, independent studies demonstrated high capillary density in nonfibrotic areas of IPF lungs and reduced numbers of capillaries in areas of active fibrosis, especially in those surrounding fibroblastic foci, which lack blood vessels (49, 52). In addition to the heterogeneous capillary remodeling, other vascular abnormalities accompany IPF progression, such as increased vascular permeability and altered pulmonary vascular volume (46, 48, 54). In this regard, a recent imaging study reported a direct correlation between reduced small-vessel volume and increased risk of developing ILDs, suggesting that alterations of the pulmonary vasculature may represent an early indicator of vasculopathy-associated ILD (55).
Our recent study reporting histological and transcriptional abnormalities in a subpopulation of lung capillary ECs, the gCap ECs, in fibrotic aged mouse lungs as well as in IPF lungs further supports the hypothesis that limited vascular regeneration in IPF lungs may be directly responsible for the fibrosis progression in patients with IPF (56).
Vascular Remodeling in Fibrotic Lungs
Although ECs rarely proliferate and are largely quiescent, they can respond to stressful conditions such as tissue injury and inflammation by coordinating a series of events leading to angiogenesis, the formation of new blood vessels starting from preexisting ones. This process is tightly regulated by a fine balance between angiogenic and angiostatic factors that can promote or inhibit angiogenesis, respectively (57).
Because lung angiogenesis is difficult to capture using imaging methods, and longitudinal studies documenting the onset of fibrosis are limited, investigating lung vascular remodeling during the progression of IPF is challenging. Thus, several studies have indirectly investigated angiogenesis in IPF lungs by measuring angiogenic and angiostatic factors released in the blood or by assessing the presence of these factors in bronchoalveolar lavage (BAL) fluid as well as in lung biopsies of patients with IPF. Circulating levels of the angiogenic factor VEGF were reported to be increased in the serum of patients with IPF with advanced disease (58). However, another study reported a different trend, with high levels of VEGF in patients with IPF exhibiting slow disease progression (59), suggesting that assessing circulating VEGF in IPF may not be a reliable approach to predicting lung vascular remodeling and disease progression. Cosgrove and colleagues assessed the levels of the angiogenic factor VEGF and the angiostatic factor PEDF (pigment epithelium–derived factor) in IPF lungs and found increased PEDF and reduced VEGF levels within the fibroblastic foci of IPF lungs as well as in BAL fluid of patients with IPF (60). These latter findings were also confirmed by other studies showing the same trend of PEDF and VEGF levels in the BAL fluid of patients with IPF (61, 62). These divergent results suggest that the contribution of VEGF to IPF progression remains largely unclear. Future studies addressing the implications of VEGF signaling on the pulmonary vasculature and fibrosis overall will be of great translational value, particularly considering that nintedanib, a U.S. Food and Drug Administration–approved therapeutic agent for the treatment of IPF, targets the kinase activity of VEGFR2, the receptor of VEGF (63). Although data from in vitro and in vivo studies have demonstrated that nintedanib interferes with fibroblast proliferation and secretion of extracellular matrix proteins by inhibiting multiple signaling pathways, including PDGF and FGF signaling pathways (64), the contribution of inhibition of VEGF signaling to the overall antifibrotic effect of nintedanib is not fully elucidated.
Several studies showed that angiopoietin-1 (Ang-1) and angiopoietin-2 (Ang-2), two critical regulators of endothelial quiescence and vascular stability, are abnormally expressed in IPF lungs. Ang-1 is best known to promote endothelial survival and vascular maturation, whereas Ang-2 antagonizes the activity of Ang-1 and induces vascular destabilization (65), which can result in inhibition or stimulation of angiogenesis depending on VEGF availability. Margaritopoulos and colleagues demonstrated reduced Ang-1 and increased Ang-2 protein levels in BAL fluid from patients with IPF (66), which would be an indicator of poor vascular stability and maturation in IPF lungs. In another study, Uehara and colleagues found a strong reduction of Ang-1 levels in the serum of patients with IPF; however, they found no difference in the levels of Ang-2 in these patients compared with healthy individuals. Intriguingly, this study also reported that, based on Ang-2 levels, patients with IPF exhibiting high Ang-2 levels had worse lung function and poorer prognosis compared with those with moderate Ang-2 expression (67).
Aberrant vascular remodeling in patients with IPF may also lead to abnormal changes in pulmonary arteries, resulting in pulmonary hypertension (PH) (68), a common comorbidity that contributes significantly to morbidity and mortality in IPF (69). As PH negatively impacts the diffusing capacity for carbon monoxide (70), an excessive reduction of this parameter in a patient with IPF may suggest concomitant PH, thus supporting a link between aberrant IPF-associated vascular remodeling and PH development (70). In addition, a recent paper by Yanagihara and colleagues showed that the expression of BMPR2 (Bone Morphogenetic Protein Receptor Type 2), an endogenous inhibitor of TGF-β (transforming growth factor-β) signaling (71), was reduced in ECs and vascular smooth muscle cells in the lungs of patients with IPF and concomitant PH, resulting in altered vascular–mesenchymal cross-talk and profibrotic effects on fibroblasts (71). Another IPF comorbidity with underlying aberrant vascular remodeling is chronic obstructive pulmonary disease (COPD) (72). In patients with IPF-associated COPD, a decreased number of pulmonary arteries, increased wall thickness, and excessive elastin deposition have been reported (73). These vascular aberrations could also be critical for the development of PH (73), suggesting that targeting vascular abnormalities in IPF lungs may have a positive impact and limit disease progression in these patients.
During the past decade, mouse models of lung fibrogenesis induced via intratracheal injection of bleomycin have been employed in several laboratories to gain mechanistic insights into the contribution of vascular remodeling to lung fibrosis progression. Two independent studies investigated the role of VEGF signaling during bleomycin-induced lung fibrosis by inhibiting this pathway using a VEGFA neutralizing antibody (CBO-P11) (74) or a VEGFR inhibitor (SU5416) (75). Both studies reported reduced lung collagen deposition and proinflammatory markers in mice treated with these inhibitors, suggesting that, during the acute phase after injury, angiogenic signals and vascular remodeling are essential to the inflammatory and fibrogenic response of the lung. However, other studies showed that VEGF signaling exerts beneficial effects during bleomycin-induced lung fibrosis. In one study, Murray and colleagues showed that lung-specific overexpression of Vegfa in epithelial cells reduced mortality and lung fibrosis after bleomycin challenge in mice and that this protective effect was mediated by the endothelium (59). The authors showed that epithelial-derived VEGF induces secretion of endothelial-derived thrombospondin 1, a soluble molecule that was previously shown to induce differentiation of BASCs (33), which in turn exerts antiapoptotic effects on the lung epithelium. In another study, Stockmann and colleagues used a transgenic mouse model to delete Vegfa from myeloid cells and showed increased collagen deposition and reduced neovascularization in mutant animals challenged with bleomycin (76).
Interestingly, recent studies have shown that AT1 cells secrete high levels of VEGF to support Car4-high EC survival and function under physiological conditions (18, 24), suggesting that other proangiogenic factors beyond or in addition to VEGF may be responsible for angiogenesis in response to injury. Indeed, many inflammatory cytokines, including TNF-α, have the ability to stimulate angiogenesis (77, 78) so the neoformed vessels can deliver oxygen and nutrients to the site of injury, remove catabolites, and deploy proreparative angiocrine factors, contributing to the restoration of homeostatic functions. Altogether, these studies reflect the complexity of pathways and cellular cross-talk involved in lung vascular remodeling after injury. Additional research work is needed to conclusively determine the contribution of the pulmonary vasculature to lung remodeling, not only in fibrotic areas but also in those not yet affected by excessive matrix deposition. In addition, investigating whether hypoxia and/or other pathogenic cues could influence VEGF signaling and promote aberrant angiogenesis may provide further insights into the involvement of this angiogenic pathway in the pathogenesis of pulmonary fibrosis, as well as in the mechanisms of action of nintedanib.
Even though the single-dose bleomycin model of fibrosis provided critical insights into the lung repair process in response to injury, it does not recapitulate the human disease, in which fibrosis is progressive. Mouse models of persistent lung fibrosis, including bleomycin delivery in aged mice and repetitive bleomycin doses in young mice (3,79–82), have been used in multiple laboratories, including our own, to study pathogenic vascular mechanisms associated with impaired resolution and progressive lung fibrogenesis.
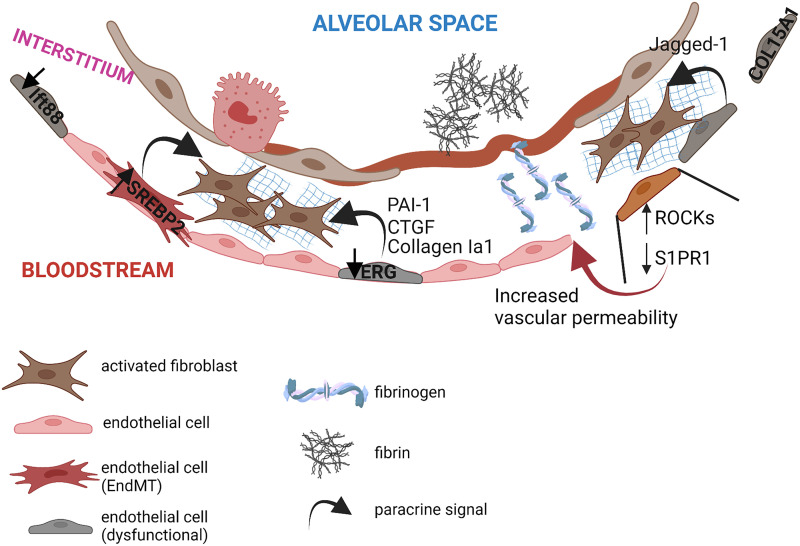
In an attempt to identify endothelial pathways that promote lung repair following injury, Cao and colleagues compared vascular responses to a single dose of bleomycin versus repetitive doses of bleomycin (79). They discovered that the endothelial chemokine receptor CXCR7 plays a critical role in promoting epithelial repair and in ameliorating fibrosis following a single dose of bleomycin. Interestingly, this endothelial reparative mechanism failed when repetitive doses of bleomycin were delivered to the mouse lungs, causing repression of CXCR7 and upregulation of the Notch ligand Jagged1 in ECs. Secreted Jagged1 then activates the profibrotic Notch pathway in fibroblasts, enhancing lung fibrosis (79) (Figure 3).
Figure 3.
Vascular alterations in the fibrotic lung. Fibrotic lungs manifest several vascular alterations, including endothelial transcriptional alterations, increased vascular permeability, intraalveolar accumulation of fibrin, and secretion of endothelial-derived paracrine factors with profibrotic effects on neighboring fibroblasts. EndMT = endothelial-to-mesenchymal transition.
More recently, we demonstrated that bleomycin-induced lung fibrosis in aged mice recapitulates fibrogenic and vascular features of IPF lungs, including myofibroblast accumulation and extensive vascular rarefaction in fibrotic areas (3). Additional studies revealed that the young pulmonary vasculature responds to injury, increasing chromatin accessibility and activating transcriptional programs, leading to vascular repair and fibrosis resolution, whereas the aged lung endothelium fails to activate these programs, resulting in vascular rarefaction and sustained fibrosis. Further studies identified the ETS (endothelial transcription factor)–related gene ERG as an orchestrator of endothelial chromatin remodeling and transcriptional activity in response to injury. Intriguingly, mice lacking endothelial ERG fail to properly respond to bleomycin injury, resulting in unresolved fibrosis, as seen in aged mice and human IPF lungs (56). Of note, scRNA-seq analysis performed on uninjured lungs from wild-type and endothelial-specific ERG-knockout mice revealed a reduction of gCap ECs in lungs lacking endothelial ERG, recapitulating vascular abnormalities observed in IPF lungs (56).
In summary, these studies demonstrated that perturbations of the pulmonary vasculature combined with acute lung injury led to disrepair and progressive fibrosis. These observations suggest that the limited vascular regeneration and increased deterioration of the pulmonary vasculature with aging may represent a predisposing factor for the progression of fibrosis, especially in those individuals who are chronically exposed to lung injury resulting from detrimental environmental cues such as cigarette smoke. Future studies aimed at restoring endothelial-specific programs to facilitate vascular regeneration in elderly patients with progressive lung fibrosis may provide potential therapeutic utility to slow the progression of this disease.
Vascular Leakage in Lung Fibrosis
Vascular leak is associated with tissue inflammation, and its transient increase following injury is necessary for the recruitment of inflammatory cells to the injured area and for the extravasation of clotting factors, which provide a provisional fibrin scaffold to reestablish tissue homeostasis (83). Failure to restore a functional vascular barrier following injury leads to vascular leakage, which perpetuates tissue inflammation and fibrosis (84, 85).
The first studies reporting an altered alveolar–capillary barrier in the lungs of patients with pulmonary fibrosis were conducted by measuring lung clearance of a radiolabeled molecule, [99mTc]diethylenetriamine pentaacetate, as an approach to evaluate vascular permeability (86, 87). These studies showed that the clearance rate of [99mTc]diethylenetriamine pentaacetate was shorter in patients with IPF than in normal subjects, and this alteration was often observed in patients with IPF exhibiting a fast disease progression (86, 87). Another study investigated alveolar–capillary permeability in IPF lungs by assessing the protein permeability index, calculated as the ratio of total proteins in BAL fluid versus total proteins in the plasma (88). Using this approach, the authors confirmed previous studies and found that increased lung vascular permeability was associated with reduced survival in patients with IPF (88). Intriguingly, by using advanced magnetic resonance in combination with a radiolabeled contrast agent that binds reversibly to serum albumin, Montesi and colleagues (89) were able to show that vascular leak in IPF lungs was not limited to fibrotic regions, but was also observed in radiographically normal areas (89), suggesting that increased vascular permeability may be an event that precedes the onset of fibrosis and can directly contribute to disease progression.
Early pioneering work conducted in mice provided useful information regarding the contribution of pulmonary vascular leak to fibrosis progression. Numerous mechanistic studies have elucidated the function of cytoskeleton-associated proteins in endothelial barrier perturbations during bleomycin-induced lung fibrosis. For example, activation of ROCK1 (Rho-associated coiled-coil–forming protein kinase-1) and ROCK2 following lung injury was shown to promote cytoskeleton remodeling and vascular leak (90), and inhibition of ROCK signaling via pharmacological or genetic approaches has been shown to inhibit vascular leak and prevent the development of lung fibrosis (90–92). Additional studies also showed that fibrosis resolution was significantly enhanced, and vascular permeability reduced when ROCK signaling was inhibited during the peak of fibrosis (90–92).
In a recent work, Knipe and colleagues explored another pathway implicated in endothelial permeability, the S1P (sphingosine-1-phosphate)–mediated signaling pathway (93). In this elegant work, the authors demonstrated that mice lacking S1PR1 (S1P receptor), from the endothelium, exhibited increased pulmonary vascular permeability in the absence of injury, and bleomycin administration to the lungs of these mice led to exacerbated lung fibrosis, suggesting that preserving pulmonary endothelial barrier integrity is critical to limit fibrogenic responses following lung injury (93). In the same study, the authors also employed a preventive approach to test whether enhancing S1P signaling is protective. Surprisingly, transgenic mice overexpressing the S1P chaperone protein apolipoprotein M (ApoM Tg+ mice), which had increased circulating S1P, did not show protective effects after bleomycin-induced lung fibrosis (93), suggesting that adaptative mechanisms may emerge to shield the pulmonary endothelium from excessive S1P signaling.
Given the role of S1P in angiogenesis (94), it would be interesting to assess whether loss of endothelial S1P during the onset of lung fibrosis affects endothelial growth and overall lung capillary density. Future studies would be needed to further dissect this pathway and its regulatory mechanisms during vascular repair and fibrosis.
In line with this study, we also recently found that lung endothelial integrity is essential to maintain an efficient vascular barrier. Indeed, vascular permeability is markedly increased in mice lacking endothelial ERG compared with wild-type mice in the absence of injury. Cytofluorimetric and histological analyses of ERG-knockout mouse lungs, compared with wild-type lungs, demonstrated increased neutrophil infiltration, aberrant perivascular remodeling, and red blood cell extravasation as consequences of the vascular leakage observed in ERG-knockout mice (56). Similarly to the mice lacking endothelial S1PR1, mice lacking endothelial ERG manifested exacerbated lung fibrosis after exposure to bleomycin (56), further indicating that pulmonary endothelial dysfunction plays a role in sustaining pathogenic mechanisms of myofibroblast activation, thus preventing lung fibrosis resolution.
As a result of the increased vascular permeability, several plasma proteins, including fibrinogen, leak into the alveoli and accumulate over time if not properly removed, causing inflammation and tissue damage (Figure 3). Extravascular fibrinogen is rapidly converted into fibrin by thrombin to form a provisional matrix that facilitates tissue repair (95). When the injury has resolved, fibrin is degraded by plasmin, a plasminogen-derived enzyme that is proteolytically activated by two serine proteases, tissue plasminogen activator and urokinase. The activity of these two proteases is tightly controlled by specific inhibitors (96), including plasminogen activator inhibitor-1 (PAI-1) and α2-antiplasmin. Interestingly, lungs of patients with IPF exhibit increased levels of PAI-1 and α2-antiplasmin, suggesting that limited plasmin activity in these pathogenic lungs may lead to reduced fibrinolysis, fibrin accumulation in the alveolar space, and inflammation (97). In another study, Shea and colleagues demonstrated an alternative pathway through which increased vascular permeability may cause lung fibrosis. The authors used a mouse model of lung fibrosis induced by a combination of a low dose of bleomycin and a nonselective S1PR modulator as an endothelial-disrupting agent. In this work, the authors demonstrated that vascular leakage promotes the development of lung fibrosis through increased profibrotic thrombin/PAR1 (proteinase-activated receptor 1)/αvβ6/TGF-β axis (98). Intriguingly, this effect was abrogated by the thrombin inhibitor dabigatran, suggesting that the activation of this pathway, and the subsequent fibrosis, is dependent on this enzyme (98). Altogether, these studies indicate that pulmonary vascular leak creates profibrotic milieus that facilitate the progression of lung fibrosis and that limiting vascular leakage in IPF lungs may be resolutive for fibrosis.
Endothelial Transcriptional Alterations and Their Implications in the Fibrotic Lung
Cellular identity is critical to support and maintain organ-specific functions (99). Different organs have distinct cell repertoires to achieve specific tasks, which are maintained by epigenetic and transcriptional programs in different lung cell types (100). Alterations of these cell identity programs often lead to disrupted organ homeostasis and to the development of a broad range of human diseases (100). The vascular endothelium exhibits remarkable transcriptional plasticity to adapt to different tissue requirements under physiological and pathological conditions (101). For example, when exposed to stressful stimuli, ECs have been reported to undergo endothelial-to-mesenchymal transition (EndMT), a biological process in which ECs partially lose their endothelial lineage markers and acquire mesenchyme-like features (102). Although EndMT plays an important physiologic role during embryogenesis (103), it has also been implicated in the progression of numerous human diseases, including cancer, atherosclerosis, hypertension, and fibrosis (104–107).
Previous studies in mice, including our own, have shown that ECs acquire mesenchymal features, including collagen-I and fibronectin expression, during experimentally induced lung fibrosis (3, 108, 109). Studies in humans, including those reported in a recent paper by Gaikwad and colleagues, showed an increased expression of mesenchymal markers, including N-cadherin, A100A4, and vimentin, in ECs of vessels of IPF lungs (70, 110), suggesting that this phenomenon may have adverse consequences in human disease.
A recent study by Martin and colleagues explored the contribution of dysfunctional ECs to the overall collagen deposition in the fibrotic lung (19). By carrying out lineage tracing studies in combination with imaging analysis, the authors demonstrated that lung ECs only partially undergo EndMT and do not fully differentiate into mesenchymal cells or migrate into the interstitial space, further supporting the concept that ECs are not the major source of myofibroblasts during bleomycin-induced lung fibrosis and that other factors associated with endothelial dysfunction may contribute to aggravate fibrosis (19). Additional work showed that the endothelial transcription factor SREBP2 (sterol regulatory element–binding protein 2), a potent driver of EndMT and endothelial dysfunction in the lung, is implicated in the release of proinflammatory cytokines with profibrotic and proliferative effects on lung-resident fibroblasts (19) (Figure 3). In line with these observations, our recent data show that lung endothelial dysfunction and transcriptional alterations caused by a lack of the endothelial transcription factor ERG lead to the release of proinflammatory and profibrotic factors, including PAI-1, CTGF, and Collagen Iα1, enhancing lung fibroblast activation in vitro (56) (Figure 3). These findings are also shown in vivo using scRNA-seq in lungs of wild-type and endothelial ERG-knockout mice in the absence of injury. Intriguingly, lack of endothelial ERG alone led to increased expression of myofibroblast markers in lung fibroblasts from knockout mice and increased expression of fibrogenic markers in lung immune cells from knockout mice (56), reinforcing the concept that the pulmonary vasculature provides a tissue niche that is a crucial regulator of the phenotype and function of neighboring cells.
In a setting of persistent fibrosis, our group has also found that, after a single intratracheal dose of bleomycin, the lung endothelium of aged mice acquires a transcriptional signature resembling EndMT (3). Following injury, aged lung ECs, but not young lung ECs, exhibited increased expression of mesenchymal and inflammatory genes and reduced expression of endothelial-specific genes (3). Among the endothelial-specific genes, we found that the expression of Nos3, encoding for endothelial nitric oxide synthase 3, the enzyme that produces soluble nitric oxide, was upregulated in young lung ECs during the early resolution phase after bleomycin injury, but not in injured lung ECs from aged mice, in which Nos3 gene expression was repressed. These observations strongly suggest that endothelial nitric oxide plays an important role during lung fibrosis resolution. Global deletion of Nos3 in mice leads to lung endothelial dysfunction and unresolved lung fibrosis following a single dose of bleomycin (3), further supporting the concept that lung ECs can sense and respond to the surrounding pathogenic microenvironment by secreting mediators that can signal to activated fibroblasts to promote their quiescence (3).
Endothelial transcriptional aberrations are also observed in the lungs of patients with IPF. Indeed, a recent study using scRNA-seq on IPF and normal lungs revealed the expansion of an ectopic endothelial subpopulation in the distal lung parenchyma of patients with IPF that was transcriptionally indistinguishable from ECs restricted to the bronchial vasculature in normal lungs (referred as peribronchial vascular ECs) (111). This population is characterized by the expression of COL15A1, encoding the α-chain of type XV collagen, an extracellular matrix component primarily located in the basement membrane of blood vessels (112). Histological analyses revealed that, in IPF lungs, peribronchial vascular ECs are highly abundant in areas of bronchiolization and fibrosis and are never seen in the lung parenchyma within the normal lung. Additional studies conducted on healthy human lungs showed that pEVs were characterized by the expression of typical venous EC markers (25), revealing a new scenario whereby the peribronchial vein may acquire the ability to migrate and invade the alveolar space during IPF. In addition, the appearance of this ectopic EC population in the distal lung of patients with IPF is intriguing because it may be related to the previous observation by Turner-Warwick, who first described an expansion of the systemic vasculature throughout the lungs and the formation of anastomoses between the systemic and the pulmonary microvasculature of the distal lung in fibrotic lungs (51).
Although many questions remain regarding the mechanisms involved in transcriptional states associated with endothelial responses to lung injury, mounting evidence suggests that understanding how such changes relate to cellular activation is critical to the identification of endothelial cellular programs promoting regenerative healing versus fibrotic scarring to be harnessed as potential therapeutic targets to treat lung fibrosis.
Conclusions and Perspectives
Recent investigations have established the functional role of microvascular ECs in organ repair and regeneration (28–30). Studies focusing on the lung have highlighted the importance of the alveolar capillary vasculature in promoting repair and regeneration of the injured lung tissue through the release of paracrine factors active on neighboring cells (2, 27, 35, 79). The use of scRNA-seq technology has significantly expanded our knowledge of lung endothelial heterogeneity (18, 20, 24) and revealed the presence of specialized lung capillary ECs that perform distinct gas-exchange or homeostatic and regenerative functions. Future studies designed to identify lung endothelial signaling pathways that specifically promote capillary regeneration and restore lung homeostasis would be of great interest in the context of diseases with heterogeneous patterns such as IPF. Novel therapeutic strategies aimed at boosting EC function in lung areas that are not severely compromised by ECM deposition could represent an innovative tool to promote lung repair and halt fibrosis progression.
As reviewed here, evidence from mouse models of lung fibrosis and analysis of human fibrotic lungs have revealed the presence of multiple vascular alterations that may contribute to sustaining profibrotic stimuli in the fibrotic lung. These aberrations include limited vascular regeneration, capillary rarefaction, and disrupted alveolar–vascular barrier with intraalveolar leakage. The appearance of peribronchial veins expressing COL15A1 in the alveolar space of the distal lung raises questions regarding the contribution of ECs with a venous origin to disease progression. Although this observation resembles the known phenomenon of cellular proximalization of the distal lung, which has been previously described (111), whether this phenomenon could be attributed to transcriptional changes of the distal lung capillary ECs or the invasion of peribronchial ECs into the alveolar space represents an intriguing future avenue of investigation. Furthermore, the development of novel mouse models to lineage-trace different EC populations, including venous ECs, during the peak or the resolution of lung fibrosis is paramount to shed new light on the origin of, as well as the contribution of different EC subtypes to, lung scarring.
Studies conducted in mice highlighted an important role for the pulmonary vasculature in providing a niche orchestrating lung repair and fibrosis resolution after injury. Whereas healthy pulmonary ECs secrete paracrine factors that are able to restrain fibroblast activation, dysfunctional ECs secrete paracrine factors with proinflammatory and profibrotic activities influencing neighboring cells, suggesting that endothelial dysfunction alone may act as a predisposing factor for progressive lung fibrosis.
These studies aimed at elucidating the relationship between endothelial dysfunction and promotion of lung fibrosis may also contribute to our understanding of the mechanisms behind the development of lung fibrosis in subjects with previously severe coronavirus disease (COVID-19) due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (113, 114). Indeed, endothelial dysfunction, including EndMT (115, 116), coagulation abnormalities, and vasculopathy, are hallmarks of COVID-19 (117), and ongoing studies conducted in patients who recovered from COVID-19 predicted a high rate of patients in whom interstitial lung abnormalities will develop over time (114).
In conclusion, these studies shed new light on the role of pulmonary ECs in the pathogenesis and progression of lung fibrosis, shifting their role from a bystander to an active driver of fibrosis, and suggest that endothelial-targeted therapeutic strategies to limit or resolve vascular alterations of the fibrotic lung will be paramount to allow resolution of tissue injury or prevention of chronic and degenerative fibrosis.
Acknowledgments
Acknowledgment
The authors thank Dr. Roberto F. Nicosia, University of Washington (Seattle, WA), for his careful review of the manuscript and helpful suggestions.
Footnotes
Supported by National Heart, Lung, and Blood Institute grant HL142596 (G.L), National Institutes of Health grant HL158733 (G.L.), an American Lung Association Dalsemer Research Grant (N.C.), and a Boehringer Ingelheim IPF/ILD Discovery Award (N.C.).
Author Contributions: Both authors contributed to the conceptualization and writing of this review article, and read and approved the final draft.
Originally Published in Press as DOI: 10.1165/rcmb.2022-0431TR on May 1, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Wagner PD. The physiological basis of pulmonary gas exchange: implications for clinical interpretation of arterial blood gases. Eur Respir J . 2015;45:227–243. doi: 10.1183/09031936.00039214. [DOI] [PubMed] [Google Scholar]
- 2. Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell . 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Caporarello N, Meridew JA, Aravamudhan A, Jones DL, Austin SA, Pham TX, et al. Vascular dysfunction in aged mice contributes to persistent lung fibrosis. Aging Cell . 2020;19:e13196. doi: 10.1111/acel.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torrisi SE, Kahn N, Wälscher J, Polke M, Lee JS, Molyneaux PL, et al. Outcomes and incidence of PF-ILD according to different definitions in a real-world setting. Front Pharmacol . 2021;12:790204. doi: 10.3389/fphar.2021.790204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wijsenbeek M, Cottin V. Spectrum of fibrotic lung diseases. N Engl J Med . 2020;383:958–968. doi: 10.1056/NEJMra2005230. [DOI] [PubMed] [Google Scholar]
- 6. Mora AL, Rojas M, Pardo A, Selman M. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat Rev Drug Discov . 2017;16:810. doi: 10.1038/nrd.2017.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caporarello N, Meridew JA, Jones DL, Tan Q, Haak AJ, Choi KM, et al. PGC1α repression in IPF fibroblasts drives a pathologic metabolic, secretory and fibrogenic state. Thorax . 2019;74:749–760. doi: 10.1136/thoraxjnl-2019-213064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Haak AJ, Kostallari E, Sicard D, Ligresti G, Choi KM, Caporarello N, et al. Selective YAP/TAZ inhibition in fibroblasts via dopamine receptor D1 agonism reverses fibrosis. Sci Transl Med . 2019;11:eaau6296. doi: 10.1126/scitranslmed.aau6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ligresti G, Caporarello N, Meridew JA, Jones DL, Tan Q, Choi KM, et al. CBX5/G9a/H3K9me-mediated gene repression is essential to fibroblast activation during lung fibrosis. JCI Insight . 2019;5:e127111. doi: 10.1172/jci.insight.127111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Puglisi S, Torrisi SE, Vindigni V, Giuliano R, Palmucci S, Mulè M, et al. New perspectives on management of idiopathic pulmonary fibrosis. Ther Adv Chronic Dis . 2016;7:108–120. doi: 10.1177/2040622315624276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suresh K, Shimoda LA. Lung circulation. Compr Physiol . 2016;6:897–943. doi: 10.1002/cphy.c140049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kandathil A, Chamarthy M. Pulmonary vascular anatomy & anatomical variants. Cardiovasc Diagn Ther . 2018;8:201–207. doi: 10.21037/cdt.2018.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weibel ER. On the tricks alveolar epithelial cells play to make a good lung. Am J Respir Crit Care Med . 2015;191:504–513. doi: 10.1164/rccm.201409-1663OE. [DOI] [PubMed] [Google Scholar]
- 14. Jayadev R, Sherwood DR. Basement membranes. Curr Biol . 2017;27:R207–R211. doi: 10.1016/j.cub.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 15. Barkaway A, Rolas L, Joulia R, Bodkin J, Lenn T, Owen-Woods C, et al. Age-related changes in the local milieu of inflamed tissues cause aberrant neutrophil trafficking and subsequent remote organ damage. Immunity . 2021;54:1494–1510.e7. doi: 10.1016/j.immuni.2021.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Basil MC, Katzen J, Engler AE, Guo M, Herriges MJ, Kathiriya JJ, et al. The cellular and physiological basis for lung repair and regeneration: past, present, and future. Cell Stem Cell . 2020;26:482–502. doi: 10.1016/j.stem.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature . 2014;507:190–194. doi: 10.1038/nature12930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gillich A, Zhang F, Farmer CG, Travaglini KJ, Tan SY, Gu M, et al. Capillary cell-type specialization in the alveolus. Nature . 2020;586:785–789. doi: 10.1038/s41586-020-2822-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martin M, Zhang J, Miao Y, He M, Kang J, Huang HY, et al. Role of endothelial cells in pulmonary fibrosis via SREBP2 activation. JCI Insight . 2021;6:e125635. doi: 10.1172/jci.insight.125635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Niethamer TK, Stabler CT, Leach JP, Zepp JA, Morley MP, Babu A, et al. Defining the role of pulmonary endothelial cell heterogeneity in the response to acute lung injury. eLife . 2020;9:e53072. doi: 10.7554/eLife.53072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stevens T, Phan S, Frid MG, Alvarez D, Herzog E, Stenmark KR. Lung vascular cell heterogeneity: endothelium, smooth muscle, and fibroblasts. Proc Am Thorac Soc . 2008;5:783–791. doi: 10.1513/pats.200803-027HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res . 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 23. Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res . 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 24. Vila Ellis L, Cain MP, Hutchison V, Flodby P, Crandall ED, Borok Z, et al. Epithelial Vegfa specifies a distinct endothelial population in the mouse lung. Dev Cell . 2020;52:617–630.e6. doi: 10.1016/j.devcel.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schupp JC, Adams TS, Cosme C, Jr, Raredon MSB, Yuan Y, Omote N, et al. Integrated single-cell atlas of endothelial cells of the human lung. Circulation . 2021;144:286–302. doi: 10.1161/CIRCULATIONAHA.120.052318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer . 2010;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rafii S, Cao Z, Lis R, Siempos II, Chavez D, Shido K, et al. Platelet-derived SDF-1 primes the pulmonary capillary vascular niche to drive lung alveolar regeneration. Nat Cell Biol . 2015;17:123–136. doi: 10.1038/ncb3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Augustin HG, Koh GY. Organotypic vasculature: from descriptive heterogeneity to functional pathophysiology. Science . 2017;357:eaal2379. doi: 10.1126/science.aal2379. [DOI] [PubMed] [Google Scholar]
- 29. Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature . 2010;468:310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rafii S, Butler JM, Ding BS. Angiocrine functions of organ-specific endothelial cells. Nature . 2016;529:316–325. doi: 10.1038/nature17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell . 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 32. Tropea KA, Leder E, Aslam M, Lau AN, Raiser DM, Lee JH, et al. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol . 2012;302:L829–L837. doi: 10.1152/ajplung.00347.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee JH, Bhang DH, Beede A, Huang TL, Stripp BR, Bloch KD, et al. Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell . 2014;156:440–455. doi: 10.1016/j.cell.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu S, Cimprich J, Varisco BM. Mouse pneumonectomy model of compensatory lung growth. J Vis Exp . 2014;94:52294. doi: 10.3791/52294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ding BS, Gomi K, Rafii S, Crystal RG, Walters MS. Endothelial MMP14is required for endothelial-dependent growth support of human airway basal cells. J Cell Sci . 2015;128:2983–2988. doi: 10.1242/jcs.168179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chakarov S, Lim HY, Tan L, Lim SY, See P, Lum J, et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science . 2019;363:eaau0964. doi: 10.1126/science.aau0964. [DOI] [PubMed] [Google Scholar]
- 37. Sabatel C, Radermecker C, Fievez L, Paulissen G, Chakarov S, Fernandes C, et al. Exposure to bacterial CpG DNA protects from airway allergic inflammation by expanding regulatory lung interstitial macrophages. Immunity . 2017;46:457–473. doi: 10.1016/j.immuni.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 38. Kalucka J, Bierhansl L, Wielockx B, Carmeliet P, Eelen G. Interaction of endothelial cells with macrophages-linking molecular and metabolic signaling. Pflugers Arch . 2017;469:473–483. doi: 10.1007/s00424-017-1946-6. [DOI] [PubMed] [Google Scholar]
- 39. Saunders DC, Aamodt KI, Richardson TM, Hopkirk AJ, Aramandla R, Poffenberger G, et al. Coordinated interactions between endothelial cells and macrophages in the islet microenvironment promote β cell regeneration. NPJ Regen Med . 2021;6:22. doi: 10.1038/s41536-021-00129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou B, Magana L, Hong Z, Huang LS, Chakraborty S, Tsukasaki Y, et al. The angiocrine Rspondin3 instructs interstitial macrophage transition via metabolic-epigenetic reprogramming and resolves inflammatory injury. Nat Immunol . 2020;21:1430–1443. doi: 10.1038/s41590-020-0764-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mammoto A, Mammoto T. Vascular niche in lung alveolar development, homeostasis, and regeneration. Front Bioeng Biotechnol . 2019;7:318. doi: 10.3389/fbioe.2019.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mammoto T, Muyleart M, Mammoto A. Endothelial YAP1 in regenerative lung growth through the angiopoietin-Tie2 pathway. Am J Respir Cell Mol Biol . 2019;60:117–127. doi: 10.1165/rcmb.2018-0105OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Link PA, Choi KM, Diaz Espinosa AM, Jones DL, Gao AY, Haak AJ, et al. Combined control of the fibroblast contractile program by YAP and TAZ. Am J Physiol Lung Cell Mol Physiol . 2022;322:L23–L32. doi: 10.1152/ajplung.00210.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu F, Lagares D, Choi KM, Stopfer L, Marinković A, Vrbanac V, et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol . 2015;308:L344–L357. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jones MG, Fabre A, Schneider P, Cinetto F, Sgalla G, Mavrogordato M, et al. Three-dimensional characterization of fibroblast foci in idiopathic pulmonary fibrosis. JCI Insight . 2016;1:e86375. doi: 10.1172/jci.insight.86375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barratt S, Millar A. Vascular remodelling in the pathogenesis of idiopathic pulmonary fibrosis. QJM . 2014;107:515–519. doi: 10.1093/qjmed/hcu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barratt SL, Flower VA, Pauling JD, Millar AB. VEGF (vascular endothelial growth factor) and fibrotic lung disease. Int J Mol Sci . 2018;19:1269. doi: 10.3390/ijms19051269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mlika M, Bacha S, Braham E, El Mezni F. The inter-connection between fibrosis and microvascular remodeling in idiopathic pulmonary fibrosis: reality or just a phenomenon. Respir Med Case Rep . 2015;17:30–33. doi: 10.1016/j.rmcr.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Renzoni EA. Neovascularization in idiopathic pulmonary fibrosis: too much or too little? Am J Respir Crit Care Med . 2004;169:1179–1180. doi: 10.1164/rccm.2403006. [DOI] [PubMed] [Google Scholar]
- 50. Renzoni EA, Walsh DA, Salmon M, Wells AU, Sestini P, Nicholson AG, et al. Interstitial vascularity in fibrosing alveolitis. Am J Respir Crit Care Med . 2003;167:438–443. doi: 10.1164/rccm.200202-135OC. [DOI] [PubMed] [Google Scholar]
- 51. Turner-Warwick M. Precapillary systemic-pulmonary anastomoses. Thorax . 1963;18:225–237. doi: 10.1136/thx.18.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ebina M, Shimizukawa M, Shibata N, Kimura Y, Suzuki T, Endo M, et al. Heterogeneous increase in CD34-positive alveolar capillaries in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2004;169:1203–1208. doi: 10.1164/rccm.200308-1111OC. [DOI] [PubMed] [Google Scholar]
- 53. Gracey DR, Divertie MB, Brown AL., Jr Alveolar-capillary membrane in idiopathic interstitial pulmonary fibrosis. Electron microscopic study of 14 cases. Am Rev Respir Dis . 1968;98:16–21. doi: 10.1164/arrd.1968.98.1.16. [DOI] [PubMed] [Google Scholar]
- 54. Jacob J, Bartholmai BJ, Rajagopalan S, Kokosi M, Nair A, Karwoski R, et al. Mortality prediction in idiopathic pulmonary fibrosis: evaluation of computer-based CT analysis with conventional severity measures. Eur Respir J . 2017;49:1601011. doi: 10.1183/13993003.01011-2016. [DOI] [PubMed] [Google Scholar]
- 55. Synn AJ, Li W, Hunninghake GM, Washko GR, San José Estépar R, O’Connor GT, et al. Vascular pruning on CT and interstitial lung abnormalities in the Framingham Heart Study. Chest . 2021;159:663–672. doi: 10.1016/j.chest.2020.07.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Caporarello N, Lee J, Pham TX, Jones DL, Guan J, Link PA, et al. Dysfunctional ERG signaling drives pulmonary vascular aging and persistent fibrosis. Nat Commun . 2022;13:4170. doi: 10.1038/s41467-022-31890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol . 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 58. Ando M, Miyazaki E, Ito T, Hiroshige S, Nureki SI, Ueno T, et al. Significance of serum vascular endothelial growth factor level in patients with idiopathic pulmonary fibrosis. Lung . 2010;188:247–252. doi: 10.1007/s00408-009-9223-x. [DOI] [PubMed] [Google Scholar]
- 59. Murray LA, Habiel DM, Hohmann M, Camelo A, Shang H, Zhou Y, et al. Antifibrotic role of vascular endothelial growth factor in pulmonary fibrosis. JCI Insight . 2017;2:e92192. doi: 10.1172/jci.insight.92192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cosgrove GP, Brown KK, Schiemann WP, Serls AE, Parr JE, Geraci MW, et al. Pigment epithelium-derived factor in idiopathic pulmonary fibrosis: a role in aberrant angiogenesis. Am J Respir Crit Care Med . 2004;170:242–251. doi: 10.1164/rccm.200308-1151OC. [DOI] [PubMed] [Google Scholar]
- 61. Koyama S, Sato E, Haniuda M, Numanami H, Nagai S, Izumi T. Decreased level of vascular endothelial growth factor in bronchoalveolar lavage fluid of normal smokers and patients with pulmonary fibrosis. Am J Respir Crit Care Med . 2002;166:382–385. doi: 10.1164/rccm.2103112. [DOI] [PubMed] [Google Scholar]
- 62. Meyer KC, Cardoni A, Xiang ZZ. Vascular endothelial growth factor in bronchoalveolar lavage from normal subjects and patients with diffuse parenchymal lung disease. J Lab Clin Med . 2000;135:332–338. doi: 10.1067/mlc.2000.105618. [DOI] [PubMed] [Google Scholar]
- 63. Kolb M, Richeldi L, Behr J, Maher TM, Tang W, Stowasser S, et al. Nintedanib in patients with idiopathic pulmonary fibrosis and preserved lung volume. Thorax . 2017;72:340–346. doi: 10.1136/thoraxjnl-2016-208710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wollin L, Wex E, Pautsch A, Schnapp G, Hostettler KE, Stowasser S, et al. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J . 2015;45:1434–1445. doi: 10.1183/09031936.00174914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Akwii RG, Sajib MS, Zahra FT, Mikelis CM. Role of angiopoietin-2 in vascular physiology and pathophysiology. Cells . 2019;8:471. doi: 10.3390/cells8050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Margaritopoulos GA, Antoniou KM, Karagiannis K, Vassalou E, Lasithiotaki I, Lambiri I, et al. Investigation of angiogenetic axis Angiopoietin-1 and -2/Tie-2 in fibrotic lung diseases: a bronchoalveolar lavage study. Int J Mol Med . 2010;26:919–923. doi: 10.3892/ijmm_00000543. [DOI] [PubMed] [Google Scholar]
- 67. Uehara M, Enomoto N, Mikamo M, Oyama Y, Kono M, Fujisawa T, et al. Impact of angiopoietin-1 and -2 on clinical course of idiopathic pulmonary fibrosis. Respir Med . 2016;114:18–26. doi: 10.1016/j.rmed.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 68. Nathan SD, Noble PW, Tuder RM. Idiopathic pulmonary fibrosis and pulmonary hypertension: connecting the dots. Am J Respir Crit Care Med . 2007;175:875–880. doi: 10.1164/rccm.200608-1153CC. [DOI] [PubMed] [Google Scholar]
- 69. Lettieri CJ, Nathan SD, Browning RF, Barnett SD, Ahmad S, Shorr AF. The distance-saturation product predicts mortality in idiopathic pulmonary fibrosis. Respir Med . 2006;100:1734–1741. doi: 10.1016/j.rmed.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 70. Gaikwad AV, Eapen MS, McAlinden KD, Chia C, Larby J, Myers S, et al. Endothelial to mesenchymal transition (EndMT) and vascular remodeling in pulmonary hypertension and idiopathic pulmonary fibrosis. Expert Rev Respir Med . 2020;14:1027–1043. doi: 10.1080/17476348.2020.1795832. [DOI] [PubMed] [Google Scholar]
- 71. Yanagihara T, Tsubouchi K, Zhou Q, Chong M, Otsubo K, Isshiki T, et al. Vascular-parenchymal crosstalk promotes lung fibrosis through BMPR2 signaling. Am J Respir Crit Care Med . 2023 doi: 10.1164/rccm.202109-2174OC. [DOI] [PubMed] [Google Scholar]
- 72. Raghu G, Amatto VC, Behr J, Stowasser S. Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review. Eur Respir J . 2015;46:1113–1130. doi: 10.1183/13993003.02316-2014. [DOI] [PubMed] [Google Scholar]
- 73. Bhattarai P, Lu W, Gaikwad AV, Dey S, Chia C, Larby J, et al. Arterial remodelling in smokers and in patients with small airway disease and COPD: implications for lung physiology and early origins of pulmonary hypertension. ERJ Open Res . 2022;8:00254-2022. doi: 10.1183/23120541.00254-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Iyer AK, Ramesh V, Castro CA, Kaushik V, Kulkarni YM, Wright CA, et al. Nitric oxide mediates bleomycin-induced angiogenesis and pulmonary fibrosis via regulation of VEGF. J Cell Biochem . 2015;116:2484–2493. doi: 10.1002/jcb.25192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ou XM, Li WC, Liu DS, Li YP, Wen FQ, Feng YL, et al. VEGFR-2 antagonist SU5416 attenuates bleomycin-induced pulmonary fibrosis in mice. Int Immunopharmacol . 2009;9:70–79. doi: 10.1016/j.intimp.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 76. Stockmann C, Kerdiles Y, Nomaksteinsky M, Weidemann A, Takeda N, Doedens A, et al. Loss of myeloid cell-derived vascular endothelial growth factor accelerates fibrosis. Proc Natl Acad Sci USA . 2010;107:4329–4334. doi: 10.1073/pnas.0912766107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Benelli R, Lorusso G, Albini A, Noonan DM. Cytokines and chemokines as regulators of angiogenesis in health and disease. Curr Pharm Des . 2006;12:3101–3115. doi: 10.2174/138161206777947461. [DOI] [PubMed] [Google Scholar]
- 78. Sainson RC, Johnston DA, Chu HC, Holderfield MT, Nakatsu MN, Crampton SP, et al. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood . 2008;111:4997–5007. doi: 10.1182/blood-2007-08-108597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cao Z, Lis R, Ginsberg M, Chavez D, Shido K, Rabbany SY, et al. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat Med . 2016;22:154–162. doi: 10.1038/nm.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hecker L, Logsdon NJ, Kurundkar D, Kurundkar A, Bernard K, Hock T, et al. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med . 2014;6:231ra47. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Podolsky MJ, Yang CD, Valenzuela CL, Datta R, Huang SK, Nishimura SL, et al. Age-dependent regulation of cell-mediated collagen turnover. JCI Insight . 2020;5:e137519. doi: 10.1172/jci.insight.137519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Redente EF, Black BP, Backos DS, Bahadur AN, Humphries SM, Lynch DA, et al. Persistent, progressive pulmonary fibrosis and epithelial remodeling in mice. Am J Respir Cell Mol Biol . 2021;64:669–676. doi: 10.1165/rcmb.2020-0542MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol . 2010;72:463–493. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- 84. Bruni C, Frech T, Manetti M, Rossi FW, Furst DE, De Paulis A, et al. Vascular leaking, a pivotal and early pathogenetic event in systemic sclerosis: should the door be closed? Front Immunol . 2018;9:2045. doi: 10.3389/fimmu.2018.02045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Rizzo AN, Sammani S, Esquinca AE, Jacobson JR, Garcia JG, Letsiou E, et al. Imatinib attenuates inflammation and vascular leak in a clinically relevant two-hit model of acute lung injury. Am J Physiol Lung Cell Mol Physiol . 2015;309:L1294–L1304. doi: 10.1152/ajplung.00031.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mogulkoc N, Brutsche MH, Bishop PW, Murby B, Greaves MS, Horrocks AW, et al. Greater Manchester Pulmonary Fibrosis Consortium Pulmonary (99m)Tc-DTPA aerosol clearance and survival in usual interstitial pneumonia (UIP) Thorax . 2001;56:916–923. doi: 10.1136/thorax.56.12.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wells AU, Hansell DM, Harrison NK, Lawrence R, Black CM, du Bois RM. Clearance of inhaled 99mTc-DTPA predicts the clinical course of fibrosing alveolitis. Eur Respir J . 1993;6:797–802. [PubMed] [Google Scholar]
- 88. McKeown S, Richter AG, O’Kane C, McAuley DF, Thickett DR. MMP expression and abnormal lung permeability are important determinants of outcome in IPF. Eur Respir J . 2009;33:77–84. doi: 10.1183/09031936.00060708. [DOI] [PubMed] [Google Scholar]
- 89. Montesi SB, Rao R, Liang LL, Goulart HE, Sharma A, Digumarthy SR, et al. Gadofosveset-enhanced lung magnetic resonance imaging to detect ongoing vascular leak in pulmonary fibrosis. Eur Respir J . 2018;51:1800171. doi: 10.1183/13993003.00171-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Knipe RS, Probst CK, Lagares D, Franklin A, Spinney JJ, Brazee PL, et al. The Rho kinase isoforms ROCK1 and ROCK2 each contribute to the development of experimental pulmonary fibrosis. Am J Respir Cell Mol Biol . 2018;58:471–481. doi: 10.1165/rcmb.2017-0075OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shimizu Y, Dobashi K, Iizuka K, Horie T, Suzuki K, Tukagoshi H, et al. Contribution of small GTPase Rho and its target protein rock in a murine model of lung fibrosis. Am J Respir Crit Care Med . 2001;163:210–217. doi: 10.1164/ajrccm.163.1.2001089. [DOI] [PubMed] [Google Scholar]
- 92. Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, et al. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest . 2013;123:1096–1108. doi: 10.1172/JCI66700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Knipe RS, Spinney JJ, Abe EA, Probst CK, Franklin A, Logue A, et al. Endothelial-specific loss of sphingosine-1-phosphate receptor 1 increases vascular permeability and exacerbates bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol . 2022;66:38–52. doi: 10.1165/rcmb.2020-0408OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Takuwa Y, Du W, Qi X, Okamoto Y, Takuwa N, Yoshioka K. Roles of sphingosine-1-phosphate signaling in angiogenesis. World J Biol Chem . 2010;1:298–306. doi: 10.4331/wjbc.v1.i10.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Probst CK, Montesi SB, Medoff BD, Shea BS, Knipe RS. Vascular permeability in the fibrotic lung. Eur Respir J . 2020;56:1900100. doi: 10.1183/13993003.00100-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Law RH, Zhang Q, McGowan S, Buckle AM, Silverman GA, Wong W, et al. An overview of the serpin superfamily. Genome Biol . 2006;7:216. doi: 10.1186/gb-2006-7-5-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Liu RM. Oxidative stress, plasminogen activator inhibitor 1, and lung fibrosis. Antioxid Redox Signal . 2008;10:303–319. doi: 10.1089/ars.2007.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shea BS, Probst CK, Brazee PL, Rotile NJ, Blasi F, Weinreb PH, et al. Uncoupling of the profibrotic and hemostatic effects of thrombin in lung fibrosis. JCI Insight . 2017;2:e86608. doi: 10.1172/jci.insight.86608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Fisher AG. Cellular identity and lineage choice. Nat Rev Immunol . 2002;2:977–982. doi: 10.1038/nri958. [DOI] [PubMed] [Google Scholar]
- 100. Eccleston A, Cesari F, Skipper M. Transcription and epigenetics. Nature . 2013;502:461. doi: 10.1038/502461a. [DOI] [PubMed] [Google Scholar]
- 101. Jambusaria A, Hong Z, Zhang L, Srivastava S, Jana A, Toth PT, et al. Endothelial heterogeneity across distinct vascular beds during homeostasis and inflammation. eLife . 2020;9:e51413. doi: 10.7554/eLife.51413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Dejana E, Hirschi KK, Simons M. The molecular basis of endothelial cell plasticity. Nat Commun . 2017;8:14361. doi: 10.1038/ncomms14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lin F, Wang N, Zhang TC. The role of endothelial-mesenchymal transition in development and pathological process. IUBMB Life . 2012;64:717–723. doi: 10.1002/iub.1059. [DOI] [PubMed] [Google Scholar]
- 104. Dufton NP, Peghaire CR, Osuna-Almagro L, Raimondi C, Kalna V, Chauhan A, et al. Dynamic regulation of canonical TGFβ signalling by endothelial transcription factor ERG protects from liver fibrogenesis. Nat Commun . 2017;8:895. doi: 10.1038/s41467-017-01169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lovisa S, Fletcher-Sananikone E, Sugimoto H, Hensel J, Lahiri S, Hertig A, et al. Endothelial-to-mesenchymal transition compromises vascular integrity to induce Myc-mediated metabolic reprogramming in kidney fibrosis. Sci Signal . 2020;13:eaaz2597. doi: 10.1126/scisignal.aaz2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rieder F, Kessler SP, West GA, Bhilocha S, de la Motte C, Sadler TM, et al. Inflammation-induced endothelial-to-mesenchymal transition: a novel mechanism of intestinal fibrosis. Am J Pathol . 2011;179:2660–2673. doi: 10.1016/j.ajpath.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med . 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 108. Hashimoto N, Phan SH, Imaizumi K, Matsuo M, Nakashima H, Kawabe T, et al. Endothelial-mesenchymal transition in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol . 2010;43:161–172. doi: 10.1165/rcmb.2009-0031OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jia W, Wang Z, Gao C, Wu J, Wu Q. Trajectory modeling of endothelial-to-mesenchymal transition reveals galectin-3 as a mediator in pulmonary fibrosis. Cell Death Dis . 2021;12:327. doi: 10.1038/s41419-021-03603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Gaikwad AV, Lu W, Dey S, Bhattarai P, Chia C, Larby J, et al. Vascular remodelling in idiopathic pulmonary fibrosis patients and its detrimental effect on lung physiology: potential role of endothelial-to-mesenchymal transition. ERJ Open Res . 2022;8:00571-2021. doi: 10.1183/23120541.00571-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Adams TS., et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv . 2020;6:eaba1983. doi: 10.1126/sciadv.aba1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bretaud S, Guillon E, Karppinen SM, Pihlajaniemi T, Ruggiero F. Collagen XV, a multifaceted multiplexin present across tissues and species. Matrix Biol Plus . 2020;6-7:100023. doi: 10.1016/j.mbplus.2020.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. George PM, Wells AU, Jenkins RG. Pulmonary fibrosis and COVID-19: the potential role for antifibrotic therapy. Lancet Respir Med . 2020;8:807–815. doi: 10.1016/S2213-2600(20)30225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Mo X, Jian W, Su Z, Chen M, Peng H, Peng P, et al. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur Respir J . 2020;55:2001217. doi: 10.1183/13993003.01217-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Eapen MS, Lu W, Gaikwad AV, Bhattarai P, Chia C, Hardikar A, et al. Endothelial to mesenchymal transition: a precursor to post-COVID-19 interstitial pulmonary fibrosis and vascular obliteration? Eur Respir J . 2020;56:2003167. doi: 10.1183/13993003.03167-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhang L, Tang C, Zhang M, Tong X, Xie Y, Yan R, et al. Single cell meta-analysis of EndMT and EMT state in COVID-19. Front Immunol . 2022;13:976512. doi: 10.3389/fimmu.2022.976512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Nicosia RF, Ligresti G, Caporarello N, Akilesh S, Ribatti D. COVID-19 vasculopathy: mounting evidence for an indirect mechanism of endothelial injury. Am J Pathol . 2021;191:1374–1384. doi: 10.1016/j.ajpath.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]