Abstract
Background:
In patients with advanced hepatocellular carcinoma (HCC) progressing after atezolizumab and bevacizumab, the optimal therapeutic sequence is still unclear and no second-line agent has proven its efficacy.
Objectives:
The aim of this retrospective multicenter real-world cohort study was to provide an evaluation of the efficacy and safety of the use of second-line tyrosine kinase inhibitors (TKIs) in this population.
Methods:
All patients with advanced HCC, treated in first-line setting by atezolizumab–bevacizumab, and who received at least one dose of treatment with TKI were included in this study. All the data were retrospectively collected from medical records. The primary outcome was progression-free survival (PFS). Secondary outcomes were overall survival (OS), overall global survival (OGS), and safety. A total of 82 patients were included in this study.
Results:
Patients were assigned to the regorafenib group (n = 29, 35.4%) or other TKI (sorafenib n = 41, lenvatinib n = 8, or cabozantinib n = 4) group (n = 53). PFS was not significantly different between the two groups [2.6 versus 2.8 months, HR 1.07 (95% CI: 0.61–1.86), p = 0.818]. Median PFS rates were 2.6, 4.4, and 2.8 months in sorafenib-, lenvatinib-, and cabozantinib group, respectively. OS was statistically different between the regorafenib group and other TKI group [15.8 versus 7.0 months, HR 0.40 (95% CI: 0.20–0.79), p = 0.023]. When adjusting on confounding factors, there was still a difference in OS favoring the regorafenib group (adjusted hazard ratio 0.35, p = 0.019). OGS of patients who received regorafenib was improved compared to other TKI [18.6 versus 15.0 months, HR 0.42 (95% CI: 0.22–0.84), p = 0.036]. Twenty percent of patients had grade 3 and none had grade 4 or 5 adverse events. In patients who experienced disease progression and fit for a third-line treatment, 80% and 50% received cabozantinib in regorafenib group and other TKI group, respectively.
Conclusion:
Efficacy of any TKI in the second-line setting was not affected by atezolizumab–bevacizumab treatment as first-line therapy. The safety profile in the second-line setting was consistent with the results shown in pivotal studies. PFS rates of patients were similar, regardless of TKI type. Regorafenib was associated with better OS and OGS rates compared to other TKI. These data need to be confirmed in prospective comparative studies.
Keywords: advanced hepatocellular carcinoma, HCC, regorafenib, sorafenib, tyrosine kinase inhibitors
Introduction
Hepatocellular carcinoma (HCC) is the most common type of liver tumor and the fourth leading cause of cancer mortality and liver transplantation worldwide. In 2018, its incidence increased to reach 854,000 new cases and about 810,000 deaths. 1 Most of the cases occur in patients with liver cirrhosis.
Both prognosis and the therapeutic approach depend on the tumor stage and the underlying liver function which can be difficult to evaluate reliably. The liver function should be estimated beyond Child-Pugh score using Model for End-Stage Liver Disease in decompensated cirrhosis, or alpha-fetoprotein concentration and albumin–bilirubin score in compensated liver disease. 2 Performance status and tumor burden defined as extrahepatic spread and/or vascular invasion were previously identified as independent predictor factors of survival. 3 Patients with HCC are mainly fragile due to comorbidities and multinodular lesions [Barcelona Clinic Liver Cancer (BCLC) B or C]. 4 At least half of the patients are diagnosed at an advanced stage and are not eligible for liver-directed therapies such as surgical resection, radiofrequency ablation, liver transplantation, transarterial chemoembolization, or radioembolization. In this situation, since 2020, the validated first-line therapy is the association of atezolizumab [anti-programmed death-ligand 1 (PD-L1)] and bevacizumab [anti-vascular endothelial growth factor (VEGF)]. 5
The doublet therapy was found superior to sorafenib, an oral tyrosine kinase inhibitor (TKI) in the first-line setting in terms of overall survival (OS) [OS at 12 months: 67.2% (95% CI: 61.3–73.1) with atezolizumab–bevacizumab and 54.6% (95% CI: 45.2–64.0) with sorafenib]. 5 In the past decade, regorafenib and cabozantinib have proven their efficacy in the second-line setting after sorafenib in advanced HCC.6,7 Lenvatinib, another TKI, was approved as a noninferior first-line option compared to sorafenib. 8
So far, no therapeutic agent has proven its efficacy in patients with advanced HCC after disease progression with atezolizumab–bevacizumab. Recently, a retrospective Korean study suggested a preserved efficacy of TKI, mainly lenvatinib and sorafenib, in the second-line setting with a median OS (mOS) of 14.7 months. 9 However, no patient received regorafenib, and only one patient received cabozantinib. As the optimal therapeutic sequence is still unclear in second line, we conducted a retrospective multicenter real-life cohort study to evaluate efficacy and safety of TKI for patients with advanced HCC progressing after atezolizumab–bevacizumab treatment.
Materials and methods
Patients
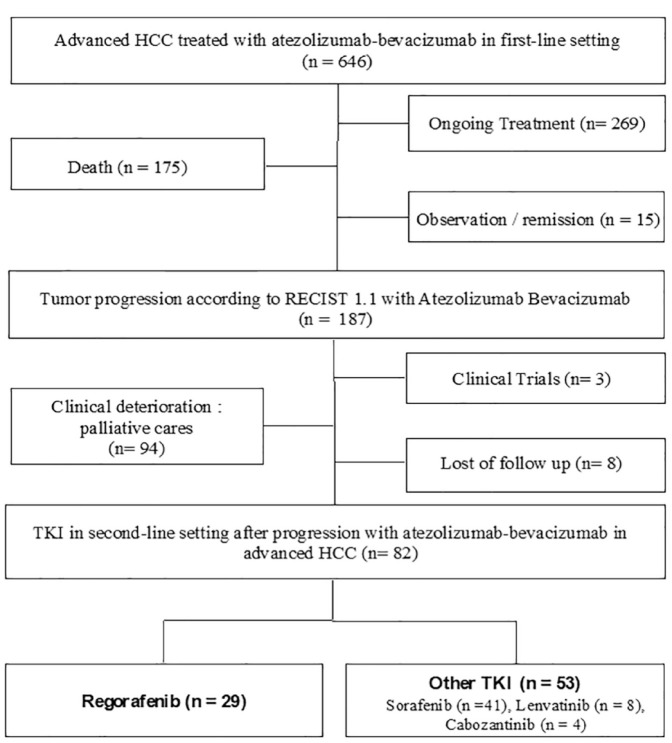
All patients treated in 11 French centers for an HCC and who received a doublet chemotherapy with atezolizumab–bevacizumab were screened (Figure 1). Patients who did not experience disease progression with atezolizumab–bevacizumab were excluded from the present study. Inclusion criteria were patients with advanced HCC, treated in the first-line setting by atezolizumab–bevacizumab, and who received at least 1 day of treatment with a TKI. All TKI were registered: regorafenib, sorafenib, lenvatinib, and cabozantinib. Advanced HCC was defined according to the 2022 updated BCLC staging system, as BCLC B or C stages corresponding to diffuse, infiltrative, bilobar liver involvement or vascular invasion and/or extrahepatic spread and a preserved liver function. Clinical data, biological and radiological features regarding patient characteristics, treatment history, tumor response, adverse events, and survival outcomes were obtained by reviewing electronic medical records.
Figure 1.
Flow chart of patients: enrollment and outcomes.
HCC, hepatocellular carcinoma; TKI, tyrosine kinase inhibitor.
Treatment schedule and toxicity assessment
All patients received at least one perfusion of atezolizumab–bevacizumab between 30th April 2020 and 1st June 2022, regardless of previous liver-directed therapy or systemic therapy, given off-trial in the daily practice setting. After tumor progression according to Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) or drug discontinuation because of adverse events (grade 3 or 4), all included patients received TKI (sorafenib, regorafenib, cabozantinib, or lenvatinib) in the second-line setting.
Regorafenib was given at the initial dose of 160 mg/day during weeks 1–3 of each 4-week cycle. Sorafenib was given at the dose of 400 mg twice daily, lenvatinib 8 or 12 mg/day according to patient’s weight (<60 or >60 kg), and cabozantinib 60 mg/day.
Dose reduction or interruption was on discretion of clinicians and local practices based on the RCP (Summary of Product Characteristics) of molecules. Body weight, performance status, and toxicities were recorded at each visit. Toxicity grading was based on the National Cancer Institute Common Toxicity Criteria (version 3.0). If non-neurological grade 3–4 toxicity occurred, the subsequent cycle was administered only after recovery and the treatment dose was adjusted based on the French national guidelines. In the event of persistent (14 days or longer) grade 3–4 toxicity, TKI was omitted.
Evaluation
Patients were evaluated by medical examination every 4–6 weeks during TKI treatment and tumor response was assessed every 8–12 weeks by multiphase tomography scan and/or MRI, or whenever there was a suspicion of disease progression. Tumor response was assessed according to RECIST v1.1.
Statistical analysis
The primary outcome of the study was progression-free survival (PFS) in patients with advanced HCC treated by TKI in second-line setting. PFS was measured from the first day of TKI intake until the date of the radiological disease progression according to RECIST v1.1 10 or death. Patients were censored to date to the last visit in case of lack of disease progression.
Secondary outcomes included OS, overall global survival (OGS), and safety. OS was measured from the first day of TKI intake until death whatever the cause. OGS was measured from the first perfusion of atezolizumab–bevacizumab until death or date of last news.
Safety was assessed in all patients receiving at least one dose of TKI, with the use of version 5.0 of the National Cancer Institute Common Terminology Criteria for adverse events. Descriptive statistics [median, ranges, 95% confidence intervals (95% CIs)] were used to report patient baseline characteristics and treatment-induced adverse events. Comparisons between TKI were done using Fisher’s exact test and the χ2 test with Yates correction. Continuous variables were expressed as medians (interquartile range 25–75) and compared using the Mann–Whitney test. Survival analyses were performed using the Kaplan–Meier method with the log-rank test. To evaluate the association of predefined characteristics (treatment group, sex, performans status, child, and extrahepatic spread) with OS and adjust on potential confounding factors, we estimated the adjusted hazard ratios (aHRs) in multivariable analysis using the Cox proportional hazard model. We tested the proportional hazard assumption for each variable in our model using the cox.zph function on R and plotting the scaled Schoenfeld residuals against time. All predefined variables were included in the final analysis. All p values were two-sided, and point estimates were presented with 95% CIs. The significance level was set at p = 0.05 for all analyses.
Calculations were done with NCSSC 2007 software (NCSS, Kaysville, UT, USA) and R software (R Version 4.0.2).
Results
Patients
A total of 646 patients in 11 centers received at least one perfusion or more of atezolizumab–bevacizumab for an advanced HCC before 1st June 2022. Among those patients, 269 were still under combination therapy, 15 had stable disease and took a break from therapy or underwent remission, and 175 died during first-line treatment. In all, 187 patients discontinued the first-line therapy due to radiologic progression according to RECIST v1.1 criteria. In all, 82 patients (44%) received TKI in second-line setting and were included in the present study (Figure 1). Table 1 summarized the baseline characteristics of patients. All patients received a TKI between September 2020 and October 2022: regorafenib (35.3%) or another TKI including sorafenib (50%), cabozantinib (4.9%), or lenvatinib (9.8%) after atezolizumab–bevacizumab failure or intolerance. Patients were assigned to regorafenib group (n = 29) or other TKI group (sorafenib, lenvatinib, or cabozantinib) (n = 53). Median patient age was 63 (range 22–88) and 62 (range 22–88) in regorafenib and other TKI groups, respectively. 92.7% of patients had BCLC C, 93.1% and 77.4% of patients had Child-Pugh A liver function in regorafenib group and other TKI group, respectively (p = 0.122). First etiology of cirrhosis was hepatitis B or C virus in both groups (regorafenib: 41.4%; other TKI: 30.2%). The median duration of atezolizumab–bevacizumab was 3.5 months (range 0.1–20.9) and 3.3 months (range 0.1–20.9) in regorafenib group and other TKI group, respectively. The median dose of regorafenib was 160 mg/day during weeks 1–3 of each 4-week cycle. The median dose of sorafenib, cabozantinib, and lenvatinib was 800 mg/day (400 mg twice daily), 8 mg/day, and 60 mg/day respectively. Patients in the regorafenib group presented more peritoneal metastasis than those in other TKI group (p = 0.029). There was no other difference between the two groups.
Table 1.
Patients’ characteristics at baseline.
| Patients’ characteristics | Total (n = 82) | Regorafenib group (n = 29) | Other TKI group (n = 53) | |||
|---|---|---|---|---|---|---|
| Total (n = 53) | Sorafenib (n = 41) | Lenvatinib (n = 8) | Cabozantinib (n = 4) | |||
| Median age, years (range) | 62 (22–88) | 63 | 62 | 62 | 65 | 46 |
| Sex | ||||||
| Male (%) | 65 (79.3) | 23 (79.3) | 42 (79.2) | 32 (78.1) | 7 (87.5) | 3 (75.0) |
| Female (%) | 17 (20.7) | 6 (20.7) | 11 (20.8) | 9 (21.9) | 1 (12.5) | 1 (25.0) |
| Median BMI, kg/m2 (range) | 23 (14.2–52.7) | 26 | 22.5 | 22.5 | 23.5 | 27.5 |
| ECOG PS | ||||||
| 0 (%) | 6 (7.3) | 1 (3.4) | 5 (9.4) | 5 (12.2) | 0 (0) | 0 (0) |
| 1 (%) | 64 (78.1) | 26 (89.7) | 38 (71.7) | 30 (73.2) | 5 (62.5) | 3 (75.0) |
| 2 (%) | 12 (14.6) | 2 (6.9) | 10 (18.9) | 6 (14.6) | 3 (37.5) | 1 (25.0) |
| Cirrhosis (%) | 62 (75.6) | 22 (75.9) | 40 (75.5) | 30 (73.2) | 7 (87.5) | 3 (75.0) |
| Child-Pugh score in cirrhotic patients (%) | ||||||
| A | 68 (82.9) | 27 (93.1) | 41 (77.4) | 34 (82.9) | 3 (37.5) | 4 (100) |
| B | 14 (17.1) | 2 (6.9) | 12 (22.6) | 7 (17.1) | 5 (62.5) | 0 (0) |
| BCLC stage B | 6 (7.3) | 2 (6.9) | 4 (7.6) | 2 (4.9) | 2 (25.0) | 0 (0) |
| BCLC stage C | 76 (92.7) | 27 (93.1) | 49 (92.4) | 39 (95.1) | 6 (75.0) | 4 (100) |
| Etiology of HCC | ||||||
| Ethylic only | 10 (12.2) | 3 (10.3) | 7 (13.2) | 4 (9.8) | 3 (37.5) | 0 |
| Hepatitis B only | 21 (25.7) | 9 (31.1) | 12 (22.6) | 10 (24.3) | 0 (0) | 2 (50.0) |
| Hepatitis C only | 6 (7.3) | 3 (10.3) | 3 (5.7) | 2 (4.9) | 1 (12.5) | 0 (0) |
| Coinfection hepatitis B and C | 1 (1.2) | 0 (0) | 1 (1.9) | 1 (2.4) | 0 (0) | 0 (0) |
| NASH only | 12 (14.6) | 3 (10.3) | 9 (17.0) | 8 (19.5) | 1 (12.5) | 0 (0) |
| NASH and ethylic | 12 (14.6) | 5 (17.3) | 7 (13.2) | 4 (9.8) | 2 (25.0) | 1 (25.0) |
| Others | 19 (23.2) | 6 (20.7) | 13 (24.5) | 12 (29.3) | 1 (12.5) | 0 (0) |
| Unknown | 1 (1.2) | 0 (0) | 1 (1.9) | 0 (0) | 0 (0) | 1 (25.0) |
| Macroscopic vascular invasion | 31 (37.8) | 8 (27.6) | 23 (43.4) | 20 (48.8) | 2 (25.0) | 1 (25.0) |
| Extrahepatic spread | ||||||
| No | 16 (19.5) | 4 (13.8) | 12 (22.6) | 7 (17.1) | 4 (50.0) | 1 (25.0) |
| Yes | 66 (80.5) | 25 (86.2) | 41 (77.4) | 34 (82.9) | 4 (50.0) | 3 (75.0) |
| Lung | 35 (42.7) | 15 (51.7) | 20 (37.7) | 18 (43.9) | 1 (12.5) | 1 (25.0) |
| Lymph node | 18 (22.0) | 4 (13.8) | 14 (26.4) | 10 (24.4) | 2 (25.0) | 2 (50.0) |
| Peritonea | 10 (12.2) | 7 (24.1) | 3 (5.7) * | 1 (2.4) | 1 (12.5) | 1 (25.0) |
| Adrenal glands | 6 (7.3) | 1 (3.4) | 5 (9.4) | 3 (7.3) | 0 (0) | 2 (50.0) |
| Bone | 12 (14.6) | 5 (17.3) | 7 (13.2) | 7 (17.1) | 0 (0) | 0 (0) |
| Cerebral | 1 (1.2) | 0 (0) | 1 (1.9) | 1 (2.4) | 0 (0) | 0 (0) |
| Macroscopic vascular invasion, extrahepatic spread, or both | ||||||
| No | 6 (7.3) | 2 (6.9) | 4 (7.6) | 2 (4.9) | 2 (25.0) | 0 (0) |
| Yes | 76 (92.7) | 27 (93.1) | 49 (92.4) | 39 (95.1) | 6 (75.0) | 4 (100) |
| Prior therapy | ||||||
| No | 24 (29.3) | 6 (20.7) | 18 (34.0) | 15 (36.6) | 2 (25.0) | 1 (25.0) |
| Yes | 58 (70.7) | 23 (79.3) | 35 (66.0) | 26 (63.4) | 6 (75.0) | 3 (75.0) |
| TACE | 37 (45.1) | 17 (58.6) | 20 (37.7) | 15 (36.6) | 4 (50.0) | 1 (25) |
| Radiofrequency | 20 (24.3) | 6 (20.7) | 14 (26.4) | 10 (24.4) | 3 (37.5) | 1 (25) |
| Radioembolization | 7 (8.5) | 4 (13.8) | 3 (5.7) | 2 (4.9) | 0 (0) | 1 (25) |
| Surgery: hepatic resection | 18 (22.0) | 6 (20.7) | 12 (22.6) | 10 (24.4) | 2 (25.0) | 0 (0) |
| Sorafenib | 7 (8.5) | 3 (10.3) | 4 (7.6) | 0 (0) | 1 (12.5) | 3 (75.0) |
| Others | 7 (8.5) | 2 (6.9) | 5 (9.4) | 5 (12.2) | 0 (0) | 0 (0) |
| Median duration of atezolizumab–bevacizumab, months (range) | 3.5 (0.1–20.9) | 3.5 | 3.3 | 3.3 | 3.9 | 3.9 |
| Median number of atezolizumab–bevacizumab injections (range) | 5 (1–27) | 5 | 4 | 4 | 4.5 | 6 |
| TKI type n (%) | ||||||
| Sorafenib | 41 (50.0) | – | 41 (77.4) | 41 (100) | – | – |
| Regorafenib | 29 (35.3) | 29 (100) | – | – | – | – |
| Cabozantinib | 4 (4.9) | – | 4 (7.6) | – | – | 4 (100) |
| Lenvatinib | 8 (9.8) | – | 8 (15.0) | – | 8 (100) | – |
| Biochemical analysis at progression with atezolizumab–bevacizumab | ||||||
| Alpha-fetoprotein (median), ng/ml | 520 | 823.5 | 423.5 | 401 | 252 | 5114 |
| Total bilirubin (median), µmol/l | 20 | 14 | 20 | 19 | 41 | 15 |
| Albumin (median), g/dl | 34 | 35 | 34 | 34 | 32 | 26 |
| Prothrombin time (median), % | 83 | 84,5 | 82 | 82,5 | 75 | 78 |
| ALBI score | ||||||
| Grade 1 (⩽ −2.60) n (%) | 15 (18.3) | 6 (20.7) | 9 (17.0) | 6 (14.6) | 1 (12.5) | 2 (50.0) |
| Grade 2 (< −2.60 and ⩽ −1.39) n (%) | 37 (45.1) | 14 (48.3) | 23 (43.4) | 20 (48.8) | 1 (12.5) | 2 (50.0) |
| Grade 3 (> −1.39) n (%) | 13 (15.9) | 4 (13.8) | 9 (17.0) | 7 (17.1) | 2 (25.0) | 0 (0) |
| Unknown | 17 (20.7) | 5 (17.2) | 12 (22.6) | 8 (19.5) | 4 (50.0) | 0 (0) |
Values denote n (%) unless specified otherwise.
Significant p value (<0.05) using χ2 or Fisher’s exact tests and Mann–Whitney comparing groups.
ALBI score, albumin–bilirubin score; BCLC, Barcelona Clinic Liver Cancer; BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group performance status; NASH, nonalcoholic steatohepatitis; TACE, transarterial chemoembolization; TKI, tyrosine kinase inhibitor.
Progression-free survival and OS
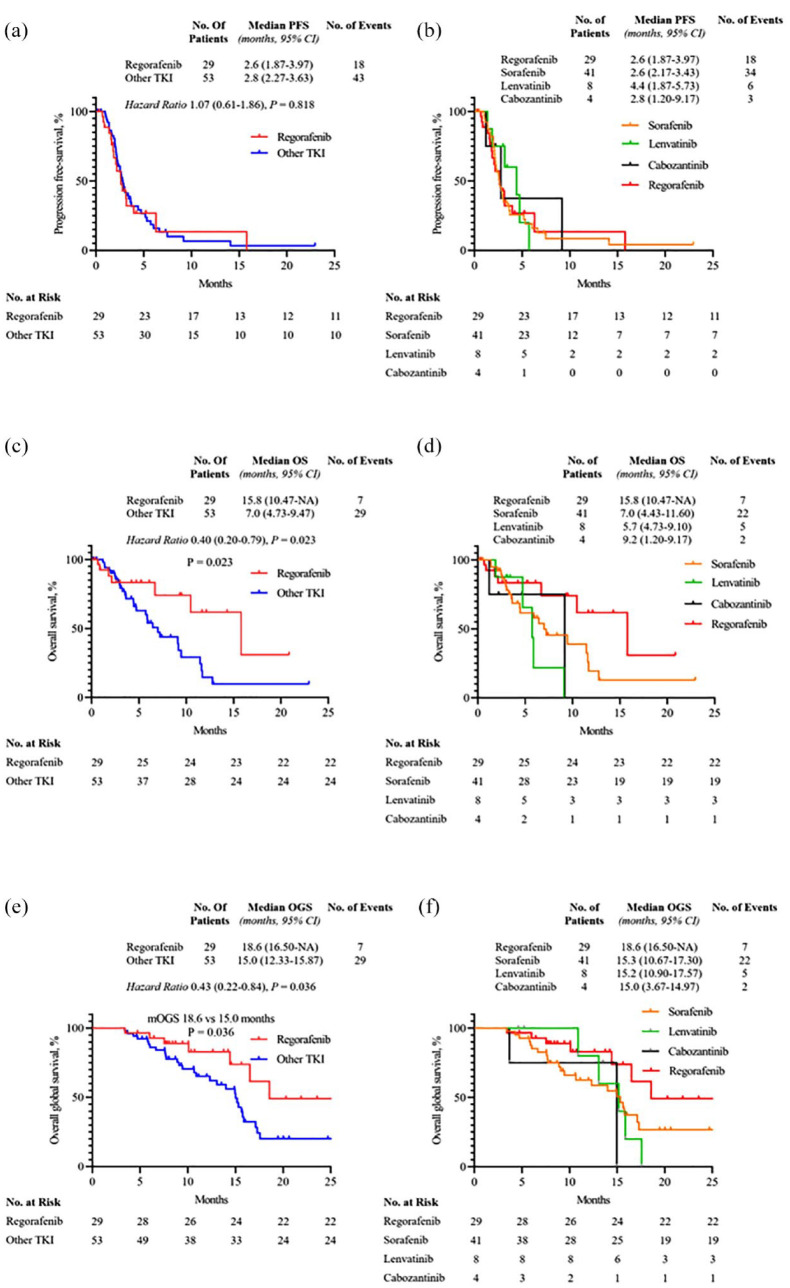
With a median follow-up of 8 months, median PFS was 2.6 months (95% CI: 1.87–3.97) versus 2.8 months (95% CI: 2.27–3.63) in the regorafenib group and other TKI group, respectively [HR 1.07 (95% CI: 0.61–1.86), p = 0.818; Figure 2(a)]. In the other TKI group, median PFS rates were 2.6 months (95% CI: 2.17–3.43), 4.4 months (95% CI: 1.87–5.73), and 2.8 months (95% CI: 1.20–9.17) for sorafenib, lenvatinib, and cabozantinib, respectively (Figure 2(b)).
Figure 2.
The Kaplan–Meier curves of progression-free survival (a), overall survival (c), and overall global survival (e) of regorafenib group and other TKI group. The Kaplan–Meier curves of progression-free survival (b), overall survival (d), and overall global survival (f) of regorafenib, sorafenib, lenvatinib, and cabozantinib groups.
mOS was 15.8 months (95% CI: 10.47–NE) versus 7.0 months (95% CI: 4.73–9.47) in regorafenib group and other TKI group, respectively [HR 0.40 (95% CI: 0.20–0.79), p = 0.023; Figure 2(c)]. In the other TKI group, mOS rates were 7.0 months (95% CI: 4.43–11.60), 5.7 months (95% CI: 4.73–9.10), and 9.2 months (95% CI: 1.20–9.17) for sorafenib, lenvatinib, and cabozantinib, respectively (Figure 2(d)). Patients treated with regorafenib had a trend for a more favorable OS than those treated by sorafenib.
In multivariate analysis, treatment with regorafenib was also associated with better OS [aHR 0.35 (95% CI: 0.14–0.84), p = 0.019] (Table 2).
Table 2.
Patient characteristics associated with overall survival (multivariate analysis).
| Patient characteristics | Overall survival |
|---|---|
| Treatment group | |
| Regorafenib versus other TKI | aHR 0.35 (95% CI: 0.14–0.84), p = 0.019 |
| Sex | |
| Women versus men | aHR 1.06 (95% CI: 0.44–2.56), p = 0.90 |
| ECOG PS | |
| ECOG PS 2 versus ECOG PS 0–1 | aHR 1.78 (95% CI: 0.63–5.04), p = 0.28 |
| Child-Pugh score | |
| Child-Pugh A versus no cirrhosis | aHR 1.81 (95% CI: 0.65–5.03), p = 0.26 |
| Child-Pugh B versus no cirrhosis | aHR 1.21 (95% CI: 0.53–2.74), p = 0.65 |
| Extrahepatic spread | |
| Yes versus no | aHR 1.88 (95% CI: 0.71–4.98), p = 0.20 |
aHR, adjusted hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; TKI, tyrosine kinase inhibitor.
Overall global survival
Median OGS was 18.6 months in the regorafenib group (95% CI: 16.50–NE) versus 15.0 months in the other TKI group (95% CI: 12.33–15.87) [HR 0.43 (95% CI: 0.22–0.84), p = 0.036] (Figure 2(e)). Median OGS rates in sorafenib, lenvatinib, and cabozantinib groups were 15.3 months (95% CI: 10.67–17.30), 15.2 months (95% CI: 10.90–17.57), and 15.0 months (95% CI: 3.67–14.97), respectively (Figure 2(f)).
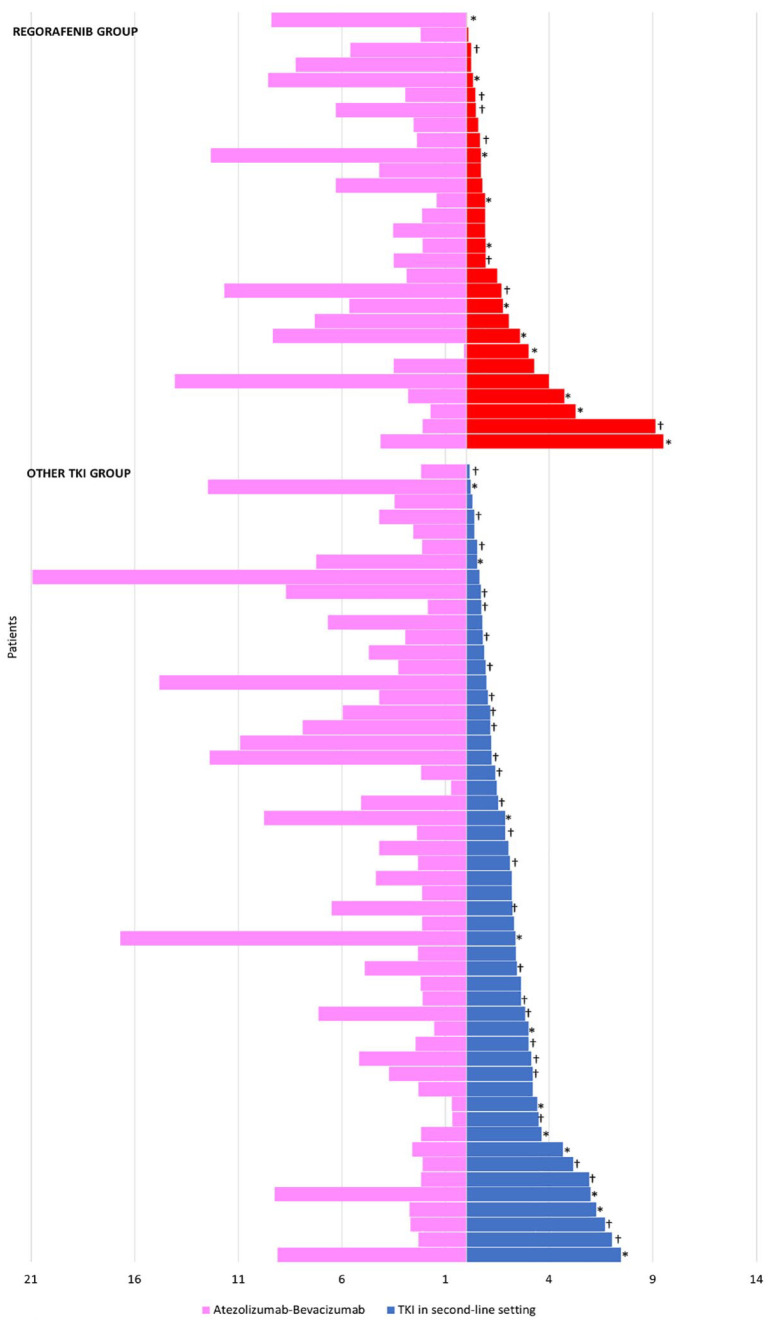
Median duration of first-line treatment was not linked to median exposure with a second-line treatment (Figure 3). Median duration of first-line treatment was 3.5 and 3.3 months in the regorafenib group and other TKI group, respectively. In patients with long duration of atezolizumab–bevacizumab treatment (⩾7.4 months), the median duration of TKI treatment was not statistically different compared to patients with short duration of atezolizumab–bevacizumab treatment (1.9 months versus 1.7 months, p = 0.330).
Figure 3.
Duration of first-line and second-line therapies in the regorafenib group and other TKI group.
*Ongoing treatment or censored, †death.
Incidence of treatment-emergent adverse events
Treatment-emergent adverse events (TEAEs) are shown in Table 3. TEAEs of any grade were observed in 68 patients. There was no significant difference between the two groups. The most common adverse events were hand–foot skin (HFS) reaction in 51.7% and 58.5%, fatigue in 58.6% and 79.2%, diarrhea in 17.2% and 28.3%, and hypertension in 3.4% and 20.8% in the regorafenib group and in the other TKI group, respectively. Grade 3 TEAE occurred in 17.2% and 28.3% in the regorafenib group and in the other TKI group, respectively. One patient treated by sorafenib presented grade 2 HFS and fatigue, and grade 1 diarrhea, hypertension, and nausea. There was no grade 4 or treatment-related deaths in both groups. None of the patients received granulocyte colony-stimulating factor or erythropoietin in primary prevention.
Table 3.
Incidence of treatment-emergent adverse events (TEAEs).
| Treatment-emergent adverse events | Total patients (n = 82) | Regorafenib (n = 29) | Other TKI (n = 53) | ||
|---|---|---|---|---|---|
| Sorafenib (n = 41) | Cabozantinib (n = 4) | Lenvatinib (n = 8) | |||
| HFSR | |||||
| Any grade | 40 (48.8%) | 13 (44.8%) | 22 (53.6%) | 1 (25.0%) | 4 (50.0%) |
| Grade 3 | 6 (7.3%) | 2 (6.9%) | 3 (7.3%) | 1 (25.0%) | 0 |
| Fatigue | |||||
| Any grade | 54 (65.8%) | 16 (55.2%) | 30 (73.0%) | 2 (50.0%) | 6 (75.0%) |
| Grade 3 | 5 (6.1%) | 1 (3.4%) | 2 (4.9%) | 1 (25.0%) | 1 (12.5%) |
| Diarrhea | |||||
| Any grade | 15 (18.2%) | 4 (13.7%) | 10 (24.4%) | 1 (25.0%) | 1 (12.5%) |
| Grade 3 | 4 (4.9%) | 1 (3.4%) | 2 (4.9%) | 1 (25.0%) | 0 |
| Nausea, vomiting | |||||
| Any grade | 7 (8.5%) | 1 (3.4%) | 3 (7.3%) | 0 | 2 (25.0%) |
| Grade 3 | 1 (1.2%) | 0 | 1 (2.4%) | 0 | 0 |
| Hypertension | |||||
| Any grade | 11 (12.2%) | 1 (3.4%) | 7 (17.0%) | 1 (25.0%) | 2 (25.0%) |
| Grade 3 | 1 (1.2%) | 0 | 1 (2.4%) | 0 | 0 |
| Liver failure (asterixis, jaundice, ascites) (yes) | 9 (10.9%) | 2 (6.9%) | 5 (12.1%) | 0 | 2 (25.0%) |
| Skin rash | |||||
| Any grade | 5 (6.0%) | 2 (6.9%) | 3 (2.4%) | 0 | 0 |
| Grade 3 | 3 (3.7%) | 1 (3.4%) | 2 (4.9%) | 0 | 0 |
HFSR, hand–foot skin reaction; TKI, tyrosine kinase inhibitor.
Patient’s outcomes
TKI treatment was definitely discontinued in 24.1% and 22.6% of patients due to adverse events in the regorafenib group and in the other TKI group, respectively. 17.2% and 22.6% of patients had dose modification due to intolerance in the regorafenib group and in the other TKI group, respectively. The median duration of treatment was 1.6 months (range 0–9.5) and 0.9 months in the regorafenib group and 2.1 months in the other TKI group, respectively.
Treatment was discontinued in 37.9% and 60.4% of patients due to radiologic or symptomatic progression in the regorafenib group and in the other TKI group, respectively. One patient in the regorafenib group was lost of follow-up before the first radiological evaluation (patient was censored at date of last news).
Therapy in the third-line setting after progression or intolerance
Out of the 62 included patients, 43 experienced radiological or symptomatic disease progression (52.4%) and 19 discontinued treatment because of adverse events (23.2%).
After disease progression or intolerance in the second-line setting, 55.6% and 31.8% of patients received a TKI as a third-line treatment in the regorafenib group and in other TKI group, respectively (Supplemental Appendix A). The use of a TKI in third line was justified due to radiological or symptomatic progression (62.5%) or intolerance (37.5%). Cabozantinib was the TKI used in third line in 80% and 50% of cases in the regorafenib group and other TKI group, respectively. Only one patient (12.5%) who experienced disease progression after another TKI than regorafenib received regorafenib as third-line therapy.
Discussion
In this retrospective real-world cohort study, efficacy and safety of TKI in the second-line setting were evaluated in advanced HCC after progression with doublet therapy with atezolizumab and bevacizumab. In our study, patients treated with regorafenib versus other TKI in the second-line setting showed better OS and OGS but not PFS.
Since 2020, the atezolizumab and bevacizumab combination therapy became the standard of care in first-line setting 5 as it showed an improvement in PFS compared with sorafenib (6.8 months versus 4.3 months). Yet, the optimal sequence of systemic treatment after progression with atezolizumab and bevacizumab is still not clear. To our knowledge, our work is the first to describe effectiveness results of second-line TKI in a large French population after atezolizumab–bevacizumab. Clinical trials have only assessed second-line TKI after progression under sorafenib. Indeed, the RESORCE phase III trial showed a median PFS of 3.1 months for regorafenib compared with 1.5 months for placebo. 6 The CELESTIAL phase III trial, assessing cabozantinib versus placebo in the second-line setting, showed a median PFS of 5.2 months versus 1.9 months. 7
Patients in our study received different types of TKI in the second-line setting including sorafenib, regorafenib, lenvatinib, or cabozantinib. Sorafenib was the most frequently systemic treatment used (50% of patients) as it was approved as first-line agent before 2020. Median PFS ranged from 2.6 months to 4.4 months. Median PFS did not significantly differ between the regorafenib group and other TKI group [HR 1.07 (95% CI: 0.61–1.86), p = 0.818]. Our PFS results are similar with those from clinical trials,6,7 and show that efficacy of TKI treatment in second-line setting is not affected after progression with atezolizumab–bevacizumab as first-line treatment.
In our work, a more favorable trend was suggested in patients treated with regorafenib (mOS 15.8 months). The regorafenib group was associated with better OS compared to the other TKI group [HR 0.40 (95% CI: 0.20–0.79), p = 0.023]. Adjusted HR in multivariate analysis still favored the regorafenib group for OS.
mOS was not reached in the IMBrave 150 trial testing atezolizumab–bevacizumab versus sorafenib. 5 Median OGS of patients who received regorafenib was improved compared to patients who received other TKI [HR 0.43 (95% CI: 0.22–0.84), p = 0.036]. Median OGS was estimated in sorafenib, lenvatinib, and cabozantinib subgroups at 15.3, 15.2, and 15.0 months, respectively. This relatively prolonged OGS, despite the short duration of combination therapy in first-line setting in our study (3.5 months), reinforces the importance of TKI in second-line setting and allowed 38.7% of patients to receive another TKI in third-line setting. To note, in patients with long duration of atezolizumab–bevacizumab treatment (⩾7.4 months), the median duration of TKI treatment was not statistically different from patients with short duration of atezolizumab–bevacizumab treatment (1.9 months versus 1.7 months).
The adverse events observed with sorafenib, regorafenib, and other TKI were mostly manageable with appropriate supportive care except for 16 patients (19.5%) who presented grade 3 TEAE. The most common TEAE was fatigue in 65.8% of patients, and HFS reaction in 48.8% of patients. There was neither grade 4 TEAE nor treatment-related death. Again, our safety results were comparable with the data published in the phase III clinical trials of each drug6–8,11 and with a recent prospective, observational study (REFINE NCT03289273) that included 1005 patients, and showed that the safety profile in real-world was consistent with the safety profile shown in the RESORCE trial.
Our study shows various limitations. First, our results should be interpreted cautiously due to the small patient sample size and the retrospective nature of our study. To adjust on potential confounding factors and limit the effect of indication and selection biases, we performed a multivariate analysis which still favored regorafenib for OS. Also there was heterogeneity regarding the population with some patients without cirrhosis, although this did not affect OS in multivariate analysis.
Treatment strategy for advanced HCC will continue to change in the future. Currently, the HIMALAYA phase III trial (NCT03298451) is in progress to compare durvalumab (anti-PD-L1) with or without tremelimumab (anti-cytotoxic T-lymphocyte-associated protein 4) to sorafenib in first-line treatment of advanced HCC and primary results are promising. The association of these two agents significantly improved OS, and durvalumab was noninferior to sorafenib with a favorable safety so this could become an alternative of atezolizumab–bevacizumab in the first-line setting. 2 The association between intensity-modulated radiotherapy and a combination therapy of anti-programmed cell death 1 or anti-PD-L1 and anti-VEGF is another therapeutic approach that could be considered in patients with advanced HCC. 12 A recent retrospective study showed that this triplet therapy could improve PFS (8.7 versus 5.4 months, p = 0.013) and OS (18.5 versus 12.6 months, p = 0.043). 13 Also, new prognostic and predictive biomarkers have been tested to improve the management of advanced HCC. For example, high plasma alkaline phosphatase, lactate dehydrogenase, and heat-shock protein 90 plasma levels, whose expression is associated with tumor growth and extrahepatic spread, seem all associated with poor OS.14,15 In patients treated by a combination therapy of anti-PD-L1 and anti-VEGF, the count of circulating tumor cells positive for PD-L1 has also been described as a potential independent predictive factor of OS. 16 Finally, challenges remain for real-world patients who are not suitable for clinical trials and for whom treatment data are scarce.
Conclusions
Efficacy of sorafenib, regorafenib, lenvatinib, and cabozantinib in the second-line setting is not affected after progression with atezolizumab–bevacizumab and PFS rates of patients are not different regardless of TKI type. Regorafenib used in the second-line setting seems associated with improved OS and OGS compared to other TKI. Prospective studies as well as other large collaborative real-world studies are still needed to better investigate the optimal sequence in second-line setting in patients with advanced HCC.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359231189425 for Beyond atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma: overall efficacy and safety of tyrosine kinase inhibitors in a real-world setting by Manon Falette-Puisieux, Jean-Charles Nault, Mohamed Bouattour, Marie Lequoy, Giuliana Amaddeo, Thomas Decaens, Frederic Di Fiore, Sylvain Manfredi, Philippe Merle, Aurore Baron, Christophe Locher, Anna Pellat and Romain Coriat in Therapeutic Advances in Medical Oncology
Acknowledgments
None.
Footnotes
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Manon Falette-Puisieux, Gastroenterology and Digestive Oncology Unit, Cochin Hospital AP-HP, 27 rue du Faubourg Saint Jacques, Paris 75014, France.
Jean-Charles Nault, Hepatology Unit, Hôpital Avicenne, Hôpitaux Universitaires Paris Seine-Saint-Denis, Assistance Publique–Hôpitaux de Paris, Bobigny, France; Functional Genomics of Solid Tumors, Centre de Recherche des Cordeliers, INSERM, Sorbonne Université, Université de Paris, Paris, France.
Mohamed Bouattour, Liver Cancer Unit, Beaujon Hospital AP-HP, Clichy, France.
Marie Lequoy, Hepatology Unit, Saint-Antoine Hospital AP-HP, Paris, France.
Giuliana Amaddeo, Hepatology Unit, Henri Mondor Hospital AP-HP, Paris Est Créteil University, Créteil, France.
Thomas Decaens, Hepatology Unit, Grenoble Alpes Hospital, Grenoble, France.
Frederic Di Fiore, Hepatology and Gastroenterology Unit, Charles-Nicolle Hospital, Rouen, France.
Sylvain Manfredi, Hepatology and Gastroenterology Unit, Dijon Hospital, Dijon, France.
Philippe Merle, Hepatology Unit, Croix-Rousse Hospital, Lyon, France.
Aurore Baron, Hepatology and Gastroenterology Unit, Sud-Francilien Hospital, Corbeil-Essonne, France.
Christophe Locher, Hepatology and Gastroenterology Unit, Est-Francilien Hospital, Meaux, France.
Anna Pellat, METHODS Team, UMR 1153, Centre d’épidémiologie clinique de l’Hôtel Dieu, Université Paris Cité, Paris, France.
Romain Coriat, Gastroenterology and Digestive Oncology Unit, Cochin Hospital AP-HP, Paris, France; Institut Cochin, INSERM U 1016 CNRS UMR 8104, Université Paris Cité, Paris, France.
Declarations
Ethics approval and consent to participate: The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Cochin Hospital, Paris, France (protocol code AAA-2023-09006, 26 January 2023). Informed consent was obtained from all subjects involved in the study.
Consent for publication: Not applicable.
Author contributions: Manon Falette-Puisieux: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Visualization; Writing – original draft; Writing – review & editing.
Jean-Charles Nault: Conceptualization; Methodology; Resources; Validation; Writing – review & editing.
Mohamed Bouattour: Conceptualization; Methodology; Resources; Validation; Writing – review & editing.
Marie Lequoy: Conceptualization; Methodology; Resources; Validation; Writing – review & editing.
Giuliana Amaddeo: Conceptualization; Methodology; Resources; Validation; Writing – review & editing.
Thomas Decaens: Conceptualization; Methodology; Resources; Validation; Writing – review & editing.
Frederic Di Fiore: Conceptualization; Methodology; Resources; Validation; Writing – review & editing.
Sylvain Manfredi: Conceptualization; Methodology; Resources; Validation; Writing – review & editing.
Philippe Merle: Conceptualization; Methodology; Resources; Validation; Writing – review & editing.
Aurore Baron: Conceptualization; Methodology; Resources; Validation; Writing – review & editing.
Christophe Locher: Conceptualization; Methodology; Resources; Validation; Writing – review & editing.
Anna Pellat: Conceptualization; Formal analysis; Investigation; Methodology; Resources; Software; Validation; Writing – review & editing.
Romain Coriat: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Akinyemiju T, Abera S, Ahmed M, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level. JAMA Oncol 2017; 3: 1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Falette Puisieux M, Pellat A, Assaf A, et al. Therapeutic management of advanced hepatocellular carcinoma: an updated review. Cancers (Basel) 2022; 14: 2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology 1999; 29: 62–67. [DOI] [PubMed] [Google Scholar]
- 4. Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 2022; 76: 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med 2020; 382: 1894–1905. [DOI] [PubMed] [Google Scholar]
- 6. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 389: 56–66. [DOI] [PubMed] [Google Scholar]
- 7. Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 2018; 379: 54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamashita T, Kudo M, Ikeda K, et al. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol 2020; 55: 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yoo C, Kim JH, Ryu MH, et al. Clinical outcomes with multikinase inhibitors after progression on first-line atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma: a multinational multicenter retrospective study. Liver Cancer 2021; 10: 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–247. [DOI] [PubMed] [Google Scholar]
- 11. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378–390. [DOI] [PubMed] [Google Scholar]
- 12. Zhong L, Wu D, Peng W, et al. Safety of PD-1/PD-L1 inhibitors combined with palliative radiotherapy and anti-angiogenic therapy in advanced hepatocellular carcinoma. Front Oncol 2021; 11: 686621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Su K, Guo L, Ma W, et al. PD-1 inhibitors plus anti-angiogenic therapy with or without intensity-modulated radiotherapy for advanced hepatocellular carcinoma: a propensity score matching study. Front Immunol 2022; 13: 972503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Su K, Liu Y, Wang P, et al. Heat-shock protein 90α is a potential prognostic and predictive biomarker in hepatocellular carcinoma: a large-scale and multicenter study. Hepatol Int 2022; 16: 1208–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Su K, Huang W, Li X, et al. Evaluation of lactate dehydrogenase and alkaline phosphatase as predictive biomarkers in the prognosis of hepatocellular carcinoma and development of a new nomogram. J Hepatocell Carcinoma 2023; 10: 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Su K, Guo L, He K, et al. PD-L1 expression on circulating tumor cells can be a predictive biomarker to PD-1 inhibitors combined with radiotherapy and antiangiogenic therapy in advanced hepatocellular carcinoma. Front Oncol 2022; 12: 873830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359231189425 for Beyond atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma: overall efficacy and safety of tyrosine kinase inhibitors in a real-world setting by Manon Falette-Puisieux, Jean-Charles Nault, Mohamed Bouattour, Marie Lequoy, Giuliana Amaddeo, Thomas Decaens, Frederic Di Fiore, Sylvain Manfredi, Philippe Merle, Aurore Baron, Christophe Locher, Anna Pellat and Romain Coriat in Therapeutic Advances in Medical Oncology