ABSTRACT
Pneumococcal serogroups consist of structurally related serotypes, and serotype-specific antibodies can cross-react against other serotypes within the same serogroup. Cross-reactivity of vaccine-induced serotype 6A antibodies, and, to a lesser extent, serotype 6B antibodies, to serotype 6C has been demonstrated following receipt of the 13-valent pneumococcal conjugate vaccine (PCV13), which contains serotypes 6A and 6B. V114 is a 15-valent PCV containing the 13 PCV13 serotypes plus two additional serotypes, 22F and 33F. This study assessed cross-reactivity to serotype 6C in recipients of V114 and PCV13 as well as specificity of opsonophagocytic activity (OPA) responses in serogroup 6. Following receipt of V114 or PCV13, the observed OPA geometric mean titers to serotypes 6A, 6B, and 6C were comparable across both vaccination groups (post-single dose in adults ≥50 years of age [n = 250] and from pre- to post-dose 4 in pediatric participants 12–15 months of age [n = 150]). Based on OPA inhibition studies, V114 induced cross-reactive antibodies to serotype 6C in adult and pediatric populations that were specific and comparable to those induced by PCV13. Based on experience with PCV13, V114 may also provide comparable protection against pneumococcal disease caused by serotype 6C; however, this will have to be evaluated in real-world studies.
KEYWORDS: Streptococcus pneumoniae, pneumococcal vaccine, V114, 15-valent PCV, pneumococcal disease, cross-reactive antibodies, opsonophagocytic activity, OPA
Serotypes of Streptococcus pneumoniae are characterized by the structure of their capsular polysaccharides (PS), with each pneumococcal serogroup consisting of structurally related serotypes.1 Serotype-specific antibodies can cross-react against other serotypes with similar capsular composition to induce immune responses, but the quality of cross-reactive antibodies can differ between serotypes.1,2 Pneumococcal vaccines are designed to cover serotypes associated with the highest burden of disease,3,4 and data on serotype distribution following widespread implementation of these vaccines suggested that these vaccines may have an impact on non-vaccine serotypes within a serogroup.5 A 7-valent pneumococcal conjugate vaccine (PCV7; Prevnar7®; Pfizer Inc.) was the first pneumococcal conjugate vaccine (PCV) licensed in the United States in 2000 and included serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F.6 Following the widespread use of PCV7, reductions in pneumococcal disease and colonization attributable to serotype 6A were observed despite serotype 6A not being contained in PCV7, likely due to cross-protection with vaccine-induced antibodies against serotype 6B.7,8 Serotype 6C shares many epitopes with serotype 6A and was indistinguishable from serotype 6A using conventional serotyping reagents until it was independently characterized in 2007.9 As serotype 6C has structural similarity with serotype 6A and, to a lesser extent, serotype 6B,1,2,9,10 cross-reactivity was anticipated between these serotypes. However, surveillance studies in the post-PCV7 era have shown little impact on serotype 6C colonization and, in fact, showed that rates of colonization and invasive pneumococcal disease (IPD) caused by serotype 6C increased in individuals ≥5 years of age.1,2,8 Retrospective studies carried out using specific methodologies to distinguish between serotypes 6A and 6C have demonstrated a similar increase in the prevalence of serotype 6C in the United States.8,11–14 In 2010, a 13-valent PCV (PCV13; Prevnar 13™; Pfizer Inc.) was introduced and expanded the serotype coverage to contain six additional serotypes, including serotype 6A.15,16 The incidence of IPD due to serotype 6A following the introduction of PCV13, already reduced in the post-PCV7 era, has decreased further in adults in most regions and is consistently low in children.7 Cross-reactivity of vaccine-induced antibodies to serotype 6C has been demonstrated following receipt of PCV13,1,2 and the use of PCV13 has been associated with an observed decrease in the incidence of disease caused by serotype 6C in some populations.17–19 However, serotype 6C remains a significant contributor to the residual burden of pneumococcal disease in some populations and regions.7,11,20,21
V114 (VAXNEUVANCE™, Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA) is a 15-valent PCV containing the 13 serotypes in PCV13 (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F) and two additional serotypes (22F and 33F). V114 was first approved for use in adults in 2021 and then for use in infants and children 6 weeks through 17 years of age in 2022.22–24 Opsonophagocytic activity (OPA) of antibodies against S. pneumoniae induced by pneumococcal vaccines has been shown to correlate with immune protection in various studies of pneumococcal vaccines.25 Moreover, levels of cross-reactive immunoglobulin G (IgG) against a vaccine-related serotype may not reflect the opsonic capacity of antibodies against this serotype,26 supporting the use of OPA in evaluating responses to vaccine-related serotypes. The objectives of this post-hoc study were to assess cross-reactive antibody responses to serotype 6C in adult and pediatric recipients of V114 and PCV13, and to evaluate the specificity of OPA responses in serogroup 6. This study was presented, in part, at the 12th International Symposium on Pneumococci and Pneumococcal Diseases (ISPPD-12), Toronto, Canada, in 2022 (abstract #235).
The study design is described in Figure 1. The sera from a subset of adult and pediatric participants from two Phase II randomized, double-blind, proof-of-concept V114 trials were randomly selected using the RANUNI function in SAS© software, version 9.4 of the SAS System for Unix (Cary, NC, USA). The adult trial (NCT02547649) compared the safety, tolerability, and immunogenicity of a single dose of two different formulations of V114 with PCV13 in pneumococcal vaccine-naïve adults ≥50 years of age.27 The sera from 250 adult participants (V114 = 150; PCV13 = 100) with the remaining pre- and post-vaccination samples were randomly selected and stratified by age group (50–64, 65–74, and ≥75 years of age), based on the proportion of participants in each age subgroup in the original study. The pediatric trial (NCT02987972) compared the safety, tolerability, and immunogenicity profiles of two different lots of V114 compared with PCV13 in healthy infants 6–12 weeks of age at enrollment; vaccines were administered at approximately 2, 4, 6, and 12–15 months of age.28 In total, the sera from 150 pediatric participants (V114 = 100; PCV13 = 50) with available pre- and post-dose 4 samples were randomly selected. Written informed consent for post-hoc testing and analyses using the remaining sera samples had been obtained from all participants or parents/guardians before the participants were enrolled in the original clinical studies.
Figure 1.
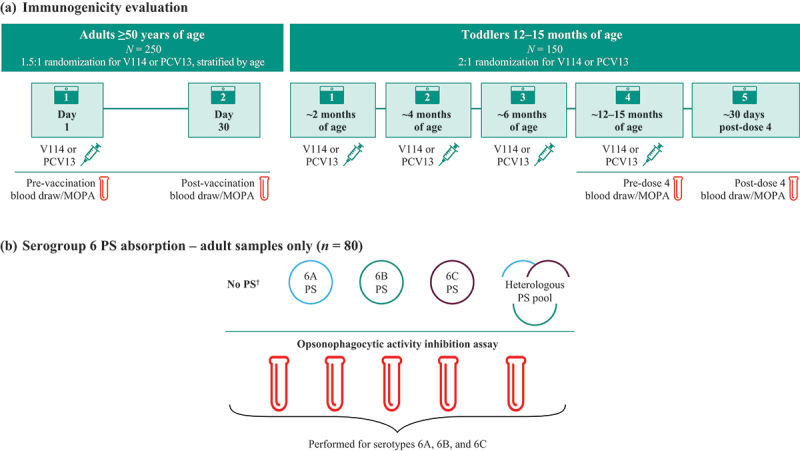
Study design. (a) For the 250 adult participants (V114 = 150; PCV13 = 100) with available pre- and post-vaccination sera samples, OPA GMTs against serotypes 6A, 6B, and 6C were measured prior to, and 30 days following, vaccination with a single dose of V114 or PCV13. For the 150 pediatric participants (V114 = 100; PCV13 = 50) with available pre- and post-dose 4 sera samples, OPA GMTs against serotypes 6A, 6B, and 6C were measured pre-dose 4 and 30 days post-dose 4. OPA GMTs were calculated by exponentiating the estimates of the mean of the natural log values. The within-group CIs were obtained by exponentiating the CIs of the mean of the natural log values based on the t-distribution. (b) Eighty adult participants (40 in each vaccination group) were further tested for the additional inhibition analysis for serogroup 6. Sera were tested for 6A, 6B, and 6C opsonophagocytic antibody activity after no PS absorption (control), after absorption with homologous 6A, 6B, or 6C PS, or after absorption with a heterologous PS pool. The 6A, 6B, and 6C PS stock reagents were diluted from 400 mg/L in the MOPA assay to final concentrations of 100 mg/mL for each PS. Stocks of the heterologous PS pools were mixed at 4.8 mg/mL, and equal volumes of each were combined to make 400 mg/L of each PS. The solutions were then diluted 4-fold when added to the sera to achieve final 100 mg/mL concentrations of each PS in the MOPA. The average inhibition rate was calculated by 1 − exponentiating the mean of the natural log ratio of post- and pre-absorption MOPA titer. The within-group CIs were obtained by 1 − exponentiating the corresponding CIs based on the t-distribution. Owing to limitations in the blood volume, pre-absorption tests were not performed in pediatric samples.
†No PS absorption to provide uninhibited control.
CI = confidence interval; GMT = geometric mean titer; MOPA = multiplexed opsonophagocytic killing assay; OPA = opsonophagocytic activity; PCV13 = 13-valent pneumococcal conjugate vaccine; PS = polysaccharide; V114 = 15-valent pneumococcal conjugate vaccine.
For the immunogenicity evaluation, serotype-specific immune responses induced by V114 and PCV13 to serotypes 6A, 6B, and 6C were each evaluated separately via multiplexed opsonophagocytic killing assay (MOPA) to quantify OPA or functional antibodies 30 days after vaccination.29
To assess the relative contributions of serotype-specific antibodies to cross-reactivity, post-vaccination samples from 80 adults were re-tested by OPA after absorbing sera with purified PS of serogroup 6 serotypes (6A, 6B, and 6C); the endpoint was the degree of inhibition of OPA antibody activity following absorption with these three serotypes. Sera were tested for 6A, 6B, and 6C antibody activity after no PS absorption (control), after absorption with homologous serogroup 6 PS, or after absorption with a heterologous control PS pool (which included all V114 serotypes except for those that may potentially cross-react with serogroup 6 [serotypes 6A, 6B, 6C, and 19A]).30
Demographic and baseline characteristics were generally comparable across the two intervention groups for both adult and pediatric participants (Table 1). In the pediatric study, more males were selected in the PCV13 group (62%) and more females in the V114 group (61%) due to sample availability.
Table 1.
Demographics of (a) adult and (b) pediatric participants.
| V114 (n = 150) n (%) |
PCV13 (n = 100) n (%) |
|
|---|---|---|
| (a) Adult participants | ||
| Sex | ||
| Male | 60 (40.0) | 46 (46.0) |
| Female | 90 (60.0) | 54 (54.0) |
| Age (years) | ||
| 50–64 | 77 (51.3) | 51 (51.0) |
| 65–74 | 58 (38.7) | 38 (38.0) |
| ≥75 | 15 (10.0) | 11 (11.0) |
| Mean, years (range) | 63.0 (50–81) | 63.3 (51–85) |
| Race | ||
| White | 123 (82.0) | 85 (85.0) |
| Black or African American | 25 (16.7) | 14 (14.0) |
| American Indian/Alaska Native | 0 | 1 (1.0) |
| Asian | 1 (0.7) | 0 |
| Multiple | 1 (0.7) | 0 |
| Ethnicity | ||
| Not Hispanic/Latino | 109 (72.7) | 73 (73.0) |
| Hispanic/Latino | 40 (26.7) | 27 (27.0) |
| Not reported |
1 (0.7) |
0 |
| |
V114 (n = 100) n (%) |
PCV13 (n = 50) n (%) |
| (b) Pediatric participants | ||
| Sex | ||
| Male | 39 (39.0) | 31 (62.0) |
| Female | 61 (61.0) | 19 (38.0) |
| Age at enrollment (weeks) | ||
| 6 | 3 (3.0) | 0 |
| 7 | 4 (4.0) | 2 (4.0) |
| 8 | 43 (43.0) | 17 (34.0) |
| 9 | 37 (37.0) | 19 (38.0) |
| 10 | 7 (7.0) | 9 (18.0) |
| 11 | 5 (5.0) | 2 (4.0) |
| 12 | 1 (1.0) | 1 (2.0) |
| Mean, weeks (range) | 8.6 (6.0–12.0) | 8.9 (7.0–12.0) |
| Race | ||
| White | 85 (85.0) | 42 (84.0) |
| Black or African American | 7 (7.0) | 3 (6.0) |
| American Indian/Alaska Native | 2 (2.0) | 0 |
| Multiple | 6 (6.0) | 5 (10.0) |
| Ethnicity | ||
| Not Hispanic/Latino | 95 (95.0) | 49 (98.0) |
| Hispanic/Latino | 5 (5.0) | 1 (2.0) |
PCV13 = 13-valent pneumococcal conjugate vaccine; V114 = 15-valent pneumococcal conjugate vaccine.
In both the adult and pediatric populations, the observed OPA geometric mean titers (GMTs) to serotypes 6A, 6B, and 6C were comparable across both vaccination groups (post-single dose in adults and post-dose 4 in pediatric participants; Figure 2). The cross-reactive response to serotype 6C, as assessed by levels of OPA GMTs, was relatively stronger in toddlers than in adults, while the responses to serotypes 6A and 6B were relatively stronger in adults than in toddlers. While the immunological mechanisms underlying these differences and their clinical implications are not known, the evaluation of cross-reactivity in children followed the fourth dose of the PCV, reflecting a memory response, whereas adults received only one dose of V114 or PCV13; furthermore, pneumococcal vaccine-induced antibodies in adults build on the B-cell repertoire developed due to natural exposure and nasopharyngeal colonization over time31 and may therefore be different than the antibodies induced in children.
Figure 2.
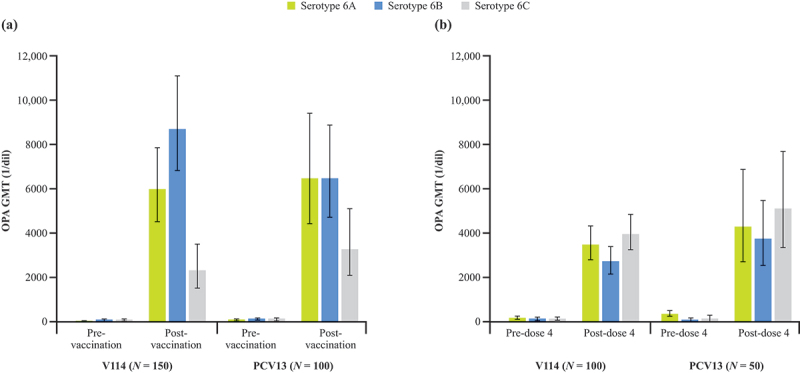
Opsonophagocytic antibody activity at (a) pre-vaccination and 30 days post-vaccination in adults ≥50 years of age and (b) at pre- and 30 days post-dose 4 in toddlers 12–15 months of age.
GMT = geometric mean titer; OPA = opsonophagocytic activity; PCV13 = 13-valent pneumococcal conjugate vaccine; V114 = 15-valent pneumococcal conjugate vaccine.
In adults ≥50 years of age, the degrees of inhibition following absorption of a given serogroup 6 PS were comparable between both vaccination groups (Figure 3). For all three serotypes, antibody activity was inhibited the most following absorption with the homologous PS. However, for serotype 6C responses, inhibition by serotype 6A PS was almost as much as that caused by serotype 6C PS in both vaccination groups. Conversely, the inhibition of serotype 6B response by serotype 6C PS was closer to that seen following absorption with the heterologous PS pool than inhibition associated with 6B PS. For all three serotypes, the antibody activity was least inhibited following absorption with the heterologous PS pool (containing all V114 serotypes except serogroup 6 and serotype 19A).
Figure 3.
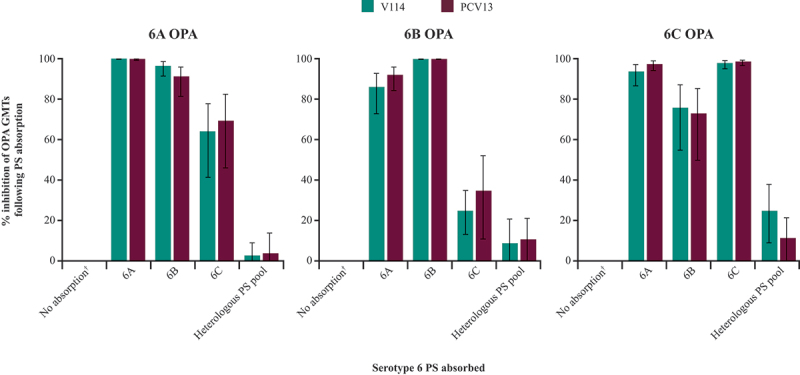
Inhibition of opsonophagocytic antibody activity for serotypes 6A, 6B, and 6C after absorption of serogroup 6 PS at 30 days post-vaccination in adults ≥50 years of age.
†No PS absorption to provide uninhibited control.
GMT = geometric mean titer; OPA = opsonophagocytic activity; PCV13 = 13-valent pneumococcal conjugate vaccine; PS = polysaccharide; V114 = 15-valent pneumococcal conjugate vaccine.
The results of this post-hoc study demonstrate that V114 induces cross-reactive functional antibodies to serotype 6C that are specific and at levels comparable to those induced by PCV13 in adults and toddlers. Serotype 6C emerged as the predominant serotype associated with serogroup 6 IPD in the pre-PCV13 period following the widespread use of PCV7, while cases associated with serotypes 6A and 6B decreased.11 Our results align with those from studies on the cross-reactivity of serotypes 6A- and 6B-induced antibodies in recipients of PCV13, which have demonstrated the vaccine-elicited induction of cross-reactive antibodies to serotype 6C.1,2 Observational data following the implementation of PCV13 into routine vaccination recommendations have shown a decrease in the incidence of disease caused by serotype 6C,17–19,32 providing evidence of PCV13-induced cross-protection against serotype 6C.
The specificity of these cross-functional OPA responses was demonstrated using the OPA inhibition analysis, with the greatest inhibition of antibody activity for a given serotype occurring after absorption with the homologous PS and the least inhibition occurring after absorption with the heterologous PS pool. Notably, the inhibition of serotype 6C by serotype 6A PS was almost the same as the inhibition by serotype 6C PS in both vaccination groups, while the inhibition of serotype 6B response by serotype 6C PS was less than the inhibition associated with 6A and 6B PS and closer to that seen following absorption with the heterologous PS pool. This can be explained by the fact that serotype 6A has greater structural similarity to serotype 6C than to serotype 6B, with one galactose residue in serotype 6A being exchanged for a glucose residue in serotype 6C.1,9
The OPA inhibition results are consistent with real-world evidence that cross-functional antibody responses to serotype 6C seem to be primarily elicited by the serotype 6A antigen.17,32 In a European surveillance study comparing vaccine effectiveness of PCV13 and 10-valent PCV (PCV10) (which contains serotype 6B but not 6A) in children <5 years of age from 2012 to 2018, PCV13 provided protection against serotype 6C, whereas PCV10 effectiveness against serotype 6C was not observed, despite the inclusion of serotype 6B in the vaccine.32 In a US surveillance study comparing IPD incidence in the pre-PCV13 era (2007–2008) to the period following PCV13 introduction (2013–2014), a significant decrease in non-vaccine-type IPD was seen in healthy adults 19–64 years of age. As serotype 6C was one of the main non-vaccine-type serotypes causing IPD in the pre-PCV13 era, this decrease was likely attributable to cross-protection of serotype 6A against serotype 6C.17
This post-hoc study was primarily limited by its small sample size. While the cross-reactive response to serotype 6C was quantitatively higher in toddlers than in adults, the sample size for toddlers (n = 150) was relatively smaller than the sample size for adults (n = 250). In addition, owing to limitations in blood volume in toddlers, pre-absorption tests were only performed on adult samples.
In conclusion, V114 induces cross-reactive functional antibodies to serotype 6C in adult and pediatric populations that are specific and comparable to those induced by PCV13, as demonstrated by observed OPA responses to serotypes 6A, 6B, and 6C, as well as the results of the OPA inhibition assays. Based on experience with PCV13, V114 may also have a beneficial impact on disease caused by serotype 6C; however, this will have to be evaluated in real-world studies.
Acknowledgments
The authors would like to thank the patients, their families, and all investigators involved in this study. Medical writing support, including assisting the authors with the development of the initial draft and incorporation of comments, was provided by Cindy Cheung, MBBS (MD), of Scion, London; and editorial support, including figure preparation, formatting, proofreading, and submission, was provided by Ian Norton, PhD, of Scion, London, UK, supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, according to Good Publication Practice guidelines (Link). The sponsor was involved in the study design and collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
Funding Statement
This study was supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author contributions
All authors are responsible for the work described in this paper and meet ICMJE authorship criteria. All authors were involved in at least one of the following: conception, design of work or acquisition, analysis, interpretation of data, drafting the manuscript and/or revising/reviewing the manuscript for important intellectual content. All authors provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure statement
R.B. is an employee and co-founder of SunFire Biotechnologies LLC, which received funding from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA to perform this project. R.D.M. and L.M. were employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA at the time of the study and may own stock and/or stock options in Merck & Co., Inc., Rahway, NJ, USA. All other authors are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may own stock and/or stock options in Merck & Co., Inc., Rahway, NJ, USA.
Data sharing
The data sharing policy, including restrictions, of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA is available at http://engagezone.msd.com/ds_documentation.php through the EngageZone site or via e-mail to dataaccess@merck.com.
References
- 1.Cooper D, Yu X, Sidhu M, Nahm MH, Fernsten P, Jansen KU.. The 13-valent pneumococcal conjugate vaccine (PCV13) elicits cross-functional opsonophagocytic killing responses in humans to Streptococcus pneumoniae serotypes 6C and 7A. Vaccine. 2011;29:7207–6. doi: 10.1016/j.vaccine.2011.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant LR, O’Brien SE, Burbidge P, Haston M, Zancolli M, Cowell L, Johnson M, Weatherholtz RC, Reid R, Santosham M, et al. Comparative immunogenicity of 7 and 13-valent pneumococcal conjugate vaccines and the development of functional antibodies to cross-reactive serotypes. PLoS One. 2013;8:e74906. doi: 10.1371/journal.pone.0074906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Pink Book: epidemiology and prevention of vaccine-preventable diseases. 14th ed. Chapter 17: pneumococcal disease [Internet]. Centers for Disease Control and Prevention; 2021. [accessed 2023 June 12]. https://www.cdc.gov/vaccines/pubs/pinkbook/pneumo.html. [Google Scholar]
- 4.Pneumococcal disease [Internet]. World Health Organization; 2021. [accessed 2021 Nov 29]. https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/pneumococcal-disease.
- 5.Current epidemiology of pneumococcal disease and pneumococcal vaccine coverage among children, U.S. [Internet]. Gierke R; 2022. [accessed 2022 Sept 8]. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-02-23-24/02-pneumococcal-gierke-508.pdf.
- 6.Pneumococcal 7-valent conjugate vaccine (diphtheria CRM197 protein) Prevnar® [Internet]. Pfizer; 2009. [accessed 2021 Aug 16]. http://labeling.pfizer.com/showlabeling.aspx?id=134. [Google Scholar]
- 7.Lochen A, Croucher NJ, Anderson RM. Divergent serotype replacement trends and increasing diversity in pneumococcal disease in high income settings reduce the benefit of expanding vaccine valency. Sci Rep. 2020;10:18977. doi: 10.1038/s41598-020-75691-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park IH, Moore MR, Treanor JJ, Pelton SI, Pilishvili T, Beall B, Shelly MA, Mahon BE, Nahm MH. Active bacterial core surveillance team. Differential effects of pneumococcal vaccines against serotypes 6A and 6C. J Infect Dis. 2008;198:1818–22. doi: 10.1086/593339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, Nahm MH. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol. 2007;45:1225–33. doi: 10.1128/JCM.02199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park IH, Park S, Hollingshead SK, Nahm MH. Genetic basis for the new pneumococcal serotype, 6C. Infect Immun. 2007;75:4482–9. doi: 10.1128/IAI.00510-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carvalho Mda G, Pimenta FC, Gertz RE, Jr., Joshi HH, Trujillo AA, Keys LE, Findley J, Moura IS, Park IH, Hollingshead SK, et al. PCR-based quantitation and clonal diversity of the current prevalent invasive serogroup 6 pneumococcal serotype, 6C, in the United States in 1999 and 2006 to 2007. J Clin Microbiol. 2009;47:554–9. doi: 10.1128/JCM.01919-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs MR, Bajaksouzian S, Bonomo RA, Good CE, Windau AR, Hujer AM, Massire C, Melton R, Blyn LB, Ecker DJ, et al. Occurrence, distribution, and origins of Streptococcus pneumoniae serotype 6C, a recently recognized serotype. J Clin Microbiol. 2009;47:64–72. doi: 10.1128/JCM.01524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nahm MH, Lin J, Finkelstein JA, Pelton SI. Increase in the prevalence of the newly discovered pneumococcal serotype 6C in the nasopharynx after introduction of pneumococcal conjugate vaccine. J Infect Dis. 2009;199:320–5. doi: 10.1086/596064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nunes S, Valente C, Sa-Leao R, de Lencastre H. Temporal trends and molecular epidemiology of recently described serotype 6C of Streptococcus pneumoniae. J Clin Microbiol. 2009;47:472–4. doi: 10.1128/JCM.01984-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.PREVNAR 13 (Pneumococcal 13-valent conjugate vaccine [Diphtheria CRM197 Protein]) [Internet]. Pfizer; 2017. [accessed 2022 Sept 29]. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM574852.pdf. [Google Scholar]
- 16.Prevnar 13 summary of product characteristics [Internet]. Pfizer; 2020. [accessed 2022 Nov 16]. https://www.medicines.org.uk/emc/medicine/22689#DOCREVISION. [Google Scholar]
- 17.Ahmed SS, Pondo T, Xing W, McGee L, Farley M, Schaffner W, Thomas A, Reingold A, Harrison LH, Lynfield R, et al. Early impact of 13-valent pneumococcal conjugate vaccine use on invasive pneumococcal disease among adults with and without underlying medical conditions-United States. Clin Infect Dis. 2020;70:2484–92. doi: 10.1093/cid/ciz739. [DOI] [PubMed] [Google Scholar]
- 18.Porat N, Benisty R, Givon-Lavi N, Trefler R, Dagan R. The impact of pneumococcal conjugate vaccines on carriage of and disease caused by Streptococcus pneumoniae serotypes 6C and 6D in southern Israel. Vaccine. 2016;34:2806–12. doi: 10.1016/j.vaccine.2016.04.043. [DOI] [PubMed] [Google Scholar]
- 19.Jayasinghe S, Menzies R, Chiu C, Toms C, Blyth CC, Krause V, McIntyre P. Long-term impact of a “3 + 0” schedule for 7- and 13-valent pneumococcal conjugate vaccines on invasive pneumococcal disease in Australia, 2002–2014. Clin Infect Dis. 2017;64:175–83. doi: 10.1093/cid/ciw720. [DOI] [PubMed] [Google Scholar]
- 20.Fenoll A, Ardanuy C, Linares J, Cercenado E, Marco F, Fleites A, Rodriguez-Mayo M, Lopez-Hontangas JL, Palop B, Aller AI, et al. Serotypes and genotypes of S. pneumoniae isolates from adult invasive disease in Spain: a 5-year prospective surveillance after pediatric PCV13 licensure. The ODIN study. Vaccine. 2018;36:7993–8000. doi: 10.1016/j.vaccine.2018.10.098. [DOI] [PubMed] [Google Scholar]
- 21.Narvaez PO, Gomez-Duque S, Alarcon JE, Ramirez-Valbuena PC, Serrano-Mayorga CC, Lozada-Arcinegas J, Bastidas A, Gomez S, Vargas H, Feldman C, et al. Invasive pneumococcal disease burden in hospitalized adults in Bogota, Colombia. BMC Infect Dis. 2021;21:1059. doi: 10.1186/s12879-021-06769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.VAXNEUVANCE™ (Pneumococcal 15-valent conjugate vaccine) prescribing information [Internet]. Food And Drug Administration; 2021. [accessed 2023 Apr 4]. https://www.fda.gov/vaccines-blood-biologics/vaccines/vaxneuvance.
- 23.Vaxneuvance summary of product characteristics [Internet]. Merck & Co. I; 2022. [accessed 2022 Nov 29]. https://www.ema.europa.eu/en/documents/product-information/vaxneuvance-epar-product-information_en.pdf.
- 24.VAXNEUVANCE® product monograph [Internet]. Health Canada; 2022. [accessed 2022 Aug 26]. https://www.merck.ca/en/wp-content/uploads/sites/20/2022/06/VAXNEUVANCE-PM_E.pdf.
- 25.Song JY, Moseley MA, Burton RL, Nahm MH. Pneumococcal vaccine and opsonic pneumococcal antibody. J Infect Chemother. 2013;19:412–25. doi: 10.1007/s10156-013-0601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H, Nahm MH, Burton R, Kim KH. Immune response in infants to the heptavalent pneumococcal conjugate vaccine against vaccine-related serotypes 6A and 19A. Clin Vaccine Immunol. 2009;16:376–81. doi: 10.1128/CVI.00344-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stacey HL, Rosen J, Peterson JT, Williams-Diaz A, Gakhar V, Sterling TM, Acosta CJ, Nolan KM, Li J, Pedley A, et al. Safety and immunogenicity of 15-valent pneumococcal conjugate vaccine (PCV-15) compared to PCV-13 in healthy older adults. Hum Vaccin Immunother. 2019;15:530–9. doi: 10.1080/21645515.2018.1532249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Platt HL, Greenberg D, Tapiero B, Clifford RA, Klein NP, Hurley DC, Shekar T, Li J, Hurtado K, Su SC, et al. A phase II trial of safety, tolerability and immunogenicity of V114, a 15-valent pneumococcal conjugate vaccine, compared with 13-valent pneumococcal conjugate vaccine in healthy infants. Pediatr Infect Dis J. 2020;39:763–70. doi: 10.1097/INF.0000000000002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burton RL, Nahm MH. Development of a fourfold multiplexed opsonophagocytosis assay for pneumococcal antibodies against additional serotypes and discovery of serological subtypes in Streptococcus pneumoniae serotype 20. Clin Vaccine Immunol. 2012;19:835–41. doi: 10.1128/CVI.00086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park S, Parameswar AR, Demchenko AV, Nahm MH. Identification of a simple chemical structure associated with protective human antibodies against multiple pneumococcal serogroups. Infect Immun. 2009;77:3374–9. doi: 10.1128/IAI.00319-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou J, Lottenbach KR, Barenkamp SJ, Reason DC. Somatic hypermutation and diverse immunoglobulin gene usage in the human antibody response to the capsular polysaccharide of Streptococcus pneumoniae Type 6B. Infect Immun. 2004;72:3505–14. doi: 10.1128/IAI.72.6.3505-3514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savulescu C, Krizova P, Valentiner-Branth P, Ladhani S, Rinta-Kokko H, Levy C, Mereckiene J, Knol M, Winje BA, Ciruela P, et al. Effectiveness of 10 and 13-valent pneumococcal conjugate vaccines against invasive pneumococcal disease in European children: SpIDnet observational multicentre study. Vaccine. 2022;40:3963–74. doi: 10.1016/j.vaccine.2022.05.011. [DOI] [PubMed] [Google Scholar]


