Abstract
Lack of perforin renders the relatively resistant mouse strain C57BL/6 highly susceptible to the natural mouse pathogen ectromelia virus, a cytopathic orthopoxvirus. This is indicated by increased mortality, elevated virus titers and pathology in liver and spleen, and increased levels of liver enzymes in blood. Cowpox virus on the other hand is more virulent in the presence of perforin than in its absence. An additional lack of granzyme A which together with perforin is a constituent of cytoplasmic granules from cytotoxic T cells increases the virulence of cowpox virus.
Cytototoxic T (Tc) cells are of primary importance in the recovery of mice from infection with mousepox ectromelia virus (ECT) (1, 2). Tc cells exert their effector function by two very different mechanisms, with one being mediated by cytokines such as gamma interferon and interleukins (11) and the other being mediated by cytotoxic molecules. To date two major pathways of target cell killing by cytolytic leukocytes (mainly natural killer [NK] and Tc cells) have been described. Firstly, the granule exocytosis pathway mediated by perforin or cytolysin and serine proteases or granzymes (Gzm) (5, 13). This is generally believed to be the dominant mechanism by which Tc and NK cells eliminate virus-infected cells (7). The second mechanism, called the Fas-mediated pathway, requires the interaction of the Fas receptor on the target cell with the Fas ligand on the killer cell (14) and is supposedly involved in immunregulation and tolerance (12).
From the three main constituents of the granules involved in the exocytosis pathway—perforin and the two Gzm, GzmA and GzmB—only GzmA has been thoroughly investigated so far for its role in recovery of mice from ECT infection. By comparing gzmA−/− knockout (KO) mice with wild-type C57BL/6 (B6) mice (10) it was found that the lack of GzmA does not affect the cytolytic potential of ECT-immune Tc cells but leads to increased mortality and morbidity, as well as higher virus titers and tissue damage in liver and spleen. The actual role of GzmA in recovery from ECT infection is still elusive but possibly involves reduction of progeny virus infectivity by its own proteolyic activity or via secondary mediators (10). As for the role of perforin, conclusions have been reached only in regard to the non-mouse pathogen vaccinia virus (VV). In their study, concerning a comparison of the role of perforin on protection against cytopathic and noncytopathic viruses, Kägi et al. (8) came to the conclusion that cytopathic viruses are not controlled by perforin, as mice survived infection with this virus in the absence of perforin. However, it was already known that mice survive even high doses of VV in the absence of CD8+ T cells (16).
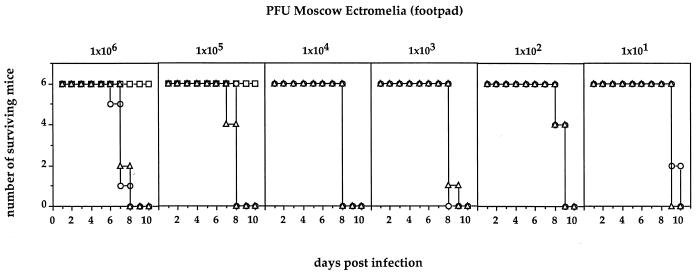
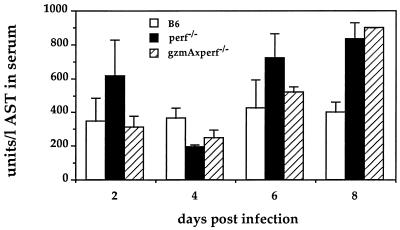
Thus, it was of interest to us to have a fresh look at the role of perforin in the survival of mice to two additional poxvirus infections, one being the natural pathogen ECT and the other being cowpox virus (CPV). The virulent Moscow strain of ECT was grown in mice and prepared from infected spleens and titrated as described previously (10). CPV was grown on CV-1 cell monolayers and titrated as was ECT. B6 animals are relatively resistant to ECT administered via the hind footpad. Doses of >106 PFU of virulent Moscow strain are required to cause disease ending in mortality. We used three strains of mice, wild-type B6, the perforin-defective KO strain (6) (perf−/−), and the double-KO mouse lacking both the perforin and gzmA genes (gzmA−/− × perf−/−). The latter strain was obtained by crossing the perf−/− mice with the gzmA−/− mice (4) and breeding to homozygosity. All animals were monitored for the correct genotype by PCR analysis as has been described (see reference 15 and the legend to Fig. 1). In Fig. 1 the results of a dose-response experiment using ECT, ranging from 101 to 106 PFU/mouse (administered via the footpad), are illustrated. B6 mice were only infected with the two highest doses, and although morbidity was noticed no mortality occurred. On the other hand, mice of the two KO strains started to die at the highest dose 6 to 7 days postinfection and all had died at day 8. Even at the lowest inoculum of only 10 PFU, the mutant mice started to die at day 9 and all had succumbed by day 10. These results indicate that the animals are as susceptible to ECT as the least-resistant strains known (3) and clearly point to perforin’s being of paramount importance in the recovery from primary ECT infection. No statistically significant differences were found between the single perf−/− KO mice and the double-KO mice also defective in GzmA, again suggesting perforin is the overriding prerequisite for survival. However as shown before, the presence of GzmA contributes to control of ECT infection by a mechanism(s) other than cytolytic activity (10). To obtain a more detailed analysis, a kinetic study was undertaken using the three mouse strains and an infectious dose of 102 PFU of ECT. Three individual mice of each strain were sacrificed 2, 4, 6, and 8 days after infection. Livers and spleens were analyzed for virus titers (Table 1) and histology and blood samples were assayed for liver enzyme (Fig. 2). Virus was not detectable 2 days postinfection in any of the mouse strains. In B6 mice virus titers in liver and spleen reached a maximum by day 6 and then declined in liver to undetectable levels by day 8. In the perf−/− and gzmA−/− × perf−/− mice virus titers were at least 1 log higher in both organs on day 6. By day 8 the difference was at least 3 logs in liver and up to 2 logs in spleen. One animal of the perf−/− genotype had died by day 8 (Table 1). Histological examinations of liver and spleen mirror virus load, with increased necrosis and cellular infiltration in the KO mice from day 6 on compared to B6 wild-type mice (data not shown). Using an objective assay of liver damage, namely levels in the blood of the liver-derived enzyme aspartate aminotransferase (AST) (10), and using the same animals for which virus titer determinations (Table 1) and histology examinations were undertaken, we found that at day 8 postinfection, despite the generally observed high variability in this assay (10, 17) the perf−/− and gzmA−/− × perf−/− mice had significantly higher liver enzyme levels than B6 mice (Fig. 2).
FIG. 1.
Dose-response curves for ECT infection via the footpad of C57BL/6 (□), perforin-deficient (○), and GzmA-plus perforin-deficient (▵) mice. Surviving mice were monitored for 21 days. For detection of the respective mutations, DNA of all individual mice was analyzed by PCR, as described previously (15), using the following primers: for gzmA−/− mice, 5′-AGG AGC AAT ATA TAC CAA TGG-3′ and 5′-AGG TAG GTG AAG GAT AGC CAC-3′ (neo-primer 5′-CGG AGA ACC TGC GTG CAA TC-3′); for perf−/− mice, 5′-CCA CTC CAC CTT GAC TTC AAA AAG GCG-3′ and 5′-TGG GCA GCA GTC CTG GTT GGT GAC CTT-3′.
TABLE 1.
Kinetics of virus titers in liver and spleen of individual micea infected with ECT
| Time postinfection (day) | Virus titer in indicated organ of mouse strain
|
|||||
|---|---|---|---|---|---|---|
| B6
|
perf−/−
|
gzmA−/− × perf−/−
|
||||
| Liver | Spleen | Liver | Spleen | Liver | Spleen | |
| 2 | <102 | <102 | <102 | <102 | <102 | <102 |
| <102 | <102 | <102 | <102 | <102 | <102 | |
| <102 | <102 | <102 | <102 | <102 | <102 | |
| 4 | 3 × 103 | 3 × 104 | 3 × 102 | 3 × 103 | 3 × 103 | 2 × 105 |
| 1 × 102 | 1 × 103 | <102 | 2 × 103 | 2 × 103 | 3 × 105 | |
| 2 × 102 | 1 × 104 | <102 | 1 × 103 | 4 × 103 | 2 × 105 | |
| 6 | 2 × 103 | 2 × 104 | 1 × 105 | 2 × 107 | 6 × 105 | 5 × 107 |
| 2 × 105 | 9 × 105 | 3 × 105 | 4 × 107 | 1 × 106 | 3 × 107 | |
| 2 × 105 | 2 × 106 | 3 × 105 | 6 × 107 | 4 × 105 | 6 × 107 | |
| 8 | <102 | 2 × 104 | 6 × 105 | 8 × 106 | 4 × 105 | 4 × 106 |
| <102 | 3 × 105 | 2 × 105 | 9 × 106 | 6 × 105 | 2 × 107 | |
| <102 | 2 × 105 | †b | † | 5 × 105 | 4 × 107 | |
Mice were infected via the footpad with 102 PFU of ECT (Moscow strain). At the indicated time postinfection, livers and spleens of individual mice were removed and virus titers were determined as described previously (10).
†, dead.
FIG. 2.
Liver enzyme levels in serum of ECT-infected mice. Shown are mean AST levels in units per liter of serum (± standard deviations [error bars]) from three individual animals—B6 (□), perf−/− (■), and gzmA−/− × perf−/− (□)—immunized via the footpad with 102 PFU of ECT (Moscow strain). Values are enzyme levels from a single surviving animal.
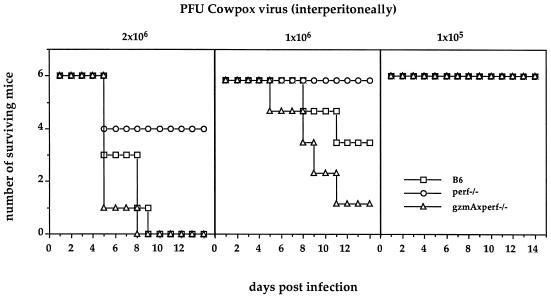
In contrast to ECT, CPV is much less virulent in mice, and mortality is obtained most consistently after intraperitoneal infections. The same three strains of mice were infected with 105, 1 × 106, and 2 × 106 PFU of CPV (Fig. 3). None of the animals died as a result of receiving the lowest concentration. At the two higher doses it was found that the lack of both GzmA and perforin made the double-KO mice more susceptible than wild-type B6 mice. This was especially significant at 106 PFU. Comparing perf−/− mice with wild-type B6 mice, the absence of perforin provided a significant protective effect, with 0 versus 50% mortality at 106 PFU of CPV and only 30 versus 100% mortality at the highest dose, respectively. This striking contrast in virulence between these two closely related cytopathic orthopoxviruses, ECT and CPV, in the presence or absence of perforin must reflect totally different pathogenic mechanisms which control these two viruses. Thus, for a meaningful interpretation of host-parasite relationships only natural pathogens will uncover strategies of either host or virus which are of evolutionary significance.
FIG. 3.
Dose-response curves for (intraperitoneally induced) CPV infection of C57BL/6 (□), perforin-deficient (○), and the GzmA-plus perforin-deficient (▵) mice. Surviving mice were monitored for 21 days.
The finding that perforin is essential in the recovery of mice from ECT infection questions the proposed role of poxvirus-encoded serpins, one of interfering in the death pathway, in the evasion of poxviruses from the Tc cell response (9). Thus, perforin is an essential element in the survival strategy of mice to recover from cytopathic and noncytopathic viruses.
Acknowledgments
This work was supported in part by a grant from the Deutsche Forschungsgemeinschaft (Si 214/7-1).
REFERENCES
- 1.Blanden R V. Mechanisms of recovery from a generalized viral infection: mousepox. II. Passive transfer of recovery mechanisms with immune lymphoid cells. J Exp Med. 1971;133:1074–1089. doi: 10.1084/jem.133.5.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanden R V. Progress in immunology. II. Clinical aspects. In: Brent L, Holborow J, editors. Mechanisms of cell-mediated immunity in viral infection. Amsterdam, The Netherlands: North Holland; 1974. pp. 117–125. [Google Scholar]
- 3.Blanden R V, Deak B D, McDevitt H O. Immunology of virus diseases, 125–138. In: Blanden R V, editor. Strategies in virus-host interactions. Curtin, Australia: Brolga Press; 1989. [Google Scholar]
- 4.Ebnet K, Hausmann M, Lehmann-Grube F, Müllbacher A, Kopf M, Lamers M, Simon M M. Granzyme A-deficient mice retain potent cell-mediated cytotoxicity. EMBO J. 1995;14:4230–4239. doi: 10.1002/j.1460-2075.1995.tb00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henkart P A. Lymphocyte-mediated cytotoxicity: two pathways and multiple effector molecules. Immunity. 1994;1:343–346. doi: 10.1016/1074-7613(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 6.Kägi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 7.Kägi D, Ledermann B, Bürki K, Zinkernagel R M, Hengartner H. Lymphocyte-mediated cytotoxicity in vitro and in vivo: mechanisms and significance. Immunol Rev. 1995;146:95–115. doi: 10.1111/j.1600-065x.1995.tb00686.x. [DOI] [PubMed] [Google Scholar]
- 8.Kägi D, Seiler P, Pavlovic P, Ledermann B, Bürki K, Zinkernagel R M, Hengartner H. The roles of perforin- and fas-dependent cytotoxicity in protection against cytopathic and non cytopathic viruses. Eur J Immunol. 1995;25:3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- 9.Macen J L, Garner R S, Musy P Y, Brooks M A, Turner P C, Moyer R W, McFadden G, Bleackley R C. Differential inhibition of Fas- and granule-mediated cytolysis pathways by the orthopoxvirus cytokine response modifier A/SPI-2 and SPI-1 protein. Proc Natl Acad Sci USA. 1996;93:9108–9113. doi: 10.1073/pnas.93.17.9108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Müllbacher A, Ebnet K, Blanden R V, Stehle T, Museteanu C, Simon M M. Granzyme A is essential for recovery of mice from infection with the natural cytopathic viral pathogen, ectromelia. Proc Natl Acad Sci USA. 1996;93:5783–5787. doi: 10.1073/pnas.93.12.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nabholz M, MacDonald H R. Cytolytic T lymphocytes. Annu Rev Immunol. 1983;1:273–306. doi: 10.1146/annurev.iy.01.040183.001421. [DOI] [PubMed] [Google Scholar]
- 12.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 13.Podack E R, Hengartner H, Lichtenheld M G. A central role of perforin in cytolysis? Annu Rev Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- 14.Rouvier R, Luciani M-F, Golstein P. Fas involvement in Ca2+-independent T-cell-mediated cytotoxicity. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simon M M, Hausmann M, Tran T, Ebnet K, Tschopp J, Tha Hla R, Müllbacher A. In vitro and ex vivo-derived cytolytic leukocytes from granzyme AxB double knockout mice are defective in granule-mediated apoptosis but not lysis of target cells. J Exp Med. 1997;186:1781–1786. doi: 10.1084/jem.186.10.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spriggs M K, Koller B H, Sato T, Morrissey P J, Fanslow W C, Smithies O, Voice R F, Widmer M B, Maliszewski C R. Beta 2-microglobulin-, CD8+ T-cell-deficient mice survive inoculation with high doses of vaccinia virus and exhibit altered IgG responses. Proc Natl Acad Sci USA. 1992;89:6070–6074. doi: 10.1073/pnas.89.13.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zinkernagel R M, Haenseler E, Leist T, Cerny A, Hengartner H, Althage A. T cell-mediated hepatitis in mice infected with lymphocytic choriomeningitis virus. Liver cell destruction by H-2 class I-restricted virus-specific cytotoxic T cells as a physiological correlate of the 51-Cr-release assay? J Exp Med. 1986;164:1075–1092. doi: 10.1084/jem.164.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]