ABSTRACT
Objectives:
Laparoscopic hepatectomy (LH) is still technically challenging for patients with previous nonhepatectomy abdominal surgery (AS). Therefore, this study aimed to assess the difficulty of performing LH for patients with hepatocellular carcinoma (HCC) and a history of nonhepatectomy AS during the initial developing period of LH.
Materials and Methods:
The retrospective study enrolled patients who were newly diagnosed with HCC receiving LH from January 2013 to June 2021. Demographic characteristics, perioperative variables, and surgical complications were prospectively collected.
Results:
One hundred patients were reviewed consecutively, comprising 23 in the AS group and 77 in the non-AS group. No significant differences were observed in median IWATE score (5 vs. 5, P = 0.194), operative time (219 vs. 200 min, P = 0.609), blood loss (100.0 vs. 200.0 mL, P = 0.734), transfusion rate (4.3% vs. 10.4%, P = 0.374), duration of parenchyma transection (90.0 vs. 72.4 min, P = 0.673), and mean nonparenchymal transection time (191.0 vs. 125.0 min, P = 0.228), without increasing the conversion rate (0.0% vs. 3.9%, P = 0.336), postoperative complications (30.3% vs. 33.8%, P = 0.488), and postoperative hospital stay (6 vs. 7 days, P = 0.060) in AS group and non-AS groups.
Conclusion:
History of previous nonhepatectomy AS can lead to longer nonparenchymal transection time instead of conversion and did not increase the difficulty. Prolonged nonparenchymal transection time did not increase the surgical complications, prolong the postoperative hospital stay, and compromise the survival outcomes.
KEYWORDS: Abdomen/surgery, hepatectomy, laparoscopy, operative time, outcome
INTRODUCTION
Hepatocellular carcinoma (HCC) was ranked as the sixth most common neoplasm and the third leading cause of cancer death worldwide in 2020 with 905,677 diagnosed cases and 830,180 deaths [1]. Surgical resection is an effective treatment for lesions limited to an acceptable condition and well-preserved liver function under the suggestion of Barcelona Clinic Liver Cancer strategy [2]. Traditionally, open hepatectomy for liver malignancy is a common surgical procedure, especially in Asian countries. In the past 20 years, laparoscopic hepatectomy (LH) has been gradually performed in liver surgery. Although several favorable results of LH have emerged [3-5], this approach has still not been widely developed, especially in patients with a history of various abdominal surgeries.
Some reasons that may explain why LH is not widely accepted in earlier decades are as follows [6]: difficulty in approaching the posterosuperior lesions, vascular control, inability to perform manual compression or suture for the bleeding, working with the deep intrahepatic areas, and intraoperative hazards, such as gas embolism, massive bleeding, and bile duct injury [7,8]. Moreover, in patients with a history of upper abdominal surgery (AS), postoperative changes such as adhesions at the liver surface and hepatoduodenal ligament may increase the difficulties and challenges during the laparoscopic approach [9]. Recently, several reports focused on how to overcome the current limitations of LH based on tumor location and underlying liver cirrhosis [10,11]. However, only a few reports discussed the condition of patients with a history of whole AS and then underwent LH. The importance and application of liver resection in this group of patients have gradually increased annually.
Therefore, the current study aimed to evaluate the difficulty and perioperative and postoperative outcomes of LH in patients diagnosed with primary HCC and with a history of nonhepatectomy AS.
MATERIALS AND METHODS
Patients and categorization
Data of patients with primary HCC undergoing LH at a tertiary referral center in eastern Taiwan from January 2013 to June 2021 were retrospectively collected. Patients diagnosed with recurrent HCC and synchronous malignancy were all excluded. A history of nonhepatectomy AS was defined as any operation entering the peritoneal cavity. Upper AS included previous abdominal surgeries with scars over the midline or paramedian incisions above the umbilicus and lower AS included surgeries with scars located below the umbilicus [12]. Transverse or oblique abdominal incisions were also classified as upper or lower AS based on their umbilical level. The history of cholecystectomy was highlighted from the upper AS as it would make more tissue reaction around the hepatoduodenal ligament and interfere with the Pringle maneuver preparation. Indocyanine green (ICG) 15-min retention rate was measured noninvasively on the day before liver resection. An ICG (25 mg) dissolved in saline (10 mL) was injected through a peripheral vein. The injected ICG dosage was 0.5 mg per kg of the patient. Patients were classified into the following two groups depending on the history of nonhepatectomy AS: patients with a history of nonhepatectomy (AS group, n = 23) and patients without a history of any AS (non-AS group, n = 77). The overall median follow-up duration was 29 months. The clinical data for these patients were retrospectively collected using medical records.
Terminology and definitions
The nomenclature from Brisbane 2000 Guidelines for liver anatomy and resection was used to describe the extent of hepatic resection [13]. Major resection was defined as resection of ≥3 segments, otherwise defined as minor resection. The IWATE criteria were used to evaluate the difficulty of laparoscopic liver resection [14]. The parenchymal transection time was defined as the duration of the Pringle maneuver plus the resting time during the Pringle maneuver, whereas the nonparenchymal transection time was defined as the total operation minus parenchymal transection time. The surgical video was also reviewed to calculate the parenchymal transection time for patients without the Pringle maneuver. The Clavien–Dindo classification was used to grade postoperative complications [15].
The definite diagnosis of diabetes mellitus (DM) was glycated hemoglobin level of >6.5% or under regular insulin control. Hypertension (HTN) was defined as systolic blood pressure of ≥140 mmHg or diastolic blood pressure of ≥90 mmHg or taking any antihypertensive medications. Coronary artery disease (CAD) was defined as a history of CAD and undergoing treatment or even treatment based on CAD diagnosis.
Operative technique
First, a 12-mm trocar was inserted at the periumbilical area or virgin zone of the surgical scar in patients with a history of AS. After establishing pneumoperitoneum through the first insertion trocar, a 10-mm laparoscope was introduced, and then, additional 3–4 ports were made based on the adhesion condition and hepatectomy laterality. Adhesiolysis was performed by electrocautery, ultrasonic device (Harmonic scalpel®, Ethicon, Cincinnati, OH, USA), or vessel sealing device (Ligasure, Medtronic, Minneapolis, MN, USA) when the surgical field would be compromised. After adhesiolysis, liver mobilization and hepatoduodenal ligament wrapping would be performed. Active and cycling Pringle maneuver was routinely performed to reduce blood loss and possible hepatocyte protection before bleeding during the parenchymal transection [16]. However, if the Pringle maneuver preparation would be interfered by severe adhesion around the hepatoduodenal ligament, the Pringle maneuver would not be adopted by the surgeon intraoperatively. Intraoperative sonography would be applied to identify and determine the tumor location, margin, and associated vascular distribution in the liver. Parenchymal transection was performed by electrocautery, ultrasonic device, or vessel sealing device under performing the Pringle maneuver. After completing the liver resection, meticulous hemostasis for the resection plane and the placement of a closed drainage tube was performed. The specimen was placed into the commercial tissue bag and extracted through the incision extension as per the surgeon’s preference before wound closure. No hand-assisted technique was used in our series.
Statistical analysis
The Chi-square test was used to analyze for categorical variables, which were presented as numbers and percentages. Continuous variables were presented as medians with interquartile ranges and were analyzed with the Mann–Whitney U-test. The Kaplan–Meier curve with the log-rank test was used for the survival analysis. SPSS for MAC ver. 26 (SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. P < 0.05 was considered statistically significant.
Ethic declaration
Ethical approval for this study (Research Ethics Committee, REC No. IRB 109-074-B) was provided by the Research Ethics Committee of Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation. Written informed consent was waived because the study was a retrospective data analysis.
RESULTS
The general characteristics of patients are indicated in Table 1, including 23 (23.0%) and 77 (77.0%) patients in the AS and non-AS groups, respectively. Male patients were dominant, and comorbidity rates, including DM, HTN, and CAD were not significantly different in both the groups. The mean age of patients was similar in both the groups (67 vs. 66 in the AS and non-AS groups, respectively; P = 0.684). The preoperative cardiopulmonary function tests showed that the mean left ventricular ejection fraction was similar in the AS and non-AS groups (76.6% vs. 73.5%, P = 0.816), and the mean ratio of forced expiratory volume in 1 s to forced vital capacity was worse in the AS group (69.7% vs. 78.8%, P = 0.020). The majority of patients in both the groups were classified Child–Pugh class A. The median MELD-Na score (7 vs. 8, P = 0.321), albumin–bilirubin grade (P = 0.295), and preoperative ICG 15-min retention rate (9.3% vs. 11.0%, P = 0.600) were not significantly different in the AS and non-AS groups. The viral hepatitis status of both the groups was also similar with 53.0% HBV, 21.0% HCV, 13.0% HBV/HCV, and 13.0% non-HBV/HCV. The distribution of previous abdominal surgeries is listed in Table 2. The majority of patients (43.5% in both the groups) underwent previous upper and lower abdominal surgeries.
Table 1.
Patient characteristics
| Characteristics | Whole cohort (n=100) | AS group (n=23) | Non-AS group (n=77) | P |
|---|---|---|---|---|
| Age* | 66±9 | 67±10 | 66±9 | 0.684 |
| Gender, male (%) | 80.0 (80/100) | 78.3 (18/23) | 80.5 (62/77) | 0.812 |
| BMI (kg/m2)§ | 25.6 (23.1-28.8) | 25.5 (22.7-28.0) | 25.6 (23.2-29.5) | 0.258 |
| Comorbidity (%) | ||||
| DM | 44.0 (44/100) | 34.8 (8/23) | 46.8 (36/77) | 0.347 |
| HTN | 55.0 (55/100) | 43.5 (10/23) | 58.4 (45/77) | 0.238 |
| CAD | 8.0 (8/100) | 8.7 (2/23) | 7.8 (6/77) | 0.889 |
| FEV1 (%)* | 89.1±19.7 | 87.0±24.7 | 89.6±18.4 | 0.135 |
| FEV1/FVC (%)* | 77.0±9.7 | 69.7±13.7 | 78.8±7.6 | 0.020 |
| LVEF (%)* | 74.3±9.0 | 76.6±9.2 | 73.5±8.8 | 0.816 |
| Child-Pugh classification Stage A (%) | 99.0 (96/97) | 100.0 (21/21) | 98.7 (75/76) | 0.597 |
| MELD-Na score§ | 8 (7-10) | 7 (7-8) | 8 (7-10) | 0.010 |
| ALBI grade | ||||
| Grade I | 61.9 (60/97) | 76.2 (16/21) | 57.9 (44/76) | 0.295 |
| Grade II | 37.1 (36/97) | 23.8 (5/21) | 40.8 (31/76) | |
| Grade III | 1.0 (1/97) | 0.0 (0/21) | 1.3 (1/76) | |
| ICG-15 (%)§ | 10.5 (5.1-16.6) | 9.3 (4.0-17.2) | 11.0 (6-17) | 0.600 |
| Viral hepatitis status (%) | ||||
| HBV | 53.0 (53/100) | 65.2 (15/23) | 49.4 (38/77) | 0.538 |
| HCV | 21.0 (21/100) | 13.0 (3/23) | 23.4 (18/77) | |
| Non-B, Non-C | 13.0 (13/100) | 13.0 (3/23) | 13.0 (10/77) | |
| HBV pulse HCV | 13.0 (13/100) | 8.7 (2/23) | 14.3 (11/77) |
*Normal distribution, §Nonnormal distribution, AS: Abdominal surgery group, DM: Diabetes mellitus, HTN: Hypertension, CAD: Coronary artery disease, FEV1: Forced expiratory volume in 1 s, FVC: Forced vital capacity, LVEF: Left ventricular ejection fraction, MELD-Na: Model for end-stage liver disease-Na, ALBI grade: Albumin-bilirubin grade, ICG: Indocyanine green, BMI: Body mass index, HBV: Hepatitis B virus, HCV: Hepatitis C virus
Table 2.
Types of previous abdominal surgeries
| Type | n (%) |
|---|---|
| Upper abdominal surgery | 10 (43.5) |
| Cholecystectomy | 3 (13) |
| Lower abdominal surgery | 10 (43.5) |
No differences were observed in the distribution of major hepatectomy between the two groups [Table 3]. The median IWATE difficulty score for LH was 5 (3–7) and 5 (3–8) in the AS and non-AS groups, respectively (P = 0.194). No significant difference was also observed in the median operative time (219 vs. 200 min, P = 0.609), median intraoperative blood loss (100.0 vs. 200.0 mL, P = 0.734), intraoperative transfusion rate (4.3% vs. 10.4%, P = 0.374), median duration of parenchymal transection (90.0 vs. 72.4 min, P = 0.673), and median duration of nonparenchymal transection (191.0 vs. 125.0 min, P = 0.228) between the AS and non-AS groups. The conversion rate was 3.9% (n = 3) in the non-AS group (one experienced uncontrollable bleeding from inferior hepatic vein with unstable hemodynamic status during parenchyma transection; the second one experienced difficulty in approach to inferior vena cava zone with massive bleeding during parenchyma transection; the third one experienced lymphadenopathy at hilar region and group eight region with suspicious cholangiocarcinoma and converted to laparotomy for further lymph nodes dissection before performing parenchyma transection) and none of the patients in the AS group were converted to laparotomy (P = 0.336). The pathology report showed no significant difference of the median tumor size (2.5 vs. 3.0 cm; P = 0.167) and the marginal status (0.3 vs. 0.5 cm; P = 0.217) in the AS and non-AS groups. Most of the tumor was grade II (58.0%). No significant was noted over the ratio of angiolymphatic invasion (52.2% vs. 55.8%, in the AS group and in non-AS group, respectively; P = 0.814). The surgical complication rate did not differ in both the groups (30.3% vs. 33.8% in the AS and non-AS groups, respectively; P = 0.488) mainly in grades 1 and 2, and no 30-day mortality occurred in this series. The postoperative hospital stay was nearly identical in both the groups. No significant difference was observed in the 2-year and overall survival (82.4% vs. 85.9%; P = 0.820) and disease-free survival (65.8% vs. 56.8%; P = 0.155) between AS and non-AS groups [Figures 1 and 2].
Table 3.
Perioperative results, pathology findings, and postoperative results
| Characteristics | Whole cohort (n=100) | AS group (n=23) | Non-AS group (n=77) | P |
|---|---|---|---|---|
| Major resection (%)* | 14.0 (14/100) | 4.3 (1/23) | 16.9 (13/77) | 0.128 |
| IWATE score§ | 5 (3-8) | 5 (3-7) | 5 (3-8) | 0.194 |
| Operative time (min)§ | 204 (157-274) | 219 (156-344) | 200 (158-267) | 0.609 |
| Pringle maneuver (%) | 70.0 (70/100) | 39.1 (9/23) | 79.2 (61/77) | 0.001 |
| Pringle duration (min)§ | 55.5 (32.2-85.8) | 69.2 (41.0-101.5) | 55.0 (31.0-85.0) | 0.424 |
| Parenchymal transection time (min)§ | 80.0 (45.0-122.2) | 90.0 (50.0-136.5) | 72.4 (43.7-118.6) | 0.673 |
| Nonparenchymal transection time (min)§ | 129.0 (87.0-185.0) | 191.0 (75.6-322.8) | 125.0 (90.1-174.5) | 0.228 |
| Blood loss (mL)§ | 200.0 (50.0-500.0) | 100.0 (50.0-900.0) | 200.0 (50.0-425.0) | 0.734 |
| Conversion (%) | 3.0 (3/100) | 0.0 (0/23) | 3.9 (3/77) | 0.336 |
| Intraoperative transfusion (%) | 9.0 (9/100) | 4.3 (1/23) | 10.4 (8/77) | 0.374 |
| Drainage tube placement (%) | 78.0 (78/100) | 78.3 (18/23) | 77.9 (60/77) | 1.000 |
| Solitary tumor (%) | 86.0 (86/100) | 82.6 (19/23) | 87.0 (67/77) | 0.593 |
| Size (cm)§ | 3.0 (2.2-4.1) | 2.5 (1.5-4.0) | 3.0 (2.4-4.3) | 0.167 |
| Margin (cm)§ | 0.5 (0.2-1.2) | 0.3 (0.1-0.9) | 0.5 (0.2-1.4) | 0.217 |
| Histology grading (%) | ||||
| Grade I | 8.0 (8/100) | 13.1 (3/23) | 6.5 (5/77) | 0.107 |
| Grade II | 58.0 (58/100) | 39.1 (9/23) | 63.6 (49/77) | |
| Grade III | 34.0 (34/100) | 47.8 (11/23) | 29.9 (23/77) | |
| Angiolymphatic invasion (%) | 55.0 (55/100) | 52.2 (12/23) | 55.8 (43/77) | 0.814 |
| Ishak score§ | 4 (2-5) | 3 (2-4) | 4 (2-6) | 0.055 |
| Complications (%) | ||||
| 1+2 | 30.0 (30/100) | 26.0 (6/23) | 31.2 (24/77) | 0.488 |
| 3a | 1.0 (1/100) | 0.0 (0/23) | 1.3 (1/77) | |
| 3b | 0.0 (0/100) | 0.0 (0/23) | 0.0 (0/77) | |
| 4a | 1.0 (1/100) | 4.3 (1/23) | 0.0 (0/77) | |
| 4b | 1.0 (1/100) | 0.0 (0/23) | 1.3 (1/77) | |
| B | 0.0 (0/100) | 0.0 (0/23) | 0.0 (0/77) | |
| Time to remove drainage tube (days)§ | 6 (4-7) | 5 (4-6) | 6 (5-7) | 0.142 |
| Postoperative hospital stay (days)§ | 7 (5-8) | 6 (5-7) | 7 (6-8) | 0.060 |
*Normal distribution, §Nonnormal distribution. AS: Abdominal surgery group
Figure 1.
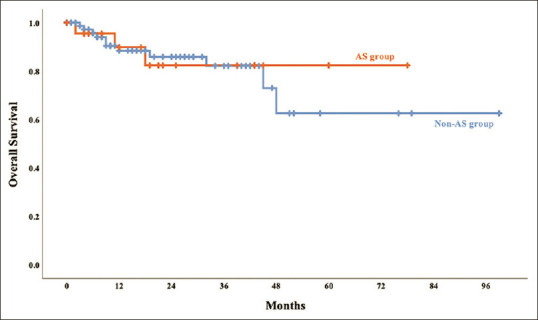
The Kaplan–Meier curve of overall survival in the AS and non-AS groups. The 12-and 24-month overall survival rates were 89.8% and 82.4% in the AS group and 88.4% and 85.9% in the non-AS group. Log-rank test, P = 0.820. AS: Abdominal surgery, non-AS: Nonabdominal surgery
Figure 2.
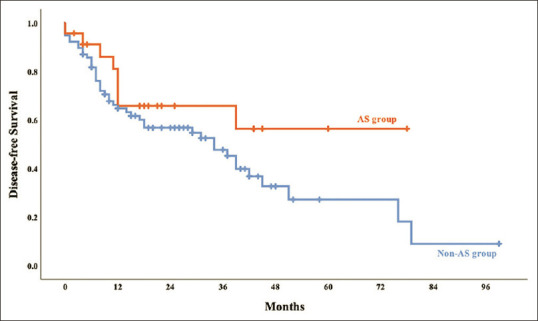
The Kaplan–Meier curve of disease-free survival in the AS and non-AS groups. The 12-and 24-month disease-free survival rates were 81.0% and 65.8% in the AS group and 64.8% and 56.8% in the non-AS group. Log-rank test, P = 0.155. AS: Abdominal surgery, non-AS: Nonabdominal surgery
DISCUSSION
The incidence of peritoneal adhesions is 70%–95% after laparotomy, although 10%–25% of the general population may have peritoneal adhesions even without previous surgery [17-20]. In laparoscopic surgeries, there is an increased risk of conversion to laparotomy, intraoperative complications, and longer operative time [21]. Therefore, a history of AS has been considered a relative contraindication for laparoscopic surgery during the early years of this kind of procedure. However, as the skills, instruments, and video systems of laparoscopy gradually improved annually, laparoscopic interventions, such as cholecystectomy, colectomy, and even gastrectomy, have been safely used in patients with a history of AS [22-25]. As LH is still a technically demanding procedure, only a few reports have demonstrated laparoscopic liver resection performed on patients with a history of abdominal surgical intervention. Therefore, this study focused on analyzing the cohort of patients who underwent LH for primary HCC with a history of nonhepatectomy abdominal surgical intervention.
The LH procedure is performed in the following steps: trocar insertion, possible adhesiolysis, liver mobilization, performing intraoperative sonography for lesion localization, Pringle maneuver preparation, parenchyma transection, and hemostasis. Several studies had already reported that a history of AS would prolong the LH total operative time, including all of the abovementioned procedures within 15–91 min [9,12,26-29]. Overall, the duration for trocar insertion, liver mobilization, performing intraoperative sonography for lesion localization, and the Pringle maneuver preparation did not significantly vary in each operation. However, the varied operative time would be caused by difficulty in performing adhesiolysis, tumor location, and size of the transection plan. Consequently, prolonged operative time cannot be only attributed to adhesion caused by previous AS.
Unlike previous studies that only determined the overall operative time, this is the first study that divided the operative time into parenchymal and nonparenchymal transection phases. The total operative time was divided into two phases to identify the duration and complexity of adhesiolysis during the nonparenchymal transection phase, which may indicate the difficulty caused by previous nonhepatectomy surgery and specifically attribute the difficulty of tumor resection to parenchymal transection phase. Besides, we also calculated the IWATE difficult score to identify the difficulty of tumor resection. Under this condition, a previous AS led to longer nonparenchymal transection time of up to 66 min specifically for the adhesiolysis procedure. Conversely, the duration of parenchymal transection and the mean IWATE difficulty score were not significantly different in patients who underwent AS or not. In addition, based on learning from extensive experiences of adhesiolysis using the laparoscopic intraperitoneal onlay mesh technique in our daily practice, no adhesiolysis-related conversion occurred in the AS group although prolonged nonparenchymal transection time, with the conversion rate of approximately 4.5%–14.3% mainly caused adhesion according to previous reports [9,12,28].
With regard to other parameters, such as blood loss, transfusion rate, and postoperative hospital stay, previous surgery was not interfered by but was compatible with several previous studies [9,12,27,30-33]. When comparing complications reported in a previous study, i.e., 13.2%–31.0% and 13.5%–17.0% in AS and non-AS groups [9,12,28], major complications (above grade 3) were 4.3% and 2.6% in the AS and non-AS groups, respectively, without mortality in our series. With the adequate and delicate adhesiolysis during the operation, it will not increase the difficulty in parenchyma transection. It will not increase the short-term complication and will not compromise the long-term survival.
This study has some limitations. First, this retrospective and nonrandomized review had a relatively small sample size. Second, the specific grading system for adhesion severity was not used in this study. Third, the effects of the interval between previous nonhepatectomy AS and LH index cannot be investigated. Fourth, most of the patients in AS group received minor resection, though not achieved significant, but was not similar to the ratio of minor resection of non-AS group.
CONCLUSIONS
A history of nonhepatectomy abdominal operation leads to a longer nonparenchymal transection time of approximately 66 min. Prolonged nonparenchymal transection time indicated more delicate and complicated adhesiolysis and did not indicate the LH difficulty and also did not cause adhesion-related conversion. Surgical complications, postoperative hospital stay, and survival outcomes were not compromised. LH did not increase the difficulty in patients with previous nonhepatectomy AS without causing a conversion.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Financial support and sponsorship
Nil.
Conflicts of interest
Dr. Ming-Che Lee, an editorial board member at Tzu Chi Medical Journal, had no role in the peer review process of or decision to publish this article. The other authors declared no conflicts of interest in writing this paper.
REFERENCES
- 1.International Agency for Research on Cancer. Liver. Globocan: World Health Organization; 2020. Available from:http://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf . [Google Scholar]
- 2.Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation:The 2022 update. J Hepatol. 2022;76:681–93. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009;250:831–41. doi: 10.1097/SLA.0b013e3181b0c4df. [DOI] [PubMed] [Google Scholar]
- 4.Cherqui D, Husson E, Hammoud R, Malassagne B, Stéphan F, Bensaid S, et al. Laparoscopic liver resections:A feasibility study in 30 patients. Ann Surg. 2000;232:753–62. doi: 10.1097/00000658-200012000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sasaki A, Nitta H, Otsuka K, Takahara T, Nishizuka S, Wakabayashi G. Ten-year experience of totally laparoscopic liver resection in a single institution. Br J Surg. 2009;96:274–9. doi: 10.1002/bjs.6472. [DOI] [PubMed] [Google Scholar]
- 6.Ishii J, Otsuka Y, Tsuchiya M, Kubota Y, Katagiri T, Maeda T, et al. Application of microwave tissue coagulator in laparoscopic hepatectomy for the patients with liver cirrhosis. J Microwave Surg. 2012;30:213–7. [Google Scholar]
- 7.Vibert E, Perniceni T, Levard H, Denet C, Shahri NK, Gayet B. Laparoscopic liver resection. Br J Surg. 2006;93:67–72. doi: 10.1002/bjs.5150. [DOI] [PubMed] [Google Scholar]
- 8.Buell JF, Thomas MJ, Doty TC, Gersin KS, Merchen TD, Gupta M, et al. An initial experience and evolution of laparoscopic hepatic resectional surgery. Surgery. 2004;136:804–11. doi: 10.1016/j.surg.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Ahn KS, Han HS, Yoon YS, Cho JY, Kim JH. Laparoscopic liver resection in patients with a history of upper abdominal surgery. World J Surg. 2011;35:1333–9. doi: 10.1007/s00268-011-1073-z. [DOI] [PubMed] [Google Scholar]
- 10.Yoon YS, Han HS, Cho JY, Ahn KS. Totally laparoscopic central bisectionectomy for hepatocellular carcinoma. J Laparoendosc Adv Surg Tech A. 2009;19:653–6. doi: 10.1089/lap.2009.0012. [DOI] [PubMed] [Google Scholar]
- 11.Kaneko H, Tsuchiya M, Otsuka Y, Yajima S, Minagawa T, Watanabe M, et al. Laparoscopic hepatectomy for hepatocellular carcinoma in cirrhotic patients. J Hepatobiliary Pancreat Surg. 2009;16:433–8. doi: 10.1007/s00534-009-0123-5. [DOI] [PubMed] [Google Scholar]
- 12.Cai LX, Tong YF, Yu H, Liang X, Liang YL, Cai XJ. Is laparoscopic hepatectomy a safe, feasible procedure in patients with a previous upper abdominal surgery? Chin Med J (Engl) 2016;129:399–404. doi: 10.4103/0366-6999.176068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pang YY. The Brisbane 2000 terminology of liver anatomy and resections HPB. 2000;2:333–39. doi: 10.1080/136518202760378489. HPB (Oxford) 2002;4:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wakabayashi G. What has changed after the Morioka consensus conference 2014 on laparoscopic liver resection? Hepatobiliary Surg Nutr. 2016;5:281–9. doi: 10.21037/hbsn.2016.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications:Five-year experience. Ann Surg. 2009;250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 16.Lei GY, Shen L, Junnarkar SP, Huey CT, Low J, Shelat VG. Predictors of 90-day mortality following hepatic resection for hepatocellular carcinoma. Visc Med. 2021;37:102–9. doi: 10.1159/000510811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beck DE, Ferguson MA, Opelka FG, Fleshman JW, Gervaz P, Wexner SD. Effect of previous surgery on abdominal opening time. Dis Colon Rectum. 2000;43:1749–53. doi: 10.1007/BF02236862. [DOI] [PubMed] [Google Scholar]
- 18.Ellis H. The causes and prevention of intestinal adhesions. Br J Surg. 1982;69:241–3. doi: 10.1002/bjs.1800690502. [DOI] [PubMed] [Google Scholar]
- 19.Menzies D. Peritoneal adhesions. Incidence, cause, and prevention. Surg Annu. 1992;24(Pt 1):27–45. [PubMed] [Google Scholar]
- 20.Weibel MA, Majno G. Peritoneal adhesions and their relation to abdominal surgery. A postmortem study. Am J Surg. 1973;126:345–53. doi: 10.1016/s0002-9610(73)80123-0. [DOI] [PubMed] [Google Scholar]
- 21.Wiebke EA, Pruitt AL, Howard TJ, Jacobson LE, Broadie TA, Goulet RJ, Jr, et al. Conversion of laparoscopic to open cholecystectomy. An analysis of risk factors. Surg Endosc. 1996;10:742–5. doi: 10.1007/BF00193048. [DOI] [PubMed] [Google Scholar]
- 22.Karayiannakis AJ, Polychronidis A, Perente S, Botaitis S, Simopoulos C. Laparoscopic cholecystectomy in patients with previous upper or lower abdominal surgery. Surg Endosc. 2004;18:97–101. doi: 10.1007/s00464-003-9001-4. [DOI] [PubMed] [Google Scholar]
- 23.Law WL, Lee YM, Chu KW. Previous abdominal operations do not affect the outcomes of laparoscopic colorectal surgery. Surg Endosc. 2005;19:326–30. doi: 10.1007/s00464-004-8114-8. [DOI] [PubMed] [Google Scholar]
- 24.Curet MJ. Special problems in laparoscopic surgery. Previous abdominal surgery, obesity, and pregnancy. Surg Clin North Am. 2000;80:1093–110. doi: 10.1016/s0039-6109(05)70215-2. [DOI] [PubMed] [Google Scholar]
- 25.Nunobe S, Hiki N, Fukunaga T, Tokunaga M, Ohyama S, Seto Y, et al. Previous laparotomy is not a contraindication to laparoscopy-assisted gastrectomy for early gastric cancer. World J Surg. 2008;32:1466–72. doi: 10.1007/s00268-008-9542-8. [DOI] [PubMed] [Google Scholar]
- 26.Shelat VG, Serin K, Samim M, Besselink MG, Al Saati H, Gioia PD, et al. Outcomes of repeat laparoscopic liver resection compared to the primary resection. World J Surg. 2014;38:3175–80. doi: 10.1007/s00268-014-2728-3. [DOI] [PubMed] [Google Scholar]
- 27.Isetani M, Morise Z, Kawabe N, Tomishige H, Nagata H, Kawase J, et al. Pure laparoscopic hepatectomy as repeat surgery and repeat hepatectomy. World J Gastroenterol. 2015;21:961–8. doi: 10.3748/wjg.v21.i3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cipriani F, Ratti F, Fiorentini G, Catena M, Paganelli M, Aldrighetti L. Effect of previous abdominal surgery on laparoscopic liver resection:Analysis of feasibility and risk factors for conversion. J Laparoendosc Adv Surg Tech A. 2018;28:785–91. doi: 10.1089/lap.2018.0071. [DOI] [PubMed] [Google Scholar]
- 29.Ome Y, Hashida K, Yokota M, Nagahisa Y, Yamaguchi K, Okabe M, et al. The feasibility and efficacy of pure laparoscopic repeat hepatectomy. Surg Endosc. 2018;32:3474–9. doi: 10.1007/s00464-018-6066-7. [DOI] [PubMed] [Google Scholar]
- 30.Belli G, Cioffi L, Fantini C, D'Agostino A, Russo G, Limongelli P, et al. Laparoscopic redo surgery for recurrent hepatocellular carcinoma in cirrhotic patients:Feasibility, safety, and results. Surg Endosc. 2009;23:1807–11. doi: 10.1007/s00464-009-0344-3. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen KT, Laurent A, Dagher I, Geller DA, Steel J, Thomas MT, et al. Minimally invasive liver resection for metastatic colorectal cancer:A multi-institutional, international report of safety, feasibility, and early outcomes. Ann Surg. 2009;250:842–8. doi: 10.1097/SLA.0b013e3181bc789c. [DOI] [PubMed] [Google Scholar]
- 32.Shafaee Z, Kazaryan AM, Marvin MR, Cannon R, Buell JF, Edwin B, et al. Is laparoscopic repeat hepatectomy feasible?A tri-institutional analysis. J Am Coll Surg. 2011;212:171–9. doi: 10.1016/j.jamcollsurg.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Tsuchiya M, Otsuka Y, Maeda T, Ishii J, Tamura A, Kaneko H. Efficacy of laparoscopic surgery for recurrent hepatocellular carcinoma. Hepatogastroenterology. 2012;59:1333–7. doi: 10.5754/hge12302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


