Abstract
Central metabolic pathways control virulence and antibiotic resistance, and constitute potential targets for antibacterial drugs. In Staphylococcus aureus the role of the pentose phosphate pathway (PPP) remains largely unexplored. Mutation of the 6-phosphogluconolactonase gene pgl, which encodes the only non-essential enzyme in the oxidative phase of the PPP, significantly increased MRSA resistance to β-lactam antibiotics, particularly in chemically defined media with physiologically-relevant concentrations of glucose, and reduced oxacillin (OX)-induced lysis. Expression of the methicillin-resistance penicillin binding protein 2a and peptidoglycan architecture were unaffected. Carbon tracing and metabolomics revealed extensive metabolic reprogramming in the pgl mutant including increased flux to glycolysis, the TCA cycle, and several cell envelope precursors, which was consistent with increased β-lactam resistance. Morphologically, pgl mutant cells were smaller than wild-type with a thicker cell wall and ruffled surface when grown in OX. The pgl mutation reduced resistance to Congo Red, sulfamethoxazole and oxidative stress, and increased resistance to targocil, fosfomycin and vancomycin. Levels of lipoteichoic acids (LTAs) were significantly reduced in pgl, which may limit cell lysis, while the surface charge of pgl cells was significantly more positive. A vraG mutation in pgl reversed the increased OX resistance phenotype, and partially restored wild-type surface charge, but not LTA levels. Mutations in vraF or graRS from the VraFG/GraRS complex that regulates DltABCD-mediated d-alanylation of teichoic acids (which in turn controls β-lactam resistance and surface charge), also restored wild-type OX susceptibility. Collectively these data show that reduced levels of LTAs and OX-induced lysis combined with a VraFG/GraRS-dependent increase in cell surface positive charge are accompanied by significantly increased OX resistance in an MRSA pgl mutant.
Author summary
High-level resistance to penicillin-type (β-lactam) antibiotics significantly limits the therapeutic options for patients with MRSA infections necessitating the use of newer agents, for which reduced susceptibility has already been described. Here we report for the first time that the central metabolism pentose phosphate pathway controls MRSA resistance to penicillin-type antibiotics. We comprehensively demonstrated that mutation of the PPP gene pgl perturbed metabolism in MRSA leading to increased flux to cell envelope precursors to drive increased antibiotic resistance. Moreover, increased resistance was associated with reduced levels of the lipoteichoic acids in the cell envelope, reduced rates of cell lysis under β-lactam stress and was dependent on the VraRG/GraRS multienzyme membrane complex that controls d-alanylation of teichoic acids and cell surface charge. Our data provide new insights on MRSA mechanisms of β-lactam resistance, which will support efforts to expand the treatment options for infections caused by this and other antimicrobial resistant pathogens.
Introduction
The World Health Organization (WHO) recently reported a dramatic increase in antimicrobial resistance (AMR) among human pathogens [1, 2]. Exacerbation of the AMR crisis is driven by the misuse and overuse of last-resort antibiotics, the decline in new antimicrobial drugs being approved for clinical use and a lack of mechanistic understanding of AMR in bacterial pathogens [1, 2]. Staphylococcus aureus, which is among the most challenging AMR human pathogens, can cause a variety of infections. Skin and soft tissue infections can be localised or enter the vasculature [3, 4], whereas osteomyelitis, septic arthritis, infective endocarditis and pneumonia are deep-seated and systemic [5–13].
Introduction of penicillin to treat S. aureus bacteraemia patients in the early 1940s was immediately followed by isolation of penicillin resistant S. aureus strains [14]. In S. aureus, penicillin resistance is mediated by the β-lactamase enzyme encoded by blaZ, which cleaves the β-lactam ring, thus disrupting the activity of the β-lactam antibiotic [14, 15]. Methicillin, a penicillin derivative resistant to β-lactamase hydrolysis, was introduced in 1960s, but was quickly followed by the emergence of methicillin resistant S. aureus (MRSA) [16]. Methicillin resistance was driven to the acquisition of the mecA gene on Staphylococcus cassette chromosome mec (SCCmec) elements, which encodes an alternative penicillin-binding protein, PBP2a, with a decreased affinity to β-lactams [17–21]. In addition to mecA, auxiliary factors also contribute to high-level MRSA β-lactam resistance [22–36], including several involved in the synthesis of cell wall precursors, as well other physiological processes.
The ability of S. aureus to adapt to diverse host environments is linked to its ability to obtain essential nutrients from host tissues [37, 38], which in turn is dependent on metabolic reprogramming. A growing body of literature links central metabolic pathways to the pathogenicity of S. aureus, from its capacity to proliferate within the host, to the control of antibiotic resistance [22, 37–41]. Thus, the identification of new drug targets and antibacterial strategies is reliant on first understanding virulence mechanisms associated with reprogramming of central metabolic pathways and their role in pathogenesis and antimicrobial resistance.
Bacteria synthesize macromolecules from 13 biosynthetic intermediates derived from glycolysis, the pentose phosphate pathway (PPP) and the tricarboxylic acid (TCA) cycle [42]. S. aureus has the complete enzyme set for all three pathways, although it lacks a glyoxylate shunt [42]. In addition to producing pentose precursors for biosynthesis of nucleotides and several amino acids, the PPP plays a critical role in cellular metabolism, maintaining carbon homeostasis by glucose turnover and contributing to the regeneration of reducing power in the form of NADPH [43–48]. There are two branches in the PPP: the oxidative branch contributes to oxidative stress tolerance by generating reducing power in the form of NADPH/H+, and the non-oxidative branch produces ribose-5-P used in the de novo purine synthesis and the generation of nucleotide pools (ATP, ADP, AMP, c-di-AMP, GTP, GDP, GMP, ppGpp, pppGpp, IMP, XMP, etc.) for repair and synthesis of aromatic amino acids and peptidoglycan [47, 48]. PPP activity is increased by environmental stress in Gram-positive organisms [48, 49].
Even though the contribution of glycolysis/gluconeogenesis and the PPP to intracellular persistence of S. aureus has been the subject of numerous studies [37, 38, 40, 45, 46, 48, 49], the role of these major glucose metabolism pathways in the antibiotic resistance of S. aureus remains largely unstudied. Mutations in PPP enzymes have been previously identified in slow growing-vancomycin intermediate S. aureus isolates [50].
We and others have previously reported that purine nucleotide homeostasis plays a key role in the regulation of β-lactam resistance in MRSA [49–53]. Mutations in the pur operon and purine salvage pathway were associated with increased resistance, whereas exposure of MRSA to the purine nucleosides guanosine or xanthosine reduced β-lactam resistance [53]. The purine-derived second messenger signalling molecules (p)ppGpp and c-di-AMP regulate β-lactam resistance, and exposure to exogenous guanosine downregulated c-di-AMP levels in S. aureus [53].
In this study, we investigated if mutations upstream of purine biosynthesis also control β-lactam resistance focusing on pgl, which is the only mutable gene in the oxidative phase of the PPP. We show that a pgl mutation in MRSA strain JE2, which leads to a slight growth defect in laboratory growth media, increased β-lactam resistance, but did not cause changes in PBP2a levels or peptidoglycan architecture. Carbon tracing and metabolomics experiments revealed increased flux to glycolysis and several cell envelope precursors. The susceptibility of wild-type JE2 to β-lactam antibiotics was dramatically increased in chemically defined medium containing glucose (CDMG), and accompanied by extensive cell lysis, whereas the pgl mutant remained highly resistant, exhibited a thick cell wall, intact septa and had a ruffled cell surface. Lipoteichoic acid (LTA) levels were reduced in the pgl mutant and the surface charge of pgl cells was significantly increased. β-lactam resistance in the pgl mutant reverted to wild-type levels by mutations in the ABC transporter VraFG and cognate two-component regulatory system GraRS. These data reveal that metabolic reprogramming in an MRSA pgl mutant increases β-lactam resistance via VraFG/GraRS-dependent changes in cell envelope biogenesis.
Results
β-lactam resistance is increased in a MRSA pgl mutant
Extrapolating from previous data showing that purine metabolism controls β-lactam resistance [26, 41, 53–56], we turned our attention to the PPP, which produces ribose-5-P, a major substrate for purine and pyrimidine biosynthesis (Fig 1). Given the important role of the PPP in central metabolism and production of reducing power, it is perhaps not surprising that mutations in the key enzymes in this pathway, including zwf and gnd, are not available in the Nebraska Transposon Mutant library (NTML) [57]. However, the NTML does contain a mutation in the monocistronic pgl gene (SAUSA300_1902, NE202), which encodes 6-phosphogluconolactonase, the second enzyme in the oxidative phase of the PPP that converts 6-P-gluconolactone to gluconate-6-P.
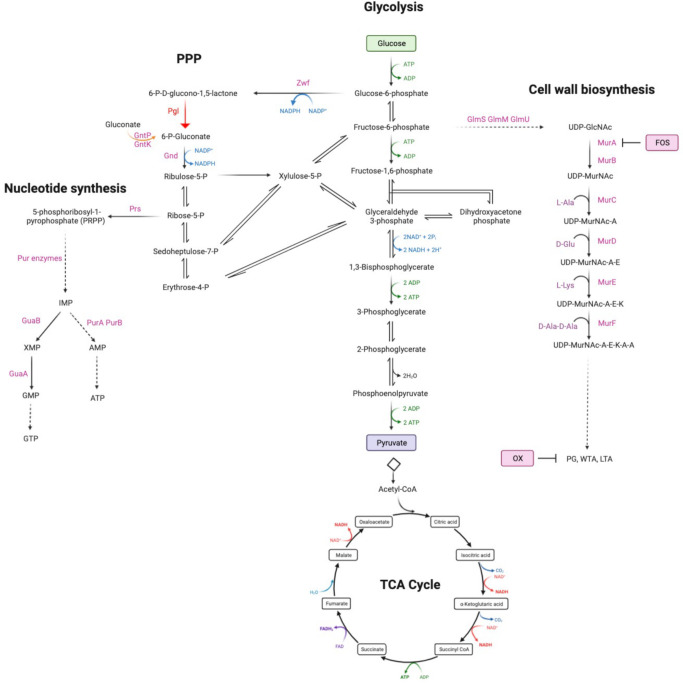
Fig 1. Summary of the oxidative phase of the pentose phosphate pathway including 6-phosphogluconolactonase (Pgl), that converts 6-P-gluconololactone to gluconate-6-P.
For reference, key glycolysis, TCA cycle, nucleotide and cell wall biosynthetic pathway intermediates are also shown. Fructose-6-P is fluxed from glycolysis to peptidoglycan (PG), wall teichoic acid (WTA) and lipoteichoic acid (LTA) via UDP-N-acetylglucosamine (UDP-GlcNAc) and UDP-N-acetylmuramic acid (UDP-MurNAc). Fosfomycin (FOS) targets MurA which together with MurB is required for the conversion of UDP-GlcNAc to UDP-MurNAc. Oxacillin (OX) targets the transpeptidase activity of the penicillin binding proteins required for PG crosslinking. The putative gluconate shunt involves the export of 6-phosphogluconolactone, which spontaneously degrades to gluconate before being transported into the cell by the gluconate permease GntP and phosphorylated by the gluconate kinase GntK. Schematic made using Biorender.com.
When grown in Mueller Hinton 2% NaCl broth (MHB) the pgl mutant NE202 exhibited significantly increased resistance to cefoxitin in disk diffusion assays (zone diameters were 11mm for JE2 versus 8mm for pgl) and oxacillin (OX) in broth dilution assays (Table 1). Comparative whole genome sequencing analysis confirmed the absence of unexpected secondary mutations outside the pgl locus in NE202. The NE202 phenotype was verified by (i) showing that increased OX resistance was acquired by wild-type following transduction of the pgl::Ermr allele and (ii) complementation of NE202 with the wild-type pgl gene (pglcomp) (Table 1). A pgl/mecA mutant was OX susceptible (Table 1) and Western immunoblotting revealed no differences in PBP2a expression between wild-type JE2, pgl and pglcomp grown in TSB supplemented with OX 0.5 μg/ml (S1A Fig) or MHB 2% NaCl supplemented with OX 32 μg/ml (S1B Fig). Thus, high-level OX resistance in pgl was dependent on mecA but was not associated with increased PBP2a expression.
Table 1. Minimum inhibitory concentrations (μg/ml; % for Congo Red) of strains used in this study to oxacillin (OX), targocil (TG), tunicamycin (TM), fosfomycin (FOS), D-cycloserine (DCS), Congo Red (CR), vancomycin (VAN), amsacrine (AMS), sulfamethoxazole (SMX) and polymyxin B (PMB) in Mueller Hinton Broth (+ 2% NaCl for OX).
| Strain | OX | TG | TM | FOS | DCS | CR | VAN | AMS | SMX | PMB |
|---|---|---|---|---|---|---|---|---|---|---|
| JE2 | 64 | 1–2 | 4 | 32–64 | 16–32 | 0.25% | 1 | >256 | 128–256 | 128 |
| pgl | 128–256 | 4–8 | 4 | 64–128 | 32 | 0.03% | 2–4 | 32–64 | 16–32 | 64 |
| pgl comp | 64 | 1–2 | 4 | 32–64 | 32 | 0.25% | 1–2 | >256 | 128–256 | n/d |
| pgl::Kmr | 128–256 | 4–8 | 2–4 | 64–128 | 32 | n/d | 2–4 | n/d | 16–32 | n/d |
| pgl/mecA | 0.5 | n/d | n/d | n/d | n/d | n/d | n/d | n/d | n/d | n/d |
| mecA | 0.25 | n/d | n/d | n/d | n/d | n/d | n/d | n/d | n/d | n/d |
| JE2 pgl::tn | 128–256 | n/d | n/d | n/d | n/d | n/d | n/d | n/d | n/d | n/d |
| vraG | 64 | n/d | n/d | n/d | n/d | n/d | 0.5 | n/d | n/d | 2–4 |
| vraF | 64 | n/d | n/d | n/d | n/d | n/d | n/d | n/d | n/d | n/d |
| pgl/vraG | 128–256 | n/d | n/d | n/d | n/d | n/d | 0.5 | n/d | n/d | 4 |
| pgl/vraF | 128–256 | n/d | n/d | n/d | n/d | n/d | n/d | n/d | n/d | n/d |
n/d—not determined
The pgl OX resistance phenotype is glucose-dependent and unrelated to changes in peptidoglycan (PG) structure
Colonies of pgl were smaller than JE2 on MHA plates (S2A Fig) and, in the absence of antibiotics, the pgl mutation negatively impacted growth in MHB (S2B Fig), but to a lesser extent in LB, TSB and BHI (S2C–S2E Fig). A pgl growth defect was also measured in chemically defined media with glucose (CDMG), but not in CDM without glucose (S2F and S2G Fig). Growth of the complemented pglcomp mutant was indistinguishable from the wild-type JE2 under all culture conditions tested (S2B–S2F Fig). The mild growth defects of pgl in MHB and CDMG correlated with significantly increased OX MICs (Table 1, Fig 2A), whereas the MIC of pgl in CDM (32–64 μg/ml) was more similar to wild-type JE2 (16–32 μg/ml; Fig 2A). Notably, not only was pgl more resistant than wild-type JE2 in CMDG, but wild-type JE2 OX resistance was significantly reduced in this growth medium (MIC = 0.5–1 μg/ml; Fig 2A). Wild-type JE2 and pgl grew similarly in CDM OX 10 μg/ml (Fig 2B), whereas only pgl was able to grow in CDMG OX 10 μg/ml (Fig 2C). Doubling dilutions of the CDMG glucose concentration from 5 g/l (28 mM) further revealed that even at the lowest glucose concentration tested, 0.07 g/l (0.43 mM), JE2 growth in OX was significantly impaired (S3A Fig). Similarly, the OX MIC of JE2 was reduced from 32–64 to ≤1 μg/ml in CDMG at glucose concentrations >0.3 g/l (1.75 mM) (S3B Fig). These data indicate that mutation of pgl significantly increased the glucose-dependent reduction of OX resistance in JE2 at physiologically relevant concentrations. Unlike wild-type JE2, the pgl mutant was able to grow at OX concentrations >1 μg/ml in 25 or 70% human serum (S4 Fig), and exhibited a higher OX MIC in CDMG or MHB supplemented with up to 70% human serum (S1 Table) further demonstrating the in vivo relevance of this phenotype. The pgl mutation increased sensitivity to oxidative stress (H2O2) in CDMG (S5 Fig), similar to previous observations in Listeria monocytogenes using BHI media [58].
Fig 2. Mutation of pgl increases resistance to oxacillin.
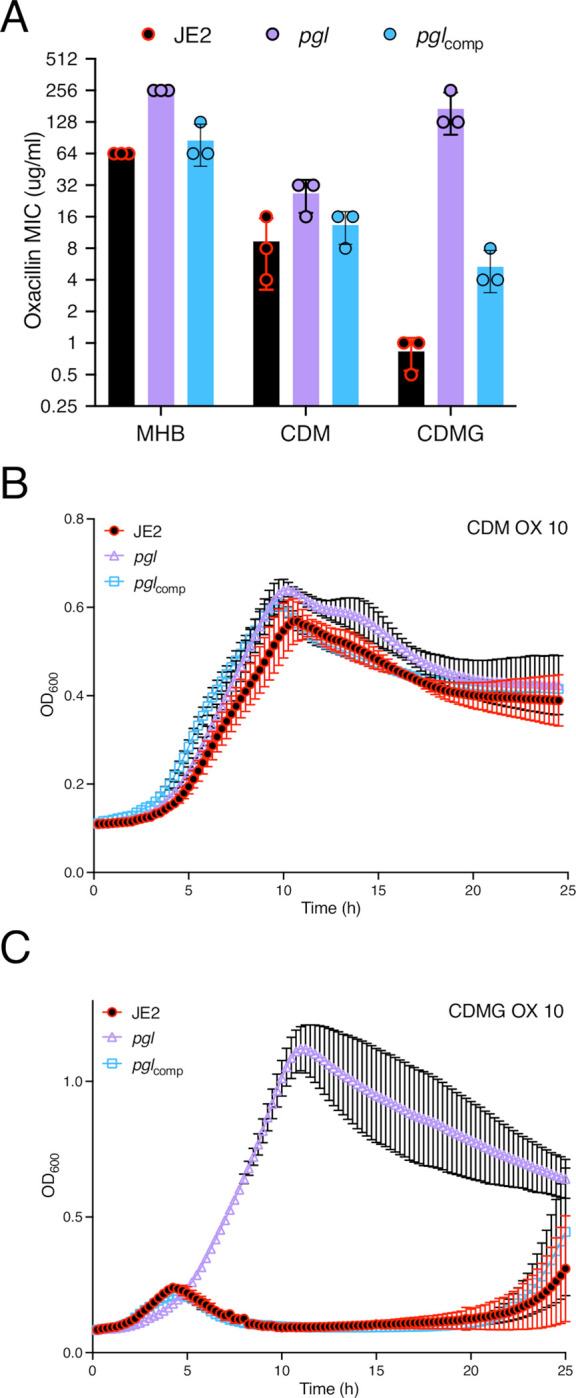
A. Oxacillin MICs of JE2, pgl and the complemented pgl mutant in Mueller Hinton broth with 2% NaCl (MHB), chemically defined media (CDM) and CDM with glucose (CDMG). Note that the Y axis (Oxacillin MIC) is a log2 scale. B and C. Growth of JE2, pgl and pglcomp for 25 hrs at 35°C in CDM (B) and CDMG (C) supplemented with OX 10 μg/ml. Growth (OD600) was measured at 15 min intervals in a Tecan plate reader. Data are the average of 3 independent experiments and error bars represent standard deviation.
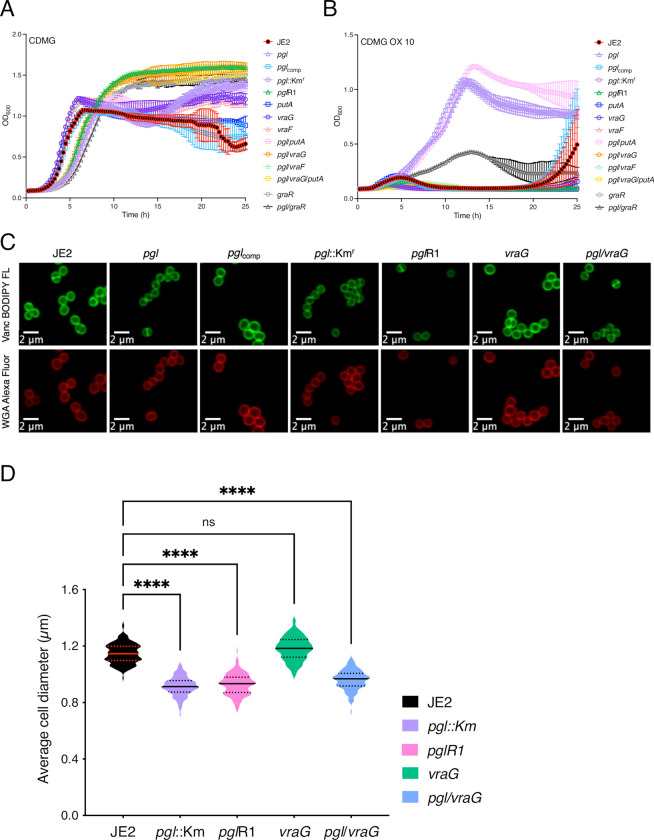
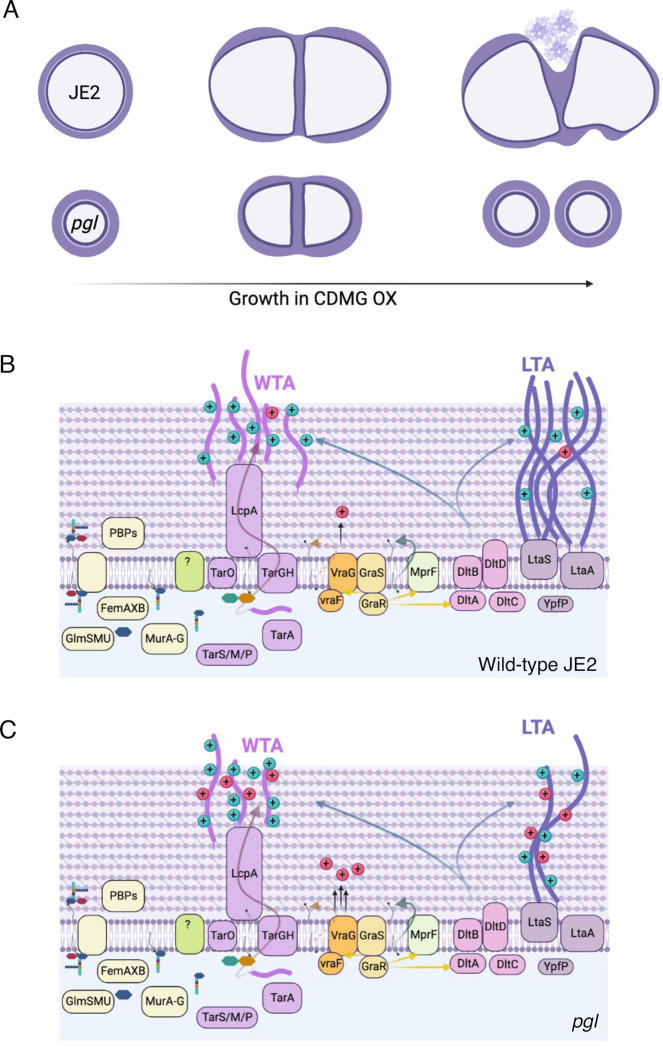
Confocal microscopy revealed that the diameter of pgl cells from overnight CDMG grown cultures was significantly smaller than wild-type JE2 or pglcomp cells (Fig 3A,B). The significant OX-induced increase in MRSA cell size, which we and others have previously reported [31, 53, 59–61], was more pronounced in wild-type JE2 and pglcomp than the pgl mutant (Fig 3C). Furthermore, the increased cell size of wild-type JE2 and pglcomp in CDMG OX was associated with a dramatic increase in the number of cells undergoing visible lysis (Fig 3D), an observation consistent with the abrupt decline in the OD600 of wild-type JE2 and pglcomp cultures after 4–5 h growth under these growth conditions (Fig 2C). Quantitative PG compositional analysis of muramidase-digested muropeptide fragments revealed similar oligomerisation profiles and crosslinking for wild-type JE2, pgl and the pglcomp strains grown in CDMG, or CDMG supplemented with sub-inhibitory 0.05 μg/ml OX, MHB 2% NaCl, MHB 2% NaCl supplemented with 0.5 μg/ml OX (S6A–S6D Fig). The total PG content was also similar for all three strains under these growth conditions (S6E–S6H Fig). Thus, in addition to the unchanged PBP2a expression (S1 Fig), increased pgl OX resistance was unrelated to changes in PG structure or amount (S6 Fig).
Fig 3. Mutation of pgl reduces cell size and prevents OX-induced cell lysis in CDMG.
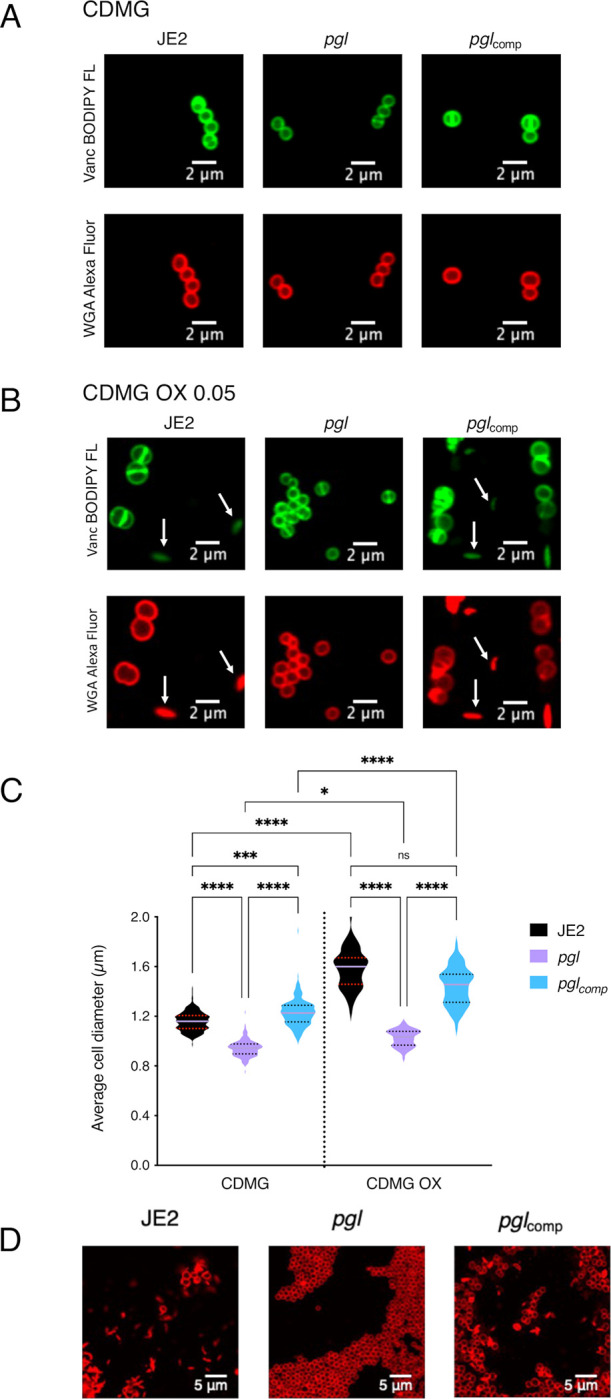
A and B. Representative microscopic images of JE2, pgl and pglcomp cells grown in CDMG (A) or CDMG supplemented with OX 0.05 μg/ml (B) and labelled with vancomycin BODIPY FL, which binds to the terminal d-ala-d-ala in the peptidoglycan stem peptide (green, top panel) or WGA Alexa Fluor 594, which binds to GlcNAc and other sugars in the cell envelope (red, bottom panel). C. Average diameter of JE2, pgl and pglcomp cells grown in CDMG or CDMG OX. Images of cells from four biological replicates were acquired using Fv3000 confocal microscope and software, 50 cells measured per biological replicate (200 cells in total) for CDMG and 60 cells in total counted for CDMG OX (due to cell lysis), and the violin plots for the four biological replicates were generated using GraphPad Prism V9. Asterisks indicate statistically significant difference according to using a Kruskal-Wallis test followed by a Dunn’s multiple comparison test. Adjusted p-values * p<0.05, *** p<0.001 and **** p<0.0001 are indicated. D. Extensive lysis of JE2 and pglcomp (but not pgl) in CDMG OX 0.05 μg/ml cultures. Cells were labelled with WGA Alexa Fluor 594 and representative microscopic images are shown.
Exogenous d-gluconate or mutation of the gntPK gluconate shunt genes did not restore wild-type OX resistance in the pgl mutant
In Escherichia coli and L. monocytogenes, 6-phosphogluconolactone that accumulates in pgl mutants is dephosphorylated to labile gluconolactone, which is exported out of the cell where it spontaneously hydrolyses to gluconate [58, 62]. In S. aureus, the predicted gluconate shunt genes gntP (SAUSA300_2442) and gntK (SAUSA300_2443) are co-located on the chromosome with the gntR regulator. In a previous RNAseq analysis, we reported that gntP was upregulated by OX [63]. Growth and OX resistance of wild-type JE2, pgl and pglcomp were similar in CDMG supplemented with 5 g/l d-gluconate and CDMG OX d-gluconate (S7A and S7B Fig). Inactivation of gntP or gntK in the pgl mutant was accompanied by a modest growth delay in CDMG OX but did not restore wild-type levels of OX susceptibility (S7C Fig). Therefore, exogenous d-gluconate and the gluconate shunt genes are not notably involved in the increased OX resistance phenotype of the pgl mutant.
Inactivation of pgl reduces carbon flux through PPP
Liquid chromatography-tandem mass spectrometry analysis was used to trace [1,2-13C2] glucose flux through glycolysis and the PPP in wild-type JE2, pgl and pglcomp. As described previously [64], six-carbon [1,2-13C] glucose can be metabolised via glycolysis and the PPP to produce three-carbon 13C2-pyruvate (M+2) and 13C1-pyruvate (M+1), respectively (Fig 4A). The M+1 fraction is produced following a decarboxylation reaction in the PPP that releases 13CO2 (Fig 4A). M+1 pyruvate levels were reduced in pgl, indicative of reduced PPP activity (Fig 4B), whereas M+2 pyruvate levels derived primarily from glycolysis, were similar (Fig 4B). The M+2/M+1 ratio further illustrated the impaired PPP activity of pgl and showed >2-times more pyruvate generated directly from glucose entering glycolysis in pgl than in wild-type JE2 or pglcomp (Fig 4C).
Fig 4. PPP activity is impaired in the pgl mutant.
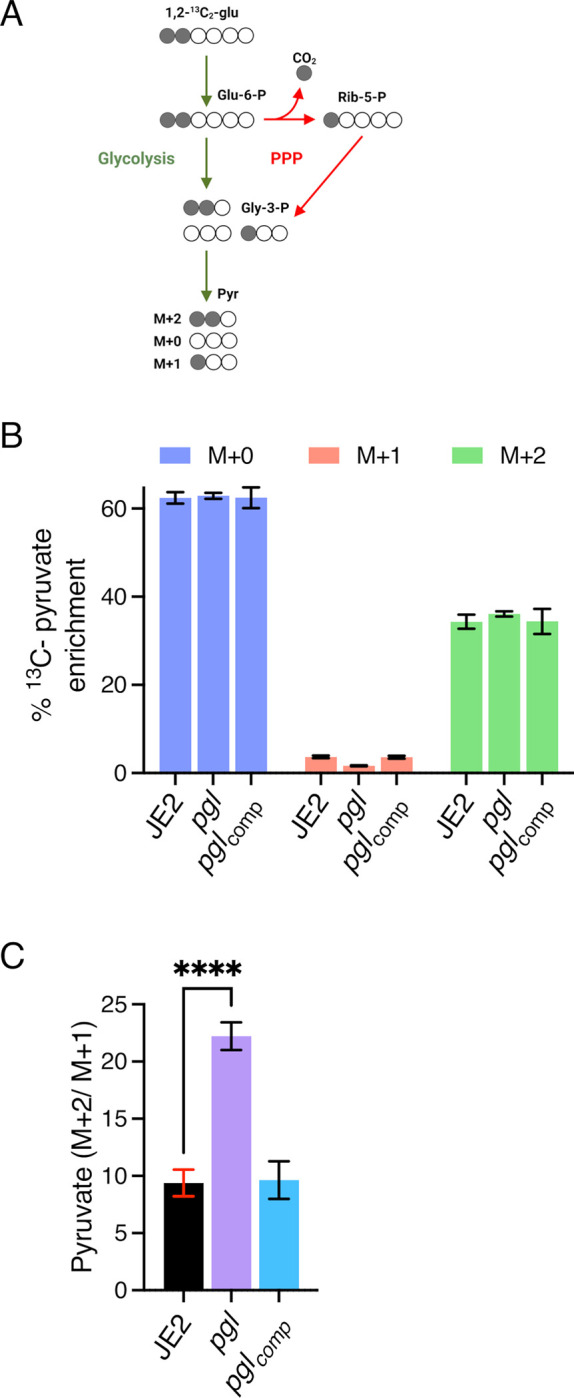
A. JE2, pgl and the complemented pgl mutant (pglcomp) were grown in CDM [1,2-13C]Glucose and fluxes via glycolysis and the pentose phosphate pathway (PPP) were compared as described previously [64]. The M+2 pyruvate is unique to glycolysis and the M+1 pyruvate to PPP. Thus, the M+2/M+1 ratio is indicative of carbon flux through glycolysis relative to PPP. The M+0 pyruvate can arise from different sources including the unlabeled part of the [1,2-13C]Glucose and pyruvogenic amino acids that are consumed alongside glucose. B. Relative levels of M+1 pyruvate indicative of PPP activity and M+2 pyruvate indicative of glycolytic activity in JE2, pgl and pglcomp. C. The M+2/M+1 ratio indicative of pyruvate produced directly from glucose flux through glycolysis in JE2, pgl and pglcomp. Data are the average of three independent experiments and standard deviations are shown. Significant differences were determined using ordinary one-way ANOVA with Dunnett’s multiple comparison using GraphPad Prism V9 and adjusted p-value **** p<0.0001 is indicated.
OX resistance in the pgl mutant is independent of the TCA cycle or glucogenic and ketogenic amino acids
HPLC was used to investigate if redirected glucose flux from the PPP to glycolysis impacted consumption of amino acids in CDMG, and revealed that levels of threonine, the branched chain amino acids (BCAAs) valine, leucine and isoleucine, as well as phenylalanine, tryptophan and tyrosine, histidine, methionine and aspartic acid were increased in the supernatant of pgl cultures compared to JE2 or pglcomp after 7.5 h growth (S8A Fig). Interestingly, the levels of the TCA cycle intermediates malate, succinate and particularly α-ketoglutarate were also increased in CDMG supernatants of pgl (S8B Fig), which may be consistent with a reduced requirement for glucogenic and ketogenic amino acids. To investigate this proline dehydrogenase (putA::Ermr) and glutamate dehydrogenase (gudB::Ermr) mutations, predicted to interfere with the flux of amino acids to α-ketoglutarate, were transduced from the NTML [57] into pgl::Kmr. Growth of the resulting pgl/putA and pgl/gudB mutants in CDMG and CDMG OX was similar to pgl::Kmr (S8C and S8D Fig). Similarly the pgl TCA cycle double mutants pgl/sdhA, pgl/sucA and pgl/sucC remained capable of growing in CDMG OX (S8C and S8D Fig), although pgl/sucC exhibited an extended lag phase in keeping with our previous report that sucC mutation re-sensitizes MRSA to β-lactam antibiotics due to increased accumulation of succinyl CoA [39]. Collectively, these data indicate that an intact TCA cycle or the accumulation of TCA cycle intermediates and ketogenic amino acids in culture supernatants was not associated with the increased β-lactam resistance of the pgl mutant.
Increased resistance to β-lactam antibiotics in pgl is promoted by redirected carbon flux to cell wall precursors
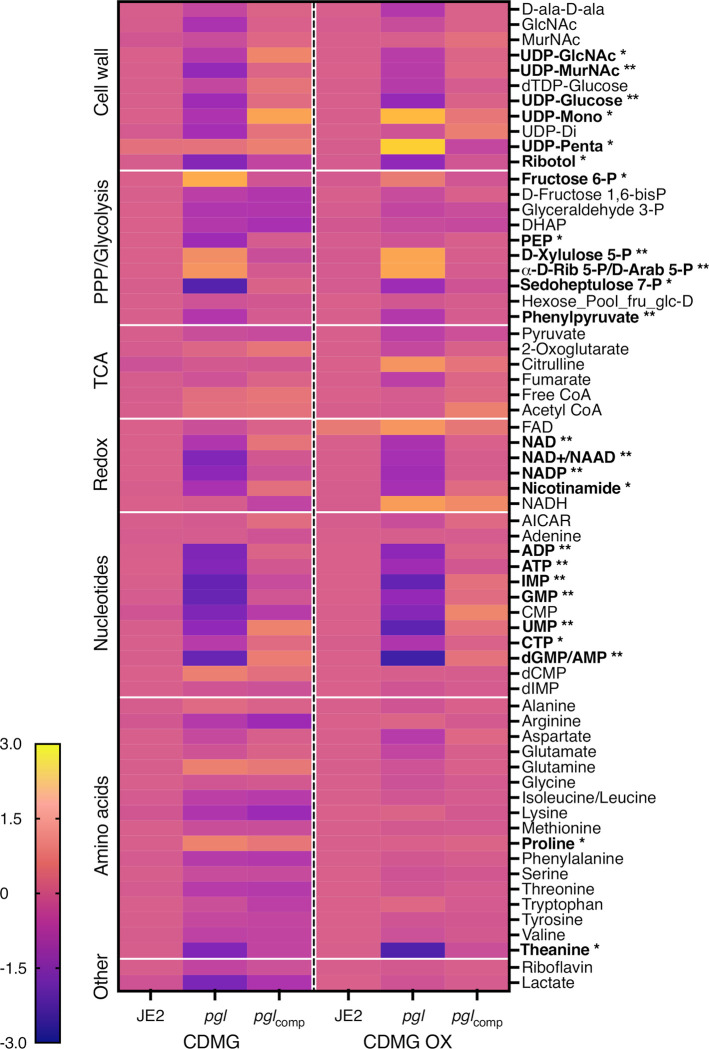
Whole cell metabolomics was performed on JE2, pgl and pglcomp grown in CDMG or CDMG OX (Fig 5). Consistent with the important role of the PPP in the generation of reducing power and nucleotide biosynthesis, levels of key redox carriers and six nucleotides were significantly reduced in pgl and restored to JE2 levels in the complemented mutant (Fig 5). Interestingly, reduced nucleotide levels correlated with a 2-4-fold increase in the susceptibility of pgl mutant to sulfamethoxazole, which inhibits dihydropteroate synthetase in the folate synthesis pathway (Table 1). Levels of sedoheptulose 7-P which is downstream of Pgl in the PPP was also reduced in pgl, reaching significance in CDMG, whereas ribose 5-P and erythrose 5-P were significantly increased (Fig 5), indicative of complex metabolic reprogramming in the pgl mutant.
Fig 5. Heatmap comparison of cell wall, pentose phosphate pathway (PPP)/glycolysis, TCA cycle, redox, nucleotides and amino acid metabolites in JE2, pgl and pglcomp.
Whole cell metabolomics was performed on JE2, pgl and pglcomp grown in CDMG and CDMG OX 10 μg/ml and the cells collected after 4–5 hours (early exponential phase). Data presented are the average of three biological replicates (2 biological replicates for FAD) analysed using GraphPad Prism V9. Individual metabolite levels that were significantly different using a one-way ANOVA with Turkey’s post-hoc in pgl grown in CDMG, CDMG OX or both are highlighted in bold text. * significant difference in either CDMG or CDMG OX. ** significant difference in both CDMG and CDMG OX.
Consistent with the [1,2-13C] glucose tracing experiments, accumulation of fructose 6-P from which cell wall precursors are derived, was increased in CDMG OX and significantly increased in CDMG (Fig 5). Furthermore, the downstream glycolytic intermediates fructose 1,6-bis-P, dihydroxyacetone phosphate (DHAP), glyceraldehyde 3-P and phosphoenolpyruvate (PEP) were reduced (Fig 5). Although there are several possible explanations for this, one possibility is that the accumulated fructose 6-P may be fluxed to the PPP or cell wall. Indeed, significantly increased levels of UDP-mono and UDP-penta were measured in pgl grown in CDMG OX, but not in CDMG (Fig 5). In contrast, the levels of UDP-GlcNAc and UDP-MurNAc were significantly decreased (Fig 5), perhaps reflecting increased consumption of these substrates in the production of UDP-mono and UDP-penta in CDMG OX. Increased accumulation of UDP-mono and UPD-penta correlated with the increased resistance of the pgl mutant to fosfomycin (FOS) (Table 1, S9 Fig), an antibiotic that inhibits the MurA enzyme, which together with MurB catalyses the conversion of UDP-GlcNAc to UDP-MurNAc (Fig 1). Furthermore, pgl exhibited significantly increased resistance to an OX/FOS combination compared to wild-type JE2 in a checkerboard dilution assay (S9 Fig). Broth microdilution susceptibility experiments revealed that the pgl mutant was 1-2-fold more resistant to vancomycin (VAN), which targets the terminal d-ala-d-ala of the PG stem peptide (Table 1).
Taken together, these data indicate that redirected carbon flux to cell wall precursors in pgl contributes to the increased resistance to β-lactam antibiotics. Furthermore, pgl viability appears to be underpinned by a complex and regulated interconversion of glycolytic and PPP intermediates, which may also explain why the glycolytic shunt genes are dispensable for the growth of the pgl mutant under these culture conditions.
Mutation of pgl alters susceptibility to antimicrobial agents targeting wall teichoic acids (WTAs) and lipoteichoic acids (LTAs) and is accompanied by morphological changes in the cell envelope
The MICs of wild-type JE2 and pgl to the TarO inhibitor tunicamycin were the same, whereas pgl was more resistant to the TarGH inhibitor targocil and more susceptible to the d-alanylation inhibitor amsacrine (Table 1), revealing different effects of antimicrobial agents targeting distinct steps in WTA biosynthesis. TarO catalyzes the transfer of N-acetylglucosamine-1-phosphate from UDP-GlcNAc to undecaprenyl-P to initiate WTA synthesis [65]. The TarGH ABC transporter transports WTAs across the cytoplasmic membrane [66], and the polymer is then d-alanylated by the DltABCD complex [67]. The pgl mutant was also more sensitive to Congo Red which targets the LTA synthase LtaS [68] (Table 1). Importantly LTA is also d-alanylated by DltABCD. The susceptibility of pgl to d-cycloserine, which targets the alanine racemase and ligase enzymes in the d-ala-d-ala pathway was unchanged when compared to wild-type, and the metabolomic analysis also showed no significant differences in the levels of d-ala-d-ala in wild-type JE2, pgl and pglcomp (Fig 5).
Transmission electron microscopy (TEM) revealed that pgl cells grown in CDMG OX had visibly ruffled surface characteristics, and thick, intact septa compared to JE2 cells (Fig 6). Consistent with previous microscopic analysis (Fig 3), TEM revealed defective/truncated septa in dividing wild-type cells, as well as cells undergoing lysis (Fig 6). In contrast wild-type and pgl cells grown in the absence of OX were largely similar (S10 Fig). Taken together these data suggest that cell envelope changes in the pgl mutant are the result of altered activity of the TarGH, LtaAS-YpfP and DltABCD membrane complexes involved in export and d-alanylation of WTAs and LTAs that collectively contribute to increased OX resistance.
Fig 6. Increased OX resistance in the pgl mutant is associated with a ruffled surface morphology, a thicker cell wall and thicker septa between dividing cells.
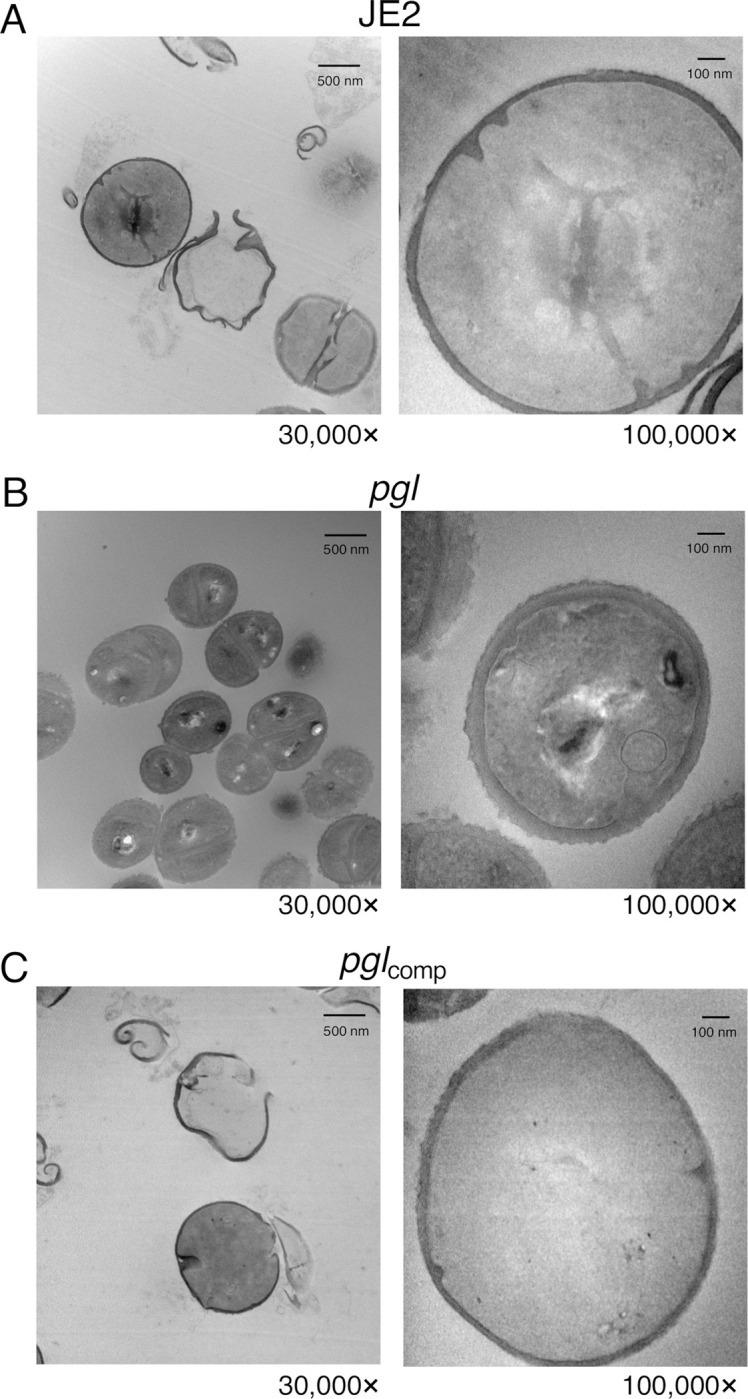
Transmission electron microscopy at 30,000× (left) and 100,000× (right) magnification was performed on JE2 (A), pgl (B) and pglcomp (C) cells collected from exponential phase cultures grown for 4.5 h in CDMG OX 1 μg/ml normalized to OD600 = 1 in PBS before being fixed and thin sections prepared. Representative cells from each strain are shown. Scale bars represent 500 nm at 30,000× or 100 nm at 100,000× magnification.
OX resistance in the pgl mutant is dependent on vraF and vraG
During experiments to remove the Ermr marker in NE202 a pgl markerless transposon mutant that had reverted to wild-type patterns of growth, particularly in CDMG OX, was isolated (Fig 7A and 7B). Whole genome sequencing of this mutant, designated pglR1, identified a thymine nucleotide deletion 73bp upstream of putA and a Gln394STOP substitution in VraG. Construction of pgl double and triple mutants revealed that the pgl OX resistance phenotype was dependent on vraG and not putA (Fig 7A and 7B). vraG encodes a membrane permease, which together with its cognate ATPase, VraF, comprises an ABC efflux pump previously implicated in resistance to cationic antimicrobial peptides, polymyxin B and vancomycin [69–73], potentially via the export of cell wall/teichoic acid precursors or modifying subunits [71]. Consistent with this, a pgl/vraF mutant grew similarly to pgl/vraG and JE2 in CDMG and CDMG OX (Fig 7A and 7B). VraFG is also part of a multicomponent complex with the glycopeptide resistance-associated GraRS two component system, that regulates vraFG as well as the dltABCD operon and mprF [69–71]. A pgl/graR mutant exhibited an intermediate phenotype when grown in CDMG OX compared to JE2 and the pgl/vraF or pgl/vraG mutants (Fig 7B) revealing a role for the entire VraFG/GraRS complex in the pgl OX resistance phenotype. In contrast, consistent with published data [74], mutation of vraG was associated with significantly increased susceptibility to polymyxin B, independent of pgl (Table 1).
Fig 7. Mutation of vraG restores wild-type OX resistance, but not cell size, in the pgl mutant grown in CDMG.
A and B. Growth of JE2, pgl::Kmr, pglR1, putA, graR, vraG, pgl/putA, pgl/graR, pgl/vraG, pgl/putA/vraG for 25 hrs at 35°C in CDMG (A) and CDMG supplemented with OX 10 μg/ml (B). Growth (OD600) was measured at 15 min intervals in a Tecan plate reader. Data are the average of 3 independent experiments and error bars represent standard deviation. C. Representative microscopic images of JE2, pgl, pglcomp, pgl::Kmr, pglR1, vraG and pgl/vraG cells grown in CDMG and labelled with vancomycin BODIPY FL (green, top panel) or WGA Alexa Fluor 594 (red, bottom panel). D. Average diameter of JE2, pgl::Kmr, pglR1, vraG and pgl/vraG cells grown in CDMG. Images of cells from three biological replicates were acquired using Fv3000 confocal microscope and software, 50 cells measured per biological replicate (150 cells in total) and the violin plots for the three biological replicates were generated using GraphPad Prism V9. Asterisks indicate statistically significant difference according to using a Kruskal-Wallis test followed by a Dunn’s multiple comparison test. Adjusted p-values **** p<0.0001 or ns, not significant are indicated.
Interestingly, the increased VAN MIC of the pgl mutant grown in MHB was reduced from 2–4 μg/ml to 0.5 μg/ml in pgl/vraG (Table 1) further indicating a reversal of cell envelope changes. However, pgl/vraG and pgl cells were the same size in CDMG and CDMG OX (Fig 7C and 7D) demonstrating that the vraG mutation does not reverse other central metabolism-related phenotypes in the pgl mutant.
In CDMG, the OX MICs of putA, vraF, vraG and pgl/putA were the same as JE2, whereas pgl/graR was reduced to 32–64 μg/ml and pgl/putA/vraG, pgl/vraF and pgl/vraG were reduced to 8 μg/ml compared to 128–256 μg/ml for pgl and pgl::Kmr (S11 Fig). Interestingly, in MHB 2% NaCl, the increased OX MIC of the pgl mutant (128–256 μg/ml) was not reversed in the pgl/vraG or pgl/vraF mutants (Table 1), underlining the importance of exogenous glucose in pgl-related phenotypes and indicating that VraFG-dependent OX resistance in the pgl mutant is environmentally regulated.
Lipoteichoic acids are reduced in the pgl mutant
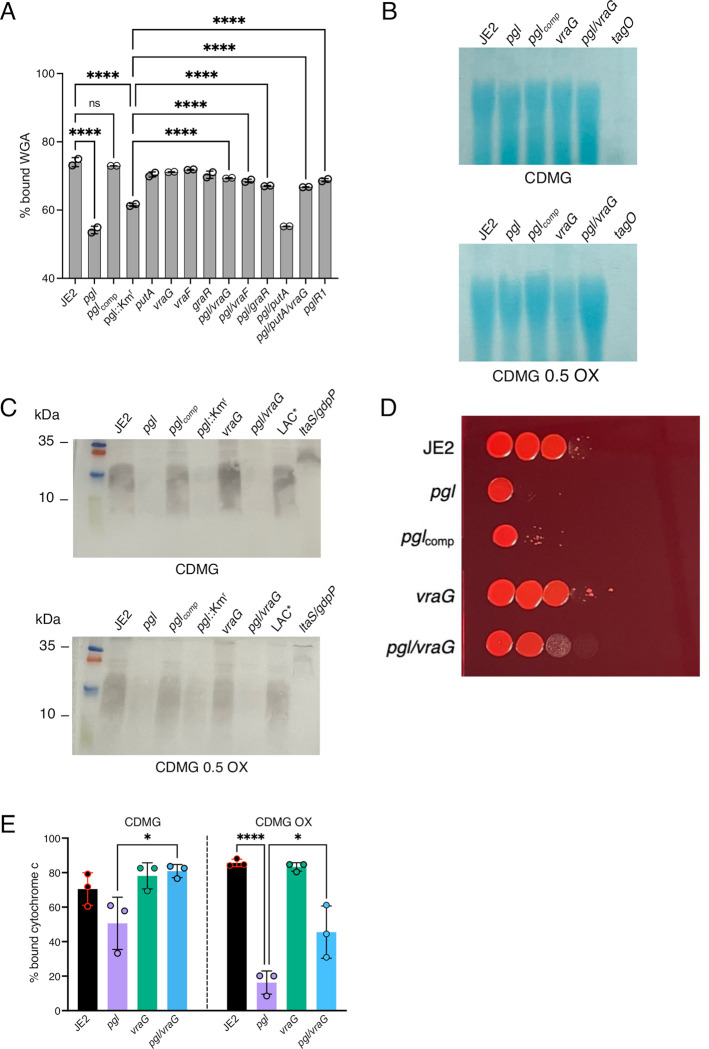
Levels of teichoic acids in the pgl, vraF, vraG and graR strains were compared using several assays. Binding of wheat germ agglutinin (WGA) Alexa Fluor 594 to GlcNAc and other sugars in WTA, LTA and PG was significantly reduced in pgl and pgl::Kmr compared to the JE2, vraF, vraG and graR strains (Fig 8A). Furthermore, WGA binding was restored to wild-type levels in pglR1, pgl/vraF, pgl/vraG, pgl/putA/vraG and pgl/graR (Fig 8A). Levels of ribitol, the backbone for WTAs and LTAs, were also reduced in pgl grown in CDMG OX and significantly reduced in CDMG (Fig 5). Levels of WTAs visualised on Alcian blue stained WTA gels were similar in all strains grown in CDMG (Fig 8B). In CDMG OX, WTAs in the pgl mutant were marginally reduced in total amount compared to JE2 (Fig 8B), but ImageJ densitometry analysis from 3 independent experiments revealed that this was not significant. Analysis of LTA immunoblots by ImageJ densitometry revealed a significant reduction (p <0.05) in the relative levels of LTAs in pgl and pgl/vraG compared to JE2 (Fig 8C). The reduced levels of LTAs in pgl correlated with the reduction in WGA binding (Fig 8A) and the increased susceptibility of the pgl mutant to Congo Red (Fig 8D), which targets LtaS. LTA accumulates at the site of cell division [75], and reduced levels of this glycopolymer may contribute to the defective cell division and reduced cell size phenotypes of pgl (Fig 6). Furthermore, because high levels of LTA have been implicated in increased cell lysis [76], these data may also explain in part the increased susceptibility of the wild-type to lysis in CDMG OX compared to pgl.
Fig 8. Lipoteichoic acids are reduced and the cell surface is more positively charged in the pgl mutant.
A. Comparison of wheat germ agglutinin (WGA) Alexa Fluor 594 binding to JE2, pgl, pglcomp, pgl::Kmr, vraG, vraF, graR, putA, pgl/vraG, pgl/vraF, pgl/graR, pgl/putA, pgl/putA/vraG and pglR1 cells grown for 4.5 h in CDMG OX using fluorescence microscopy at 594nm excitation/618nm detection. The data are the average of 2 independent experiments and error bars represent standard deviation. Significant differences were determined using a two-way ANOVA with Turkey’s post-hoc analysis. Adjusted p-values **** p<0.0001 or ns, not significant are indicated. B. Comparison of relative wall teichoic acid (WTA) levels in JE2, pgl, pglcomp, vraG, pgl/vraG and tagO (negative control) grown in CDMG and CDMG OX 0.05 mg/ml. Purified WTA samples were separated on 20% native polyacrylamide (PAA) gels before being stained with 0.1% Alcian blue. A representative image from 3 independent biological repeats is shown. C. Comparison of relative lipoteichoic acid (LTA) levels in JE2, pgl, pglcomp, pgl::Kmr, vraG, pgl/vraG, USA300 LAC* and ltaS/gdpP (negative control) grown in CDMG and CDMG OX 0.05 mg/ml (the ltaS/gdpP mutant is OX susceptible and was only grown in CDMG). Extracted LTAs were separated on 15% PAA gels, transferred to a PVDF membrane and probed with LTA antibody (1:5000), followed by HRP-conjugated protein G (1:2000) and colorimetric detection with Opti-4CN substrate kit. Three independent experiments were performed, and a representative blot is shown. D. Mutation of vraG partially restores Congo Red resistance in the pgl mutant. 10-fold serial dilutions of JE2, pgl, pglcomp, vraG and pgl/vraG inoculated onto TSA supplemented with 0.125% Congo Red and grown for 24 h at 37°C. This experiment was repeated three times and a representative plate is shown. E. Comparison of cell surface charge using a cyctochrome c binding assay in JE2, pgl, vraG and pgl/vraG grown in CDMG and CDMG OX 0.05 mg/ml. Positively charged cytochrome c binds more strongly to negatively charged cells. The data are the average of 3 independent experiments and error bars represent standard deviation Significant differences were determined using ordinary one-way ANOVA followed by Turkey’s multiple comparison post-hoc test (* p<0.05, **** p<0.0001).
Evidence that VraG-dependent control of cell surface charge is altered in pgl
The reduced LTA levels in the pgl mutant raise questions about the impact of the altered WTA:LTA ratio on the pgl cell envelope and/or the post-translational modification(s) of teichoic acids (TAs). WTA glycosylation, which has previously been implicated in increased OX resistance [63, 77], was ruled out because the OX MIC of the pgl mutant was unaffected by mutations in the WTA α and β glycosylase genes tarM and tarS (S11 Fig). Because TAs contribute to cell surface charge, cyctochrome c binding assays [74] were performed and revealed that pgl cells were significantly more positively charged in CDMG OX (Fig 8E). A similar trend was observed in CDMG, but did not reach significance. Consistent with published data [74], the vraG mutant was more negatively charged than wild-type, albeit not reaching significance (Fig 8E). The vraG mutation partially restored the surface charge of the pgl mutant. Together with the reduction in LTA levels, the increased positive charge is further evidence of the altered composition and decoration of the cell envelope in pgl. Moreover, these observations are consistent with increased VraFG/GraRS-dependent, DltABCD-mediated d-alanylation of TAs and increased OX resistance in pgl. Future studies to characterise VraFG/GraRS-controlled WTA/LTA polymer length and decoration [76, 78, 79], and patterns of LTA release [27, 80], in pgl will be informative. In summary, the data presented here reveal that extensive metabolic reprogramming in a MRSA pgl mutant is accompanied by increased OX resistance, which is associated with redirected carbon flux to cell envelope precursors, reduced levels of LTAs and cell lysis, and a significantly more positive cell surface charge which is VraFG/GraRS-dependent.
Discussion
Beyond the central role of the oxidative phase of the PPP in producing reducing power and 5 carbon sugars for nucleotide and amino acid biosynthesis, its contribution to other phenotypes in bacteria remains much less understood due to the essentiality of most enzymes in the pathway. One exception to this is 6-phosphogluconlactonase (Pgl). Here, for the first time, we report a role for pgl in the control of MRSA β-lactam antibiotic resistance, growth, cell size and cell surface morphology. Our analysis revealed pleiotropic effects of the pgl mutation on (i) the PPP itself and downstream nucleotide biosynthesis, (ii) glycolysis and the TCA cycle and (iii) flux to cell wall, WTA and LTA precursors. All three of these pathways have previously been implicated in the control of MRSA β-lactam resistance providing a multifaceted explanation for the increased OX resistance phenotype of the pgl mutant.
Although OX MICs of the wild-type JE2 and pgl mutant were dependent on the culture media, the pgl mutant was always significantly more resistant and the most striking difference between the two strains was measured in chemically defined media with glucose (CDMG), which is the substrate for the PPP. Strikingly, the wild-type JE2 OX MIC was reduced to 1 μg/ml in CDMG, compared to 64 μg/ml in MHB 2% NaCl, whereas the pgl OX MIC was similar in both culture media (128–256 μg/ml). Given that the JE2 OX MIC was 4–16 μg/ml in CDM, in which growth of S. aureus is dependent on amino acid catabolism, it appears that glucose plays a significant role in controlling OX susceptibility in JE2 but not in the pgl mutant in which central metabolism is significantly perturbed. MHB 2% NaCl is the standard culture medium used to measure the susceptibility of S. aureus clinical isolates to oxacillin in diagnostic laboratories, and these experiments raise the question of whether CDMG may be more physiologically relevant in terms of predicting the in vivo effectiveness of β-lactams in patients with MRSA infections.
The pgl gene has previously been mutated in E. coli and L. monocytogenes [58, 62], leading to an accumulation of gluconate, which can be transported back into the cell and phosphorylated, thus potentially bypassing the Pgl-catalysed reaction in the PPP [58, 62]. However, in S. aureus the slower growth and OX resistance phenotypes of the pgl mutant were not dependent on the gluconate transport and catabolism genes gntPK or the presence of exogenous d-gluconate in the culture media. Thus, questions remain about the importance and regulation of the gluconate shunt in S. aureus.
In CDMG, pgl mutant cell size was significantly reduced compared to wild-type. Reduction in cell size may correlate with increased β-lactam resistance of pgl, as previously reported for a c-di-AMP phosphodiesterase gdpP mutant [41]. In addition to the previously reported OX-induced increase in cell size [31, 53, 59, 61], a dramatic cell lysis phenotype was also observed in wild-type JE2 grown in CDMG with sub-inhibitory OX (0.05 μg/ml), and not in the pgl mutant.
Not unexpectedly, the pgl mutation significantly perturbed the metabolome. However, accumulation of several individual metabolites within the PPP and glycolysis was not uniformly affected suggesting that the restoration of homeostasis required to enable growth in the absence of Pgl was complex. For example, downstream of Pgl in the PPP, levels of ribose-5-P were increased whereas sedoheptulose 7-P and nucleotide levels were decreased. The accumulation of ribose-5-P in a mutant lacking the transketolase tkt gene from the non-oxidative phase of the PPP was also accompanied by decreased sedoheptulose 7-P, although levels of inosine-5-monophosphate, xanthosine-5-monophosphate, and hypoxanthine were increased in the tkt mutant [48]. The reduction in purine (and pyrimidine) nucleotide accumulation in the pgl mutant is consistent with its sensitivity to sulfamethoxazole, and with previous studies showing that mutations in the purine biosynthesis and salvage pathways are accompanied by increased OX resistance [26, 53]. The metabolomics data presented here suggest that mutation of pgl was accompanied by a complex and intricately regulated interconversion of glycolytic and PPP intermediates to ensure maintenance of key central metabolites needed to support growth.
Analysis of PG architecture, crosslinking and overall concentration revealed no differences between the wild-type and pgl strains suggesting that other changes in the cell envelope are responsible for increased pgl OX resistance. However, reduced WGA binding and teichoic acid levels, particularly LTAs, is of particular interest. Hesser et al [76] recently proposed that the production of long and abundant LTAs in S. aureus promotes cell lysis, whereas abundant WTA levels have the opposite effect and limit cell lysis. Our data appear to be consistent with this hypothesis and revealed a correlation between significantly reduced LTA levels in pgl and reduced cell lysis under OX stress. In turn, the reduced cell lysis of pgl may also contribute to increased OX resistance. The reduced levels of UDP-Glucose and increased resistance to Congo Red, which targets LtaS were also consistence with the reduced LTA levels in pgl.
Strikingly, mutations in VraFG/GraSR reversed the increased OX resistance phenotype of pgl in CDMG, as well as increased VAN resistance in MHB and reduced Congo Red resistance. Meehl et al previously proposed that because mutation of vraG increased susceptibility to the structurally dissimilar vancomycin and polymyxin B in S. aureus strains Mu50 and COL, VraFG may play a broader role in the export of cell wall/teichoic acid precursors or modifying subunits, rather than specific antimicrobial agents [71]. d-alanylation of WTAs was also shown to be reduced in a graRS mutant [72], further implicating this multienzyme membrane complex in cell envelope biogenesis. In contrast to its effect on OX resistance, mutation of vraG did not reverse the impact of the pgl mutation on LTA levels or cell size suggesting that the broader metabolic consequences of the disrupted PPP on cell size are distinct from the more precise VraFG/GraRS-dependent increase in OX resistance.
The VraG-independent reduction in LTA levels in pgl raised questions about the impact of the altered WTA:LTA ratio on the pgl cell envelope and/or the post-translational modification(s) of teichoic acids. While construction of pgl/tarM and pgl/tarS double mutants excluded a role for α and β glycosylation of WTAs, respectively, the pgl mutant was significantly more positively charged in OX and this was partially reversed by the vraG mutation. Thus, despite the reduction in the levels of LTAs, these observations are consistent with increased d-alanylation of both LTAs and WTAs, and increased OX resistance in pgl.
GraSR was also shown to regulate the transcription of mprF, which codes for the LysPG flippase implicated in resistance to CAMPs and daptomycin [81–83]. Notably, a mprF missense mutation associated with increased cell size and daptomycin resistance was also shown to reduce MRSA OX resistance [84] raising the possibility that altered MprF activity could contribute to pgl phenotypes in a VraFG/GraRS-dependent manner.
The GraRS/VraFG complex shares significant amino acid sequence similarity with BceRS/BceAB in Bacillus subtilis, which plays an important role in bacitracin resistance. Bacitracin targets the lipid II cycle intermediate undecaprenyl-pyrophosphate (UPP), which is believed to be flipped/transported across the membrane, potentially by the BecAB ABC transporter, during PG biosynthesis [85, 86]. Upregulation of bceAB expression by the BceR response regulator and changes in the conformation of BceAB appear to protect UPP from bacitracin inhibition [85]. Thus, while PG structure and crosslinking is unaffected by the pgl mutant, it is tempting to speculate that UPP flipping across the membrane by VraFG may be of particular importance for PG biosynthesis in the pgl mutant under OX stress in CDMG, which is detected by the GraRS two component system. GraRS is known to be required for S. aureus growth at high temperatures and under oxidative stress [87], supporting the conclusion that the vraFG-dependent increase in OX resistance in pgl is also environmentally-regulated, as evidenced by changes in OX MICs in different culture media.
Taken together, our data reveal dramatically increased OX susceptibility and lysis of wild-type JE2 in CDMG, which may be associated with relatively higher LTA levels compared to pgl. This vulnerability is apparently reversed by the reduced LTA levels and higher positive cell surface charge of pgl mutant cells, which are smaller and have a ruffled surface morphology, thicker cell walls and intact septa. The pgl OX resistance phenotype and increased positive surface charge is, in turn, dependent on VraFG and GraRS. However mutation of vraG in the pgl background did not restore LTA levels or result in an increase in cell size. We propose a possible model (Fig 9), in which the combined effects of reduced levels of LTAs and VraFG/GraRS-dependent DltABCD-mediated d-alanylation of WTAs and LTAs in pgl results in increased β-lactam resistance and prevents the extensive OX-induced lysis evident in the wild-type JE2.
Fig 9. Suggested model for VraFG-dependent high-level β-lactam resistance in the MRSA pgl mutant.
A. Illustration of JE2 and pgl cell division during growth in CDMG OX. pgl cells are smaller than wild-type JE2 when grown in CDMG and undergo normal cell division, whereas extensive lysis is evident among wild-type cells. B and C. Illustration of peptidoglycan (PG), wall teichoic acid (WTA) and lipoteichoic acid (LTA) biosynthesis in wild-type JE2 (B) and pgl (C). Mutations in vraF, vraG and to a lesser extent graR reverse the increased OX resistance phenotype of the pgl mutant. Metabolic reprogramming in the pgl mutant increases carbon flux to cell envelope precursors and β-lactam resistance via a mechanism dependent on VraFG/GraRS-controlled regulation of WTA/LTA biosynthesis, export or posttranslational modification. Previous studies have implicated the VraFG/GraRS complex in resistance to cationic antimicrobial peptides and regulation of dltABCD and mprF transcription, and it has also been proposed to play a role in the export of peptidoglycan or teichoic acid precursors or modifying subunits. Reduced levels of LTAs in pgl (C) compared to JE2 (B) may contribute to reduced cell lysis under OX stress. The significantly increased positive charge in the pgl mutant (C) compared to JE2 (B) was partially reversed by the vraG mutation suggesting that reduced levels of LTAs and increased VraFG/GraRS-dependent d-alanylation of WTAs and LTAs in the pgl mutant combine to increase OX resistance. Figure made using Biorender.com.
Materials and methods
Bacterial strains and growth conditions
Bacterial strains and plasmids used in this study can be found in S2 Table. Escherichia coli strains were grown in lysogeny broth /Luria Bertani broth (LB) broth or agar (LBA) and Staphylococcus aureus strains were grown in Tryptic Soy Broth (TSB), Tryptic Soy Agar (TSA), Mueller-Hinton Broth (MHB) supplemented with 2% NaCl where indicated, Mueller-Hinton Agar (MHA) supplemented with 2% NaCl where indicated, Brain Heart Infusion (BHI) broth, LB broth, chemically defined medium (CDM) [38] or chemically defined medium supplemented with glucose (5 g/L) (CDMG). Culture media were supplemented with erythromycin (Erm) 10 μg/ml, chloramphenicol (Cm) 10 μg/ml, ampicillin (Amp) 100 μg/ml, kanamycin (Km) 90 μg/ml, oxacillin (OX) at varying concentrations as indicated.
Genetic manipulation of S. aureus, complementation of NE202 (pgl) and construction of pgl double and triple mutants
To verify the increased OX resistance phenotype of NE202, phage 80α was used to transduce the pgl::Ermr allele into wild-type JE2, as described previously [39, 57]. The presence of the pgl::Ermr allele in NE202 and the transductant was verified by PCR amplification with primers NE202_check_F, NE202_check_R, Martn_ermF, and Martn_ermR (S3 Table).
To complement NE202, the pgl gene including its promoter and upstream regulatory sequences was first amplified from JE2 genomic DNA using pgl_Fwd and pgl_Rev primers (S3 Table), cloned into pDrive (Qiagen) in E. coli TOP10 (Invitrogen), verified by Sanger sequencing (Source Biosciences) before being sub-cloned on an EcoRI restriction fragment into the E. coli-Staphylococcus shuttle plasmid pLI50 [88] and transformed into E. coli HST08 (Takara Bio). The pLI50_pgl plasmid was then isolated and transformed by electroporation into the restriction-deficient strain RN4220, and subsequently into NE202. All plasmid-harbouring strains were cultured in media supplemented with 100 μg/ml ampicillin (E. coli) or 10 μg/ml chloramphenicol (S. aureus) to maintain plasmid selection.
To generate the pgl::Kmr mutant, pKAN plasmid [57] was isolated from IM08B and electroporated into NE202 (pgl::Ermr) and the Ermr marker swapped for the Kmr marker using allelic exchange [57]. To construct a markerless Δpgl mutant, the pTNT plasmid [57] from RN4220 pTNT was isolated and electroporated into NE202 (pgl::Ermr) and the Ermr marker swapped out for a shorter, markerless version of the transposon insertion leaving a small deletion in the pgl gene. The pgl::Kmr and Δpgl mutants were verified by PCR using primers NE202_check_F, NE202_check_R, KanR_fwd and KanR_rev (S3 Table)
To construct pgl double mutants phage 80α used to transduce the Ermr-marked alleles from the following Nebraska transposon library [57] mutants into pgl::Kmr: NE1868 (mecA), NE952 (gntP), NE1124 (gntK), NE569 (sucC), NE547 (sucA), NE76 (leuB), NE239 (putA), NE1518 (gudB), NE70 (vraG), NE645 (vraF), NE481 (graR), NE942 (tarS), NE611 (tarM) NE626 (sdhA). The presence of transposon insertions in the genes was confirmed by PCR using primers listed in S3 Table.
To construct the pgl/vraG/putA triple mutant the NE239 putA::Ermr strain was first transformed with pSPC plasmid [57] isolated from IM08B pSPC and allelic exchange performed as previously described [57] to generate putA::Specr. The putA::Specr allele was then transduced into the pgl/vraG double mutant using phage 80α. The presence of transposon insertions in pgl, vraG and putA genes were confirmed by PCR using primers listed in S3 Table.
Tecan growth curves
A Tecan Sunrise microplate instrument equipped with Magellan software was used to record data from growth experiments performed in 96-well plates. Cultures were streaked on TSA plates supplemented with antibiotics where needed and grown at 37°C for 24 hours. The next day, colonies were resuspended in 1 ml of PBS, before being washed in PBS. The PBS washed cell suspensions were adjusted to an OD600 of 0.2 in 1 ml of PBS and 10 μl inoculated into wells containing 190 μl growth media (MHB, LB, TSB, BHI, CDM, CDMG, CDM 10 μg/ml OX, CDMG 10 μg/ml OX, CDMG supplemented with potassium d-Gluconate (5 g/L) (with or without OX 10 μg/ml) (starting OD600 = 0.01) and were then incubated at 35–37°C for 24 h with shaking and OD600 recorded every 15 min intervals. For H2O2 sensitivity assays (S5 Fig), CDMG and CDMG containing 500 μM H2O2 were inoculated at a starting OD600 of 0.05. For human serum growth curves, human serum (from human male AB plasma, Merck) was mixed with CDMG v/v (70%-30%, 50%-50%, 25%-75%, and 10%-90% respectively). Strains were first grown at 37°C on TSA 2% NaCl for 24 h and 5–10 colonies were resuspended in 0.85% saline before being adjusted to 0.5 McFarland standard (A600 = 0.1). The cell suspension was then diluted 1:20 in PBS and 10 μl used to inoculate 100 μl human serum-CDMG mixture and inoculated at a starting OD600 of 0.005. Three independent biological replicates were performed for each strain and the resulting data plotted using GraphPad Prism software V9.
Antibiotic disc diffusion susceptibility assays
Disk diffusion susceptibility testing was performed in accordance with Clinical Laboratory Standards Institute (CLSI) guidelines [89] and as previously described [53] with the slight modifications. Briefly, overnight cultures were diluted into 5 ml fresh TSB and grown for 3 h at 37°C with shaking at 200 rpm. The 3 h grown cultures were then adjusted to A600 = 0.5 and 150 μl of this suspension was swabbed evenly 3 times across the surface of an MHA plate (4 mm agar thickness). Six mm blank discs (OXOID) were spotted with 20 μl antibiotics (cefoxitin 1.5 mg/ml stock). Once dried, the discs were applied onto the MHA plates spread with culture suspension before incubation for times specified by CLSI guidelines for stated antibiotics at 37°C. Three independent measurements were performed for each strain and zone of inhibition was measured and recorded.
Antibiotic minimum inhibitory concentration (MIC) measurements and synergy/checkerboard assays
MIC measurements by broth microdilutions were performed in accordance with CLSI methods for dilution susceptibility testing of staphylococci [90] with modifications. Briefly, strains were first grown at 37°C on MHA 2% NaCl for 24 h and 5–10 colonies were resuspended in 0.85% saline before being adjusted to 0.5 McFarland standard (A600 = 0.1). The cell suspension was then diluted 1:20 in PBS and 10 μl used to inoculate 100 μl media (MHB/ MHB 2% NaCl / CDM / CDMG) containing serially diluted antibiotics (oxacillin, fosfomycin, targocil, tunicamycin, Congo Red, amsacrine, DCS, vancomycin, sulfamethoxazole and polymyxin B) in 96-well plates. For human serum MICs, Human serum (from human male AB plasma, Merck) was mixed with CDMG or MHB 2% NaCl v/v (70%-30%, 50%-50%, 25%-75%, and 10%-90%) and inoculated as described above. The 96-well plates were incubated at 35°C for 24 h and MIC values were recorded as the lowest antibiotic concentration where no growth was observed. Checkerboard/synergy assays were performed as previously described, using (0–128 μg/ml) fosfomycin and (0–256 μg/ml) oxacillin as indicated in S9 Fig.
Genomic DNA (gDNA) extraction and Whole Genome Sequencing (WGS)
Genomic DNA (gDNA) extractions were performed using the Wizard Genomic DNA Purification Kit (Promega) following pre-treatment of S. aureus cells with 10 μg/ml lysostaphin (Ambi Products LLC) at 37°C for 30 min. The genome sequencing for NE202 (pgl) was performed by MicrobesNG using an Illumina HiSeq platform and a 250-bp paired end read kit. DNA libraries for pgl::Kmr and pglR1 were prepared using an Illumina Nextera XT DNA Library Prep kit, validating size distribution by gel electrophoresis, and bead-normalizing the libraries. An Illumina MiSeq v2 600 cycle kit was used for genome sequencing, generating 300-bp paired end reads. PhiX was used as a sequencer loading control. The CLC Genomics Workbench software (Qiagen Version 20) was used for genome sequencing analysis of the different strains, as described previously [91]. As a reference genome, a contig was produced for wild-type JE2 by mapping Illumina reads onto the closely related USA300 FPR3757 genome sequence (RefSeq accession number NC_007793.1). The Illumina short read sequences from NTML mutants [57] of interest were then mapped onto the assembled JE2 sequence, and the presence of the transposon insertion confirmed. Single Nucleotide Polymorphisms (SNPs), deletions or insertions were identified where present.
PBP2a Western blot analysis
PBP2a Western blots were performed as previously described [92] with slight modifications. Briefly, single colonies from wild-type JE2, pgl and pglcomp, MSSA strain 8325–4 (negative control) and HoR MRSA strain BH1CC (positive control) were inoculated in TSB overnight and grown at 37°C with 200 rpm shaking. The next day, day cultures were started at OD600 0.05 in 50 ml TSB supplemented with 0.5 μg/ml OX except for 8325–4 which was grown with no OX supplementation, and BHICC with 50 or 75 μg/ml OX, and grown for 6 hours, with shaking (200 rpm). For MHB 2% NaCl grown cells, single colonies from wild-type JE2, and pgl were inoculated in MHB overnight and grown at 37°C with 200 rpm shaking. The next day, day cultures were started at OD600 0.05 in 50 ml MHB 2% NaCl w/wo 32 μg/ml OX and grown for 6 hours with shaking (200 rpm). Samples were pelleted and resuspended in PBS to an A600 = 10. Six μl of lysostaphin (10 μg/ml) and 1 μl of DNase (10 μg/ml) was added to 500 μl of this concentrated cell suspension before being incubated at 37°C for 40 min. Next, 50 μl of 10% SDS was added, and the incubation continued for a further 20 min. The lysed cells were then pelleted in a microcentrifuge for 15 min, following which the protein-containing supernatant was collected and total protein concentration determined using the Pierce BCA Protein Assay Kit. For each sample, 8 μg total protein was run on a 7.5% Tris-Glycine gel, transferred to a PVDF membrane, and probed with anti-PBP2a (1:1000), followed by HRP-conjugated protein G (1:2000) and colorimetric detection with Opti-4CN Substrate kit. Three independent experiments were performed, and a representative image is shown.
Peptidoglycan (PG) analysis
Wild-type JE2, pgl and pglcomp were grown in MHB and MHB supplemented with oxacillin 0.5 μg/ml, CDMG and CDMG supplemented with OX 0.05 μg/ml. For each strain and growth condition tested, independent quadruplicate 50 ml cultures were grown in flasks at 37°C with 200 rpm shaking overnight and cell pellets were collected at 4°C at 7000 rpm. The pellets were then resuspended in PBS, pelleted at 10000 rpm and snap frozen in liquid nitrogen in 1.5 ml tubes. Peptidoglycan (PG) was extracted from wild-type JE2, pgl and pglcomp from boiled samples as described previously [93]. Once boiled, cell wall material was pelleted by ultracentrifugation and washed with water. Clean sacculi were digested with muramidase (100 μg/ml) and soluble muropeptides reduced using 0.5 M sodium borate pH 9.5 and 10 mg/mL sodium borohydride. The pH of the samples was then adjusted to 3.5 with phosphoric acid. UPLC analyses were performed on a Waters-UPLC system equipped with an ACQUITY UPLC BEH C18 Column, 130Å, 1.7 μm, 2.1 mm × 150 mm (Waters Corporation, USA) and identified at Abs. 204 nm. Muropeptides were separated using a linear gradient from buffer A (0.1% formic acid in water) to buffer B (0.1% formic acid in acetonitrile). Identification of individual peaks was assigned by comparison of the retention times and profiles to validated chromatograms. The relative amount of each muropeptide was calculated by dividing the peak area of a muropeptide by the total area of the chromatogram. The abundance of PG (total PG) was assessed by normalizing the total area of the chromatogram to the OD600. The degree of cross-linking was calculated as described previously [94].The data for at least three independent experiments were plotted using GraphPad Prism software V9.
Confocal microscopy and cell size determination
For microscopy experiments, JE2, pgl and pglcomp were grown overnight at 37°C in CDMG w/wo 0.05 μg/ml OX. The next day, the cultures were washed and normalized to an OD600 of 1 in PBS and 75 μl of these cultures were double stained for 30 mins at 37°C with vancomycin-BODIPY FL at a final concentration of 2 μg/ml and WGA Alexa Fluor 594 at a final concentration of 25 μg/ml. Bacteria were then collected by centrifugation for 2 mins at 14,000 xg. The cells were resuspended with 100 μl of PBS, pH 7.4, and 5 μl of this sample was spotted onto a thin 1.5% agarose gel patch prepared in PBS. Stained bacteria were then imaged at X1000 magnification using Olympus LS FLUOVIEW Fv3000 Confocal Laser Scanning Microscope. Cell size was measured as previously described [54] using ImageJ software (Fiji v.1.0). Images of cells from three/four biological replicates were acquired, 50 cells measured per biological replicate (150–200 cells in total per condition), and the average and standard deviations for the three/four biological replicates were plotted using GraphPad Prism version 9.2 and significant differences were determined using a Kruskal-Wallis test followed by a Dunn’s multiple comparison test. Only 60 cells could be measured for OX treated cells due to cell lysis.
Transmission Electron Microscopy (TEM) and cell morphology analysis
Overnight cultures of JE2, pgl and pglcomp were grown overnight in 5 ml CDMG at 37°C shaking at 200 rpm. The next day, OD600 values were measured, and cultures were used to inoculate 5 ml day cultures in CDMG 1 μg/ml OX to OD600 of 0.06. The day cultures were grown for 4.5 hours at 35°C shaking at 200 rpm, before being pelleted down, and normalised to OD600 of 1 in PBS. Cells pellets were resuspended in 0.2M sodium cacodylate buffer pH 7.2. Fixed bacteria were dehydrated, embedded in resin, and thin sectioned in the University of Galway Centre for Microscopy & Imaging. Images were acquired using Hitachi H7500 Transmission Electron Microscope. Representative cells from each strain were imaged at 30,000× and 100,000× magnification.
Congo Red susceptibility spotting assays
S. aureus strains JE2, pgl, pglcomp, vraG and pgl/vraG were streaked onto TSA plates containing appropriate antibiotics, and the plates were incubated overnight at 37°C. The next day, overnight cultures of the strains from single colonies were grown in 5 ml TSB, at 37°C shaking at 200 rpm. The next day, PBS washed cells were normalised to an OD600 of 1 per ml in PBS and serial dilutions prepared from 10−1 until 10−8 in a 96-well plate. Five μl of the serially diluted cell suspensions was spotted onto TSA plates containing 0.125% Congo Red. The plates were dried in a flow hood and were incubated at 37°C for 24 hours. Plates were visualised and photos were taken for three biological replicates. Representative image is shown in Fig 8D.
Quantification of Wheat Germ Agglutinin (WGA) binding
Overnight cultures of S. aureus strains were grown in 3 ml CDMG at 37°C shaking at 200 rpm. The next day, OD600 values were measured, and cultures were used to inoculate 5 ml day cultures in CDMG 0.1 μg/ml OX to OD600 of 0.06. The day cultures were grown for 4.5 hours at 35°C shaking at 200 rpm, before being pelleted down, washed with PBS, and normalised to OD600 of 1 in PBS. One hundred μl of this cell suspension was incubated with WGA Alexa Fluor 594 at a final concentration of 25 μg/ml for 30 minutes at 37°C. After the incubation with the dye, the cells were pelleted at 14,000 rpm for 3 minutes, and the supernatant was used for fluorescence measurements in Polarstar plate reader (Excitation/Emission 590/617 nm). PBS containing WGA Alexa Fluor 594 at a final concentration of 25 μg/ml was used as a positive control, and PBS was used as a blank control. The reduction in WGA Alexa Fluor 594 from the positive control was calculated per sample, and % bound WGA plotted using were plotted and significant differences were determined for two biological repeats using two-way ANOVA with Turkey’s post-hoc. using GraphPad Prism version 9.2
Culture supernatant sample preparation for LC-MS/MS
Overnight cultures of S. aureus strains were grown in 3 ml CDMG at 37°C shaking at 250 rpm. The next day, 250 ml flasks containing 25 ml CDMG were inoculated to an OD600 of 0.06 and were grown for 7.5 h (OD600 = 4.22–4.96). One ml from the cultures were collected and centrifuged at 12,000 rpm, 10 min at 4°C, and supernatant collected. These samples were diluted 1:100 v/v using 10 mM NH4OAc + 10mM NH4OH + 5% acetonitrile. 5 μl was injected into the LC-MS/MS (details below).
Sample preparation intracellular metabolite analysis by LC-MS/MS
Overnight cultures of S. aureus strains were grown in 3 ml CDMG at 37°C shaking at 250 rpm. The next day, 250 ml flasks containing 25 ml CDMG (with or without 1 μg/ml OX) were inoculated at a starting OD600 of 0.06 and grown for 4–5 hours (until exponential phase was reached) at 37°C shaking at 250 rpm. Culture volumes corresponding to OD600 of 10 were then harvested and rapidly filtered through a membrane (0.45 μm, Millipore). The cells on the membrane were washed twice with 5 ml cold saline and immediately quenched in ice-cold 60% ethanol containing 2 μM Br-ATP as an internal control. The cells were mechanically disrupted using a bead homogenizer set to oscillate for 3 cycles (30 s) of 6800 rpm with a 10 s pause between each cycle. Cell debris was separated by centrifugation at 12,000 rpm. The supernatant containing intracellular metabolites were lyophilized and stored at -80°C. These samples were reconstituted in 100 μl of 50% MeOH.
Analysis of PPP flux using 1,2-13C glucose
The S. aureus strains were inoculated in 25 ml CDM containing either unlabelled or 1,2-13C-labeled glucose at a starting OD600 of 0.06. The cultures were grown at 37°C with shaking at 250 rpm until the OD600 reached 1.0. The culture volume corresponding to an OD600 of 10 was then harvested and immediately filtered through a 0.45 μm Millipore membrane before being subjected to further processing as outlined in the previous section.
LC-MS/MS mass spectrometry
A triple-quadrupole-ion trap hybrid mass spectrometer (QTRAP6500+ by Sciex, USA) connected to an ultra-performance liquid chromatography I-class (UPLC) system (Waters, USA) was utilized for metabolomics analysis. The chromatographic separation was performed using the UPLC on a XBridge Amide analytical column (150 mm x 2.1 mm ID, 3.5 μm particle size by Waters, USA) and a binary solvent system with a flow rate of 0.3 ml/min. The analytical column was preceded by a guard XBridge Amide column (20 mm x 2.1 mm ID, 3.5 μm particle size by Waters, USA). The mobile phase A consisted of 10 mM ammonium acetate and 10 mM ammonium hydroxide with 5% acetonitrile in LC-MS grade water (pH adjusted to 8.0 with glacial acetic acid), while mobile phase B was 100% LC-MS grade acetonitrile. The column was maintained at 40°C and the autosampler temperature was kept at 5°C throughout the sample run. The gradient conditions were as follows: A/B ratio of 15/85 for 0.1 minute, 16/84 for 3.0 minutes, 35/65 for 4.0 minutes, 40/60 for 5.0 minutes, 45/55 for 3.0 minutes, 50/50 for 5.5 minutes, 30/70 for 1.5 minutes, and finally equilibrated at 15/85 for 5.0 minutes before the next run. The needle was washed with 1000 μL of strong wash solvent (100% acetonitrile) and 1000 μL of weak wash solvent (10% aqueous methanol) prior to injection, with an injection volume of 5 μL. The QTRAP6500+ operated in polarity switching mode for the targeted quantitation of amino acids through the Multiple Reaction Monitoring (MRM) process. The electrospray ionization (ESI) parameters were optimized, with an electrospray ion voltage of -4200 V in negative mode and 5500V in positive mode, a source temperature of 400°C, a curtain gas of 35 and gas 1 and 2 of 40 and 40 psi, respectively. Compound-specific parameters were optimized for each compound through manual tuning, with declustering potential (DP) of 65V in positive mode and -60V in negative mode, entrance potential (EP) of 10V in positive mode and -10V in negative mode, and collision cell exit potential (CXP) of 10V in positive mode and -10V in negative mode.
Extraction and visualisation of WTA
The WTA extraction and visualization was performed as previously described [34, 95, 96] with modifications. Briefly, wild-type JE2, pgl, vraG, pgl/vraG, tagO (LAC* ΔtagO, ANG4759 [97]) were grown in CDMG and CDMG supplemented with OX 0.5 μg/ml (except tagO) overnight. For each strain and growth condition tested, independent triplicate 50 ml cultures were grown in flasks at 35°C with 200 rpm shaking overnight. Cell pellets (OD600 of 50) were collected at 10°C at 7800 rpm. The pellets were then washed with 1 ml of 50 mM MES, pH 6.5, resuspended in 4% SDS and 50 mM MES, pH 6.5 and incubated at 100°C for 1 hr. The boiled samples were washed twice in 4% SDS and 50 mM MES, once with 2% NaCl and 50 mM MES, and once with 50 mM MES before being resuspended in 1 mL 20 mM Tris-HCl (pH 8), 0.5% SDS, and 20 μg/ml proteinase K. The samples were incubated for 4 hrs at 50°C (shaking at 1,400 rpm in a thermomixer). The pellet was recovered, washed with 2% NaCl and 50 mM MES, and washed three times with water before being resuspended in 100 μl 0.1 M NaOH and incubated at 20°C for 12 h (shaking at 1,400 rpm). Samples were then heated to 65°C for 1 hr, followed by centrifugation at 14,000 rpm for 1 min. The supernatant was removed into a fresh 1.5 ml Eppendorf tube, and neutralized with 25 μL 1 M Tris-HCl, pH 8, and stored at −20°C.
Purified WTA samples were mixed with 2 M Sucrose (1:4 ratio), and 12.5 μl loaded on 20% native polyacrylamide gels. The gels were run at 120 V in Tris-Tricine running buffer (0.1 M Tris, 0.1 M Tricine) before being stained with 0.1% Alcian blue in 3% acetic acid for 30 minutes at RT on a shaker, covered in aluminium foil. The gels were de-stained with water and a representative image from 3 independent biological repeats are shown.
Extraction of LTA and detection by Western blot Analysis
LTA extraction and visualization was performed as previously described [34, 95, 96], with modifications. Briefly, wild-type JE2, pgl, pglcomp, pgl::Kmr, vraG, pgl/vraG, wild-type LAC* [98] and ltaS/gdpP (ANG2434) [41] were grown in CDMG and CDMG supplemented with OX 0.05 μg/ml (except ltaS/gdpP) overnight. For each strain and growth condition tested, independent triplicate 5 ml cultures were grown in 30 ml universals at 35°C with 200 rpm shaking overnight. Cell pellets (OD600 of 3) were collected at 10°C at 7800 rpm. The pellets were then resuspended in 1 ml PBS and transferred to screw cap tubes containing approximately 100 μL of 100 μm glass beads. The cells were homogenised at room temperature using a FastPrep-24 (MP Biomedicals) for 3 cycles of 6 m/s for 40 s each, followed by a 4 mins rest. The tubes were then centrifuged at 200 × g for 1 min to settle the beads, and 700 μl of the supernatant transferred into a new tube, and the cell debris were pelleted at 14,000 rpm for 15 minutes. The supernatant was removed, and the remaining pellet containing LTAs was resuspended in 80 μL 2× Laemmli sample buffer. The samples were incubated at 95°C for 20 mins, centrifuged at 14,000 rpm for 5 mins, and the supernatant stored at −20°C.
LTA extracts (20 μl) were run on 15% polyacrylamide gels and transferred to PVDF membranes using a Trans-Blot Turbo transfer system (Bio-Rad). After blocking with 5% milk and 1% BSA in TBST for 3 hrs, LTA was detected with an anti-LTA primary antibody (MAb 55; HycultBiotech; 1:5000 dilution), followed by HRP-conjugated protein G (1:2000) and colorimetric detection with Opti-4CN Substrate kit. Three independent experiments were performed, and a representative image is shown.
Cytochrome c binding assay
Cytochrome c binding assay was performed as previously described [67, 99] with modifications. Briefly, wild-type JE2, pgl, vraG and pgl/vraG were grown in CDMG and CDMG supplemented with OX 0.5 μg/ml overnight. For each strain and growth condition tested, independent triplicate 50 ml cultures were grown in flasks at 35°C with 200 rpm shaking overnight. Cell pellets (OD600 of 10) were collected by centrifugation at 7800 rpm. The pellets were then washed twice and resuspended in 20 mM MOPS buffer (pH 7.0), mixed with cytochrome c (0.5 mg/ml final concentration) and incubated at room temperature for 10 minutes. The cells were then centrifuged, and the amount (% bound) of cytochrome c left in the supernatant was determined by measuring the OD410 and comparing it to the relative (100%) cytochrome c control (0.5 mg/ml).
Supporting information
(DOCX)
(DOCX)
(DOCX)
A. JE2, pgl (NE202), pgl::Kmr, and pglcomp grown for 6 h in TSB supplemented with OX 0.5 mg/ml. HoR MRSA strain BH1CC (positive control) was grown in TSB OX 50 and OX 75 mg/ml, and MSSA strain 8325–4 (negative control) was grown in TSB alone. (B) JE2 and pgl grown for 6 h in MHB 2% NaCl alone or supplemented with OX 32 mg/ml. For each sample, 8 μg total protein was run on a 7.5% Tris-Glycine gel, transferred to a PVDF membrane and probed with anti-PBP2a (1:1000), followed by HRP-conjugated protein G (1:2000) and colorimetric detection with Opti-4CN Substrate kit. Three independent experiments were performed, and representative blots are shown.
(TIF)
A. Isolated colonies of JE2 (left) and pgl (right) after growth on MHA for 24 h at 37°C. B-G. Growth of JE2, pgl and the complemented pgl mutant for 25 hrs at 37°C in Mueller Hinton broth, MHB (B), Luria Bertani, LB (C), Tryptic Soya broth, TSB (D), Brain Heart Infusion, BHI (E), Chemically defined media with glucose, CDMG (F) and chemically defined media with no glucose, CDM (G). Growth (OD600) was measured at 15 min intervals in a Tecan plate reader. Data are the average of 3 independent experiments plotted using GraphPad Prism V9 and error bars represent standard deviation.
(TIFF)
A. Growth of wild-type JE2 and pgl for 24 hrs at 37°C in chemically defined media (CDM) supplemented with 0.07 g/l (0.43 mM) glucose alone or with OX 10 mg/ml. Growth (OD600) was measured at 15 min intervals in a Tecan plate reader. Data are the average of 3 independent experiments plotted using GraphPad Prism V9 and error bars represent standard deviation. B. Oxacillin MICs of JE2 and pgl in CDM with no glucose (CDM), or CDM supplemented with glucose concentrations from 0.03–5 g/l (1.75–28 mM). Note that the Y axis (Oxacillin MIC) is a log2 scale.
(TIF)
A and B. Growth of JE2 (A) and pgl (B) for 24 hrs at 35°C in CMDG 25% human serum supplemented with OX 1, 32 and 256 mg/ml. C and D. Growth of JE2 (C) and pgl (D) for 24 hrs at 35°C in CMDG 70% human serum supplemented with OX 1, 32 and 256 mg/ml. Growth (OD600) was measured at 15 min intervals in a Tecan plate reader. Data are the average of 3 independent experiments plotted using GraphPad Prism V9 and error bars represent standard deviation.
(TIF)
Growth of wild-type JE2 and pgl::Kmr for 24 hrs at 37°C in CDMG or CDMG supplemented with 500 mM H2O2. Growth (OD600) was measured at 15 min intervals in a Tecan plate reader. Data are the average of 3 independent experiments plotted using GraphPad Prism V9 and error bars represent standard deviation.
(TIFF)
A-D. Relative proportions of cell wall muropeptide fractions based on oligomerization relative cross-linking efficiency in peptidoglycan extracted from JE2, pgl and pglcomp grown to exponential phase in CDMG (A), CDMG supplemented with OX 0.05 μg/ml (B), MHB (C) and MHB supplemented with OX 0.5 mg/ml (D). E-H. Total peptidoglycan (PG) extracted from normalised cell extracts of JE2, pgl and pglcomp grown to exponential phase in CDMG (E), CDMG supplemented with OX 0.05 μg/ml (F), MHB (G) and MHB supplemented with OX 0.5 mg/ml (H). The total PG content was calculated as the area below the chromatogram peaks/OD600 and mean and standard deviation from three/four biological repeats plotted using GraphPad Prism V9.
(TIFF)
A and B. Growth of JE2, pgl and the complemented pgl mutant for 25 hrs at 35°C in CDMG supplemented with 5g/l potassium D-gluconate (0.5%) and no OX (A) or with both D-gluconate (0.5%) and OX 10 mg/ml (B). C. Growth of JE2, pgl, pglcomp pgl::Kmr, gntP (NE952), gntK (NE1124), pgl/gntP and pgl/gntK for 25 hrs at 35°C in CDMG supplemented with OX 10 mg/ml. Growth (OD600) was measured at 15 min intervals in a Tecan plate reader. Data are the average of 3 independent experiments using GraphPad Prism V9 and error bars represent standard deviation.
(TIFF)
A and B. Comparison of amino acid (A) and TCA cycle metabolites (B) in supernatants of JE2, pgl and pglcomp cultures grown for 7.5 h in CDMG measured by HPLC. Cell densities (OD600) were normalized to each other before the cells were pelleted and supernatants collected. The data (CPS) shown are the average of three independent experiments and standard deviations are shown‡. Statistical significance was determined using a 2-way Anova with Dunnett’s multiple comparison test; * p<0.05, ** p<0.01, ***p<0.001, ****p<0.0001. C and D. Growth of JE2, pgl, pgl::Kmr, pglcomp, putA, gudB, sdhA, sucA, sucC, pgl/putA, pgl/gudB, pgl/sdhA, pgl/sucA and pgl/sucC for 25 hrs at 35°C in CDMG (C) and CDMG supplemented with OX 10 mg/ml (D). Growth (OD600) was measured at 15 min intervals in a Tecan plate reader. Data are the average of 3 independent experiments using GraphPad Prism V9 and error bars represent standard deviation.
(TIF)
Checkerboard titration assays were conducted using fosfomycin and oxacillin with (A) JE2 and (B) pgl, grown for 24 h in Mueller Hinton 2% NaCl broth in 96-well plates. The data shown are the OD600 values for each well. The experiments were repeated at least three times and the data from a representative 96-well plate is shown. Green shaded boxes indicated wells in which significant growth was measured.
(TIFF)
Comparison of wild-type JE2 (A), pgl (B) and pglcomp (C) cells grown in CDMG without oxacillin (OX) using transmission electron microscopy at 30,000× (left) and 100,000× (right) magnification. Cells were collected from exponential phase cultures grown for 4.5 h in CDMG normalized to OD600 = 1 in PBS before being fixed and thin sections prepared. Representative cells from each strain are shown. Scale bars represent 500 nm at 30,000× or 100 nm at 100,000× magnification.
(TIFF)
OX MICs (mg/ml) of JE2, pgl, pglcomp pgl::Kmr, vraG, vraF, putA, graR, tarM, tarS, pgl/tarS, pgl/tarM, pgl/vraG, pgl/vraF, pgl/putA, pgl/graR, pgl/putA/vraG and pglR1 were measured by the broth microdilution method in CDMG. The MIC was measured in two independent experiments for each strain and variation plotted using GraphPad Prism V9 shown. Note that the Y axis (Oxacillin MIC) is a log2 scale.
(TIFF)
(ZIP)
Acknowledgments
We are grateful to Dr Peter Owens and Dr Emma McDermott from the University of Galway Centre for Microscopy & Imaging (https://imaging.universityofgalway.ie) for their technical and scientific assistance with confocal and electron microscopy analysis, and to Volkhard Kaever, Hannover Medical School for preliminary metabolomic analysis.
M.S.Z. and J.P.O’G. conceptualized the study. Formal analysis was performed by M.S.Z., L.A.G., E.B., A.C.N., J.A., E.O’N., F.R., P.D.F., F.C., V.C.T and J.P.O’G. The investigation and methodology were performed by M.S.Z., L.A.G., E.B., A.C.N., J.A., D.S., M.S., Ó.B. and F.R. The data was curated by M.S.Z. The original draft of the manuscript was written by M.S.Z. and J.P.O’G. and was reviewed, edited, and approved by all authors. Funding was acquired by E.O’N., P.D.F., F.C., V.C.T. Ó.B. and J.P.O’G. The project was administered by J.P.O’G.
Data Availability
Whole-genome sequence data is available from the European Nucleotide Archive (https://www.ebi.ac.uk/ena), Registered Project PRJEB59981, sample accession numbers ERS14733509-ERS14733512 and run accession numbers ERR10960504-ERR10960507. The SAUSA300_FRP3757 (TaxID: 451515) reference genome sequence is available from NCBI (www.ncbi.nlm.nih.gov).
Funding Statement
This research was supported by the Health Research Board (ILP-POR-2019-102 and HRA-POR-2015-1158 to J.P.O’G.), Science Foundation Ireland (19/FFP/6441 to J.P.O’G.), Irish Research Council (GOIPG/2019/2011 to J.P.O’G.), National Institute of Allergy and Infectious Diseases (P01 AI083211 and R01 AI125588 to V.C.T), Swedish Research Council (to F.C.), Laboratory for Molecular Infection Medicine Sweden (to F.C.), Knut and Alice Wallenberg Foundation (to F.C.) and Kempe Foundation (to F.C.). The funders had no role in study design, data collection and interpretation, manuscript writing or the decision to submit the work for publication.
References
- 1.Khouja T, Mitsantisuk K, Tadrous M, Suda KJ. Global consumption of antimicrobials: impact of the WHO Global Action Plan on Antimicrobial Resistance and 2019 coronavirus pandemic (COVID-19). J Antimicrob Chemother. 2022;77(5):1491–9. doi: 10.1093/jac/dkac028 [DOI] [PubMed] [Google Scholar]
- 2.Mendelson M, Matsoso MP. The World Health Organization Global Action Plan for antimicrobial resistance. S Afr Med J. 2015;105(5):325. doi: 10.7196/samj.9644 [DOI] [PubMed] [Google Scholar]
- 3.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339(8):520–32. doi: 10.1056/NEJM199808203390806 [DOI] [PubMed] [Google Scholar]
- 4.Kluytmans J, van Belkum A, Verbrugh H. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev. 1997;10(3):505–20. doi: 10.1128/CMR.10.3.505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellington JK, Reilly SS, Ramp WK, Smeltzer MS, Kellam JF, Hudson MC. Mechanisms of Staphylococcus aureus invasion of cultured osteoblasts. Microb Pathog. 1999;26(6):317–23. doi: 10.1006/mpat.1999.0272 [DOI] [PubMed] [Google Scholar]
- 6.Peacock SJ, Day NPJ, Thomas MG, Berendt AR, Foster TJ. Clinical isolates of Staphylococcus aureus exhibit diversity in fnb genes and adhesion to human fibronectin. J Infection. 2000;41(1):23–31. doi: 10.1053/jinf.2000.0657 [DOI] [PubMed] [Google Scholar]
- 7.Foster TJ, Hook M. Surface protein adhesins of Staphylococcus aureus. Trends in Microbiology. 1998;6(12):484–8. doi: 10.1016/s0966-842x(98)01400-0 [DOI] [PubMed] [Google Scholar]
- 8.Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28(3):603–61. doi: 10.1128/CMR.00134-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5(12):751–62. [DOI] [PubMed] [Google Scholar]
- 10.Peacock SJ, Foster TJ, Cameron BJ, Berendt AR. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiol-Uk. 1999;145:3477–86. doi: 10.1099/00221287-145-12-3477 [DOI] [PubMed] [Google Scholar]
- 11.Fey PD, Ulphani JS, Gotz F, Heilmann C, Mack D, Rupp ME. Characterization of the relationship between polysaccharide intercellular adhesin and hemagglutination in Staphylococcus epidermidis. J Infect Dis. 1999;179(6):1561–4. doi: 10.1086/314762 [DOI] [PubMed] [Google Scholar]
- 12.Washburn RG, Durack DT. Efficacy of ampicillin plus a beta-lactamase inhibitor (CP-45,899) in experimental endocarditis due to Staphylococcus aureus. J Infect Dis. 1981;144(3):237–43. doi: 10.1093/infdis/144.3.237 [DOI] [PubMed] [Google Scholar]
- 13.Ziebuhr W, Krimmer V, Rachid S, Lossner I, Gotz F, Hacker J. A novel mechanism of phase variation of virulence in Staphylococcus epidermidis: evidence for control of the polysaccharide intercellular adhesin synthesis by alternating insertion and excision of the insertion sequence element IS256. Molecular Microbiology. 1999;32(2):345–56. doi: 10.1046/j.1365-2958.1999.01353.x [DOI] [PubMed] [Google Scholar]
- 14.Barber M. Staphylococcal infection due to penicillin-resistant strains. Br Med J. 1947;2(4534):863–5. doi: 10.1136/bmj.2.4534.863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hackbarth CJ, Chambers HF. blaI and blaR1 regulate beta-lactamase and PBP 2a production in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1993;37(5):1144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barber M. Methicillin-resistant staphylococci. J Clin Pathol. 1961;14:385–93. doi: 10.1136/jcp.14.4.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barberis-Maino L, Ryffel C, Kayser FH, Berger-Bachi B. Complete nucleotide sequence of IS431mec in Staphylococcus aureus. Nucleic Acids Res. 1990;18(18):5548. doi: 10.1093/nar/18.18.5548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger-Bachi B, Barberis-Maino L, Strassle A, Kayser FH. FemA, a host-mediated factor essential for methicillin resistance in Staphylococcus aureus: molecular cloning and characterization. Mol Gen Genet. 1989;219(1–2):263–9. [DOI] [PubMed] [Google Scholar]
- 19.Ryffel C, Tesch W, Birch-Machin I, Reynolds PE, Barberis-Maino L, Kayser FH, et al. Sequence comparison of mecA genes isolated from methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. Gene. 1990;94(1):137–8. doi: 10.1016/0378-1119(90)90481-6 [DOI] [PubMed] [Google Scholar]
- 20.Pinho MG, de Lencastre H, Tomasz A. An acquired and a native penicillin-binding protein cooperate in building the cell wall of drug-resistant staphylococci. Proc Natl Acad Sci U S A. 2001;98(19):10886–91. doi: 10.1073/pnas.191260798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinho MG, Filipe SR, de Lencastre H, Tomasz A. Complementation of the essential peptidoglycan transpeptidase function of penicillin-binding protein 2 (PBP2) by the drug resistance protein PBP2A in Staphylococcus aureus. J Bacteriol. 2001;183(22):6525–31. doi: 10.1128/JB.183.22.6525-6531.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baek KT, Gründling A, Mogensen RG, Thogersen L, Petersen A, Paulander W, et al. beta-Lactam resistance in methicillin-resistant Staphylococcus aureus USA300 is increased by inactivation of the ClpXP protease. Antimicrob Agents Chemother. 2014;58(8):4593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panchal VV, Griffiths C, Mosaei H, Bilyk B, Sutton JAF, Carnell OT, et al. Evolving MRSA: High-level beta-lactam resistance in Staphylococcus aureus is associated with RNA Polymerase alterations and fine tuning of gene expression. PLoS Pathog. 2020;16(7):e1008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim C, Mwangi M, Chung M, Milheirico C, de Lencastre H, Tomasz A. The mechanism of heterogeneous beta-lactam resistance in MRSA: key role of the stringent stress response. PLoS One. 2013;8(12):e82814. doi: 10.1371/journal.pone.0082814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peacock SJ, Paterson GK. Mechanisms of Methicillin Resistance in Staphylococcus aureus. Annu Rev Biochem. 2015;84:577–601. doi: 10.1146/annurev-biochem-060614-034516 [DOI] [PubMed] [Google Scholar]
- 26.Dordel J, Kim C, Chung M, Pardos de la Gandara M, Holden MT, Parkhill J, et al. Novel determinants of antibiotic resistance: identification of mutated loci in highly methicillin-resistant subpopulations of methicillin-resistant Staphylococcus aureus. MBio. 2014;5(2):e01000. doi: 10.1128/mBio.01000-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mikkelsen K, Sirisarn W, Alharbi O, Alharbi M, Liu H, Nohr-Meldgaard K, et al. The Novel Membrane-Associated Auxiliary Factors AuxA and AuxB Modulate beta-lactam Resistance in MRSA by stabilizing Lipoteichoic Acids. Int J Antimicrob Agents. 2021:106283. [DOI] [PubMed] [Google Scholar]
- 28.Wu S, de Lencastre H, Sali A, Tomasz A. A phosphoglucomutase-like gene essential for the optimal expression of methicillin resistance in Staphylococcus aureus: molecular cloning and DNA sequencing. Microb Drug Resist. 1996;2(2):277–86. doi: 10.1089/mdr.1996.2.277 [DOI] [PubMed] [Google Scholar]
- 29.Gardete S, Ludovice AM, Sobral RG, Filipe SR, de Lencastre H, Tomasz A. Role of murE in the Expression of beta-lactam antibiotic resistance in Staphylococcus aureus. J Bacteriol. 2004;186(6):1705–13. doi: 10.1128/JB.186.6.1705-1713.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aedo S, Tomasz A. Role of the Stringent Stress Response in the Antibiotic Resistance Phenotype of Methicillin-Resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2016;60(4):2311–7. doi: 10.1128/AAC.02697-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thalso-Madsen I, Torrubia FR, Xu L, Petersen A, Jensen C, Frees D. The Sle1 Cell Wall Amidase Is Essential for beta-Lactam Resistance in Community-Acquired Methicillin-Resistant Staphylococcus aureus USA300. Antimicrob Agents Chemother. 2019;64(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jousselin A, Manzano C, Biette A, Reed P, Pinho MG, Rosato AE, et al. The Staphylococcus aureus Chaperone PrsA Is a New Auxiliary Factor of Oxacillin Resistance Affecting Penicillin-Binding Protein 2A. Antimicrob Agents Chemother. 2015;60(3):1656–66. doi: 10.1128/AAC.02333-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Memmi G, Filipe SR, Pinho MG, Fu Z, Cheung A. Staphylococcus aureus PBP4 is essential for beta-lactam resistance in community-acquired methicillin-resistant strains. Antimicrob Agents Chemother. 2008;52(11):3955–66. doi: 10.1128/AAC.00049-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karinou E, Schuster CF, Pazos M, Vollmer W, Gründling A. Inactivation of the Monofunctional Peptidoglycan Glycosyltransferase SgtB Allows Staphylococcus aureus To Survive in the Absence of Lipoteichoic Acid. J Bacteriol. 2019;201(1). doi: 10.1128/JB.00574-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SJ, Singh M, Sharif S, Schaefer J. Cross-link formation and peptidoglycan lattice assembly in the FemA mutant of Staphylococcus aureus. Biochemistry. 2014;53(9):1420–7. doi: 10.1021/bi4016742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blake KL, O’Neill AJ, Mengin-Lecreulx D, Henderson PJ, Bostock JM, Dunsmore CJ, et al. The nature of Staphylococcus aureus MurA and MurZ and approaches for detection of peptidoglycan biosynthesis inhibitors. Mol Microbiol. 2009;72(2):335–43. [DOI] [PubMed] [Google Scholar]
- 37.Potter AD, Butrico CE, Ford CA, Curry JM, Trenary IA, Tummarakota SS, et al. Host nutrient milieu drives an essential role for aspartate biosynthesis during invasive Staphylococcus aureus infection. Proc Natl Acad Sci U S A. 2020;117(22):12394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halsey CR, Lei S, Wax JK, Lehman MK, Nuxoll AS, Steinke L, et al. Amino Acid Catabolism in Staphylococcus aureus and the Function of Carbon Catabolite Repression. mBio. 2017;8(1). doi: 10.1128/mBio.01434-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell C, Fingleton C, Zeden MS, Bueno E, Gallagher LA, Shinde D, et al. Accumulation of Succinyl Coenzyme A Perturbs the Methicillin-Resistant Staphylococcus aureus (MRSA) Succinylome and Is Associated with Increased Susceptibility to Beta-Lactam Antibiotics. mBio. 2021;12(3):e0053021. doi: 10.1128/mBio.00530-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nuxoll AS, Halouska SM, Sadykov MR, Hanke ML, Bayles KW, Kielian T, et al. CcpA regulates arginine biosynthesis in Staphylococcus aureus through repression of proline catabolism. PLoS Pathog. 2012;8(11):e1003033. doi: 10.1371/journal.ppat.1003033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corrigan RM, Abbott JC, Burhenne H, Kaever V, Gründling A. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog. 2011;7(9):e1002217. doi: 10.1371/journal.ppat.1002217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Somerville GA, Proctor RA. At the crossroads of bacterial metabolism and virulence factor synthesis in Staphylococci. Microbiol Mol Biol Rev. 2009;73(2):233–48. doi: 10.1128/MMBR.00005-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harper L, Balasubramanian D, Ohneck EA, Sause WE, Chapman J, Mejia-Sosa B, et al. Staphylococcus aureus Responds to the Central Metabolite Pyruvate To Regulate Virulence. mBio. 2018;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vitko NP, Grosser MR, Khatri D, Lance TR, Richardson AR. Expanded Glucose Import Capability Affords Staphylococcus aureus Optimized Glycolytic Flux during Infection. mBio. 2016;7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vitko NP, Spahich NA, Richardson AR. Glycolytic dependency of high-level nitric oxide resistance and virulence in Staphylococcus aureus. mBio. 2015;6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Purves J, Cockayne A, Moody PC, Morrissey JA. Comparison of the regulation, metabolic functions, and roles in virulence of the glyceraldehyde-3-phosphate dehydrogenase homologues gapA and gapB in Staphylococcus aureus. Infect Immun. 2010;78(12):5223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stincone A, Prigione A, Cramer T, Wamelink MM, Campbell K, Cheung E, et al. The return of metabolism: biochemistry and physiology of the pentose phosphate pathway. Biol Rev Camb Philos Soc. 2015;90(3):927–63. doi: 10.1111/brv.12140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan X, Ramond E, Jamet A, Barnier JP, Decaux-Tramoni B, Dupuis M, et al. Transketolase of Staphylococcus aureus in the Control of Master Regulators of Stress Response During Infection. J Infect Dis. 2019;220(12):1967–76. [DOI] [PubMed] [Google Scholar]
- 49.Gardner SG, Marshall DD, Daum RS, Powers R, Somerville GA. Metabolic Mitigation of Staphylococcus aureus Vancomycin Intermediate-Level Susceptibility. Antimicrob Agents Chemother. 2018;62(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katayama Y, Azechi T, Miyazaki M, Takata T, Sekine M, Matsui H, et al. Prevalence of Slow-Growth Vancomycin Nonsusceptibility in Methicillin-Resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2017;61(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sause WE, Balasubramanian D, Irnov I, Copin R, Sullivan MJ, Sommerfield A, et al. The purine biosynthesis regulator PurR moonlights as a virulence regulator in Staphylococcus aureus. Proc Natl Acad Sci U S A. 2019;116(27):13563–72. doi: 10.1073/pnas.1904280116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goncheva MI, Flannagan RS, Sterling BE, Laakso HA, Friedrich NC, Kaiser JC, et al. Stress-induced inactivation of the Staphylococcus aureus purine biosynthesis repressor leads to hypervirulence. Nat Commun. 2019;10(1):775. doi: 10.1038/s41467-019-08724-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nolan AC, Zeden MS, Kviatkovski I, Campbell C, Urwin L, Corrigan RM, et al. Purine Nucleosides Interfere with c-di-AMP Levels and Act as Adjuvants To Re-Sensitize MRSA To beta-Lactam Antibiotics. mBio. 2022:e0247822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeden MS, Schuster CF, Bowman L, Zhong Q, Williams HD, Gründling A. Cyclic di-adenosine monophosphate (c-di-AMP) is required for osmotic regulation in Staphylococcus aureus but dispensable for viability in anaerobic conditions. J Biol Chem. 2018;293(9):3180–200. doi: 10.1074/jbc.M117.818716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhawini A, Pandey P, Dubey AP, Zehra A, Nath G, Mishra MN. RelQ Mediates the Expression of beta-Lactam Resistance in Methicillin-Resistant Staphylococcus aureus. Front Microbiol. 2019;10:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Geiger T, Kastle B, Gratani FL, Goerke C, Wolz C. Two small (p)ppGpp synthases in Staphylococcus aureus mediate tolerance against cell envelope stress conditions. J Bacteriol. 2014;196(4):894–902. doi: 10.1128/JB.01201-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, et al. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. MBio. 2013;4(1):e00537–12. doi: 10.1128/mBio.00537-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crimmins GT, Schelle MW, Herskovits AA, Ni PP, Kline BC, Meyer-Morse N, et al. Listeria monocytogenes 6-Phosphogluconolactonase mutants induce increased activation of a host cytosolic surveillance pathway. Infect Immun. 2009;77(7):3014–22. doi: 10.1128/IAI.01511-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salamaga B, Kong L, Pasquina-Lemonche L, Lafage L, von Und Zur Muhlen M, Gibson JF, et al. Demonstration of the role of cell wall homeostasis in Staphylococcus aureus growth and the action of bactericidal antibiotics. Proc Natl Acad Sci U S A. 2021;118(44). doi: 10.1073/pnas.2106022118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lorian V. Low concentrations of antibiotics. J Antimicrob Chemother. 1985;15 Suppl A:15–26. doi: 10.1093/jac/15.suppl_a.15 [DOI] [PubMed] [Google Scholar]
- 61.Pinho MG, Errington J. Dispersed mode of Staphylococcus aureus cell wall synthesis in the absence of the division machinery. Mol Microbiol. 2003;50(3):871–81. doi: 10.1046/j.1365-2958.2003.03719.x [DOI] [PubMed] [Google Scholar]
- 62.Kupor SR, Fraenkel DG. Glucose metabolism in 6 phosphogluconolactonase mutants of Escherichia coli. J Biol Chem. 1972;247(6):1904–10. [PubMed] [Google Scholar]
- 63.Waters EM, Rudkin JK, Coughlan S, Clair GC, Adkins JN, Gore S, et al. Redeploying beta-lactam antibiotics as a novel antivirulence strategy for the treatment of methicillin-resistant Staphylococcus aureus infections. J Infect Dis. 2017;215(1):80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antoniewicz MR. A guide to (13)C metabolic flux analysis for the cancer biologist. Exp Mol Med. 2018;50(4):1–13. doi: 10.1038/s12276-018-0060-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Campbell J, Singh AK, Santa Maria JP Jr, Kim Y, Brown S, Swoboda JG, et al. Synthetic lethal compound combinations reveal a fundamental connection between wall teichoic acid and peptidoglycan biosyntheses in Staphylococcus aureus. ACS Chem Biol. 2011;6(1):106–16. doi: 10.1021/cb100269f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rajagopal M, Martin MJ, Santiago M, Lee W, Kos VN, Meredith T, et al. Multidrug Intrinsic Resistance Factors in Staphylococcus aureus Identified by Profiling Fitness within High-Diversity Transposon Libraries. mBio. 2016;7(4). doi: 10.1128/mBio.00950-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Gotz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem. 1999;274(13):8405–10. doi: 10.1074/jbc.274.13.8405 [DOI] [PubMed] [Google Scholar]
- 68.Vickery CR, Wood BM, Morris HG, Losick R, Walker S. Reconstitution of Staphylococcus aureus Lipoteichoic Acid Synthase Activity Identifies Congo Red as a Selective Inhibitor. J Am Chem Soc. 2018;140(3):876–9. doi: 10.1021/jacs.7b11704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li M, Cha DJ, Lai Y, Villaruz AE, Sturdevant DE, Otto M. The antimicrobial peptide-sensing system aps of Staphylococcus aureus. Mol Microbiol. 2007;66(5):1136–47. [DOI] [PubMed] [Google Scholar]
- 70.Li M, Lai Y, Villaruz AE, Cha DJ, Sturdevant DE, Otto M. Gram-positive three-component antimicrobial peptide-sensing system. Proc Natl Acad Sci U S A. 2007;104(22):9469–74. doi: 10.1073/pnas.0702159104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meehl M, Herbert S, Gotz F, Cheung A. Interaction of the GraRS two-component system with the VraFG ABC transporter to support vancomycin-intermediate resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2007;51(8):2679–89. doi: 10.1128/AAC.00209-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herbert S, Bera A, Nerz C, Kraus D, Peschel A, Goerke C, et al. Molecular basis of resistance to muramidase and cationic antimicrobial peptide activity of lysozyme in staphylococci. PLoS Pathog. 2007;3(7):e102. doi: 10.1371/journal.ppat.0030102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Falord M, Karimova G, Hiron A, Msadek T. GraXSR proteins interact with the VraFG ABC transporter to form a five-component system required for cationic antimicrobial peptide sensing and resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 2012;56(2):1047–58. doi: 10.1128/AAC.05054-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cho J, Costa SK, Wierzbicki RM, Rigby WFC, Cheung AL. The extracellular loop of the membrane permease VraG interacts with GraS to sense cationic antimicrobial peptides in Staphylococcus aureus. PLoS Pathog. 2021;17(3):e1009338. doi: 10.1371/journal.ppat.1009338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reichmann NT, Picarra Cassona C, Monteiro JM, Bottomley AL, Corrigan RM, Foster SJ, et al. Differential localization of LTA synthesis proteins and their interaction with the cell division machinery in Staphylococcus aureus. Mol Microbiol. 2014;92(2):273–86. doi: 10.1111/mmi.12551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hesser AR, Matano LM, Vickery CR, Wood BM, Santiago AG, Morris HG, et al. The length of lipoteichoic acid polymers controls Staphylococcus aureus cell size and envelope integrity. J Bacteriol. 2020;202(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brown S, Xia G, Luhachack LG, Campbell J, Meredith TC, Chen C, et al. Methicillin resistance in Staphylococcus aureus requires glycosylated wall teichoic acids. Proc Natl Acad Sci U S A. 2012;109(46):18909–14. doi: 10.1073/pnas.1209126109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Do T, Page JE, Walker S. Uncovering the activities, biological roles, and regulation of bacterial cell wall hydrolases and tailoring enzymes. J Biol Chem. 2020;295(10):3347–61. doi: 10.1074/jbc.REV119.010155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Do T, Schaefer K, Santiago AG, Coe KA, Fernandes PB, Kahne D, et al. Staphylococcus aureus cell growth and division are regulated by an amidase that trims peptides from uncrosslinked peptidoglycan. Nat Microbiol. 2020;5(2):291–303. doi: 10.1038/s41564-019-0632-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ohta K, Komatsuzawa H, Sugai M, Suginaka H. Triton X-100-induced lipoteichoic acid release is correlated with the methicillin resistance in Staphylococcus aureus. FEMS Microbiol Lett. 2000;182(1):77–9. doi: 10.1111/j.1574-6968.2000.tb08877.x [DOI] [PubMed] [Google Scholar]
- 81.Ernst CM, Kuhn S, Slavetinsky CJ, Krismer B, Heilbronner S, Gekeler C, et al. The lipid-modifying multiple peptide resistance factor is an oligomer consisting of distinct interacting synthase and flippase subunits. mBio. 2015;6(1). doi: 10.1128/mBio.02340-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Slavetinsky CJ, Hauser JN, Gekeler C, Slavetinsky J, Geyer A, Kraus A, et al. Sensitizing Staphylococcus aureus to antibacterial agents by decoding and blocking the lipid flippase MprF. Elife. 2022;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ernst CM, Staubitz P, Mishra NN, Yang SJ, Hornig G, Kalbacher H, et al. The bacterial defensin resistance protein MprF consists of separable domains for lipid lysinylation and antimicrobial peptide repulsion. PLoS Pathog. 2009;5(11):e1000660. doi: 10.1371/journal.ppat.1000660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen FJ, Lauderdale TL, Lee CH, Hsu YC, Huang IW, Hsu PC, et al. Effect of a Point Mutation in mprF on Susceptibility to Daptomycin, Vancomycin, and Oxacillin in an MRSA Clinical Strain. Front Microbiol. 2018;9:1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.George NL, Schilmiller AL, Orlando BJ. Conformational snapshots of the bacitracin sensing and resistance transporter BceAB. Proc Natl Acad Sci U S A. 2022;119(14):e2123268119. doi: 10.1073/pnas.2123268119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kingston AW, Zhao H, Cook GM, Helmann JD. Accumulation of heptaprenyl diphosphate sensitizes Bacillus subtilis to bacitracin: implications for the mechanism of resistance mediated by the BceAB transporter. Mol Microbiol. 2014;93(1):37–49. doi: 10.1111/mmi.12637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Falord M, Mader U, Hiron A, Debarbouille M, Msadek T. Investigation of the Staphylococcus aureus GraSR regulon reveals novel links to virulence, stress response and cell wall signal transduction pathways. PLoS One. 2011;6(7):e21323. doi: 10.1371/journal.pone.0021323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee CY, Buranen SL, Ye ZH. Construction of single-copy integration vectors for Staphylococcus aureus. Gene. 1991;103(1):101–5. doi: 10.1016/0378-1119(91)90399-v [DOI] [PubMed] [Google Scholar]
- 89.CLSI. Performance standards for antimicrobial disk susceptibility tests. Approved standard, 12th ed. CLSI standard M02-A13 (3, pp 15–40). Clinical and Laboratory Standards Institute, Wayne, PA. Wayne, PA.2018. [Google Scholar]
- 90.CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 11th ed. CLSI standard M07-A11 (3, pp 27–50). Clinical and Laboratory Standards Institute, Wayne, PA. 2018. [Google Scholar]
- 91.Bowman L, Zeden MS, Schuster CF, Kaever V, Gründling A. New Insights into the Cyclic Di-adenosine Monophosphate (c-di-AMP) Degradation Pathway and the Requirement of the Cyclic Dinucleotide for Acid Stress Resistance in Staphylococcus aureus. J Biol Chem. 2016;291(53):26970–86. doi: 10.1074/jbc.M116.747709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fingleton C, Zeden MS, Bueno E, Cava F, O’Gara JP. Mutation of lipoprotein processing pathway gene lspA or inhibition of LspA activity by globomycin increases MRSA resistance to β-lactam antibiotics. bioRxiv. 2021:2021.02.03.429649. [Google Scholar]
- 93.Alvarez L, Hernandez SB, de Pedro MA, Cava F. Ultra-Sensitive, High-Resolution Liquid Chromatography Methods for the High-Throughput Quantitative Analysis of Bacterial Cell Wall Chemistry and Structure. Methods Mol Biol. 2016;1440:11–27. doi: 10.1007/978-1-4939-3676-2_2 [DOI] [PubMed] [Google Scholar]
- 94.Desmarais SM, De Pedro MA, Cava F, Huang KC. Peptidoglycan at its peaks: how chromatographic analyses can reveal bacterial cell wall structure and assembly. Mol Microbiol. 2013;89(1):1–13. doi: 10.1111/mmi.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ledger EVK, Edwards AM. Growth Arrest of Staphylococcus aureus Induces Daptomycin Tolerance via Cell Wall Remodelling. mBio. 2023;14(1):e0355822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ledger EVK, Mesnage S, Edwards AM. Human serum triggers antibiotic tolerance in Staphylococcus aureus. Nat Commun. 2022;13(1):2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schuster CF, Wiedemann DM, Kirsebom FCM, Santiago M, Walker S, Grundling A. High-throughput transposon sequencing highlights the cell wall as an important barrier for osmotic stress in methicillin resistant Staphylococcus aureus and underlines a tailored response to different osmotic stressors. Mol Microbiol. 2020;113(4):699–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boles BR, Thoendel M, Roth AJ, Horswill AR. Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS One. 2010;5(4):e10146. doi: 10.1371/journal.pone.0010146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Raafat D, Leib N, Wilmes M, Francois P, Schrenzel J, Sahl HG. Development of in vitro resistance to chitosan is related to changes in cell envelope structure of Staphylococcus aureus. Carbohydr Polym. 2017;157:146–55. [DOI] [PubMed] [Google Scholar]