Abstract
Roost selection by insectivorous bats in temperate regions is presumably influenced by roost microclimates in relation to thermoregulatory strategies, but few studies have included temperature measurements in habitat selection models. Rocky landscape features are an important source of roosts that provide both shelter from predators and beneficial microclimates for bats. Most information about rock-roosting bats has been derived from western North America. We studied microhabitat selection by the Eastern Small-footed Myotis (Myotis leibii) on natural talus slopes and human-made stone structures in the Appalachian Mountains of Virginia and New Hampshire, relative to thermal and structural characteristics of rock crevices. Roosts were located with a combination of radiotelemetry and randomized visual surveys. Roost-switching behavior and structural characteristics of roosts did not appear to be influenced by the methods we used to locate roosts. Compared to random crevices, both sexes selected crevices with narrow openings, likely to provide protection from predators. Reproductive females also selected rocks that were larger and more thermally stable than random crevices, whereas males selected crevices that were structurally similar to random crevices but warmed more during the day. Rock size and other structural characteristics influenced temperatures of roosts and random crevices alike by inhibiting excessive daytime heating and nighttime cooling. Because large rocks were important for reproductive females, and talus slopes with large rocks could be limited, we recommend including rock size as a variable in landscape scale habitat assessments for Eastern Small-footed Myotis. Protecting or managing for habitat features with large rocks that receive high solar exposure could benefit Eastern Small-footed Myotis, and perhaps other rock-roosting species.
Keywords: microclimate, microhabitat, Myotis leibii, riprap, rock-roosts, roost selection, scree, talus, temperature, thermoregulation
Selection of diurnal roosts is one of the most important ecological aspects of insectivorous bats. Roosts provide shelter from predators, help manage thermoregulatory costs, and can facilitate sociality and mating (Dechmann et al. 2005; Klug et al. 2012; Russo et al. 2017). Availability and diversity of suitable roosting habitat also shape regional bat faunas and can be a limiting factor for individual species (Humphrey 1975; USFWS 2007). In temperate regions, insectivorous bats are endothermic when foraging at night, but when roosting they typically become torpid to conserve energy (Altringham 1996). However, use of torpor by pregnant and lactating bats may slow growth rates of young, and this likely explains why thermoregulatory strategies typically differ between sexes and female reproductive phases (Racey and Swift 1981; Barclay 1991; Speakman and Thomas 2003). A common finding that has been documented in the literature is for pregnant and lactating females to roost communally in crevices with microclimates that favor homeothermy, to speed development of young (Barclay 1982; Solick and Barclay 2006). Males and nonreproductive females may roost alone and tolerate comparatively cooler roosts that allow greater use of daily torpor (Hamilton and Barclay 1994; Kunz and Hood 2000; Cryan and Wolf 2003; Muñoz-Garcia et al. 2012). Roosting communally can create a warmer microclimate and reduce energetic costs of maintaining homeothermy, whereas roosting alone likely facilitates use of torpor (Trune and Slobodchikoff 1976; Chruszcz and Barclay 2002; Pretzlaff et al. 2010; Russo et al. 2017). Thus, decisions bats make about where to roost and whether to do so communally may reflect thermoregulatory strategies, and they are made in the context of factors including reproductive stage, roost characteristics, and environmental conditions (Solick and Barclay 2007; Encarnação et al. 2012; Russo et al. 2017; Bergeson et al. 2021).
Roost selection and thermoregulatory strategies are sometimes more nuanced than stereotypical scenarios predict. For example, pregnant and lactating females of the Long-eared Myotis (Myotis evotis) in the prairies roost alone in crevices of large boulders that likely offer broad thermal gradients conducive to either homeothermy or heterothermy, whereas those in the Rocky Mountains roost alone during pregnancy but in groups during lactation, in crevices on talus slopes that warm quickly and are well-suited for homeothermy (Solick and Barclay 2007). Although roost selection is usually assumed to be influenced by microclimate and thermoregulatory strategies, few studies have gone so far as to include temperature measurements when modeling roost selection. Most information about the relationship between microclimates and roost selection comes from studies of bats in western North America that roost in rocky structures (Chruszcz and Barclay 2002; Lausen and Barclay 2003; Solick and Barclay 2006).
Rocks provide particularly important roosting habitat for many species of bats in temperate regions (Michaelsen et al. 2013; Andrews 2021). In North America, over 40% of bat species roost in aboveground rocky habitat features in at least a portion of their range (Wilson and Ruff 1999). In some cases, bats primarily known to roost in snags in other parts of their range use rocks instead when they have the option to do so (Rancourt et al. 2005; O’Shea et al. 2011). Rock-roosts come in a diversity of forms and the factors that govern roost selection among the different types that are available are unclear (O’Shea et al. 2011; Andrews 2021). Studies from North America have reported bats roosting in cavernous openings in cliffs and deep interstitial voids between talus blocks or boulders (Lacki et al. 1994; Hurst and Lacki 1999; Reid et al. 2010); in relatively narrow crevices or tubes in cliffs, bluffs, and other large masses of emergent bedrock (Lausen and Barclay 2003; Rancourt et al. 2005; Randall et al. 2014); and in crevices between or under rocks at the surface of talus, scree, shale barrens, and under rocks sitting directly on bedrock (Baker and Lacki 2006; Lacki and Baker 2007; Whitby et al. 2013). The few studies that have directly assessed selection of microclimates by rock-roosting bats collectively report at least two kinds of thermal profiles–some rock-roosts have pronounced daily cyclical temperature fluctuations, whereas others have relatively stable thermal profiles that may be warmer or cooler than ambient air (Chruszcz and Barclay 2002; Lausen and Barclay 2003; Solick and Barclay 2006, 2007; Rambaldini and Brigham 2008; Schorr and Siemers 2013). Rock-roosting bats also vary in the degree to which they roost communally, across and within species. Some rock-roosting bats cluster in groups–but others roost alone–when pregnant and lactating (Chruszcz and Barclay 2002; Lausen and Barclay 2002; Solick and Barclay 2006; Snider et al. 2013).
The Eastern Small-footed Myotis (M. leibii) roosts in a variety of rocky habitat features including under flat rocks laying on bedrock, in narrow crevices of vertical cliff faces, and between rocks on shale barrens and talus slopes–they also use human-made structures (Saugey et al. 1993; Roble 2004; Whitby et al. 2013; Fagan et al. 2016; Moosman et al. 2017; Loeb and Jodice 2018). Most published information about behavior and ecology of this species is anecdotal and roost selection has only received rudimentary study, but aspects of their life history raise interesting questions about how or if selection differs between sexes and female reproductive phases (Johnson and Gates 2008; Johnson et al. 2011). Notably, the sexes of Eastern Small-footed Myotis roost separately from one another, but often only meters apart within the same rock formations (Johnson et al. 2011; Moosman et al. 2015). Unlike some other species of insectivorous bats, female Eastern Small-footed Myotis may roost alone until late pregnancy, when they begin to cluster into groups of pregnant or lactating adults with their pups (Moosman et al. 2015). Thus, Eastern Small-footed Myotis provide a unique opportunity to assess patterns in roost selection that have been suggested by studies of rock-roosting bats in other regions.
We studied roost selection by reproductive female and adult male Eastern Small-footed Myotis on natural talus slopes and artificial rocky structures in the Appalachian Mountains of Virginia and New Hampshire, United States. Our goals were to understand: (1) what structural and thermal characteristics of rock crevices corresponded to selection by reproductive females and male bats; and (2) what structural attributes most influenced microclimates of rock crevices. We hypothesized that: reproductive females would preferentially select crevices between larger rocks that would provide greater thermal stability; and adult males would be less selective with respect to structural and thermal characteristics of crevices, making microclimates of their roosts relatively labile.
Materials and Methods
Study sites
We studied roosting behavior of Eastern Small-footed Myotis at six talus slopes, two riprap-covered dams, and a human-made pile of boulders (Supplementary Data SD1 and SD2). When describing rocky habitat features, we follow terminology proposed by Andrews (2021). Sites represented a range of latitudes, elevations, geologic compositions, and natural or human-made origins. All sites were surrounded by relatively mature, closed-canopy, eastern deciduous forests. The rock formations we studied were predominantly south-facing aggregations of natural rocks or quarried stones that ranged from 0.12 to 6.72 ha of canopy-free habitat, creating high densities of potential roosts. Elevations of rock formations ranged from 149 to 1,016 m, the latter of which was relatively high and steep for topography of the region, which had nearby peaks of 472 to 1,111 m. Geologic composition included naturally fragmented accumulations of sedimentary quartzite and metamorphic (greenstone) rock, and quarried limestone and granite. Sites in Virginia included talus slopes in the Blue Ridge and Appalachian Valley and Ridge Physiographic Provinces: Humpback (0.14 ha; 1,016 m elev.; N37.96°, W78.90°); Sherando (3.25 ha; 734 m elev.; N37.93°, W79.00°); Slacks (0.13 ha; 991 m elev.; N37.91°, W79.05°); Saint Mary (0.12 ha; 604 m elev.; N37.93°, W79.11°); Marbleyard (3.00 ha; 737 m elev.; N37.58°, W79.47°); and Cascades (0.01 ha; 963 m elev.; N37.35°, W80.59°). Most of these talus slopes also had regions with sediment sizes small enough to be considered scree (Andrews 2021). Additionally, we studied bats at three artificial rocky structures: Surry Mountain Dam (hereafter, Surry; 6.72 ha; 149 m elev.; N43.00°, W72.31°) in the New England Upland Physiographic Province of New Hampshire; Gathright Dam (hereafter, Gathright; 4.31 ha; 507 m elev.; N37.95°, W79.96°) in the Valley and Ridge Physiographic Province of Virginia; and Conway, a 996 m long pile of boulders (357 m elev.; N38.42°, W78.44°) in the Blue Ridge Mountains of Virginia. Both dams were covered in riprap that had been quarried from adjacent ridges and placed on the dams to prevent erosion. The Conway boulder pile was an ornamental landscape feature composed of round rocks gathered from a local river valley.
Locating roosts
We located diurnal roosts by radio-tracking bats and conducting visual surveys between May and October from 2009 to 2018. We captured bats for radio-tracking in mist-nets or by hand. Methods of handling bats followed guidelines of the American Society of Mammalogists (Sikes et al. 2016), were permitted under scientific collecting permits by the New Hampshire Department of Game and Inland Fisheries and Virginia Department of Wildlife Resources, and were approved by the Animal Subjects Committee at the Virginia Military Institute. Mist-nets were placed across travel corridors and foraging areas such as trails and streams, perpendicular to forested edges near rock formations, or directly on rock formations to capture bats soon after emergence. We captured bats by hand with two lengths of insulated wires with solid cores that served as malleable probes to extract bats from their roost. One probe was used to block the bat from retreating deeper into the crevice; the other probe was bent at the end and used to coax the bat to the opening where it could be reached by hand.
We examined captured bats to determine sex, age (based on condition of phalangeal joints), and female reproductive status (judged by palpating the abdomen and examining condition of the teats). We also weighed bats with a spring scale to the nearest 0.1 g (Pesola, Schindellegi, Switzerland) and gave each a uniquely numbered 2.4-mm-wide aluminum band with a rounded lip (Porzana Ltd, Thetford, Norfolk, United Kingdom) on the forearm. Bands were placed on the right forearm of males and the left forearm of females to allow us to determine sex from a distance, in the event that we encountered them again during visual surveys. We affixed a 0.23-g (LB-2XT; Holohil Systems, Ltd, Carp, Ontario, Canada) or 0.22-g (PIP41; Lotek Wireless, Inc., Newmarket, Ontario, Canada) radio transmitter to the interscapular region of each bat, using medical adhesive, after trimming the fur with cuticle scissors. Radio transmitters were 4–5% of body weight of bats. Each bat was radio-tracked to its roost, starting the day after it was captured, with a handheld receiver (R1000; Communications Specialists, Inc., Orange, California) and 3-element directional antenna.
Because we were concerned that human activity at roosts might disturb bats and cause them to switch roosts, we studied this possibility during the first year of fieldwork by alternating between radio-tracking bats to their roost versus only triangulating their position from >5 m away. We kept track of whether bats switched roosts on days after we visited them at the roost, compared to the days after they were triangulated or not radio-tracked because they had left the study area. After assessing results of this portion of the study, we tracked bats daily over the life of each transmitter at Surry, Sherando, and Gathright between 2010 and 2012. From 2013 to 2018 we located roosts either using radiotelemetry or with randomized visual searches that were part of population monitoring efforts (see detailed methods in Moosman et al. 2020). We also included data from bats encountered incidentally while moving between random plots.
Visual surveys provide information about roosts at lower costs than radiotelemetry, allowing larger sample sizes and no handling of bats. Disadvantages of using visual surveys to locate roosts include overlooking bats–although this kind of detection error was relatively low at our sites (Moosman et al. 2020)–and loss of information on sex, age, and reproductive condition of some individuals because bats are not captured. Uncertainty over sex and reproductive status of some bats led us to categorize roosts based on a combination of information obtained at the time of capture (for radio-tracked bats or those hand-captured from roosts) and information visible from outside the roost, such as number of bats present and presence of juveniles or lactating bats. During visual surveys we distinguished between adult females and pups based on pelage and size. Pelage of adults was typically copper-colored and contrasted strongly with their dark skin and facial fur, whereas pelage of pups was dull gray and had less contrast with their skin, even late into development. Furthermore, because we always observed males roosting alone, we assumed that the few bats of unknown sex observed roosting in groups were reproductive females. Cumulatively this provided five categories of roosts: 87 roosts used by solitary males; eight roosts used by solitary females that were likely in early stages of pregnancy; 31 roosts with solitary bats of unknown sex; 28 roosts with groups of females and pups during late pregnancy or lactation; and four roosts with solitary females during the postlactating or nonreproductive period.
Measuring crevice characteristics
Bats usually roosted in crevices formed by one rock sitting on top of or leaning against another rock. We used a measuring tape to determine physical dimensions of the two rocks creating the crevice and of the crevice itself. For the sake of consistency, we designated rocks based on their position relative to one another as upper versus lower rock for horizontal or diagonal crevices, or upslope versus downslope rock for vertically oriented crevices. We measured the maximum size of each rock along three dimensions to approximate its length, depth, and height. Rocks occurred in many different shapes and arrangements, so we defined the longest dimension of each as the length, and the second and third longest dimensions as depth and height, respectively. For crevices, we defined length as the longest dimension of the opening and depth as the maximum distance from the opening to the back of the crevice (measured perpendicular to length). Crevice width was defined as the distance between the two planar surfaces that formed the opening. Measurements were made to the nearest cm, except crevice width that was measured to the nearest 0.1 cm. We also categorized the orientation of each crevice based on whether the plane of its depth more closely approximated a horizontal (0–22°), diagonal (23–68°), or vertical (69–90°) angle.
We characterized potential roosts by measuring the same variables at randomly selected crevices within the same rock formations. These crevices were chosen randomly from grid intersections, transects, or points generated by GIS software (ArcMap; ESRI, Redlands, California). Methods used to select random points evolved over the course of the study as the number and diversity of sites grew. We initially used grid intersections to select random points by overlaying a 10 × 10 m grid onto aerial photos and randomly selecting from the intersections using SPSS software (IBM Corp., Armonk, New York). For linear-shaped rock formations, we sampled at random intervals (≥10 m apart) along an adaptive transect designed to maximize coverage of the rock formation. From 2016 onward we used GIS to select random points by creating a polygon bounded by the forested border of each rock formation and creating a pool of random points separated by a 10-m radius buffer within the polygon. We randomly selected GIS-generated points for sampling using SPSS. For each point, we navigated to within 5 m of its location using a handheld global positioning (GPS) unit and measured the nearest crevice. Random crevices were defined as any space formed by two adjacent rocks that created a cavity wide enough for an eastern small-footed bat to fit within it (≥0.7 cm). These were usually formed by rocks that were in contact with one another, but maximum width was 30 cm.
In addition to quantifying structural characteristics of crevices, we revisited a subset of 66 roosts the summer after they were first identified to record their temperature profiles with Thermocron iButton sensors (NexSens Technology, Inc., Fairborn, Ohio). We recorded temperatures for crevices at both riprap-covered dams (Surry and Gathright) and at three talus slopes (Sherando, Marbleyard, and Slacks). Temperatures were recorded in each crevice on an hourly basis for three consecutive days, within a year and 2 weeks of when they were originally occupied. Thus, temperature readings should be understood to at best depict general thermal profiles of crevices, but not the specific temperatures when occupied by bats. We placed sensors in the part of each crevice where bats had roosted previously–typically close to the surface and in the part of each crevice that narrowed to about 1 cm. Although we did not check if roosts were occupied during the temperature monitoring period, based on how frequently bats switched roosts we presumed most were vacant. Only one exception to this was observed, when a maternity group moved into a roost during the 3 days of temperature being recorded. Because the thermal profile of this roost did not appear to change suddenly during the time that it was monitored, and it resembled thermal profiles of other roosts of reproductive females, we included it in the analysis.
We also deployed sensors in an equal number of random crevices at each site and recorded data simultaneously with that of roosts, though sample sizes were not identical because some sensors were removed by rodents or people. We placed each sensor in the recess of the crevice that most closely approximated places that bats tended to use, where the crevice was 1 cm wide (or the narrowest part of the crevice if it was wider than 1 cm). Sensors were mounted in a plastic fob that was held in place by an insulated wire wedged into the crevice. Crevices were narrow enough that sensors were in contact with one or both rocks that formed the walls of the crevice. Ambient air temperature (Tamb) at each site was recorded simultaneously with crevice temperature, using a sensor placed 2.5 m above the ground in the shade of the forest canopy nearby and downslope from each rock formation. To quantify the degree to which crevices deviated from ambient temperatures, we calculated the difference between the crevice temperatures and the corresponding ambient temperatures, and averaged values across the three days of monitoring. These variables quantified how much warmer crevices were compared to ambient air overall (ΔTavg) and at the hottest (ΔTmax) and coldest (ΔTmin) moments in the day. We used these relative temperature measurements instead of absolute temperatures to help remove some of the variation caused by nonsimultaneous readings within and across sites.
Statistical analyses
In order to guide analysis of roost selection and identify potentially redundant variables, we first conducted bivariate correlations between the nine continuous variables measuring dimensions of rocks and crevices, followed by a principal components analysis (PCA) of these same variables (Supplementary Data SD3). This portion of the analysis drew from a pool of 156 random crevices and 126 roosts. PCA is useful because it uses correlations among observed independent variables to estimate latent variables (i.e., components) that capture much of the variation present in the data set. Components can reveal important patterns within the data set and their scores can be used in lieu of individual variables to streamline analyses. Because PCA can be sensitive to unequal variances, we examined versions with and without z-transforming variables, but there was no difference in results. Based on structuring evident in the first PCA, we conducted a second analysis with crevice dimensions removed to generate component scores that quantified rock size better than individual length, depth, or height measurements. Component scores were used in lieu of raw measurements of rock size during subsequent modeling.
We also tested two assumptions of our methods before proceeding with further analysis. First, we used a binary logistic model to test the assumption that visits to roosts by researchers did not influence probability of bats moving to a different roost the next day using data from the nine bats we tracked during the first year of the study (n = 61 total days). This test used a dichotomous dependent variable (coded as ‘0’ when a bat stayed in the roost and ‘1’ when it switched roosts) and a categorical independent variable to account for whether we visited the roost or not on the preceding day (coded ‘1’ or ‘0,’ respectively). Second, we examined whether method of locating roosts (radiotelemetry versus visual surveys) influenced the structural characteristics of roosts (principal component scores) using a multivariate analysis of variance (MANOVA). Because these questions addressed distinct a priori hypotheses, we used P-values to identify significant differences at α = 0.05.
We studied relationships between selection of roosts by males and reproductive females separately, using binary logistic models in the generalized linear models (GLM) procedure of SPSS. Roosts used by bats of unknown sex and solitary late-season females were excluded from the analysis. We compared the relative support for different roost selection models (Supplementary Data SD4) with Akaike Information Criterion corrected for small sample size (AICc) and model weights (ωi). Models were considered potentially informative if they were within 2 AICc units of the highest-ranked alternative model. Influence of individual variables was judged using summed model weights (∑ω) and parameter estimates (β ± SE), following Burnham and Anderson (2002).
Roost selection was first assessed with separate sets of models focused on: (1) structural variables (components 1 and 2, crevice width, and orientation); and (2) thermal variables (∆Tavg, ∆Tmin, and ∆Tmax; Supplementary Data SD4). These models assessed the effects of habitat variables on the dichotomous dependent variable “crevice type,” with random crevices coded as ‘0’ and roosts coded as ‘1.’ To assess whether inferences were affected by site differences, we compared regression coefficients and AICc values in models with and without a site interaction term. For males, we modeled site interactions using a nominal variable that included seven sites–data from Conway and Cascades were excluded because of insufficient sample size. For reproductive females, we had observations from only five sites, with low sample size at four (10 roosts at Sherando, two at St. Mary, and one each at Slacks and Humpback). Because the Marbleyard site had both larger sample size (20 roosts) and larger boulders than other sites, we were concerned this might have an outsized influence on model inferences. This led us to pool all roosts but those at the Marbleyard and use a dichotomous site variable coded to indicate the Marbleyard versus other sites. Site interaction terms were generated by multiplying the site variable with other independent variables. We concluded by comparing models with combinations of the structural and thermal variables that were informative in earlier comparisons.
We used GLM with normally distributed error to understand how temperature profiles of crevices corresponded to their structural characteristics (Supplementary Data SD5). This portion of the analysis drew from the entire pool of 122 crevices that had temperature measurements (including random crevices and roosts). All models included a site effect to account for variation due to factors such as elevation, latitude, geology, and weather. We also included an intercept in all models. All results are reported as mean (±SE).
Results
We located 164 roosts, including 115 on talus slopes, 42 on riprap-covered dams, six on vertical outcrops, and one in a human-made rock pile. Seventy-eight roosts were located by telemetry and 86 were found with visual surveys. We were able to document or estimate the number of bats visible in 153 roosts (93%), of which 130 (85%) held solitary bats and the remainder were used by groups of 2–25 bats. Of bats we observed roosting alone, 82 (66%) were males, 31 (25%) were bats of unknown sex, eight (6%) were females during pregnancy, and four (3%) were females during the postlactation or nonreproductive period. Groups of reproductive females were observed from 29 May to 26 July and evidence of lactation occurred from 29 May to 19 July. Of the 34 roosts that held multiple bats, we could estimate group size for 28 roosts. Average number of bats visible in groups was five ± six bats. The smallest groups held one adult female with one pup, although we once observed a lactating female roosting with three pups. Other groups were composed entirely of adults or a mixture of adults and juveniles. Grouped bats were usually positioned close to the entrance of the crevice, or sometimes just outside of it. Roosts used by reproductive females appeared to occur in discrete patches at some sites (Fig. 1).
Fig. 1.
Locations of rock crevices used as roosts by reproductive female (shaded symbols) and male (white symbols) Eastern Small-footed Myotis (Myotis leibii) at two relatively large (≥3 ha) talus slopes in the Appalachian Mountains of Virginia, 2012–2018. Reproductive females at Sherando (bottom) were radio-tracked to discrete patches that had the largest rocks at the site; at Marbleyard (top) reproductive females and large rocks were widespread.
Most roosts occurred on predominantly south-facing talus slopes and riprap that were devoid of canopy cover, in crevices formed by one rock sitting on top of or leaning against another rock. Occasionally roosts occurred in crevices of bedrock, cliffs, or vertical outcrops that were too big to measure. Radio-tagged bats of both sexes changed roosts daily, but they almost always (93.5% of days) moved to a nearby roost within the same primary rock formation. On two occasions, at Surry, we tracked solitary males to secondary vertical outcrops that were west-facing and under closed-canopy forest, 1.5 and 3.2 km from the primary rock formation, as well as to an adjacent east-facing cliff face where bats were inaccessible to researchers. Bats also occasionally moved outside the range of our receiver for one or more days, sometimes returning to the primary rock formation during the life of the radio transmitter.
Overall size (averaged length, depth, and height) of the upper and lower rocks that formed roosts was 48.7 ± 3.1 cm and 62.6 ± 3.5 cm, respectively. Corresponding sizes for upper and lower rocks at randomly selected crevices were somewhat smaller: 41.8 ± 2.5 cm and 58.1 ± 3.6 cm, respectively. Average width of crevices was 1.6 cm (range: 0.5–8.0 cm) for roosts and 3.5 cm (range: 0.5–30.0 cm) for random crevices.
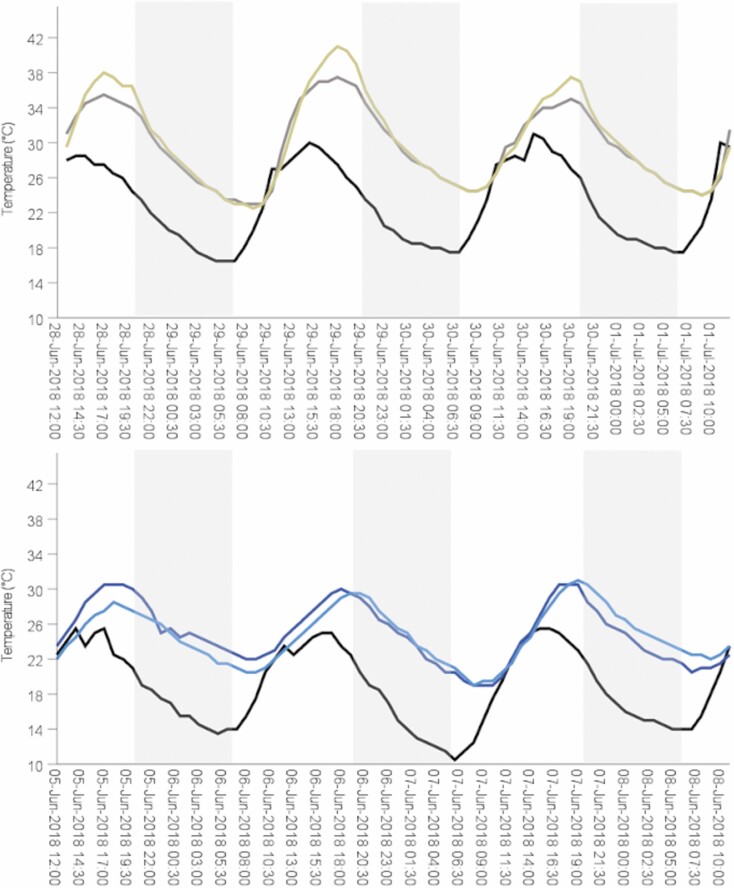
Average ambient air temperatures for all sites pooled was 18.1 ± 0.3°C. Ambient temperatures started at a minimum of 14.1 ± 0.3°C in the morning and warmed to a maximum of 23.4 ± 0.4°C in the afternoon. Ambient air temperatures also varied among sites, with the coolest conditions at the northernmost site (Surry; min. Tamb = 12.8 ± 0.4, max. Tamb = 21.1 ± 0.6°C) and the warmest conditions at the southernmost site at which temperature data were available (Marbleyard; min. Tamb = 16.4 ± 0.4, max. Tamb = 28.1 ± 0.4°C). All rock crevices (roosts and random combined) warmed and cooled with ambient air over a daily cycle, but rock crevices were usually warmer than ambient air (average Tcrev was 23.2 ± 0.5°C for roosts and 22.0 ± 0.4°C for random crevices). Across all sites, roosts averaged 17.5 ± 0.5°C at their coldest point in the morning and warmed to an average of 30.4 ± 0.6°C at the hottest point in the afternoon. Respective conditions in random crevices were cooler at the coldest point in the morning (16.4 ± 0.5°C) and at the hottest point in the afternoon (29.1 ± 0.7°C). Roosts of reproductive females typically had less variable temperature profiles, with warmer ∆Tmin and cooler ∆Tmax, than those of males (Fig. 2).
Fig. 2.
Examples of temperature profiles in rock crevices used by Eastern Small-footed Myotis (Myotis leibii), including two solitary males (top) and two groups of reproductive females (bottom), at a talus slope (Marbleyard) in the Appalachian Mountains of Virginia, June and July 2018. Black lines indicate ambient temperature recorded in nearby closed-canopy forest. Shaded regions represent night.
Data reduction and bias assessment
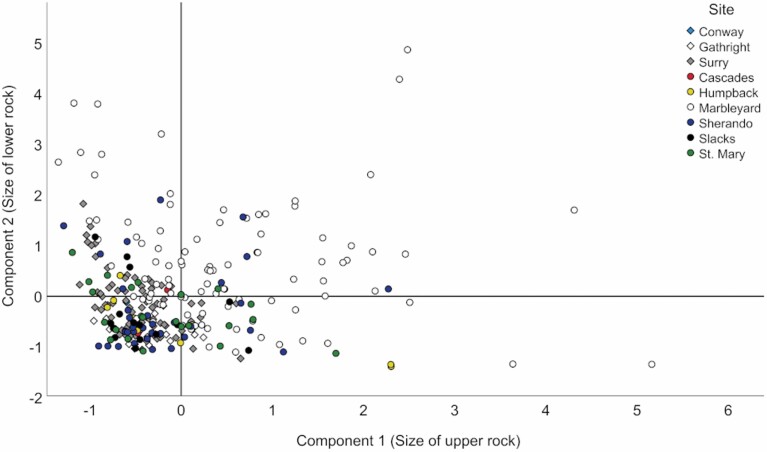
Bivariate correlations identified significant relationships between 31 pairs of structural variables, including 20 pairs with strong correlations (Pearson r > 0.5) and 11 pairs with weak correlations (Pearson r < 0.5). The initial PCA based on all nine rock and crevice variables extracted two components that cumulatively explained 73% of variance in the data set. Measurements from the upper and lower rocks loaded separately onto components 1 and 2, respectively, with loading scores > 0.8. However, crevice length and depth were cross-loaded onto both components, with loadings ranging between 0.56 and 0.63, whereas crevice width did not load strongly onto either component. These results indicated that crevice length and depth were functions of the size of the two rocks forming the crevice; thus, we considered them to be redundant. The second PCA based only on rock variables exhibited a similar pattern of loadings, with unique loadings > 0.89 and with no strong cross-loadings. The two components extracted by this PCA cumulatively explained 88% of the variance. Strong positive loading scores suggested that component 1 (44.1% of variance explained) was a proxy for size of the upper rock and component 2 (43.8% of variance explained) represented size of the lower rock. Component scores varied considerably within and between sites, with the Marbleyard site accounting for the largest boulders (Fig. 3). For reference, component scores corresponded to a pooled average rock dimension (length, width, and depth) for roosts and random crevices combined that ranged from 28.6 ± 4.5 cm at Gathright to 83.0 ± 6.7 cm at Marbleyard.
Fig. 3.
Variation in principal component scores within and among rock formations where we studied roost selection by Eastern Small-footed Myotis (Myotis leibii), in New Hampshire and Virginia, 2009–2018. Scores were derived from a principal components analysis of six different rock measurements taken at 156 randomly selected crevices and 164 roosts. Components 1 and 2 correspond to the size of the upper and lower rock forming each crevice, respectively, with the largest rocks represented by strong positive scores.
The MANOVA used to test whether structural characteristics of roosts (components 1 and 2) were affected by method of location suggested similarity between the two methods (P = 0.96), although component scores for roosts varied substantially by site (Wilks’ lambda F10, 428 = 6.22, P < 0.001) for both component 1 (Univariate F5, 215 = 4.86,P < 0.001) and component 2 (Univariate F5, 215 = 5.40,P < 0.001). Bonferroni-adjusted multiple comparisons indicated that site effects resulted from the Marbleyard site having larger component 1 scores than Surry (P < 0.001) and Slacks (P = 0.012)–and larger component 2 scores than Gathright (P = 0.047), Slacks (P = 0.010), St. Mary (P = 0.003), and Surry (P = 0.045). Data from the first year of study suggested that movements between roosts were not associated with disturbances from researchers visiting the roost (β = 0.11 ± 0.54, P = 0.85).
Roost selection based on crevice structure
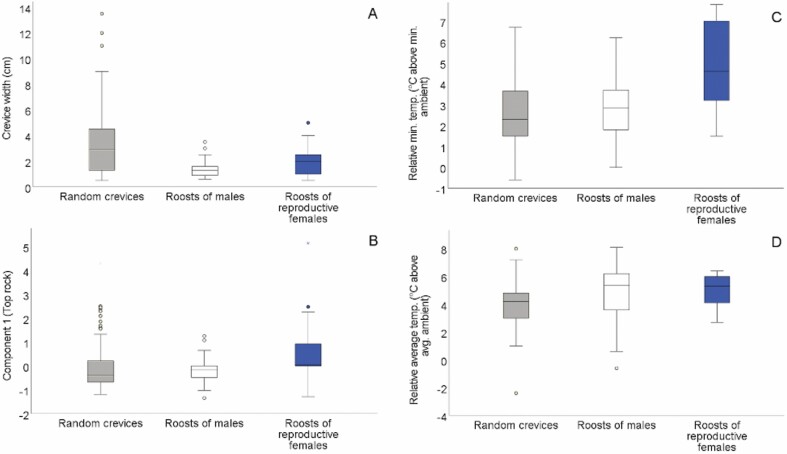
Models of roost selection suggested that crevice width was an important structural characteristic for both sexes (Fig. 4A). For models including only structural variables, models with crevice width had greater support than those based on other structural variables (Table 1). The regression coefficient for crevice width was substantial in the top-ranked model (β = −1.73 ± 0.52) as well as in a simpler model containing crevice width and a site interaction (β = −1.18 ± 0.44). Average crevice width was 2.0 ± 0.2 cm for roosts of reproductive females, 1.4 ± 0.1 cm for roosts of males, and 3.4 ± 0.2 cm for random crevices. There was some evidence that roost selection by females was also more likely for crevices with larger component 1 scores (Fig. 4B). Component 1 was included in the top-ranked model (ωi = 0.95) and the regression coefficient relating the term to the probability of crevice selection was positive for both the model with all structural variables (β = 0.77 ± 0.24) and the model with component 1 alone (β = 0.49 ± 0.20). Reproductive females appeared to select for crevices with larger component 1 scores at all sites, but this pattern was most pronounced at the Marbleyard. For reference, the average length of upper rocks used by reproductive females was 107 ± 11 cm compared to 63 ± 4 cm for random crevices. Models that either incorporated component 2 or crevice orientation had the least support of the variables included in the full model, and coefficients for both variables had confidence intervals that overlapped zero.
Fig. 4.
Structural (A and B) and thermal (C and D) characteristics of roosts used by Eastern Small-footed Myotis (Myotis leibii) compared to random crevices within the same rock formations in the Appalachian Mountains of Virginia and New Hampshire, 2009–2018. Component 1 is a latent variable (principal component) describing overall size of the upper rock that formed each crevice. Strong positive component scores indicate the largest rocks.
Table 1.
Comparison of models assessing relationships between structural characteristics of rock crevices and roost selection by Eastern Small-footed Myotis (Myotis leibii) in the Appalachian Mountains of Virginia and New Hampshire, 2009–2018, based on Akaike Information Criterion corrected for small sample sizes (AICc) and model weights (ωi). Models with less support (ΔAICc) than the intercept-only model are not shown. The intercept was included in all models.
| Model | LogLikelihood | AICc | ΔAICc | ωi |
|---|---|---|---|---|
| Reproductive females | ||||
| Crevice width + Component 1 + Component 2 + Orientation | −52.0 | 114.5 | 0.0 | 0.948 |
| Crevice width | −58.8 | 121.7 | 7.2 | 0.026 |
| Crevice width + Crevice width * Site | −57.9 | 122.1 | 7.6 | 0.020 |
| Component 1 | −61.0 | 126.1 | 11.6 | 0.003 |
| Component 1 + Component 1 * Site | −60.5 | 127.2 | 12.7 | 0.002 |
| Intercept only | −64.4 | 130.7 | 16.2 | <0.001 |
| Males | ||||
| Crevice width | −104.2 | 212.4 | 0.0 | 0.839 |
| Crevice width + Crevice width * Site | −100.1 | 215.7 | 3.3 | 0.161 |
| Intercept only | −133.0 | 268.0 | 55.6 | <0.001 |
For models of roost selection by males that focused on structural variables, there was substantially more support for models including crevice width than other models. These models indicated that male bats selected for crevices with substantially narrower openings (β = −2.53 ± 0.46) than random crevices and including a site interaction did not change this inference. Models of roost selection by males that incorporated other structural variables all had less support than the intercept-only model.
Roost selection based on crevice microclimate
Roost selection models based on thermal variables suggested further differences between sexes. For reproductive females, roost selection was strongly associated with higher ∆Tmin (Fig. 4C) and moderately associated with higher ∆Tavg (Fig. 4D). Model comparisons indicated the greatest overall support for a model with ∆Tmin and a site interaction (ωi = 0.91; β = 1.41 ± 0.49), relative to a version of this model with no site interaction or to models with other thermal variables (ωi ≤ 0.04; Table 2). Roost selection models based on ∆Tavg were less supported than those based on ∆Tmin, but they had greater support than the intercept-only model. The effects of ∆Tmin and ∆Tavg on roost selection by females were most pronounced at the Marbleyard site, but they occurred at other sites as well. Female roost selection models based on ∆Tmax alone (with or without a site interaction) had less support than the null model, and coefficients for this thermal variable were close to zero. In contrast, ∆Tmin had little support among models for roost selection by male bats based on thermal variables. Instead, the model with ∆Tavg as the sole habitat variable had the greatest support (ωi = 0.62), although the associated coefficient was modest (β = 0.33 ± 0.13). Model comparisons indicated that the influence of ∆Tavg on male roost selection likely was not subject to a site interaction (Table 2).
Table 2.
Comparison of models assessing relationships between thermal characteristics of rock crevices and roost selection by Eastern Small-footed Myotis (Myotis leibii) in the Appalachian Mountains of Virginia and New Hampshire, 2009–2018, based on Akaike Information Criterion corrected for small sample sizes (AICc) and model weights (ωi). Thermal measurements represent how much warmer crevices were compared to ambient air, at the coldest (ΔTmin) and hottest (ΔTmax) part of the day, and on average (ΔTavg). Models with less support (ΔAICc) than the intercept-only model are not shown. The intercept was included in all models.
| Model | LogLikelihood | AICc | ΔAICc | ωi |
|---|---|---|---|---|
| Reproductive females | ||||
| ΔTmin + ΔTmin * Site | −17.5 | 41.6 | 0.0 | 0.906 |
| ΔTmin | −21.8 | 48.0 | 6.4 | 0.037 |
| ΔTmin + ΔTmax + ΔTavg | −19.5 | 48.2 | 6.6 | 0.033 |
| ΔTavg | −22.7 | 49.7 | 8.1 | 0.016 |
| ΔTavg + ΔTavg * Site | −22.5 | 51.6 | 10.0 | 0.006 |
| Intercept only | −26.5 | 55.0 | 13.4 | 0.001 |
| Males | ||||
| ΔTavg | −70.9 | 145.9 | 0.0 | 0.615 |
| ΔTmax | −72.5 | 149.1 | 3.2 | 0.124 |
| ΔTavg + ΔTavg * Site | −68.5 | 149.7 | 3.8 | 0.092 |
| ΔTmin + ΔTmax + ΔTavg | −70.9 | 150.2 | 4.3 | 0.072 |
| Intercept only | −74.8 | 151.6 | 5.7 | 0.036 |
Roost selection relative to combined structural and thermal characteristics
When we considered models of roost selection by reproductive female bats that combined structural and thermal variables, two of the best models included ∆Tmin, an interaction between ∆Tmin and site, and component 1 (Table 3). Models that lacked component 1 but included crevice width also had some support. Summed model weights for each of the three habitat variables in these models showed that ∆Tmin was particularly influential (1.00), followed by component 1 (0.65) and crevice width (0.27). For male bats, a model with crevice width and ∆Tavg had greater support (ωi = 0.92) than the next most competitive model, which included only crevice width (ωi = 0.08). Across the model set for male bats, summed model weights for crevice width (1.00) were only slightly higher than that of ∆Tavg (0.92).
Table 3.
Comparison of models using combinations of thermal and structural characteristics of rock crevices as predictors of roost selection by Eastern Small-footed Myotis (Myotis leibii) in the Appalachian Mountains of Virginia and New Hampshire, 2009–2018, based on Akaike Information Criterion corrected for small sample sizes (AICc) and model weights (ωi). Thermal measurements represent how much warmer crevices were compared to ambient air, at the coldest (ΔTmin) part of the day, and on average (ΔTavg). Component 1 is a proxy for overall size of the upper rock forming each crevice. Models with less support (ΔAICc) than the intercept-only model are not shown. The intercept was included in all models.
| Model | LogLikelihood | AICc | ΔAICc | ωi |
|---|---|---|---|---|
| Reproductive females | ||||
| Component 1 + ΔTmin + ΔTmin * Site | −15.6 | 40.3 | 0.0 | 0.476 |
| ΔTmin + ΔTmin * Site | −17.5 | 41.6 | 1.3 | 0.249 |
| Component 1 + Crevice width + ΔTmin + ΔTmin * Site | −15.3 | 42.4 | 2.1 | 0.167 |
| Crevice width + ΔTmin + ΔTmin * Site | −17.1 | 43.3 | 3.0 | 0.106 |
| Component 1 | −23.8 | 52.0 | 11.7 | 0.001 |
| Component 1 + Crevice width | −23.8 | 54.3 | 14.0 | <0.001 |
| Intercept only | −26.5 | 55.0 | 14.7 | <0.001 |
| Males | ||||
| Crevice width + ΔTavg | 56.9 | 120.0 | 0.0 | 0.924 |
| Crevice width | 60.4 | 125.0 | 5.0 | 0.076 |
| ΔTavg | 71 | 146.1 | 26.1 | <0.001 |
| Intercept only | 74.9 | 151.9 | 31.9 | <0.001 |
Factors influencing crevice microclimates
Substantial variation in absolute (i.e., unadjusted) crevice temperatures was evident across sites. Crevices at our northernmost site, Surry, had the coolest absolute average temperatures (21.4 ± 0.5°C) and crevices at the southernmost site, Marbleyard, had the warmest (25.8 ± 0.4°C). The Slacks site had the coolest absolute maximum crevice temperatures (25.4 ± 1.0°C) and Gathright had the warmest (33.5 ± 0.8°C). However, crevice temperatures adjusted for ambient air temperature did not necessarily follow the same patterns. For example, despite cooler ambient temperatures at Surry, crevices at this site had higher ∆Tavg (4.8 ± 0.2°C) than those at more southerly sites, such as the Marbleyard site (4.1 ± 0.5°C) and Slacks site (1.9 ± 0.5°C).
Comparisons of models indicated that combinations of structural variables were informative for understanding thermal profiles of crevices. In models of ∆Tmin, combinations of three or four structural variables and site had greater support than simpler models, but all models had greater support than the site and intercept-only models (Table 4). Summed model weights for structural variables, in decreasing order, were 1.00 for components 1 and 2, 0.90 for crevice orientation, and 0.61 for crevice width. In the global model, crevices with larger component 2 scores (β = 0.84 ± 0.21), larger component 1 scores (β = 0.70 ± 0.21), relatively vertical orientations (β = 0.41 ± 0.15), and wider openings (β = 0.36 ± 0.19) were associated with higher values for ΔTmin.
Table 4.
Comparison of models assessing relationships between structural and thermal characteristics of rock crevices, including random crevices and roosts of Eastern Small-footed Myotis (Myotis leibii) in the Appalachian Mountains of Virginia and New Hampshire, 2009–2018, based on Akaike Information Criterion corrected for small sample sizes (AICc) and model weights (ωi). Thermal characteristics described how much warmer crevices were than ambient air at the coldest (ΔTmin) and hottest (ΔTmax) parts of the day, and on average (ΔTavg). Models with lower ΔAICc rank than a site or intercept-only model are not shown. All models include the intercept.
| Model | LogLikelihood | AICc | ΔAICc | ωi |
|---|---|---|---|---|
| Models explaining ΔTmin | ||||
| Component 1 + Component 2 + Crevice width + Orientation + Site | −252.4 | 528.8 | 0.0 | 0.562 |
| Component 1 + Component 2 + Orientation + Site | −254.1 | 529.9 | 1.1 | 0.324 |
| Component 1 + Component 2 + Site | −256.9 | 533.2 | 4.4 | 0.062 |
| Component 1 + Component 2 + Crevice width + Site | −256.2 | 534.1 | 5.3 | 0.040 |
| Site | −267.7 | 550.3 | 21.5 | <0.001 |
| Intercept only | −291.2 | 586.4 | 57.6 | <0.001 |
| Models explaining ΔTmax | ||||
| Component 1 + Component 2 + Crevice width + Site | −367.4 | 754.8 | 0.0 | 0.328 |
| Component 1 + Component 2 + Site | −368.0 | 755.5 | 0.7 | 0.231 |
| Component 1 + Site | −370.0 | 757.1 | 2.3 | 0.104 |
| Component 1 + Component 2 + Orientation + Site | −368.0 | 757.8 | 3.0 | 0.073 |
| Site | −372.4 | 759.6 | 4.8 | 0.030 |
| Intercept only | −389.1 | 782.3 | 27.5 | <0.001 |
| Models explaining ΔTavg | ||||
| Orientation + Site | −253.8 | 524.8 | 0.0 | 0.193 |
| Site | −255.0 | 524.8 | 0.0 | 0.193 |
| Component 2 + Site | −254.8 | 526.7 | 1.9 | 0.075 |
| Component 1 + Site | −254.8 | 526.7 | 1.9 | 0.075 |
| Intercept only | −264.7 | 533.4 | 8.6 | <0.003 |
Comparisons of models assessing ΔTavg indicated that, although all models had greater support than the intercept-only model, no single combination of structural variables was particularly informative. A model with orientation had the same level of support as a site-only model (ωi = 0.19), and both models were supported over models with other structural variables (ωi = 0.08; ΔAICc = 1.9). Crevices with orientations closest to vertical were somewhat associated (β = 0.23 ± 0.15) with higher ΔTavg than those with more horizontal orientations. Summed model weights were relatively dispersed among structural variables, and were 0.48 for crevice orientation, 0.30 for component 2, 0.28 for component 1, and 0.26 for crevice width.
Comparisons of models predicting ∆Tmax indicated greatest support for a model that combined components 1 and 2 with crevice width (ωi = 0.33). However, a version of this model that did not account for crevice width had similar support (ωi = 0.23; ΔAICc = 0.7). Summed model weights for structural variables were 0.88 for component 1, 0.73 for component 2, 0.49 for crevice width, and 0.20 for crevice orientation. The top-ranked model suggested that crevices with smaller component 1 scores (β = −1.23 ± 0.48) and smaller component 2 scores (β = −1.00 ± 0.48) reached higher ΔTmax values.
Discussion
Roosting habits of Eastern Small-footed Myotis paralleled those of western rock-roosting bat species in several ways. Rock formations used by Eastern Small-footed Myotis were predominantly south-facing, devoid of tree canopy, and received direct solar radiation for much of the day. Similar conditions have been reported for rocky features used by Spotted Bat (Euderma maculatum; Chambers et al. 2011), Townsend’s Big-eared Bat (Corynorhinus townsendii; Reid et al. 2010), Pallid Bat (Antrozous pallidus; Schorr and Siemers 2013), Fringed Myotis (M. thysanodes; Lacki and Baker 2007), Western Long-eared Myotis (Rancourt et al. 2005), Western Small-footed Myotis (M. ciliolabrum; Rodhouse and Hyde 2014), and Canyon Bat (Parastrellus hesperus; Cross 1965). Rocks have high thermal inertia and when exposed to direct sunlight sometimes create microclimates that are warmer and more thermally stable than their surroundings (Lausen and Barclay 2002). This phenomenon was perceptible at the primary rock formations we studied and we suspect it is one of the reasons these sites supported aggregations of Eastern Small-footed Myotis. However, Eastern Small-footed Myotis also occasionally roosted on rock formations that did not face south or were under closed-canopy forest. Such rock formations presumably would have had stable but cooler temperatures than the crevices we monitored at primary sites.
Within primary rock formations, male and reproductive female Eastern Small-footed Myotis appeared to differentially select from among potential roosts based on structural and thermal characteristics of rock crevices. Bats of both sexes selected crevices with substantially narrower openings than random crevices. This was most noticeable for males, which tended to use crevices that were barely wide enough for an Eastern Small-footed Myotis to enter (≤1 cm wide). Such narrow crevices probably offer substantial protection from predators. Records of predation on insectivorous bats in their roosts are not common but include predation by arthropods and vertebrates (Molinari et al. 2005; Carver and Lereculeur 2013; Lima and O’Keefe 2013). At our study sites we commonly saw Five-lined Skink (Eumeces inexpectatus), Eastern Fence Lizard (Sceloporus undulatus), Eastern Milksnake (Lampropeltis triangulum), Eastern Ratsnake (Pantherophis allegheniensis), Timber Rattlesnake (Crotalus horridus), and Common Raven (Corvus corax), but it is unclear the extent to which any of these species could prey on Eastern Small-footed Myotis in crevices ≤1 cm wide. Selection for narrow rock crevices has also been reported for some western bat species, although none were as narrow as those used by Eastern Small-footed Myotis (Lausen and Barclay 2002; Rancourt et al. 2005). Narrow crevice openings conceivably might also prevent competition with larger species of bats, such as the Big Brown Bat (Eptesicus fuscus), which we observed roosting in lower densities on the same talus slopes (Rancourt et al. 2005).
Roosts of reproductive females, particularly those roosting in groups, had narrower openings than random crevices but were wider than crevices used by males. The reason for this difference in crevice widths is uncertain, but we note that Lausen and Barclay (2002) observed a similar pattern for groups of lactating big brown bats compared to solitary postlactating bats. Crevice width had a modest influence on microclimates, but wider crevices might be important for other reasons, such as providing room for bats to move within crowded crevices or increasing airflow. In the largest groups that we observed (≥10 bats), bats were often crowded near the crevice opening and appeared to jostle for position. Some bats even roosted outside of the crevice, a behavior we observed on hot days as well as cool days. We wonder how such behaviors factor into predation risk for reproductive females and pups. Female Eastern Small-footed Myotis and other species of insectivorous bats tend to have lower survivorship than males, but this has been attributed to unequal costs of reproduction (Keen and Hitchcock 1980; Hitchcock et al. 1984).
Bats are believed to switch roosts frequently to reduce risk of predation, parasitism, and to aid in thermoregulation (Lewis 1995; Reckardt and Kerth 2007). The daily movements that Eastern Small-footed Myotis made during our study were consistent with results of studies on this species elsewhere in their range (Johnsons and Gates 2008; Johnson et al. 2011), and were comparable to behaviors of other species of rock-roosting bats, such as Fringed Myotis and Western Long-eared Myotis (Solick and Barclay 2006; Snider et al. 2013). Behaviors of radio-tracked bats from the first year of our study suggest that such frequent roost-switching was part of the normal behavioral repertoire of Eastern Small-footed Myotis and not the result of our methods. Bats that roost in talus and other low outcrops have among the highest reported rates of roost-switching for insectivorous bats (Lausen and Barclay 2002; Lacki and Baker 2007; Snider et al. 2013; Rodhouse and Hyde 2014). Frequent roost-switching on talus slopes, where crevices are at ground level, could be important for mitigating risk of discovery by terrestrial predators. Talus slopes and their artificial counterparts also facilitate frequent movements because they contain such high densities of potential roosts. Moosman et al. (2020) recorded average surface densities of 6,268 ± 257 crevices per ha at talus sites in Virginia.
Protection from predators, however, was unlikely to be the only factor driving roost selection by Eastern Small-footed Myotis. Even though males and reproductive females shared the same rock formation and sometimes roosted within meters of one another, they selected different types of roosts. Roosts of reproductive females were most often associated with crevices formed by the largest rocks available at each site, despite substantial variation in rock sizes among sites. Microclimate models indicated that large rocks acted as heat sinks during the warmest parts of the day and heat sources throughout the night and coolest parts of the day, resulting in relatively stable thermal profiles. Selection of crevices with similar thermal profiles has been reported for lactating Western Long-eared Myotis and big brown bats in Alberta–which use thick-walled crevices that retain more heat at night and had have cooler maximum daytime temperatures than crevices used by pregnant bats (Chruszcz and Barclay 2002; Lausen and Barclay 2002). Large rocks have been experimentally shown to have more stable microclimates than small rocks because their greater volume increases thermal inertia (Lausen and Barclay 2002).
Given the positive association that roosts of reproductive female Eastern Small-footed Myotis had with large rocks in our study, future work should investigate whether substrate size influences suitability of rock formations for use as maternity habitat at larger spatial scales. In the Appalachian Mountains, sites with rocks of the size used by reproductive females could be rarer than those dominated by smaller substrates (e.g., scree), raising the possibility that rock size is a limiting factor. Selection for large rocks by reproductive females might also explain the nonrandom spatial patterns Johnson et al. (2011) observed for female Eastern Small-footed Myotis in West Virginia. Rock sizes often have heterogeneous distributions within talus slopes (Bones 1973; Vittorio de Blasio and Sæter 2010) and at some of our study sites, the largest rocks predominated the upslope edges of talus slopes, closest to the exposed masses of parent material. For example, this pattern occurred at Sherando, where we only radio-tracked reproductive females to two discrete upslope patches that had larger rocks than the rest of the site. At Marbleyard, large rocks and the reproductive females they supported were relatively widespread.
Male bats largely matched our prediction that they would be less selective than reproductive females with regards to both structural and thermal characteristics of crevices. Roost selection by male bats has not often been described in the literature, but there is evidence for sex-specific behaviors in other rock-roosting bat species. For example, male Western Long-eared Myotis in Oregon were more likely than lactating females to roost in trees rather than rocks (Anthony and Sanchez 2018). Male Eastern Small-footed Myotis sometimes roosted between surprisingly small rocks. The smallest rock that a male bat roosted under–for which we obtained measurements–was a 12 cm long, 7 cm deep, and 6 cm high fragment sitting on top of a 41 cm long, 25 cm deep, and 19 cm high rock. We also twice found males in crevices that were not deep enough to entirely enclose them at Sherando and Gathright. Such small roosts were rare, however, and were probably close to the minimum size needed to provide suitable roosting habitat for Eastern Small-footed Myotis. Sherando also had an extensive area of small scree on its lower slopes where we never observed bats roost using either radiotelemetry or visual surveys.
Roosts of males also tended to reach higher relative maximum temperatures in the hottest part of the day and cool off more at night than crevices used by reproductive females. Microclimate models supported the idea that these greater thermal extremes were associated with the small rocks that males selected. Similarly labile thermal profiles have been reported for other solitary rock-roosting bats, including pregnant Western Long-eared Myotis and male Pallid Bat (Chruszcz and Barclay 2002; Rambaldini and Brigham 2008; Schorr and Siemers 2013). Such microclimates conceivably provide opportunities for torpor early in the day, followed by the opportunity to return to active body temperature using passive rewarming in the afternoon. Use of torpor by bats reduces energetic demands and total evaporative water loss (Thomas 1995; Muñoz-Garcia et al. 2012). Passive rewarming reduces the extent to which bats need to actively arouse before nightly emergence, potentially reducing other physiological costs such as oxidative and cardiovascular stress (Currie et al. 2015). However, skin temperature data are needed to confirm thermoregulatory strategies used by Eastern Small-footed Myotis.
In addition to the effects of rock size, microclimate models indicated that thermal profiles of crevices were influenced by crevice orientation and width, as well as by site. Crevices with orientations close to vertical and with moderate widths were somewhat better at retaining heat at night than other kinds of crevices. Previous studies have shown that vertical rock crevices were less prone to nocturnal heat loss and were more likely to be selected by reproductive females (Chruszcz and Barclay 2002; Lausen and Barclay 2002; Rancourt et al. 2005). Vertical crevices could also provide thermal gradients that bats can take advantage of by moving up or down within the roost (Vaughn and O’Shea 1976, 1977; Lausen and Barclay 2003; Solick and Barclay 2007).
Microclimates were heavily influenced by site effects, and we suspect also by within-site characteristics that we did not measure. Different rock formations, and locations across the surfaces of larger rock formations, seemed to heat and cool in spatially and temporally dynamic manners. We suspect that such thermal heterogeneity was influenced by variation in absorption of solar radiation, due to undulations in terrain (e.g., slope angle and aspect), and time of day and season. Lausen and Barclay (2002) discussed the possibility that shifts in roosting behavior by big brown bats along rocky river valleys in Alberta were related to solar absorption.
Differences in geologic composition also probably contributed to variation in microclimates among sites. Some of the hottest crevice temperatures we measured occurred at our northernmost site (Surry), which has a relatively cold and wet climate. The highest absolute roost temperature at Surry was 45.5°C for a single day and 41.3°C for a 3-day period. These maximum temperature readings appeared to be part of a continuous distribution, rather than outliers; thus, we doubt they were caused by sunlight striking sensors directly. Surry also had among the coldest absolute morning roost temperatures (10.0°C). Relatively high variability in crevice temperatures at Surry might have been because the riprap was granite, giving it lower specific heat and higher thermal emissivity than the sedimentary rocks at the sites in Virginia (Roberston 1988). This may have contributed to rapid cooling in the cold New Hampshire nights.
Thermal profiles of Eastern Small-footed Myotis roosts closely resembled those described for reproductive female Western Long-eared Myotis in the Rocky Mountains of Alberta, which had higher daytime temperatures but cooled quickly at night compared to roosts in the prairies (Solick and Barclay 2006, 2007). The thermoregulatory strategy of Western Long-eared Myotis is thought to have been driven by constraints from a cooler and shorter growing season in the Rocky Mountains, and by greater needs to reduce evaporative water loss in the prairies (Solick and Barclay 2007). Our results suggest that Eastern Small-footed Myotis also may mitigate climatic constraints by selecting rock crevices with appropriate thermal regimes.
In some cases, rock crevices used by Eastern Small-footed Myotis demonstrated the potential to create physiologically challenging microclimates during hot weather. Crevices sometimes warmed as much as 10 to 15°C above the active skin temperature (Tskin = 30.1–38.1°C) of Eastern Small-footed Myotis (Moosman et al. 2015). How rock-roosting bats manage the costs of keeping cool during extreme heat is not well understood, but it has implications for their ecology and conservation. Water loss from evaporative cooling can be substantial for bats, and severity of water loss can differ based on roosting habitat (Noakes et al. 2021). The costs of evaporative water loss for bats in rock-roosts compared to other roost types might explain why proximity to water has often corresponded with roost selection at the landscape scale, and perhaps why some species select different roost types in some parts of their range (Solick and Barclay 2007; Snider et al. 2013; Anthony and Sanchez 2018). Additional study of this topic is warranted given that many species of rock-roosting bats occur in arid regions and declining water availability is expected to impact their populations (Adams and Hayes 2008; O’Shea et al. 2011). Overall, our results highlight the importance of structural and thermal characteristics of roosts and suggest some broad similarities between Eastern Small-footed Myotis and western species of rock-roosting bats.
Supplementary Data
Supplementary data are available at Journal of Mammalogy online.
Supplementary Data SD1.—Examples of talus slopes in the Appalachian Mountains of Virginia where Eastern Small-footed Myotis (Myotis leibii) roosted, 2011–2018. Roosts were usually in crevices between two rocks and occurred in a variety of orientations. Arrows indicate position of bats. Size and shape of rocks varied among and within sites.
Supplementary Data SD2.—Human-made rock formations used as roost sites by Eastern Small-footed Myotis (Myotis leibii), including a riprap-covered dam (Surry) in the New England Physiographic Province of New Hampshire, and a riprap-covered dam (Gathright) in the Valley and Ridge Physiographic Province, and linear pile of boulders (Conway) in the Blue Ridge Mountains of Virginia, 2009–2018.
Supplementary Data SD3.—Statistical analyses used to assess potential methodological bias, identify redundant variables, and guide later analyses of roost selection by Eastern Small-footed Myotis (Myotis leibii) in the Appalachian Mountains of Virginia and New Hampshire, 2009–2018.
Supplementary Data SD4.—Models used to assess roost selection by Eastern Small-footed Myotis (Myotis leibii) in the Appalachian Mountains of Virginia and New Hampshire, 2009–2018, relative to structural and thermal characteristics rock crevices. Components 1 and 2 are latent variables corresponding to overall size of the upper and lower rocks forming each crevice. Thermal measurements represent how much warmer crevices were compared to ambient air, at the coldest (ΔTmin) part of the day, and on average (ΔTavg). The intercept was included in all models.
Supplementary Data SD5.—Generalized linear models used to understand factors that influenced microclimates of rock crevices (roosts and random crevices combined) at sites used by Eastern Small-footed Myotis (Myotis leibii) in the Appalachian Mountains of Virginia and New Hampshire, June and July 2009–2018. We modeled effects of different combinations of structural variables on temperatures of crevices relative to ambient air, at the coldest and hottest part of the day, and overall, over 3 days. Components 1 and 2 are latent variables corresponding to overall size of the upper and lower rocks forming each crevice. The intercept was included in all models.
Acknowledgments
We thank M. Hawes, H. Thomas, J. Veilleux, M. Dannon, D. Warner, H. Hendren, D. Pody, A. Barlow, S. Sell, D. Moosman, J. Stuart, and W. Siple for help with this project. Additionally, we thank the USDA Forest Service and U.S. Army Corp of Engineers for providing access to field sites. Funding was provided, in part, by a wildlife restoration grant provided by the U.S. Fish and Wildlife Service through the Virginia Department of Wildlife Resources, and by the Center for Undergraduate Research at the Virginia Military Institute.
Contributor Information
Paul R Moosman, Jr., Department of Biology, Virginia Military Institute, Lexington, Virginia 24450, USA
David M Marsh, Department of Biology, Washington and Lee University, Lexington, Virginia 24450, USA.
Emily K Pody, Department of Biology, Virginia Military Institute, Lexington, Virginia 24450, USA; EDGE Engineering and Science, LLC, Houston, Texas 77084, USA.
Timothy J Brust, Department of Biology, Virginia Military Institute, Lexington, Virginia 24450, USA; EDGE Engineering and Science, LLC, Houston, Texas 77084, USA.
Conflict of Interest
The authors declare no conflict of interest.
Data Availability
Raw data describing structural characteristics of rocks and crevices, summarized thermal measurements for crevices, and information about the bats we observed roosting have been deposited in Dryad.
Literature Cited
- Adams R.A., Hayes M.A.. 2008. Water availability and successful lactation by bats as related to climate change in arid regions of western North America. Journal of Animal Ecology 77:1115–1121. [DOI] [PubMed] [Google Scholar]
- Altringham J.D. 1996. Bats: biology and behaviour. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- Andrews H. 2021. Bat roosts in rock, a guide to identification and assessment for climbers, cavers and ecology professionals: bat rock habitat key. Pelagic Publishing, Exeter, United Kingdom. [Google Scholar]
- Anthony C.R., Sanchez D.M.. 2018. Roost site selection of western long-eared myotis in a western juniper woodland. Journal of Wildlife Management 82:618–628. [Google Scholar]
- Baker M.D., Lacki M.J.. 2006. Day-roosting habitat of female long-legged myotis in ponderosa pine forests. Journal of Wildlife Management 70:207–215. [Google Scholar]
- Barclay R.M.R. 1982. Night roosting behavior of the little brown bat, Myotis lucifugus. Journal of Mammalogy 63:464–474. [Google Scholar]
- Barclay R.M.R. 1991. Population structure of temperate zone insectivorous bats in relation to foraging behaviour and energy demand. Journal of Animal Ecology 60:165–178. [Google Scholar]
- Bergeson S.M., Brigham R.M., O’Keefe J.M.. 2021. Free-ranging bats alter thermoregulatory behavior in response to reproductive stage, roost type, and weather. Journal of Mammalogy 102:705–717. [Google Scholar]
- Bones T.G. 1973. Process and sediment size arrangement on high arctic talus, Southwest Devon Island, N.W.T. Canada. Arctic and Alpine Research 5:29–40. [Google Scholar]
- Burnham K.P., Anderson D.R.. 2002. Model selection and multimodel inference: a practical information-theoretic approach. 2nd ed. Springer, New York, USA. [Google Scholar]
- Carver B.D., Lereculeur A.E.. 2013. Predation on a northern long-eared myotis by a gray rat snake. Southeastern Naturalist 12:N6–N8. [Google Scholar]
- Chambers C.L., Herder M.J., Yasuda K., Mikesic D.G., Dewhurst S.M., Masters W.M., Vleck D.. 2011. Roosts and home range of spotted bats (Euderma maculatum) in northern Arizona. Canadian Journal of Zoology 89:1256–1267. [Google Scholar]
- Chruszcz B.J., Barclay R.M.R.. 2002. Thermoregulatory ecology of a solitary bat, Myotis evotis, roosting in rock crevices. Functional Ecology 16:18–26. [Google Scholar]
- Cross S.P. 1965. Roosting habits of Pipistrellus hesperus. Journal of Mammalogy 46:270–279. [Google Scholar]
- Cryan P.M., Wolf B.O.. 2003. Sex differences in the thermoregulation and evaporative water loss of a heterothermic bat, Lasiurus cinereus, during its spring migration. The Journal of Experimental Biology 206:3381–3390. [DOI] [PubMed] [Google Scholar]
- Currie S.E., Noy K., Geiser F.. 2015. Passive rewarming from torpor in hibernating bats: minimizing metabolic costs and cardiac demands. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 308:R34–R41. [DOI] [PubMed] [Google Scholar]
- Dechmann D.K.N., Kalko E.K.V., König B., Kerth G.. 2005. Mating system of a neotropical roost-making bat: the white-throated, round-eared bat, Lophostoma silvicolum (Chiroptera: Phyllostomidae). Behavioral Ecology and Sociobiology 58:316–325. [Google Scholar]
- Encarnação J.A., Otto M.S., Becker N.I.. 2012. Thermoregulation in male temperate bats depends on habitat characteristics. Journal of Thermal Biology 37:564–569. [Google Scholar]
- Fagan K.E., Wilcox E.V., Bernard R.F., Stiver W.H.. 2016. Myotis leibii (eastern small-footed myotis) roosting in buildings of Great Smoky Mountains National Park, Tennessee. Northeastern Naturalist 15:N23–N27. [Google Scholar]
- Hamilton I.M., Barclay R.M.R.. 1994. Patterns of daily torpor and day-roost selection by male and female big brown bats (Eptesicus fuscus). Canadian Journal of Zoology 72:744–749. [Google Scholar]
- Hitchcock H.B., Keen R., Kurta A.. 1984. Survival rates of Myotis leibii and Eptesicus fuscus in southeastern Ontario. Journal of Mammalogy 65:126–130. [Google Scholar]
- Hurst T.E., Lacki M.J.. 1999. Roost selection, population size, and habitat use by a colony of Rafinesque’s big-eared bats (Corynorhinus rafinesquii). American Midland Naturalist 142:363–371. [Google Scholar]
- Humphrey S.R. 1975. Nursery roosts and community diversity of Nearctic bats. Journal of Mammalogy 56:321–346. [Google Scholar]
- Johnson J.B., Gates J.E.. 2008. Spring migration and roost selection of female Myotis leibii in Maryland. Northeastern Naturalist 15:453–460. [Google Scholar]
- Johnson J.S., Kiser J.D., Watrous K.S., Peterson T.S.. 2011. Day-roosts of Myotis leibii in the Appalachian Ridge and Valley of West Virginia. Northeastern Naturalist 18:95–106. [Google Scholar]
- Klug B.J., Goldsmith D.A., Barclay R.M.R.. 2012. Roost selection by the solitary, foliage-roosting hoary bat (Lasiurus cinereus) during lactation. Canadian Journal of Zoology 90:329–336. [Google Scholar]
- Keen R., Hitchcock H.B.. 1980. Survival and longevity of the little brown bat (Myotis lucifugus) in southeastern Ontario. Journal of Mammalogy 61:1–7. [Google Scholar]
- Kunz T.H., Hood. W.R.. 2000. Parental care and postnatal growth in the Chiroptera. In: Krichton E.G., Krutzsch P.H., editors. Reproductive biology of bats. Academic Press, London, United Kingdom; p. 415–468. [Google Scholar]
- Lacki M.J., Adam M.D., Shoemaker L.G.. 1994. Observations of seasonal cycle, population patterns and roost selection in summer colonies of Plecotus townsendii virginianus in Kentucky. American Midland Naturalist 131:34–42. [Google Scholar]
- Lacki M.J., Baker M.D.. 2007. Day roosts of female fringed myotis (Myotis thysanodes) in xeric forests of the Pacific Northwest. Journal of Mammalogy 88:967–973. [Google Scholar]
- Lausen C.L., Barclay R.M.R.. 2002. Roosting behaviour and roost selection of female big brown bats (Eptesicus fuscus) roosting in rock crevices in southeastern Alberta. Canadian Journal of Zoology 80:1069–1076. [Google Scholar]
- Lausen C.L., Barclay R.M.R.. 2003. Thermoregulation and roost selection by reproductive female big brown bats (Eptesicus fuscus) roosting in rock crevices. Journal of the Zoological Society of London 260:235–244. [Google Scholar]
- Lewis S.E. 1995. Roost fidelity of bats: a review. Journal of Mammalogy 76:481–496. [Google Scholar]
- Lima S.L., O’Keefe J.M.. 2013. Do predators influence the behaviour of bats? Biological Reviews 88:626–644. [DOI] [PubMed] [Google Scholar]
- Loeb S.C., Jodice G.R.. 2018. Activity of southeastern bats along sandstone cliffs used for rock climbing. Journal of Fish and Wildlife Management 9:255–265. [Google Scholar]
- Michaelsen T.C., Olsen O., Grimstad K.J.. 2013. Roosts used by bats in late autumn and winter at northern latitudes in Norway. Folia Zoologica 62:297–303. [Google Scholar]
- Molinari J., Gutiérrez E.E., de Acsenção A.A., Nassar J.M., Ahrens A., Márquez R.J.. 2005. Predation by giant centipedes, Scolopendra gigantea, on three species of bats in a Venezuelan cave. Caribbean Journal of Science 41:340–346. [Google Scholar]
- Moosman P.R. Jr., Anderson P.R., Frasier M.G.. 2017. Use of rock crevices in winter by big brown bats and eastern small-footed bats in the Appalachian Ridge and Valley of Virginia. Banisteria 48:9–13. [Google Scholar]
- Moosman P.R. Jr., Warner D.P., Hendren R.H., Hosler M.J.. 2015. Potential for monitoring eastern small-footed bats on talus slopes. Northeastern Naturalist 22:NENHC-1–NENHC-13. [Google Scholar]
- Moosman P.R. Jr., Marsh D.M., Pody E.K., Dannon M.P., Reynolds R.J.. 2020. Efficacy of visual surveys for monitoring populations of talus-roosting bats. Journal of Fish and Wildlife Management 11:597–608. [Google Scholar]
- Muñoz-Garcia A., Ben-Hamo M., Penshow B., Williams J.B., Korine C.. 2012. The relationship between cutaneous water loss and thermoregulatory state in Kuhl’s pipistrelle Pipistrelluskuhlii, a vespertilionid bat. Physiological and Biochemical Zoology 85:516–525. [DOI] [PubMed] [Google Scholar]
- Noakes M.J., McKechnie A.E., Brigham R.M.. 2021. Interspecific variation in heat tolerance and evaporative cooling capacity among sympatric temperate-latitude bats. Canadian Journal of Zoology 99:480–488. [Google Scholar]
- O’Shea T.J., Cryan P.M., Snider E.A., Valdez E.W., Ellison L.E., Neubaum D.J.. 2011. Bats of Mesa Verde National Park, Colorado: composition, reproduction, and roosting habits. Monographs of the Western North American Naturalist 5:1–19. [Google Scholar]
- Pretzlaff I., Kerth G., Dausmann K.H.. 2010. Communally breeding bats use physiological and behavioural adjustments to optimize daily energy expenditure. Naturwissenschaften 97:353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racey P.A., Swift S.M.. 1981. Variations in gestation length in a colony of pipistrelle bats (Pipistrellus pipistrellus) from year to year. Journal of Reproduction and Fertility 1:123–129. [DOI] [PubMed] [Google Scholar]
- Rambaldini D.A., Brigham R.M.R.. 2008. Torpor use by free-ranging pallid bats (Antrozous pallidus) at the northern extent of their range. Journal of Mammalogy 89:933–941. [Google Scholar]
- Rancourt S.J., Rule M.I., O’Connell M.A.. 2005. Maternity roost site selection of long-eared myotis, Myotis evotis. Journal of Mammalogy 86:77–84. [Google Scholar]
- Randall L.A., Jung T.S., Barclay R.M.R.. 2014. Roost-site selection and movements of little brown myotis (Myotis lucifugus) in southwestern Yukon. Northwestern Naturalist 95:312–317. [Google Scholar]
- Reckardt K., Kerth G.. 2007. Roost selection and roost switching of female Bechstein’s bats (Myotis bechsteinii) as a strategy of parasite avoidance. Oecologia 154:581–588. [DOI] [PubMed] [Google Scholar]
- Reid A., Hill T., Clarke R., Gwilliam J., Krebs J.. 2010. Roosting ecology of female Townsend’s big-eared bats (Corynorhinus townsendii) in south-eastern British Columbia: implications for conservation management. Northwestern Naturalist 91:215–218. [Google Scholar]
- Roble S. 2004. Notes on an autumn roost of an eastern small-footed bat (Myotis leibii). Banisteria 23:42–44. [Google Scholar]
- Roberston E.C. 1988. Thermal properties of rocks. U.S. Department of Interior, Geological Survey, Reston, Virginia, USA, Open-file Report 88-441:1–106. [Google Scholar]
- Rodhouse T.J., Hyde K.J.. 2014. Roost and forage site fidelity of western small-footed myotis (Myotis ciliolabrum) in an Oregon desert canyon. Western North American Naturalist 74:241–248. [Google Scholar]
- Russo D., Cistrone L., Budinski I., Console G., Corte M.D., Milighetti C., Di Salvo I., Nardone V., Brigham R.M., Ancillotto L.. 2017. Sociality influences thermoregulation and roost switching in a forest bat using ephemeral roosts. Ecology and Evolution 7:5310–5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saugey D.A., McDaniel V.R., England D.R., Rowe M.C., Chandler-Mozisek L.R., Cochran B.G.. 1993. Arkansas range extensions of the eastern small-footed bat (Myotis leibii) and northern long-eared bat (Myotis septentrionalis) and additional county records for the silver-haired bat (Lasionycteris noctivagans), hoary bat (Lasiurus cinereus), southeastern bat (Myotis austroriparius), and Rafinesque’s big-eared bat (Plecotus rafinesquii). Journal of the Arkansas Academy of Science 47:102–106. [Google Scholar]
- Schorr R.A., Siemers J.L.. 2013. Characteristics of roosts of male pallid bats (Antrozous pallidus) in southeastern Colorado. The Southwestern Naturalist 58:470–474. [Google Scholar]
- Sikes R.S.,. and the Animal Care and Use Committee of the American Society of Mammalogists. 2016. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. Journal of Mammalogy 97:663–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider E.A., Cryan P.M., Wilson K.R.. 2013. Roost selection by western long-eared myotis (Myotis evotis) in burned and unburned piñon-juniper woodlands of southwestern Colorado. Journal of Mammalogy 94:640–649. [Google Scholar]
- Solick D.I., Barclay R.M.R.. 2006. Thermoregulation and roosting behaviour of reproductive and nonreproductive female western long-eared bats (Myotis evotis) in the Rocky Mountains of Alberta. Canadian Journal of Zoology 84:589–599. [Google Scholar]
- Solick D.I., Barclay R.M.R.. 2007. Geographic variation in the use of torpor and roosting behaviour of female western long-eared bats. Journal of Zoology 272:358–366. [Google Scholar]
- Speakman J.R., Thomas D.W.. 2003. Physiological ecology and energetics of bats. In: Kunz T.H., Fenton M.B., editors. Bat ecology. University of Chicago Press, Chicago, Illinois, USA; p. 430–490. [Google Scholar]
- Trune D.R., Slobodchikoff C.N.. 1976. Social effects of roosting on the metabolism of the pallid bat (Antrozous pallidus). Journal of Mammalogy 57:656–663. [PubMed] [Google Scholar]
- Thomas D.W. 1995. Physiological ecology of hibernation in vespertilionid bats. In: Racey P.A., Swift S., editors. Ecology, evolution and behaviour of bats (Symposia of the Zoological Society of London, 67). p. 233–244. [Google Scholar]
- U.S. Fish and Wildlife Service (USFWS). 2007. Indiana bat (Myotis sodalis) draft recovery plan: first revision. U.S. Fish and Wildlife Service, Fort Snelling, Minnesota, USA. 258 pp. [Google Scholar]
- Vaughn T.A., O’Shea T.J.. 1976. Roosting ecology of the pallid bat, Antrozous pallidus. Journal of Mammalogy 57:19–42. [PubMed] [Google Scholar]
- Vaughn T.A., O’Shea T.J.. 1977. Nocturnal and seasonal activities of the pallid bat, Antrozous pallidus. Journal of Mammalogy 58:269–284. [Google Scholar]
- Vittorio de Blasio F., Sæter M.. 2010. Properties of talus slopes composed of flat blocks. Norwegian Journal of Geography 64:85–93. [Google Scholar]
- Whitby M., Bergeson S., Carter T., Rutan S., McClanahan R.. 2013. The discovery of a reproductive population of eastern small-footed bat, Myotis leibii, in southern Illinois using a novel survey method. American Midland Naturalist 169:229–233. [Google Scholar]
- Wilson D.E., Ruff S., editors. 1999. The Smithsonian Book of North American Mammals. Smithsonian Institution Press, Washington, District of Columbia, USA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data describing structural characteristics of rocks and crevices, summarized thermal measurements for crevices, and information about the bats we observed roosting have been deposited in Dryad.