Abstract
Constitutive activation of signal transducers and activators of transcription (STATs) has been associated with oncogenesis. Previously, a protein required for T-cell transformation by the DNA tumor virus herpesvirus saimiri (HVS) strain 484, designated tyrosine kinase-interacting protein (Tip-484), was shown to interact with and dramatically upregulate the activity of the STATs in an Lck-dependent manner. The minimal region of Tip-484 responsible for binding Lck was defined as a 10-residue C-terminal Src-related kinase homology domain, an 18-amino-acid spacer, and a 10-residue potential SH3 binding domain. This region is termed the LBD (for Lck binding domain). The present data show that only the LBD of Tip-484 is needed to activate Lck in vitro and in vivo. Finally, the LBD was shown to form a complex with STAT3 in vitro, and expression of the LBD in T cells led to STAT3 activation equal to that of full-length Tip-484. These studies demonstrate that the 48-amino-acid LBD of Tip-484 can perform as effectively as the full-length protein in vitro and in vivo.
Signal transducers and activators of transcription (STATs) are cytoplasmic transcription factors that remain latent until required for signaling by a ligand-receptor interaction. Generally, the cascade of signaling occurs as follows. When any one of a variety of cytokines binds and induces dimerization of its receptor, a receptor-associated member of the Janus family of tyrosine kinases (Jaks) is activated to phosphorylate the receptor on tyrosine residues. The phosphorylated tyrosines then serve as docking sites for latent STATs, which bind and themselves become tyrosine phosphorylated by Jaks. STATs are released, dimerize, and translocate to the nucleus to regulate the transcription of target genes. STAT3 has been shown to be induced by many cytokines which induce cellular proliferation (for reviews, see references 16 and 17).
The transforming oncoprotein v-src of the Rous sarcoma virus, the prototypical member of the Src family of tyrosine kinases, has been shown to constitutively activate STATs (6, 40). Recently, it has been shown that the transforming ability of v-src is dependent on the association and activation of STAT3 (5, 36). Lck, another Src family kinase, may also be involved with STAT activation. Lck is a lymphocyte-specific member of the Src family and is essential for normal signaling through the T-cell receptor (TCR) (3). Cross-linking of the TCR induces rapid activation of Lck, which phosphorylates the TCR zeta chain on tyrosine residues. The phosphorylated tyrosines then serve as a docking site for the recruitment of ZAP70, which is activated via tyrosine phosphorylation by Lck. Activated Lck and ZAP70 then propagate activation signaling downstream of the TCR. Other downstream Lck targets may include phospholipase Cγ1, protein kinase C, and phosphoinositide-3 kinase (for reviews, see references 31 and 35). Lck may also be involved in signaling through the interleukin-2 (IL-2) receptor (21).
Herpesvirus saimiri (HVS) is a gammaherpesvirus isolated from the South American squirrel monkey. Group C strains of HVS have the ability to transform monkey and human T cells to an IL-2-independent phenotype (27, 28). Two viral proteins have been found to be essential for transformation: saimiri transforming protein and tyrosine kinase-interacting protein (Tip), a membrane-bound protein (9, 27). Tip from HVS strain 484 (Tip-484) has been shown to interact with and to be a potent activator of Lck in T cells (22, 23). Recently, it was shown that Tip-484 also binds to and constitutively activates STATs in an Lck-dependent manner (24).
The minimal region of Tip responsible for binding Lck has been previously described (19, 23). We now term this region the LBD (for Lck binding domain). The LBD is composed of a 10-residue C-terminal Src-related kinase homology domain (CSKH), an 18-amino-acid spacer, and a 10-residue potential SH3 binding domain (SH3B). These two domains, along with the spacer, have been shown to be essential as the minimal region of Tip required for Lck interaction in vitro (19). Here we show that the LBD of Tip-484 can augment the protein kinase activity of a glutathione S-transferase (GST)–Lck fusion protein in vitro. The LBD alone can also upregulate Lck kinase activity in vivo. Furthermore, we show that LBD can bind to STAT3 in the presence of Lck and is fully capable of activating STAT3 DNA binding activity in T cells to the same extent as full-length Tip-484.
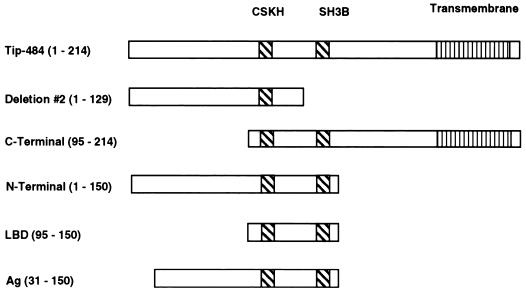
To elucidate the various regions of Tip-484 that might be important in activating Lck and STATs, several recombinants were constructed (Fig. 1). The full-length GST–Tip-484 construct has been previously described (23). Additional GST constructs were created by amplifying the region of interest by PCR and cloning it downstream of GST in the vector GST 5x-3 (Invitrogen, San Diego, Calif.). Deletions were made in a previously described construct of Tip-484 cloned into the eukaryotic expression vector pZeo (Invitrogen) (22). All constructs were sequenced and verified for protein production.
FIG. 1.
Summary of deletion mutants constructed from HVS Tip-484. Deletion 2 was constructed by deleting a restriction fragment from a GST–Tip-484 construct and religating the DNA (22). The N-terminal, C-terminal, LBD, and antigenic-region (Ag) constructs were made by amplifying gene fragments from TIP-484 by PCR and cloning them downstream of the GST gene as fusion proteins. All constructs were verified by restriction analysis, PCR, and fusion protein production. The hydrophobic transmembrane domain is indicated by vertical lines. The CSKH and SH3B regions are indicated with diagonal lines.
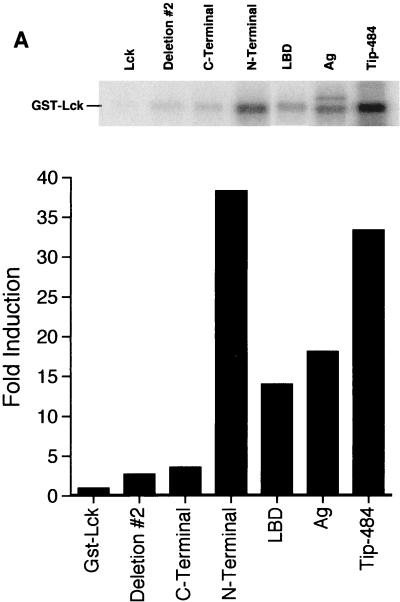
To determine which region of Tip affects Lck activity, a GST fusion protein system was utilized as an initial approach. lck was cloned downstream of the gene encoding GST and expressed in Escherichia coli, and the protein was harvested, affinity purified, and used in an in vitro kinase assay. Fusion proteins precipitated on glutathione-agarose beads were washed twice with kinase buffer (20 mM Tris [pH 7.4], 5 mM MgCl2). The beads were then suspended in a 1:1 slurry with kinase buffer. [γ-32P]ATP (20 μCi; Dupont) was added, and the beads were incubated at 30°C for 15 min. They were then washed one time with kinase buffer and boiled in sodium dodecyl sulfate (SDS) loading buffer (24). Radiolabeled proteins were separated on an SDS-polyacrylamide gel. Visualization and quantification were carried out with a PhosphorImager and ImageQuant software (Molecular Dynamics). GST-Lck was found to be partially active, as determined by autophosphorylation (Fig. 2A). If GST-Lck was allowed to interact with GST–Tip-484 prior to performance of the kinase assay, a significant increase in kinase activity was seen (Fig. 2A). The use of various regions of Tip-484 showed that the LBD of Tip, as well as the N-terminal region of Tip, could significantly activate GST-Lck. Conversely, the C-terminal region of Tip-484, which contains the LBD and also the downstream transmembrane region of this protein, did not enhance GST-Lck activity. Deletion mutant no. 2 has only one of the domains required to bind Lck, and it did not activate GST-Lck. No kinase activity was seen when any of the Tip fusion proteins were incubated with native GST (data not shown). It should be noted that the extra band seen in the antigenic-region construct lane is probably due to a nonspecific E. coli protein which appears intermittently in fusion protein purifications. These data show that the LBD alone can significantly activate Lck and that the addition of the downstream transmembrane region to LBD inhibits activation of GST-Lck.
FIG. 2.
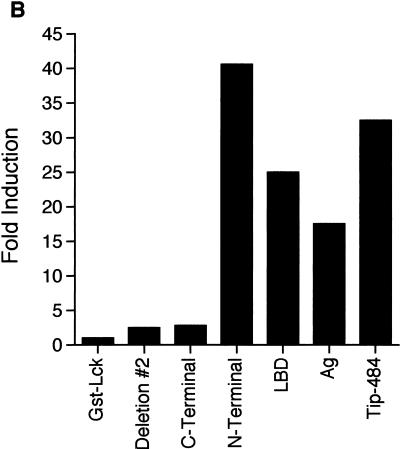
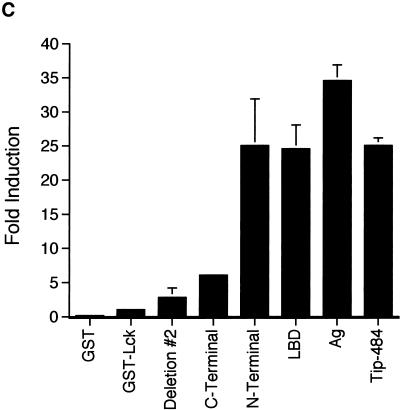
GST–Tip-484 fusion proteins can activate autokinase activity of GST-Lck. Lysates normalized for the amount of fusion protein produced (GST-Lck and various GST-Tip constructs) were mixed and precipitated on glutathione-agarose beads. An in vitro kinase assay was performed, and radiolabeled proteins were separated by SDS-PAGE. (A) GST-Lck autokinase activity was visualized by autoradiography (top). Quantitation of phosphorylation of GST-Lck was done with a PhosphorImager and ImageQuant software (Molecular Dynamics) (bottom). Data are displayed as fold induction over the kinase activity of GST-Lck mixed with native GST. The data are representative of one of several experiments giving similar results. Ag, antigenic-region construct. (B) An in vitro kinase assay was performed as for panel A with the addition of 1 μg of acid-denatured rabbit enolase. (C) A protein-tyrosine kinase assay system (Gibco BRL) (22) employing a synthetic peptide specific for Src family kinases was used for in vitro kinase assays according to the manufacturer’s protocol. Experiments were performed in triplicate.
We next looked at phosphorylation of a substrate by GST-Lck in the presence of Tip-484. Kinase assays were done as described above except that enolase was included as a substrate. Radiolabeled proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) (data not shown), and phosphorylated enolase was quantified by radiodensitometry. Figure 2B shows that, as before, the n-terminal region of Tip-484, its LBD, and full-length Tip-484 were best at activating Lck. Again, the LBD in combination with the downstream transmembrane domain did not activate Lck. Therefore, the LBD of Tip activates both autokinase and kinase activities of Lck toward a substrate.
To evaluate Tip-484 activation of Lck by a different method, a synthetic peptide specific for Src family kinases was used in the Protein-Tyrosine Kinase Assay System (Gibco BRL, Grand Island, N.Y.) (22). The substrate, derived from the amino acid sequence surrounding the phosphorylation site in pp60src (R-R-L-I-E-D-A-E-Y-A-A-R-G), is specific for tyrosine kinases. Using this system, fusion proteins were prepared as described above and resuspended in kinase buffer with the peptide substrate and [γ-32P]ATP. Figure 2C shows that expression of the LBD greatly enhanced the ability of GST-Lck to phosphorylate the peptide substrate. These data corroborate those obtained by the previous kinase assays (Fig. 2A and B).
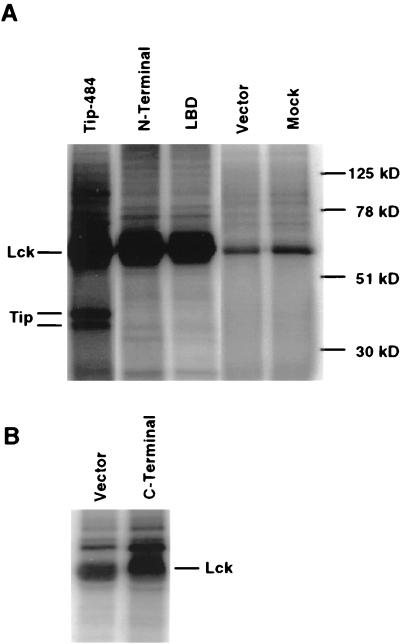
Fusion proteins can provide information about potential interactions, but to confirm that the LBD could function as well as full-length Tip-484 in vivo, constructs were expressed in a eukaryotic expression vector. Jurkat T cells were transfected with wild-type Tip-484, the N-terminal region of Tip-484, its LBD, or its C-terminal region or with vector alone. Forty-eight hours posttransfection, cell lysates were prepared. Lysates normalized for protein content were subjected to Lck immunoprecipitation followed by an in vitro kinase assay. Figure 3A shows the marked activation of Lck by Tip-484; it also shows that the N-terminus and LBD constructs activated Lck just as well as Tip-484. We have previously identified the two lower bands as two differentially phosphorylated forms of Tip-484 (22). The N-terminal region of Tip is inconsistently seen as a significantly smaller, slightly phosphorylated protein (data not shown). The LBD, at only 56 residues in length, was generally too small to be visualized by SDS-PAGE. Figure 3B shows that the C-terminus construct was able to activate Lck only twofold, as determined by radiodensitometry. The inability of this construct to significantly activate Lck agrees with the previous data obtained with GST fusion proteins. These data show that the LBD, like full-length Tip-484, can function as a potent activator of Lck in vivo.
FIG. 3.
The LBD of Tip-484 activates Lck kinase activity. (A) Jurkat T cells (107) were transfected with 20 μg of expression vector. Forty-eight hours posttransfection, the cells were harvested and equal amounts of cell extracts were used for immunoprecipitation with anti-Lck antibody (UBI, Lake Placid, N.Y.). An in vitro kinase assay was performed on immunoprecipitates, using 20 μCi of [γ-32P]ATP. Phosphorylated proteins were visualized by SDS-PAGE followed by autoradiography. The positions of molecular mass markers are indicated on the right (in kilodaltons). Mock, no DNA was added to mock-transfected cells. (B) A similar experiment was performed with the C-terminus construct. The exposure was significantly longer than for panel A. Vector, cells were transfected with pZeo vector DNA.
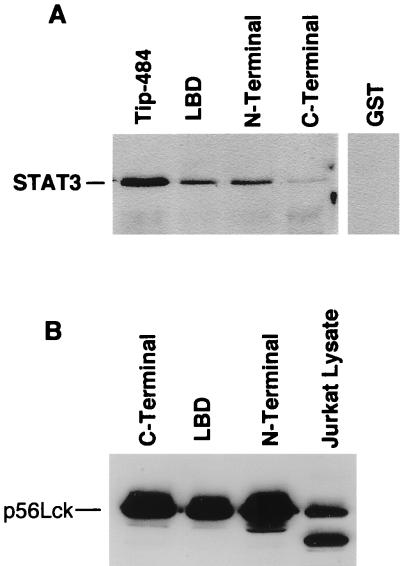
We have previously shown that Tip-484 binds to STAT3 in a multiprotein complex and that this binding is dependent on the presence of Lck (24). Because the LBD is the minimal region required for binding of Lck and it activates Lck activity in vitro and in vivo, we were interested in investigating whether LBD could also bind STAT3. To do so, fusion proteins were prepared, normalized, and used to bind proteins from Jurkat T-cell extracts. Bound proteins were separated by SDS-PAGE, and immunoblotting was performed to identify STAT3. Figure 4A shows that the LBD as well as the N-terminal region of Tip-484 can effectively bind STAT3, although not as well as the full-length Tip-484. Figure 4A also shows that the C-terminal region of Tip has a greatly diminished capacity to bind STAT3. To determine whether the C-terminal region’s lack of Lck activation seen in the previous experiments was due to the absence of an interaction with Lck, a similar binding experiment was done and immunoblotting for Lck was performed. Figure 4B shows that all constructs tested bound Lck effectively in vitro. GST alone did not bind any detectable proteins. These data indicate that the inability of the C-terminal region of Tip-484 to activate Lck in vivo is not due to an inability to bind Lck. Although the specific mechanism by which the C terminus inhibits Lck activation is unknown, we speculate that the addition of the hydrophobic C-terminal region to the LBD interferes with a specific conformational change required for Lck activation by the LBD.
FIG. 4.
The LBD of Tip-484 interacts with STAT3 and Lck. Equal amounts of lysate from 107 Jurkat T cells were incubated with 1 μg of fusion protein purified on glutathione-agarose beads as previously described (22). Interacting proteins were separated by SDS-PAGE and analyzed by Western blotting with anti-STAT3 antibody (A) or anti-Lck antibody (B).
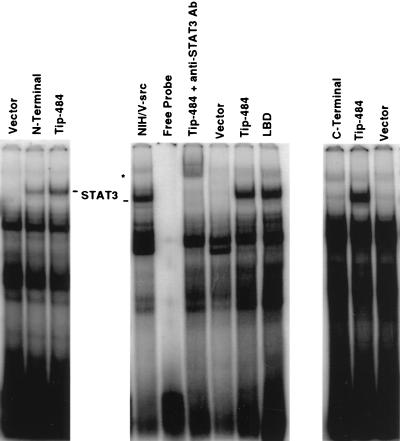
We have previously shown that STAT activation occurs in HVS-transformed T cells (24). We also found that Tip-484 alone is a potent activator of STATs (24). Since the LBD of Tip-484 can activate Lck to the same extent as the full-length protein and can bind STAT3 in vitro, we were interested in determining whether the LBD could also activate STAT3 in vivo. As in the previous experiments, Tip-484 N-terminus- and LBD-expressing vectors were transfected into Jurkat T cells. Forty-eight hours posttransfection, nuclear extracts were prepared. Normalized nuclear extracts were subjected to an electrophoretic mobility shift assay (EMSA) using an end-labeled sis-inducible element (SIE) probe which contains binding sites for STAT1 and -3 as described previously (24, 37). v-src-transformed NIH 3T3 cells, which have constitutively activated STAT3, were used as a positive control (40). Figure 5 shows that the LBD was indeed able to activate STAT3 as well as does Tip-484. This was confirmed by using an antibody to STAT3 in a supershift assay. Also, the results of an SIE affinity binding assay of total-cell lysates were similar to those obtained by EMSA (data not shown). A separate experiment showed that the N-terminal region of Tip is also a potent activator of STAT3. These data indicate that the LBD is a potent activator of both Lck and STAT3 in vivo. We speculate that the activation of STAT3 occurs via the LBD’s strong upregulation of Lck, which may be directly responsible for STAT3 activation. The C-terminus construct did not significantly activate STAT3 (Fig. 5) and, concurrently, did not activate Lck in vivo. Although we do not know the specific inhibitory mechanism of the C terminus, the data support the notion that Lck activation can lead to STAT3 activation in vivo.
FIG. 5.
The LBD of Tip-484 activates DNA binding activity of STAT3. Jurkat T cells (107) were transfected with 20 μg of expression vector. Forty-eight hours posttransfection, nuclear extracts were prepared. Normalized extracts were incubated with a 32P-radiolabeled SIE oligonucleotide probe derived from the c-fos gene promoter (sense strand, 5′ AGCTTCATTTCCCGTAAATCCCTA) that binds STAT1 and -3 (24). Protein-DNA complexes were resolved by nondenaturing PAGE and detected by autoradiography as described previously (24). Arrows indicate STAT3 homodimer DNA complexes. Anti-STAT3 polyclonal antibodies (Santa Cruz Biotechnology Inc.) were used in supershift assays. One microliter of antibody was incubated with nuclear extract for 20 min at room temperature prior to addition of radiolabeled probe and performance of PAGE. Addition of a nonspecific antibody had no effect on DNA binding activity (data not shown). Nuclear proteins (4 μg) extracted from NIH 3T3 cells transformed with the v-src oncogene were used as a positive control. The asterisk indicates supershifted STAT3. Vector, cells were transfected with pZeo vector DNA.
Lck activation in T cells occurs when Lck is both myristylated and palmitoylated (2, 32, 43). This suggests that Lck must be localized to the plasma membrane, perhaps for interaction with other proteins. However, here we showed that activation of Lck by Tip-484 occurs in the absence of cell membrane and other eukaryotic proteins. GST-Lck fusion proteins were used to determine the regions of Tip-484 which can activate Lck in vitro. In this study, a GST-Lck construct in which the N terminus of Lck was fused to GST was used; therefore, it could not be acylated. Fusion proteins were produced in E. coli, which lacks most of the posttranslational activity found in eukaryotic cells. We found no evidence of activation of Lck by E. coli lysates, and the use of affinity-purified fusion proteins allowed for easier interpretation of results. Earlier studies showed that the LBD is the only region of Tip-484 responsible for interaction with Lck (19, 23). In the present study, we found that the LBD is the only domain required for activation of Lck and that it is just as potent an activator as full-length Tip-484. This provided evidence that Tip can activate Lck in the absence of all other components of T cells.
Members of the Src kinase family are activated via a dephosphorylation event at the C-terminal tyrosine residue (tyrosine 505 of Lck) and autophosphorylation of a tyrosine located near the middle of the protein (tyrosine 394 of Lck) (for a review, see reference 8). Recent evidence suggests that phosphorylation at tyrosine 394 is the more potent regulator of activation, since inactive non-membrane-bound forms of Lck can be fully activated by phosphorylation at this tyrosine residue (15, 42). It is thought that activation of Lck by phosphorylation of tyrosine 394 is an intermolecular event, as opposed to intramolecular phosphorylation. Lck can transphosphorylate tyrosine 394, but it is also possible that other tyrosine kinases can perform a similar function. We speculate that the LBD binds Lck and induces a conformational change that makes Lck a better substrate for transphosphorylation of tyrosine 394 by a neighboring Lck protein and that this leads to activation of Lck kinase activity. Further studies will have to be done to verify this specific mechanism of activation.
A Tip-484 homologue termed Tip-488 exists in HVS strain 488. The function of Tip-488 has been the subject of some debate, since data indicative of both activation and downregulation of Lck have been reported (20, 30, 39). Tip-488 is highly homologous to Tip-484 but contains a 51-amino-acid N-terminal duplication. While Tip-484 has been consistently shown to activate Lck, the duplication in Tip-488 may alter its conformation and, hence, its binding affinity for other proteins, allowing it to function differently from Tip-484. Mutations in either the CSKH or SH3B region of Tip-488 abolish binding to Lck (19). A recent study showed that a mutated SH3B region of Tip-488 does not activate Lck but that an HVS-488 recombinant containing the mutant Tip-488 was still able to transform primate lymphocytes and give rise to lymphomas in New World monkeys (10). Tip-484 has not been tested in this manner. Another possible explanation for the difference in data involves the specificity of Lck antibodies. We have found that in vitro kinase assays are highly dependent on the antibody used for immunoprecipitation. There are significant differences between lots as well as commercial preparations of antibodies. Some antibodies interfere with Tip-484 binding and activation of Lck (data not shown). We routinely test our antibodies to ensure that they do not interfere with kinase activation of Lck. The presence of Tip-484 or Tip-488 has been shown to be required for viral transformation (9, 24). Therefore, evaluation of activation of Lck by Tip-488 with several different antibodies is warranted.
Our previous studies found an association between transformation by HVS and Lck-mediated STAT activation (24). HVS Tip-484 alone was shown to activate STAT DNA binding activity in vivo (24). In the present study, we showed that the only region of Tip-484 required for activation of STAT3 in vivo is the LBD. Other transforming viruses have been shown to constitutively activate STATs. Human T-cell lymphotropic virus type 1-transformed cells exhibit constitutive activation of the Jak-STAT pathway (29). Epstein-Barr virus transformation has also been shown to lead to uncontrolled STAT activation (38). Perhaps the best-characterized activation of STATs is that by the Rous sarcoma virus v-src oncoprotein. v-src has been shown to phosphorylate and activate STAT3 (6, 41). Recently, it was found that the transforming ability of v-src is dependent on its interaction with and activation of STAT3 (5, 36). Thus, like v-src, the LBD seems to be the critical domain of Tip-484 for activation of Lck and STAT3.
The evaluation of the C-terminus construct proved to be invaluable since this construct did not activate Lck either as a fusion protein or when expressed in vivo. Although it was fully capable of binding to Lck in vitro, STAT3 binding was hindered. The lack of Lck activation in vivo was accompanied by a similar lack of stimulation of STAT3 activity. This provided strong evidence of a link between the Lck pathway and STAT3 signaling. We hypothesize that the presence of the hydrophobic C terminus linked to the LBD caused an unnatural and unfavorable conformational change in the LBD. This possibly disrupted protein-protein interdynamics required for activation of Lck. We speculate that this hinderance does not occur in the native form of Tip-484 since Tip-484 is folded correctly into a normal Lck-activating conformation.
The constitutive activation of nonreceptor protein-tyrosine kinases (NRPTKs), which include the Src family kinases, has been shown to be associated with transformation (for a review, see references 7 and 11). Disregulated NRPTKs have been linked to the constitutive activation of STATs that is found in several human malignancies of T- and B-cell origin (12, 26, 33, 34, 41). It is thought that unregulated STAT activation is a key element in the transformation process. v-src, the prototypical member of the Src family, is a constitutively activated form of a cellular protein, has the ability to transform fibroblasts, and, as mentioned earlier, directly activates STAT3 (6). Another example is the oncogenic fusion construct containing the B-cell receptor (Bcr) and NRPTK c-abl. Bcr-abl is a hallmark of chronic myelogenous leukemia (4). This NRPTK fusion protein has constitutively activated kinase activity. Bcr-abl has also been shown to constitutively activate STATs (13, 18). Finally, Lck is overexpressed in the murine T-cell line LSTRA (14). A recent study has shown the LSTRA cell line also has constitutive Jak-STAT activity (41). In vivo overexpression of Lck kinase activity has also been linked to murine and human lymphoid malignancies (1, 25, 26). Therefore, constitutive activation of NRPTKs and STATs appears to be a significant event in carcinogenesis.
Acknowledgments
This work was supported by Public Health Service grants CA 43264 and CA 75895.
We thank Cynthia Coleman and Susan Pross for assistance in the preparation of the manuscript.
REFERENCES
- 1.Abraham K, Levin S D, Marth J D, Forbush K A, Perlmutter R M. Thymic tumorigenesis induced by overexpression of p56lck. Proc Natl Acad Sci USA. 1991;88:3977–3981. doi: 10.1073/pnas.88.9.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham N, Veillette A. Activation of p56lck through mutation of a regulatory carboxy-terminal tyrosine residue requires intact sites of autophosphorylation and myristylation. Mol Cell Biol. 1990;10:5197–5206. doi: 10.1128/mcb.10.10.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson S J, Levin S D, Perlmutter R M. Involvement of the protein tyrosine kinase p56lck in T cell signaling and thymocyte development. Adv Immunol. 1994;56:151–178. doi: 10.1016/s0065-2776(08)60451-4. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Neriah Y, Daley G Q, Mes-Masson A-M, White O N, Baltimore D. The chronic myelogenous leukemia-specific P210 protein is the product of the bcr-abl hybrid gene. Science. 1986;233:212–214. doi: 10.1126/science.3460176. [DOI] [PubMed] [Google Scholar]
- 5.Bromberg J F, Horvath C M, Besser D, Lathem W W, Darnell J E., Jr Stat3 activation is required for cellular transformation by v-src. Mol Cell Biol. 1998;18:2553–2558. doi: 10.1128/mcb.18.5.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao X, Tay A, Guy G R, Tan Y H. Activation and association of Stat3 with Src in v-Src-transformed cell lines. Mol Cell Biol. 1996;16:1595–1603. doi: 10.1128/mcb.16.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collette Y, Olive D. Non-receptor protein tyrosine kinases as immune targets of viruses. Immunol Today. 1997;8:393–400. doi: 10.1016/s0167-5699(97)01104-3. [DOI] [PubMed] [Google Scholar]
- 8.Cooper J A, Howell B. The when and how of Src regulation. Cell. 1993;73:1051–1054. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- 9.Duboise S M, Guo J, Czajak S, Desrosiers R C, Jung J U. STP and Tip are essential for herpesvirus saimiri oncogenicity. J Virol. 1998;72:1308–1313. doi: 10.1128/jvi.72.2.1308-1313.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duboise S M, Lee H, Guo J, Choi J-K, Czajak S, Simon M, Desrosiers R C, Jung J U. Mutation of the Lck-binding motif of Tip enhances lymphoid cell activation by herpesivrus saimiri. J Virol. 1998;72:2607–2614. doi: 10.1128/jvi.72.4.2607-2614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunan N, Ballmer-Hofer K. Signalling by Src family kinases: lessons learnt from DNA tumor viruses. Cell Signalling. 1997;9:385–393. doi: 10.1016/s0898-6568(97)00040-5. [DOI] [PubMed] [Google Scholar]
- 12.Frank D A, Mahajan S, Ritz J. B lymphocytes from patients with chronic lymphocytic leukemia contain signal transducer and activator of transcription (STAT) 1 and STAT3 constitutively phosphorylated on serine residues. J Clin Investig. 1997;100:3140–3148. doi: 10.1172/JCI119869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frank D A, Varticovski L. BCR/abl leads to the constitutive activation of Stat proteins and shares an epitope with tyrosine phosphorylated Stats. Leukemia. 1996;10:1724–1730. [PubMed] [Google Scholar]
- 14.Garvin A M, Pawar S, Marth J D, Perlmutter R M. Structure of the murine lck gene and its rearrangement in a murine lymphoma cell line. Mol Cell Biol. 1988;8:3058–3064. doi: 10.1128/mcb.8.8.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardwick J S, Sefton B M. The activated form of the Lck tyrosine protein kinase in cells exposed to hydrogen peroxide is phosphorylated at both Tyr-394 and Tyr-505. J Biol Chem. 1997;272:25429–25432. doi: 10.1074/jbc.272.41.25429. [DOI] [PubMed] [Google Scholar]
- 16.Heim M H. The Jak-STAT pathway: specific signal transduction from cell membrane to nucleus. Eur J Clin Investig. 1996;26:1–12. doi: 10.1046/j.1365-2362.1996.103248.x. [DOI] [PubMed] [Google Scholar]
- 17.Ihle J N. STATs: signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 18.Ilaria R L, Jr, Van Etten R A. P210 and P190(BCR/ABL) induce the tyrosine phosphorylation and DNA binding activity of multiple specific STAT family members. J Biol Chem. 1996;271:31704–31710. doi: 10.1074/jbc.271.49.31704. [DOI] [PubMed] [Google Scholar]
- 19.Jung J U, Lang S M, Friedrich U, Jun T, Roberts T M, Desrosiers R C, Biesinger B. Identification of Lck-binding elements in Tip of herpesvirus saimiri. J Biol Chem. 1995;270:20660–20667. doi: 10.1074/jbc.270.35.20660. [DOI] [PubMed] [Google Scholar]
- 20.Jung J U, Lang S M, Jun T, Roberts T M, Veillette A, Desrosiers R C. Downregulation of Lck-mediated signal transduction by tip of herpesvirus saimiri. J Virol. 1995;69:7814–7822. doi: 10.1128/jvi.69.12.7814-7822.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karnitz L, Sutor S L, Torigoe T, Reed J C, Bell M P, McKean D J, Leibson P J, Abraham R T. Effects of p56lck deficiency on the growth and cytolytic effector function of an interleukin-2-dependent cytotoxic T-cell line. Mol Cell Biol. 1992;12:4521–4530. doi: 10.1128/mcb.12.10.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lund T, Medveczky M M, Medveczky P G. Herpesvirus saimiri Tip-484 membrane protein markedly increases p56lck activity in T cells. J Virol. 1997;71:378–382. doi: 10.1128/jvi.71.1.378-382.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lund T, Medveczky M M, Neame P J, Medveczky P G. A herpesvirus saimiri membrane protein required for interleukin-2 independence forms a stable complex with p56lck. J Virol. 1996;70:600–606. doi: 10.1128/jvi.70.1.600-606.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lund T C, Garcia R, Medveczky M M, Jove R, Medveczky P G. Activation of STAT transcription factors by herpesvirus saimiri Tip-484 requires p56lck. J Virol. 1997;71:6677–6682. doi: 10.1128/jvi.71.9.6677-6682.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marth J, Disteche C, Pravtcheva D, Ruddle F, Krebs E, Perlmutter R. Localization of a lymphocyte-specific protein tyrosine kinase gene (lck) at a site of frequent chromosomal abnormalities in human lymphomas. Proc Natl Acad Sci USA. 1986;83:7400–7404. doi: 10.1073/pnas.83.19.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marth J D, Cooper J A, King C S, Ziegler S F, Tinker D A, Overell R W, Krebs E G, Perlmutter R M. Neoplastic transformation induced by an activated lymphocyte-specific protein tyrosine kinase (pp56lck) Mol Cell Biol. 1988;8:540–550. doi: 10.1128/mcb.8.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medveczky M M, Geck P, Sullivan J L, Serbousek D, Djeu J Y, Medveczky P. IL-2 independent growth and cytotoxicity of herpesvirus saimiri-infected human CD8 cells and involvement of two open reading frame sequences of the virus. Virology. 1993;196:402–412. doi: 10.1006/viro.1993.1495. [DOI] [PubMed] [Google Scholar]
- 28.Medveczky P G. Oncogenic transformation of T cells by Herpesvirus saimiri. In: Barbanti-Brodano G, et al., editors. DNA tumor viruses: oncogenic mechanisms. New York, N.Y: Plenum Press; 1995. pp. 239–252. [Google Scholar]
- 29.Migone T-S, Lin J-X, Ceresto A, Mulloy J C, O’Shea J J, Franchini G, Leonard W J. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-1. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- 30.Noraz N, Saha K, Ottones F, Smith S, Taylor N. Constitutive activation of TCR signaling molecules in IL-2-independent herpesvirus saimiri-transformed cells. J Immunol. 1998;160:2042–2045. [PubMed] [Google Scholar]
- 31.Ravichandran K S, Collins T L, Burakoff S J. CD4 and signal transduction. Curr Top Microbiol Immunol. 1996;205:47–62. doi: 10.1007/978-3-642-79798-9_3. [DOI] [PubMed] [Google Scholar]
- 32.Resh M D. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994;76:411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 33.Shuai K, Halpern J, Ten Hoeve J, Rao X, Sawyers C L. Constitutive activation of STAT5 by the BCR-ABL oncogene in chronic myelogenous leukemia. Leukemia. 1996;13:247–254. [PubMed] [Google Scholar]
- 34.Takemoto S, Mulloy J C, Ceresto A, Migone T-S, Patel B K R, Matsuoka M, Yamagushi K, Takatsuki K, Kamihira S, White J D, Leonard W J, Waldmann T, Franchini G. Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc Natl Acad Sci USA. 1997;94:13897–13902. doi: 10.1073/pnas.94.25.13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thome M, Germain V, DiSanto J P, Acuto O. The p56lck SH2 domain mediates recruitment of CD8/p56lck to the activated T cell receptor/CD3/zeta complex. Eur J Immunol. 1996;26:2093–2100. doi: 10.1002/eji.1830260920. [DOI] [PubMed] [Google Scholar]
- 36.Turkson J, Bowman T, Garcia R, Caldenhoven E, De Groot R P, Jove R. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol Cell Biol. 1998;18:2545–2552. doi: 10.1128/mcb.18.5.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner B J, Hayes T E, Hoban C J, Cochran B H. The sif binding element confers sis/PDGF inducibility onto the c-fos promoter. EMBO J. 1990;9:4477–4484. doi: 10.1002/j.1460-2075.1990.tb07898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber-Nordt R M, Egen C, Wehinger J, Ludwig W, Gouilleux-Gruart R, Mertelsmann R, Finke J. Constitutive activation of stat proteins in primary lymphoid and myeloid leukemia cells and in Epstein-Barr virus (EBV)-related lymphoma cell lines. Blood. 1996;88:809–816. [PubMed] [Google Scholar]
- 39.Weise N, Tsygankov A Y, Klauenberg U, Bolen J B, Fleischer B, Broker B M. Selective activation of T cell kinase p56lck by herpesvirus saimiri protein Tip. J Biol Chem. 1996;271:847–852. doi: 10.1074/jbc.271.2.847. [DOI] [PubMed] [Google Scholar]
- 40.Yu C-L, Meyer D J, Campbell G S, Larner A C, Carter-Su C, Schwartz J, Jove R. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science. 1995;269:81–83. doi: 10.1126/science.7541555. [DOI] [PubMed] [Google Scholar]
- 41.Yu C-L, Jove R, Burakoff S J. Constitutive activation of the Janus kinase-STAT pathway in T lymphoma overexpressing the Lck protein tyrosine kinase. J Immunol. 1997;159:5206–5210. [PubMed] [Google Scholar]
- 42.Yurchak L K, Hardwick J S, Amrein K, Pierno K, Sefton B M. Stimulation of phosphorylation of Tyr-394 by hydrogen peroxide reactivates biologically inactive, non-membrane-bound forms of Lck. J Biol Chem. 1996;271:12549–12554. doi: 10.1074/jbc.271.21.12549. [DOI] [PubMed] [Google Scholar]
- 43.Yurchak L K, Sefton B M. Palmitoylation of either Cys-3 or Cys-5 is required for the biological activity of the Lck tyrosine protein kinase. Mol Cell Biol. 1995;15:6914–6922. doi: 10.1128/mcb.15.12.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]