Abstract
Background:
Pediatric musculoskeletal (MSK) infections broadly include isolated osteomyelitis (OM), septic arthritis (SA), and combined infections (OM+SA). These diagnoses are often monitored with serum inflammatory markers and serial radiographs to monitor treatment response and development of negative sequelae, despite limited data supporting these practices. The purpose of this study is to evaluate the utility of obtaining serial radiographic follow-up for pediatric osteoarticular infections.
Methods:
An institutional review board–approved retrospective review was completed. Children 18 years and below admitted to a single institution with a culture/biopsy-proven diagnosis of OM, SA, or OM+SA. All postdischarge radiographs were reviewed and retrospectively categorized as either routine (scheduled) or reactive. Routine radiographs were obtained regardless of clinical presentation. Reactive radiographs were obtained in patients presenting with the sign of an altered clinical course. Negative sequelae, defined as growth arrest/disturbance, pathologic fracture, recurrent MSK infection, and underlying neoplastic process, were recorded and tracked. Descriptive statistics were used to summarize demographic and outcome variables. Number needed to screen (NNS) was defined as the inverse of the incidence of negative sequelae detected.
Results:
A total of 131 patients were included for analysis, with a mean age of 11.9 years (SD: 4.96 y). Ninety (69%) patients were diagnosed and treated for OM, 25 (19%) for SA, and 16 (12%) for combined infections. A total of 329 radiographs were obtained following discharge. Of those obtained, 287 (88%) were routine, resulting in the detection of 2 (0.7%) negative sequelae and a resultant NNS of 143 radiographs (95% confidence interval: 36–573). The remaining 39 were reactive radiographs, resulting in the detection of 2 (5.1%) negative sequelae with an NNS of 20 radiographs (95% confidence interval: 5–78).
Conclusions:
While radiographs remain a widely utilized tool to screen for the development of negative sequelae in pediatric osteoarticular infections, they rarely alter management in the absence of other concerning clinical signs or symptoms such as recurrent fevers, swelling of the extremity, or limb deformity. Moreover, routine radiographic surveillance should be replaced with a reactive radiographic protocol.
Level of Evidence:
Level III—retrospective comparative study.
Keywords: musculoskeletal infection, osteomyelitis, septic arthritis, radiographic monitoring
Musculoskeletal (MSK) infection, including osteomyelitis (OM), septic arthritis (SA), and combined infections (OM+SA), are common diagnoses in the pediatric population, comprising roughly 1.8/1000 of all pediatric emergency department visits per year.1 While early diagnosis and treatment has reduced the morbidity and mortality associated with these conditions, there remains a significant health burden in this populations given the persistent risk of developing negative sequelae, including recurrent infection, pathologic fracture, and growth disturbance or arrest.2–5 Given the prolonged time course for recovery from MSK infections, these negative sequelae often occur long after the index hospitalization. Approximately 20% of negative sequelae are detected after 12 months following initial diagnosis.6 In an effort to identify negative sequelae early and avoid catastrophic outcomes, long-term clinical follow-up is often accompanied by ongoing monitoring of serial serum inflammatory markers and serial radiographic follow-up.
While magnetic resonance imaging (MRI) remains widely utilized for diagnosis of osteoarticular infections given its high sensitivity (97% to 100%), high specificity (92%), and its ability to detect evidence of early infection within 2 to 5 days of disease onset,7,8 radiographs are routinely ordered at initial presentation, before obtaining advanced imaging, given their wide accessibility and relatively low cost. Further, plain radiographs are often obtained during long-term follow-up to monitor the infectious course and evaluate for adverse events.9,10 However, the time to resolution of imaging findings remain unclear.11
Moreover, the utility of obtaining serial radiographic imaging in this patient population and its ability to detect negative sequelae before the onset of clinical findings is unknown. As such, this study aims to examine the value of obtaining serial radiographs in the setting of pediatric MSK infections.
METHODS
An institutional review board–approved, level III retrospective review was completed. Children 18 years and below who were admitted to a single institution with a culture/biopsy-proven diagnosis of OM, SA, or OM+SA between 2012 and 2018 were included. Patients were excluded for follow-up of < 6 weeks following an acute hospitalization, lack of postdischarge radiographs, and for diagnosis of chronic recurrent multifocal OM. All post-discharge radiographs were reviewed and retrospectively categorized as either routine (scheduled) or reactive. Routine radiographs were defined as those obtained regardless of clinical presentation. Reactive radiographs were defined as those obtained in patients presenting with persistent or recurrent pain, injury, fever, swelling, limb deformity, or elevation of inflammatory markers. Radiographs were evaluated for negative sequelae by 2 separate authors in a blinded fashion to the patient’s history.
Negative sequelae, defined as growth arrest/disturbance, pathologic fracture, recurrent MSK infection, and underlying neoplastic process identified via radiographs, were recorded and tracked in all included patients. Descriptive statistics were utilized to summarize demographic and outcome variables. Number needed to screen (NNS) was defined as the inverse of the incidence of negative sequelae detected via routine and reactive radiographs. Two-tailed Student t tests and Mann-Whitney rank-sum tests were used to analyze differences within normally and non-normally distributed variables, respectively. Statistical significance was defined as P-value < 0.05. Statistical analyses were performed using R 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
A total of 168 patients were initially identified, of which 131 patients were included for analysis (36 excluded for lack of plain radiographs, 3 excluded for diagnosis of chronic recurrent multifocal OM, and 1 excluded for concomitant screening MRIs). The resultant cohort had a mean age of 11.9 years (SD = 4.99 y), of which 93 were male (71%), and 90 (69%) had a diagnosis of OM, 25 (19%) SA, and 16 (12%) combined infections. A total of 329 screening radiographic series were obtained of the affected area following discharge (average: 2.5 per patient). Forty-eight (37%) patients had 1 follow-up radiograph, 34 (26%) had 2, 21 (16%) had 3, and 28 (21%) had 4 or more (Table 1). Patients with OM had an average of 2.57 radiographs per patient, those with combined infections had 3.43, and those with SA had 1.72. Patients with SA had significantly fewer follow-up radiographs than those with OM or combined infections (P = 0.007).
TABLE 1.
Patients’ Demographic Information
| Variables | Patients [n (%)] |
|---|---|
| Infection | |
| OM | 90 (69) |
| SA | 25 (19) |
| SA+OM | 16 (12) |
| No. follow-up radiographs | |
| 1 | 48 (37) |
| 2 | 34 (26) |
| 3 | 21 (16) |
| 4+ | 28 (21) |
| Routine monitoring | |
| No. radiographs | 287 |
| Negative sequelae | 2 |
| Reactive monitoring | |
| No. radiographs | 39 |
| Negative sequelae | 2 |
OM indicates osteomyelitis; SA, septic arthritis.
Patients were clinically followed for an average of 10.2 months (SD = 16.3 mo) by an orthopaedic surgeon, or infectious disease specialist focused on the evaluation of the index infection. There was great variability in follow-up duration (0.4 to 85.6 mo). Likewise, the duration of radiographic follow-up was quite variable, with an average of 8.9 months (SD = 14.8 mo). At a minimum, all patients had 1 follow-up visit, and all were followed for 6 or more weeks following discharge; however, 29 (22%) patients were lost to follow-up during their treatment course.
Of the 329 total screening radiographic series, a total of 287 (88%) routine radiographs were obtained, resulting in the detection of 2 (0.7%) negative sequelae. The number of routine radiographs required to detect 1 adverse outcome was 143 [95% confidence interval (CI): 36–573]. Routine monitoring was performed in 127 patients, resulting in number of patients required to monitor with routine radiographs to detect 1 adverse outcome of 64 (95% CI: 16–253). Routine radiographs detected 1 pathologic fracture and 1 instances of growth arrest (Fig. 1), which were detected on days 9 and 198 postdischarge, respectively.
FIGURE 1.
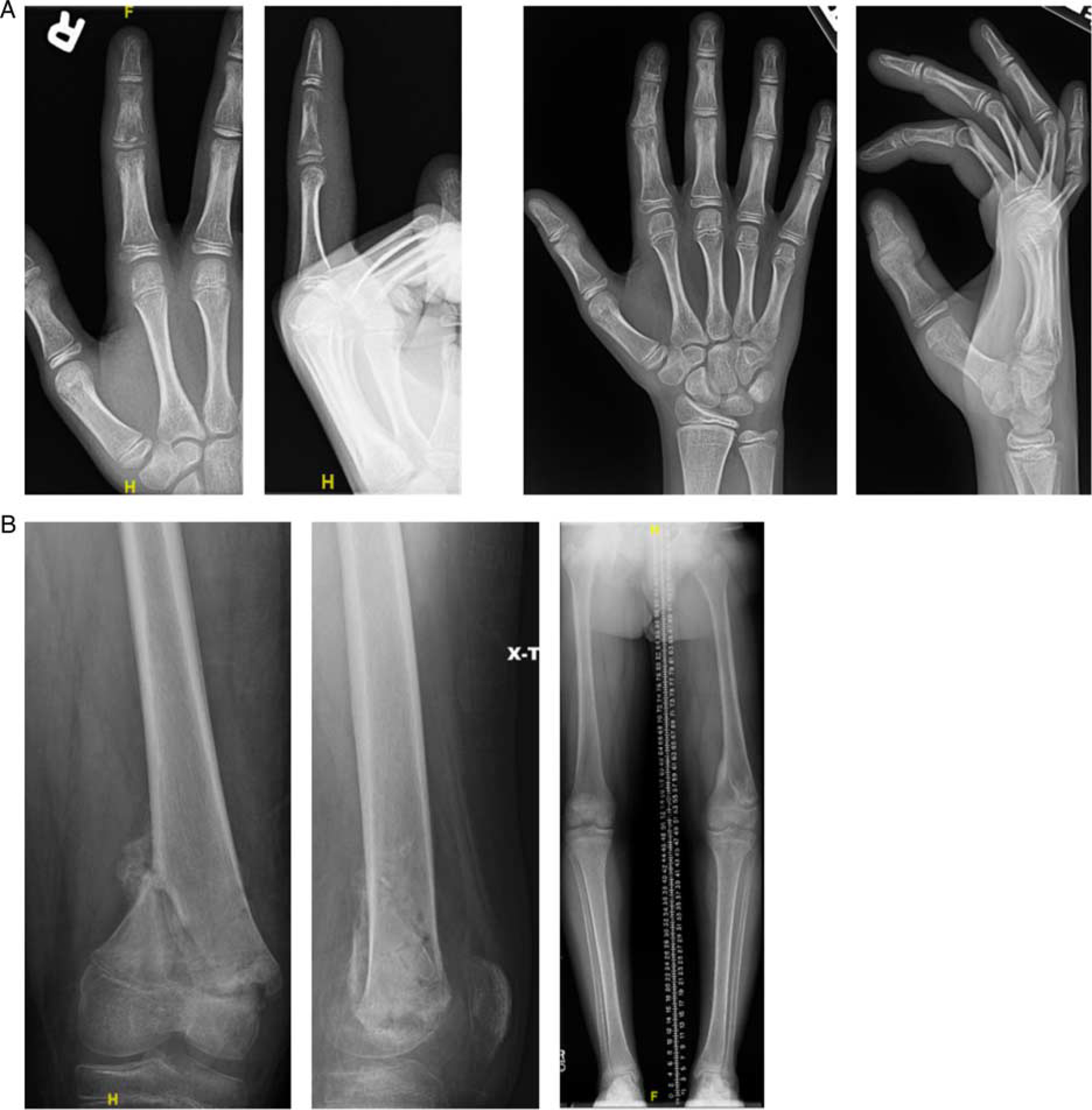
Negative sequelae of routine radiographs. A, Index finger osteomyelitis (left) and resultant ulnar deviation from middle phalanx growth arrest (right). B, Distal femoral osteomyelitis (left) and resultant leg length discrepancy (right).
The remaining 39 radiographic series obtained were reactive, resulting in the detection of 2 (5.1%) negative sequelae. Factors that prompted obtaining reactive radiographs included recurrent pain, limping, fevers, swelling, and local erythema. The resultant number of reactive radiographs required to detect 1 adverse outcome was 20 (95% CI: 5–78). These 39 reactive radiographs were obtained in 26 patients, resulting in an NNS of 13 (95% CI: 4–52). Reactive radiographs identified 1 recurrent infection and 1 Ewing sarcoma (Fig. 2), which were detected on days 56 and 546 postdischarge, respectively.
FIGURE 2.

A, Mid-diaphyseal Ewing sarcoma. B, Recurrent distal tibia osteomyelitis.
DISCUSSION
Pediatric MSK infections remain a significant concern for orthopaedic surgeons, given the potential for adverse events including growth arrest, recurrent infection, and pathologic fracture. The prevalence of these negative sequelae is decreasing despite an increase in MSK infections in this population, in large part due to early detection and aggressive treatment.12 As seen in this population, these adverse events often occur months after the index infection. As such, long-term clinical follow-up is necessary following acute eradication of infection. Stratifying which patients require longer follow-up poses a challenge to physicians given the heterogeneity in location, microorganism, and disease severity. Recent work has demonstrated the utility of considering risk factors such as C-reactive protein elevation, age, and persistent fevers to predict disease severity and guide follow-up duration, but given the limited number of adverse events identified in this cohort, this series cannot guide the length of clinical follow-up.13 Rather, its aim is to determine the utility of routine radiographic follow-up in these patients, regardless of duration. In fact, while the diagnostic utility of plain radiographs and inflammatory markers in this setting is well-established, there is no consensus on the role of obtaining routine radiographs in monitoring for treatment response or the presence of adverse outcomes in this patient population, despite their common use. In our series of 131 patients, we found that routine radiographs in the absence of clinical findings rarely identified adverse outcomes or negative sequelae and that laboartory values are not necessarily predictive of radiographic adverse outcomes, despite their utility in monitoring acute and subacute response to treatment.
In fact, the diagnosis of pediatric MSK infections remains largely clinical. While there is ultrasonography and MRI are effective at confirming the diagnosis, the utility of plain radiographs in diagnosing acute OM is limited as they do not commonly reveal osseous changes within the first week of symptom onset.14 Moreover, the osseous changes that become apparent on plain radiographs (periosteal thickening, lytic lesions, and loss of trabecular architecture) are persistently present for weeks following the initiation of treatment.15 As such, their usefulness in monitoring response to treatment is questionable. Clinical presentation was the most sensitive marker for an altered course necessitating further workup including radiographs as all adverse events presented with clinical findings concerning for an altered clinical course. Differentiation of an active versus an inactive lesion on plain radiographs is difficult and rarely alters management in the absence of additional imaging or changes in symptoms.16 Despite this, plain radiographs are often included as part of the initial workup and follow-up for this patient population.
In this current cohort, only 2 adverse events were detected via routine imaging with an NNS of 143 radiographic series. The pathologic fracture was treated with splint immobilization, which went onto union without further sequelae. The growth arrest was treated with drill epiphysiodesis of the contralateral limb to prevent subsequent leg length discrepancy. Of the 2 adverse events detected via routine radiographs, both presented with notable clinical signs and symptoms (persistent pain and gross deformity) of an altered clinical course detected during the clinical examination after radiographs had been obtained. These clinical findings would have prompted further investigation (reactionary radiographs) and would resultantly have been identified in the absence of a routine radiographic protocol. In that light, we recommend obtaining reactive radiographs when there is evidence of an altered clinical course as opposed to following all patients with routine radiographs indiscriminately. Of note, the 1 patient excluded for screening MRI was found to have a recurrent infection; the MRIs were obtained for persistent pain noted during clinical follow-up.
There are notable limitations impacting the design and results of this study. As mentioned above, the small number of negative sequelae detected limited the power and generalizability of the gathered data. While the average length of follow-up was adequate to capture most adverse events, there was significant variability in this patient population and a 22% loss of follow-up. This lack of consistent follow-up may have limited the ability to capture and account for all negative sequelae. There was also heterogeneity within the adverse events, which can differently impact the radiographic, laboratory, and clinical presentation. Most importantly, this was a retrospective review, and radiographs were obtained based on the preference of 5 treating pediatric orthopaedic surgeons, and there was much heterogeneity in the follow-up schedule and protocol. A larger randomized control study and cost analysis are necessary to definitively determine the value of routine radiographs in this patient population. However, these results are the first step in identifying probable overutilization in this patient population.
Plain radiographs remain a widely utilized tool to monitor response to treatment in osteoarticular infections, but they rarely alter management in the absence of other signs or symptoms of negative sequelae and significantly increase radiation burden, health care utilization, and costs. In our relatively large series of pediatric MSK infections, all patients with radiographically detected negative sequelae had clinical signs and/or symptoms consistent with an altered course, negating the need for routine radiographic surveillance and highlighting the utility of reactive radiographs. As such, treating orthopaedic surgeons are encouraged to shift from a routine to a reactive radiographic protocol following osteoarticular pediatric infections.
Footnotes
All authors involved in the preparation of this manuscript received no outside funding or support and have nothing to disclose.
The authors declare no conflicts of interest.
REFERENCES
- 1.Koehler R, Baldwin KD, Copley LA, et al. The epidemiology and regional burden of musculoskeletal infection in pediatric orthopaedics. Pediatrics 2018;142(1 Meeting Abstract):259. [Google Scholar]
- 2.Cole WG, Dalziel RE, Leitl S. Treatment of acute osteomyelitis in childhood. J Bone Joint Surg Br 1982;64:218–223. [DOI] [PubMed] [Google Scholar]
- 3.Craigen MAC, Watters J, Hackett JS. The changing epidemiology of osteomyelitis in children. J Bone Joint Surg Br 1992;74:541–545. [DOI] [PubMed] [Google Scholar]
- 4.Malcius D, Barauskas V, Ukuraite R. Some aspects of long-term results of treatment of acute hematogenous osteomyelitis. Medicina (Kaunas) 2007;43:472–477. [PubMed] [Google Scholar]
- 5.Wang CL, Wang SM, Yanq YJ, et al. Septic arthritis in children: relationship of causative pathogens, complications, and outcome. J Microbiol Immunol Infect 2003;36:41–44. [PubMed] [Google Scholar]
- 6.Gillespie R, Mayo KM. The management of acute hematogenous osteomyelitis in the antibiotic era: a study of the outcome. J Bone Joint Surg Br 1981;63:126–131. [DOI] [PubMed] [Google Scholar]
- 7.Iliadis AD, Ramachandran M. Paediatric bone and joint infections. EFORT Open Rev 2017;2:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saavedra-Lozano J, Falup-Pecurariu O, Faust SN, et al. Bone and joint infections. Pediatr Infect Dis J 2017;36:788–799. [DOI] [PubMed] [Google Scholar]
- 9.Frank G, Mahoney HM, Eppes SC. Musculoskeletal infections in children. Pediatr Clin North Am 2005;52:1083–1106; ix. [DOI] [PubMed] [Google Scholar]
- 10.Ranson M Imaging of pediatric musculoskeletal infection. Semin Musculoskelet Radiol 2009;13:277–279. [DOI] [PubMed] [Google Scholar]
- 11.Peltola H, Pääkkönen M. Acute osteomyelitis in children. N Engl J Med 2014;370:352–360. [DOI] [PubMed] [Google Scholar]
- 12.Pääkkönen M Septic arthritis in children: diagnosis and treatment. Pediatric Health Med Ther 2017;8:65–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manz N, Krieg AH, Buettcher M, et al. Long-term outcomes of acute osteoarticular infections in children. Front Pediatr 2020;8:587740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodley DR. Managing musculoskeletal infections in children in the era of increasing bacterial resistance. JAAPA 2015;28:24–29. [DOI] [PubMed] [Google Scholar]
- 15.Pineda C, Espinosa R, Pena A. Radiographic imaging in osteomyelitis: the role of plain radiography, computed tomography, ultrasonography, magnetic resonance imaging, and scintigraphy. Semin Plast Surg 2009;23:080–089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Schuppen J, van Doorn MMAC, van Rijn RR. Childhood osteomyelitis: imaging characteristics. Insights Imaging 2012;3:519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]


