ABSTRACT
Eosinophilic colitis is a rare condition characterized by histologic findings of high eosinophilic infiltrate in the gut wall, typically presenting with diarrhea and abdominal pain. The etiology of this entity remains unclear because it can be primary or can occur secondarily to infections, drugs, or even in association with immune-mediated diseases. We present the case of a woman referred to our outpatient clinic for chronic diarrhea that had been worsening for months. Colonoscopy with biopsies was performed, and eosinophilic colitis associated with the use of clopidogrel was diagnosed. After clopidogrel discontinuation, a complete remission of the clinical and histological picture was observed.
KEYWORDS: eosinophilic gastrointestinal disease, antiplatelet drugs, histology
INTRODUCTION
Eosinophilic colitis (EC) is a rare condition included in the broader eosinophilic gastrointestinal disorders (EGIDs) and is characterized by an abnormal infiltrate of the colonic wall by eosinophilic polymorphonuclear cells in symptomatic patients. Symptoms reported by patients diagnosed with EC include diarrhea, abdominal pain, weight loss, and malabsorption.
The etiology of this condition remains unclear: The primary form is likely due to the interaction between genetic and environmental factors because allergic diseases are strictly associated with EGIDs in most patients. While primary EC is a diagnosis of exclusion, secondary forms of EC can occur in association with various conditions such as infections, drugs, hematologic disorders, inflammatory bowel diseases, celiac disease, and other immune-mediated conditions. In this report, we present a case of EC secondary to the use of clopidogrel.
CASE REPORTS
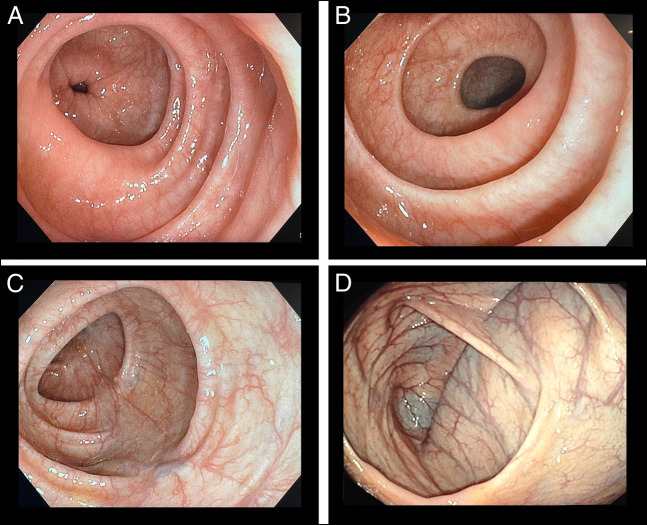
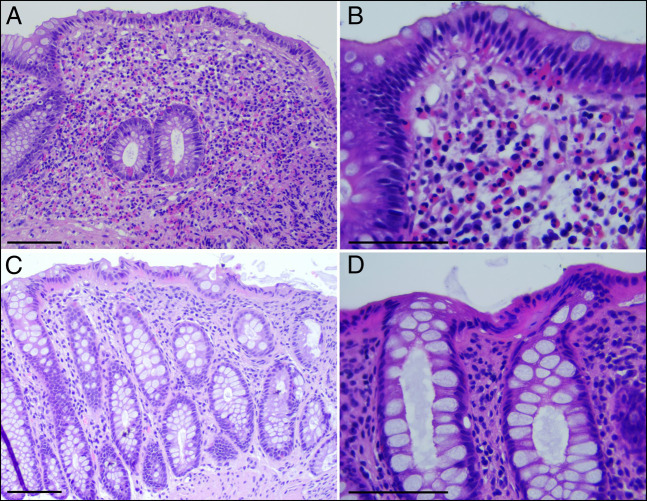
In April 2022, a 67-year-old woman was referred to our outpatient clinic for chronic diarrhea and occasional abdominal pain present for several years, showing a worsening trend since July 2021. In the past, the presence of bacterial and parasitic infections, inflammatory bowel diseases, celiac disease, and pancreatic disease had been ruled out. Complete laboratory workup was unremarkable with a normal eosinophilic count (180 cells/μL). Colonoscopy showed macroscopically normal appearance of the mucosa, and random biopsies were obtained in the ascending, transverse, descending, and sigmoid colon to exclude microscopic colitis (Figure 1). Histological examination reported features suggestive of EC, with preserved mucosal architecture and lamina propria with no lymphoplasmacellular infiltrate, but with a marked increase in eosinophils (ie, >60/high-power field [HPF]; transverse colon: 197 HPF; descending colon: 147 HPF; 1 HPF corresponds to 0.196 mm2) and focal eosinophilic cryptitis (Figure 2).
Figure 1.
Normal mucosa appearance in the (A) sigmoid, (B) ascending, (C) transverse, and (D) cecum colon.
Figure 2.
(A and B) A hematoxylin and eosin-stained section of the large bowel mucosa obtained from the first colonoscopy. Biopsies are from the transverse colon showing numerous eosinophils in the lamina propria as well as in the surface and crypt epithelium. Scale bar 50 μm (A) and 25 μm (B). (C and D) Hematoxylin and eosin-stained sections of biopsies from the descending colon obtained during the second colonoscopy, after clopidogrel suspension, showing marked reduction of lamina propria eosinophils; these are rare and are never associated with crypts or surface epithelium. Scale bar 50 μm (A) and 25 μm (B).
Histology was diagnostic for EC, and before planning treatment, she also underwent upper digestive endoscopy with gastric, duodenal, and esophageal biopsies, whose results were completely normal, thus excluding the possible diagnosis of eosinophilic gastroenteritis. Although the patient's history was unremarkable, she also underwent skin allergy tests that excluded atopic disease. Hence, a potential iatrogenic etiology of EC was explored, with coprescriptions including levotiroxine for Plummer disease, bisoprolol for arterial hypertension, teriflunomide for multiple sclerosis, and clopidogrel for prophylaxis of cerebrovascular events (this last comedication was started in April 2021).
A literature review identified a potential association between clopidogrel use and occurrence of EC, and in this patient, there was also a temporary concomitance between the introduction of the drug and the onset of diarrhea.1 Thus, following the advice of the treating cardiologist, clopidogrel was discontinued, without prescription of additional treatment of abdominal pain and diarrhea. After clopidogrel discontinuation, the patient experienced resolution of diarrhea and remission of abdominal pain. Moreover, histology obtained in the course of endoscopic examination performed 6 months after the withdrawal of clopidogrel found no significant inflammatory or structural mucosal changes, notably with no evidence of significant eosinophilic infiltrate (Figure 2).
DISCUSSION
Despite the increasing amount of literature concerning eosinophilic esophagitis, other EGIDs represent a rare group of conditions, and according to the little data available about their epidemiology, they can occur at all ages with a large spectrum of clinical presentation. Among them, EC is the least common with an estimated prevalence of 3 in 100,000.2 The presentation of EGIDs strongly depends on the location of the aberrant eosinophilic infiltrate. Eosinophilic gastritis, for example, presents with nausea and vomiting while symptoms of EC are mostly diarrhea and abdominal pain.3
The etiology and pathogenesis of primary EGIDs are not fully understood, but an exaggerated TH2-type immune response to airborne and food antigens seems to play a key role in the occurrence of these diseases, resulting in tissue damage, repair, remodeling, and disease persistence.4,5 A significant interaction between genetic and environmental factors likely plays a role in determining this group of diseases because approximately 16% of patients have family members with similar conditions.6,7 Laboratory testing has a limited role in EC diagnosis, although, unlike in patients with eosinophilic esophagitis, up to 80% of these patients can have peripheral eosinophilia and a low albumin level.5 Contrarily, colonoscopy could be useful because, despite a normal-appearing mucosa, histology may help provide a diagnosis. As a fact, although a clear cutoff for normal eosinophilic infiltrate in the gut wall is not yet available and pathologic criteria are not standardized, a key role is represented by expertise and knowledge of the pathologist: Number of eosinophilic infiltration in the lamina propria >50/HPF in the right colon, >35/HPF in the transverse colon, and >25/HPF in the left colon seems to represent a valid cutoff to support a diagnosis of EC.7,8
In the case of positive histology, the differential diagnostic process includes infections; use of drugs; and the presence of inflammatory bowel diseases, autoimmune diseases, or hematological diseases.6 In our case, all the potential comorbidities were excluded and a form secondary to the use of drugs was hypothesized. Known drug triggers of EC reported in the literature are nonsteroidal anti-inflammatory drugs, antiplatelet drugs, tacrolimus, and carbamazepine.1,7–11 A study by Casella et al demonstrated an association between the use of antiplatelets (and other drugs) and an increased eosinophilic infiltration limited to the descending and sigmoid colon, even without symptoms, and in our patient, we decided—after specialist consultation—to discontinue clopidogrel.11 After drug withdrawal, our patient experienced a rapid remission of symptoms that supported our suspicion of clopidogrel-induced EC, and histology obtained during endoscopy performed 3 months after drug discontinuation confirmed this hypothesis.
Owing to the rarity of EC, randomized trials are currently not available to base therapeutic strategies on. Diet modification and the use of corticosteroids are the most common treatments prescribed to these patients, so far. In this case, drug withdrawal led to both symptomatic and histological resolution, and no specific pharmacological therapy was additionally required.
In conclusion, EC is a gastrointestinal disease that should be included in the differential diagnosis when a patient treated with clopidogrel or another antiplatelet drug reports chronic diarrhea and abdominal pain. Adequate histological evaluation is of essence to confirm the diagnosis, and a trial of drug discontinuation—if possible—is suggested before the prescription of corticosteroids.
DISCLOSURES
Author contributions: conception: S. Djahandideh Sheijani and E. Marabotto; supervision: E. Marabotto, EV Savarino, V. Savarino, and EG Giannini; data collection and processing: F. Calabrese, A. Pasta, G. Bodini, and M. Furnari; analysis and interpretation: S. Djahandideh Sheijani, E. Marabotto, F. Grillo, L. Mastracci, EV Savarino, V. Savarino, and EG Giannini; literature review: S. Djahandideh Sheijani and E. Marabotto; written original draft: S. Djahandideh Sheijani, F. Calabrese, A. Pasta, and E. Marabotto; and critical review: EV Savarino, V. Savarino, and EG Giannini. EG Giannini is the article guarantor.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Contributor Information
Shirin Djahandideh Sheijani, Email: shirindjahandideh@hotmail.it.
Francesco Calabrese, Email: calabrese.francesco.93@gmail.com.
Andrea Pasta, Email: andreapasta93@gmail.com.
Elisa Marabotto, Email: elisa.marabotto@unige.it.
Giorgia Bodini, Email: Giorgia.Bodini@unige.it.
Manuele Furnari, Email: Manuele.Furnari@unige.it.
Federica Grillo, Email: Federica.Grillo@unige.it.
Luca Mastracci, Email: Luca.Mastracci@unige.it.
Edoardo V. Savarino, Email: Edoardo.Savarino@unipd.it.
Vincenzo Savarino, Email: vsavarin@unige.it.
REFERENCES
- 1.Wang F, Han J. Delayed eosinophilic gastroenteritis, a possible side effect of clopidogrel? Int J Cardiol. 2013;165(3):e53–4. [DOI] [PubMed] [Google Scholar]
- 2.Dellon ES, Spergel JM. Biologics in eosinophilic gastrointestinal diseases. Ann Allergy Asthma Immunol. 2023;130(1):21–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinbach EC, Hernandez M, Dellon ES. Eosinophilic esophagitis and the eosinophilic gastrointestinal diseases: Approach to diagnosis and management. J Allergy Clin Immunol Pract. 2018;6(5):1483–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wechsler ME, Munitz A, Ackerman SJ, et al. Eosinophils in health and disease: A state-of-the-art review. Mayo Clinic Proc. 2021;96(10):2694–707. [DOI] [PubMed] [Google Scholar]
- 5.Kinoshita Y, Yahata S, Oouchi S. Eosinophilic gastrointestinal diseases: The pathogenesis, diagnosis, and treatment. Intern Med. 2023;62(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alfadda AA, Storr MA, Shaffer EA. Eosinophilic colitis: Epidemiology, clinical features, and current management. Therap Adv Gastroenterol. 2011;4(5):301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarthy AJ, Sheahan K. Classification of eosinophilic disorders of the small and large intestine. Virchows Arch. 2018;472(1):15–28. [DOI] [PubMed] [Google Scholar]
- 8.Walker MM, Potter M, Talley NJ. Eosinophilic gastroenteritis and other eosinophilic gut diseases distal to the oesophagus. Lancet Gastroenterol Hepatol. 2018;3(4):271–80. [DOI] [PubMed] [Google Scholar]
- 9.Campora M, Mastracci L, Carlin L, et al. Pathologist's approach to paediatric and neonatal eosinophilic gastrointestinal disorders. Pathologica. 2022;114:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macaigne G. Eosinophilic colitis in adults. Clin Res Hepatol Gastroenterol. 2020;44(5):630–7. [DOI] [PubMed] [Google Scholar]
- 11.Casella G, Villanacci V, Fisogni S, et al. Colonic left-side increase of eosinophils: A clue to drug-related colitis in adults. Aliment Pharmacol Ther. 2009;29(5):535–41. [DOI] [PubMed] [Google Scholar]