ABSTRACT
Squamous cell carcinoma of the biliary tract is a rare disease, comprising just 2% of all biliary malignancies. The exact etiology is poorly understood but believed to be secondary to chronic inflammation. We present a case of a patient with recurrent cholecystitis and cholangitis who developed invasive biliary squamous cell carcinoma.
KEYWORDS: biliary squamous cell carcinoma, cholangiocarcinoma, adenosquamous
INTRODUCTION
Squamous cell carcinoma (SCC) of the bile ducts is extremely rare. On review of the literature, 34 cases have been reported, of which only 24 have been confirmed histologically. The pathogenesis of biliary SCC is not well-described but is believed to be secondary to chronic inflammation. Prognosis is generally poor because patients are usually asymptomatic until advanced stages. There is no standardized treatment of biliary SCC, and treatment is based on the extent of disease. Surgery is reserved for local disease, whereas chemoradiation is recommended for metastatic disease.1–3 Nearly all patients who do not undergo surgery die within 1 year of diagnosis. We present a case of an elderly man with recurrent cholecystitis and cholangitis who was diagnosed with biliary SCC.
CASE REPORT
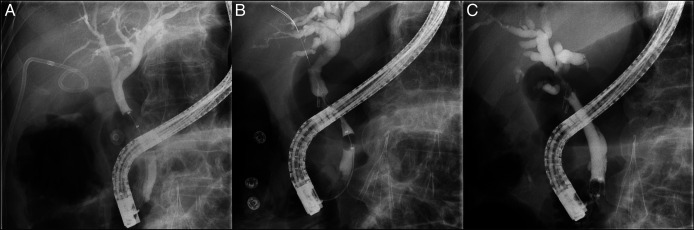
A 76-year-old man with a medical history of hypertension, hyperlipidemia, coronary artery disease, and colon cancer (status post left colectomy) presented with cholecystitis and sepsis. He was treated with a percutaneous cholecystostomy tube and antibiotics. One month later, his percutaneous cholecystectomy tube was dislodged and not replaced. Two months after his initial presentation, he presented with another episode of acute cholecystitis. A new percutaneous cholecystostomy tube was placed. Two weeks later, he returned with cholangitis. Magnetic resonance imaging showed normal contour and caliber of intrahepatic and extrahepatic biliary tree and miniscule layered stones in the distal common bile duct (CBD) without biliary ductal dilatation. Endoscopic retrograde cholangiopancreatography (ERCP) demonstrated an occluded cystic duct and mildly dilated CBD; a 10 Fr × 5 cm plastic stent was placed in the CBD (Figure 1). He underwent cholecystectomy, and gallbladder pathology showed marked acute and chronic cholecystitis. The CBD stent was removed 6 weeks after placement. Two months later, he presented with another episode of acute cholangitis. A right upper quadrant ultrasound showed new intrahepatic and extrahepatic ductal dilatation, and the CBD measured 10 mm. ERCP showed narrowing of the CBD in the region of the prior cholecystectomy clip, concerning for extrinsic compression vs narrowing due to ischemia (Figure 1).
Figure 1.
A, Fluoroscopic image from the first ERCP showing an occluded cystic duct and a mildly dilated CBD up to 8 mm. B, Fluoroscopic image from the second ERCP showing mid-CBD narrowing to 2 mm in the region of prior cholecystectomy clip placement along with upstream dilation to 8–9 mm. C, Fluoroscopic image from the third ERCP showing a 2 cm area of narrowing just below the bifurcation, with a small distance between the upper end of the structure and bifurcation, and diffusely dilated left and right main ducts, demonstrating the area of the lesion. CBD, common bile duct; ERCP, endoscopic retrograde cholangiopancreatography.
Brushings revealed atypical glandular and squamous cells in a background of acute inflammation. A 10 mm × 60 mm fully covered self-expanding metal stent was placed in the CBD.
The patient presented 1 month later with jaundice and dark urine. Laboratory test results were notable for aspartate aminotransferase 242 IU/L, alanine aminotransferase 263 IU/L, alkaline phosphatase 1,016 IU/L, total bilirubin 9.5 mg/dL (direct bilirubin 6.5 mg/dL), and white blood cell count 8.88 K/μL. ERCP demonstrated a 2 cm area of narrowing just below the bifurcation suspicious for granulation tissue or neoplasia (Figure 1). The previously placed stent was replaced with a 9 cm plastic stent. Pathology showed invasive SCC with associated glandular atypia (Figure 2). Tumor cells stained positive for p40 (confirming squamous differentiation) and CK19; CK7 and CK20 stains were negative (Figure 3).
Figure 2.
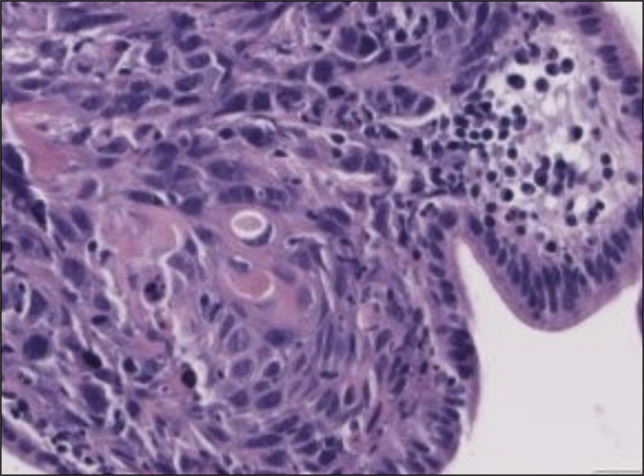
Pathology showing evidence of glandular atypia and squamous cell carcinoma (image at ×400).
Figure 3.
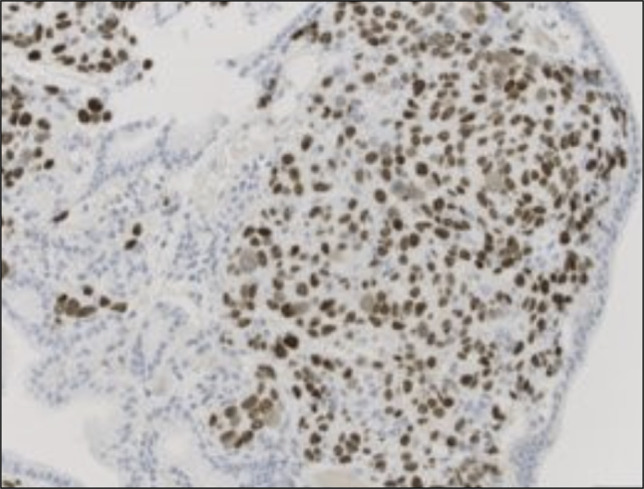
Tumor cells staining positive (brown) for p40 (confirming squamous differentiation) (image at ×200).
Immunohistochemistry showed programmed death-ligand 1 positivity at 30%. TP53 mutation and Jak 2 amplification were present. A staging computed tomography-positron emission tomography (CT-PET) scan showed intense hypermetabolic uptake in the distal CBD and evidence of hepatic and possible parotid gland metastases, consistent with stage IV disease. He was treated with gemcitabine and carboplatin with improvement in his energy and appetite. A follow-up CT-PET scan 6 months later showed less uptake in the CBD and periportal region with resolution of prior hypermetabolic lesions about the porta hepatis and liver; parotid gland uptake was unchanged. Repeat CT-PET 6 months later showed increased activity along the intrahepatic portion of the internal biliary stent, concerning for disease recurrence. He was started on radiation therapy for progression of disease and received 5 rounds of radiation. He currently remains on chemotherapy with gemcitabine every 2 weeks. He was not a surgical candidate because of metastatic disease.
DISCUSSION
Bile duct malignancies are extremely rare, making up less than 1% of all cancers.2 Tumors are categorized based on location as intrahepatic, perihilar (including the gallbladder and extrahepatic bile ducts), and distal, which includes the ampulla of Vater.4 Adenocarcinoma is the most common biliary malignancy, making up over 90%. SCC is exceedingly rare with only a few dozen case reports in the literature since the 1930s.2,3,5
The etiology of biliary SCC is unclear, and multiple theories have been proposed. It is suspected that the normal columnar epithelium undergoes squamous metaplasia with eventual development of dysplasia after recurrent episodes of inflammation.2 It is known that inflammatory marker upregulation triggers epithelial cell signaling, which can lead to transition factor activity and epigenetic changes, which can eventually lead to metaplastic and dysplastic changes.6,7 Other theories for the development of SCC include the presence of heterotopic squamous tissue that undergoes malignant transformation, metaplasia of adenocarcinoma, and pluripotent stem cell malignant transformation.1 It has also been reported that patients with a history of hepatolithiasis, recurrent pyogenic cholangitis, or clonorchiasis may be predisposed to biliary SCC, possibly because of chronic inflammation of the biliary tree.1,8 Our patient experienced recurrent episodes of cholecystitis and cholangitis, which likely lead to biliary ductal transformation to SCC.
Biliary SCC most commonly occurs in men and is most prevalent in the fifth through seventh decades of life. Rare cases have been reported in patients as young as 24 years.1 The diagnosis requires ERCP with tissue sampling. Histologically, the diagnosis of adenosquamous carcinoma is made when SCC makes up more than 25% of tumor cell type, and a diagnosis of SCC requires the absence of glandular elements.1 Metastatic disease is uncommon but has been reported in a few cases, most notably with hepatic lesions, which were seen in our patient.9 The overall prognosis is poor, partly because of its difficult diagnosis and often late presentation because of vague symptomatology.1,4,10 Currently, there is no standard therapy, but treatment options depend on the extent of disease. Surgery is recommended for local disease, with pancreatoduodenectomy being the most commonly performed procedure. Chemoradiation therapy is recommended for metastatic disease, with gemcitabine plus oxaliplatin or gemcitabine plus cisplatin being the most commonly used chemotherapy regimens.1,3,8
DISCLOSURES
Author contributions: J. Tantum, R. Schneider, S. Gallagher, and K. Leroy conducted the literature review, wrote, and edited the manuscript. J. Lander and P. Wong edited the manuscript. J. Tantum is the article guarantor.
Financial disclosure: None to report.
Prior presentation: This case was previously presented at the American College of Gastroenterology Annual Scientific Meeting; October 2021; Las Vegas, Nevada.
Informed consent was obtained for this case report.
Contributor Information
Rachael Schneider, Email: schneiderr@mlhs.org.
Stefanie Gallagher, Email: gallaghers@mlhs.org.
Kyley Leroy, Email: Leroyk@mlhs.org.
Jared Lander, Email: landerj@mlhs.org.
Patricia Wong, Email: pwongca1@gmail.com.
REFERENCES
- 1.Kang M, Kim NR, Chung DH. Squamous cell carcinoma of the extrahepatic common hepatic duct. J Pathol TranslMed. 2019;53(2):112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gatof D, Chen YK, Shah RJ. Primary squamous cell carcinoma of the bile duct diagnosed by transpapillary cholangioscopy: Case report and review. Gastrointest Endosc. 2004;60(2):300–4. [DOI] [PubMed] [Google Scholar]
- 3.Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: An update. Gut. 2012; 61:1657–69. [DOI] [PubMed] [Google Scholar]
- 4.Hennedige TP, Neo WT, Venkatesh SK. Imaging of malignancies of the biliary tract- an update. Cancer Imaging. 2014;14(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabot RC, Painter FM. Case records of the Massachusetts General Hospital: Case 16261: Four months' jaundice and rectal pain. N Eng J Med. 1930;202(26):1260–2. [Google Scholar]
- 6.Giroux V, Rustgi AK. Metaplasia: Tissue injury adaptation and a precursor to the dysplasia-cancer sequence. Nat Rev Cancer. 2017;17(10):594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen L, Deng H, Cui H, et al. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 2017;9(6):7204–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sewkani A, Kapoor S, Sharma S, et al. Squamous cell carcinoma of the distal common bile duct. J Pancreas. 2005;6(2):162–5. [PubMed] [Google Scholar]
- 9.Bacha D, Hajri M, Ferjaoui W, et al. Primary squamous cell carcinoma of the common bile duct with liver metastases. Arq Bras Cir Dig. 2021;34(1):1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan SA, Thomas HC, Davidson BR, Taylor-Robinson SD. Cholangiocarcinoma. Lancet. 2005;366(9493):1303–14. Erratum in: Lancet. 2006;367(9523):1656. [DOI] [PubMed] [Google Scholar]