Keywords: cardiovascular, echocardiography, hemodialysis
Abstract
Significance Statement
Hemodialysis (HD) can lead to acute left ventricular (LV) myocardial wall motion abnormalities (myocardial stunning) due to segmental hypoperfusion. Exercise during dialysis is associated with favorable effects on central hemodynamics and BP stability, factors considered in the etiology of HD-induced myocardial stunning. In a speckle-tracking echocardiography analysis, the authors explored effects of acute intradialytic exercise (IDE) on LV regional myocardial function in 60 patients undergoing HD. They found beneficial effects of IDE on LV longitudinal and circumferential function and on torsional mechanics, not accounted for by cardiac loading conditions or central hemodynamics. These findings support the implementation of IDE in people with ESKD, given that LV transient dysfunction imposed by repetitive HD may contribute to heart failure and increased risk of cardiac events in such patients.
Background
Hemodialysis (HD) induces left ventricular (LV) transient myocardial dysfunction. A complex interplay between linear deformations and torsional mechanics underlies LV myocardial performance. Although intradialytic exercise (IDE) induces favorable effects on central hemodynamics, its effect on myocardial mechanics has never been comprehensively documented.
Methods
To evaluate the effects of IDE on LV myocardial mechanics, assessed by speckle-tracking echocardiography, we conducted a prospective, open-label, two-center randomized crossover trial. We enrolled 60 individuals with ESKD receiving HD, who were assigned to participate in two sessions performed in a randomized order: standard HD and HD incorporating 30 minutes of aerobic exercise (HDEX). We measured global longitudinal strain (GLS) at baseline (T0), 90 minutes after HD onset (T1), and 30 minutes before ending HD (T2). At T0 and T2, we also measured circumferential strain and twist, calculated as the net difference between apical and basal rotations. Central hemodynamic data (BP, cardiac output) also were collected.
Results
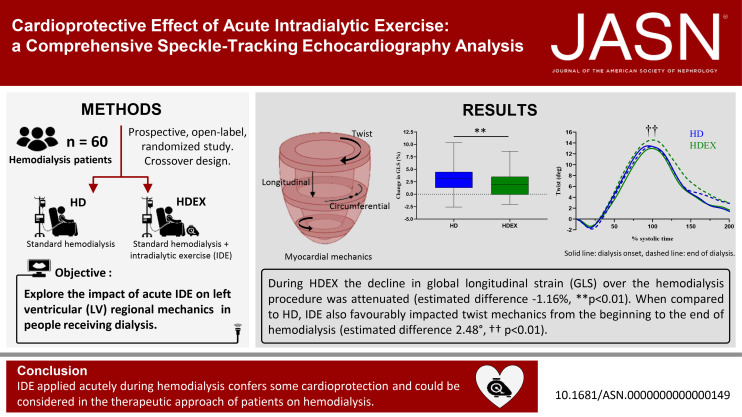
The decline in GLS observed during the HD procedure was attenuated in the HDEX sessions (estimated difference, −1.16%; 95% confidence interval [95% CI], −0.31 to −2.02; P = 0.008). Compared with HD, HDEX also demonstrated greater improvements from T0 to T2 in twist, an important component of LV myocardial function (estimated difference, 2.48°; 95% CI, 0.30 to 4.65; P = 0.02). Differences in changes from T0 to T2 for cardiac loading and intradialytic hemodynamics did not account for the beneficial effects of IDE on LV myocardial mechanics kinetics.
Conclusions
IDE applied acutely during HD improves regional myocardial mechanics and might warrant consideration in the therapeutic approach for patients on HD.
Introduction
ESKD is a global public health issue, with nearly 2.6 million people worldwide on hemodialysis (HD).1 Individuals with ESKD have a substantially increased incidence of cardiovascular events and mortality.2 LV geometry and structure (e.g., fibrosis) abnormalities as well as both global systolic and diastolic dysfunctions are frequent components of ESKD.3–6 The pathogenesis of heart disease in ESKD is not yet fully understood but remains multifactorial. Traditional cardiovascular risk factors and comorbidities (e.g., hypertension, diabetes, dyslipidemia) and factors related to ESKD itself, such as uremia and inflammation, contribute undoubtedly to the development and progression of ESKD-associated pathological cardiac remodeling.7 HD is also recognized as an independent risk factor.8 It is associated with intradialytic hypotension (IDH) and disturbances in myocardial segmental perfusion,9,10 leading to acute myocardial wall motion abnormalities and LV transient dysfunction, well known as myocardial stunning.11,12 Repetitive episodes of myocardial stunning during HD may, in the long term, precipitate individuals with ESKD to heart failure and thus contribute to the high rate of cardiovascular mortality in this population.13 Therapeutic strategies to mitigate cardiovascular disorders associated with HD are, therefore, mandatory. Acute IDE seems promising because it transiently increases central hemodynamics and BPs and can potentially reduce IDH, although this is not consensual.14 Data on its effect on myocardial function are, however, scarce. Two exploratory studies, performed on a small number of individuals with ESKD, documented a reduction in myocardial stunning identified by speckle-tracking echocardiography when IDE was applied acutely compared with standard HD.15,16 LV regional myocardial function results from a complex interplay between multidirectional (e.g., longitudinal and circumferential) deformations and torsional mechanics due to a complex interaction between myocardial fiber architecture and geometry. The LV twist motion in systole aids in ventricular ejection while early diastolic untwist generates suction and facilitates diastolic filling.17 Assessment of not only multidirectional deformations but also twist is, therefore, crucial for a comprehensive understanding of the effect of acute IDE on LV regional myocardial function in ESKD. To the best of our knowledge, no previous studies have made such measurements.
Accordingly, this study aims to explore the effect of acute IDE on LV linear deformations and torsional mechanics in HD individuals.
Methods
Study Design and Participants
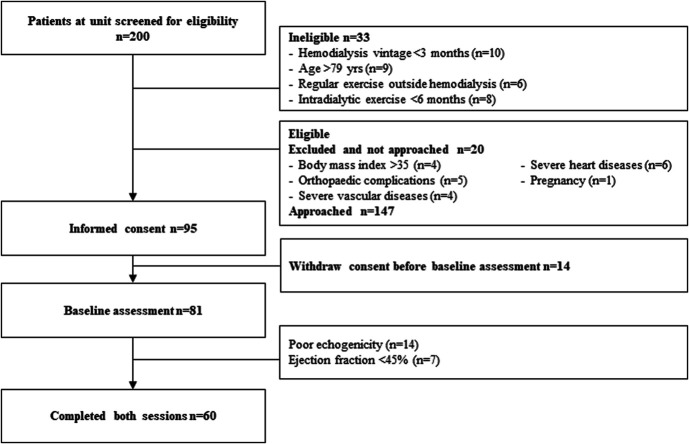
This pilot study was performed as a prelude to a larger study (EX-CHRODIAL for Chronic IDE: a Cardioprotective Role) designed to determine whether IDE improves long-term well-being and cardiovascular health. This study was a prospective, open-label, randomized trial performed in two HD units in France. It aimed to investigate the effect of acute IDE on LV myocardial mechanics over the course of HD. The ethics committee East II (clinicaltrials.gov NCT04697459) approved this study. All participants provided written informed consent. Adults, aged 20–79 years, with no regular exercise outside of dialysis, with no exposure to IDE before the study (<6 months), and undertaking maintenance HD for >3 months were eligible to participate. Exclusion criteria were poor echogenicity; contraindication to exercise; orthopaedic complications; severe heart (ischemic, valvular, etc.), vascular, or respiratory diseases; ejection fraction <45%; body mass index >35; and pregnancy. Figure 1 shows a flowchart of patient enrollment. This study was a crossover design (Figure 2), in which all individuals participated in two HD sessions in a random order: a standard HD and a session incorporating 30 minutes of aerobic exercise (HDEX). Patients were randomized by a computer using IBM SPSS statistics version 26.0 (IBM, Armonk, NY). Randomization assignment was not provided to patients until completion of the baseline study session. Exercise was performed in a semirecumbent position on a calibrated cycle ergometer (OxyCycle 3—PhysioMed) fixed to each patient's bed. The participants performed a 30-minute cycling session (50–70 rpm), starting 30 minutes after HD onset, at moderate intensity (11–14 on the Borg 20-point scale18). Echocardiography was performed three times during each session: immediately before HD (T0), after 90 minutes of HD (T1), and 30 minutes before ending HD (T2) while central hemodynamic data (BP; cardiac output [CO]) were collected before HD and every 30 minutes throughout dialysis (Figure 2). For consistency, HD and HDEX were performed 1-week apart from the first HD day of the week.
Figure 1.
Study consort diagram. Study screening, consent, echo window, participation, and dropout.
Figure 2.
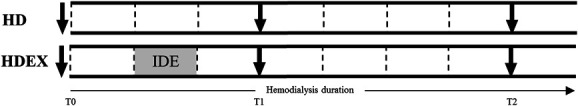
Schematic for HD and HDEX sessions. Dotted lines (⋮) indicate BP and CO measurements. Arrows (↓) indicate transthoracic echocardiography. Gray box indicates IDE during HDEX.
Clinical Examination and Dialysis Settings
In the first session, a comprehensive clinical examination was conducted and blood samples were collected from the arterial line of the dialysis circuit at the start of HD (Table 1). Biological data included hemoglobin, creatinine, calcium, potassium, sodium, high-sensitivity C-reactive protein, and urea levels. All participants were dialyzing three times weekly for 4–4.5 hours per session. HD parameters were collected at the end of each session (Table 2). Net ultrafiltration was determined clinically on the basis of ideal dry weight. For each participant, medication was not changed throughout the study period.
Table 1.
Participant characteristics
| Variable | ESKD (n=60) |
|---|---|
| Age (yr) | 63±14 |
| Sex, n (male/female) | 43/17 |
| Dry weight (kg) | 73.1±15.8 |
| Body mass index (kg/m2) | 25.2±4.6 |
| Dialysis vintage (yr) | 5.7±6.0 |
| Hemoglobin (g/100 ml) | 11.7±2.1 |
| Calcium (mmol/L) | 2.2±0.2 |
| Potassium (mmol/L) | 4.9±0.8 |
| Sodium (mmol/L) | 138±2.5 |
| Creatinine (μmol/L) | 722±274 |
| C-reactive protein ultrasensitive (mg/L) | 36.5±45.6 |
| Urea (mmol/L) | 21.3±5.0 |
| Comorbidities. n (%) | |
| Diabetes | 10 (17) |
| Hypertension | 34 (57) |
| Coronary artery disease | 6 (10) |
| Heart failure | 10 (17) |
| Hyperparathyroidism | 9 (15) |
| Peripheral vascular disease | 13 (22) |
| Chronic kidney disease etiology. n (%) | |
| Glomerular nephritis | 12 (20) |
| Immunoglobulin A nephropathy | 5 (9) |
| Hypertensive nephropathy | 11 (19) |
| Diabetic nephropathy | 6 (10) |
| Congenital | 2 (4) |
| Indeterminate | 8 (14) |
| Other | 14 (24) |
| Medication, n (%) | |
| Antiplatelet | 1 (2) |
| Anticoagulants | 14 (23) |
| Nitrates | 1 (2) |
| Statins | 11 (18) |
| Diuretics | 13 (22) |
| Anti-arrhythmic | 3 (5) |
| Calcium channel blockers | 8 (13) |
| β-blockers | 19 (32) |
| Erythropoietin | 3 (5) |
| Corticosteroids | 3 (5) |
| Thyroxine | 1 (2) |
Table 2.
Hemodialysis parameters
| Variable | HD | HDEX | P Value |
|---|---|---|---|
| Weight (kg) | |||
| Pre | 74.3±15.6 | 74.3±15.4 | 0.99 |
| Post | 71.8±15.7 | 71.7±15.4 | 0.67 |
| Ultrafiltrated volume (L) | 2.63±0.95 | 2.61±0.92 | 0.81 |
| Duration (min) | 232±17 | 232±15 | 0.78 |
| Ultrafiltration rate (ml/h) | 682±224 | 645±248 | 0.33 |
| Kt/V | 1.51±0.30 | 1.53±0.30 | 0.68 |
HD, hemodialysis.
Echocardiography
Transthoracic echocardiography was performed using a Vivid Q system (GE Healthcare, Horten, Norway) with a 3.5-MHz transducer. Cine loops in apical 4, 2, and 3-chamber views were recorded at T0, T1, and T2, respectively, while loops in parasternal long- and short-axis (basal, papillary muscle and apical levels) views were only obtained at T0 and T2, respectively. The apical plane was acquired with the transductor in a caudal position (i.e., below the papillary muscles) to improve LV apical rotation measurement.19 Images were analyzed in cine loops triggered by the QRS complex and saved for blinded offline analysis, which was performed using dedicated software (EchoPAC 203TM—GE Healthcare, Chicago). Three cycles with the best image quality were used for analysis.
Standard Echocardiography
Standard acquisitions were recorded before the first session in accordance with the guidelines from the American Society of Echocardiography.20 LV internal diameters and wall thicknesses were measured from the parasternal long-axis view. LV mass was calculated by the Devereux formula and indexed to body surface area. LV volume and ejection fraction were measured using the Simpson biplane method. LV diastolic function was assessed using early (E) and atrial transmitral flow velocities, recorded in the apical 4-chamber view. Myocardial systolic, early diastolic (e'), and atrial velocities were measured with a color-coded Doppler Tissue Imaging at the mitral annular level in the apical 4-chamber view. Peak e’ (i.e., average recorded on the LV septal and lateral wall) and the E/e’ ratio were used as indexes of LV relaxation and filling pressure, respectively.21
HD Echocardiography and Central Hemodynamics
LV volumes, ejection fraction, as well as internal dimensions and wall thicknesses were measured as aforementioned. End-diastolic volumes (EDVs) were obtained as the preload index. The systolic meridional wall stress (σes) was calculated and used as an index of afterload.22 Analysis of linear strains and twist/untwist mechanics was conducted as previously described in our laboratory.23,24 Global longitudinal strain (GLS) was calculated using a 18-segment model from apical 4, 2, and 3-chamber views. Circumferential strain (CS, at the papillary muscle level) as well as apical and basal rotations were assessed from parasternal short-axis views. Increased negative values for GLS and CS indicate greater deformation. Data were processed with a specific toolbox (Scilab 4.1) and normalized to the percentage of systole (i.e., aortic valve closure representing 100% of systolic duration) using interpolations. Net LV twist was calculated as the instantaneous difference between apical and basal rotations. The LV untwisting rate was considered as an index of LV relaxation.25
Regular monitoring of cardiovascular hemodynamics was set up (Figure 2), with measurements staggered every 30 minutes from HD onset for aortic blood flow, heart rate (HR), and BP. Aortic blood flow velocity was measured in the ascending aorta using a 2.0-MHz continuous-wave Doppler transducer (Pedof P2D CW; GE Vingmed Ultrasound) placed at the suprasternal notch for stroke volume (SV) determination.26 CO was calculated as SV×HR. Brachial BP was measured on the nonaccess arm during the HD session using an automated BP cuff integrated into the dialysis unit. Initial BP values were obtained at the start of HD. IDH was defined as a 20-mm Hg decrease in systolic BP (SBP) and/or a 10-mm Hg decrease in mean arterial pressure (MAP) associated with symptoms (e.g., abdominal discomfort, nausea, vomiting, muscle cramps, dizziness or fainting and anxiety) compared with initial BP values.27
Outcomes
The primary outcome was the GLS trajectory from T0 through T2 (T0, T1, and T2). Secondary outcomes included trajectories in all other speckle-tracking echocardiography–derived parameters and central hemodynamic variables as well as the number of IDH episodes per 100 hours of HD.
Statistical Analyses
Statistical analysis was performed using IBM SPSS statistics version 26.0 (IBM, Armonk, NY). Values are expressed as mean±SD. Statistical significance was defined as P value <0.05. No previous studies have examined the effect of IDE on GLS measured at baseline and at the end of HD (see Discussion). Sample size calculation was, therefore, based on the results from Penny et al.15 on GLS obtained at the end of HD in the two conditions. We estimated that a total of 53 individuals would be required, considering an effect size of 0.34 with a statistical power of 0.80 and an α (two-sided) of 0.05. All analyses regarding the effect of IDE were based on the intention-to-treat principle. The primary outcome was analyzed using a linear mixed-effects regression model, with condition (HD versus HDEX) as well as session sequence and time (T0, T1, T2) as a fixed effect and random effect, respectively, for patients. Analyses of prespecified secondary outcomes and HD parameters were undertaken using the same analysis methods as the primary outcome. A generalized linear mixed model (using a negative binomial regression model) was used to analyze the effect of IDE on the number of IDH episodes. Pearson correlations were used to investigate the association between differences in T0 to T2 changes between HD and HDEX of deformation imaging variables with indexes of cardiac loading conditions or the number of IDH.
Results
Baseline Characteristics
The trial recruited from December 2020 to February 2022. A total of 200 people receiving HD were screened. Of the 147 individuals eligible and approached, 95 gave initial consent and 14 subsequently dropped out. After baseline assessments, 21 were also excluded for poor echogenicity (n=14) and ejection fraction lower than 45% (n=7). Sixty individuals completed both HD and HDEX sessions (Figure 1). Baseline characteristics are presented in Table 1. The mean age was 63±14 years, and 72% of the participants were male. Body weight before and after dialysis as well as all dialysis parameters were strictly similar between HD and HDEX (Table 2). Standard echocardiographic data are presented in Table 3.
Table 3.
Echocardiographic characteristics
| Variable | ESKD (n=60) |
|---|---|
| 2D | |
| LV EDV (ml) | 107±28 |
| LV ESV (ml) | 48±15 |
| Relative wall thickness | 0.34±0.10 |
| LV mass index (g/m2) | 115±33 |
| LV ejection fraction (%) | 55±8 |
| Pulsed wave Doppler | |
| E (m/s) | 0.8±0.2 |
| A (m/s) | 0.9±1.0 |
| E/A | 1.1±0.3 |
| TDI parameters | |
| s’ (cm/s) | 8.5±2.1 |
| e’ (cm/s) | 9.2±2.7 |
| a’ (cm/s) | 9.9±2.8 |
| E/e’ ratio | 9.5±4.2 |
LV, left ventricular; EDV, end-diastolic volume; ESV, end-systolic volume; E, peak early transmitral flow velocity; A, peak late transmitral flow velocity; s’, peak systolic mitral annular velocity; e’, peak early diastolic mitral annular velocity; a’, peak late diastolic mitral annular velocity.
Cardiac Function During HD and HDEX
Data are presented in detail in Table 4. For all myocardial mechanics and loading condition parameters, there was no condition by sequence interaction, highlighting the absence of carryover effect between HDEX and HD. For the primary outcome, IDE significantly affected the GLS trajectory from T0 through T2 (condition by time interaction P = 0.008). When compared with HD, HDEX attenuated the decline in GLS by −0.59% per time point (95% confidence interval [95% CI], −0.15 to −1.01). Conducting the same statistical analysis with time in hours (T0=0 hour, T1=1.5 hours, T2=HD duration −0.5 hour), the reduction in GLS decline in HDEX compared with HD was of −0.33% per hour of dialysis (95% CI, −0.08 to −0.57, condition by time interaction P = 0.009). A subsequent analysis at each time point (i.e., from T0 to T2 and T0 to T1) reveals that the beneficial effect of HDEX on GLS change from baseline was more important at T2 (estimated difference −1.16%, 95% CI, −0.31 to −2.02, P = 0.008) than at T1 (estimated difference −0.55%, 95% CI, 0.30 to −1.46, P = 0.20). Regarding the secondary outcomes, IDE influenced also the trajectory from T0 to T2 in CS, apical rotation, and torsional mechanics, as evidenced by significant condition by time interactions (Table 4). Examination of estimated differences of T0 to T2 changes between HD and HDEX, and their 95% CIs clearly highlighted the beneficial effect of IDE on CS, apical rotation, and twisting motion. When compared with HD, HDEX yielded to greater improvement from T0 to T2 in twist, a very important component of LV myocardial function (estimated difference 2.48°, 95% CI, 0.30 to 4.65, P = 0.02). A modest effect of IDE on T0 to T2 changes was shown for basal rotation (estimated difference −0.94°, 95% CI, −2.39 to 0.51), with no significant condition by time interaction (P = 0.20).
Table 4.
Myocardial mechanics and loading conditions during hemodialysis
| Variable | Linear Mixed-Effects Model | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HD | HDEX | Condition×Sequence | Condition×Time | |||||||||
| T0 | T1 | T2 | T0 | T1 | T2 | P Value | Estimated Differencea | 95% CI | P Value | |||
| Primary outcome | ||||||||||||
| GLS (%) | −18.2±3.1 | −16.2±3.3 | −15.2±3.7 | −18.1±3.0 | −16.8±3.5 | −16.3±3.4 | 0.94 | −1.16 | −0.31 to −2.02 | 0.008 | ||
| <0.001 | <0.001 | |||||||||||
| P valueb | <0.001 | 0.007 | ||||||||||
| <0.001 | <0.001 | |||||||||||
| Secondary outcomes | T0 | T2 | T0 | T2 | ||||||||
| Myocardial mechanics | ||||||||||||
| CS (%) | −15.7±3.7 | −13.0±3.9 | −15.6±3.8 | −14.9±3.2 | 0.65 | −1.68 | −3.21 to −0.15 | 0.03 | ||||
| P valueb | <0.001 | 0.07 | ||||||||||
| Basal rotation (°) | −7.8±3.5 | −8.5±3.9 | −7.4±2.9 | −9.0±3.4 | 0.13 | −0.94 | −2.39 to 0.51 | 0.20 | ||||
| P valueb | 0.11 | <0.001 | ||||||||||
| Apical rotation (°) | 9.4±4.4 | 7.9±3.9 | 9.2±4.0 | 9.6±3.9 | 0.99 | 1.82 | 0.32 to 3.33 | 0.01 | ||||
| P valueb | 0.02 | 0.44 | ||||||||||
| Twist (°) | 14.7±5.7 | 14.6±5.2 | 14.2±5.7 | 16.3±5.4 | 0.12 | 2.48 | 0.30 to 4.65 | 0.02 | ||||
| P valueb | 0.72 | 0.01 | ||||||||||
| Twisting rate (°/s) | 94±29 | 107±43 | 89±28 | 120±44 | 0.26 | 16.0 | 0.03 to 31.9 | 0.04 | ||||
| P valueb | 0.03 | <0.001 | ||||||||||
| Untwisting rate (°/s) | −104±37 | −106±42 | −106±45 | −123±49 | 0.73 | −14.1 | −32.6 to 4.4 | 0.13 | ||||
| P valueb | 0.65 | 0.03 | ||||||||||
| Loading conditions | ||||||||||||
| EDV (ml) | 106±27 | 91±24 | 108±28 | 92±22 | 0.84 | 0.32 | −7.8 to 8.4 | 0.93 | ||||
| P valueb | <0.001 | <0.001 | ||||||||||
| σes (g/cm2) | 91±42 | 76±38 | 90±42 | 78±39 | 0.98 | 3.3 | −12.9 to 19.5 | 0.68 | ||||
| P valueb | 0.03 | 0.03 | ||||||||||
HD, hemodialysis; 95% CI, 95% confidence interval; CS, circumferential strain; EDV, end-diastolic volume; GLS, global longitudinal strain; σes, meridional wall stress.
Estimated difference of change from T0 to T2 between standard hemodialysis and HDEX generated by the linear mixed-effects regression model, with standard hemodialysis and T0 as reference factors. Negative estimated differences for negative data, such as global longitudinal strain, circumferential strain, basal rotation, or untwisting rate, and positive estimated differences for positive data, such as apical rotation, twist, or twisting rate, indicate the superiority and cardioprotective effect of HDEX. The parameters were estimated by maximum likelihood and P values generated by Wald tests.
P value for change from T0 to T2 within the same condition, analyzed using the one-sample t test.
No condition by time interaction and small estimated differences were obtained for loading conditions, but 95% CI revealed between-patient variations. However, when examining differences in T0 to T2 changes between HD and HDEX, no correlations were obtained between EDV with GLS (r=−0.17 P = 0.27), CS (r=−0.09 P = 0.59), apical rotation (r=0.20 P = 0.22), and twist (r=−0.17 P = 0.30). Similar results were obtained for σes with GLS (r=−0.29 P = 0.08), CS (r=0.02 P = 0.90), apical rotation (r=−0.13 P = 0.43), and twist (r=0.16 P = 0.35).
Hemodynamics
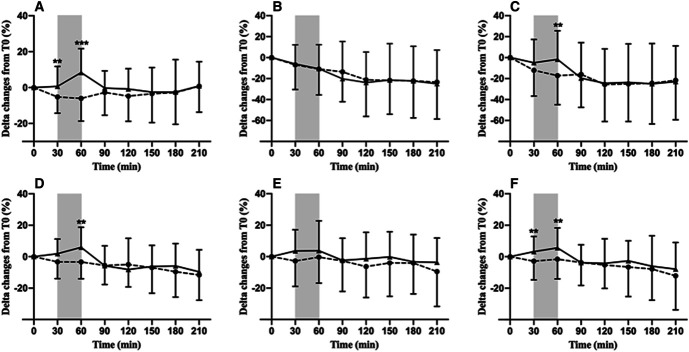
Figure 3 shows the time course of changes from baseline in HR, SV, CO, SBP, DBP, and MAP during both sessions. Significant condition by time interactions were noticed for SBP (P < 0.001), HR (P < 0.001), and CO (P = 0.02), underlining an effect of IDE. Estimated differences in changes from baseline between HD and HDEX were only significant at 60 minutes of HD (i.e., at the end of IDE) for SBP (P = 0.006) and CO (P = 0.003) and at 30 and 60 minutes of the procedure for HR (P = 0.01; P < 0.001, respectively). IDE did not modify IDH episodes per 100 hours of HD (HD=58±53 versus HDEX=51±56, P = 0.20; estimated difference: 2.2; 95% CI, −19 to +14). Furthermore, no correlations were found between HD and HDEX differences in the rate of IDH episodes with T0 to T2 changes in GLS (r=0.18, P = 0.17), CS (r=0.11, P = 0.51), apical rotation (r=−0.03, P = 0.85), and twist (r=−0.17, P = 0.29).
Figure 3.
Delta changes over the HD procedure (%) for HR (A), SV (B), CO (C), SBP (D), diastolic BP (E), and mean BP (F) during HD (dashed line) and HDEX (solid line) sessions. Gray box indicates 30 minutes of exercise during HDEX. Estimated difference of change between HD and HDEX: **P < 0.01; ***P < 0.001.
Discussion
This study is, to the best of our knowledge, the first to explore in a large sample of people receiving HD the effect of acute IDE on LV linear deformations and torsional mechanics. In people with ESKD, conventional HD is mandatory to supply the failing renal function. However, the procedure can be detrimental to the myocardium. Acute reductions of LV myocardial perfusion, sufficient in magnitude to lead to transient LV myocardial dysfunction (i.e., myocardial stunning), have indeed been clearly documented using intradialytic positron emission tomography scanning or cardiac magnetic resonance imaging.10,12,28 Speckle-tracking echocardiography provides accurate and angle-independent measurements of LV linear deformations and twist,29,30 allowing a fully comprehensive evaluation of LV regional myocardial function. In our study, GLS and CS progressively declined during HD (Table 4), agreeing with previous studies using either 2D31 or 3D32,33 speckle-tracking echocardiography. Twist was, however, maintained (Table 4), in accordance with most of the available data, which may be viewed as a compensatory mechanism maintaining overall systolic function (i.e., ejection fraction) when longitudinal shortening (i.e., GLS) is depressed.34,35 The repetitive nature of myocardial ischemic insults and transient dysfunction due to dialysis may, in the long term, yield to permanent regional myocardial dysfunction, conferring an increased risk of cardiac events and mortality,13 motivating, therefore, the application of countermeasures during HD. Acute IDE is an interesting prophylactic measure because it transiently increases central hemodynamics and BPs, factors considered in the etiology of HD-induced myocardial transient dysfunction,8 and can also potentially reduce IDH, although this is not consensual.14 However, the effects of acute IDE on LV regional myocardial mechanics remain extremely poorly documented. To the best of our knowledge, only longitudinal function has been investigated in two nonrandomized exploratory studies, performed on a small number (n<21) of individuals with ESKD.15,16 In these studies, despite a significant reduction in the number of myocardial stunned segments during the HD session with acute IDE compared with standard HD, a similar decrease in GLS during the HD procedure was reported in both conditions, results that clearly differ from ours where GLS changes from T0 through T2 were attenuated in HDEX compared with HD (Table 4). Several methodological aspects may potentially explain such discrepancies and also preclude any direct comparison between these and the current results: the low numbers of participants in both prior studies conferring weak statistical power and an increased risk of type II error, the last echocardiogram being performed 1.5 hours before the end of HD,16 or GLS changes being reported only from post-IDE to the end of HD as well as individuals with ESKD having undergone routine exercise participation during HD before the sessions during which echocardiography was performed.15 No data are available in the literature regarding the circumferential function or torsional mechanics, despite their key roles in cardiac performance. Indeed, twist is a major component of LV mechanics reflecting the helical orientation of myocardial fibers. The LV twist motion induced by apical counterclockwise and basal clockwise rotations in systole aids in ventricular ejection while early diastolic untwist generates suction and facilitates diastolic filling.17 A salient feature, not previously demonstrated, was the cardioprotective benefit of IDE on circumferential function and torsional mechanics. The results from the mixed-effects model and the one-sample t test clearly indicated the superiority of HDEX, by canceling the HD-induced deterioration of CS and apical rotation and enhancing twisting motion (Table 4).
This study was not specifically designed to resolve the underlying mechanisms responsible for the beneficial effects of IDE on LV regional myocardial function, but several hypotheses may be discarded or put forward. The etiology of HD-induced transient LV dysfunction is incompletely understood, but hemodynamic instability has been presented as a potential contributor. By cardiac magnetic resonance imaging, Buchanan et al. reported indeed negative correlations between the number of dysfunctional LV segments and changes during dialysis in indexed SV and CO or minimum SBP.28 In our study, the time courses in SV and CO as well as SBP were similar during both HD and HDEX sessions, except during the 30-minute exercise, in agreement with previous data.16 Owing to impaired baroreflex sensitivity, individuals with ESKD present with defective BP control and exhibit significant IDH during dialysis.14 IDH is independently associated with regional myocardial function.13 In our study, we did not observe differences in the rate of IDH episodes per 100 hours of HD between HD and HDEX. However, we recognize that the study was not powered (i.e., too small sample size) to detect a statistically significant difference in this outcome. However, no correlations were found between differences in changes from T0 to T2 between HD and HDEX in deformation imaging variables with differences in IDH. Altogether, these results do not speak in favor of a role of hemodynamic factors in the explanation of the beneficial effects of IDE on LV regional myocardial function.
The load dependency of speckle-tracking echocardiography–derived parameters, including linear deformations and twisting mechanics, has been established.36–39 In our study, as expected, significant declines in both cardiac preload (EDV) and afterload (σes) during HD were found (Table 4). However, acute IDE did not affect the time course of loading indexes during dialysis. Despite small estimates, quite wide 95% CIs were noticed for the difference of change from T0 to T2 between HD and HDEX, revealing interindividual variations and a potential effect of IDE. However, no correlations were found between these differences for speckle-tracking echocardiography–derived parameters with those in loading conditions, arguing for an absence of a role of cardiac preload and afterload on the cardioprotective effect of IDE.
Speckle-tracking echocardiography allows quantifying the regional function of the stunned myocardium and is able to objectively detect segmental myocardial ischemia.22,29,40 Longitudinal function is mainly set by vertically oriented subendocardial fibers. The midmyocardial fibers run circumferentially and most significantly contribute to circumferential function while the subepicardial fibers, longitudinally oriented in an oblique counterclockwise direction compared with the subendocardial layer, are the predominant source of torsional motion.41 Owing to its greater rotational radius, the epicardial layer dominates indeed the overall direction of rotation. A substantial body of evidence shows that subendocardial fibers are more sensitive to hemodynamic variations and acute ischemia than mid and subepicardial ones.42,43 In this context, it was not surprising that IDE attenuated but did not totally repress the HD-induced decline in GLS, while it preserved CS and improved twist mechanics. Considering that our speckle-tracking echocardiography–derived parameters were controlled for loading conditions, our results obtained in stable individuals with ESKD free from severe epicardial coronary artery disease strongly support the possibility of an IDE cardioprotection through a reduction in HD-induced microvascular perfusion abnormalities. Mechanisms involving exercise-induced ischemic/nonischemic preconditioning44 or changes in whole-blood viscosity45 might be advanced to explain such an improvement in tissue perfusion. Future studies will be needed to determine the role of tissue perfusion in IDE-induced cardioprotection and to precise its underlying determinants.
Clinical Implication
GLS is currently the most widely used deformation imaging parameter in clinical practice. It provides independent and incremental prognostic information regarding long-term risk of cardiovascular events and mortality.46,47 The attenuation of GLS decline from T0 to T2 in HDEX compared with HD (estimated difference −1.16%, 95% CI, −0.31 to −2.02) gives preliminary evidence of cardiac protection provided by IDE. This finding may be clinically relevant because a reduction in GLS is associated with worse outcomes and predictive of poor prognosis among stable HD patients with preserved ejection fraction.48 Furthermore, in chronic kidney disease individuals with successful kidney transplant, the hazard ratio for cardiovascular events or death after adjusting for age, sex, and race-ethnicity increased by 28% per 1% decrement in the absolute value of GLS. In patients with various cardiovascular risk factors or presenting with cardiovascular diseases, as seen in people with ESKD, each 1% decrease in GLS magnitude was also associated with an 11.3% increase in cardiovascular mortality risk.46 The prognostic value of torsional mechanics indexes in the setting of kidney disease is unknown. Whether the transient improvement in myocardial mechanics conferred by IDE when repeated on each dialysis translates into improved clinical outcomes remains to be determined.
Limitations
Our study had limitations. Exercise intensity during IDE was based on the rate of perceived exertion rather than on the results of a previous triangular maximal cardiopulmonary exercise test. Not all the participants were, therefore, exercising at strictly the same intensity within the moderate-intensity zone expected. However, the rate of perceived exertion scores correlate relatively well with physiological measures of stress and arousal (e.g., HR, ventilatory thresholds).18 In addition, the prescription of exercise intensity on the basis of the perceived exertion rate is well accepted by people with ESKD and broadens the applicability of the protocol. Our very restrictive exclusion criteria (especially ejection fraction <45%, severe heart disease and body mass index >35) helped control confounding factors, such as severe obesity or disease respiratory disorders, and minimize any potential cardiovascular risk from moderate exercise. Patients with orthopaedic complications affecting the lower limb, for whom completion of IDE was compromised, were also excluded. We recognize that these restrictions obviously affected the generalizability of our results. Only 28% of the patients were women. This gender bias constitutes a limitation regarding gender generalization of the results. A significant proportion of participants declined to participate in the study, so our findings cannot necessarily be generalized to all individuals on dialysis. However, most refused not because of IDE but because of repetitive echo measurements throughout dialysis. We believe, therefore, that the adoption of IDE in the dialysis population is not disputed. The intervention was delivered after the long interdialytic interval where patients tended to be more fluid overloaded and have higher cardiac events. Whether the effect of IDE on myocardial mechanics would have been different if applied after the short interdialytic interval is unknown. Only a continuous exercise of moderate intensity was applied in this study, and it remains unknown whether higher intensities on a continuous or interval mode would have produced greater benefits on cardiac function. Future work should attempt to investigate the optimum mode (continuous versus interval), intensity, timing, and duration of exercise to induce the greatest benefits on LV myocardial mechanics. Finally, we were unable to assess long-term outcomes because this was beyond the scope of our study. Future studies are needed to determine the cardiovascular effect of IDE when applied chronically and its effect on cardiovascular events and mortality in individuals with ESKD.
This pilot study is the first to identify the beneficial effects of IDE on LV multidirectional deformation and torsional mechanics during dialysis in people with stable ESKD. Such cardioprotection conferred by IDE has significant clinical relevance because recurrent LV transient dysfunction imposed by repetitive HD can translate in the long term into overt cardiac dysfunction and an increased risk of cardiac events and mortality.
Disclosures
J. Cristol reports Research Funding: Siemens; Honoraria: Fresenius Medical Care, Radiometer, and Servier; and Other Interests or Relationships: AIDER Santé. M. Isnard reports Employer: MEDIPOLE. C. Maufrais reports Research Funding: French Society of Cardiology. S. Nottin reports Research Funding: French Society of Cardiology. All remaining authors have nothing to disclose.
Funding
This study was supported by the “Société Française de Cardiologie,” grant number SFC21EXCRO.
Acknowledgments
We thank Axelife and the PACA region to support the scholarship of M. Josse. The authors express our sincere thanks to the volunteers involved in the study as well as the technical, administrative, and medical staff of AIDER Santé and ATIR Avignon.
Author Contributions
Conceptualization: Claire Maufrais, Philippe Obert.
Data curation: Antoine Grandperrin, Matthieu Josse, Claire Maufrais, Stéphane Nottin, Philippe Obert.
Formal analysis: Matthieu Josse, Claire Maufrais, Philippe Obert.
Funding acquisition: Antoine Grandperrin, Matthieu Josse, Claire Maufrais, Philippe Obert.
Investigation: Matthieu Josse, Claire Maufrais, Stéphane Nottin, Philippe Obert.
Methodology: Claire Maufrais, Philippe Obert.
Project administration: Myriam Isnard, Matthieu Josse, Claire Maufrais, Philippe Obert, Laure Patrier, Cécile Turc-Baron.
Resources: Jean-Paul Cristol, Myriam Isnard, Stéphane Mandigout, Claire Maufrais, Philippe Obert, Laure Patrier, Cécile Turc-Baron.
Software: Claire Maufrais, Philippe Obert.
Supervision: Jean-Paul Cristol, Myriam Isnard, Claire Maufrais, Philippe Obert, Laure Patrier.
Validation: Jean-Paul Cristol, Myriam Isnard, Claire Maufrais, Philippe Obert, Laure Patrier, Cécile Turc-Baron.
Visualization: Claire Maufrais, Philippe Obert, Cécile Turc-Baron.
Writing – original draft: Matthieu Josse, Claire Maufrais, Philippe Obert.
Writing – review & editing: Matthieu Josse, Claire Maufrais, Philippe Obert.
Data Sharing Statement
Anonymized data reported in this paper of type Analyzable Data have been deposited to Figshare, 10.6084/m9.figshare.21960083. The raw data supporting the conclusions of this article will be made available after publication by the authors, without undue reservation.
Footnotes
C.M. and P.O. are senior co-authors.
References
- 1.Bello AK, Okpechi IG, Osman MA, et al. Epidemiology of haemodialysis outcomes. Nat Rev Nephrol. 2022;18(6):378–395. doi: 10.1038/s41581-022-00542-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- 3.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. Impact of hypertension on cardiomyopathy, morbidity and mortality in end-stage renal disease. Kidney Int. 1996;49(5):1379–1385. doi: 10.1038/ki.1996.194 [DOI] [PubMed] [Google Scholar]
- 4.Cibulka R, Racek J. Metabolic disorders in patients with chronic kidney failure. Physiol Res. 2007;56(6):697–705. doi: 10.33549/physiolres.931128 [DOI] [PubMed] [Google Scholar]
- 5.Plawecki M, Morena M, Kuster N, et al. sST2 as a new biomarker of chronic kidney disease-induced cardiac remodeling: impact on risk prediction. Mediators Inflamm. 2018;2018:3952526. doi: 10.1155/2018/3952526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser SDS, Roderick PJ, May CR, et al. The burden of comorbidity in people with chronic kidney disease stage 3: a cohort study. BMC Nephrol. 2015;16(1):193. doi: 10.1186/s12882-015-0189-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wanner C, Amann K, Shoji T. The heart and vascular system in dialysis. Lancet. 2016;388(10041):276–284. doi: 10.1016/s0140-6736(16)30508-6 [DOI] [PubMed] [Google Scholar]
- 8.McIntyre CW. Effects of hemodialysis on cardiac function. Kidney Int. 2009;76(4):371–375. doi: 10.1038/ki.2009.207 [DOI] [PubMed] [Google Scholar]
- 9.McGuire S, Horton EJ, Renshaw D, Jimenez A, Krishnan N, McGregor G. Hemodynamic instability during dialysis: the potential role of intradialytic exercise. Biomed Res Int. 2018;2018:8276912. doi: 10.1155/2018/8276912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dasselaar JJ, Slart RHJA, Knip M, et al. Haemodialysis is associated with a pronounced fall in myocardial perfusion. Nephrol Dial Transplant. 2008;24(2):604–610. doi: 10.1093/ndt/gfn501 [DOI] [PubMed] [Google Scholar]
- 11.Loutradis C, Sarafidis PA, Papadopoulos CE, Papagianni A, Zoccali C. The ebb and flow of echocardiographic cardiac function parameters in relationship to hemodialysis treatment in patients with ESRD. J Am Soc Nephrol. 2018;29(5):1372–1381. doi: 10.1681/ASN.2017101102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McIntyre CW, Burton JO, Selby NM, et al. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. 2008;3(1):19–26. doi: 10.2215/CJN.03170707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4(5):914–920. doi: 10.2215/CJN.03900808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanbay M, Ertuglu LA, Afsar B, et al. An update review of intradialytic hypotension: concept, risk factors, clinical implications and management. Clin Kidney J. 2020;13(6):981–993. doi: 10.1093/ckj/sfaa078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Penny JD, Salerno FR, Brar R, et al. Intradialytic exercise preconditioning: an exploratory study on the effect on myocardial stunning. Nephrol Dial Transplant. 2019;34(11):1917–1923. doi: 10.1093/ndt/gfy376 [DOI] [PubMed] [Google Scholar]
- 16.McGuire S, Horton EJ, Renshaw D, et al. Cardiac stunning during haemodialysis: the therapeutic effect of intra-dialytic exercise. Clin Kidney J. 2019;14(5):1335–1344. doi: 10.1093/ckj/sfz159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK. Twist mechanics of the left ventricle. JACC Cardiovasc Imaging. 2008;1(3):366–376. doi: 10.1016/j.jcmg.2008.02.006 [DOI] [PubMed] [Google Scholar]
- 18.Borg G. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. doi: 10.1249/00005768-198205000-00012 [DOI] [PubMed] [Google Scholar]
- 19.Van Dalen BM, Vletter WB, Soliman OII, ten Cate FJ, Geleijnse ML. Importance of transducer position in the assessment of apical rotation by speckle tracking echocardiography. J Am Soc Echocardiogr. 2008;21(8):895–898. doi: 10.1016/j.echo.2008.02.001 [DOI] [PubMed] [Google Scholar]
- 20.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 21.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(12):1321–1360. doi: 10.1093/ehjci/jew082 [DOI] [PubMed] [Google Scholar]
- 22.Jamal F, Strotmann J, Weidemann F, et al. Noninvasive quantification of the contractile reserve of stunned myocardium by ultrasonic strain rate and strain. Circulation. 2001;104(9):1059–1065. doi: 10.1161/hc3501.093818 [DOI] [PubMed] [Google Scholar]
- 23.Doucende G, Schuster I, Rupp T, et al. Kinetics of left ventricular strains and torsion during incremental exercise in healthy subjects: the key role of torsional mechanics for systolic-diastolic coupling. Circ Cardiovasc Imaging. 2010;3(5):586–594. doi: 10.1161/circimaging.110.943522 [DOI] [PubMed] [Google Scholar]
- 24.Maufrais C, Schuster I, Doucende G, et al. Endurance training minimizes age-related changes of left ventricular twist-untwist mechanics. J Am Soc Echocardiogr. 2014;27(11):1208–1215. doi: 10.1016/j.echo.2014.07.007 [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Khoury DS, Yue Y, Torre-Amione G, Nagueh SF. Left ventricular untwisting rate by speckle tracking echocardiography. Circulation. 2007;116(22):2580–2586. doi: 10.1161/circulationaha.107.706770 [DOI] [PubMed] [Google Scholar]
- 26.Vinet A, Nottin S, Lecoq AM, Guenon P, Obert P. Reproducibility of cardiac output measurements by Doppler echocardiography in prepubertal children and adults. Int J Sports Med. 2001;22(6):437–441. doi: 10.1055/s-2001-16241 [DOI] [PubMed] [Google Scholar]
- 27.Stefánsson BV, Brunelli SM, Cabrera C, et al. Intradialytic hypotension and risk of cardiovascular disease. Clin J Am Soc Nephrol. 2014;9(12):2124–2132. doi: 10.2215/CJN.02680314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buchanan C, Mohammed A, Cox E, et al. Intradialytic cardiac magnetic resonance imaging to assess cardiovascular responses in a short-term trial of hemodiafiltration and hemodialysis. J Am Soc Nephrol. 2017;28(4):1269–1277. doi: 10.1681/ASN.2016060686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amundsen BH, Helle-Valle T, Edvardsen T, et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography. J Am Coll Cardiol. 2006;47(4):789–793. doi: 10.1016/j.jacc.2005.10.040 [DOI] [PubMed] [Google Scholar]
- 30.Helle-Valle T, Crosby J, Edvardsen T, et al. New noninvasive method for assessment of left ventricular rotation. Circulation. 2005;112(20):3149–3156. doi: 10.1161/circulationaha.104.531558 [DOI] [PubMed] [Google Scholar]
- 31.Choi JO, Shin DH, Cho SW, et al. Effect of preload on left ventricular longitudinal strain by 2D speckle tracking. Echocardiography. 2008;25(8):873–879. doi: 10.1111/j.1540-8175.2008.00707.x [DOI] [PubMed] [Google Scholar]
- 32.Ahn HS, Kim YK, Song HC, et al. The impact of preload on 3-dimensional deformation parameters: principal strain, twist and torsion. Cardiovasc Ultrasound. 2017;15(1):22. doi: 10.1186/s12947-017-0111-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guler HS, Tulunay Kaya C, Kumru G, et al. Acute stunning effect of hemodialysis on myocardial performance: a three-dimensional speckle tracking echocardiographic study. Artif Organs. 2020;44(10):1081–1089. doi: 10.1111/aor.13698 [DOI] [PubMed] [Google Scholar]
- 34.Yip A, Naicker S, Peters F, et al. Left ventricular twist before and after haemodialysis: an analysis using speckle-tracking echocardiography. Cardiovasc J Afr. 2018;29(4):231–236. doi: 10.5830/cvja-2018-019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu BT, Tsai-Pai MA, Hsu HY, Lin PS, Lin CH, Chen YT. Effect of preload reduction by hemodialysis on left ventricular mechanical parameters by three-dimensional speckle tracking echocardiography. Acta Cardiol Sin. 2012;28:25–33. https://www.semanticscholar.org/paper/Effect-of-Preload-Reduction-by-Hemodialysis-on-Left-Wu-Tsai-Pai/ae7da983097066139fcbd2c83b4abc578c5c7de0 [Google Scholar]
- 36.Park SJ, Nishimura RA, Borlaug BA, Sorajja P, Oh JK. The effect of loading alterations on left ventricular torsion: a simultaneous catheterization and two-dimensional speckle tracking echocardiographic study. Eur J Echocardiogr. 2010;11(9):770–777. doi: 10.1093/ejechocard/jeq064 [DOI] [PubMed] [Google Scholar]
- 37.Ferferieva V, Van den Bergh A, Claus P, et al. The relative value of strain and strain rate for defining intrinsic myocardial function. Am J Physiol Heart Circ Physiol. 2012;302(1):H188–H195. doi: 10.1152/ajpheart.00429.2011 [DOI] [PubMed] [Google Scholar]
- 38.Schlangen J, Petko C, Hansen JH, et al. Two-Dimensional global longitudinal strain rate is a preload independent index of systemic right ventricular contractility in hypoplastic left heart syndrome patients after fontan operation. Circ Cardiovasc Imaging. 2014;7(6):880–886. doi: 10.1161/circimaging.114.002110 [DOI] [PubMed] [Google Scholar]
- 39.Burns AT, Gerche AL, Prior DL, MacIsaac AI. Left ventricular torsion parameters are affected by acute changes in load: CME. Echocardiography. 2010;27(4):407–414. doi: 10.1111/j.1540-8175.2009.01037.x [DOI] [PubMed] [Google Scholar]
- 40.Voigt JU, Exner B, Schmiedehausen K, et al. Strain-rate imaging during dobutamine stress echocardiography provides objective evidence of inducible ischemia. Circulation. 2003;107(16):2120–2126. doi: 10.1161/01.cir.0000065249.69988.aa [DOI] [PubMed] [Google Scholar]
- 41.Sengupta PP, Korinek J, Belohlavek M, et al. Left ventricular structure and function. J Am Coll Cardiol. 2006;48(10):1988–2001. doi: 10.1016/j.jacc.2006.08.030 [DOI] [PubMed] [Google Scholar]
- 42.Algranati D, Kassab GS, Lanir Y. Why is the subendocardium more vulnerable to ischemia? A new paradigm. Am J Physiol Heart Circ Physiol. 2011;300(3):H1090–H1100. doi: 10.1152/ajpheart.00473.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toyota E, Ogasawara Y, Hiramatsu O, et al. Dynamics of flow velocities in endocardial and epicardial coronary arterioles. Am J Physiol Heart Circ Physiol. 2005;288(4):H1598–H1603. doi: 10.1152/ajpheart.01103.2003 [DOI] [PubMed] [Google Scholar]
- 44.Thijssen DHJ, Redington A, George KP, Hopman MTE, Jones H. Association of exercise preconditioning with immediate cardioprotection: a review. JAMA Cardiol. 2018;3(2):169. doi: 10.1001/jamacardio.2017.4495 [DOI] [PubMed] [Google Scholar]
- 45.Vaisman S, Kensey K, Cho YI. Effect of hemodialysis on whole blood viscosity. Int J Artif Organs. 2009;32(6):329–335. doi: 10.1177/039139880903200603 [DOI] [PubMed] [Google Scholar]
- 46.Medvedofsky D, Maffessanti F, Weinert L, et al. 2D and 3D echocardiography-derived indices of left ventricular function and shape. JACC Cardiovasc Imaging. 2018;11(11):1569–1579. doi: 10.1016/j.jcmg.2017.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biering-Sorensen T, Biering-Sørensen SR, Olsen FJ, et al. Global longitudinal strain by echocardiography predicts LongTerm risk of cardiovascular morbidity and mortality in a low risk general population: the copenhagen city heart study. J Am Coll Cardiol. 2016;67(13):1584. doi: 10.1016/s0735-1097(16)31585-6 [DOI] [Google Scholar]
- 48.Liu YW, Su CT, Sung JM, et al. Association of left ventricular longitudinal strain with mortality among stable hemodialysis patients with preserved left ventricular ejection fraction. Clin J Am Soc Nephrol. 2013;8(9):1564–1574. doi: 10.2215/CJN.10671012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data reported in this paper of type Analyzable Data have been deposited to Figshare, 10.6084/m9.figshare.21960083. The raw data supporting the conclusions of this article will be made available after publication by the authors, without undue reservation.