Abstract
The P gene of measles virus (MV) encodes the phosphoprotein, a component of the virus ribonucleoprotein complex, and two nonstructural proteins, C and V, with unknown functions. Growth of recombinant MV, defective in C or V expression, was explored in human peripheral blood mononuclear cells (PBMC). The production of infectious recombinant MV V− was comparable to that of parental MV tag in simian Vero fibroblasts and in PBMC. In contrast, MV C− progeny was strongly reduced in PBMC but not in Vero cells. Consistently, the expression of both hemagglutinin and fusion proteins, as well as that of nucleoprotein mRNA, was lower in MV C−-infected PBMC. Thus, efficient replication of MV in natural host cells requires the expression of the nonstructural C protein. The immunosuppression that accompanies MV infection is associated with a decrease in the in vitro lymphoproliferative response to mitogens. MV C− was as potent as MV tag or MV V− in inhibiting the phytohemagglutinin-induced proliferation of PBMC, indicating that neither the C protein nor the V protein is directly involved in this effect.
Measles virus (MV), a member of the Morbillivirus genus of the Paramyxoviridae family, is an enveloped virus with a negative-strand RNA genome of 15,894 nucleotides. The genome encodes six structural proteins: the nucleoprotein (N; 60 kDa), the phosphoprotein (P; 70 kDa), the matrix (M) protein (37 kDa), the hemagglutinin (H) protein (80 kDa), the fusion (F) protein (made of two subunits, F1 [40 kDa] and F2 [20 kDa], by cleavage of the F0 precursor [60 kDa]), and the large (L) protein (250 kDa) (2). The P cistron also encodes the nonstructural proteins C (21 kDa), V (46 kDa), and R (40 kDa), but their functions are at present unclear (2, 14).
All members of the Paramyxovirus and Morbillivirus genera express the C proteins, which are relatively small basic proteins (less than 220 residues). They are expressed from an alternate open reading frame overlapping the N-terminal portion of the P gene. The translation of MV C protein is initiated at the second AUG, which lies 22 nucleotides downstream of the P start codon (1). The MV C protein has been localized by immunofluorescence to the nucleus and cytoplasmic inclusions within infected cells (1). A recombinant MV mutant with abrogated expression of the C protein, MV C−, has been shown to replicate as efficiently as wild-type MV in simian Vero fibroblasts (15).
The V protein, expressed by all members of the Paramyxoviridae family except the Pneumovirus genus and human parainfluenza virus type 1, results from an RNA editing mechanism of the P gene. An insertion of a nontemplated G residue at position 751 in the MV P cistron leads to the synthesis of the V protein, which has 231 N-terminal amino acids in common with the P protein and 68 unique C-terminal amino acids rich in cysteine residues (4). The V protein is localized in the cytoplasm and, like the P protein, is phosphorylated (23). Because of their common sequence with the P protein and their well-conserved zinc finger-like cysteine-rich domains, V proteins are suspected to play a role in RNA synthesis. However, a recombinant MV defective in V protein, MV V−, grows as well as the wild-type virus in simian Vero cells (19).
To delineate the roles of C and V proteins in other members of the Paramyxoviridae family, a similar approach using recombinant virus with inactivated C or V genes has been undertaken. The P gene of the Sendai virus, which belongs to the Respirovirus genus, encodes four C proteins (C′, C, Y1, and Y2). Although a recombinant Sendai virus with a point mutation in C proteins was found to grow much better than its progenitor virus in cell culture, it was avirulent when inoculated into mice (8). In this virus, two RNA edition processes of the P gene result in the synthesis of V and W proteins. A recombinant V− W+ Sendai virus replicated normally in BHK cells and embryonated chicken eggs (6). Replication of recombinant Sendai virus defective in both V and W protein is enhanced in some cell lines but not in others (12) and is strongly attenuated in vivo (12). A virus encoding V protein with its unique C-terminal sequence deleted displayed a wild-type phenotype in vitro and almost the same phenotype as V− Sendai virus in vivo (13), suggesting an important role for the cysteine-rich domain of V in Sendai virus virulence.
The discrepancies observed in vitro and in vivo in the roles of Sendai virus C and V proteins in virus replication raised the possibility that their effects are restricted to some tissues. MV infection of peripheral blood mononuclear cells (PBMC) is likely to play a crucial role in the MV-induced pathogenesis observed in humans. Indeed, PBMC are thought to mediate the in vivo spreading of MV to lymphoid organs (24). In vitro, the lymphoproliferative response to mitogens of MV-infected cells is strongly decreased (20). This impaired cellular response might participate in the immunodepression observed in infected patients (cf. reference 10 for a review). This led us to investigate the replication of recombinant MV C− and MV V− in human PBMC.
Production of infectious MV C− particles is restricted in human PBMC but not in Vero cells.
Human PBMC were isolated from heparinized venous blood of healthy donors by density gradient centrifugation on Ficoll and cultured in RPMI 1640 supplemented with 10% fetal calf serum, 50 μg of gentamicin/ml, and 5 mM glutamine. Cells were infected at a multiplicity of infection (MOI) of 1 for 1 h at 37°C with either parental recombinant MV tag (derived from the Edmonston strain), MV C−, or MV V− (15, 16, 19). Unadsorbed virus was washed out once with fresh medium, and the cells were incubated at 37°C in a 7% CO2 incubator in the presence of 20 μg of phytohemagglutinin (PHA; Sigma)/ml and 50 U of human interleukin 2/ml. At the indicated times, the microplates were frozen and thawed once. After centrifugation at 280 × g for 2 min, the supernatants were collected and titrated by the method of 50% tissue culture infective doses (TCID50) on a Vero cell monolayer. MV tag and MV V− showed similar increases in the number of infectious units and reached 25,000 TCID50/ml at 5 days postinfection (p.i.) (Fig. 1). However, MV C− reached a maximum production of 39 TCID50/ml at day 3 p.i. and then declined. Since no differences in viral production of these MV mutants have been reported when Vero cells are used (15, 19), a parallel experiment using this cell line was performed. An MOI of 1 TCID50/cell was used to infect PBMC in order to obtain an optimal level of virus progeny, but this concentration of virus induced premature destruction of the cell monolayer on Vero cells. Therefore, an MOI of 0.01 TCID50/cell was used to infect Vero cells. In agreement with the previous reports, no difference in viral production was found in Vero cells, and the increase in the number of infectious units was similar no matter which virus was used (Fig. 1). Because of massive destruction of the monolayer at day 4 p.i., the kinetics were analyzed up to day 3. The difference observed for MV C− between PBMC and Vero cells was not related to the different MOIs used, because the infection of PBMC with an MOI of 0.01 TCID50/cell recapitulated the results obtained with an MOI of 1 TCID50/cell, although with a very low level of virus progeny (data not shown). A specific block of the entry of MV C− particles into PBMC is unlikely because (i) the same virus stock readily infected Vero cells and (ii) the C protein is not a component of the mature virion (1). These data indicated that the lack of C protein expression strongly impaired the infection of human PBMC, whereas it had no major influence on the infection of Vero cells.
FIG. 1.
Kinetics of infectious virus production by human PBMC and Vero cells infected with MV tag (diamonds), MV C− (circles), or MV V− (triangles). The activated PBMC and Vero cells were infected at MOIs of 1 and 0.01, respectively.
Restricted virus protein and mRNA synthesis after MV C− infection in PBMC.
To determine whether the differences observed in virus production were correlated with virus protein synthesis, the cell surface expression of the H protein on activated PBMC and on Vero cells was analyzed by flow cytometry at day 2 p.i. The immunofluorescence labelling procedure has been described elsewhere (9). Infection of PBMC with MV C− resulted in 9% of cells expressing the H protein, whereas infection with MV tag and MV V− resulted in 31 and 43% of cells expressing the H protein, respectively (Fig. 2A). In contrast, 93, 94, and 99% of Vero cells expressed H when infected with MV C−, MV tag, and MV V−, respectively (Fig. 2A). Interestingly, compared to MV tag infection, MV V− infection resulted in a consistent 5 to 10% increase in the numbers of H-expressing cells among both PBMC and Vero cells, suggesting that in the absence of V protein, MV may replicate more efficiently. Similar results were obtained when F protein expression was measured (data not shown).
FIG. 2.
Cell surface expression of the H protein after infection of human PBMC and Vero cells with MV tag, MV C−, or MV V− at MOIs of 1 and 0.01, respectively. (A) Histogram profile of H expression 2 days after mock infection (solid histograms) or MV infection (open histograms). (B) Kinetics of H expression after infection of PBMC with no virus (open squares), MV tag (solid diamonds), MV C− (solid circles), or MV V− (solid triangles).
To confirm the lower efficiency of virus protein expression in PBMC infected with MV C−, the kinetics of H expression were studied. Even after 7 days of culture, fewer than 20% of PBMC were found to express detectable H at their surfaces after infection with MV C− (Fig. 2B). In contrast, MV tag and MV V− infections resulted in H expression on 18 and 12% of cells, respectively, as early as 1 day p.i.; these levels increased to 58 and 70% at 5 days p.i.
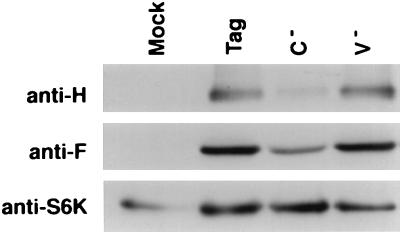
The expression of the H and F proteins was also analyzed by immunoblotting (Fig. 3). Briefly, the infected cells were lysed in 1% Nonidet P-40 buffer containing 150 mM NaCl, 50 mM Tris-HCl (pH 8.5), 5 mM EDTA, 10 mM NaF, and anti-proteases (20 μM E64, 100 μM DCI, 100 μM phenanthroline; Boehringer Mannheim and Sigma). After a 15-min incubation at 4°C, lysates were centrifuged for 15 min at 12,000 × g. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions and transferred to polyvinylidene difluoride membranes (Boehringer Mannheim). Membranes were blocked with 5% nonfat dried milk in TBS-T (20 mM Tris [pH 7.6], 150 mM NaCl, 0.1% Tween 20) and incubated for 1 h with specific antibodies in TBS-T (monoclonal antibody anti-H BH164 [7] or polyclonal anti-F cytoplasmic tail Pocono [3]). Immunoreactive bands were visualized by using secondary horseradish peroxidase-conjugated antibodies (Promega) and enhanced chemiluminescence (Pierce). The expression levels of H and F glycoproteins were almost identical in PBMC infected with MV tag and PBMC infected with MV V−. In contrast, the levels of these proteins in PBMC infected with MV C− were lower. Control immunoblotting with anti-S6 kinase antibody showed that comparable amounts of protein were loaded in each lane (Fig. 3).
FIG. 3.
Western blot analysis of the expression of H and F proteins by human PBMC infected with MV tag, MV C−, or MV V− at an MOI of 1 at day 3 p.i. S6K, S6 kinase.
Since the expression of the H and F glycoproteins was reduced both at the cell surface (as measured by cytofluorometry) and intracellularly (as measured by Western blotting with whole-cell extracts), we could exclude any impairment of MV glycoprotein maturation due to the lack of C protein.
Because of its high positive charge at physiological pH and its colocalization with the nucleoprotein in infected cells (1), the C protein was proposed to interact with RNA and play a role during viral transcription and/or replication (11). Therefore, the synthesis of MV N mRNA and MV genome RNA was evaluated in PBMC infected with MV tag or MV C−. By using the SV Total RNA Isolation System kit (Promega), total RNA was extracted from activated PBMC and either left uninfected or infected for 4 days with MV tag or MV C−. MV N mRNA and MV genome RNA were specifically quantified by densitometry of a dot blot. After hybridization with digoxigenin (DIG)-labelled N riboprobes of negative and positive polarities, the dot blot was revealed with anti-DIG–alkaline phosphatase conjugate and CDP-Star substrate (Roche Diagnostic). These probes were done by using the DIG RNA labelling kit SP6/T7 (Roche Diagnostic) from the plasmid pGEM-N in which the N nucleotide sequence 825 to 1676 was cloned between the initiation sites of SP6 and T7 polymerase (5). A control DIG-labelled actin RNA probe (Roche Diagnostic) was used. The contribution of the antigenome to the N mRNA signal was found to be negligible when the negative-polarity riboprobe was used. In the absence of C protein, the amounts of MV genome RNA and N mRNA were reduced by 25 and 66%, respectively. Thus, in PBMC, the MV C protein seemed to be required for optimal transcription, whereas it affected viral genome replication only modestly.
Almost no virus progeny was recovered from MV C−-infected human PBMC, and this correlated well with the low expression level of the viral proteins. Since the infection of the PBMC was performed at a high MOI of 1 TCID50/cell, most, if not all, virus protein expression was from the initial infectious virus input. Indeed, when the secondary cell-to-cell virus spreading was inhibited by the addition of a fusion tripeptide inhibitor, Z-d-Phe-l-Phe-Gly, 2 h after the infection (17), no significant decrease was observed in the percentage of PBMC expressing H and F glycoproteins or in their expression levels, no matter which recombinant MV was used (data not shown).
The reductions in virus protein synthesis and virus progeny after MV C− infection observed in human PBMC but not in Vero cells suggest that the C protein function might be restricted to certain cell types. Restriction of the replication of Sendai V− recombinant virus in some, but not all, cell lines has also been observed (12). A role for certain accessory proteins might then be to compensate for a missing cellular function in some cells. Importantly, PBMC are responsible for the MV spreading into the lymphoid organs (24). Therefore, our findings predict an essential role of the MV C protein for the virulence of MV in humans.
Inhibitory effect of recombinant MV on the proliferative response of PBMC.
The reduced replication of MV C− in activated PBMC led us to explore the role of the C protein in MV-induced inhibition of PHA-stimulated PBMC proliferation. Infected and mock-infected human PBMC were cultured in 0.2 ml (final volume) (5 × 104 cells/well) in 96-well flat-bottom microwell plates at 37°C. After 48 h of incubation, cells were pulsed with [3H]thymidine for 12 h and then harvested on glass fiber filters. The radioactivity was counted in a liquid scintillation beta counter. Surprisingly, MV C− infection was as inhibitory as parental MV tag infection (Fig. 4), leading to an average of 50% inhibition of thymidine uptake in three independent experiments. MV V− was consistently more efficient in inhibiting PBMC proliferation, leading to an average of 65% inhibition of proliferation. Thus, the C and V proteins are dispensable for the MV-induced inhibition of the PBMC proliferative response. These results further indicate that proliferation inhibition by MV does not require maximum virus replication. In support of this are the observations that even a small number of MV-infected PBMC can inhibit the proliferation of uninfected PBMC (18).
FIG. 4.
MV-induced inhibition of PBMC proliferation. PBMC were stimulated with PHA and interleukin 2 and infected with the indicated virus at an MOI of 1. The proliferation level was determined by [3H]thymidine incorporation, and results are expressed as counts per minute (means of triplicates ± standard deviations).
In conclusion, the infection of human PBMC with recombinant MV C− resulted in impaired synthesis of viral proteins and infectious virus production, indicating an important role for C in the natural host cells. In contrast, and as previously reported, no differences were observed in Vero cells. Further studies on the role of the C protein would allow us to determine whether any cellular components can compensate for the function of C in Vero cells, and whether the C protein plays a crucial role during an in vivo infection, as recently suggested by the reduced replication of MV C− in human thymic xenografts (22). The V protein does not seem to play a major role in MV replication in PBMC. However, this does not preclude the importance of V in the in vivo replication of MV, as illustrated by the reduced replication of MV V− observed in human thymic xenografts (22) and in cotton rats (21).
Acknowledgments
C.E. and S.M. contributed equally to this work.
We acknowledge R. Cattaneo for the kind gifts of anti-F rabbit antisera and pGEM-N plasmid and Dale Christiansen for help in writing the manuscript.
This work was partially supported by a grant from the Ministère de l’Enseignement Supérieur et de la Recherche.
REFERENCES
- 1.Bellini W J, Englund G, Rozenblatt S, Arnheiter H, Richardson C D. Measles virus P gene codes for two proteins. J Virol. 1985;53:908–919. doi: 10.1128/jvi.53.3.908-919.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bellini W J, Rota J S, Rota P A. Virology of measles virus. J Infect Dis. 1994;170:S15–S23. doi: 10.1093/infdis/170.supplement_1.s15. [DOI] [PubMed] [Google Scholar]
- 3.Cathomen T, Naim H Y, Cattaneo R. Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J Virol. 1998;72:1224–1234. doi: 10.1128/jvi.72.2.1224-1234.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cattaneo R, Kaelin K, Baczko K, Billeter M A. Measles virus editing provides an additional cysteine-rich protein. Cell. 1989;56:759–764. doi: 10.1016/0092-8674(89)90679-x. [DOI] [PubMed] [Google Scholar]
- 5.Cattaneo R, Rebman G, Schmid A, Baczko K, ter Meulen V, Billeter M. Altered transcription of a defective measles virus genome derived from a diseased human brain. EMBO J. 1987;6:681–688. doi: 10.1002/j.1460-2075.1987.tb04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delenda C, Hausmann S, Garcin D, Kolakofsky D. Normal cellular replication of Sendai virus without the trans-frame, nonstructural V protein. Virology. 1997;228:55–62. doi: 10.1006/viro.1996.8354. [DOI] [PubMed] [Google Scholar]
- 7.Fournier P, Brons N H, Berbers G A, Wiesmuller K H, Fleckenstein B T, Schneider F, Jung G, Muller C P. Antibodies to a new linear site at the topographical or functional interface between the haemagglutinin and fusion proteins protect against measles encephalitis. J Gen Virol. 1997;78:1295–1302. doi: 10.1099/0022-1317-78-6-1295. [DOI] [PubMed] [Google Scholar]
- 8.Garcin D, Itoh M, Kolakofsky D. A point mutation in the Sendai virus accessory C proteins attenuates virulence for mice, but not virus growth in cell culture. Virology. 1997;238:424–431. doi: 10.1006/viro.1997.8836. [DOI] [PubMed] [Google Scholar]
- 9.Gerlier D, Loveland B, Varior-Krishnan G, Thorley B, Mckenzie I F C, Rabourdin-Combe C. Measles virus receptor properties are shared by several CD46 isoforms differing in extracellular regions and cytoplasmic tails. J Gen Virol. 1994;75:2163–2171. doi: 10.1099/0022-1317-75-9-2163. [DOI] [PubMed] [Google Scholar]
- 10.Griffin D E. Immune responses during measles virus infection. Curr Top Microbiol Immunol. 1995;191:117–134. doi: 10.1007/978-3-642-78621-1_8. [DOI] [PubMed] [Google Scholar]
- 11.Horikami S M, Moyer S A. Structure, transcription and replication of measles virus. Curr Top Microbiol Immunol. 1995;191:35–50. doi: 10.1007/978-3-642-78621-1_3. [DOI] [PubMed] [Google Scholar]
- 12.Kato A, Kiyotani K, Sakai Y, Yoshida T, Nagai Y. The paramyxovirus, Sendai virus, V protein encodes a luxury function required for viral pathogenesis. EMBO J. 1997;16:578–587. doi: 10.1093/emboj/16.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato A, Kiyotani K, Sakai Y, Yoshida T, Shioda T, Nagai Y. Importance of the cysteine-rich carboxyl-terminal half of V protein for Sendai virus pathogenesis. J Virol. 1997;71:7266–7272. doi: 10.1128/jvi.71.10.7266-7272.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liston P, Briedis D J. Ribosomal frameshifting during translation of measles virus P protein mRNA is capable of directing synthesis of a unique protein. J Virol. 1995;69:6742–6750. doi: 10.1128/jvi.69.11.6742-6750.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radecke F, Billeter M A. The nonstructural C protein is not essential for multiplication of Edmonston B strain measles virus in cultured cells. Virology. 1996;217:418–421. doi: 10.1006/viro.1996.0134. [DOI] [PubMed] [Google Scholar]
- 16.Radecke F, Spielhofer P, Schneider H, Kaelin K, Huber M, Dotsch C, Christiansen G, Billeter M A. Rescue of measles viruses from cloned DNA. EMBO J. 1995;14:5773–5784. doi: 10.1002/j.1460-2075.1995.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson C D, Scheid A, Choppin P W. Specific inhibition of paramyxovirus and myxovirus replication by oligopeptides with amino acid sequences similar to those at the N termini of the F1 or HA2 viral polypeptides. Virology. 1980;105:205–222. doi: 10.1016/0042-6822(80)90168-3. [DOI] [PubMed] [Google Scholar]
- 18.Schlender J, Schnorr J J, Spielhofer P, Cathomen T, Cattaneo R, Billeter M A, ter Meulen V, Schneider-Schaulies S. Interaction of measles virus glycoproteins with the surface of uninfected peripheral blood lymphocytes induces immunosuppression in vitro. Proc Natl Acad Sci USA. 1996;93:13194–13199. doi: 10.1073/pnas.93.23.13194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider H, Kaelin K, Billeter M A. Recombinant measles viruses defective for RNA editing and V protein synthesis are viable in cultured cells. Virology. 1997;227:314–322. doi: 10.1006/viro.1996.8339. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan J L, Barry D W, Albrecht P, Lucas S J. Inhibition of lymphocyte stimulation by measles virus. J Immunol. 1975;114:1458–1461. [PubMed] [Google Scholar]
- 21.Tober C, Seufert M, Schneider H, Billeter M A, Johnston I C D, Niewesk S, ter Meulen V, Schneider-Schaulies S. Expression of measles virus V protein is associated with pathogenicity and control of viral RNA synthesis. J Virol. 1998;72:8124–8132. doi: 10.1128/jvi.72.10.8124-8132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valsamakis A, Schneider H, Auwaerter P G, Kaneshima H, Billeter M, Griffin D E. Recombinant measles virus with mutations in the C, V or F gene have altered growth phenotypes in vivo. J Virol. 1998;72:7754–7761. doi: 10.1128/jvi.72.10.7754-7761.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wardrop E A, Briedis D J. Characterization of V protein in measles virus-infected cells. J Virol. 1991;65:3421–3428. doi: 10.1128/jvi.65.7.3421-3428.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White R G, Boyd J F. The effect of measles on the thymus and other lymphoid tissues. Clin Exp Immunol. 1973;13:343–357. [PMC free article] [PubMed] [Google Scholar]