Abstract
This survey aims to understand the current UK practice for non-small cell lung carcinoma (NSCLC) and identify barriers that may impact patient treatment and outcomes. In March–June 2021, 57 interviews were conducted with healthcare professionals involved in the secondary care management of patients with NSCLC. Most respondents performed genetic testing at onsite and non-genomic laboratory hub (GLH) offsite locations. The most common genetic tests were EGFR T790M variant (100%), EGFR exon 18-21 covered (95%) and BRAF (93%). No targeted therapy (TT) available (69%), lack of access to a TT (54%) or excessive molecular testing turnaround times (39%) were the most common reasons for using an immuno-oncology therapy over a TT in the first-line setting. The survey highlights variation in mutation testing practices across the UK, which may impact treatment decisions and contribute to health outcome inequality.
Keywords: non-small cell lung carcinoma, real world practice, management, treatment, oncogenic genetic testing
This brief communication reports current practice for non-small cell lung carcinoma in the UK and identifies barriers that could affect patient treatment and outcomes.
Introduction
The treatment landscape of non-small cell lung carcinoma (NSCLC) has evolved with the discovery of new oncogenic mutations and the introduction of immunotherapy and targeted therapy (TT).1,2 In the UK, molecular profiling, including genetic testing, has become standard practice in the management of NSCLC to help determine suitable treatment options. The national genomic testing service is delivered through a network of Genomic Laboratory Hubs (GLHs) to address variations in quality and access to genetic testing across England.3 However, the extent to which GLHs are utilized for NSCLC within the UK at the time of the survey is unclear. Despite the standardization of testing infrastructure offered by GLHs, genetic testing rates remain suboptimal across the UK.4,5 This is likely due to prolonged turnaround time (TAT), primarily affected by delays in sample request and delivery, analysis and reporting of testing results.5,6 The aim of the Lung Adjuvant and Metastatic Pathway Survey (LAMPS) was to understand the current genetic testing practices for NSCLC in the UK.
Methods
The LAMPS survey was conducted with healthcare professionals (HCPs) known to be involved in the secondary care management of patients with NSCLC between April 2021 and July 2021. HCPs were identified from general and specialist NHS Trusts of varying sizes and levels of research expertise, based at geographically dispersed centers across the UK. The Steering Committee invited 150 potential respondents from an existing UK database of secondary lung cancer care across the UK to participate by email; those who provided consent and were available for interview were contacted by the Novartis Medical Team to take part in the survey. A steering committee consisting of 3 external clinical leads (medical oncologist, respiratory physician, and senior clinical nurse specialist) collaborated with Novartis to develop the structured questionnaire used in the survey. Interviews were carried out remotely by members of the Novartis Medical Team. Descriptive analysis was performed.
Results
Demographics and Respondent and Center Profile
In total, 57 HCPs completed the survey, including medical oncologists (40%, n = 23), clinical oncologists (26%, n = 15), clinical nurse specialist (16%, n = 9), pathologists (9%, n = 5), respiratory physicians (5%, n = 3), oncology middle grade (2%, n = 1) and research nurse (2%, n = 1) (Supplementary Fig. S1). Respondents were from geographically distributed centers across the UK (England [84%, n = 48], Northern Ireland [7%, n = 4], Scotland [5%, n = 3], Wales [4%, n = 2]; Supplementary Table S1) with representation from university teaching hospitals (84%, n = 48) and district general hospitals (16%, n = 9).
Genetic Testing Practices
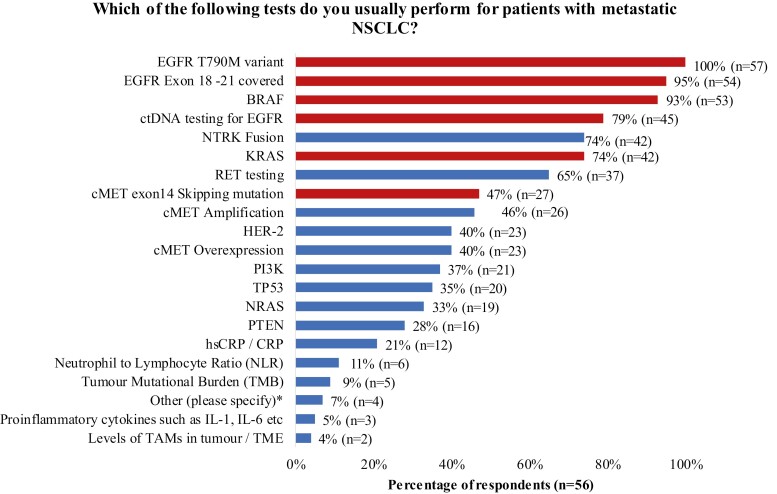
All respondents (100%, n = 57) stated that their standard genetic testing panel included ALK (anaplastic lymphoma kinase), PD-L1 (programmed death-ligand 1) and EGFR (epidermal growth factor receptor) tests; 96% (n = 55) of respondents included ROS-1 (ROS proto-oncogene 1) test. Most respondents performed their standard panel genetic testing at onsite (47%, n = 27) and non-GLH offsite (30%, n = 17) locations; 23% (n = 13) of respondents performed genetic testing at a GLH. In the standard testing panel used by onsite and non-GLH offsite centers, the top 3 most common tests were EGFR T790M variant (100%, n = 57), EGFR exon 18-21 covered (95%, n = 54), and BRAF (93%, n = 53) (Fig. 1). However, cMET exon 14 skipping (cMETex14) mutation was tested by 47% (n = 27) of onsite and offsite non-GLH locations at the time of the survey (Fig. 1) in contrast to GLHs where it forms part of their standard testing panel (Supplementary Table S2; S3).
Figure 1.
Genetic tests currently performed for patients with metastatic NSCLC at onsite and offsite non-GLH centers. Figure depicts tests performed in real world practice either onsite or at offsite non-GLH locations at the time of survey completion. Red bars in the figure indicate the percentage of respondents currently testing for mutation drivers that feature as part of the standard GLH panel (see Supplementary Tables S2 and S3 for the GLH panel). *Four HCPs responded that other tests were performed, including ALK (n = 2), PDL1 (n = 2), ROS1 (n = 2), and liquid biopsy (n = 1). BRAF, B-Raf proto-oncogene; cMET, tyrosine-protein kinase Met; ctDNA, circulating tumor deoxyribonucleic acid; EGFR, epidermal growth factor receptor; HER-2, human epidermal growth factor receptor-2; IL, interleukin; KRAS, Kirsten rat sarcoma viral oncogene homolog; NRAS, neuroblastoma RAS viral oncogene homolog; NTRK, neurotrophic tropomyosin or tyrosine receptor kinase; PI3K, phosphoinositide 3-kinases; PTEN, phosphatase and tensin homolog; RB1, RB transcriptional corepressor 1; RET, REarranged during Transfection proto-oncogene; TAM, tumor-associated macrophages; TP53, tumor protein p53.
Current Treatment Practices
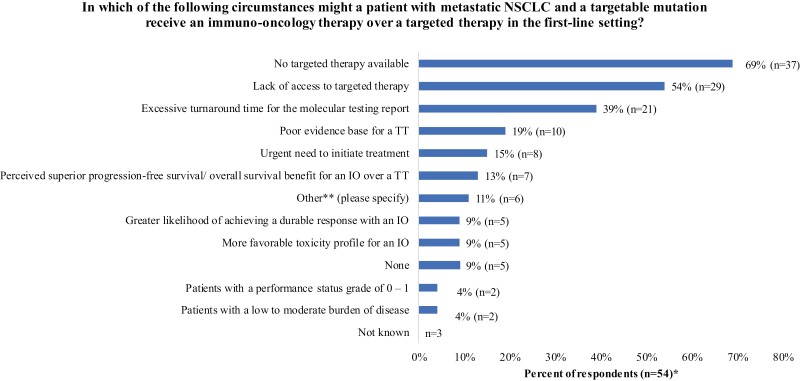
In the survey, HCPs were asked about their preferences toward an effective TT (if available) versus an immuno-oncology (IO) treatment in the first-line setting. For patients with high PD-L1 expression and a driver mutation, all respondents (100%, n = 57) preferred to use TT if available over an IO-based treatment in the first-line setting. Additionally, HCPs were asked about their view on current barriers in clinical practice that could impact a treatment choice for a specific patient group. No TT available (69%, n = 37/54), lack of access to a TT (54%, n = 29/54) and excessive molecular testing turnaround times (TAT) (39%, n = 21/54) were the most common circumstances where HCPs might treat a patient with metastatic NSCLC and a targetable mutation with an IO-based therapy over a TT in the first-line setting (Fig. 2).
Figure 2.
Circumstances in which HCPs might treat a patient with metastatic NSCLC who has a targetable mutation with an immuno-oncology therapy over a targeted therapy in the first-line setting. *Responses are not mutually exclusive. **Other circumstances were reported, including lack of tissue for biomarker testing (n = 1); administration problems (n = 1); counter indication to TT (n = 1); 2 weeks for genetic test turnaround (n = 1); for ROS-1 that is tested sequentially after common mutation (n = 1); and EGFR ex20 mutation would receive IO (n = 1). EGFR, epidermal growth factor receptor; HCPs, healthcare professions; IO, immuno-oncology; ROS-1, ROS proto-oncogene 1; TT, targeted therapy.
Discussion
This survey provides an overview of the genetic testing practices for NSCLC across the UK and highlights potential barriers that may impact patient treatment and outcomes. The results showed variation in mutation testing practices; this may impact diagnosis and contribute to health outcome inequality. With the emergence of TT options in recent years,7,8 oncogenic driver testing will become increasingly important in selecting appropriate treatments to achieve optimal clinical outcomes. However, less than half of respondents’ centers performed genetic testing for newer targets (HER2, cMET). Centers (conducting onsite and non-GLH offsite testing) are encouraged to seek access to a broad panel of genetic testing for NSCLC. Moreover, cMETex14 mutation testing has recently been included in the National Genomic Test Directory and has been introduced in other country guidelines (Supplementary Table S2; S3); it may be adopted across non-GLH centers.
The survey underlines potential barriers to accessing TT (availability, access) as a first-line treatment in the metastatic NSCLC setting, implying unmet medical needs for the discovery of novel molecular targets. Excessive testing TAT was also perceived as a key barrier to accessing TT as a first-line treatment, signifying the urgent need for improvement in availability and efficiency of molecular testing services to inform treatment choices.
These results are consistent with research suggesting variations in molecular testing practices in other European countries.9,10 Using external laboratories for biomarker testing is mainly due to a lack of structured access to testing and limited reimbursement/funding, as well a lack of public health system support.9,10 However, molecular testing is expected to become more centralized in the future to improve patient access, testing efficiency and quality while reducing costs.6
Taken together, our findings highlight the need to address barriers to accessing molecular testing in order to facilitate patient access to molecular-driven therapy and improve clinical outcomes.
Limitations to this survey design include HCP selection methodology, limited geographical representation from the devolved nations, and most participating centers being larger specialist and clinical research focused centers. Responses were based on the interviewed HCPs (limited to a small sample size, n = 57) and may be subject to recall bias. The views of a sample of HCPs involved in the management of NSCLC may not represent the wider UK treating population. There have been changes to real-world practice since the time of the survey (eg, novel TT approved for systemic management7,8; METex14 mutation included in the testing panel [Supplementary Table S2; S3]). This survey was conducted during the first wave of COVID-19 which may influence some of the responses (eg, testing TATs). The descriptive analysis limits data interpretation. Future work may focus on how these survey findings vary by geographic location or respondent speciality.
Supplementary Material
Acknowledgments
We thank all the healthcare professionals who participated in the survey and also the Novartis UK Medical Team, in particular Adrienne Gallant Lanctot, Stephen McCormack, Victoria Ross, Melanie Hovey, and Stuart Ferguson, who contributed to the design of the survey and collection of data. Joe Eva (Senior Data Analyst) of OPEN Health conducted the data analysis, and Fiona Glen (Principal Scientific Consultant) and Hui-Hsuan Liu (Associate Consultant Writer) of OPEN Health provided medical writing support for this manuscript, funded by Novartis.
Contributor Information
Shobhit Baijal, University Hospital Birmingham NHS Trust, Birmingham, UK.
Philip Crosbie, Manchester University NHS Foundation Trust, Manchester, UK; Division of Infection, Immunity and Respiratory Medicine, Faculty of Biology, Medicine and Health, University of Manchester, UK.
Jackie Fenemore, The Christie NHS Foundation Trust, Manchester, UK.
Ketul Desai, Novartis Pharmaceuticals UK Limited.
Funding
The study was supported by Novartis.
Conflict of Interest
Shobhit Baijal reports honoraria from Abbvie, Amgen, AstraZeneca, Boehringer Ingleheim, Bristol Myers-Squibb, Chugai, Daiichi Sankyo, FoundationOne, GlaxoSmithKline, Lilly, Merck Serono, Novartis, Pierre Fabre, Pfizer, Roche, Servier, Sanofi, and Takeda. Philip Crosbie has received honoraria from Novartis and AstraZeneca, consultancy fees from Everest Detection and NorthWest EHealth, and has share options with Everest Detection. Jackie Fenemore has received honoraria from Amgen, AstraZeneca, Lilly, and Novartis for advisory board meetings and educational input. Ketul Desai is a medical employee at Novartis.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Imyanitov EN, Iyevleva AG, Levchenko EV.. Molecular testing and targeted therapy for non-small cell lung cancer: current status and perspectives. Crit Rev Oncol Hematol. 2021;157(10):103194. 10.1016/j.critrevonc.2020.103194. [DOI] [PubMed] [Google Scholar]
- 2. Mielgo-Rubio X, Uribelarrea EA, Cortés LQ, Moyano MS.. Immunotherapy in non-small cell lung cancer: update and new insights. J Clin Transl Res 2021;7(1):1-21. [PMC free article] [PubMed] [Google Scholar]
- 3. NHS England. Genomic Laboratory Hubs. 2022. Available at https://www.england.nhs.uk/genomics/genomic-laboratory-hubs/. Accessed February 21, 2022.
- 4. Royal College of Physicians. National lung cancer audit. Annual report 2018. 2020. Available at https://www.rcplondon.ac.uk/projects/outputs/nlca-annual-report-2018.
- 5. Adizie JB, Tweedie J, Khakwani A, et al. Biomarker testing for people with advanced lung cancer in England. JTO Clin Res Rep 2021;2(6):100176. 10.1016/j.jtocrr.2021.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kerr KM, Bibeau F, Thunnissen E, et al. The evolving landscape of biomarker testing for non-small cell lung cancer in Europe. Lung Cancer. 2021;154(10):161-175. 10.1016/j.lungcan.2021.02.026. [DOI] [PubMed] [Google Scholar]
- 7. Li BT, Smit EF, Goto Y, et al. Trastuzumab deruxtecan in HER2-mutant non–small-cell lung cancer. NEJM 2022;386(3):241-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paik PK, Felip E, Veillon R, et al. Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med. 2020;383(10):931-943. 10.1056/NEJMoa2004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thunnissen E, Weynand B, Udovicic-Gagula D, et al. Lung cancer biomarker testing: Perspective from Europe. Transl Lung Cancer Res 2020;9(3):887-897. 10.21037/tlcr.2020.04.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ryska A, Berzinec P, Brcic L, et al. NSCLC molecular testing in Central and Eastern European countries. BMC Cancer 2018;18(1):269. 10.1186/s12885-018-4023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.