Abstract
Inflammatory myofibroblastic tumors (IMTs) are intermediate-grade mesenchymal neoplasms commonly characterized by chromosomal rearrangements causing constitutive activation of anaplastic lymphoma kinase (ALK) and/or ALK mutations causing reduced sensitivity to ALK tyrosine kinase inhibitors (TKI). We present a patient with an IMT who initially responded to first-line alectinib, but who later suffered disease relapse and presently survives with moderate residual disease after receiving second-line lorlatinib. Biopsy specimens were analyzed using next generation sequencing (DNA-seq and RNA-seq) and reverse phase protein microarray (RPPA) as part of an institutional Molecular Tumor Board (MTB) study. An EML4-ALK rearrangement and EGFR activation (pEGFRY1068) were present in both the primary and recurrent tumors, while a secondary ALK I1171N mutation was exclusive to the latter. EGFR signaling in the background of a secondary ALK mutation is correlated with reduced ALK TKI sensitivity in vitro, implicating an important mechanism of drug resistance development in this patient. The RPPA results also critically demonstrate that ALK signaling (ALKY1604) was not activated in the recurrent tumor, thereby indicating that standard-of-care use of third- or fourth-line ALK TKI would not likely be efficacious or durable. These results underscore the importance of real-time clinical integration of functional protein drug target activation data with NGS in the MTB setting for improving selection of patient-tailored therapy.
Keywords: inflammatory myofibroblastic tumor, reverse phase protein microarray, DNA sequencing, proteogenomics, proteomics
This case report illustrates the need for real-time integration of proteogenomic data from laser microdissected tumor specimens and dynamic biopsy testing in a molecular tumor board setting to comprehensively identify the evolution of disease resistance mechanisms and toward improved precision oncology
Key Points.
Proteomic evidence supporting EGFR-associated ALK inhibitor resistance in the inflammatory myofibroblastic tumor (IMT) patient presented here exemplifies the critical need for real-time clinical integration of multi-omic data in the molecular tumor board (MTB) setting.
Iterative biopsy testing by next generation sequencing (DNA-seq and RNA-seq) revealed an EML4-ALK rearrangement in primary (TKI-naïve) and recurrent (post-alectinib, on-lorlatinib) biopsies with a secondary ALK mutation exclusively in the latter, but the reverse phase protein microarray (RPPA) proteomic data critically revealed a background activation of EGFR signaling.
The proteomic data also critically revealed that the standard-of-care drug target, the ALK protein, was not activated in the recurrent specimen, suggesting that continued use of second- or third-line ALK inhibitor therapy is unlikely to be efficacious or durable.
Introduction
Inflammatory myofibroblastic tumors (IMTs) are rare intermediate-grade mesenchymal neoplasms comprised of myofibroblastic and fibroblastic spindle cells and high immune infiltration which predominantly originate in the lungs, liver, or gastrointestinal tract. Rarely, they occur in the female genital tract or maxillofacial regions.1,2 Nearly half (40-60%) of IMTs express anaplastic lymphoma kinase (ALK), and chromosomal rearrangements between ALK and a variety of fusion partners resulting in constitutive activation of ALK are common.3-11 First-line treatment of ALK-positive IMTs generally involves surgical resection.1,12,13 Crizotinib (a first-generation ALK tyrosine kinase inhibitor, TKI) was recently approved by the U.S. Food and Drug Administration (FDA) for unresectable, recurrent, or refractory ALK-positive IMT.14 Recurrent IMTs with ALK rearrangements often develop resistance to crizotinib and are thus treated with second- or third-line ALK inhibitors with varying levels of responsiveness.11,15,16 We present a case report for a patient who presented with an ALK-positive epithelioid IMT (a rare malignant IMT subtype17) who initially benefited from a second-generation ALK kinase inhibitor (alectinib) given as a first-line therapy with a near-complete radiographic response, but who later suffered disease recurrence and progression while continuing to receive alectinib and was thus started on second-line lorlatinib (a third-generation ALK TKI). At the time of last follow-up, the patient remains surviving with moderate residual disease after continuing 3.5 months of treatment with lorlatinib. Dynamic biopsy testing and proteogenomic analyses using commercially available multi-omic analysis via DNA-sequencing (DNA-seq), gene fusion detection by RNA-sequencing (RNA-seq), and reverse phase protein microarray (RPPA) protein activation mapping as part of an institutional Molecular Tumor Board (MTB) study were performed. The integration of functional protein activation data and clinical NGS (DNA-seq and RNA-seq) data suggested that the mechanism of ALK inhibitor resistance was likely related to persistent EFGR signaling in the background of a secondary ALK I1171N mutation, as previously described in vitro.18
Patient Story
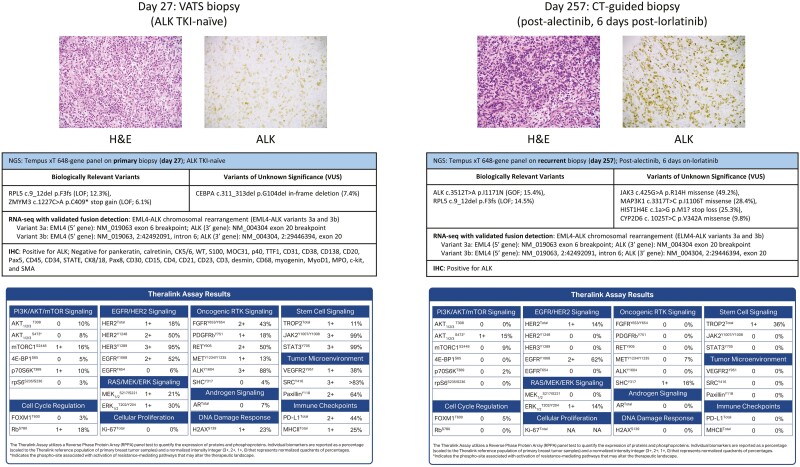
A 49-year-old woman who was a former light-smoker (0.25 packs per day) with no additional significant personal clinical history presented with shortness of breath, and a chest x-ray revealed a large left pleural effusion with atelectasis. A complete timeline of the patient’s relevant clinical history is reported in Fig. 1. Computed tomography (CT) of the chest showed left pleural masses mixed with a small-to-moderately sized pleural effusion and mass-like left lower lobe infrahilar consolidation (6.3 × 2.8 cm; Fig. 2). The patient had mildly prominent mediastinal lymph nodes measuring up to 1.4 cm. A video-assisted thoracoscopic (VATS) biopsy revealed an epithelioid neoplasm with prominent inflammatory infiltration and positive ALK (distinctive nuclear membranous reactivity) staining by immunohistochemistry (IHC; Fig. 3). Additional clinical IHC revealed negative reactivity for pankeratin, calretinin, CK5/6, WT, S100, MOC31, p40, TTF1, CD31, CD38, CD138, CD20, Pax5, CD45, CD34, STATE, CK8/18, Pax8, CD30, CD15, CD4, CD21, CD23, CD3, desmin, CD68, myogenin, MyoD1, MPO, c-kit, and SMA. Clinical next generation DNA sequencing (DNA-seq) was performed on a left pleural mass biopsy specimen (~20% tumor cellularity) by Tempus Labs, Inc. (Chicago, IL) using the xT gene panel, targeting a panel of 648 genes.19 Biologically relevant (potentially actionable) mutations identified by DNA-seq included a c.9_12del p.F3fs (NM_000969) frameshift mutation leading to loss-of-function (LOF) in RPL5 (variant allele frequency (VAF) = 12.3%) and a c.1227C>A p.C409* mutation leading to the gain of a stop codon in ZMYM3 (VAF = 6.1%). An in-frame deletion variant of unknown significance (VUS) was also measured in CEBPA (c.311_313del p.G104del; VAF = 7.4%). Whole transcriptome RNA sequencing (RNA-seq) with validated fusion detection was also conducted with the Tempus xT panel, which revealed a clinically-relevant EML4-ALK chromosomal rearrangement (Fig. 3). The EML4-ALK fusion coordinates in the primary tumor specimen correspond to EML4-ALK variants 3a and 3b.20 Specifically, variant 3a has an EML4 (5′ gene) exon 6 breakpoint (NM_019063) and an ALK (3′ gene) exon 20 breakpoint (NM_004304). Variant 3b has an EML4 (5′ gene) intron 6 breakpoint (NM_019063; 2:42492091) and an ALK (3′ gene) exon 20 breakpoint (NM_004304; 2:29446394).
Figure 1.
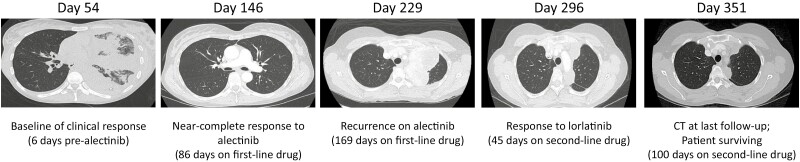
Timeline of patient’s clinical history. Timeline depicts relevant clinical events during the patient’s disease history, beginning at day 0 when the patient presented in the clinic with symptomatic complaint and ending at day 351 as the time of last follow-up with the patient surviving with moderate residual disease. VATS = video-assisted thoracoscopic. CT = computed tomography. TKI = tyrosine kinase inhibitor. CLIA testing (NGS) = next generation DNA-sequencing (DNA-seq) and RNA-sequencing (RNA-seq) with validated fusion analysis performed on solid tissue and/or liquid biopsy specimens by Tempus Labs, Inc. RPPA = reverse phase protein microarray analysis.
Figure 2.
Representative computed tomography (CT) scans obtained throughout the patient’s clinical history. CT scan images depict the tumor size at baseline assessment 6 days prior to initiation of alectinib (day 54), near-complete response to alectinib (day 146), tumor size at disease recurrence while receiving alectinib (day 229), tumor size at recurrent biopsy surgery occurring 6 days after initiation of lorlatinib (day 257), and tumor size at the time of last follow-up (day 351).
Figure 3.
Proteogenomic clinical and research testing performed on biopsy tissue specimens. Biopsy tissue specimens were obtained from a left pleural mass from the patient’s primary (day 27; ALK TKI-naïve) and recurrent (day 257; post-alectinib, 6 days on-lorlatinib) tumors. Representative immunohistochemistry (IHC), next generation DNA- and RNA-sequencing, and reverse phase protein microarray (RPPA) results are shown for each biopsy specimen. IHC images (micrographs, top row) show the representative hematoxylin and eosin (H&E) stained tissue and tissue which was immunohistochemically stained for ALK (200× magnification). NGS results (summary tables, middle row) from DNA-sequencing using the Tempus xT 648-gene panel highlight biologically relevant gene variants, variants of unknown significance (VUS), and chromosomal rearrangements identified by RNA-sequencing with validated fusion detection. RPPA results (summary tables, bottom row) using the Theralink RPPA assay represent the total protein and phosphoprotein abundances of several known cancer-related targets.
The patient was ultimately diagnosed with an ALK-positive IMT (Fig. 3) with atypical features nearly 1 month after initial presentation of symptoms. She was admitted to the hospital shortly thereafter due to respiratory distress and was noted to have complete opacification of the left lung. The patient was treated with a thoracentesis with a chest tube placement. Surgical resection (extrapleural pneumonectomy) was entertained however due to the progressive nature of the tumor, a systemic control was attempted prior to considering surgical resection. She initiated alectinib therapy (baseline tumor measuring 12.7 × 5.5 cm) which was selected after receiving FDA approval for first-line use in lung cancers with ALK fusions based on the ALEX trial (NCT02075840).21 Given the rarity of IMTs and lack of specific FDA approval therapies for this tumor type at the time of this patient’s primary disease diagnosis, we extrapolated from the ALEX trial data and selected alectinib for first-line use in this patient. A restaging chest CT scan obtained after nearly 3 months of alectinib administration revealed a near-complete radiographic response with only a remaining indeterminate density along the left fissure (Fig. 2, Day 146), and she was thus continued on alectinib. Given the remarkable response, surgery was deferred.
After 5.5 months of receiving alectinib, a chest CT scan revealed a 10.6 × 6.5 cm mass in the superior medial left hemithorax with a small pleural effusion increased from her prior presentation (Fig. 2, Day 229). DNA-seq was performed on a peripheral blood cell-free DNA (cfDNA) sample (post-alectinib) using the Tempus xF liquid biopsy test, targeting a panel of 105 genes.22 No biologically relevant variants or VUS were measured. The patient declined surgery and began lorlatinib after a final cumulative 6.4 months on alectinib, and a CT-guided biopsy with onsite assessment of a left pleural mass was performed within the first week of lorlatinib administration, revealing a recurrent/residual IMT. Immunohistochemistry (IHC) on the recurrent/residual biopsy confirmed ALK-positivity (Fig. 3). DNA-seq using the 648-gene Tempus xT targeted panel was performed using a post-alectinib, on-lorlatinib left pleura biopsy specimen (~40% tumor cellularity) from the recurrent/residual tumor. The 648-gene panel identified a biologically relevant c.3512T>A p.I1171N (NM_004304) gain-of-function (GOF) mutation in ALK (VAF = 15.4%), and the same c.9_12del p.F3fs frameshift LOF mutation in RPL5 (VAF = 14.5%) as previously measured in the TKI-naïve specimen. Other VUS detected in the recurrent specimen included a JAK3 c.425G>A p.R14H missense variant (VAF = 49.2%), a MAP3K1 c.3317T>C p.I1106T missense variant (VAF = 28.4%), a HIST1H4E c.1a>G p.M1? loss of a start codon (VAF = 25.3%), and a CYP2D6 c.1025T>C p.V342A missense variant (VAF = 9.8%). RNA-seq analysis with validated fusion detection measured the same EML4-ALK rearrangements (variants 3a and 3b) as detected in the TKI-naïve specimen.20
A CT scan obtained 1.5 months after initiation of lorlatinib showed the left pleural effusion had nearly resolved (Fig. 2, Day 296). After approximately 3.5 cumulative months of lorlatinib administration, a CT was repeated, and revealed an interval decrease in size of the pleural bi-lobed mass, with a final size measuring 5.6 × 1.9 cm (Fig. 2, Day 351). The linear left anterior basilar atelectasis and linear nodular thickening along the major fissure remained unchanged, and no new sites of disease were identified.
Molecular Tumor Board
This study was conducted under a central IRB-approved protocol and the patient provided informed consent and was enrolled in Inova Schar Cancer Institute’s Molecular Tumor Board (MTB) study, which included the provision to publish the results of the precision medicine testing, including the RPPA test results. Under this research protocol, research use only (RUO)-based analysis of the expression and/or phosphorylation (“activation”) levels of 32 proteins that are the known direct drug targets of FDA-approved and experimental therapeutics was performed using reverse phase protein microarray (RPPA) by Theralink Technologies, Inc. (Golden, CO).23 The patient’s TKI-naïve primary (pre-alectinib, pre-lorlatinib) and recurrent (post-alectinib, on-lorlatinib) biopsy tissue specimens were processed via laser microdissection (LMD) to isolate tumor epithelial cells from the tumor microenvironment,24 which were analyzed by RPPA. Of note, while the recurrent biopsy specimen was received only 6 days after beginning lorlatinib, precedent exists to suggest this interval represents sufficient time to see a visible clinical effect from a TKI targeting a clear driver mutation.25 Critically, the real-time integration of RPPA-generated protein/phosphoprotein data as a lab-developed test (LDT) with clinical NGS (DNA-seq and RNA-seq) data enables the evaluation of the actionability of targeting genomic alterations at the level of direct functional measurement of the protein drug target activation/expression from quantitative proteome-level data, representing complementary and synergistic data levels with the potential to improve the selection of patient-tailored therapy regimens.26,27 The Theralink RPPA assay reports the abundances of individual biomarkers as a percentage scaled to a referent consisting of the values of each analyte derived from a population of laser microdissected epithelium from tumors of a reference population of cancer patients along with co-arrayed protein and phosphoprotein calibration standards that provide a means of interpolation. The level of activation/expression of each protein or phosphoprotein analyte is reported as a percentage ranging from non-expressed/non-activated (0%) to highly expressed/highly activated (100%), and as a normalized intensity integer representing normalized quadrants of percentages as 0 (non-expressed/non-activated), 1+ (low expression/activation), 2+ (moderately expressed/activated), or 3+ (highly expressed/activated).26
RPPA analysis of LMD enriched tumor cells collected from the TKI-naïve IMT biopsy sampled from the same left pleural mass as was used for initial clinical NGS (DNA-seq and RNA-seq fusion analysis) revealed activation of EGFR/HER2 signaling, oncogenic RTK signaling, and JAK/STAT signaling (Fig. 3). Consistent with the observation of ALK expression measured by IHC, activation of ALK (measured by phosphorylated (p) ALKY1604) was also highly elevated in LMD enriched tumor cells (normalized intensity integer = 3+; 88% relative to reference samples). Comparatively, RPPA analysis of LMD enriched tumor cells collected from an IMT biopsy sampled after exposure to first and second-line ALK inhibitors (from a different left pleural mass tissue block than was used for NGS) found essentially no activation of ALK (pALKY1604; normalized intensity integer = 0; 0% relative to reference samples) (Fig. 3), likely owing in part to suppression of activity by alectinib and/or lorlatinib. The recurrent tumor additionally had complete loss of measurable phosphoprotein levels of pRBS780, pHER2Y1248, pHER3Y1289, pFGFRY653/Y654, pPDGFRbY751, pRETY905, pJAKY1007/Y1008, pSTATY705, pVEGFR2Y951, pSRCY416, and pPaxillinY118, as well as no measurable expression of PD-L1 (total), or MHCII (total) (Fig. 3), all of which were moderately to highly elevated, ranging from 1+ to 3+, or 13% to 99%, prior to TKI therapy, implicating ALK-independent factors as mediators of ALK TKI resistance. Evidence of substantial EGFR signaling (ie, pEGFRY1068) was found in both the primary and recurrent tumor specimens (2+; 52% and 62%, respectively). EGFR signaling in vitro is associated with resistance to ALK inhibitors in cell lines possessing a secondary ALK mutation,18 such as the I1171N mutation detected in this patient’s recurrent tumor.
Patient Update
At the time of last follow-up, the patient continues to derive benefit from receiving lorlatinib, though the response is not as remarkable as the initial response to alectinib. The patient remains surviving with moderate residual disease, with radiographic measurement via CT scan revealing the final tumor size measuring 5.6 × 1.9 cm (Fig. 2, Day 351).
Discussion
ALK gene mutations and chromosomal rearrangements resulting in fusion variants encoding ALK are associated with differential sensitivities to first- or second-line ALK-inhibitors in ALK-positive tumors such as IMT, non-small cell lung cancer (NSCLC), neuroblastoma, and anaplastic large cell lymphoma (ALCL).6,7,11,18,28 Administration of crizotinib as a monotherapy in ALK-positive IMTs only recently gained FDA approval in July 2022.14 The I1171N ALK mutation measured in the IMT patient presented here is associated with reduced sensitivity to crizotinib and alectinib, as nonsynonymous mutations that convert this residue from isoleucine to asparagine (N), threonine (T), or serine (S) are known to distort the αC helix, thereby interfering with TKI binding.6,29,30 Patients with the I1171N mutation may be alternatively sensitive to ceritinib and/or brigatinib based on results from NSCLC and neuroblastoma studies.7,11,31,32 EML4-ALK fusion proteins (of which at least 15 variants have been identified, differing by the point of fusion within the EML4 gene) contain the complete intracellular kinase domain of ALK and the trimerization domain (TD) of EML4, leading to constitutive activation of ALK through oligomerization and autophosphorylation.33-40
Protein drug target activation mapping analysis using RPPA on LMD enriched tumor samples from this patient revealed significant activation of EGFR signaling (pEGFRY1068) in both the ALK inhibitor-naïve (2+; 52%) and ALK inhibitor-treated (2+; 62%) specimens. Notably, no EGFR mutation was detected in any of the clinical NGS results. Activation of EGFR signaling with the occurrence of a secondary ALK mutation is associated with ALK TKI resistance, including in patients with EML4-ALK rearrangements.18,41 Specifically, Sasaki et al demonstrate in vitro that cells co-dependent on ALK and EGFR possess ALK TKI resistance due to having both a background of EGFR signaling and a secondary ALK mutation.18 While the precise mechanism by which EGFR signaling confers resistance remains unknown, it has been suggested to be related to ligand-mediated autocrine activation, as elevated abundances of EGFR ligands are present in conditioned media from ALK TKI resistant cell cultures.18 In our patient, the change in responsiveness to alectinib coincided with the development of the ALK I1171N mutation which occurred in a pre-existing background of significant (2+) EGFR expression. ALKTotal was retained in both the primary and recurrent biopsy specimens. ALK signaling (pALKY1064) was not activated in the TKI-treated tumor, likely reflecting some clinical benefit/response from ALK TKI therapy as well as implicating additional ALK-independent factors as mediators of ALK TKI resistance (ie, the persistent residual disease). Collectively, these data suggest that the persistent EGFR signaling measured in both primary and recurrent tumors in combination with the secondary ALK I1171N mutation detected in the recurrent tumor underlie the development of ALK TKI resistance in the IMT patient presented here. In vitro results from cell line-based proliferation and growth assays have demonstrated that concurrent inhibition of both EGFR and ALK is therapeutically effective in ALK inhibitor-resistant models.18
There are currently no approvals for concurrent administration of multiple inhibitors targeting ALK and EGFR for use in any cancers, although brigatinib has known multi-RTK inhibitory activity against ALK and EGFR (and ROS1).10,42,43 A previous phase I dose-escalation trial (NCT01121575) investigating concurrent use of dacomitinib and crizotinib in NSCLC patients demonstrated greater toxicity than either used as monotherapy alone, and the maximum tolerated dose was lower than the clinical use of either as a monotherapy.44 In the same clinical trial, crizotinib was selected for its inhibition of MET and relevance to MET-amplified NSCLCs, so ALK-positivity was not the focus of the study and the investigators reported that there were no samples positive for ALK rearrangement. Therefore, it remains to be seen whether concurrent targeting of ALK and EGFR in ALK-positive IMTs will overcome the development of TKI resistance in a clinical setting, but the increased combinatorial multidrug-associated toxicity remains an important consideration.
In this case study, we present a patient with an ALK-positive IMT which was initially responsive to ALK TKI using alectinib, but who later relapsed with the recurrent tumor demonstrating reduced sensitivity to second-line lorlatinib. At the time of last follow-up, the patient continues to derive benefit from receiving lorlatinib, although the response is not as remarkable as the initial response to alectinib. Critically, the analysis of protein signaling and protein drug target activation in the LMD enriched tumor cells from tissue specimens using RPPA analysis revealed evidence supporting important mechanistic associations relating to EGFR signaling for reduced drug sensitivity, which would not have been measured using standard clinical testing relying on NGS and IHC alone. The IHC results from this patient demonstrate ALK positivity in both the TKI-naïve and post-ALK TKI treated tumors, but the RPPA results critically demonstrate that the activation of the drug target itself is entirely absent, as indicated by lack of any measurable pALKY1604 in the post-alectinib, on-lorlatinib sample. Standard-of-care therapy would normally support the continued administration of second and third-line ALK TKI in this patient, which we show by proteomics would not likely be efficacious or durable. The RPPA results instead support concurrent targeting of both ALK and EGFR as a means of overcoming previously observed EGFR-associated mechanisms of TKI resistance.18 We additionally suggest these data support a critical need for iterative biopsy testing, LMD enrichment of tumor tissue specimens, and proteomic/phosphoproteomic analysis to directly measure the functional protein signaling pathways that will lead to improved clinical outcomes. Since NGS-based profiling provides inferential information about protein expression and activation, real-time clinical integration of functional protein drug target activation mapping data with NGS in the molecular tumor board setting will address this unmet need and lead to improved success of patient-tailored treatments supporting personalized cancer therapy.45-48
Acknowledgments
The authors thank Justin Davis, Brian Corgiat, Sherri Saturley, Kianne Kiel, and Jessica Wyman at Theralink, Inc. for their contributions. The views expressed herein are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or U.S. Government.
Contributor Information
Allison L Hunt, Women’s Health Integrated Research Center, Women’s Service Line, Inova Health System, Annandale, VA, USA; Gynecologic Cancer Center of Excellence, Gynecologic Surgery and Obstetrics, Uniformed Services University and Walter Reed National Military Medical Center, Bethesda, MD, USA.
Aratara Nutcharoen, Department of Pathology, Inova Fairfax Hospital, Falls Church, VA, USA.
Jamie Randall, Inova Schar Cancer Institute, Inova Health System, Fairfax, VA, USA.
Alyssa Papazian, Inova Schar Cancer Institute, Inova Health System, Fairfax, VA, USA.
John Deeken, Inova Schar Cancer Institute, Inova Health System, Fairfax, VA, USA.
G Larry Maxwell, Women’s Health Integrated Research Center, Women’s Service Line, Inova Health System, Annandale, VA, USA; Gynecologic Cancer Center of Excellence, Gynecologic Surgery and Obstetrics, Uniformed Services University and Walter Reed National Military Medical Center, Bethesda, MD, USA; The John P. Murtha Cancer Center Research Program, Department of Surgery, Uniformed Services University, Bethesda, MD, USA.
Nicholas W Bateman, Gynecologic Cancer Center of Excellence, Gynecologic Surgery and Obstetrics, Uniformed Services University and Walter Reed National Military Medical Center, Bethesda, MD, USA; The John P. Murtha Cancer Center Research Program, Department of Surgery, Uniformed Services University, Bethesda, MD, USA; The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, MD, USA.
Emanuel F Petricoin, Center for Applied Proteomics and Molecular Medicine, George Mason University, Manassas, VA, USA; Theralink Technologies, Inc., Golden, CO, USA.
Amin Benyounes, Inova Schar Cancer Institute, Inova Health System, Fairfax, VA, USA.
Thomas P Conrads, Women’s Health Integrated Research Center, Women’s Service Line, Inova Health System, Annandale, VA, USA; Gynecologic Cancer Center of Excellence, Gynecologic Surgery and Obstetrics, Uniformed Services University and Walter Reed National Military Medical Center, Bethesda, MD, USA; The John P. Murtha Cancer Center Research Program, Department of Surgery, Uniformed Services University, Bethesda, MD, USA.
Timothy L Cannon, Inova Schar Cancer Institute, Inova Health System, Fairfax, VA, USA.
Funding
This work was supported in part by funds from the Inova Health Foundation and the ExxonMobil Spouse’s Club.
Informed Consent
This study was conducted under a central IRB-approved protocol under which the patient provided informed consent and was enrolled in Inova Schar Cancer Institute’s Molecular Tumor Board (MTB) study. The written informed consent included the provision to analyze and publish information and data regarding the patient’s clinical presentation, and results and data from precision medicine/next generation genomic sequencing testing, including the RPPA proteomics test results.
Conflict of Interest
Emanuel F. Petricoin reported consulting and advisory role with Theralink Technologies, Inc., Perthera, Inc., and Ceres Nanosciences, Inc., and research funding from Deciphera Therapeutics, Springwork Therapeutics, Genentech, AbbVie, and Peytant Solutions. Thomas P. Conrads reported consulting or advisory role with ThermoFisher Scientific, Inc., and research funding from AbbVie. Timothy L. Cannon is a paid member of the molecular tumor board for Intermountain Health. The other authors indicated no financial relationships.
Author Contributions
A.L.H.: data generation, data analysis, manuscript writing. A.N.: administrative support, data generation, data analysis. J.R.: administrative support, data analysis. A.P.: administrative support. J.D.: data analysis. G.L.M.: data analysis. N.W.B.: data analysis. E.F.P.: data analysis. A.B.: provision of patient, data analysis. T.P.C.: conceptualization, data analysis, manuscript writing. T.L.C.: conceptualization, data analysis, provision of patient, manuscript writing. All authors gave final approval of the completed work and are accountable for accuracy and integrity.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Siemion K, Reszec-Gielazyn J, Kisluk J, et al. What do we know about inflammatory myofibroblastic tumors? – a systematic review. Adv Med Sci. 2022;67(1):129-138. 10.1016/j.advms.2022.02.002. [DOI] [PubMed] [Google Scholar]
- 2. Coffin CM, Watterson J, Priest JR, Dehner LP.. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19(8):859-872. 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 3. Griffin CA, Hawkins AL, Dvorak C, et al. Recurrent involvement of 2p23 in inflammatory myofibroblastic tumors. Cancer Res. 1999;59(12):2776-2780. [PubMed] [Google Scholar]
- 4. Lawrence B, Perez-Atayde A, Hibbard MK, et al. Tpm3-alk and tpm4-alk oncogenes in inflammatory myofibroblastic tumors. Am J Pathol. 2000;157(2):377-384. 10.1016/S0002-9440(10)64550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coffin CM, Patel A, Perkins S, et al. Alk1 and p80 expression and chromosomal rearrangements involving 2p23 in inflammatory myofibroblastic tumor. Mod Pathol. 2001;14(6):569-576. 10.1038/modpathol.3880352. [DOI] [PubMed] [Google Scholar]
- 6. Lin JJ, Riely GJ, Shaw AT.. Targeting alk: precision medicine takes on drug resistance. Cancer Discov. 2017;7(2):137-155. 10.1158/2159-8290.CD-16-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Siaw JT, Wan H, Pfeifer K, et al. Brigatinib, an anaplastic lymphoma kinase inhibitor, abrogates activity and growth in alk-positive neuroblastoma cells, drosophila and mice. Oncotarget. 2016;7(20):29011-29022. 10.18632/oncotarget.8508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Butrynski JE, D’Adamo DR, Hornick JL, et al. Crizotinib in alk-rearranged inflammatory myofibroblastic tumor. N Engl J Med. 2010;363(18):1727-1733. 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Antonescu CR, Suurmeijer AJ, Zhang L, et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent alk and ros1 gene fusions and rare novel ret rearrangement. Am J Surg Pathol. 2015;39(7):957-967. 10.1097/PAS.0000000000000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in alk-rearranged non-small-cell lung cancer and other malignancies: A single-arm, open-label, phase 1/2 trial. Lancet Oncol. 2016;17(12):1683-1696. 10.1016/S1470-2045(16)30392-8. [DOI] [PubMed] [Google Scholar]
- 11. Sharma GG, Mota I, Mologni L, et al. Tumor resistance against alk targeted therapy-where it comes from and where it goes. Cancers (Basel). 2018;10(3):62. 10.3390/cancers10030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trahair T, Gifford AJ, Fordham A, et al. Crizotinib and surgery for long-term disease control in children and adolescents with alk-positive inflammatory myofibroblastic tumors. JCO Precis Oncol. 2019;1(3):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dishop MK, Warner BW, Dehner LP, et al. Successful treatment of inflammatory myofibroblastic tumor with malignant transformation by surgical resection and chemotherapy. J Pediatr Hematol Oncol. 2003;25(2):153-158. 10.1097/00043426-200302000-00014. [DOI] [PubMed] [Google Scholar]
- 14. FDA. FDA approves crizotinib for alk-positive inflammatory myofibroblastic tumor: https://bit.ly/3o10Eij, 2022
- 15. Childress MA, Himmelberg SM, Chen H, et al. ALK fusion partners impact response to ALK inhibition: differential effects on sensitivity, cellular phenotypes, and biochemical properties. Mol Cancer Res. 2018;16(11):1724-1736. 10.1158/1541-7786.MCR-18-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sasaki T, Okuda K, Zheng W, et al. The neuroblastoma-associated f1174l ALK mutation causes resistance to an ALK kinase inhibitor in ALK-translocated cancers. Cancer Res. 2010;70(24):10038-10043. 10.1158/0008-5472.CAN-10-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mariño-Enríquez A, Wang WL, Roy A, et al. Epithelioid inflammatory myofibroblastic sarcoma: An aggressive intra-abdominal variant of inflammatory myofibroblastic tumor with nuclear membrane or perinuclear ALK. Am J Surg Pathol. 2011;35(1):135-144. 10.1097/PAS.0b013e318200cfd5. [DOI] [PubMed] [Google Scholar]
- 18. Sasaki T, Koivunen J, Ogino A, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 2011;71(18):6051-6060. 10.1158/0008-5472.CAN-11-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beaubier N, Tell R, Lau D, et al. Clinical validation of the tempus xT next-generation targeted oncology sequencing assay. Oncotarget. 2019;10(24):2384-2396. 10.18632/oncotarget.26797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wong DW-S, Leung EL-H, So KK-T, et al. ; University of Hong Kong Lung Cancer Study Group. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115(8):1723-1733. 10.1002/cncr.24181. [DOI] [PubMed] [Google Scholar]
- 21. Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated alk-positive non–small-cell lung cancer. N Engl J Med. 2017;377(9):829-838. 10.1056/nejmoa1704795. [DOI] [PubMed] [Google Scholar]
- 22. Finkle JD, Boulos H, Driessen TM, et al. Validation of a liquid biopsy assay with molecular and clinical profiling of circulating tumor DNA. NPJ Precis Oncol. 2021;5(1):63. 10.1038/s41698-021-00202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wulfkuhle JD, Speer R, Pierobon M, et al. Multiplexed cell signaling analysis of human breast cancer applications for personalized therapy. J Proteome Res. 2008;7(4):1508-1517. 10.1021/pr7008127. [DOI] [PubMed] [Google Scholar]
- 24. Mitchell D, Hunt AL, Conrads KA, et al. Industrialized, artificial intelligence-guided laser microdissection for microscaled proteomic analysis of the tumor microenvironment. J Vis Exp. 2022;(184):e641–71.. [DOI] [PubMed] [Google Scholar]
- 25. Heinicke T, Wardelmann E, Sauerbruch T, et al. Very early detection of response to imatinib mesylate therapy of gastrointestinal stromal tumours using 18fluoro-deoxyglucose-positron emission tomography. Anticancer Res. 2005;25(6C):4591-4594. [PubMed] [Google Scholar]
- 26. Wulfkuhle JD, Yau C, Wolf DM, et al. Evaluation of the her/pi3k/akt family signaling network as a predictive biomarker of pathologic complete response for patients with breast cancer treated with neratinib in the i-spy 2 trial. JCO Precis Oncol. 2018;1:20(2):1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pierobon M, Ramos C, Wong S, et al. Enrichment of pi3k-akt-mtor pathway activation in hepatic metastases from breast cancer. Clin Cancer Res. 2017;23(16):4919-4928. 10.1158/1078-0432.CCR-16-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Heuckmann JM, Balke-Want H, Malchers F, et al. Differential protein stability and alk inhibitor sensitivity of EML4-ALK fusion variants. Clin Cancer Res. 2012;18(17):4682-4690. 10.1158/1078-0432.ccr-11-3260. [DOI] [PubMed] [Google Scholar]
- 29. Katayama R, Friboulet L, Koike S, et al. Two novel ALK mutations mediate acquired resistance to the next-generation ALK inhibitor alectinib. Clin Cancer Res. 2014;20(22):5686-5696. 10.1158/1078-0432.CCR-14-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Toyokawa G, Hirai F, Inamasu E, et al. Secondary mutations at i1171 in the alk gene confer resistance to both crizotinib and alectinib. J Thorac Oncol. 2014;9(12):e86-e87. 10.1097/JTO.0000000000000358. [DOI] [PubMed] [Google Scholar]
- 31. Friboulet L, Li N, Katayama R, et al. The alk inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4(6):662-673. 10.1158/2159-8290.CD-13-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Katayama R, Sakashita T, Yanagitani N, et al. P-glycoprotein mediates ceritinib resistance in anaplastic lymphoma kinase-rearranged non-small cell lung cancer. EBioMedicine. 2016;3:54-66. 10.1016/j.ebiom.2015.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sabir SR, Yeoh S, Jackson G, et al. EML4-ALK variants: biological and molecular properties, and the implications for patients. Cancers (Basel). 2017;9(9):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Richards MW, O’Regan L, Roth D, et al. Microtubule association of eml proteins and the EML4-ALK variant 3 oncoprotein require an n-terminal trimerization domain. Biochem J. 2015;467(3):529-536. 10.1042/BJ20150039. [DOI] [PubMed] [Google Scholar]
- 35. Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561-566. 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 36. Sanders HR, Li HR, Bruey JM, et al. Exon scanning by reverse transcriptase-polymerase chain reaction for detection of known and novel EML4-ALK fusion variants in non-small cell lung cancer. Cancer Genet. 2011;204(1):45-52. 10.1016/j.cancergencyto.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 37. Choi YL, Takeuchi K, Soda M, et al. Identification of novel isoforms of the EML4-ALK transforming gene in non-small cell lung cancer. Cancer Res. 2008;68(13):4971-4976. 10.1158/0008-5472.CAN-07-6158. [DOI] [PubMed] [Google Scholar]
- 38. Takeuchi K, Choi YL, Soda M, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res. 2008;14(20):6618-6624. 10.1158/1078-0432.CCR-08-1018. [DOI] [PubMed] [Google Scholar]
- 39. Horn L, Pao W.. EML4-ALK: Honing in on a new target in non-small-cell lung cancer. J Clin Oncol. 2009;27(26):4232-4235. 10.1200/JCO.2009.23.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Takeuchi K, Choi YL, Togashi Y, et al. Kif5b-alk, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for alk-positive lung cancer. Clin Cancer Res. 2009;15(9):3143-3149. 10.1158/1078-0432.CCR-08-3248. [DOI] [PubMed] [Google Scholar]
- 41. Mizuta H, Okada K, Araki M, et al. Gilteritinib overcomes lorlatinib resistance in alk-rearranged cancer. Nat Commun. 2021;12(1):1261. 10.1038/s41467-021-21396-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rashdan S, Gerber DE.. A crowded, but still varied, space: Brigatinib in anaplastic lymphoma kinase-rearranged non-small cell lung cancer. Transl Cancer Res. 2017;6(Suppl 1):S78-S82. 10.21037/tcr.2017.02.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang S, Anjum R, Squillace R, et al. The potent alk inhibitor brigatinib (ap26113) overcomes mechanisms of resistance to first- and second-generation alk inhibitors in preclinical models. Clin Cancer Res. 2016;22(22):5527-5538. 10.1158/1078-0432.CCR-16-0569. [DOI] [PubMed] [Google Scholar]
- 44. Jänne PA, Shaw AT, Camidge DR, et al. Combined pan-her and alk/ros1/met inhibition with dacomitinib and crizotinib in advanced non-small cell lung cancer: Results of a phase I study. J Thorac Oncol. 2016;11(5):737-747. 10.1016/j.jtho.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 45. Behel V, Noronha V, Choughule A, et al. Impact of molecular tumor board on the clinical management of patients with cancer. JCO Glob Oncol. 2022;8:e2200030. 10.1200/GO.22.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Letai A. Functional precision medicine: putting drugs on patient cancer cells and seeing what happens. Cancer Discov. 2022;12(2):290-292. 10.1158/2159-8290.CD-21-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kornauth C, Pemovska T, Vladimer GI, et al. Functional precision medicine provides clinical benefit in advanced aggressive hematologic cancers and identifies exceptional responders. Cancer Discov. 2022;12(2):372-387. 10.1158/2159-8290.CD-21-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Malani D, Kumar A, Brück O, et al. Implementing a functional precision medicine tumor board for acute myeloid leukemia. Cancer Discov. 2022;12(2):388-401. 10.1158/2159-8290.CD-21-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.