Each year, an estimated 1.3 million persons living with the human immunodeficiency virus (HIV) become pregnant, and approximately 1.1 million (85%) of them receive antiretroviral therapy (ART) during pregnancy.1,2 The vast majority of pregnant persons living with HIV reside in low- and middle-income countries.3 In the absence of ART, 15 to 30% of infants born to persons with HIV acquire HIV antenatally or perinatally, with additional transmission during breast-feeding.4 The landmark 1994 Pediatric AIDS Clinical Trials Group (PACTG) 076 trial showed the efficacy of maternal zidovudine (ZDV) monotherapy in decreasing the rate of vertical transmission, from 25.5% without ZDV monotherapy to 8.3% with it.5 Vertical HIV transmission is essentially eliminated in non–breast-feeding persons who have sustained viral suppression (HIV RNA level, <50 copies per milliliter) with a three-drug ART regimen taken from conception throughout pregnancy,6 and transmission through breast-feeding is very rare with maternal viral suppression from ART (<0.2% per month of breast-feeding).7 The global scale-up of ART has led to a 50% decline in new perinatal infections between 2010 (320,000 cases) and 2021 (160,000 cases) — a remarkable public health success, although still short of the ultimate global targets for the elimination of vertical transmission.1
The ideal ART regimen during pregnancy depends on multiple factors, including pharmacokinetics, virologic efficacy, safety, side effects, drug resistance, dosing convenience, local cost, and availability. As noted above, the three-drug ART regimen is remarkably effective at preventing vertical transmission (with rare exceptions),8 and no apparent differences in prevention and efficacy have been observed to date among the current commonly used regimens.9 ART before and throughout pregnancy is strongly recommended for the improvement of maternal, fetal, and child health outcomes.
The main questions pertain to which ART regimens have the best safety and side-effect profiles when used during pregnancy. As improved regimens are adopted in the general population, guidelines for ART during pregnancy are evolving. ART regimens currently recommended during pregnancy include two nucleoside reverse-transcriptase inhibitors in combination with ART from a different class10 (classified as “preferred or recommended” or “alternative” in pregnancy) (Table 1). However, because of the absence or delayed availability of pharmacokinetic and safety data associated with the use of ART during pregnancy, recommendations for ART often lag behind guidelines for nonpregnant adults living with HIV.8 As a result, pregnant persons often receive older, less desirable regimens.8
Table 1.
Antiretroviral Therapy (ART) Regimens for Use in Pregnant Persons Living with Human Immunodeficiency Virus (HIV).*
| Guideline | Nucleoside Reverse-Transcriptase Inhibitors |
Nonnucleoside Reverse-Transcriptase Inhibitors |
Integrase Strand-Transfer Inhibitors |
Protease Inhibitors |
Monoclonal Antibodies |
|---|---|---|---|---|---|
| Preferred or recommended antiretroviral agents | |||||
| WHO perinatal HIV guideline (2021) | TDF Emtricitabine Lamivudine |
None | Dolutegravir | None | None |
| DHHS guideline (2023) | TAF Emtricitabine TDF Lamivudine Abacavir† |
None | Dolutegravir | Darunavir–ritonavir (600 mg/100 mg twice daily) | None |
| EACS perinatal HIV guideline (2022) | TAF (after 14 wk of gestation) Emtricitabine TDF Lamivudine Abacavir† |
None | Dolutegravir Raltegravir (400 mg twice daily) |
Darunavir–ritonavir (600 mg/100 mg twice daily) | None |
| Alternative regimens | |||||
| WHO (2021) | None | Low-dose efavirenz (400 mg) | None | Atazanavir–ritonavir Darunavir–ritonavir Lopinavir–ritonavir |
None |
| DHHS (2023) | Zidovudine | Efavirenz Oral rilpivirine |
Raltegravir | Atazanavir–ritonavir | None |
| EACS (2022) | None | Efavirenz Oral rilpivirine |
None | None | None |
| Not recommended | |||||
| DHHS (2023) | None | None | Elvitegravir–cobicistat | Darunavir–cobicistat Atazanavir–cobicistat Lopinavir–cobicistat |
None |
| Insufficient data to recommend use | |||||
| DHHS (2023) | None | Doravirine Intramuscular rilpivirine |
Bictegravir Cabotegravir |
None | Ibalizumab |
All the guidelines recommend a three-drug ART regimen during pregnancy with a combination of two nucleoside reverse-transcriptase inhibitors (tenofovir disoproxil fumarate [TDF] or tenofovir alafenamide [TAF] or abacavir, plus lamivudine or emtricitabine) and one integrase strand-transfer inhibitor (or plus one protease inhibitor, or plus efavirenz). In most cases, persons who are already taking antiretroviral agents that are associated with an acceptable side-effect profile and that reduce the viral load to undetectable levels can keep taking them throughout their pregnancy but with more frequent virologic surveillance. DHHS denotes Department of Health and Human Services, EACS European AIDS Clinical Society, and WHO World Health Organization.
Abacavir should not be used if HLA-B*57:01 is positive. Even if HLA-B*57:01 is negative, counseling regarding the risk of a hypersensitivity reaction is still required.
This review focuses on contemporary ART regimens but includes some data on “legacy” antiretroviral agents that are still used by many persons in low- and middle-income countries. We summarize key available evidence on the effect of ART used during pregnancy on birth and maternal and child health outcomes (Table 2). Use of antiretroviral agents during breast-feeding or for preexposure prophylaxis is also important but is not addressed in this review.
Table 2.
Adverse Pregnancy Outcomes Associated with ART during Pregnancy.*
| Adverse Outcomes | ART Regimen | Good Practice Points |
|---|---|---|
| Excess weight gain, metabolic syndrome | Dolutegravir and TAF | Measure prepregnancy weight; evaluate for other causes of weight gain during pregnancy (e.g., hypothyroidism and Cushing’s syndrome); monitor weight and blood pressure during pregnancy; discuss optimal weight gain and ways to maintain adequate weight in pregnancy through diet and exercise; consider nutrition consultation early in pregnancy; consider screening for gestational diabetes |
| Insufficient weight gain | TDF (particularly in combination with EFV) | Measure prepregnancy weight; evaluate for other causes of insufficient weight gain during pregnancy; monitor weight and blood pressure during pregnancy; discuss reasonable pregnancy weight gain and ways to maintain adequate weight in pregnancy through diet and exercise; consider nutrition consultation early in pregnancy |
| Anemia | Zidovudine | Evaluate for and treat other causes of anemia; avoid or discontinue zidovudine if anemia is severe (hemoglobin, <7 g/dl) |
| Depression, suicidal thoughts, psychiatric adverse events | EFV, rilpivirine, dolutegravir | Routine screening for and treatment of prenatal and postnatal depression recommended; consult mood disorders specialist |
| IUGR, SGA, and low birth weight | Protease inhibitors (especially lopinavir–ritonavir); EFV–FTC–TDF | Evaluate for other causes of IUGR; perform serial assessments of fetal growth with fundal height measurements and ultrasound |
| Gestational diabetes mellitus | Protease inhibitor–based ART (lopinavir–ritonavir) | Consider first-trimester screening with oral glucose tolerance testing, especially if additional risk factors for diabetes are present (e.g., history of gestational diabetes, obesity, known history of glucose intolerance, or family history); otherwise, screen according to national guidelines |
| ART-associated liver injury | Protease inhibitor– and nonnucleoside reverse-transcriptase inhibitor–based ART | Rule out other causes of liver dysfunction; perform serial monitoring of liver function during pregnancy |
| Indirect hyperbilirubinemia | Atazanavir | Consider pharmacogenomics for UGT1A1*28 allele |
| Preterm birth | Lopinavir–ritonavir, EFV in combination with TDF | Evaluate for other causes of preterm labor; institute preterm labor precautions; avoid initiating lopinavir–ritonavir with tenofovir-based ART; use darunavir–ritonavir or atazanavir–ritonavir if protease inhibitor–based ART is indicated |
| ART-associated renal injury | Tenofovir-based regimen | Evaluate for other causes of renal dysfunction; monitor renal function during pregnancy; for deteriorating renal function, consider switching from tenofovir to an alternative agent |
EFV denotes efavirenz, FTC emtricitabine, IUGR intrauterine growth restriction, and SGA small for gestational age.
RESEARCH CHALLENGES
Challenges remain in our understanding of adverse pregnancy outcomes related to ART use during pregnancy. The greatest limitation is that pregnancy studies either are not done at all or are completed years after drug registration. Analyses of the effect of the timing of ART initiation in pregnancy are susceptible to selection bias, and confounders are often inadequately controlled for in observational studies. Large numbers of ART exposures are needed to detect differences in low-frequency end points. Background rates of adverse birth outcomes vary markedly among populations, time periods, and settings, making comparisons with background rates challenging to interpret in observational studies. In addition, end point definitions vary among studies, and gestational age is not accurately determined in the absence of fetal ultrasonography.
ADVERSE FETAL AND BIRTH OUTCOMES
With the exception of congenital anomalies, adverse birth outcomes (specifically fetal loss, preterm birth, intrauterine growth restriction, small for gestational age, and low birth weight) receive much less attention than prevention of HIV transmission, despite the large effect of these outcomes on the population. Preterm birth can cause poor health and developmental outcomes and is the leading cause of death worldwide among children under the age of 5 years.11 Low birth weight is also an important predictor of infant mortality in low-income areas.12
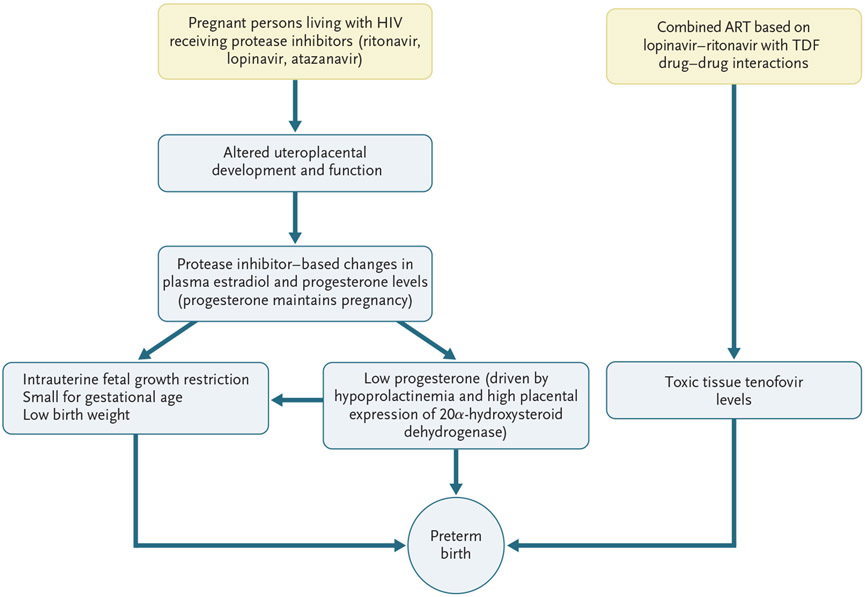
In the pre-ART era, persons living with HIV infection had worse birth outcomes than uninfected persons, including twice the rates of preterm birth, low birth weight, and stillbirth.13 Although birth outcomes are better in pregnant persons with HIV who are receiving ART than in those not receiving ART, specific adverse pregnancy outcomes vary according to the regimen. In this section, we summarize major studies and findings related to ART and birth outcomes. Figure 1 shows several proposed mechanisms for the development of preterm birth and measures of low birth weight in pregnant persons with HIV who are receiving protease inhibitor–based ART.
Figure 1. Possible Mechanisms for Adverse Fetal and Birth Outcomes in Pregnant Persons Living with Human Immunodeficiency Virus (HIV) Who Are Receiving Protease Inhibitors.
The data on low progesterone levels are from Papp et al.,14,15 and the data on drug interactions are from Kearney et al.16 ART denotes antiretroviral therapy, and TDF tenofovir disoproxil fumarate.
MISCARRIAGE
Data on miscarriage (early fetal loss, generally before 20 weeks of gestation) are limited. Many miscarriages are undetected, most are unreported in clinical settings and observational studies, and it is difficult to determine the effect of ART on miscarriage rates in randomized trials. Most pregnant persons living with HIV are already receiving ART at the time of conception (rather than starting ART during pregnancy), which is ideal for overall maternal health and prevention of vertical transmission. The multicenter Women’s Interagency HIV Study (WIHS) showed a lower risk of miscarriage for persons receiving ART than for those not receiving it.17 Among pregnant persons receiving ART in the WIHS cohort, lower rates of miscarriage were reported with protease inhibitor–based ART than with a single nucleoside reverse-transcriptase inhibitor or no ART. In contrast, data from the Promoting Maternal and Infant Safety Everywhere (PROMISE) trial showed that persons who conceived while receiving ART (predominantly protease inhibitor–based) were more likely to have a miscarriage or stillbirth than those who started to receive ART during pregnancy.18,19 Twenty percent of persons who conceived while receiving ART in AIDS Clinical Trials Group (ACTG) trials20 and 37% of those who conceived while receiving ART in the WIHS cohort17 had early fetal loss, percentages that are at the higher end of those reported in the general U.S. population.
In summary, the effect of first-trimester ART on miscarriage remains unclear. Additional high-quality data are needed, particularly for comparing ART regimens. Most important, on the basis of what we know, the advantages to mother and child of uninterrupted maternal ART outweigh the risk of miscarriage.
INTRAUTERINE FETAL DEATH AND STILLBIRTH
A meta-analysis of studies from the pre-ART era showed higher rates of stillbirth (late fetal loss, generally at or after 20 weeks of gestation) among persons living with HIV than among those without HIV (relative risk, 1.7; 95% confidence interval [CI], 1.1 to 2.7).13 Subsequent studies suggest that maternal ART during pregnancy reduces but does not eliminate the excess risk of stillbirth, as compared with the risk among persons who are not living with HIV.21,22 Stillbirth rates may vary according to the maternal ART regimen, the timing of ART exposure in pregnancy, and maternal HIV disease status, and many studies have not been able to adequately control for potential confounding variables.
High rates of stillbirth were observed in early studies of persons who had conceived while receiving ART (e.g., 6.3% with nevirapine [NVP]–based ART).23 However, stillbirth rates are lower with the transition to regimens containing efavirenz (EFV) or dolutegravir (DTG) in combination with lamivudine (3TC) or emtricitabine (FTC) plus tenofovir disoproxil fumarate (TDF) (range, 2 to 5%).24,25 Despite these reassuring findings, stillbirth rates are reportedly still higher among persons living with HIV and receiving EFV–3TC–TDF than among those without HIV in some studies.21
The relationship between the timing of ART initiation and stillbirth is uncertain and may depend on the regimen. Two large cohort studies (one from Ireland and the United Kingdom and one from Malawi), involving more than 18,000 women living with HIV, did not show an association between the timing of ART (before vs. after conception) and stillbirth26,27; a large observational study from Botswana also did not find such an association.28 In contrast, data from the smaller PROMISE trial showed that stillbirths were more likely to occur in persons who conceived while receiving ART than in those who were not receiving ART at the time of conception, with no significant differences according to the ART regimen.19
SPONTANEOUS PRETERM BIRTH
Data from the pre-ART era showed that persons living with HIV were more than twice as likely to have a preterm birth (at <37 weeks of gestation) as persons without HIV (21.1% vs. 9.4%, P<0.001).29 With advances in ART, HIV treatment during pregnancy appears to partially mitigate this excess risk,30 but the risk reduction differs according to the ART regimen.31 Most (but not all) studies have shown an increased risk of preterm birth with an ART regimen based on lopinavir and ritonavir.31-33 The rate of very preterm birth (at <34 weeks of gestation) was also increased with lopinavir–ritonavir–3TC–TDF as compared with lopinavir–ritonavir–3TC–ZDV in the PROMISE randomized trial, which used an increased dose of lopinavir and ritonavir in the third trimester.34
Tenofovir alafenamide (TAF), a prodrug of tenofovir, is characterized by lower plasma (but higher intracellular) levels of tenofovir than is TDF.24 In the randomized IMPAACT 2010/VESTED trial, which compared three ART regimens started during pregnancy, rates of preterm birth were lowest with TAF–DTG–FTC, highest with EFV–FTC–TDF, and intermediate with DTG–FTC–TDF.24 In two randomized trials of EFV–FTC–TDF during pregnancy, one comparing this regimen with integrase inhibitor–based ART containing DTG (DolPHIN-2 trial)35 and the other comparing the regimen with raltegravir (RAL; National Institute of Child Health and Human Development [NICHD] P1081 trial),36 rates of preterm birth were similar, although TAF was not used in these studies.
The effect of timing in the initiation of ART (before or after conception) on preterm birth is also uncertain. Some studies have shown an increased risk of preterm birth among pregnant persons who started receiving ART before conception,37,38 whereas other studies have not shown an increase in risk.39,40 As noted above, such associations may be due to biases, and ART is recommended for all persons living with HIV, including those trying to conceive who are concerned about preterm birth.
SMALL FOR GESTATIONAL AGE AND LOW BIRTH WEIGHT
As with other adverse birth outcomes, a small-for-gestational-age (birth weight, <10th percentile) or low-birth-weight (<2500 g) neonate is more common among untreated persons living with HIV than among persons without HIV infection.13 Some studies have shown lower risks of these outcomes among those living with HIV who are receiving ART during pregnancy than among those not receiving ART,41 whereas other studies have suggested that the risks are higher with ART.42,43 A meta-analysis of 34 cohort studies showed that protease inhibitor–based regimens were associated with an increased risk of a small-for-gestational-age outcome, as compared with an ART regimen without a protease inhibitor (relative risk, 1.24; 95% CI, 1.08 to 1.43), with no apparent difference between protease inhibitors.44 Data regarding the timing of ART exposure during pregnancy and the risk of a small-for-gestational-age or low-birth-weight neonate are conflicting.21,45
NEONATAL MORTALITY
Neonatal mortality (the rate of death within 28 days after birth) has varied according to the maternal ART regimen during pregnancy. In a Botswana surveillance study of approximately 11,000 pregnancies, neonatal mortality was higher with ART based on lopinavir–ritonavir or with ZDV–3TC–NVP and was lower with EFV–TDF–FTC.28 In the PROMISE randomized trial, early neonatal death occurred more frequently with TDF–FTC–lopinavir–ritonavir than with ZDV–3TC–lopinavir–ritonavir (in 4.4% of pregnancies vs. 0.6%, P = 0.001), mostly in fetuses delivered before 34 weeks of gestation as compared with those delivered between 34 and 37 weeks of gestation (27% vs. 3.3%, P<0.001).34 In the more recent IMPAACT 2010/VESTED trial, neonatal mortality was significantly higher with EFV–FTC–TDF than with DTG-based regimens.24 The mechanisms for these differences in neonatal mortality are not yet known.
CONGENITAL ANOMALIES
Detection of an association between drug exposure during pregnancy and congenital malformations requires analysis of data from a large number of persons with medication exposure during early pregnancy. If the background malformation rate in the general population is 3%, approximately 200 first-trimester exposures must be analyzed to detect an increase by a factor of 2 in any anomaly; for a rare anomaly with a prevalence of 0.1%, such as neural-tube defects, analysis of 2000 exposures is needed to detect an increase by a factor of 3.46 By necessity, such data are available only from postmarketing surveillance. True teratogens are very rare, despite the common focus on potential medication teratogenicity.
Data from the Antiretroviral Pregnancy Registry47 and the European Pregnancy and Pediatric HIV Cohort Collaboration rule out an increase in the baseline risk of a birth defect by a factor of 2 or higher for most approved antiretroviral agents. Other large studies have shown a similar prevalence of major congenital anomalies among participants commencing ART in the first trimester and those starting ART later in pregnancy.48,49
EFV and DTG deserve special mention. Early nonhuman primate studies suggested the possibility of central nervous system malformations with EFV, but large cohort studies and antiretroviral pregnancy registries have not shown this association in humans.50,51 Concern about a possible early signal of an increase in neural-tube defects with DTG-based ART from a birth surveillance system in Botswana was first reported in 2019.52 However, as more data were analyzed, the prevalence of neural-tube defects with periconception DTG exposure declined and is now similar to that in the general population.53,54 As a result of concern regarding congenital anomalies with EFV and DTG, pregnant persons have been denied EFV and DTG regimens that were both safer and more effective than older regimens, highlighting the need for the establishment of surveillance systems to rapidly provide pregnant persons with high-quality data so that they can make informed decisions. It is also important to view concern about a potential association with rare anomalies in the larger context of the safety and efficacy of drugs for both maternal and fetal health.55
RISKS TO HEALTH, GROWTH, AND NEURODEVELOPMENTAL OUTCOMES
Infants who do not acquire HIV infection despite having been born to mothers with HIV are at higher risk for illness and death than HIV-unexposed infants. However, this excess risk is sub-stantially mitigated with maternal ART and breast-feeding in low-resource settings.56 Children who were exposed to HIV but are uninfected are at risk for impaired growth, including stunting (height or length for age <2 SD below the mean), underweight (weight for age <2 SD below the mean), and wasting (weight for height or length <2 SD below the mean).57 Maternal EFV-based ART was associated with stunting, as compared with DTG-based regimens, in the IMPAACT 2010/VESTED trial.57
Most (but not all) studies have shown similar postnatal neurodevelopmental outcomes in HIV-exposed but uninfected children and HIV-unexposed children.58,59 In addition, most studies have failed to find causal links between maternal ART exposure and poor neurodevelopment in exposed but uninfected children.60-62 Some exceptions include a possible association between in utero exposure to EFV and microcephaly,63 neurologic conditions,63 and impairment in certain developmental domains64 and between atazanavir–ritonavir and lower language and social–emotional scores.65 The neurodevelopmental outcomes for uninfected children exposed to many contemporary antiretroviral agents are as yet unknown.66 Additional neurodevelopmental data are needed, particularly for in utero exposure to newer antiretroviral agents and for outcomes in older children, given the large and increasing number of HIV-exposed children.
ADVERSE MATERNAL HEALTH OUTCOMES
The adverse-effect profile of most medications is similar in pregnant and nonpregnant persons. We focus here on the effects of drugs that may be expected to differ between pregnant and nonpregnant persons (or effects that may have particular implications for birth or child health outcomes).
WEIGHT GAIN AND METABOLIC COMPLICATIONS DURING PREGNANCY
In nonpregnant adults, ART that includes integrase strand-transfer inhibitors such as DTG and RAL, particularly in combination with TAF, is associated with greater weight gain than ART with EFV–3TC–TDF or EFV–FTC–TDF. This effect may be due to suppression of weight gain by TDF, excess weight gain with integrase strand-transfer inhibitors (heightened by TAF), or both.67-69 Among nonpregnant persons, weight gain appears to be greatest in Black persons and persons with low pretreatment CD4 counts and high viral loads.69 Large observational studies in Botswana showed that although persons commencing DTG-based ART during pregnancy gained more weight than those starting EFV-based ART, neither group gained as much weight during pregnancy as persons without HIV.70,71 Greater weight gain with DTG-based ART that was started during pregnancy, particularly in combination with TAF, was also found in the randomized IMPAACT 2010/VESTED trial.24 Other pregnancy studies have shown similar patterns of greater weight gain with TAF–DTG.72,73 In the DolPHIN-235 and NICHD P1081 trials,74 pregnancy and postpartum weight gain were greater with DTG- and RAL-based regimens than with EFV-based regimens (in combination with TDF).
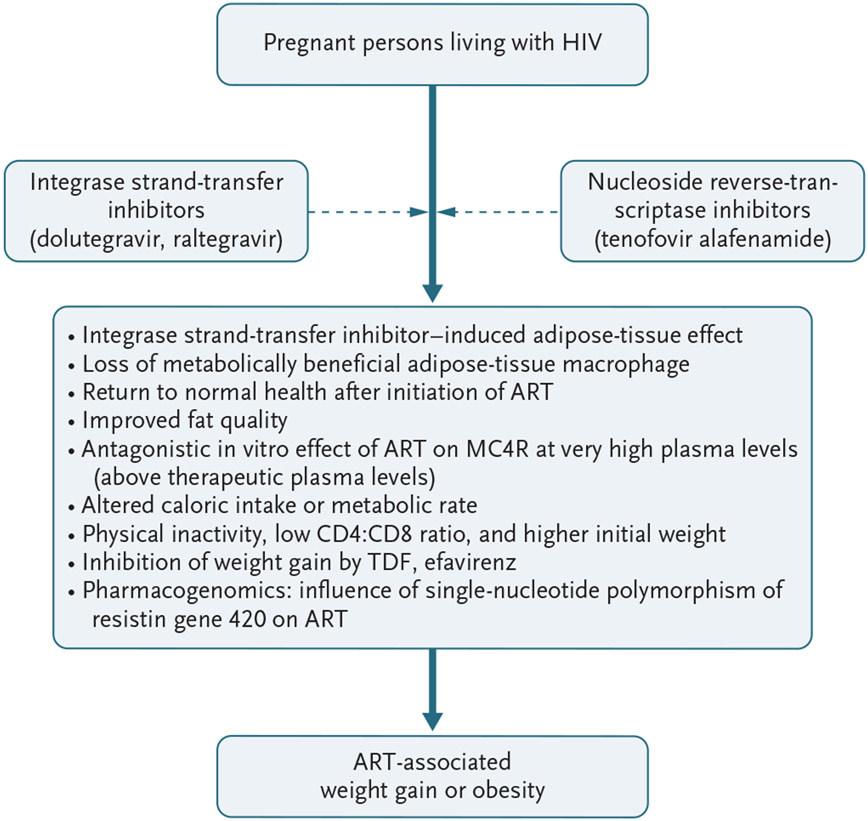
Figure 2 shows some theories or pathways posited to explain weight gain in pregnant persons living with HIV and receiving ART. In both the observational Botswana study and the randomized trials, the average weekly weight gain during pregnancy in persons taking integrase inhibitors was less than the gestational weight gain recommended by the U.S. National Academy of Medicine. Furthermore, insufficient or low weight gain, which is more common than excessive weight gain during pregnancy in low-resource settings, was associated with adverse birth outcomes and was more frequent with ART that did not include integrase inhibitors.36,74 However, the longer-term health effects of greater maternal weight gain with DTG and TAF and the outcomes of subsequent pregnancies are unknown and need to be evaluated.
Figure 2. Proposed Theories to Explain Excessive Weight Gain in Pregnant Persons Living with HIV Who Are Receiving ART.
The proposed theories are from Gorwood et al.75 (adipose-tissue effect), Vakili et al.76 (loss of metabolically beneficial adipose-tissue macrophages), Guaraldi et al.77 (improved fat quality), McMahon et al.78 (antagonistic in vitro effect of ART on melanocortin 4 receptor [MC4R] at very high plasma levels), Eckard et al.79 (altered caloric intake or metabolic rate), Guaraldi et al.80 (physical inactivity, low CD4:CD8 ratio, and higher initial weight), Shah et al.81 (inhibition of weight gain), and Minami et al.82 (pharmacogenomics).
GESTATIONAL AND PREGESTATIONAL DIABETES
Pregnancy is associated with increased insulin resistance and glucose intolerance. A recent meta-analysis of studies involving nonpregnant adults showed variations in the effects of different protease inhibitor–based ART regimens on the onset of diabetes.83 In a multicenter trial involving 1407 pregnant persons living with HIV, the prevalence of gestational diabetes was 4.6% with protease inhibitor–based ART that was started early in pregnancy, as compared with 1.7% with other ART regimens.84 A 2017 meta-analysis showed that older protease inhibitor–based ART regimens might be linked with gestational diabetes if strict diagnostic criteria are used.85 There are limited data on gestational diabetes with newer (second-generation) protease inhibitor–based regimens.83
Although TAF–DTG–FTC ART would be expected to be associated with an increased risk of diabetes,86 this was not reflected in maternal glucose and glycated hemoglobin levels in the IMPAACT 2010/VESTED trial.24 The risk of observed gestational diabetes was actually lower with DTG-based ART than with EFV-based ART in a prospective pregnancy cohort study in Botswana.87 Additional studies are needed, and screening for gestational diabetes in pregnant persons living with HIV who are receiving ART should be considered (Table 2).
HYPERTENSIVE DISORDERS OF PREGNANCY
ART has not been associated with an increased risk of hypertensive disorders of pregnancy (gestational hypertension, chronic hypertension, HELLP [hemolysis, elevated liver-enzyme levels, and a low platelet count] syndrome, and preeclampsia) in most studies, including a 2015 meta-analysis,88 although a 2019 meta-analysis of 28 studies did show an increased risk of hypertensive disorders, particularly with the older protease inhibitors lopinavir–ritonavir and indinavir–ritonavir.89 Surveillance studies in Botswana showed an association between NVP-based ART and hypertension during pregnancy90 and a possible association of prepregnancy baseline weight and weight gain during pregnancy with hypertensive disorders of pregnancy in persons receiving DTG-based ART.71 There is currently insufficient evidence to recommend preventive therapies (e.g., low-dose aspirin) to mitigate the risk of preeclampsia in pregnant persons living with HIV who are receiving ART and do not have other known risk factors for preeclampsia.
ART-INDUCED TOXIC EFFECTS ON RENAL TUBULES
Data from studies involving nonpregnant adults have shown an association between TDF and renal dysfunction, with a mild-to-moderate, reversible decline in the estimated glomerular filtration rate.91 However, clinically significant toxic effects of TDF on the kidney are very rare in young, healthy persons, including pregnant persons24 (who have a physiologic increase in the glomerular filtration rate).
ART-INDUCED LIVER INJURY
The protease inhibitor atazanavir is associated with indirect hyperbilirubinemia as a result of uridine diphosphate glucuronosyltransferase inhibition, though kernicterus has not yet been described.92 In a large French cohort of pregnant persons living with HIV, severe elevations in liver-enzyme levels developed in 2% of the participants. The risk of elevated liver-enzyme levels was lower with nonnucleoside reverse-transcriptase inhibitor–based regimens than with protease inhibitor–based regimens.93 Regular liver-function tests should be considered for pregnant persons with HIV who are receiving ART (Table 3).
Table 3.
Common Tests for Diagnosis, Management, and Monitoring of ART-Associated Adverse Effects during Pregnancy.*
| Test | Timing in Pregnancy | Benefits | Drawbacks |
|---|---|---|---|
| Biochemical methods | |||
| Noninvasive prenatal testing, cell-free fetal DNA | Performed at >10 wk of gestation | High sensitivity in aneuploidy screening for detection of congenital anomalies (trisomy 21, 18, and 13) in all trimesters of pregnancy; accuracy of detection >98% | Not diagnostic, so follow-up testing (e.g., chorionic villus sampling, amniocentesis) recommended in persons with a positive screen; chorionic villus sampling and amniocentesis can be associated with risk of perinatal HIV transmission if viral load is not suppressed |
| First-trimester serum analytes (PAPP-A, free-beta hCG) | Performed when crown-to-rump length is 45–84 mm | Low PAPP-A (<0.4 MoM) can be predictive of adverse outcomes, including preeclampsia, IUGR, and stillbirth | Limited sensitivity and specificity in congenital anomaly screening; interpret together with nuchal translucency imaging |
| HLA-B*5701 | Performed before administration of abacavir | Valuable genetic test for avoiding abacavir-related hypersensitivity reactions | Testing specific to abacavir; HLA-B*5701 testing not available in many LMICs |
| Complete blood count | Performed at ART initiation or entry into prenatal care, at 28–36 wk of gestation, and if anemia is suspected (especially with zidovudine-based regimens) | Useful for diagnosis and monitoring of anemia, leukopenia, thrombocytopenia, and other cytopenias and cytosis during pregnancy in persons receiving ART | Results can be affected by dehydration, preeclampsia; additional testing or workup needed in cases of anemia and thrombocytopenia to rule out other potential causes |
| Comprehensive metabolic panel or basic metabolic panel with liver-function tests | Performed at ART initiation or entry into prenatal care and within 2–4 wk after initiating or changing ART, with checks every 3 months afterward or as needed | Screening for and diagnosis of hepatic and renal ART-related complications during pregnancy | Additional testing (e.g., prothrombin time, international normalized ratio) needed to complement comprehensive or basic metabolic panel for diagnosis and management of hepatic dysfunction or injury; testing for UGT1A1*28 allele may not be readily available in LMICs |
| Glucose screening for diabetes based on country-specific protocols | Performed in first or early third trimester or both (with older protease inhibitors) | Early diagnosis of diabetes in pregnancy improves maternal and neonatal outcomes in pregnant persons living with HIV | Screening for diabetes twice during pregnancy (first and third trimesters) or a two-step screening in pregnant persons whose initial glucose test results are abnormal could be cumbersome and create anxiety |
| Biophysical methods | |||
| First-trimester nuchal translucency ultrasound scan | Performed when crown-to-rump length is 45–84 mm | Approximately 75% sensitivity in screening for Down’s syndrome | Has to be interpreted together with first-trimester serum analytes for a prognostic score; 5% false positive rate in screening for Down’s syndrome |
| Fetal anatomical survey (level II) ultrasound scan | Preferably performed at 18–24 wk of gestation, but can be performed at any time during gestation | Useful in the diagnosis of fetal anomalies, growth issues (e.g., IUGR), and amniotic fluid, placental, and umbilical cord abnormalities | Can be difficult to complete, especially in obese patients and with advanced (>24 wk) gestation |
| Doppler evaluation (umbilical artery, middle cerebral artery) | Performed at diagnosis of IUGR, after confirmation of fetal viability | Early detection of changes in fetal blood flow associated with IUGR increases fetal surveillance, alters delivery timing, and improves fetal outcomes | Usefulness of umbilical artery Doppler evaluation for pregnancy complications other than IUGR not established |
| Serial growth sonograms, fetal non-stress testing, biophysical profile | Performed after confirmation of fetal viability in pregnant persons with coexisting maternal or fetal complications or diagnosis of IUGR, with sonograms obtained every 2–4 wk for fetal growth assessment | Early diagnosis of IUGR or fetal heart rate abnormalities increases fetal surveillance, alters delivery timing, and improves fetal outcomes | A minimum of 2 wk is recommended between fetal growth sonograms to reduce inherent variations in ultrasound measurements |
The abbreviation hCG denotes human chorionic gonadotropin, LMICs low- and middle-income countries, MoM multiples of the median, and PAPP-A pregnancy-associated plasma protein A.
PERIPARTUM DEPRESSION
Persons living with HIV are more likely to have depressive symptoms during pregnancy and in the postpartum period than are those without HIV infection.94,95 Perinatal depression in persons living with HIV has been linked to increased chances of suicidal thoughts, self-harm, gestational diabetes, and preterm birth and to low birth weight and cognitive issues in their offspring.95 Some antiretroviral agents (e.g., EFV96 and, less frequently, rilpivirine and DTG) have been associated with a risk of depression and suicidal thoughts in adults. Screening for antepartum and postpartum depression is advised (Table 2).
RECOMMENDATIONS FOR FUTURE STUDIES
Standardized definitions of end points (e.g., miscarriage and small for gestational age), fetal ultrasonography for dating gestation, unified approaches to screening for gestational diabetes, and systematic assessment for congenital anomalies would improve comparability among studies. Careful attention to enrollment criteria and analyses could reduce confounding and biases (e.g., immortal time bias) that often complicate observational pregnancy studies.97 Finally, well-powered, randomized pregnancy safety trials during phase 3 or early in the postapproval period should be conducted for high-priority regimens that will be used by large numbers of pregnant persons living with HIV. Given the possibility of adverse pregnancy outcomes among persons receiving ART, increased prenatal fetal surveillance should be considered (Tables 2 and 3).
CONCLUSIONS
The known benefits of ART in pregnant persons living with HIV for optimizing maternal and infant health and preventing transmission of HIV to the infant clearly outweigh potential adverse outcomes. Newer ART regimens are associated with better pregnancy outcomes than the older antiretroviral agents. For these reasons, pregnant persons should start or continue treatment with preferred or alternative ART regimens that result in adequate viral suppression and are associated with an acceptable adverse-effect profile. The data on most of the newer ART regimens remain reassuring, but pharmacovigilance should be maintained. Although there are no particular concerns, current data on the safety of darunavir–ritonavir during pregnancy are limited, and pharmacokinetic and safety data for bictegravir, doravirine, and the recently licensed long-acting injectable agents cabotegravir and rilpivirine are even more limited (Table 1). Earlier (including prelicensure) pharmacokinetic and limited safety studies of new antiretroviral agents during pregnancy are needed, so that patients and providers do not have to wait years for sufficient data to inform their use of new HIV drugs during pregnancy. In the meantime, clinicians should continue to monitor pregnant persons living with HIV for potential pregnancy complications and manage their care and the care of their babies.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Ahizechukwu C. Eke, Division of Maternal Fetal Medicine, Department of Gynecology and Obstetrics, and the Division of Clinical Pharmacology, Johns Hopkins University School of Medicine, Baltimore
Mark Mirochnick, Department of Pediatrics, Boston University Chobanian & Avedisian School of Medicine
Shahin Lockman, Division of Infectious Diseases, Brigham and Women’s Hospital; Department of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health
REFERENCES
- 1.United Nations Program on HIV/AIDS (UNAIDS). AIDSinfo fact sheets (http://aidsinfo.unaids.org/).
- 2.Mother-to-child transmission of HIV. World Health Organization (https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/prevention/mother-to-child-transmission-of-hiv).
- 3.Shao Y, Williamson C. The HIV-1 epidemic: low- to middle-income countries. Cold Spring Harb Perspect Med 2012;2:a007187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teasdale CA, Marais BJ, Abrams EJ. HIV: prevention of mother-to-child transmission. BMJ Clin Evid 2011;2011:0909. [PMC free article] [PubMed] [Google Scholar]
- 5.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med 1994;331:1173–80. [DOI] [PubMed] [Google Scholar]
- 6.Sibiude J, Le Chenadec J, Mandelbrot L, et al. Update of perinatal HIV-1 transmission in France: zero transmission for 5482 mothers on continuous ART from conception and with undetectable viral load at delivery. Clin Infect Dis 2022. August 29 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 7.Shapiro RL, Hughes MD, Ogwu A, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med 2010;362:2282–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eke AC, Olagunju A, Momper J, et al. Optimizing pharmacology studies in pregnant and lactating women using lessons from HIV: a consensus statement. Clin Pharmacol Ther 2021;110:36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davey S, Ajibola G, Maswabi K, et al. Mother-to-child HIV transmission with in utero dolutegravir vs. efavirenz in Botswana. J Acquir Immune Defic Syndr 2020;84:235–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panel on Treatment of HIV During Pregnancy and Prevention of Perinatal Transmission. Recommendations for the use of antiretroviral drugs during pregnancy and interventions to reduce perinatal HIV transmission in the United States. Department of Health and Human Services, 2022. (https://clinicalinfo.hiv.gov/en/guidelines/perinatal/whats-new-guidelines). [Google Scholar]
- 11.Jones AJ, Eke UA, Eke AC. Prediction and prevention of preterm birth in pregnant women living with HIV on antiretroviral therapy. Expert Rev Anti Infect Ther 2022;20:837–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blencowe H, Krasevec J, de Onis M, et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health 2019;7(7):e849–e860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wedi COO, Kirtley S, Hopewell S, Corrigan R, Kennedy SH, Hemelaar J. Perinatal outcomes associated with maternal HIV infection: a systematic review and meta-analysis. Lancet HIV 2016;3(1):e33–48. [DOI] [PubMed] [Google Scholar]
- 14.Papp E, Mohammadi H, Loutfy MR, et al. HIV protease inhibitor use during pregnancy is associated with decreased progesterone levels, suggesting a potential mechanism contributing to fetal growth restriction. J Infect Dis 2015;211:10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papp E, Balogun K, Banko N, et al. Low prolactin and high 20-α-hydroxysteroid dehydrogenase levels contribute to lower progesterone levels in HIV-infected pregnant women exposed to protease inhibitor-based combination antiretroviral therapy. J Infect Dis 2016;213:1532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kearney BP, Mathias A, Mittan A, Sayre J, Ebrahimi R, Cheng AK. Pharmacokinetics and safety of tenofovir disoproxil fumarate on coadministration with lopinavir/ritonavir. J Acquir Immune Defic Syndr 2006;43:278–83. [DOI] [PubMed] [Google Scholar]
- 17.Wall KM, Haddad LB, Mehta CC, et al. Miscarriage among women in the United States Women’s Interagency HIV Study, 1994–2017. Am J Obstet Gynecol 2019;221(4):347.e1–347.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman RM, Brummel SS, Britto P, et al. Adverse pregnancy outcomes among women who conceive on antiretroviral therapy. Clin Infect Dis 2019;68:273–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theron G, Brummel S, Fairlie L, et al. Pregnancy outcomes of women conceiving on antiretroviral therapy (ART) compared to those commenced on ART during pregnancy. Clin Infect Dis 2021;73(2):e312–e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stringer EM, Kendall MA, Lockman S, et al. Pregnancy outcomes among HIV-infected women who conceived on antiretroviral therapy. PLoS One 2018;13(7):e0199555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tukei VJ, Hoffman HJ, Greenberg L, et al. Adverse pregnancy outcomes among HIV-positive women in the era of universal antiretroviral therapy remain elevated compared with HIV-negative women. Pediatr Infect Dis J 2021;40:821–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Liu J, Tan D, et al. Maternal HIV infection and risk of adverse pregnancy outcomes in Hunan province, China: a prospective cohort study. Medicine (Baltimore) 2020;99(8):e19213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen JY, Ribaudo HJ, Souda S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis 2012;206:1695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lockman S, Brummel SS, Ziemba L, et al. Efficacy and safety of dolutegravir with emtricitabine and tenofovir alafenamide fumarate or tenofovir disoproxil fumarate, and efavirenz, emtricitabine, and tenofovir disoproxil fumarate HIV antiretroviral therapy regimens started in pregnancy (IMPAACT 2010/VESTED): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet 2021;397:1276–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zash R, Jacobson DL, Diseko M, et al. Comparative safety of antiretroviral treatment regimens in pregnancy. JAMA Pediatr 2017;171(10):e172222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Favarato G, Townsend CL, Peters H, et al. Stillbirth in women living with HIV delivering in the United Kingdom and Ireland: 2007–2015. J Acquir Immune Defic Syndr 2019;82:9–16. [DOI] [PubMed] [Google Scholar]
- 27.Msukwa MT, Keiser O, Jahn A, et al. Timing of combination antiretroviral therapy (cART) initiation is not associated with stillbirth among HIV-infected pregnant women in Malawi. Trop Med Int Health 2019;24:727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zash R, Rough K, Jacobson DL, et al. Effect of gestational age at tenofoviremtricitabine-efavirenz initiation on adverse birth outcomes in Botswana. J Pediatric Infect Dis Soc 2018;7(3):e148–e151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Temmerman M, Chomba EN, Ndinya-Achola J, Plummer FA, Coppens M, Piot P. Maternal human immunodeficiency virus-1 infection and pregnancy outcome. Obstet Gynecol 1994;83:495–501. [DOI] [PubMed] [Google Scholar]
- 30.Venkatesh KK, Farhad M, Fenton T, et al. Association between HIV antiretroviral therapy and preterm birth based on antenatal ultrasound gestational age determination: a comparative analysis. AIDS 2019;33:2403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Powis KM, Kitch D, Ogwu A, et al. Increased risk of preterm delivery among HIV-infected women randomized to protease versus nucleoside reverse transcriptase inhibitor-based HAART during pregnancy. J Infect Dis 2011;204:506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tshivuila-Matala COO, Honeyman S, Nesbitt C, Kirtley S, Kennedy SH, Hemelaar J. Adverse perinatal outcomes associated with antiretroviral therapy regimens: systematic review and network meta-analysis. AIDS 2020;34:1643–56. [DOI] [PubMed] [Google Scholar]
- 33.Koss CA, Natureeba P, Plenty A, et al. Risk factors for preterm birth among HIV-infected pregnant Ugandan women randomized to lopinavir/ritonavir- or efavirenz-based antiretroviral therapy. J Acquir Immune Defic Syndr 2014;67:128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fowler MG, Qin M, Fiscus SA, et al. Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med 2016;375:1726–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kintu K, Malaba TR, Nakibuka J, et al. Dolutegravir versus efavirenz in women starting HIV therapy in late pregnancy (DolPHIN-2): an open-label, randomised controlled trial. Lancet HIV 2020;7(5):e332–e339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.João EC, Morrison RL, Shapiro DE, et al. Raltegravir versus efavirenz in antiretroviral-naive pregnant women living with HIV (NICHD P1081): an open-label, randomised, controlled, phase 4 trial. Lancet HIV 2020;7(5):e322–e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sexton H, Kumarendran M, Brandon Z, Shi C, Kirtley S, Hemelaar J. Adverse perinatal outcomes associated with timing of initiation of antiretroviral therapy: systematic review and meta-analysis. HIV Med 2022. June 6 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 38.Shinar S, Agrawal S, Ryu M, et al. Perinatal outcomes in women living with HIV-1 and receiving antiretroviral therapy — a systematic review and meta-analysis. Acta Obstet Gynecol Scand 2022;101:168–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin F, Taylor GP. Increased rates of preterm delivery are associated with the initiation of highly active antiretroviral therapy during pregnancy: a single-center cohort study. J Infect Dis 2007;196:558–61. [DOI] [PubMed] [Google Scholar]
- 40.Rudin C, Spaenhauer A, Keiser O, et al. Antiretroviral therapy during pregnancy and premature birth: analysis of Swiss data. HIV Med 2011;12:228–35. [DOI] [PubMed] [Google Scholar]
- 41.Quinn MK, Williams PL, Muhihi A, et al. Timing of antiretroviral therapy. J Infect Dis 2022;226:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saleska JL, Turner AN, Maierhofer C, Clark J, Kwiek JJ. Use of antiretroviral therapy during pregnancy and adverse birth outcomes among women living with HIV-1 in low- and middle-income countries: a systematic review. J Acquir Immune Defic Syndr 2018;79:1–9. [DOI] [PubMed] [Google Scholar]
- 43.Xiao P-L, Zhou Y-B, Chen Y, et al. Association between maternal HIV infection and low birth weight and prematurity: a meta-analysis of cohort studies. BMC Pregnancy Childbirth 2015;15:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cowdell I, Beck K, Portwood C, et al. Adverse perinatal outcomes associated with protease inhibitor-based antiretroviral therapy in pregnant women living with HIV: a systematic review and meta-analysis. EClinicalMedicine 2022;46:101368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uthman OA, Nachega JB, Anderson J, et al. Timing of initiation of antiretroviral therapy and adverse pregnancy outcomes: a systematic review and meta-analysis. Lancet HIV 2017;4(1):e21–e30. [DOI] [PubMed] [Google Scholar]
- 46.Watts DH. Teratogenicity risk of antiretroviral therapy in pregnancy. Curr HIV/AIDS Rep 2007;4:135–40. [DOI] [PubMed] [Google Scholar]
- 47.Covington DL, Tilson H, Elder J, Doi P. Assessing teratogenicity of antiretroviral drugs: monitoring and analysis plan of the Antiretroviral Pregnancy Registry. Pharmacoepidemiol Drug Saf 2004;13:537–45. [DOI] [PubMed] [Google Scholar]
- 48.Williams PL, Crain MJ, Yildirim C, et al. Congenital anomalies and in utero antiretroviral exposure in human immunodeficiency virus-exposed uninfected infants. JAMA Pediatr 2015;169:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bérard A, Sheehy O, Zhao J-P, et al. Antiretroviral combination use during pregnancy and the risk of major congenital malformations. AIDS 2017;31:2267–77. [DOI] [PubMed] [Google Scholar]
- 50.Ford N, Calmy A, Mofenson L. Safety of efavirenz in the first trimester of pregnancy: an updated systematic review and meta-analysis. AIDS 2011;25:2301–4. [DOI] [PubMed] [Google Scholar]
- 51.Martinez de Tejada B, European Pregnancy and Paediatric HIV Cohort Collaboration Study Group. Birth defects after exposure to efavirenz-based antiretroviral therapy at conception/first trimester of pregnancy: a multicohort analysis. J Acquir Immune Defic Syndr 2019;80:316–24. [DOI] [PubMed] [Google Scholar]
- 52.Zash R, Holmes L, Diseko M, et al. Neural-tube defects and antiretroviral treatment regimens in Botswana. N Engl J Med 2019;381:827–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zash R, Holmes LB, Diseko M, et al. Update on neural tube defects with antiretroviral exposure in the Tsepamo Study, Botswana. Presented at the 24th International AIDS Conference, Montreal, July 29–August 2, 2022. [Google Scholar]
- 54.Pereira GFM, Kim A, Jalil EM, et al. Dolutegravir and pregnancy outcomes in women on antiretroviral therapy in Brazil: a retrospective national cohort study. Lancet HIV 2021;8(1):e33–e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dugdale CM, Ciaranello AL, Bekker L-G, et al. Risks and benefits of dolutegravir- and efavirenz-based strategies for South African women with HIV of child-bearing potential: a modeling study. Ann Intern Med 2019;170:614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arikawa S, Rollins N, Jourdain G, et al. Contribution of maternal antiretroviral therapy and breastfeeding to 24-month survival in human immunodeficiency virus-exposed uninfected children: an individual pooled analysis of African and Asian studies. Clin Infect Dis 2018;66:1668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stranix-Chibanda L, Ziemba L, Brummel S, et al. Growth of infants with perinatal exposure to maternal DTG vs EFV and TDF vs TAF: the randomized IMPAACT 2010 trial. Presented at the Conference on Retroviruses and Opportunistic Infections, virtual, February 12–16, 2022. [Google Scholar]
- 58.Kerr SJ, Puthanakit T, Vibol U, et al. Neurodevelopmental outcomes in HIV-exposed-uninfected children versus those not exposed to HIV. AIDS Care 2014;26:1327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Springer PE, Slogrove AL, Kidd M, et al. Neurodevelopmental and behavioural outcomes of HIV-exposed uninfected and HIV-unexposed children at 2–3 years of age in Cape Town, South Africa. AIDS Care 2020;32:411–9. [DOI] [PubMed] [Google Scholar]
- 60.Williams PL, Marino M, Malee K, Brogly S, Hughes MD, Mofenson LM. Neurodevelopment and in utero antiretroviral exposure of HIV-exposed uninfected infants. Pediatrics 2010;125(2):e250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piske M, Budd MA, Qiu AQ, et al. Neurodevelopmental outcomes and inutero antiretroviral exposure in HIV-exposed uninfected children. AIDS 2018;32:2583–92. [DOI] [PubMed] [Google Scholar]
- 62.Fowler MG, Hanrahan C, Yende N, et al. Neurodevelopmental outcomes of HIV/antiretroviral drug perinatally exposed uninfected children aged 3–6 years. AIDS 2022;36:1533–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams PL, Yildirim C, Chadwick EG, et al. Association of maternal antiretroviral use with microcephaly in children who are HIV-exposed but uninfected (SMARTT): a prospective cohort study. Lancet HIV 2020;7(1):e49–e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cassidy AR, Williams PL, Leidner J, et al. In utero efavirenz exposure and neurodevelopmental outcomes in HIV-exposed uninfected children in Botswana. Pediatr Infect Dis J 2019;38:828–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sirois PA, Huo Y, Williams PL, et al. Safety of perinatal exposure to antiretroviral medications: developmental outcomes in infants. Pediatr Infect Dis J 2013;32:648–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wedderburn CJ, Weldon E, Bertran-Cobo C, et al. Early neurodevelopment of HIV-exposed uninfected children in the era of antiretroviral therapy: a systematic review and meta-analysis. Lancet Child Adolesc Health 2022;6(6):393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Calmy A, Tovar Sanchez T, Kouanfack C, et al. Dolutegravir-based and low-dose efavirenz-based regimen for the initial treatment of HIV-1 infection (NAMSAL): week 96 results from a two-group, multicentre, randomised, open label, phase 3 non-inferiority trial in Cameroon. Lancet HIV 2020;7(10):e677–e687. [DOI] [PubMed] [Google Scholar]
- 68.Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019;381:803–15. [DOI] [PubMed] [Google Scholar]
- 69.Kanters S, Renaud F, Rangaraj A, et al. Evidence synthesis evaluating body weight gain among people treating HIV with antiretroviral therapy — a systematic literature review and network meta-analysis. EClinicalMedicine 2022;48:101412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caniglia EC, Shapiro R, Diseko M, et al. Weight gain during pregnancy among women initiating dolutegravir in Botswana. EClinicalMedicine 2020;29-30:100615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zash R, Caniglia EC, Diseko M, et al. Maternal weight and birth outcomes among women on antiretroviral treatment from conception in a birth surveillance study in Botswana. J Int AIDS Soc 2021;24(6):e25763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Joseph NT, Satten GA, Williams RE, et al. The effect of antiretroviral therapy for the treatment of human immunodeficiency virus (HIV)-1 in pregnancy on gestational weight gain. Clin Infect Dis 2022;75:665–72. [DOI] [PubMed] [Google Scholar]
- 73.Thimm MA, Livingston A, Ramroop R, Eke AC. Pregnancy outcomes in pregnant women with HIV on tenofovir disoproxil fumarate (TDF) compared to tenofovir alafenamide (TAF). J AIDS HIV Treat 2022;4:6–13. [PMC free article] [PubMed] [Google Scholar]
- 74.Coutinho CM, Warshaw MG, Duarte G, et al. Effects of initiating raltegravir-based versus efavirenz-based antiretroviral regimens during pregnancy on weight changes and perinatal outcomes: NICHD P1081. J Acquir Immune Defic Syndr 2022;91:403–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gorwood J, Bourgeois C, Pourcher V, et al. The integrase inhibitors dolutegravir and raltegravir exert proadipogenic and profibrotic effects and induce insulin resistance in human/simian adipose tissue and human adipocytes. Clin Infect Dis 2020;71(10):e549–e560. [DOI] [PubMed] [Google Scholar]
- 76.Vakili S, Paneru B, Guerrier CM, et al. Altered adipose tissue macrophage populations in people with HIV on integrase inhibitor-containing antiretroviral therapy. AIDS 2022;36:1493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guaraldi G, Draisci S, Milic J, et al. Fat distribution and density in people living with HIV with ≥5% weight gain. Presented at HIV Glasgow, virtual, October 5–8, 2020. (https://www.natap.org/2020/GLASGOW/GLASGOW_72.htm). [Google Scholar]
- 78.McMahon C, Trevaskis JL, Carter C, et al. Lack of an association between clinical INSTI-related body weight gain and direct interference with MC4 receptor (MC4R), a key central regulator of body weight. PLoS One 2020;15(2):e0229617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eckard AR, Sattar A, Yu J, et al. Fat gains occur after ART without changes in metabolic rate or caloric intake. Presented at the Conference on Retroviruses and Opportunistic Infections, Boston, March 8–11, 2020. (https://www.natap.org/2020/CROI/croi_39.htm). [Google Scholar]
- 80.Guaraldi G, Milic J, Malagoli A, et al. Contribution of InSTI, BMI, physical inactivity, caloric intake to weight gain in PWH. Presented at the Conference on Retroviruses and Opportunistic Infections, Boston, March 8–11, 2020. [Google Scholar]
- 81.Shah S, Pilkington V, Hill A. Is tenofovir disoproxil fumarate associated with weight loss? AIDS 2021;35:S189–S195. [DOI] [PubMed] [Google Scholar]
- 82.Minami R, Takahama S, Koyama K, et al. Resistin gene polymorphism related to weight gain and psychiatric symptoms on InSTI. Presented at the Conference on Retroviruses and Opportunistic Infections, Boston, March 8–11, 2020. (https://www.natap.org/2020/CROI/croi_257.htm). [Google Scholar]
- 83.Kajogoo VD, Gorret Atim M, Amare D, et al. HIV protease inhibitors and insulin sensitivity: a systematic review and meta-analysis of randomized controlled trials. Front Pharmacol 2021;12:635089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Watts DH, Balasubramanian R, Maupin RT Jr, et al. Maternal toxicity and pregnancy complications in human immunodeficiency virus-infected women receiving antiretroviral therapy: PACTG 316. Am J Obstet Gynecol 2004;190:506–16. [DOI] [PubMed] [Google Scholar]
- 85.Soepnel LM, Norris SA, Schrier VJMM, et al. The association between HIV, antiretroviral therapy, and gestational diabetes mellitus. AIDS 2017;31:113–25. [DOI] [PubMed] [Google Scholar]
- 86.McCann K, Shah S, Hindley L, et al. Implications of weight gain with newer anti-retrovirals: 10-year predictions of cardiovascular disease and diabetes. AIDS 2021;35:1657–65. [DOI] [PubMed] [Google Scholar]
- 87.Mmasa KN, Powis K, Sun S, et al. Gestational diabetes in women living with HIV in Botswana: lower rates with dolutegravir- than with efavirenz-based antiretroviral therapy. HIV Med 2021;22:715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Browne JL, Schrier VJMM, Grobbee DE, Peters SA, Klipstein-Grobusch K. HIV, antiretroviral therapy, and hypertensive disorders in pregnancy: a systematic review and meta-analysis. J Acquir Immune Defic Syndr 2015;70:91–8. [DOI] [PubMed] [Google Scholar]
- 89.Premkumar A, Dude AM, Haddad LB, Yee LM. Combined antiretroviral therapy for HIV and the risk of hypertensive disorders of pregnancy: a systematic review. Pregnancy Hypertens 2019;17:178–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fennell C, Seage GR, Zash R, et al. Adverse birth outcomes in Botswana among women with vertically or horizontally acquired human immunodeficiency virus. J Pediatric Infect Dis Soc 2021;10:252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fafin C, Pugliese P, Durant J, et al. Increased time exposure to tenofovir is associated with a greater decrease in estimated glomerular filtration rate in HIV patients with kidney function of less than 60 ml/min/1.73 m2. Nephron Clin Pract 2012;120:c205–14. [DOI] [PubMed] [Google Scholar]
- 92.Floridia M, Masuelli G, Ravizza M, et al. Atazanavir and darunavir in pregnant women with HIV: evaluation of laboratory and clinical outcomes from an observational national study. J Antimicrob Chemother 2018;73:1025–30. [DOI] [PubMed] [Google Scholar]
- 93.Sibiude J, Warszawski J, Tubiana R, et al. Liver enzyme elevation in pregnant women receiving antiretroviral therapy in the ANRS-French Perinatal Cohort. J Acquir Immune Defic Syndr 2019;81:83–94. [DOI] [PubMed] [Google Scholar]
- 94.Momplaisir F, Hussein M, Kacanek D, et al. Perinatal depressive symptoms, human immunodeficiency virus (HIV) suppression, and the underlying role of antiretroviral therapy adherence: a longitudinal mediation analysis in the IMPAACT P1025 Cohort. Clin Infect Dis 2021;73:1379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhu Q-Y, Huang D-S, Lv J-D, Guan P, Bai X-H. Prevalence of perinatal depression among HIV-positive women: a systematic review and meta-analysis. BMC Psychiatry 2019;19:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mollan KR, Smurzynski M, Eron JJ, et al. Association between efavirenz as initial therapy for HIV-1 infection and increased risk for suicidal ideation or attempted or completed suicide: an analysis of trial data. Ann Intern Med 2014;161:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Caniglia EC, Zash R, Jacobson DL, et al. Emulating a target trial of antiretroviral therapy regimens started before conception and risk of adverse birth outcomes. AIDS 2018;32:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]