Abstract
Advancing age and environmental stressors lead to mitochondrial dysfunction in the skin, inducing premature aging, impaired regeneration, and greater risk of cancer. Cells rely on the communication between the mitochondria and the nucleus by tight regulation of long non-coding RNAs (lncRNAs) to avoid premature aging and maintain healthy skin. LncRNAs act as key regulators of cell proliferation, differentiation, survival, and maintenance of skin structure. However, research on how the lncRNAs are dysregulated during aging and due to stressors is needed to develop therapies to regenerate skin’s function and structure. In this article, we discuss how age and environmental stressors may alter lncRNA homeodynamics, compromising cell survival and skin health, and how these factors may become inducers of skin aging. We describe skin cell types and how they depend on mitochondrial function and lncRNAs. We also provide a list of mitochondria localized and nuclear lncRNAs that can serve to better understand skin aging. Using bioinformatic prediction tools, we predict possible functions of lncRNAs based on their subcellular localization. We also search for experimentally determined protein interactions and the biological processes involved. Finally, we provide therapeutic strategies based on gene editing and mitochondria transfer/transplant (AMT/T) to restore lncRNA regulation and skin health. This article offers a unique perspective in understanding and defining the therapeutic potential of mitochondria localized lncRNAs (mt-lncRNAs) produced and AMT/T to treat skin aging and related diseases.
Keywords: Skin, aging, mitochondria, lncRNAs, gene editing, artificial mitochondrial transfer / transplant, AMT/T
Introduction
Skin aging is triggered by the accumulation of mitochondrial and nuclear damage due to sunlight overexposure and other physical, chemical, and biological stressors (Chocron et al., 2019; Krutmann and Schroeder, 2009; López-Otín et al., 2013; Parrado et al., 2019). mitochondrial DNA (mtDNA) damage appears in the form of mutations that negatively affect the translation of proteins involved in the electron transport chain (ETC), leading to an excess of reactive oxygen species (ROS) and metabolic malfunction. Additionally, mitochondrial ROS increases mtDNA mutations, thereby accelerating the production of more ROS, ultimately leading to cell senescence and apoptosis (Hahn and Zuryn, 2019; Zhang et al., 2017). The accumulation of mitochondrial damage over time reduces the skin’s regenerative capacity, deregulates the inflammatory response, promotes structural weakening, and increases the risk of cancer (Sreedhar et al., 2020; Stout and Birch-Machin, 2019). To develop therapies we need to understand the mechanisms by which skin cell mitochondria regulate their functionality in response to environmental stressors.
Skin health relies on the cross-talk between the mitochondria and the nucleus through ncRNAs and other messengers in cells. NcRNAs are present in the mammalian genome and play an essential role at various levels of nuclear and mitochondrial gene expression, thereby supporting the body’s ability to maintain homeostasis and fight off disease (Mattick and Makunin, 2006). There are two broad categories of ncRNAs: small ncRNA (sncRNA), under 200 nucleotides, and lncRNA, of over 200 nucleotides in length (L. Liu et al., 2021). Focusing on lncRNAs and their regulation, we point out questions that remain to be answered regarding how the skin maintains its health with time and how ncRNAs are affected by environmental stressors, changing normal gene expression (Cagin and Enriquez, 2015; Shteinfer-Kuzmine et al., 2021). LncRNAs act as key regulators of cell proliferation, differentiation, survival, and maintenance of skin structure (Cagin and Enriquez, 2015; Jin et al., 2020; Paralkar and Weiss, 2013; Shteinfer-Kuzmine et al., 2021; Soheilifar et al., 2022; Statello et al., 2021; Wang and Jiang, 2020). Interestingly, it has been observed that ultraviolet radiation (UVR) from sunlight is a major environmental stressor of lncRNA expression leading to photoaging (Soheilifar et al., 2022; Wang and Jiang, 2020). In consequence, altered lncRNA structure and expression hinder the proliferative ability of fibroblasts and keratinocytes, triggering an aged skin phenotype (Leucci et al., 2016; L. Tang et al., 2020).
In this article, we discuss how UVR from sunlight hampers lncRNA homeodynamics compromising cell survival, skin health, and their role in aging. To understand this subject, we provide an overview of skin cell types, their function, and dependency on mitochondrial physiology and lncRNA. Then, we discuss how lncRNAs could be a missing link in understanding skin aging and their putative role in restoring the loss of function with time. We consider therapeutic options and strategies under development to delay lncRNAs deregulation by AMT/T.
Insights into the dependence of skin cells on mitochondrial metabolism
The skin is the first barrier against environmental stressors and the most sensitive organ to mutagenic agents (Balcázar et al., 2020; Soheilifar et al., 2022). The regulation of skin cell homeostasis, differentiation, and death are fundamental to maintain tissue health. Regulation of these factors enables the skin to repair or regenerate after tissue damage, preserving its structure and function as the body ages. This can only be achieved by tight interaction between mitochondrial and nuclear metabolites with chemical messengers that affect one another. In this section, we provide insights about the main skin cell types and discuss how their mitochondria and lncRNA regulation work together to maintain homeostasis (Fig.1).
Figure 1. Insights into the dependence of skin cells on mitochondrial metabolism.

Mitochondria play a key role in maintaining the homeostasis and function of skin cells. It has been observed that keratinocytes depend on mitochondria to produce ATP, regulate calcium homeostasis, sustain hair development and tissue structure, and support wound healing. Thanks to the supporting role of mitochondria, melanocytes produce melanin, which leads to skin pigmentation. Langerhans cells have an important mitochondrial function activity rate that supports phenotypic changes from proinflammatory to immune regulatory responses. Merkel cells’ mitochondria play an important role in supporting perception, a process that may be affected by aging. Mitochondria in epithelial stem cells help regulate oxidative stress and preserve skin homeostasis and regenerative capacity. Created with BioRender.com.
The skin is composed of three layers: epidermis, dermis, and hypodermis. The epidermis is comprised of keratinocytes, melanocytes, Langerhans cells (LCs), Merkel cells, and epithelial/epidermis stem cells (EPSCs). Keratinocytes are the primary constituents of the epidermis and are involved in tissue architecture; melanocytes secrete melanin to shield cells against UVR; LCs offer immunological protection of the skin against the infection of harmful microorganisms; Merkel cells are responsible for tactile sensitivity; and EPSCs reside in the epidermis and hair follicles (HFs) and are responsible for homeostasis of adult skin cells and hair regeneration (Blanpain and Fuchs, 2006; Y. Li et al., 2017; Solanas and Benitah, 2013). Each skin cell has a mitochondrial-dependent metabolism (Balcázar et al., 2020; Sreedhar et al., 2020). Epidermal cells such as fibroblasts and keratinocytes have a short life with high metabolic and mitochondrial activity (Balcazar M. et al., 2020). In contrast, Merkel and EPSCs live longer and have a low mitochondrial metabolic rate (Balcázar et al., 2020).
Mitochondria have a crucial role in the maintenance of epidermal homeostasis and cellular organization. Basal undifferentiated epidermal keratinocytes depend on mitochondrial function to produce ATP and regulate Ca2+ and ROS for cell differentiation. Interestingly, the epidermis faces a thermal gradient where keratinocytes in the proliferating basal layer have a temperature of 37°C and 32°C on the skin surface. It has been suggested that this thermal difference is concomitant with mitochondrial oxidative metabolism with a lower activity in the cells of the basal layer, and a higher one at the top of the epidermis, inducing a reduction in keratinocyte proliferation (Viano et al., 2017). In addition, recent in vitro evidence shows that temperature is a decisive factor to consider when producing human keratinocyte sheets to treat large severe wounds (Frese et al., 2021). Keratinocytes grown at 37°C have a better proliferative capacity, and cell differentiation was induced when switched to 33°C (Frese et al., 2021). Temperature and ROS are vital factors that coregulate the differentiation process of keratinocytes, the latter being an essential mitochondrial caspase-dependent apoptotic pathway activator in the upper layer of the epidermis, the stratum corneum (SC) (Sreedhar et al., 2020). During the differentiation process, it appears that even if mitochondria are metabolically active, they lose their capacity to sustain efficient respiration, leading to glycolysis which in turn might contribute to lactate production in the SC. Lactate production is important for maintaining skin flexibility and acting as a barrier to pathogens (Wagner et al., 2021). At the SC, apoptosis is activated by ROS, and keratinocytes go through corneoptosis (Murata et al., 2022). Furthermore, ROS produced by keratinocytes is needed to maintain hair development and tissue structure and to regulate wound healing (Hamanaka and Chandel, 2013; Steen et al., 2020). LncRNAs seem to be regulated by ROS and temperatures, supporting keratinocyte differentiation. These transcripts are key regulators of keratinocyte proliferation, and their deregulation may lead to stress, psoriasis, and cancer (Villota et al., 2012; Ziegler et al., 2019). Further research regarding the right balance between temperature, mitochondrial function, ROS, and lncRNA production and regulation is needed to understand how skin fully maintains homeostasis.
Melanocytes occupy a small fraction of the cells in the epidermis, mainly constituted by keratinocytes (J. Tang et al., 2020). Even in small numbers, basal layer melanocytes fulfill a crucial role through the production of melanin, the major pigment in the skin and hair of mammals, which has the function of absorbing and protecting skin from sunlight UVR (Dumbuya et al., 2020). After UVR exposure, melanocytes produce more melanin in specialized organelles, the melanosomes, which are then transferred to the surrounding keratinocytes (Benito-Martínez et al., 2021). Melanin is composed of two indole compounds synthesized from tyrosine, insoluble black eumelanin, and yellow-reddish pheomelanin. The mix of these two is responsible for color differences in skin and hair (Ohbayashi and Fukuda, 2020). It has been observed that mitochondria and melanosomes interact closely, which is key for melanin production through Ca+2 homeostasis and ROS regulation (Dumbuya et al., 2020). Mitochondria and melanosomes connect in a similar way to mitochondria-endoplasmic-reticulum contact. This contact is modulated by the protein Mitofusin 2 (MFN2) and mitochondrial NCKX5 (Zhang et al., 2019). Interestingly, UVR physiological exposure induces ROS through mitochondrial Complex III, which leads to melanin production and skin pigmentation (Dumbuya et al., 2020). Regarding the regulation of melanogenesis by lncRNA, it has been observed that UCA1 and H19 from keratinocytes that enter melanocytes via exosomes suppress the production of melanin (N.-H. Kim et al., 2014; Pei et al., 2020). More information is needed to understand how ROS, mitochondrial calcium signaling, and lncRNA interact to produce melanin and favor melanocyte maintenance as we age to prevent cancer and other diseases.
LCs are antigen-presenting cells residing in the skin epidermis with a key role in both the innate and adaptive immune responses (Clayton et al., 2017). These cells are derived from macrophage precursors with acquired dendritic cell properties, the ability to migrate to skin-draining lymph nodes, and the ability to communicate with native T-cells (West and Bennett, 2017; Zhou et al., 2022). These mononuclear phagocytes are implanted from a macrophage precursor in the epidermis before birth (West and Bennett, 2017) and constitute a self-renewing population. However, monocytes originated in the bone marrow help to maintain their population, especially during skin inflammation (Zhou et al., 2022). Recently, the existence of two LC subsets has been described: LC1 and LC2. LC1 is related to innate immunity and antigen processing, and LC2 is phenotypically related to monocytes and myeloid dendritic cells regarding leukocyte activation (X. Liu et al., 2021). When LCs are exposed to proinflammatory cytokines, such as tumor necrosis factor α (TNF-α), their mitochondrial activity rate increases through the expression of ribosomal genes such as FBXO2, PSMC3, UCHL3, USP46, and TRIM32. LCs are long-lived cells with a low turnover rate and are highly dependent on mitochondrial function (Balcázar et al., 2020; Seré et al., 2012; Soheilifar et al., 2022). It has been proposed that an increase in the intracellular concentration of mitochondria in LCs could mediate a phenotypic change from proinflammatory into immune regulatory cells (Balcázar et al., 2020). However, no evidence has been found to our knowledge about the mitochondrial dynamics of skin LC1 and LC2 subsets. More evidence on LC metabolism is needed to understand how skin homeostasis is maintained with time.
Merkel cells are mechanosensory, long-lived, highly metabolically active constituents of the basal epidermal layer. Glabrous skin, HFs, the interfollicular epidermis (IFE), and mucosal tissues have a high number of these cells (Balcázar et al., 2020; Moll et al., 2005). Merkel cells contain spine-like structures, variable in length, with longer dendritic processes mixed in with basal and suprabasal keratinocytes. Their area of contact with the nerve fiber, very similar to that of a synapse-like zone, contains numerous mitochondria (Moll et al., 2005). A loss in the perception of physical stimuli on the skin has been associated with aging and a decreased number of mitochondria in Merkel cells (Eckhart et al., 2019). To our knowledge, there is no information on mitochondrial and lncRNA regulation in these cells, and we expect further work and evidence to be produced, especially on how aging may affect Merkel cell function.
EPSCs exist in a limited number and reside in specific niche compartments at the basal layer of the skin epidermis and HFs. EPSC compartments include the IFE, HFs, and sebaceous glands (SGs). Each compartment contains its own population of EPSCs together with differentiated cells that modulate their activity and maintain their stemness (Aponte and Caicedo, 2017). EPSCs preserve skin homeostasis, giving rise to fast-cycling transit-amplifying cells with a key role in injury repair and hair growth (Blanpain and Fuchs, 2006; Naik et al., 2017). EPSCs are long-lived cells that do not rely on their mitochondria for energy production; however, mitochondria remain functional in a cellular compartment, being activated after the beginning of differentiation (Wagner et al., 2021). Interestingly, mitochondria can accumulate damage as we age, affecting EPSC renewal and differentiation even if this organelle is not active in these cells. It has been observed that the induction of oxidative stress by tissue-specific antioxidant Sod2 deficiency accelerates wound closure in young mice; however, in old age, this deficiency hampers tissue repair. EPSCs and their mitochondria can respond to and regulate oxidative stress, adjusting the skin’s regenerative capacity to repair wounds and maintain homeostasis as we age (Velarde et al., 2015). Changes in the skin due to age and exposure to environmental stress affect mitochondria and lncRNA expression, altering homeostasis or leading to disease. It has been demonstrated that HF EPSCs depend on lncRNAs, such as PlncRNA-1, to promote their proliferation and differentiation in a mechanism regulated by TGF-ꞵ1 and mediated by the Wnt/ꞵ-catenin signaling pathway (L. Tang et al., 2020).
Long non-coding RNAs (lncRNAs): the missing link in skin aging, metabolism, and health
NcRNAs, particularly lncRNAs, mediate cellular phenotypic changes through intense communication between the nucleus and mitochondria (Mattick and Makunin, 2006); (Winkle et al., 2021). Anterograde regulation (nucleus signaling to mitochondria) and a retrograde response (mitochondria signaling to the nucleus) characterize this communication (Erdmann and Barciszewski, 2012). LncRNAs have complex properties yet to be described and are fundamental for developing preventive strategies and maintaining the health of skin and other tissues (Wang and Jiang, 2020). Therefore, this section proposes mitochondrial and nuclear lncRNA candidates based on a literature analysis with described roles in cell metabolism, differentiation, and fate. The proposed lncRNAs need to be further studied to understand skin cellular dynamics and maintenance of its functional properties.
The expression of mt-lncRNAs correlates with the replicative state of the cell and has a crucial role in cell proliferation (Villegas et al., 2007). LncRNAs exhibit diverse biological functions as they translocate from mitochondria to the cytosol or remain within mitochondria—the mitochondrial genome codes for at least eight lncRNAs, small RNAs, and hundreds of circRNAs. Key mt lncRNAs are SncmtRNA, ASncmtRNA-1 and −2, MDL1, LncCytb, LncND6, and LncND5 (Burzio et al., 2009; Liu and Shan, 2021; Li et al., 2022; Rackham et al., 2011).
Human and mouse mitochondria express two lncRNAs derived from the 16S rRNA gene: sense mitochondrial ncRNA (SncmtRNA) and antisense mitochondrial ncRNAs (ASncmtRNAs). Neither are detectable by in situ hybridization in resting or senescent cells; however, SncmtRNA is detectable in all proliferating cells (Borgna et al., 2017; Burzio et al., 2009; Lobos-González et al., 2016; Vidaurre et al., 2014; Villegas et al., 2007; Villota et al., 2012). Interestingly, only normal proliferating cells express two antisense mt-lncRNAs, ASncmtRNA-1 and −2, in contrast to cancer cells, in which these transcripts are undetectable. ASncmtRNAs are also linked to controlled cell proliferation, acting as tumor suppressors, and downregulation of these transcripts seems to be a general hallmark of cancer (Borgna et al., 2017; Burzio et al., 2009; Lobos-González et al., 2016; Vidaurre et al., 2014; Villota et al., 2012). ASncmtRNAs show retrograde signaling evidenced by electron microscopy in situ hybridization, where their presence was observed in the cytoplasm and nucleus of human and mouse cells (Kazemzadeh et al., 2015; Landerer et al., 2011). Downregulation of ASncmtRNAs seems to be a vulnerability of cancer cells since their knockdown induces apoptosis in tumor cell lines from various tissue origins, while the viability of normal cells remains unaffected (Lobos-González et al., 2016). The tight regulation of SncmtRNA and ASncmtRNAs is vital to maintain cell homeostasis and equilibrium with their environment. Nevertheless, the regulation of SncmtRNA and ASncmtRNAs warrants further studies mainly in the context of the recently described process of mitochondrial transfer between cells, from mesenchymal stem cells (MSCs) to cancer, therapeutic mitochondrial transfer, and the analysis of the effects of extracellular mitochondria in circulation (Cabrera et al., 2022, 2019; Caicedo et al., 2021b, 2021a; Padilla-Sánchez et al., 2020).
MDL1 is an essential mt lncRNA involved in the cross-talk between the nucleus and mitochondria, regulating the cell’s response to environmental stressors (Li et al., 2022). MDL1 is transcribed from the mitochondrial D-loop region. Interestingly, this lncRNA controls the movement of p53 from the nucleus to the cytoplasm. p53 is a crucial gene that helps prevent cancer by controlling cell division. This regulation occurs through the formation of a three-part complex, consisting of p53, tumorous imaginal disc 1 (TID1), and the lncRNA. TID1 is a protein that is found in mitochondria and plays a role in regulating the electrical potential across the mitochondrial membrane and keeping the mtDNA intact(Li et al., 2022). In this way, TID1 prevents the clumping together of Complex I and handles the stress response that results from a decrease in the function of ATP synthase (Ng et al., 2014). Due to its relationship with p53, MDL1 could be considered a new mediator of cell cycle regulation and response to stress (Li et al., 2022). Understanding the role of MDL1 during EPSCs differentiation, proliferation, and death is critical to develop countermeasures to the environmental stress that induces skin aging.
In the mitochondrial genome, lncRNA LncCytb, LncND6, and LncND5 are produced from the complementary regions of the genes Cytb, ND6, and ND5, respectively. These lncRNAs remain inside the mitochondrial matrix in contact with its genome, where each lncRNA forms a duplex structure with the region of its complementary gene, thereby stabilizing ND5, ND6, and Cyt b mRNA expression (Rackham et al., 2011). Cyt b, ND6, and DN5 proteins are involved in ATP production as they are part of the ETC; thus, proper ATP production requires adequate expression of these proteins (Bai and Attardi, 1998). The replicative state of skin cells consumes large amounts of ATP; therefore, inadequate production of ATP results in a senescent phenotype. Accordingly, LncCytb, LncND6, and LncND5 are promising candidates for developing therapies to avoid premature skin aging.
Nuclear lncRNAs with a significant role in skin cells are ANRC, H19, SPRIGHTLY, UCA1, PlncRNA 1, RP11–766N7.3, HOTAIR, MALAT1, WAKMAR1 and ANRIL (Kretz et al., 2012; Lin et al., 2014; C.-X. Li et al., 2017; Li et al., 2019; Pasmant et al., 2007; Pei et al., 2020; Si et al., 2018; Sun et al., 2017; L. Tang et al., 2020; Zhao et al., 2016). LncRNAs that are linked to mitochondrial metabolic dynamics are SAMMSON, AK055347, ANRIL, CARL, BATE1, CCAT2, CEROX, FAL1, GAS5, HOTAIR, H19, HOTTIP, MEG3, MPRL, PVT1, TUG1, MALAT1, and UCA1 (Goding, 2016; Gusic and Prokisch, 2020). Therefore, the nuclear lncRNAs that are vital in skin aging and have an incidence on mitochondrial function are H19, ANRIL, UCA1, and MALAT1. We analyzed some of the aforementioned lncRNAs with the BioGPS tool (Wu et al., 2009) to determine the organs that express the highest levels of each (Fig. 2). To speculate on their possible function, we then used three bioinformatics tools to predict their subcellular localization: (1) DeepLncLoc (Zeng et al., 2022), (2) Locate-R (Ahmad et al., 2020), and (3) LncLocator (Cao et al., 2018) and used their canonical sequences reported in Ensembl (Fig. 3). Finally, we searched for experimentally-determined protein interactions to expand our gene list and used these results as input to obtain the enriched Gene Ontology (GO) terms shown in Fig. 4.
Figure 2. shows the enrichment fold of available lncRNA in data sets compared to a control sample, as detected by sequencing and reported in BioGPS. Profiling the tissues in which lncRNAs are most highly expressed provides insights into their relevance in health.

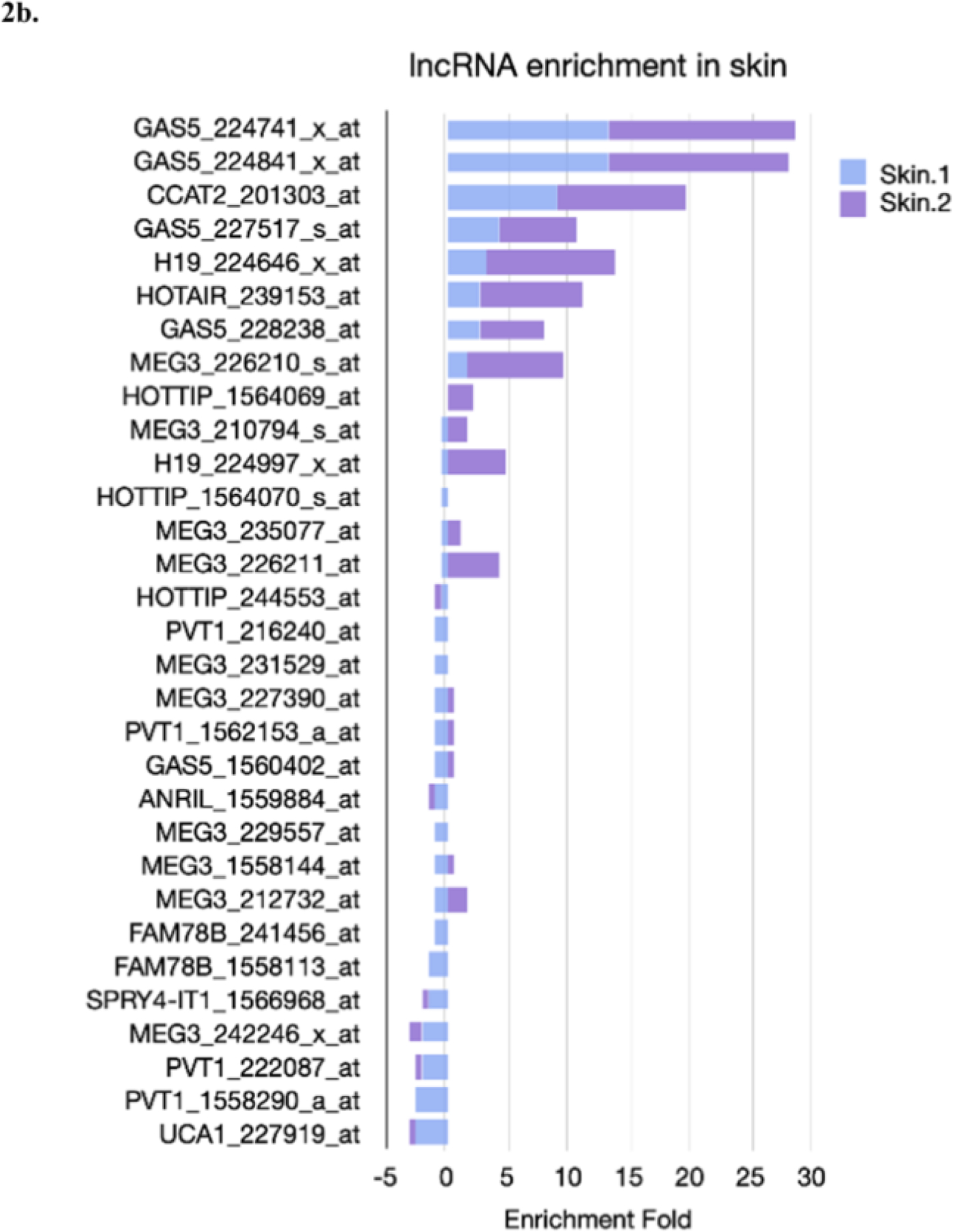
2a. Top ten tissues where each lncRNA has the highest enrichment fold, or values that are above any of the two backgrounds used. The enrichment fold is the z-score, which indicates the number of standard deviations from the mean. A 5 fold change suggests that the lncRNAs are expressed in that tissue. For each graph, there is a 5-fold reference (black line), and the identification code for the probe analyzed for each gene. The selected probe is the one with the highest values for their top ten genes. Note that each graph has been generated with an automated axis, since it is not appropriate to compare data from different sets of probes.
2b. Describes the comparison of the enrichment fold in skin for each gene and their probe sets.
Figure 3. Predicted sub-cellular localization of lncRNAs.

We predicted the sub-cellular localization of our candidate lncRNAs using three different tools based on their sequence. Specifically, we used the canonical transcript reported in Ensembl to predict their location. The three tools employed were LncLocator (shown in sky-blue), DeepLncLoc (shown in green), and Locate-R (shown in blue).
Figure 4. Putative role for lncRNAs in the protection of cells against UV radiation.

4a. Gene Ontology significantly enriched terms (p. value <0.05) for Biological Processes using GOstat version 2022–11-03 on R. The gene list corresponds to the lncRNAs, plus the protein interactions described on Arena-Idb (- Institute for Biomedical Technologies, National Research Council (CNR), Bari - Department of Computer Science, University of Verona).
4b.Directed Acyclic Graph (DAG) representation for GO results using QuickGO using 4a. terms as input. The kind of relationship is detailed in the legend of the graph.
2c. Proteins associated with the lncRNAs described in Arena-Idb as a network that were used as an input. Colored proteins represent the GO term to which they are associated.
Notably, lncRNAs are subject to a highly regulated expression that tends to be cell-type specific and may have different roles depending on their subcellular localization ((Cagin and Enriquez, 2015; Jin et al., 2020; Paralkar and Weiss, 2013; Shteinfer-Kuzmine et al., 2021; Soheilifar et al., 2022; Statello et al., 2021; Wang and Jiang, 2020)). Moreover, eight of the aforementioned lncRNAs are more likely to localize in the nucleus based on their sequence alone. This matched localization in the cell suggests that their role might be similar across different cells. The lncRNAs that are predicted to localize primarily in the nucleus in at least two sources are ANRIL, MEG3, HOTAIR, CCAT2, FAL1, and GAS5 (Fig. 3). While HOTAIR was most enriched in skin tissue (Fig. 2A), GAS5, CCAT2, MEG3, and H19 had a 5X enrichment in at least one of their analyzed probesets (Fig. 2B). Conclusively, the GO results show significantly enriched terms that are related to various stimuli responses, mainly those associated to cellular response to UVR (GO:0034644), RNA metabolic process (GO:0016070), positive regulation of nitrogen compound metabolic process (GO:10051173), positive regulation of cellular metabolic process (GO:GO:0031325), and positive regulation of macromolecule metabolic process (GO:0010604) (Fig. 4). Altogether, the evidence supports a possible role for lncRNAs in the protection of cells against UVR, which is closely related to the skin aging process.
As mentioned previously, H19, ANRIL, UCA1, and MALAT1 are the primary nuclear lncRNAs that have an effect on mitochondrial function and, in return, skin aging.Their specific functions in the subject matter are explained subsequently.
H19 is a lncRNA imprinting gene that promotes keratinocyte differentiation by binding to miR-130b-3p (Tang et al., 2020), thus increasing the translation of its target desmoglein-1 (Dsg1) (Wang et al., 2020). H19 is also the precursor of miR-675, which directly targets voltage-dependent anion channel 1 (VDAC1) (Li et al., 2016). VDAC1 is the most abundant protein located on the outer membrane of the mitochondria (Camara et al., 2017; Olajide et al., 2022). This specific protein is crucial in regulating mitochondria-mediated apoptosis and is vital for maintaining tissue structure (Li et al., 2016). However, enforced expression of H19 can reduce VDAC1 expression and inhibit apoptosis (Li et al., 2016).
ANRIL is an antisense lncRNA located in the INK4 locus and is involved in cell proliferation, migration, and senescence (Bian et al., 2021). It takes part in several biological processes with contrasting effects on cell growth, proliferation, and senescence of vascular smooth muscle cells. Its role in these processes needs to be further understood to analyze its effect on cardiovascular disease, cancers, diabetes, and other pathologies (Bian et al., 2021; Congrains et al., 2013). ANRIL-mediated homologous recombination (HR) repair of DNA leads to the promotion of cancer resistance and a large number of polymorphisms (L. Liu et al., 2021). ANRIL is highly expressed in cancerous tissues compared to normal tissue and displays the capacity to modulate the expression of cleaved-PARP and Bcl-2, both apoptosis-related proteins in the mitochondrial pathway (Xu et al., 2017).
Urothelial Carcinoma Associated 1 (UCA1) is a lncRNA with a pro-oncogenic role in bladder and skin cancers. In the bladder, UCA1 upregulates the cell cycle through the cAMP response element-binding protein (CREB)/PI3K/AKT pathway, signaling tumor growth (H.-J. Li et al., 2017). UCA1 indirectly improves mitochondrial function in this tissue and upregulates ARL2 and miR-195 genes enhancing mitochondrial fusion and boosting cancerous cell viability (H.-J. Li et al., 2017). On the other hand, in the skin, UCA1 negatively regulates the CREB/MITF/melanogenesis as it inhibits the cAMP/PKA, ERK, and JNK signaling pathways in melanocytes (Pei et al., 2020). As pigmented skin is an aged phenotype, UCA1 is a therapeutic target. In melanomas, the high expression of UCA1 has been linked to melanoma metastasis (Tian et al., 2014). Upregulation of UCA1 shows an increased expression of the tumor-promoting homeobox B3 (HOXB3) and forkhead box M1 (FOXM1) transcription factors explaining metastatic features (Han et al., 2019; Wei et al., 2016). Nevertheless, the role of UCA1 in the regulation of mitochondrial fusion and dynamics, together with melanoma aggressiveness, needs further study as the control of both processes may lead to new therapeutic strategies to hamper the advancement of cancer and aged skin.
Metastasis-associated adenocarcinoma of the lung transcript 1 (MALAT1) is expressed in the nucleus and plays a role in splicing, transcriptional activity, and numerous biological processes such as tumor proliferation, metastasis, and epithelial-mesenchymal transition (Gutschner et al., 2013; Lei et al., 2017; Tian et al., 2014; Xu et al., 2016). In skin fibroblasts, after UVR irradiation, increased MALAT1 expression was manifested, denoting a role of MALAT1 in photoaging (Lei et al., 2017). The results showed that upregulation of MALAT1 in skin fibroblasts induced a senescent phenotype (Lei et al., 2017). In lung cancer, MALAT1-deficient cells produce less ATP and show impaired cell invasion (Gutschner et al., 2013). This finding suggests that this lncRNA may play an important role in mitochondrial metabolism, as it plays a role in the senescent phenotype and cell invasion capabilities. Photoaged skin cells have high ERK/MAPK activity as a result of high ROS concentrations, and studies have suggested a relationship with MALAT1, as a link between MALAT1 and the ERK/MAPK pathway has been established in other tissues such as hepatocellular carcinoma, and cardiovascular tissues (M.-J. Kim et al., 2014; Liu et al., 2014; Wu et al., 2014; Xie et al., 2021). However, in skin cells, this relationship remains unclear and further studies are needed to determine the extent to which MALAT1 regulates mitochondrial dynamics.
The cross-talk of lncRNAs synthesized in the nucleus and mitochondria plays a fundamental role in regulating cell activity, metabolism, and proliferation (Bianchessi et al., 2015; Degirmenci and Lei, 2016). Either overexpression or underexpression, these messengers may be used to maintain tissue structure with time or to treat cancer. Control of their expression and suppression could induce cellular changes toward maintaining tissue health. By proposing a list of those lncRNAs expressed in the mitochondria and the nucleus, we aim to awaken the scientific community’s interest, especially those working on skin aging. In the future, after understanding how lncRNAs work on each skin cell, it would be possible to identify those that are upregulated and related to disease and use zinc finger nucleases (ZFNs) (Lim et al., 2022). The use of base editing in nuclear and mtDNA is constantly evolving towards better strategies, such as zinc finger deaminases (ZFDs) (Lim et al., 2022). ZFDs were developed for indel-free, precision base editing in eukaryotic cells by combining split-DddAtox to customized ZFPs. Programmable nucleases such as ZFNs, TALENs, and Cas9 can cleave DNA, leading to double-strand breaks (DSBs), and still create deletions, causing an off-target reprogramming, which in turn activates cellular stress response (Lim et al., 2022). An exciting alternative under development is the application of AMT/T, which may lead to the expression of new mt lncRNAs in recipient cells. Undoubtedly, further research is needed to understand how gene editing tools or AMT/T may affect skin aging. In the next section, we propose new therapeutic strategies that could regulate lncRNA expression and effects.
Therapeutic strategies to restore lncRNA gene deregulation
Due to the lack of tools designed to assess the damages in the structure and expression of lncRNAs, mt-lncRNA deregulation by different environmental factors and aging represents a challenge for the field. The tools mentioned below can regulate both nuclear and mt-lncRNA expression:
The ZFNs technique consists of nucleases containing monomers with Fok1 domains, which allow them to reach the mitochondrial matrix, bind to mtDNA, and selectively cleave lncRNA genes. However, toxic effects and loss of mtDNA copies may be long-term consequences of this therapy (Rai et al., 2018). Similarly, transcription activator-like effector nucleases for mitochondria (mitoTALEN) contain Fok1 restriction endonuclease activity that breaks the double mtDNA after dimerization of specific binding sites such as lncRNA loci (Rai et al., 2018).
On the other hand, chemically-modified antisense oligonucleotides (ASOs) can be designed to target specific RNAs for their RNase H-mediated degradation. Thus, these oligonucleotides are post-transcriptional elements that knock down lncRNAs through endonucleolytic cleavage (Arun et al., 2018). Additionally, there is a second generation of ASOs termed uniformly-modified ASOs, capable of inhibiting or altering the expression of genes through steric hindrance and alteration at splicing sites (Arun et al., 2018). They do not elicit RNAse H activity, but they are adequate for modulating splicing patterns of target RNAs by blocking splicing enhancers or repressor binding sites.
Additionally, the third generation of ASOs includes modifications such as locked nucleic acids (LNA) and S-constrained ethyl (cEt), which have led to promising clinical trials for cancer and neurological diseases. Currently, cEt for STAT3 and androgenic receptors are in phase II clinical trials (Arun et al., 2018). Besides acting in the cytoplasm, ASOs also work effectively in the cell nucleus and mitochondria (Lobos-González et al., 2016). The effectiveness of ASOs is possible since the nuclease RNase H is present in both the nucleus and mitochondria (Reyes et al., 2015).
Synthetic RNAs hold great potential for lncRNA modulation. Recently, an RNA interference pathway (RNAi), which consists of an exogenous double-stranded RNA system that specifically disables specific RNAs, was discovered in Caenorhabditis elegans (Winkle et al., 2021). This RNAi has a circular shape and is covalently closed, making it more resistant and longer-acting. Moreover, RNAi can silence post-transcriptional lncRNAs since synthetic RNAs can promote the activity of RNase III enzymes, RNA-induced silencing complex (RISC complex), and Endonuclease Argonaut 2 (Ago2) to degrade specific RNAs. Interestingly, a successful in vivo study was conducted using RNA-targeted RNAi in human cells and mouse models to disable Fas RNA in mouse liver cells with fulminant hepatitis (Winkle et al., 2021). The Fas gene, also known as Tnfrsf6, encodes the Fas receptor, which protects mice from liver failure and fibrosis. However, to our knowledge there are no studies supporting the application of this technique for crossing the mitochondrial barriers, and therefore its application is currently limited to the cell nucleus.
Preventive strategies, such as antioxidants, help maintain mtDNA structure. Vitamin D, among other antioxidants, affects the mitochondrial function of skin cells; therefore, low levels of Vitamin D may cause alterations in the maintenance of skin health over time. The lack of Vitamin D produces cellular damage, apoptosis, and aging (Wimalawansa, 2019). The primary source of vitamin D is the transformation of 7-dehydrocholesterol to cholecalciferol, which is triggered by UVR exposure. Vitamin D regulates erythroid-2 nuclear factor (NF-E2), related nuclear factor (NrF2) and Klotho. Transcription factors NrF2 and NF-E2 play an important and protective role within skin cells. Vitamin D and other antioxidants may prevent both oxidative stress in our daily life and extreme conditions, possibly hampering the advancement of the skin aging process (Gómez et al. 2021). The levels of these factors are inversely correlated with the accumulation of mitochondrial ROS and cell apoptosis (Wang et al., 2010). Klotho is a hormone that drives the signaling systems and the formation of antioxidants. Therefore, Klotho-deficient mice undergo a premature aging syndrome in mouse models (Kuro-o, 2009). Thus, vitamin D is vital for regulating cellular oxidations and redox. Low vitamin D levels promote the release of inflammatory cytokines such as TNF-α and 1,4,5-trisphosphate receptors (InsP3Rs), which may deregulate the anticancer response by our bodies.
AMT/T as an emerging therapeutic strategy to prevent skin aging
Cells can transfer mitochondria naturally by using nanotubes, vesicles, or freely without membranes (FreeMitos), reprogramming the phenotype of recipient cells and helping them recover from damage and function deterioration (Caicedo et al., 2021b; Velarde et al., 2022). Cell fusion, synaptosomes, and dendritic networks are recent mechanisms involving mitochondrial transfer between cells. In harmed tissue in vitro and vivo, MSCs can transfer mitochondria to damaged cells to restore homeodynamics (Sinha et al., 2016). The natural transfer of mitochondria leads to the possibility of isolating mitochondria from the donor cell and transferring them to others to induce phenotypic changes or to re-establish lost functions.
The allogeneic or xenogeneic AMT/T has been inspired by two main currents: First, mitochondria can exit and enter cells because of their endosymbiotic origin and their past as alpha-proteobacteria. Second, there is a natural transfer of mitochondria between cells, especially from MSCs to others that are damaged or phenotypically unhealthy (Caicedo et al., 2017). AMT/T has been used as a therapeutic approach to rescue damaged tissue with promising results in vitro, in vivo, and in clinical trials (Doulamis et al., 2020; Moskowitzova et al., 2020, 2019; Shin et al., 2019). This transfer may reduce ROS production in recipient cells, thus aiding in the repair of damaged cells, decreasing cell death, and reestablishing basal functions (Cabrera et al., 2019; Gollihue and Rabchevsky, 2017). After the transfer process, there is an increase in the energetic capacities of cells that receive mitochondria (Caicedo et al., 2015; Chen et al., 2011). For example, AMT/T to nerve tissue rescued neurons that had suffered acute ischemic stress (Pei et al., 2020). Additionally, transferring mitochondria to liver tissue after intoxication can reduce stress by increasing its energy production, decreasing the amount of ROS, and promoting tissue restoration (Zhao et al., 2021). In ischemic cardiomyopathy, damaged cells release damaged mitochondria as a signal for support. Mitochondria transfer improves mitochondrial biogenesis, antioxidant capacity enhancement, and reduction of apoptosis (Chen et al., 2021; Kaza et al., 2017). For these reasons, mitochondrial transfer represents a promising therapeutic strategy for the skin rejuvenation.
Mitochondria express lncRNAs such as SncmtRNA, ASncmtRNA-1 and −2, MDL1, MDL1AS, LncCytb, LncND6, and LncND5, originated from their genome (mtDNA). AMT/T could relocate new mitochondria in recipient cells with the capacity to produce mtDNA encoded lncRNAs at normal levels. As the skin ages, cancer risk increases (Sreedhar et al., 2020). Transfering mitochondria with the capacity to produce SncmtRNAs may prevent or tumor growth and recover normal proliferation. AMT/T could lead to the expression of MDL1 and mediate the response to stress due to aging. Inducing the presence of LncCytb, LncND6, and LncND5 may help to rebalance energy production and improve the overall skin capacity to maintain its function with time. Using AMT/T in vitro, in vivo, and in clinical trials, reports show that the recovery of cells could be used to repair skin damage produced by aging (Balcázar et al., 2020). MitoCeption and other AMT/T techniques have been shown to induce in vitro an increase in cell proliferation, migration, and ATP production, key processes to restore aged skin (Caicedo et al., 2015; Kitani et al., 2014; Wu et al., 2016).
Aging deregulates vital processes in the skin, such as wound healing, pigmentation, and inflammation. Previously we reported that AMT/T could repair UVR-damaged cells by restoring their metabolic activity and mitochondrial mass, replenishing mtDNA levels in recipient cells, and decreasing p53 expression (Cabrera et al., 2019). AMT/T can change the phenotype of TH17 cells to Tregs, promoting immune regulation but not immune activation (Court et al., 2020; Luz-Crawford et al., 2019; Ramirez-Barbieri et al., 2019). Based on this evidence, the use of AMT/T to induce the expression of lncRNAs with anti-cancerous properties and as metabolic regulators has shown to be a promising approach to regenerating aged skin.
Concluding Remarks
Skin aging reduces skin’s regenerative capacity and increases cancer risk likley by the deregulation of the cross-talk between nuclear and mitochondrial lncRNAs. We analyzed how skin cells use their mitochondria and lncRNA to maintain healthy levels of cell proliferation, differentiation, survival, and maintenance of skin structure. We provide a list of nuclear and mitochondrial lncRNAs to develop therapeutic approaches to restore healthy skin. Understanding the link between the deregulation of lncRNAs and aging is necessary. The bioinformatic prediction tools helped us analyze their subcellular localization and protein interactions with the biological processes involved. Based on this evidence, we propose future therapeutic options and strategies utilizing on gene editing and AMT/T to restore lncRNA regulation and skin health. We offer a unique perspective to further research needed to understand the therapeutic potential of using mitochondrial lncRNAs and AMT/T to treat skin aging.
Table 1.
Summary of the lncRNAs primary function and subcellular localization.
| LncRNA | Main Function | Localization | Original Paper | |
|---|---|---|---|---|
| Mitochondrial | SncmtRNA | Cells proliferation | Nucleus associated with the heterocromatin | (Landerer et al., 2011) |
| ASncmtRNA-1 and −2 | Tumor suppressors; retrograde signaling | Cytoplasm and nucleus, not found in cancer cells | (Landerer et al., 2011) | |
| MDL1 | Translocation of p53 | In the nucleus, forming a three-part complex with TID1 and p53 | (Li et al., 2022) | |
| lncRNA LncCytb, LncND6, and LncND5 | Each lncRNA forms a duplex structure with the region of their complementary gene, thereby stabilizing ND5, ND6, and Cyt b mRNA expression | Mitochondrial matrix in contact with its genome | (Rackham et al., 2011) | |
| NUCLEAR | Antisense non-coding RNA in the INK4 locus (ANRIL) | Cell proliferation, migration, and senescence | Nucleus and cytoplasm | (Naemura et al., 2016) |
| H19 | Promotes keratinocyte differentiation. Precursor of miR-130–3p. Reduces VDAC1 expression and inhibit apoptosis | Nucleus | (Huang et al., 2015; Tan et al., 2019) | |
| UCA1 | Pro-oncogenic role in bladder and skin cancers | Cytoplasma | (Wang et al., 2019, 2008) | |
| Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) | Rolele in photoaging, splicing, transcriptional activity, and numerous biological processes such as tumor proliferation, metastasis, and epithelial-mesenchymal transition. | Nucleus | (Ying et al., 2012) |
Highlights:
Accumulation of mitochondrial and nuclear damage induces skin aging.
Nuclear and mt-lncRNAs are tightly regulated to preserve skin health.
The study of age-related alterations on skin lncRNAs could lead to better therapies.
Gene editing and AMT/T could regulate lncRNA expression in the skin.
Acknowledgments
We thank the School of Medicine at the Universidad San Francisco de Quito USFQ, the “Instituto de Investigaciones en Biomedicina, USFQ,” and the Mito-Act Research Consortium in Quito, Ecuador for their constant support of our work and initiatives. VC thanks the ANID-Subdirección de Capital Humano/Doctorado Nacional for their support.
Funding
This work was supported by the Air Force Office of Scientific Research (AFOSR) grant (FA9550–19–S–0003; FA9550–20–1–0407, Project Leader Andrés Caicedo) and partially supported by the Escuela de Medicina, Colegio de Ciencias de la Salud COCSA, Universidad San Francisco de Quito USFQ. KKS is supported by NIH grant R21OD031970. VC is supported by ANID-Subdirección de Capital Humano/Doctorado Nacional/2022–21220897 and FONDECYT 11190998; Proyecto Centro Basal ANID IMPACT:FB210024.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
AC is the leader of the Dragon BioMed-USFQ initiative, and AC and VC are its founders. KKS is the scientific founder and chief scientfic officer of Yuva Biosciences. All other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Bibliography
- Ahmad A, Lin H, Shatabda S, 2020. Locate-R: Subcellular localization of long non-coding RNAs using nucleotide compositions. Genomics 112, 2583–2589. doi: 10.1016/j.ygeno.2020.02.011 [DOI] [PubMed] [Google Scholar]
- Aponte PM, Caicedo A, 2017. Stemness in cancer: stem cells, cancer stem cells, and their microenvironment. Stem Cells Int 2017, 5619472. doi: 10.1155/2017/5619472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Attardi G, 1998. The mtDNA-encoded ND6 subunit of mitochondrial NADH dehydrogenase is essential for the assembly of the membrane arm and the respiratory function of the enzyme. EMBO J 17, 4848–4858. doi: 10.1093/emboj/17.16.4848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcázar M, Cañizares S, Borja T, Pontón P, Bisiou S, Carabasse E, Bacilieri A, Canavese C, Diaz RF, Cabrera F, Caicedo A, 2020. Bases for treating skin aging with artificial mitochondrial transfer/transplant (AMT/T). Front. Bioeng. Biotechnol 8, 919. doi: 10.3389/fbioe.2020.00919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito-Martínez S, Salavessa L, Raposo G, Marks MS, Delevoye C, 2021. Melanin Transfer and Fate within Keratinocytes in Human Skin Pigmentation. Integr. Comp. Biol 61, 1546–1555. doi: 10.1093/icb/icab094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian M, Yu Y, Li Y, Zhou Z, Wu X, Ye X, Yu J, 2021. Upregulating the Expression of LncRNA ANRIL Promotes Osteogenesis via the miR-7–5p/IGF-1R Axis in the Inflamed Periodontal Ligament Stem Cells. Front. Cell Dev. Biol 9, 604400. doi: 10.3389/fcell.2021.604400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchessi V, Badi I, Bertolotti M, Nigro P, D’Alessandra Y, Capogrossi MC, Zanobini M, Pompilio G, Raucci A, Lauri A, 2015. The mitochondrial lncRNA ASncmtRNA-2 is induced in aging and replicative senescence in Endothelial Cells. J. Mol. Cell. Cardiol 81, 62–70. doi: 10.1016/j.yjmcc.2015.01.012 [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E, 2006. Epidermal stem cells of the skin. Annu. Rev. Cell Dev. Biol 22, 339–373. doi: 10.1146/annurev.cellbio.22.010305.104357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgna V, Villegas J, Burzio VA, Belmar S, Araya M, Jeldes E, Lobos-González L, Silva V, Villota C, Oliveira-Cruz L, Lopez C, Socias T, Castillo O, Burzio LO, 2017. Mitochondrial ASncmtRNA-1 and ASncmtRNA-2 as potent targets to inhibit tumor growth and metastasis in the RenCa murine renal adenocarcinoma model. Oncotarget 8, 43692–43708. doi: 10.18632/oncotarget.18460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzio VA, Villota C, Villegas J, Landerer E, Boccardo E, Villa LL, Martínez R, Lopez C, Gaete F, Toro V, Rodriguez X, Burzio LO, 2009. Expression of a family of noncoding mitochondrial RNAs distinguishes normal from cancer cells. Proc Natl Acad Sci USA 106, 9430–9434. doi: 10.1073/pnas.0903086106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera F, Castañeda V, Morales E, Velarde F, Ortega M, Leon-Sosa A, Jorgensen C, Caicedo A, 2022. Early evidence of the artificial transfer/transplant of mitochondria to oocytes and zygotes by MitoCeption. Mitochondrion 65, 102–112. doi: 10.1016/j.mito.2022.05.006 [DOI] [PubMed] [Google Scholar]
- Cabrera F, Ortega M, Velarde F, Parra E, Gallardo S, Barba D, Soto L, Peña G, Pedroza LA, Jorgensen C, Khoury M, Caicedo A, 2019. Primary allogeneic mitochondrial mix (PAMM) transfer/transplant by MitoCeption to address damage in PBMCs caused by ultraviolet radiation. BMC Biotechnol 19, 42. doi: 10.1186/s12896-019-0534-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagin U, Enriquez JA, 2015. The complex crosstalk between mitochondria and the nucleus: What goes in between? Int. J. Biochem. Cell Biol 63, 10–15. doi: 10.1016/j.biocel.2015.01.026 [DOI] [PubMed] [Google Scholar]
- Caicedo A, Aponte PM, Cabrera F, Hidalgo C, Khoury M, 2017. Artificial mitochondria transfer: current challenges, advances, and future applications. Stem Cells Int 2017, 7610414. doi: 10.1155/2017/7610414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Fritz V, Brondello J-M, Ayala M, Dennemont I, Abdellaoui N, de Fraipont F, Moisan A, Prouteau CA, Boukhaddaoui H, Jorgensen C, Vignais M-L, 2015. MitoCeption as a new tool to assess the effects of mesenchymal stem/stromal cell mitochondria on cancer cell metabolism and function. Sci. Rep 5, 9073. doi: 10.1038/srep09073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo A, Zambrano K, Sanon S, Gavilanes AWD, 2021a. Extracellular mitochondria in the cerebrospinal fluid (CSF): Potential types and key roles in central nervous system (CNS) physiology and pathogenesis. Mitochondrion 58, 255–269. doi: 10.1016/j.mito.2021.02.006 [DOI] [PubMed] [Google Scholar]
- Caicedo A, Zambrano K, Sanon S, Luis Vélez J, Montalvo M, Jara F, Moscoso SA, Vélez P, Maldonado A, Velarde G, 2021b. The diversity and coexistence of extracellular mitochondria in circulation: A friend or foe of the immune system. Mitochondrion 58, 270–284. doi: 10.1016/j.mito.2021.02.014 [DOI] [PubMed] [Google Scholar]
- Camara AKS, Zhou Y, Wen P-C, Tajkhorshid E, Kwok W-M, 2017. Mitochondrial VDAC1: A key gatekeeper as potential therapeutic target. Front. Physiol 8, 460. doi: 10.3389/fphys.2017.00460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Pan X, Yang Y, Huang Y, Shen H-B, 2018. The lncLocator: a subcellular localization predictor for long non-coding RNAs based on a stacked ensemble classifier. Bioinformatics 34, 2185–2194. doi: 10.1093/bioinformatics/bty085 [DOI] [PubMed] [Google Scholar]
- Chen J, Zhong J, Wang L-L, Chen Y-Y, 2021. Mitochondrial transfer in cardiovascular disease: from mechanisms to therapeutic implications. Front. Cardiovasc. Med 8, 771298. doi: 10.3389/fcvm.2021.771298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S-D, Yang D-I, Lin T-K, Shaw F-Z, Liou C-W, Chuang Y-C, 2011. Roles of oxidative stress, apoptosis, PGC-1α and mitochondrial biogenesis in cerebral ischemia. Int. J. Mol. Sci 12, 7199–7215. doi: 10.3390/ijms12107199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chocron ES, Munkácsy E, Pickering AM, 2019. Cause or casualty: The role of mitochondrial DNA in aging and age-associated disease. Biochim. Biophys. Acta Mol. Basis Dis 1865, 285–297. doi: 10.1016/j.bbadis.2018.09.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton K, Vallejo AF, Davies J, Sirvent S, Polak ME, 2017. Langerhans Cells-Programmed by the Epidermis. Front. Immunol 8, 1676. doi: 10.3389/fimmu.2017.01676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congrains A, Kamide K, Ohishi M, Rakugi H, 2013. ANRIL: molecular mechanisms and implications in human health. Int. J. Mol. Sci 14, 1278–1292. doi: 10.3390/ijms14011278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court AC, Le-Gatt A, Luz-Crawford P, Parra E, Aliaga-Tobar V, Bátiz LF, Contreras RA, Ortúzar MI, Kurte M, Elizondo-Vega R, Maracaja-Coutinho V, Pino-Lagos K, Figueroa FE, Khoury M, 2020. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep 21, e48052. doi: 10.15252/embr.201948052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degirmenci U, Lei S, 2016. Role of lncRNAs in Cellular Aging. Front Endocrinol (Lausanne) 7, 151. doi: 10.3389/fendo.2016.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulamis IP, Guariento A, Duignan T, Kido T, Orfany A, Saeed MY, Weixler VH, Blitzer D, Shin B, Snay ER, Inkster JA, Packard AB, Zurakowski D, Rousselle T, Bajwa A, Parikh SM, Stillman IE, Del Nido PJ, McCully JD, 2020. Mitochondrial transplantation by intra-arterial injection for acute kidney injury. Am. J. Physiol. Renal Physiol 319, F403–F413. doi: 10.1152/ajprenal.00255.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumbuya H, Hafez SY, Oancea E, 2020. Cross talk between calcium and ROS regulate the UVA-induced melanin response in human melanocytes. FASEB J 34, 11605–11623. doi: 10.1096/fj.201903024R [DOI] [PubMed] [Google Scholar]
- Eckhart L, Tschachler E, Gruber F, 2019. Autophagic control of skin aging. Front. Cell Dev. Biol 7, 143. doi: 10.3389/fcell.2019.00143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann VA, Barciszewski J (Eds.), 2012. From nucleic acids sequences to molecular medicine Springer; Berlin Heidelberg, Berlin, Heidelberg. doi: 10.1007/978-3-642-27426-8 [DOI] [Google Scholar]
- Frese L, Darwiche SE, von Rechenberg B, Hoerstrup SP, Giovanoli P, Calcagni M, 2021. Thermal conditioning improves quality and speed of keratinocyte sheet production for burn wound treatment. Cytotherapy 23, 536–547. doi: 10.1016/j.jcyt.2021.01.006 [DOI] [PubMed] [Google Scholar]
- Goding CR, 2016. Targeting the lncRNA SAMMSON Reveals Metabolic Vulnerability in Melanoma. Cancer Cell 29, 619–621. doi: 10.1016/j.ccell.2016.04.010 [DOI] [PubMed] [Google Scholar]
- Gollihue JL, Rabchevsky AG, 2017. Prospects for therapeutic mitochondrial transplantation. Mitochondrion 35, 70–79. doi: 10.1016/j.mito.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusic M, Prokisch H, 2020. ncRNAs: New Players in Mitochondrial Health and Disease? Front. Genet 11, 95. doi: 10.3389/fgene.2020.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutschner T, Hämmerle M, Eissmann M, Hsu J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Gross M, Zörnig M, MacLeod AR, Spector DL, Diederichs S, 2013. The noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cells. Cancer Res 73, 1180–1189. doi: 10.1158/0008-5472.CAN-12-2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Zuryn S, 2019. Mitochondrial Genome (mtDNA) Mutations that Generate Reactive Oxygen Species. Antioxidants (Basel) 8. doi: 10.3390/antiox8090392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka RB, Chandel NS, 2013. Mitochondrial metabolism as a regulator of keratinocyte differentiation. Cell. Logist 3, e25456. doi: 10.4161/cl.25456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C, Tang F, Chen J, Xu D, Li X, Xu Y, Wang S, Zhou J, 2019. Knockdown of lncRNA-UCA1 inhibits the proliferation and migration of melanoma cells through modulating the miR-28–5p/HOXB3 axis. Exp. Ther. Med 17, 4294–4302. doi: 10.3892/etm.2019.7421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Zheng Y, Jia L, Li W, 2015. Long Noncoding RNA H19 Promotes Osteoblast Differentiation Via TGF-β1/Smad3/HDAC Signaling Pathway by Deriving miR-675. Stem Cells 33, 3481–3492. doi: 10.1002/stem.2225 [DOI] [PubMed] [Google Scholar]
- Jin K-T, Yao J-Y, Fang X-L, Di H, Ma Y-Y, 2020. Roles of lncRNAs in cancer: Focusing on angiogenesis. Life Sci 252, 117647. doi: 10.1016/j.lfs.2020.117647 [DOI] [PubMed] [Google Scholar]
- Kaza AK, Wamala I, Friehs I, Kuebler JD, Rathod RH, Berra I, Ericsson M, Yao R, Thedsanamoorthy JK, Zurakowski D, Levitsky S, Del Nido PJ, Cowan DB, McCully JD, 2017. Myocardial rescue with autologous mitochondrial transplantation in a porcine model of ischemia/reperfusion. J. Thorac. Cardiovasc. Surg 153, 934–943. doi: 10.1016/j.jtcvs.2016.10.077 [DOI] [PubMed] [Google Scholar]
- Kazemzadeh M, Safaralizadeh R, Orang AV, 2015. LncRNAs: emerging players in gene regulation and disease pathogenesis. J. Genet 94, 771–784. doi: 10.1007/s12041-015-0561-6 [DOI] [PubMed] [Google Scholar]
- Kim M-J, Woo SW, Kim M-S, Park J-E, Hwang J-K, 2014. Anti-photoaging effect of aaptamine in UVB-irradiated human dermal fibroblasts and epidermal keratinocytes. J. Asian Nat. Prod. Res 16, 1139–1147. doi: 10.1080/10286020.2014.983092 [DOI] [PubMed] [Google Scholar]
- Kim N-H, Choi S-H, Kim C-H, Lee CH, Lee TR, Lee A-Y, 2014. Reduced MiR-675 in exosome in H19 RNA-related melanogenesis via MITF as a direct target. J. Invest. Dermatol 134, 1075–1082. doi: 10.1038/jid.2013.478 [DOI] [PubMed] [Google Scholar]
- Kitani T, Kami D, Matoba S, Gojo S, 2014. Internalization of isolated functional mitochondria: involvement of macropinocytosis. J. Cell. Mol. Med 18, 1694–1703. doi: 10.1111/jcmm.12316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz M, Webster DE, Flockhart RJ, Lee CS, Zehnder A, Lopez-Pajares V, Qu K, Zheng GXY, Chow J, Kim GE, Rinn JL, Chang HY, Siprashvili Z, Khavari PA, 2012. Suppression of progenitor differentiation requires the long noncoding RNA ANCR. Genes Dev 26, 338–343. doi: 10.1101/gad.182121.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutmann J, Schroeder P, 2009. Role of mitochondria in photoaging of human skin: the defective powerhouse model. J. Investig. Dermatol. Symp. Proc 14, 44–49. doi: 10.1038/jidsymp.2009.1 [DOI] [PubMed] [Google Scholar]
- Landerer E, Villegas J, Burzio VA, Oliveira L, Villota C, Lopez C, Restovic F, Martinez R, Castillo O, Burzio LO, 2011. Nuclear localization of the mitochondrial ncRNAs in normal and cancer cells. Cell Oncol (Dordr) 34, 297–305. doi: 10.1007/s13402-011-0018-8 [DOI] [PubMed] [Google Scholar]
- Lei L, Zeng Q, Lu J, Ding S, Xia F, Kang J, Tan L, Gao L, Kang L, Cao K, Zhou J, Xiao R, Chen J, Huang J, 2017. MALAT1 participates in ultraviolet B-induced photo-aging via regulation of the ERK/MAPK signaling pathway. Mol. Med. Report 15, 3977–3982. doi: 10.3892/mmr.2017.6532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucci E, Coe EA, Marine J-C, Vance KW, 2016. The emerging role of long non-coding RNAs in cutaneous melanoma. Pigment Cell Melanoma Res 29, 619–626. doi: 10.1111/pcmr.12537 [DOI] [PubMed] [Google Scholar]
- Li D, Kular L, Vij M, Herter EK, Li X, Wang A, Chu T, Toma M-A, Zhang L, Liapi E, Mota A, Blomqvist L, Gallais Sérézal I, Rollman O, Wikstrom JD, Bienko M, Berglund D, Ståhle M, Sommar P, Jagodic M, Landén NX, 2019. Human skin long noncoding RNA WAKMAR1 regulates wound healing by enhancing keratinocyte migration. Proc Natl Acad Sci USA 116, 9443–9452. doi: 10.1073/pnas.1814097116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K, Cho S-I, Kim J-S, 2022. Nuclear and mitochondrial DNA editing in human cells with zinc finger deaminases. Nat. Commun 13, 366. doi: 10.1038/s41467-022-27962-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C-M, Liu Y, Huang K, Chen X-C, Cai B-Z, Li H-H, Yuan Y-P, Zhang H, Li Y, 2014. Long noncoding RNA expression in dermal papilla cells contributes to hairy gene regulation. Biochem. Biophys. Res. Commun 453, 508–514. doi: 10.1016/j.bbrc.2014.09.119 [DOI] [PubMed] [Google Scholar]
- Liu JY, Yao J, Li XM, Song YC, Wang XQ, Li YJ, Yan B, Jiang Q, 2014. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis 5, e1506. doi: 10.1038/cddis.2014.466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Chen Y, Huang Y, Cao K, Liu T, Shen H, Cui J, Li B, Cai J, Gao F, Yang Y, 2021. Long non-coding RNA ANRIL promotes homologous recombination-mediated DNA repair by maintaining ATR protein stability to enhance cancer resistance. Mol. Cancer 20, 94. doi: 10.1186/s12943-021-01382-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Shan G, 2021. Mitochondria Encoded Non-coding RNAs in Cell Physiology. Front. Cell Dev. Biol 9, 713729. doi: 10.3389/fcell.2021.713729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu R, Luo Y, Wang S, Zhao Y, Qiu Z, Zhang Y, Xiao Liu, Yao X, Li X, Li W, 2021. Distinct human Langerhans cell subsets orchestrate reciprocal functions and require different developmental regulation. Immunity 54, 2305–2320.e11. doi: 10.1016/j.immuni.2021.08.012 [DOI] [PubMed] [Google Scholar]
- Li C-X, Li H-G, Huang L-T, Kong Y-W, Chen F-Y, Liang J-Y, Yu H, Yao Z-R, 2017. H19 lncRNA regulates keratinocyte differentiation by targeting miR-130b-3p. Cell Death Dis 8, e3174. doi: 10.1038/cddis.2017.516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H-J, Sun X-M, Li Z-K, Yin Q-W, Pang H, Pan J-J, Li X, Chen W, 2017. LncRNA UCA1 Promotes Mitochondrial Function of Bladder Cancer via the MiR-195/ARL2 Signaling Pathway. Cell. Physiol. Biochem 43, 2548–2561. doi: 10.1159/000484507 [DOI] [PubMed] [Google Scholar]
- Li J, Bai R, Yang W, Miao H, Li Y, Dai H, Li L, Zhao Y, Song X, 2022. The mitochondrial‐derived lncRNA MDL1 mediates a mitochondria‐to‐nucleus retrograde regulation by inhibiting the nuclear translocation of p53. MedComm – Oncology 1 doi: 10.1002/mog2.15 [DOI]
- Li X, Wang H, Yao B, Xu W, Chen J, Zhou X, 2016. lncRNA H19/miR-675 axis regulates cardiomyocyte apoptosis by targeting VDAC1 in diabetic cardiomyopathy. Sci. Rep 6, 36340. doi: 10.1038/srep36340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang J, Yue J, Gou X, Wu X, 2017. Epidermal stem cells in skin wound healing. Adv Wound Care (New Rochelle) 6, 297–307. doi: 10.1089/wound.2017.0728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobos-González L, Silva V, Araya M, Restovic F, Echenique J, Oliveira-Cruz L, Fitzpatrick C, Briones M, Villegas J, Villota C, Vidaurre S, Borgna V, Socias M, Valenzuela S, Lopez C, Socias T, Varas M, Díaz J, Burzio LO, Burzio VA, 2016. Targeting antisense mitochondrial ncRNAs inhibits murine melanoma tumor growth and metastasis through reduction in survival and invasion factors. Oncotarget 7, 58331–58350. doi: 10.18632/oncotarget.11110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz-Crawford P, Hernandez J, Djouad F, Luque-Campos N, Caicedo A, Carrère-Kremer S, Brondello J-M, Vignais M-L, Pène J, Jorgensen C, 2019. Mesenchymal stem cell repression of Th17 cells is triggered by mitochondrial transfer. Stem Cell Res. Ther 10, 232. doi: 10.1186/s13287-019-1307-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick JS, Makunin IV, 2006. Non-coding RNA. Hum. Mol. Genet 15 Spec No 1, R17–29. doi: 10.1093/hmg/ddl046 [DOI] [PubMed] [Google Scholar]
- Moll I, Roessler M, Brandner JM, Eispert A-C, Houdek P, Moll R, 2005. Human Merkel cells--aspects of cell biology, distribution and functions. Eur. J. Cell Biol 84, 259–271. doi: 10.1016/j.ejcb.2004.12.023 [DOI] [PubMed] [Google Scholar]
- Moskowitzova K, Orfany A, Liu K, Ramirez-Barbieri G, Thedsanamoorthy JK, Yao R, Guariento A, Doulamis IP, Blitzer D, Shin B, Snay ER, Inkster JAH, Iken K, Packard AB, Cowan DB, Visner GA, Del Nido PJ, McCully JD, 2020. Mitochondrial transplantation enhances murine lung viability and recovery after ischemia-reperfusion injury. Am. J. Physiol. Lung Cell. Mol. Physiol 318, L78–L88. doi: 10.1152/ajplung.00221.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitzova K, Shin B, Liu K, Ramirez-Barbieri G, Guariento A, Blitzer D, Thedsanamoorthy JK, Yao R, Snay ER, Inkster JAH, Orfany A, Zurakowski D, Cowan DB, Packard AB, Visner GA, Del Nido PJ, McCully JD, 2019. Mitochondrial transplantation prolongs cold ischemia time in murine heart transplantation. J. Heart Lung Transplant 38, 92–99. doi: 10.1016/j.healun.2018.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Honda T, Mostafa A, Kabashima K, 2022. Stratum corneum as polymer sheet: concept and cornification processes. Trends Mol. Med 28, 350–359. doi: 10.1016/j.molmed.2022.02.008 [DOI] [PubMed] [Google Scholar]
- Naemura M, Tsunoda T, Inoue Y, Okamoto H, Shirasawa S, Kotake Y, 2016. ANRIL regulates the proliferation of human colorectal cancer cells in both two- and three-dimensional culture. Mol. Cell. Biochem 412, 141–146. doi: 10.1007/s11010-015-2618-5 [DOI] [PubMed] [Google Scholar]
- Naik S, Larsen SB, Gomez NC, Alaverdyan K, Sendoel A, Yuan S, Polak L, Kulukian A, Chai S, Fuchs E, 2017. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 550, 475–480. doi: 10.1038/nature24271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng AC-H, Baird SD, Screaton RA, 2014. Essential role of TID1 in maintaining mitochondrial membrane potential homogeneity and mitochondrial DNA integrity. Mol. Cell. Biol 34, 1427–1437. doi: 10.1128/MCB.01021-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohbayashi N, Fukuda M, 2020. Recent advances in understanding the molecular basis of melanogenesis in melanocytes. F1000Res 9. doi: 10.12688/f1000research.24625.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olajide OJ, La Rue C, Bergdahl A, Chapman CA, 2022. Inhibiting amyloid beta (1–42) peptide-induced mitochondrial dysfunction prevents the degradation of synaptic proteins in the entorhinal cortex. Front. Aging Neurosci 14, 960314. doi: 10.3389/fnagi.2022.960314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Sánchez SD, Navarrete D, Caicedo A, Teran E, 2020. Circulating cell-free mitochondrial DNA levels correlate with body mass index and age. Biochim. Biophys. Acta Mol. Basis Dis 1866, 165963. doi: 10.1016/j.bbadis.2020.165963 [DOI] [PubMed] [Google Scholar]
- Paralkar VR, Weiss MJ, 2013. Long noncoding RNAs in biology and hematopoiesis. Blood 121, 4842–4846. doi: 10.1182/blood-2013-03-456111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrado C, Mercado-Saenz S, Perez-Davo A, Gilaberte Y, Gonzalez S, Juarranz A, 2019. Environmental stressors on skin aging. mechanistic insights. Front. Pharmacol 10, 759. doi: 10.3389/fphar.2019.00759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasmant E, Laurendeau I, Héron D, Vidaud M, Vidaud D, Bièche I, 2007. Characterization of a germ-line deletion, including the entire INK4/ARF locus, in a melanoma-neural system tumor family: identification of ANRIL, an antisense noncoding RNA whose expression coclusters with ARF. Cancer Res 67, 3963–3969. doi: 10.1158/0008-5472.CAN-06-2004 [DOI] [PubMed] [Google Scholar]
- Pei S, Chen J, Lu J, Hu S, Jiang L, Lei L, Ouyang Y, Fu C, Ding Y, Li S, Kang L, Huang L, Xiang H, Xiao R, Zeng Q, Huang J, 2020. The long noncoding RNA UCA1 negatively regulates melanogenesis in melanocytes. J. Invest. Dermatol 140, 152–163.e5. doi: 10.1016/j.jid.2019.04.029 [DOI] [PubMed] [Google Scholar]
- Rackham O, Shearwood A-MJ, Mercer TR, Davies SMK, Mattick JS, Filipovska A, 2011. Long noncoding RNAs are generated from the mitochondrial genome and regulated by nuclear-encoded proteins. RNA 17, 2085–2093. doi: 10.1261/rna.029405.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai PK, Craven L, Hoogewijs K, Russell OM, Lightowlers RN, 2018. Advances in methods for reducing mitochondrial DNA disease by replacing or manipulating the mitochondrial genome. Essays Biochem 62, 455–465. doi: 10.1042/EBC20170113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Barbieri G, Moskowitzova K, Shin B, Blitzer D, Orfany A, Guariento A, Iken K, Friehs I, Zurakowski D, Del Nido PJ, McCully JD, 2019. Alloreactivity and allorecognition of syngeneic and allogeneic mitochondria. Mitochondrion 46, 103–115. doi: 10.1016/j.mito.2018.03.002 [DOI] [PubMed] [Google Scholar]
- Seré K, Baek J-H, Ober-Blöbaum J, Müller-Newen G, Tacke F, Yokota Y, Zenke M, Hieronymus T, 2012. Two distinct types of Langerhans cells populate the skin during steady state and inflammation. Immunity 37, 905–916. doi: 10.1016/j.immuni.2012.07.019 [DOI] [PubMed] [Google Scholar]
- Shin B, Saeed MY, Esch JJ, Guariento A, Blitzer D, Moskowitzova K, Ramirez-Barbieri G, Orfany A, Thedsanamoorthy JK, Cowan DB, Inkster JA, Snay ER, Staffa SJ, Packard AB, Zurakowski D, Del Nido PJ, McCully JD, 2019. A novel biological strategy for myocardial protection by intracoronary delivery of mitochondria: safety and efficacy. JACC Basic Transl. Sci 4, 871–888. doi: 10.1016/j.jacbts.2019.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shteinfer-Kuzmine A, Verma A, Arif T, Aizenberg O, Paul A, Shoshan-Barmaz V, 2021. Mitochondria and nucleus cross-talk: Signaling in metabolism, apoptosis, and differentiation, and function in cancer. IUBMB Life 73, 492–510. doi: 10.1002/iub.2407 [DOI] [PubMed] [Google Scholar]
- Sinha P, Islam MN, Bhattacharya S, Bhattacharya J, 2016. Intercellular mitochondrial transfer: bioenergetic crosstalk between cells. Curr. Opin. Genet. Dev 38, 97–101. doi: 10.1016/j.gde.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si Y, Bai J, Wu J, Li Q, Mo Y, Fang R, Lai W, 2018. LncRNA PlncRNA‑1 regulates proliferation and differentiation of hair follicle stem cells through TGF‑β1‑mediated Wnt/β‑catenin signal pathway. Mol. Med. Report 17, 1191–1197. doi: 10.3892/mmr.2017.7944 [DOI] [PubMed] [Google Scholar]
- Soheilifar MH, Masoudi-Khoram N, Shirkavand A, Ghorbanifar S, 2022. Non-coding RNAs in photoaging-related mechanisms: a new paradigm in skin health. Biogerontology 23, 289–306. doi: 10.1007/s10522-022-09966-x [DOI] [PubMed] [Google Scholar]
- Solanas G, Benitah SA, 2013. Regenerating the skin: a task for the heterogeneous stem cell pool and surrounding niche. Nat. Rev. Mol. Cell Biol 14, 737–748. doi: 10.1038/nrm3675 [DOI] [PubMed] [Google Scholar]
- Sreedhar A, Aguilera-Aguirre L, Singh KK, 2020. Mitochondria in skin health, aging, and disease. Cell Death Dis 11, 444. doi: 10.1038/s41419-020-2649-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statello L, Guo C-J, Chen L-L, Huarte M, 2021. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol 22, 96–118. doi: 10.1038/s41580-020-00315-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen K, Chen D, Wang F, Majumdar R, Chen S, Kumar S, Lombard DB, Weigert R, Zieman AG, Parent CA, Coulombe PA, 2020. A role for keratins in supporting mitochondrial organization and function in skin keratinocytes. Mol. Biol. Cell 31, 1103–1111. doi: 10.1091/mbc.E19-10-0565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout R, Birch-Machin M, 2019. Mitochondria’s role in skin ageing. Biology (Basel) 8. doi: 10.3390/biology8020029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Cheng H, Wang G, Yu G, Zhang D, Wang Y, Fan W, Yang W, 2017. Deregulation of miR-183 promotes melanoma development via lncRNA MALAT1 regulation and ITGB1 signal activation. Oncotarget 8, 3509–3518. doi: 10.18632/oncotarget.13862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Fewings E, Chang D, Zeng H, Liu S, Jorapur A, Belote RL, McNeal AS, Tan TM, Yeh I, Arron ST, Judson-Torres RL, Bastian BC, Shain AH, 2020. The genomic landscapes of individual melanocytes from human skin. Nature 586, 600–605. doi: 10.1038/s41586-020-2785-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Liang Y, Xie H, Yang X, Zheng G, 2020. Long non-coding RNAs in cutaneous biology and proliferative skin diseases: Advances and perspectives. Cell Prolif 53, e12698. doi: 10.1111/cpr.12698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan P, Guo Y-H, Zhan J-K, Long L-M, Xu M-L, Ye L, Ma X-Y, Cui X-J, Wang H-Q, 2019. LncRNA-ANRIL inhibits cell senescence of vascular smooth muscle cells by regulating miR-181a/Sirt1. Biochem. Cell Biol 97, 571–580. doi: 10.1139/bcb-2018-0126 [DOI] [PubMed] [Google Scholar]
- Tian Y, Zhang X, Hao Y, Fang Z, He Y, 2014. Potential roles of abnormally expressed long noncoding RNA UCA1 and Malat-1 in metastasis of melanoma. Melanoma Res 24, 335–341. doi: 10.1097/CMR.0000000000000080 [DOI] [PubMed] [Google Scholar]
- Velarde F, Ezquerra S, Delbruyere X, Caicedo A, Hidalgo Y, Khoury M, 2022. Mesenchymal stem cell-mediated transfer of mitochondria: mechanisms and functional impact. Cell. Mol. Life Sci 79, 177. doi: 10.1007/s00018-022-04207-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velarde MC, Demaria M, Melov S, Campisi J, 2015. Pleiotropic age-dependent effects of mitochondrial dysfunction on epidermal stem cells. Proc Natl Acad Sci USA 112, 10407–10412. doi: 10.1073/pnas.1505675112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viano M, Alotto D, Aillon A, Castagnoli C, Silvagno F, 2017. A thermal gradient modulates the oxidative metabolism and growth of human keratinocytes. FEBS Open Bio 7, 1843–1853. doi: 10.1002/2211-5463.12303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaurre S, Fitzpatrick C, Burzio VA, Briones M, Villota C, Villegas J, Echenique J, Oliveira-Cruz L, Araya M, Borgna V, Socías T, Lopez C, Avila R, Burzio LO, 2014. Down-regulation of the antisense mitochondrial non-coding RNAs (ncRNAs) is a unique vulnerability of cancer cells and a potential target for cancer therapy. J. Biol. Chem 289, 27182–27198. doi: 10.1074/jbc.M114.558841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villegas J, Burzio V, Villota C, Landerer E, Ronny Martinez, Santander M, Rodrigo Martinez, Pinto R, Vera MI, Boccardo E, Villa LL, Burzio LO, 2007. Expression of a novel non-coding mitochondrial RNA in human proliferating cells. Nucleic Acids Res 35, 7336–7347. doi: 10.1093/nar/gkm863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villota C, Campos A, Vidaurre S, Oliveira-Cruz L, Boccardo E, Burzio VA, Varas M, Villegas J, Villa LL, Valenzuela PDT, Socías M, Roberts S, Burzio LO, 2012. Expression of mitochondrial non-coding RNAs (ncRNAs) is modulated by high risk human papillomavirus (HPV) oncogenes. J. Biol. Chem 287, 21303–21315. doi: 10.1074/jbc.M111.326694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner RN, Piñón Hofbauer J, Wally V, Kofler B, Schmuth M, De Rosa L, De Luca M, Bauer JW, 2021. Epigenetic and metabolic regulation of epidermal homeostasis. Exp. Dermatol 30, 1009–1022. doi: 10.1111/exd.14305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-J, Zhu C-C, Xu J, Wang M, Zhao W-Y, Liu Q, Zhao G, Zhang Z-Z, 2019. The lncRNA UCA1 promotes proliferation, migration, immune escape and inhibits apoptosis in gastric cancer by sponging anti-tumor miRNAs. Mol. Cancer 18, 115. doi: 10.1186/s12943-019-1032-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Li X, Xie X, Zhao L, Chen W, 2008. UCA1, a non-protein-coding RNA up-regulated in bladder carcinoma and embryo, influencing cell growth and promoting invasion. FEBS Lett 582, 1919–1927. doi: 10.1016/j.febslet.2008.05.012 [DOI] [PubMed] [Google Scholar]
- Wang Y-J, Jiang X, 2020. Insight into the roles of long non-coding RNAs in ultraviolet-induced skin diseases. Chin. Med. J 134, 398–400. doi: 10.1097/CM9.0000000000001062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Sun Q, Zhao L, Wu J, Chen X, Wang Y, Zang W, Zhao G, 2016. LncRNA UCA1-miR-507-FOXM1 axis is involved in cell proliferation, invasion and G0/G1 cell cycle arrest in melanoma. Med. Oncol 33, 88. doi: 10.1007/s12032-016-0804-2 [DOI] [PubMed] [Google Scholar]
- West HC, Bennett CL, 2017. Redefining the Role of Langerhans Cells As Immune Regulators within the Skin. Front. Immunol 8, 1941. doi: 10.3389/fimmu.2017.01941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkle M, El-Daly SM, Fabbri M, Calin GA, 2021. Noncoding RNA therapeutics - challenges and potential solutions. Nat. Rev. Drug Discov 20, 629–651. doi: 10.1038/s41573-021-00219-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, Hodge CL, Haase J, Janes J, Huss JW, Su AI, 2009. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol 10, R130. doi: 10.1186/gb-2009-10-11-r130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T-H, Sagullo E, Case D, Zheng X, Li Y, Hong JS, TeSlaa T, Patananan AN, McCaffery JM, Niazi K, Braas D, Koehler CM, Graeber TG, Chiou P-Y, Teitell MA, 2016. Mitochondrial transfer by photothermal nanoblade restores metabolite profile in mammalian cells. Cell Metab 23, 921–929. doi: 10.1016/j.cmet.2016.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X-S, Wang X-A, Wu W-G, Hu Y-P, Li M-L, Ding Q, Weng H, Shu Y-J, Liu T-Y, Jiang L, Cao Y, Bao R-F, Mu J-S, Tan Z-J, Tao F, Liu Y-B, 2014. MALAT1 promotes the proliferation and metastasis of gallbladder cancer cells by activating the ERK/MAPK pathway. Cancer Biol. Ther 15, 806–814. doi: 10.4161/cbt.28584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S-J, Diao L-T, Cai N, Zhang L-T, Xiang S, Jia C-C, Qiu D-B, Liu C, Sun Y-J, Lei H, Hou Y-R, Tao S, Hu Y-X, Xiao Z-D, Zhang Q, 2021. mascRNA and its parent lncRNA MALAT1 promote proliferation and metastasis of hepatocellular carcinoma cells by activating ERK/MAPK signaling pathway. Cell Death Discov 7, 110. doi: 10.1038/s41420-021-00497-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Zhang Z-Q, Fang Y-C, Li X-L, Sun Y, Xiong C-Z, Yan L-Q, Wang Q, 2016. Metastasis-associated lung adenocarcinoma transcript 1 promotes the proliferation of chondrosarcoma cell via activating Notch-1 signaling pathway. Onco Targets Ther 9, 2143–2151. doi: 10.2147/OTT.S100003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Mao Y, Chen K, He W, Shi W, Han Y, 2017. The long noncoding RNA ANRIL acts as an oncogene and contributes to paclitaxel resistance of lung adenocarcinoma A549 cells. Oncotarget 8, 39177–39184. doi: 10.18632/oncotarget.16640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying L, Chen Q, Wang Y, Zhou Z, Huang Y, Qiu F, 2012. Upregulated MALAT-1 contributes to bladder cancer cell migration by inducing epithelial-to-mesenchymal transition. Mol. Biosyst 8, 2289–2294. doi: 10.1039/c2mb25070e [DOI] [PubMed] [Google Scholar]
- Zeng M, Wu Y, Lu C, Zhang F, Wu F-X, Li M, 2022. DeepLncLoc: a deep learning framework for long non-coding RNA subcellular localization prediction based on subsequence embedding. Brief. Bioinformatics 23. doi: 10.1093/bib/bbab360 [DOI] [PubMed] [Google Scholar]
- Zhang R, Wang Y, Ye K, Picard M, Gu Z, 2017. Independent impacts of aging on mitochondrial DNA quantity and quality in humans. BMC Genomics 18, 890. doi: 10.1186/s12864-017-4287-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Gong J, Sviderskaya EV, Wei A, Li W, 2019. Mitochondrial NCKX5 regulates melanosomal biogenesis and pigment production. J. Cell Sci 132. doi: 10.1242/jcs.232009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Mazar J, Lee B, Sawada J, Li J-L, Shelley J, Govindarajan S, Towler D, Mattick JS, Komatsu M, Dinger ME, Perera RJ, 2016. The long noncoding RNA SPRIGHTLY regulates cell proliferation in primary human melanocytes. J. Invest. Dermatol 136, 819–828. doi: 10.1016/j.jid.2016.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Hou Y, Zhou W, Keerthiga R, Fu A, 2021. Mitochondrial transplantation therapy inhibit carbon tetrachloride-induced liver injury through scavenging free radicals and protecting hepatocytes. Bioeng. Transl. Med 6, e10209. doi: 10.1002/btm2.10209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Jiang A, Veenstra J, Ozog DM, Mi Q-S, 2022. The roles of skin langerhans cells in immune tolerance and cancer immunity. Vaccines (Basel) 10. doi: 10.3390/vaccines10091380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler C, Graf J, Faderl S, Schedlbauer J, Strieder N, Förstl B, Spang R, Bruckmann A, Merkl R, Hombach S, Kretz M, 2019. The long non-coding RNA LINC00941 and SPRR5 are novel regulators of human epidermal homeostasis. EMBO Rep 20. doi: 10.15252/embr.201846612 [DOI] [PMC free article] [PubMed] [Google Scholar]


