Abstract
Occupational lung diseases (OLD) are a group of preventable conditions caused by noxious inhalation exposure in the workplace. Workers in various industries are at a higher risk of developing OLD. Despite regulations contributing to a decreased incidence, OLD remain among the most frequently diagnosed work-related conditions, contributing to significant morbidity and mortality. A multidisciplinary discussion (MDD) is necessary for a timely diagnosis. Imaging, particularly computed tomography, plays a central role in diagnosing OLD and excluding other inhalational lung diseases. OLD can be broadly classified into fibrotic and non-fibrotic forms. Imaging reflects variable degrees of inflammation and fibrosis involving the airways, parenchyma, and pleura. Common manifestations include classical pneumoconioses, chronic granulomatous diseases (CGD), and small and large airway diseases. Imaging is influenced by the type of inciting exposure. The findings of airway disease may be subtle or solely uncovered upon expiration. High-resolution chest CT, including expiratory-phase imaging, should be performed in all patients with suspected OLD. Radiologists should familiarize themselves with these imaging features to improve diagnostic accuracy.
Keywords: Occupational lung disease, Asbestosis, Silicosis, Coal worker's pneumoconiosis, Chronic beryllium disease, Hypersensitivity pneumonitis, Asthma, Obliterative bronchiolitis
INTRODUCTION
Occupational lung diseases (OLD) comprise a heterogeneous group of preventable conditions that typically result from ongoing or recurrent noxious inhalation exposure in the workplace. Common causal agents include various organic and inorganic dust particles and chemicals, with OLD nomenclature often relevant to specific inciting exposures [1]. Industrial workers are particularly at risk. Although workplace regulations have contributed to a decreased incidence, OLD continue to be among the most frequently diagnosed work-related conditions. In developing countries, OLD are becoming increasingly prevalent as economies expand and regulations lag behind. In the United States, nearly 30 million Americans remain at risk of developing OLD, and approximately 30000 deaths annually can partially be attributed to these conditions [2]. In recent decades, the resurgence of certain forms of OLD has been reported in high-risk areas, notably within the central Appalachian region of the United States of America [3]. With new emerging technologies, modified industrial practices, and material discoveries, new sources of OLD are frequently identified, adding to the complexity of the diagnostic approach and leading to underreporting [4,5,6]. Similar to other interstitial lung diseases (ILD), multidisciplinary discussion (MDD) is key to achieving a timely diagnosis [7]. A thorough investigation of the possible inhalational exposures and their temporal relationship with symptom development is critical [1]. Long latency periods are possible in certain OLD forms, notably asbestosis [4]. While MDD is necessary, the imaging features of certain OLD may be highly suggestive. Chest high-resolution computed tomography (HRCT) plays a central role in diagnosing OLD and excluding other possible inhalational lung diseases (e.g., smoking-related lung disease) [1,8]. Therefore, radiologists should familiarize themselves with the common imaging manifestations of OLD, especially as imaging utilization increases.
Pneumoconiosis can be broadly classified into fibrotic and non-fibrotic forms, depending on the fibrogenic or non-fibrogenic nature of the inciting agent [1,4,9]. Imaging manifestations reflect variable degrees of inflammation and fibrosis, with multi-compartment involvement of the airways, parenchyma, and pleura. An overlap may be observed (Table 1). OLD can generally be categorized into classical pneumoconioses, chronic granulomatous diseases (CGD), and airways diseases [10]. Inflammation in the setting of ILD may manifest as centrilobular or perilymphatic nodularity, ground-glass opacities, and occasional consolidation, which may progress to frank fibrosis. Fibrotic features include reticulation, traction bronchiectasis, honeycombing, volume loss, and architectural distortion. Examples of occupational airway diseases include irritant asthma, asthma with latency, and obliterative bronchiolitis (OB). Imaging of airway diseases may appear mostly normal or show centrilobular nodularity, bronchial wall thickening, mosaic attenuation, or emphysema [1,11]. Expiratory phase imaging increases sensitivity for air trapping.
Table 1. List of common exposures with typical imaging findings and differentiating features.
| Exposure | Imaging findings | Differentiating feature |
|---|---|---|
| Silica dust exposure | Multiple small nodules, especially in the upper lung fields. May show eggshell calcifications in the hilar lymph nodes. | Presence of eggshell calcifications in the hilar lymph nodes is characteristic of silicosis. |
| Asbestos fiber exposure | Bilateral, irregular opacities in the lower lung fields, often with a “reticular” or “honeycomb” appearance. May show pleural plaques. | Presence of pleural plaques, calcified or thickened areas on the pleura, is highly suggestive of asbestos exposure. |
| Coal dust exposure | Small nodules or opacities in the upper lung fields. Progressive massive fibrosis (PMF) with larger, confluent opacities or masses in advanced cases. | Presence of PMF with larger opacities or masses is specific to coal worker’s pneumoconiosis. |
| Beryllium exposure | Small nodules, ground-glass opacities, or diffuse interstitial lung disease. May show hilar and mediastinal lymphadenopathy. | Presence of hilar and mediastinal lymphadenopathy is often seen in berylliosis. |
| Aluminum dust exposure | Fine, irregular opacities typically involving the upper lung zones. May show pleural effusions in severe cases. | Association with pleural effusions in severe cases. |
| Cadmium dust exposure | Ground-glass opacities, interstitial fibrosis, and emphysematous changes. May show calcium deposition in the lung parenchyma or pleura. | Presence of calcium deposition in the lung parenchyma or pleura. |
| Lead dust exposure | Basal or lower lung predominant interstitial fibrosis with possible upper lobe sparing. May show evidence of lead lines in long bones on radiography. | Presence of upper lobe sparing and evidence of lead lines in long bones on radiography. |
| Mercury vapor exposure | Diffuse ground-glass opacities or consolidations. May show pleural effusions. | Association with pleural effusions. |
| Grain dust exposure | Transient pulmonary infiltrates or ground-glass opacities. | Symptoms of acute febrile illness may be present in organic dust toxic syndrome. |
| Isocyanate exposure | Diffuse ground-glass opacities or consolidation. | History of exposure to isocyanates, may have associated bronchospasm or asthmatic symptoms. |
Classical Pneumoconiosis
The three major pneumoconioses are: asbestosis, silicosis, and coal worker’s pneumoconiosis.
Asbestosis
Asbestosis is a form of pulmonary fibrosis that results from the inhalation of asbestos fibers. Occupations previously associated with asbestosis include asbestos mining, milling, and shipyard work. Currently, asbestosis is primarily observed in construction that focuses on the maintenance and remodeling of older buildings. Exposure is typically long-standing, with a latency period of 20–30 years following exposure [4]. Asbestosis is indicative of pulmonary fibrosis. The development and severity of asbestosis are dose-dependent [12]. Although pleural disease is commonly associated with asbestos, it should be distinguished from asbestosis.
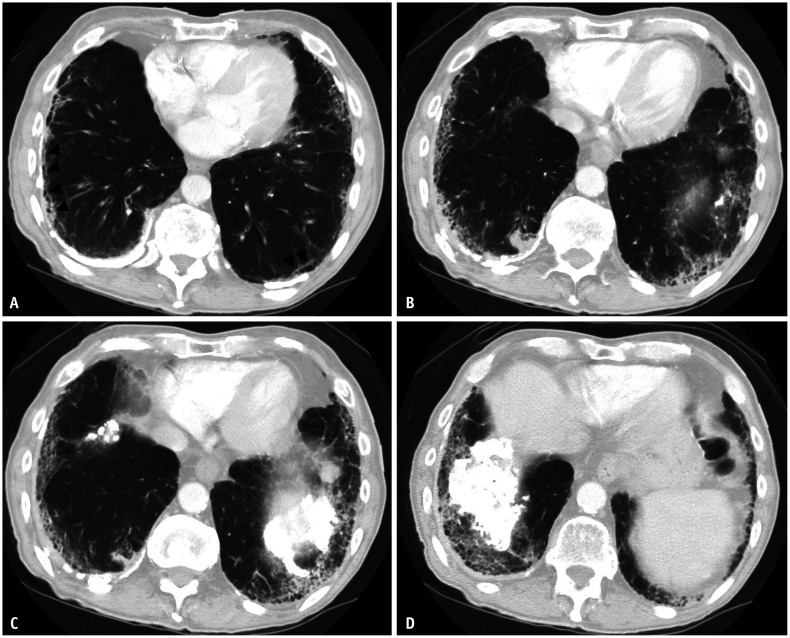
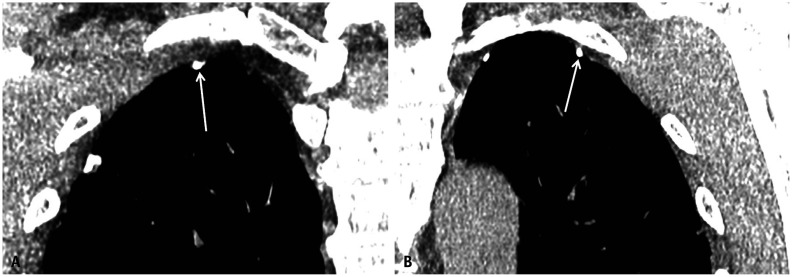
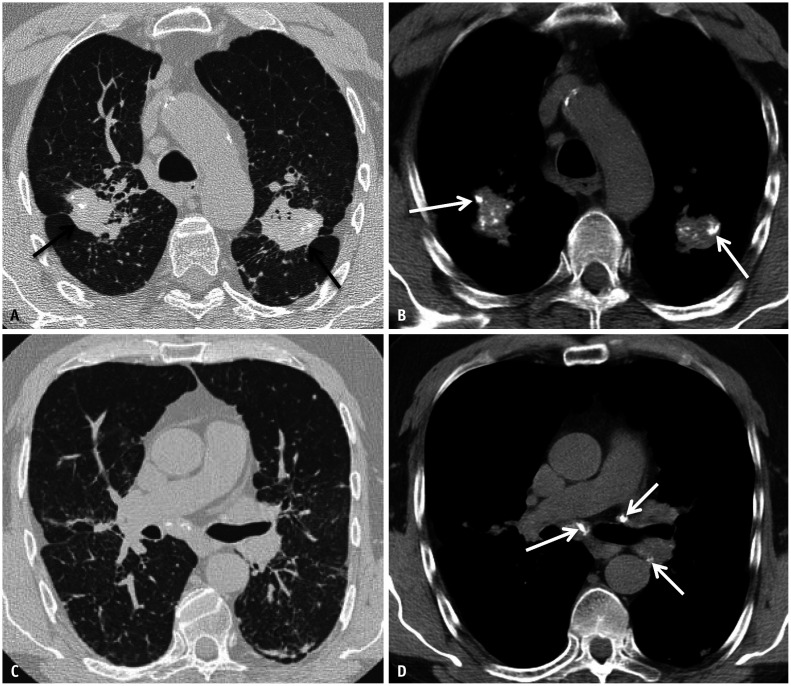
Asbestos-related pleural disease can present as either calcified or noncalcified pleural plaques, or diffuse pleural thickening. Pleural plaques often involve the lateral and diaphragmatic parietal pleura, are frequently calcified, and spare the costophrenic sulci and apices (Figs. 1, 2). When the enface is imaged, pleural plaques may resemble holly leaves (i.e., the holly leaf sign) (Fig. 3). Pleural plaques alone are unlikely to affect pulmonary function testing. In contrast, more diffuse pleural thickening can cause restrictive pulmonary physiology and is usually associated with parenchymal bands and, possibly, round atelectasis (Fig. 4A). Parenchymal bands are more frequent in the presence of pleural thickening and are markers of pleural disease rather than parenchymal fibrosis [13]. A diagnosis of round atelectasis can be confirmed when four findings are present: pleural thickening, subpleural mass-like opacity with broad-based attachment to the underlying pleural thickening, convergence of bronchovascular bundles towards the opacity (comet tail sign), and associated volume loss (Fig. 4B) [12].
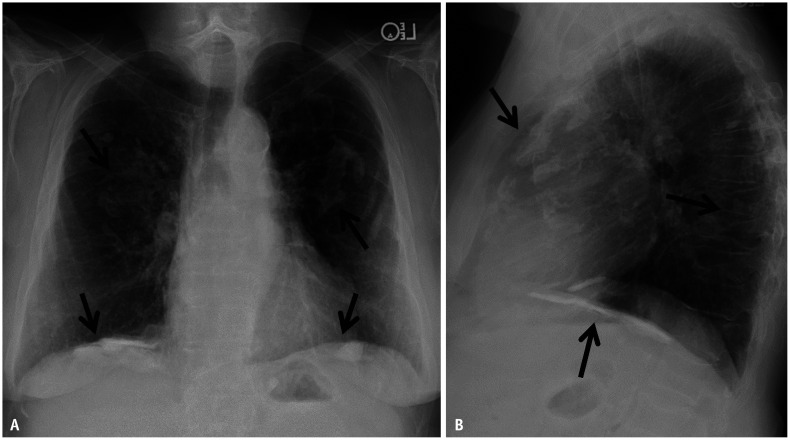
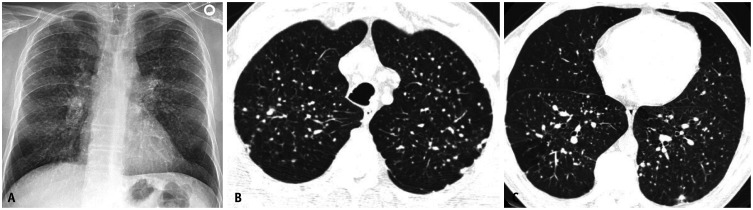
Fig. 1. Frontal (A) and lateral (B) chest radiographs showing ill-defined calcific densities along the posterior and diaphragmatic pleural surfaces (arrows) and sparing the costophrenic sulci and the apices of the lungs.
Fig. 2. Frontal chest radiograph (A) and corresponding dual energy subtraction image (B) show improved visualization of pleural plaques (arrows) provided by dual energy technique.
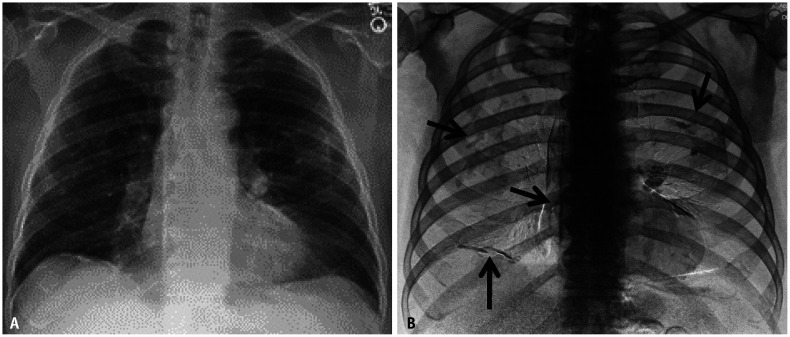
Fig. 3. Cropped down frontal chest radiograph (A) shows an asbestos-related plaque resembling a holly leaf appearance on radiography. Picture of a holly leaf (B) is provided for comparison.
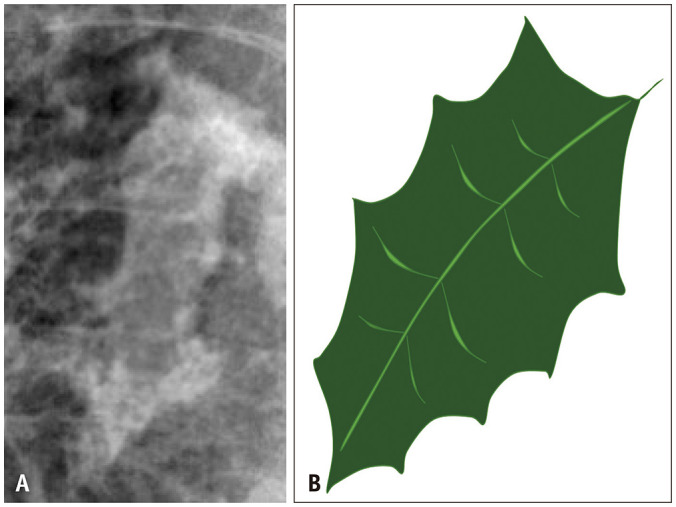
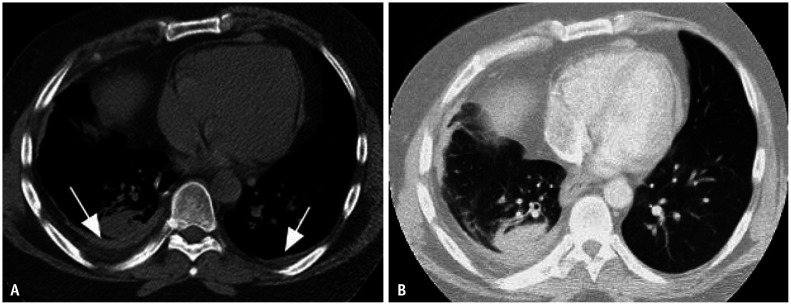
Fig. 4. Round atelectasis and asbestos related pleural disease. Non-contrast axial computed tomography (CT) image in soft tissue window (A) shows bilateral pleural thickening reflective of non-calcified pleural plaques (white arrows). Axial CT image in lung window (B) showing right lower lobe rounded consolidation with broad base attachment to adjacent pleural thickening. Associated volume loss is evidenced by posterior fissural displacement and convergence of adjacent bronchovascular structures (hurricane sign or comet tail sign). Findings are compatible with round atelectasis.
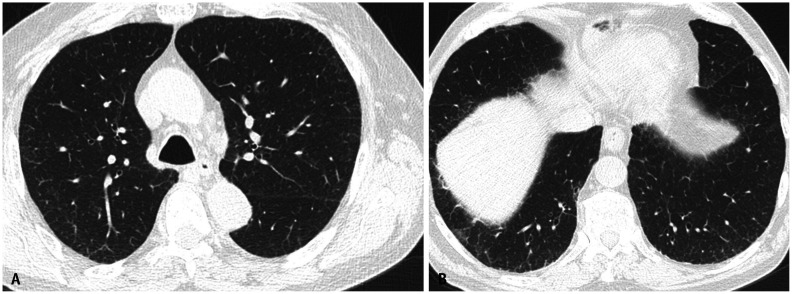
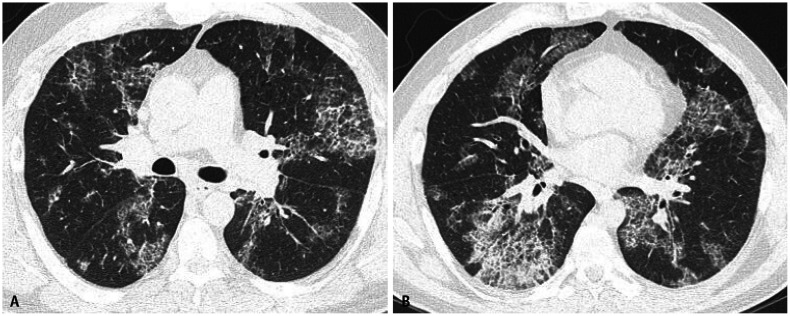
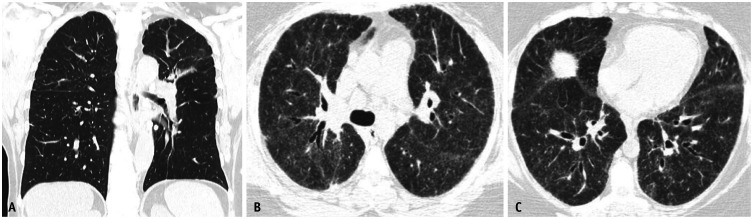
Asbestosis may manifest in its early stages as subtle peripheral lower-lobe-predominant branching reticulonodular opacities with immediate subpleural sparing. They may progressively become confluent and form peripheral curvilinear lines of peribronchiolar fibrosis parallel to the chest wall [14]. In more advanced stages, asbestosis often manifests as a usual interstitial pneumonia (UIP) pattern on HRCT. The disease is characterized by symmetrical peripheral posterobasilar fibrosis (Fig. 5), with honeycombing present in more severe cases (Fig. 6). Asbestos-related pleural disease frequently occurs concomitantly but, in 10%–15% of cases, there may be no evidence of pleural disease on imaging [15] (Fig. 7). Other imaging findings favoring asbestosis over UIP in idiopathic pulmonary fibrosis include mosaic attenuation, centrilobular reticulonodularity (Fig. 5), and peripheral curvilinear densities [14] (Fig. 6). However, for these patients, a detailed exposure history is crucial to ensure an accurate diagnosis.
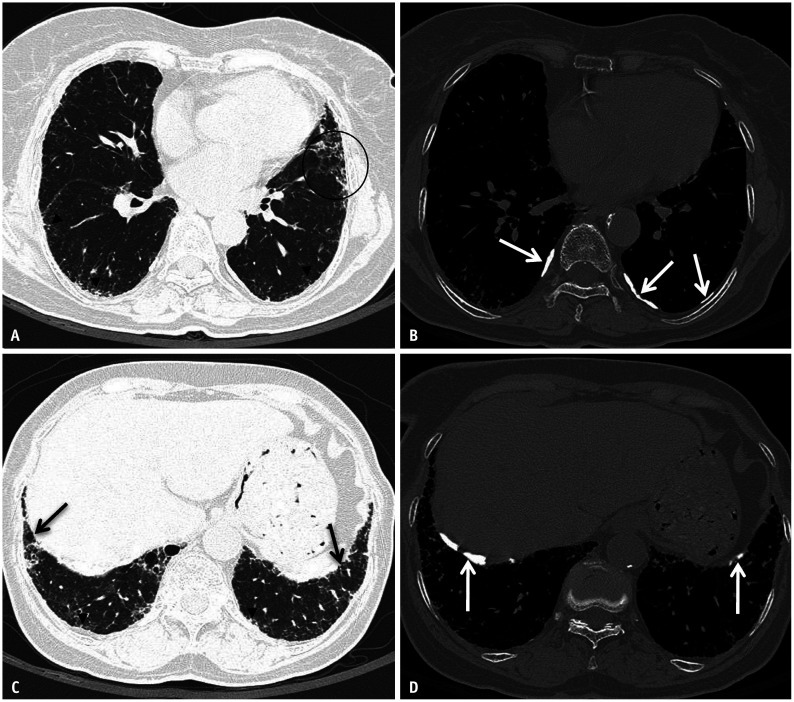
Fig. 5. Asbestos related pleural disease and asbestosis. Non-contrast chest computed tomography (CT) images in lung (A) and bone (B) windows show subpleural reticulation with subtle traction bronchiolectasis and architectural distortion (circle) and subpleural dot-like opacities (arrowheads) compatible with asbestosis. Concurrent posterolateral calcified pleural plaques are compatible with asbestos related pleural disease (white arrows in B). Non-contrast chest CT images at a lower level in lung (C) and bone (D) windows show peripheral reticulation with subtle bronchiolectasis (black arrows) and subpleural dot-like opacities (arrowheads). Calcified pleural plaques are also present (white arrows in D) indicating asbestos exposure.
Fig. 6. Severe asbestosis. Axial chest computed tomography images in lung windows (A-D) show peripheral posterobasilar fibrosis with subpleural honeycombing compatible with a usual interstitial pneumonia pattern. Subpleural curvilinear opacities are present bilaterally (arrowheads in A). Bilateral calcified pleural plaques are present indicating asbestos exposure. Findings are compatible with asbestosis.
Fig. 7. Mild asbestosis in the absence of asbestos-related pleural disease. Non-contrast axial chest computed tomography images in lung window (A, B) showing mild multifocal subpleural reticulation indicative of mild pulmonary fibrosis. Pleural plaques were not identified.
Silicosis and Coal Worker’s Pneumoconiosis
Coalworker pneumoconiosis (CWP) and silicosis result from the inhalation of coal and silica dust, respectively. The industries associated with silicosis include agriculture, mining, foundries, glass, ceramics, and construction. CWP occurs in coal mininers. Although these are distinct diseases, the imaging manifestations of these two conditions share striking similarities on HRCT [9]. Acute and chronic forms of CWP and silicosis exist, although the latter is far more common.
Chronic CWP and silicosis include simple and complex forms. Simple CWP and silicosis typically demonstrate small, upper lobe-predominant centrilobular and subpleural nodules that may be calcified (Fig. 8). Subpleural nodules in silicosis can be calcified and coalesce, resembling pleural plaques (pseudoplaques), and typically involve the lung apices (Fig. 9). Nodules are characteristically well-defined in silicosis, which is the main distinguishing feature from CWP on HRCT [9]. Calcification is also more common in patients with silicosis than those with CWP. Because the nodularity of silicosis is similar to that of sarcoidosis, the presence of centrilobular nodules is more typical of pneumoconiosis. In addition, conglomerate masses in complicated silicosis may be calcified or cavitated, which is another uncommon finding in sarcoidosis. In complicated pneumoconiosis, upper lobe conglomerates of fibrotic nodules and masses are associated with progressive volume loss, which is known as progressive massive fibrosis (PMF) [3]. Fibrosis may lead to adjacent paracicatricial emphysema (Fig. 10). These patients are at increased risk of developing a pneumothorax. In some patients, it may be difficult to distinguish PMF from a malignancy, particularly in asymmetric diseases. The PMF will have higher attenuation on non-contrast-enhanced CT than on the aortic arch (Fig. 10). T2 weighted magnetic resonance imaging may help to differentiate T2 hypointense fibrosis from T2 hyperintense neoplasms in challenging cases [16]. Intrathoracic lymphadenopathy is possible, which may be associated with the characteristic “eggshell calcifications.”
Fig. 8. Patient with simple silicosis. Frontal chest radiograph (A) showing extensive upper to mid lung nodularity with relative basilar sparing. Non-contrast computed tomography scans from upper (B) to lower (C) aspect of thorax demonstrating nodules throughout, but more numerous throughout the upper and mid lung zones. Nodules demonstrate clustering in the centrilobular and subpleural portions of the lungs.
Fig. 9. Silicotic pseudo-plaques (arrows). Cropped down non-contrast coronal chest computed tomography images (A, B) in soft tissue windows demonstrating biapical focal calcified plaque like nodularity.
Fig. 10. A 55-year-old male with complicated coal worker pneumoconiosis. Non-contrast axial computed tomography images of the upper lung in lung (A) and soft tissue (B) windows show mass like opacities with subtle relative hyperattenuation relative to the aortic blood pool (black arrows in A) and internal coarse calcification (white arrows in B), consistent with progressive massive fibrosis. There is associated architectural distortion (C). Findings are consistent with progressive massive fibrosis. Small nodules are also present in continuity and adjacent to the upper lung masses. There are calcified mediastinal and left hilar lymph nodes (white arrows in D).
Acute forms are rare. Acute silicosis (silicoproteinosis) is a severe and rapidly progressive form of silicosis that results from a relatively short-standing high-dose exposure to silica dust. The exposure periods may vary from weeks to 2 years [17]. HRCT typically shows diffuse bilateral ground-glass opacities with interlobular and intralobular septal thickening, often described as “crazy paving,” which can progress to consolidation (Fig. 11). Geographic margins, where the normal lung parenchyma separates the areas of opacification, can also be observed.
Fig. 11. Acute silicosis (silicoproteinosis) acquired from sandblasting. Axial non-contrast chest computed tomography images (A, B) in lung window show multifocal ground glass opacities with interlobular and intralobular septal thickening resulting in a “crazy paving” appearance and with geographical margination.
Chronic Granulomatous Diseases
Chronic Beryllium Disease
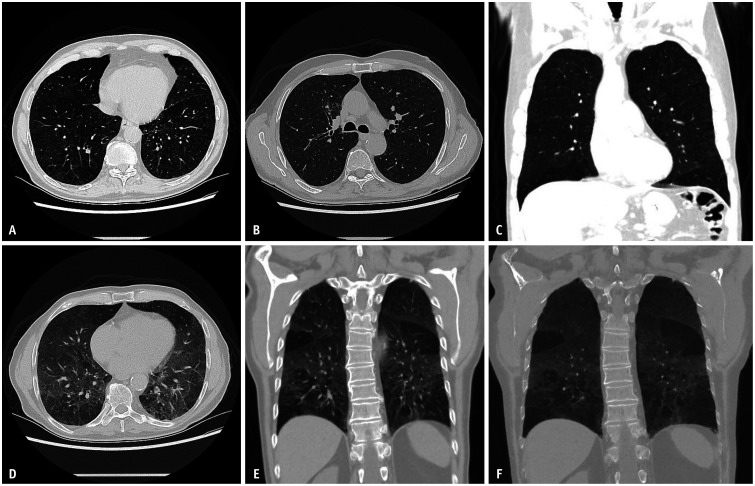
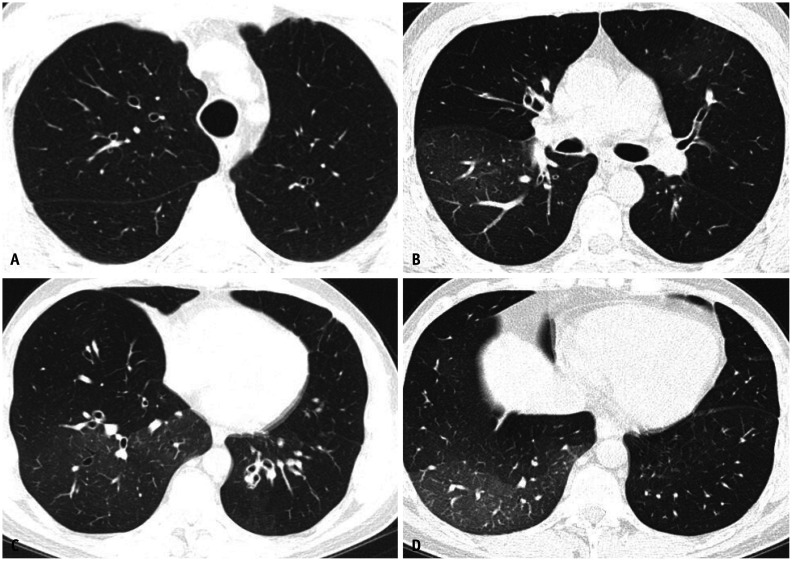
Chronic beryllium disease (CBD) is a condition caused by the inhalation of beryllium, a nonfibrogenic toxic metal found in various industries, including ceramics, aerospace, nuclear engineering, and fluorescent lamp manufacturing. The presentation is usually nonspecific, but can include a localized rash. CBD can manifest and progress slowly over the course of months to decades. Imaging manifestations mimic sarcoidosis, demonstrating preponderant perilymphatic nodularity in the upper lung with interstitial septal, peribronchovascular, and subpleural nodularity (Fig. 12) [9]. Upper lung fibrosis and honeycombing are uncommon compared with sarcoidosis. Hilar and mediastinal lymphadenopathies are present in a substantial proportion of these patients [18].
Fig. 12. Chronic beryllium disease. Coronal non-contrast chest computed tomography (CT) image in lung window (A) demonstrating upper lung preponderant perilymphatic nodularity, with nodules extending along interlobular septa and in a subpleural and peribronchovascular distribution. The lung bases are relatively preserved. Non-contrast axial chest CT in lung window (B, C) show diffuse micronodularity extending in a perifissural, subpleural and peribronchovascular distribution.
Hypersensitivity Pneumonitis
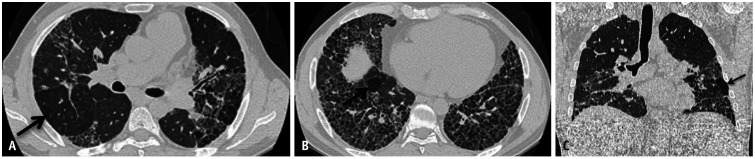
Hypersensitivity pneumonitis (HP) is an immune-mediated lung disease caused by exposure to inhaled organic dust or chemicals. Certain occupations and hobbies that involve exposure to microbial antigens, animal proteins, or low-molecular-weight chemicals have been associated with an increased risk of developing HP, although the list of potential exposures is inexhaustible [19]. Plumbers, salami or cheese workers, bird handlers, painters, and coffee workers are examples of at-risk groups. HP can be either fibrotic or nonfibrotic. Societal guidelines have attempted to streamline the diagnosis and guide the management of HP [20,21]. A high-confidence diagnosis of HP on HRCT is possible when signs of small airway disease (SAD) (i.e., mosaicism and diffuse ground glass centrilobular nodularity) are present (Fig. 13). In fibrotic HP, additional findings of parenchymal infiltration and fibrosis are also present (diffuse ground-glass opacities, reticulation, traction bronchiectasis, and architectural distortion) (Fig. 14). The three characteristic density signs are defined by juxtaposed geographic ground-glass opacity, decreased attenuation, and normal parenchyma reflect a combination of SAD and parenchymal inflammation/infiltration [19].
Fig. 13. A 75-year-old male with history of woodworking and mold exposure demonstrating non-fibrotic hypersensitivity pneumonitis. Non-contrast axial (A, B) and coronal (C) chest computed tomography images show diffuse centrilobular ground-glass nodularity with inspiratory mosaicism. Non-contrast expiratory axial (D), coronal (E), and minimum intensity projection (F) images show air trapping.
Fig. 14. Fibrotic hypersensitivity pneumonitis in a farmer. Fibrotic non-contrast expiratory axial (A, B) and coronal (C) images in lung windows show mid to lower-lobe-predominant fibrosis with reticulation, cystic change, patchy ground-glass opacities, and lobular regions of air trapping. All arrows are pointing towards lobular air trapping.
Airway Disease
Airway disease is a common complication of occupational exposure to noxious substances. SAD can occur in isolation or in association with various OLD and large airway diseases. In contrast, large airway disease is usually not a dominant feature in other OLD [1]. Asthma and OB are common subtypes of SAD, and discussed below.
Asthma
A significant proportion of patients are diagnosed with asthma, which can present as immunological asthma with latency or irritant-induced reactive airway dysfunction syndrome in the context of OLD. Immunologic asthma is characterized by chronic exposure to high- or low-molecular-weight substances, whereas irritant asthma is caused by a single inhalation of non-immunologic irritants such as strong acids or bases. Occupations commonly associated with asthma include textile workers, hairdressers, construction workers, chemical manufacturers, carpenters, farmers, and bakers. Imaging findings associated with asthma may be subtle, but can manifest as mosaicism with expiratory air trapping and bronchial wall thickening. These observations underscore the need for careful clinical and radiological assessment when evaluating these patients. Expiratory imaging is important for increased diagnostic sensitivity.
Obliterative Bronchiolitis
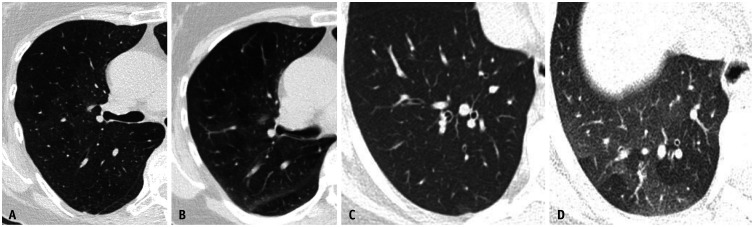
OB is commonly linked to flavoring chemicals, such as diacetyl used in popcorn flavoring, oxides of nitrogen (silo filler's lung), and post-deployment lung disease [22,23]. Imaging findings in OB are typically more pronounced than in asthma, notably revealing mosaicism with expiratory air trapping. Air trapping on HRCT manifests with a geographic lucent parenchyma contiguous to the apparent ground-glass parenchyma reflective of the spared lung. Bronchiectasis, bronchial wall thickening, and pruned vasculature resulting from hypoxemic vasoconstriction were observed in the regions of air trapping (Fig. 15). Asthma and OB are challenging to differentiate on imaging techniques. Anecdotally, a more confluent spread of air trapping was observed in OB than in asthma [24] (Fig. 16).
Fig. 15. Obliterative bronchiolitis. Non-contrast axial (A-D) images in lung windows show geographic areas of hypodensity representing air trapping associated with bronchial wall thickening, mild scattered bronchiectasis and pruning of smaller vessels due to hypoxia.
Fig. 16. Differences in imaging appearance of obliterative bronchiolitis (A, B) and asthma (C, D). Non-contrast axial in lung windows in inspiration (A, C) and expiration (B, D) demonstrating geographic air trapping appearing more confluent in obliterative bronchiolitis.
CONCLUSION
The spectrum of organic and inorganic compounds that can cause OLD is diverse and continually expanding. MDDs, including a comprehensive review of exposures, temporal relationships with symptomatology, and radiological-pathological specimens, are essential. OLD can manifest as various combinations of parenchymal and airway diseases. The imaging patterns are suggestive at a minimum. Therefore, radiologists can play a central role in optimizing diagnosis. Thus, understanding common imaging presentations is of the utmost importance.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: all authors.
- Data Curation: Lydia Chelala, Jonathan H. Chung.
- Formal Analysis: all authors.
- Investigation: all authors.
- Project Administration: Jonathan H. Chung.
- Resources: all authors.
- Supervision: Lydia Chelala, Jonathan H. Chung.
- Validation: Lydia Chelala, Jonathan H. Chung.
- Visualization: all authors.
- Writing—original draft: Alexander W. Matyga.
- Writing—review & editing: all authors.
Funding Statement: None
References
- 1.Cox CW, Rose CS, Lynch DA. State of the art: imaging of occupational lung disease. Radiology. 2014;270:681–696. doi: 10.1148/radiol.13121415. [DOI] [PubMed] [Google Scholar]
- 2.Driscoll T, Nelson DI, Steenland K, Leigh J, Concha-Barrientos M, Fingerhut M, et al. The global burden of non-malignant respiratory disease due to occupational airborne exposures. Am J Ind Med. 2005;48:432–445. doi: 10.1002/ajim.20210. [DOI] [PubMed] [Google Scholar]
- 3.Almberg KS, Halldin CN, Blackley DJ, Laney AS, Storey E, Rose CS, et al. Progressive massive fibrosis resurgence identified in U.S. coal miners filing for black lung benefits, 1970-2016. Ann Am Thorac Soc. 2018;15:1420–1426. doi: 10.1513/AnnalsATS.201804-261OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Onodera S. [Left ventricular function in pulmonary embolism] Kokyu To Junkan. 1975;23:315–323. Japanese. [PubMed] [Google Scholar]
- 5.De Matteis S, Heederik D, Burdorf A, Colosio C, Cullinan P, Henneberger PK, et al. Current and new challenges in occupational lung diseases. Eur Respir Rev. 2017;26:170080. doi: 10.1183/16000617.0080-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzei MA, Sartorelli P, Bagnacci G, Gentili F, Sisinni AG, Fausto A, et al. Occupational lung diseases: underreported diagnosis in radiological practice. Semin Ultrasound CT MR. 2019;40:36–50. doi: 10.1053/j.sult.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Dodia N, Amariei D, Kenaa B, Corwin D, Chelala L, Britt EJ, et al. A comprehensive assessment of environmental exposures and the medical history guides multidisciplinary discussion in interstitial lung disease. Respir Med. 2021;179:106333. doi: 10.1016/j.rmed.2021.106333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flors L, Domingo ML, Leiva-Salinas C, Mazón M, Roselló-Sastre E, Vilar J. Uncommon occupational lung diseases: high-resolution CT findings. AJR Am J Roentgenol. 2010;194:W20–W26. doi: 10.2214/AJR.09.2593. [DOI] [PubMed] [Google Scholar]
- 9.Chong S, Lee KS, Chung MJ, Han J, Kwon OJ, Kim TS. Pneumoconiosis: comparison of imaging and pathologic findings. Radiographics. 2006;26:59–77. doi: 10.1148/rg.261055070. [DOI] [PubMed] [Google Scholar]
- 10.Champlin J, Edwards R, Pipavath S. Imaging of occupational lung disease. Radiol Clin North Am. 2016;54:1077–1096. doi: 10.1016/j.rcl.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Sirajuddin A, Kanne JP. Occupational lung disease. J Thorac Imaging. 2009;24:310–320. doi: 10.1097/RTI.0b013e3181c1a9b3. [DOI] [PubMed] [Google Scholar]
- 12.Cha YK, Kim JS, Kim Y, Kim YK. Radiologic diagnosis of asbestosis in Korea. Korean J Radiol. 2016;17:674–683. doi: 10.3348/kjr.2016.17.5.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akira M, Yamamoto S, Inoue Y, Sakatani M. High-resolution CT of asbestosis and idiopathic pulmonary fibrosis. AJR Am J Roentgenol. 2003;181:163–169. doi: 10.2214/ajr.181.1.1810163. [DOI] [PubMed] [Google Scholar]
- 14.Akira M, Yokoyama K, Yamamoto S, Higashihara T, Morinaga K, Kita N, et al. Early asbestosis: evaluation with high-resolution CT. Radiology. 1991;178:409–416. doi: 10.1148/radiology.178.2.1987601. [DOI] [PubMed] [Google Scholar]
- 15.Gamsu G, Salmon CJ, Warnock ML, Blanc PD. CT quantification of interstitial fibrosis in patients with asbestosis: a comparison of two methods. AJR Am J Roentgenol. 1995;164:63–68. doi: 10.2214/ajr.164.1.7998570. [DOI] [PubMed] [Google Scholar]
- 16.Ogihara Y, Ashizawa K, Hayashi H, Nagayasu T, Hayashi T, Honda S, et al. Progressive massive fibrosis in patients with pneumoconiosis: utility of MRI in differentiating from lung cancer. Acta Radiol. 2018;59:72–80. doi: 10.1177/0284185117700929. [DOI] [PubMed] [Google Scholar]
- 17.Marchiori E, Souza CA, Barbassa TG, Escuissato DL, Gasparetto EL, Souza AS., Jr Silicoproteinosis: high-resolution CT findings in 13 patients. AJR Am J Roentgenol. 2007;189:1402–1406. doi: 10.2214/AJR.07.2402. [DOI] [PubMed] [Google Scholar]
- 18.Newman LS, Buschman DL, Newell JD, Jr, Lynch DA. Beryllium disease: assessment with CT. Radiology. 1994;190:835–840. doi: 10.1148/radiology.190.3.8115636. [DOI] [PubMed] [Google Scholar]
- 19.Chelala L, Adegunsoye A, Cody BA, Husain AN, Chung JH. Updated imaging classification of hypersensitivity pneumonitis. Radiol Clin North Am. 2022;60:901–913. doi: 10.1016/j.rcl.2022.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Raghu G, Remy-Jardin M, Ryerson CJ, Myers JL, Kreuter M, Vasakova M, et al. Diagnosis of hypersensitivity pneumonitis in adults. An official ATS/JRS/ALAT clinical practice cuideline. Am J Respir Crit Care Med. 2020;202:e36–e69. doi: 10.1164/rccm.202005-2032ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernández Pérez ER, Travis WD, Lynch DA, Brown KK, Johannson KA, Selman M, et al. Diagnosis and evaluation of hypersensitivity pneumonitis: CHEST guideline and expert panel report. Chest. 2021;160:e97–e156. doi: 10.1016/j.chest.2021.03.066. [DOI] [PubMed] [Google Scholar]
- 22.Banks DE, Morris MJ. Inhalational constrictive bronchiolitis: the evolution of our understanding of this disease. Lung. 2021;199:327–334. doi: 10.1007/s00408-021-00466-2. [DOI] [PubMed] [Google Scholar]
- 23.King MS, Eisenberg R, Newman JH, Tolle JJ, Harrell FE, Jr, Nian H, et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. N Engl J Med. 2011;365:222–230. doi: 10.1056/NEJMoa1101388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen SP, Lynch DA, Brown KK, Wenzel SE, Newell JD. High-resolution CT features of severe asthma and bronchiolitis obliterans. Clin Radiol. 2002;57:1078–1085. doi: 10.1053/crad.2002.1104. [DOI] [PubMed] [Google Scholar]