Abstract
A 46-year-old man was referred to our hospital for the examination of a flat elevated lesion with an erosion-like depression, located on the greater curvature of the antrum. Endoscopic submucosal dissection was performed. Histological findings of the resected specimen demonstrated a well-differentiated tubular adenocarcinoma with a diameter of 12 mm. No atrophy was observed in the tumor-adjacent mucosa. Serum Helicobacter pylori antibody estimation and 13C-urea breath tests yielded negative results. Immunohistochemical staining was positive for both gastric mucin and intestinal mucin. The final diagnosis was well-differentiated tubular adenocarcinoma with a gastrointestinal phenotype that originated in mucosa uninfected by H. pylori.
Keywords: Helicobacter pylori-negative, gastrointestinal phenotype, differentiated gastric adenocarcinoma
Introduction
The incidence of Helicobacter pylori-negative gastric cancer (HPNGC) is reported to be 0.42-0.66% (1,2). The most common types of HPNGC are signet-ring cell carcinoma and adenocarcinoma of the fundic gland. More recently, rare cases of differentiated adenocarcinoma without H. pylori infection have been reported in the gastric antrum (3-17). The carcinogenic process of this type of cancer is suspected to be different from that of gastric cancer in H. pylori-infected gastritis. However, the detailed carcinogenetic process and clinicopathological characteristics of these lesions remain unclear.
We herein report a case of early gastric adenocarcinoma with a gastrointestinal phenotype and without H. pylori infection arising in the antrum.
Case Report
A 46-year-old man was referred to our hospital for a further examination and treatment. Six years earlier, a flat elevated lesion in the antrum had been identified during a routine endoscopic examination. The lesion did not change in size or morphology over time. The biopsy specimen revealed an adenoma of the intestinal type. He had no significant history of taking drugs, alcohol or smoking, and his family history was unremarkable. Serum H. pylori antibody and 13C-urea breath tests and a histological examination were negative. H. pylori eradication therapy was not provided at that time, nor were proton-pump inhibitors or antibiotics prescribed at the time of the diagnosis of infection.
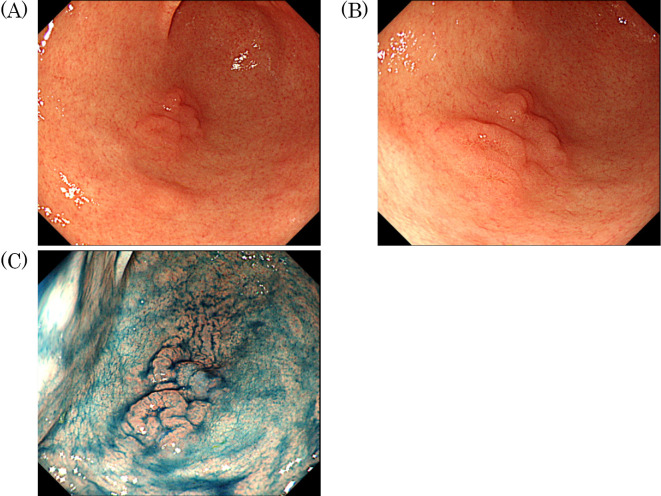
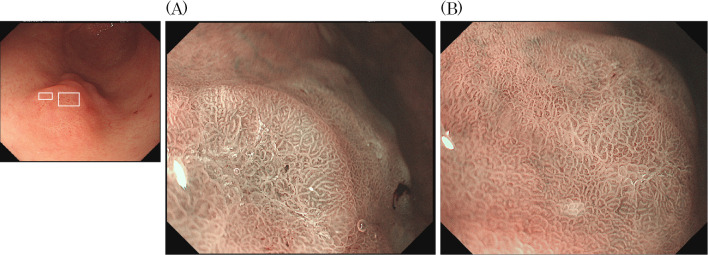
At our hospital esophagogastroduodenoscopy (EGD) showed a moderately reddish, single, flat elevated lesion with two humps and an erosion-like depression 10 mm in diameter mimicking verrucous gastritis on the greater curvature of the antrum, (Fig. 1A, B). No atrophy was observed in the tumor-adjacent mucosa. Chromoendoscopy with indigo carmine clearly revealed the tumor area (Fig. 1C). Magnifying endoscopy with narrow-band imaging (ME-NBI) revealed a slightly irregular micro surface pattern and a microvascular pattern with an unclear demarcation line (Fig. 2).
Figure 1.
Esophagogastroduodenoscopy showed a moderately reddish, single, flat elevated lesion with two humps and an erosion-like depression 10 mm in diameter mimicking verrucous gastritis on the greater curvature of the antrum. No atrophy was observed in the tumor-adjacent mucosa (A, B). Chromoendoscopy with indigo carmine clearly revealed the tumor area (C).
Figure 2.
Magnifying endoscopy with narrow-band imaging (ME-NBI) revealed a slightly irregular microsurface pattern and an irregular microvascular pattern with an unclear demarcation line (A: left square, B: right square).
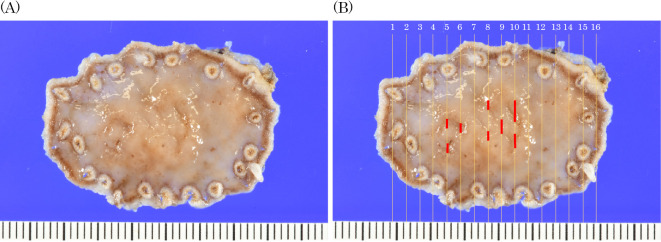
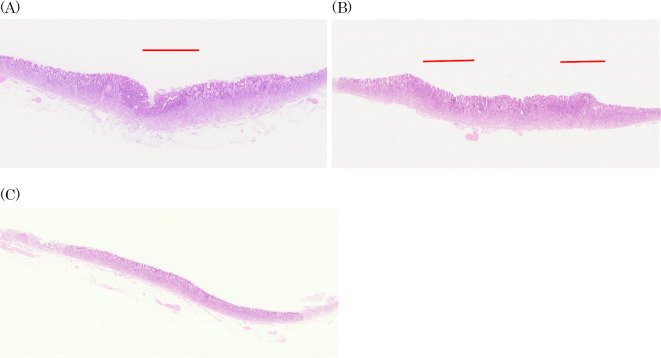
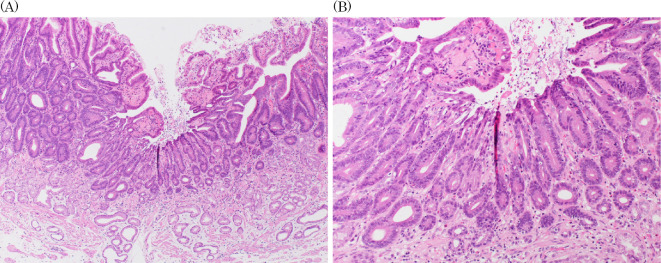
We performed endoscopic submucosal resection on suspicion of adenocarcinoma (Fig. 3). A histopathological analysis revealed that the well-differentiated tubular adenocarcinoma was confined to the mucosa of the depressed area (Fig. 4A, B, Fig. 5). There was a fibromuscular obliteration without an adenocarcinoma in the pyloric side of the flat elevated lesions indicated by chromoendoscopy with indigo carmine (Fig. 4C).
Figure 3.
Histopathological mapping of the resected specimen (A). The red line indicates tubular adenocarcinoma (B).
Figure 4.
Hematoxylin and Eosin staining. A histopathological analysis revealed that the well-differentiated tubular adenocarcinoma was confined to the mucosa of the depressed area. The lesions are the histopathological mapping of No.6 (A), No.10 (B) and No.12 (C).
Figure 5.
Hematoxylin and Eosin staining. (A) Low-power field, (B) High-power field.
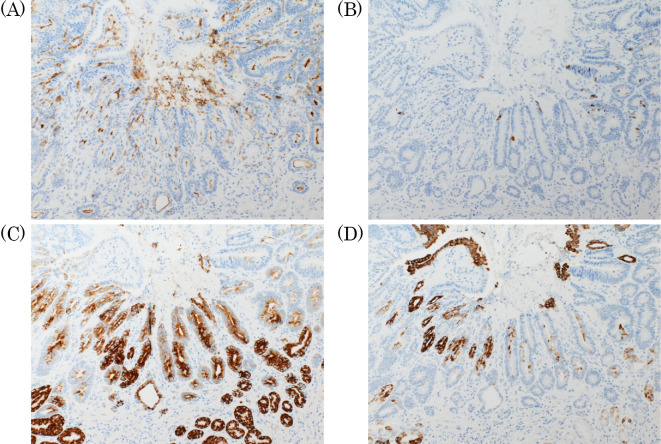
Immunohistochemical staining demonstrated that the tumor cells were positive for CD10, MUC2, MUC5AC, and MUC6 (Fig. 6). These results suggested that the tumor cells were characteristic of the gastrointestinal phenotype. The final histopathological diagnosis was a well-differentiated tubular adenocarcinoma with a gastrointestinal phenotype that originated in H. pylori-uninfected mucosa.
Figure 6.
Immunohistochemical staining demonstrated that the tumor cells were positive for CD10 (A), MUC2 (B), MUC5AC (C), and MUC6 (D).
Discussion
The incidence of HPNGC is reported to be 0.42-0.66% (1,2). The most common types of HPNGC are signet-ring cell carcinoma and adenocarcinoma of the fundic gland. Differentiated tubular adenocarcinomas without H. pylori infection arising in the gastric antrum are rare. Since the determination of H. pylori infection is affected by false negatives in each examination, the diagnostic criteria for absence of H. pylori infection have not yet been clearly established. In our case, serum H. pylori antibody, 13C-urea breath test, and a histological examination were negative; there was no history of H. pylori eradication; and there were no atrophic changes in the gastric mucosa. These met the minimum criteria for the absence of H. pylori infection of the stomach, as recommended by Yamamoto et al. (18). Furthermore, no risk factors for gastric cancer, such as type A gastritis, Epstein-Barr (EB) virus infection, genetic factors, or postoperative gastritis, were present.
In recent years, there have been 30 reported cases of differentiated adenocarcinoma arising in the antrum in the absence of H. pylori infection (Table) (3-17). The percentage of men was 56%, and the mean age was 54 (range 30-73) years old. The lesions mainly occurred at different locations of the greater curvature. Morphologically, they were flat elevated or depressed lesions mimicking verrucous gastritis. The median tumor diameter was 7.6 (range 2-18) mm. The postoperative diagnosis was well-differentiated adenocarcinoma in all cases, and the depth was intramucosal, except in one case. None of the patients had lymphovascular invasion. The phenotypes included 16 cases of the intestinal phenotype, 11 of the gastrointestinal phenotype, and 3 of the gastric phenotype. None of the patients had a medical history or family history of gastric cancer. Little information is available concerning these patients' drinking and smoking habits or body mass index.
Table.
A Summary of Previous Case Reports with Differentiated Adenocarcinoma Arising in the Antrum without Helicobacter pylori Infection.
| No. | Reference | Age | Gender | Location | Morphology | Size (mm) | Preoperative diagnosis | Differentiation | Depth | Phenotype | Lymphovascular invasion | VS classification system | Metaplasia | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 73 | Female | N/D | IIa | 4 | N/D | tub1 | M | Gastric | 0 | N/D | N/D | N/D |
| 2 | 4 | 30s | Female | Gre | IIc | 6 | Group 5 | tub1 | M | Intestinal | 0 | DL+ | MS irreg./MV irreg. | 0 |
| 3 | 5 | 67 | Male | Post | IIc | 8 | Group 3 | tub1 | M | Intestinal | 0 | DL+ | MS irreg./MV reg. | 0 |
| 4 | 6 | 30s | Female | Ant | IIa+IIc | 11 | Group 4 | tub1 | M | Gastrointestinal | 0 | DL+ | MS reg./MV reg. | 1 |
| 5 | 7 | 70s | Female | Gre | IIa+IIc | 10 | Group 5 | tub1 | M | Gastrointestinal | 0 | DL+ | MS irreg./MV irreg. | 0 |
| 6 | 8 | 54 | Male | Gre | IIa | 13 | N/D | tub1 | M | Gastrointestinal | 0 | DL+ | MS irreg./MV reg. | 0 |
| 7 | 9 | 40 | Male | Less | IIa | 9 | Group 3-4 | tub1 | M | Intestinal | 0 | DL+ | MS irreg./MV irreg. | 0 |
| 8 | 10 | 68 | Male | Post | IIc | Multiple | Group 5 | tub1 | M | Intestinal | 0 | Unclear | MS irreg./MV irreg. | 1 |
| 9 | 11 | 40s | Male | Ant | IIa | 2 | Group 5 | tub1 | M | Gastric | 0 | Unclear | MS irreg./MV irreg. | 0 |
| 10 | 60s | Female | Ant | IIa | 3 | Group 5 | tub1 | M | Gastric | 0 | DL+ | MS irreg./MV irreg. | 0 | |
| 11 | 12 | 60s | Female | Gre | IIc | 6 | Group 5 | tub1 | M | Intestinal | 0 | DL+ | MS irreg./MV irreg. | 0 |
| 12 | 13 | 40 | Male | Gre | IIc | 10 | Group 5 | tub1 | M | Gastrointestinal | 0 | DL+ | MS irreg./MV irreg. | 1 |
| 13 | 14 | 34 | Male | Post | IIa+IIc | 9 | Group 3 | tub1 | M | Intestinal | 0 | DL+ | MS irreg./MV irreg. | 0 |
| 14 | 15 | 70 | Female | Gre | IIa+IIc | 6 | Group 3 | tub1 | M | Gastrointestinal | 0 | DL+ | MS irreg./MV irreg. | 0 |
| 15 | 16 | 66 | Male | N/D | IIc | 9 | N/D | tub1 | M | Intestinal | 0 | N/D | N/D | N/D |
| 16 | 49 | Male | N/D | IIc | 5 | N/D | tub1 | M | Intestinal | 0 | N/D | N/D | N/D | |
| 17 | 65 | Female | N/D | IIa | 3 | N/D | tub1 | M | Intestinal | 0 | N/D | N/D | N/D | |
| 18 | 61 | Female | N/D | IIc | 5 | N/D | tub1 | M | Intestinal | 0 | N/D | N/D | N/D | |
| 19 | 43 | Female | N/D | IIc | 3 | N/D | tub1 | M | Intestinal | 0 | N/D | N/D | N/D | |
| 20 | 48 | Male | N/D | IIa | 7 | N/D | tub1 | M | Intestinal | 0 | N/D | N/D | N/D | |
| 21 | 52 | Female | N/D | IIa | 5 | N/D | tub1 | M | Intestinal | 0 | N/D | N/D | N/D | |
| 22 | 17 | 51 | Male | Less | IIa+IIc | 10 | Group 4 | tub1 | M | Intestinal | 0 | N/D | N/D | N/D |
| 23 | 57 | Male | Ant | IIa | 6 | Group 3 | tub1 | M | Intestinal | 0 | N/D | N/D | N/D | |
| 24 | 56 | Male | Post | IIa | 12 | Group 2 | tub1 | M | Intestinal | 0 | N/D | N/D | N/D | |
| 25 | 51 | Male | Gre | IIa | 14 | Group 3 | tub1 | M | Gastrointestinal | 0 | N/D | N/D | N/D | |
| 26 | 32 | Male | Gre | IIa | 8 | Group 5 | tub1 | M | Gastrointestinal | 0 | N/D | N/D | N/D | |
| 27 | 48 | Female | Gre | IIa | 10 | Group 3 | tub1 | M | Gastrointestinal | 0 | N/D | N/D | 0 | |
| 28 | 61 | Female | Gre | IIa+IIc | 18 | Group 5 | tub1 | SM1 | Gastrointestinal | 0 | N/D | N/D | N/D | |
| 29 | 64 | Male | Post | IIa | 2 | Group 2 | tub1 | M | Gastrointestinal | 0 | N/D | N/D | N/D | |
| 30 | 64 | Male | Gre | IIa | 3 | Group 5 | tub1 | M | Gastrointestinal | 0 | N/D | N/D | N/D | |
| 31 | Our case | 46 | Male | Gre | IIc | 12 | Group 3 | tub1 | M | Gastrointestinal | 0 | Unclear | MS irreg./MV irreg. | 0 |
Takita et al. reported nine cases of single erosion with white-light endoscopy (17). Sato et al. reported that the intestinal phenotype was characterized by a macroscopic type resembling a single verrucous erosion found in the antrum (16). Using ME-NBI, Takita et al. reported cases in which the border, surface structure, and vascular structure atypia of the tumor were indistinct (17). Conversely, Kotani et al. reported that ME-NBI could distinguish differentiated tubular adenocarcinoma from surrounding erosions (5). Wada et al. reported that the tumor border, surface structure, and vascular structure became clear after the administration of antacids (15,19). Our patient had received no antacids. Esophagogastroduodenoscopy (EGD) showed a single, flat elevated lesion with two humps. Histological mapping showed non-tumor mucosa intervening in the tumor of two depressed areas. We did not perform multiple biopsies in the past, and the non-tumor mucosa intervening in the tumor was not a scar by a biopsy. While previous reports demonstrated a wide range of characteristics, we hope that an appropriate diagnostic method will be developed in the future.
Our case occurred in H. pylori-uninfected gastric mucosa without atrophy and intestinal metaplasia, which occurred through a mechanism different from that of conventional gastric cancer; however, the process has not been elucidated. Tatsugami et al. reported the role of bile acid reflux in the stomach as a carcinogenic mechanism (20). Of the 22 previously reported cases, 11 were located in the greater curvature, which is normally more exposed to bile acids (21). Matsuhisa et al. reported that gastric mucosa uninfected by H. pylori occasionally accompanies intestinal metaplasia, in the presence of increased concentrations of bile acid in the stomach (22). Kishimoto et al. reported multiple intestinal-type gastric cancers arising from the non-atrophic gastric mucosa with sporadic intestinal metaplasia (11). Bile acid is a well-known carcinogen, and reflux may induce sporadic intestinal metaplasia in the pyloric glands in the absence of H. pylori infection (23).
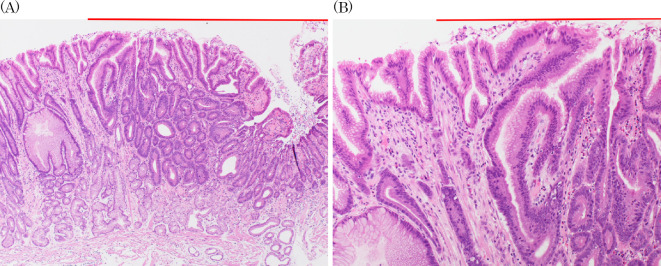
However, Takita et al. conversely reported that all patients had fibromuscular obliteration of the lamina around the lesion, similar to mucosal prolapse syndrome (MPS) (9,12,24). Our case also showed fibromuscular obliteration surrounded by noncancerous mucosa. (Fig. 7A, B). MPS causes chronic mechanical stimulation and is histologically characterized by fibromuscular obliteration, but there are few reports of complications with cancer. Yamamoto et al. reported a case of MPS complicated by adenocarcinoma after long-term follow-up by colonoscopy, suggesting the possibility of carcinogenesis due to chronic inflammation (25). In our case, there is a possibility that carcinogenesis occurred in the protuberant part that caused fibromuscular obliteration due to physical stimulation by peristalsis. Furthermore, the two cancers may have developed simultaneously in a multicentric manner. Since these cancers occurs in area affected by physical stimulation by peristalsis and bile acids reflex, we speculated that both should be involved in carcinogenesis. Our lesion occurred in areas that were strongly affected by peristaltic stimulation and bile acids. However, the relationship between these chronic stimulations and carcinogenic mechanisms is still unclear.
Figure 7.
Fibromuscular obliteration of the lamina propria was observed in the background mucosa. (A) Low-power field, (B) High-power field.
This case was an extremely well-differentiated adenocarcinoma with a gastrointestinal phenotype (26). In general, it has been reported that the superficial elevated type of gastric cancer is mostly intramucosal with low atypia and shows very slow growth. Most previous reports concerned intramucosal cancer, except for one case, and lymphovascular invasion was not confirmed in any cases. However, it is difficult to detect such lesions, since they often have non-neoplastic epithelium inside the lesion, as in gastric cancers detected after H. pylori eradication (11,18).
The histological features of this phenotype may be useful for the tumor diagnosis, although the further accumulation of cases is necessary. Thus far, it has been difficult to diagnose cancer using only biopsy specimens. Therefore, it is necessary to work closely with a pathologist to make an accurate diagnosis.
Conclusion
We reported a case of H. pylori-negative gastric adenocarcinoma with a gastrointestinal phenotype arising in the antrum. The incidence of HPNGC may increase in the future with the decline in H. pylori infection, making it essential that we fully understand the endoscopic findings and characteristics of HPNGC arising in the antrum.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Ono S, Kato M, Suzuki M, et al. Frequency of Helicobacter pylori-negative gastric cancer and gastric mucosal atrophy in a Japanese endoscopic submucosal dissection series including histological, endoscopic and serological atrophy. Digestion 86: 59-65, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Matsuo T, Ito M, Tanaka S, et al. Low prevalence of Helicobacter pylori-negative gastric cancer among Japanese. Helicobacter 16: 415-419, 2011. [DOI] [PubMed] [Google Scholar]
- 3.Yaita H, Kurahara K, Kawasaki K, et al. Clinicopathological feature of Helicobacter pylori-negative gastric cancer. Stomach Intest 49: 863-873, 2014. (in Japanese). [Google Scholar]
- 4.Ozaki Y, Suto H, Nasaka T, et al. A case of Helicobacter pylori-negative intramucosal well-differentiated gastric adenocarcinoma with intestinal phenotype. Clin J Gastroenterol 8: 18-21, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Kotani S, Miyaoka Y, Fujiwara A, et al. Intestinal-type gastric adenocarcinoma without Helicobacter pylori infection successfully treated with endoscopic submucosal dissection. Clin J Gastroenterol 9: 228-232, 2016. [DOI] [PubMed] [Google Scholar]
- 6.Tsuruta F, Kawai K, Amano T, et al. Well-diffierentiated tubular adenocaricinoma with a predominantly intestinal phenotype pattern in the gastric antrum of a Helicobacter pylori-negative pateient, report of a case. Stomach Intest 51: 946-958, 2016. (in Japanese). [Google Scholar]
- 7.Shibukawa N, Wakamatsu S, Ouchi S, et al. A well-differentiated early gastric cancer in a patient confirmed negative for Helicobacter pylori. Nihon Shokakibyo Gakkai Zasshi 114: 78-83, 2017. (in Japanese). [DOI] [PubMed] [Google Scholar]
- 8.Yoshii S, Hayashi Y, Takehara T. Helicobacter pylori-negative early gastric adenocarcinoma with complete intestinal mucus phenotype mimicking verrucous gastritis. Dig Endosc 29: 235-236, 2017. [DOI] [PubMed] [Google Scholar]
- 9.Nakauchi S, Tanaka H, Takata R, et al. A case of well-differentiated tubular adenocarcinoma with intestinal phenotype at the gastric antrum of a patient who was not infected with Helicobacter pylori. Gastroenterol Endosc 60: 223-229, 2018. (in Japanese). [Google Scholar]
- 10.Kishimoto K, Shibagaki K, Itawaki A, et al. Synchronously multiple gastric adenocarcimona with intestinal mucin phenotype in a patient not infected with Helicobacter pylori, showing a gastritis-like appearance. Intern Med 59: 3155-3159, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nawata Y, Ichiba S, Hirasawa D, et al. H. pylori-negative intramucosal well-differentiated tubular adenocarcinoma of the gastric antrum with a gastric phenotype, report of two cases. Stomach Intest 55: 1090-1097, 2020. (in Japanese). [Google Scholar]
- 12.Horiuchi Y, Shiroma S, Yamamoto N, et al. Helicobacter pylori uninfected intestinal-type well-differentiated adenocarcinoma in early gastric cancer, report of a case. Stomach Intest 55: 1085-1089, 2020. (in Japanese). [Google Scholar]
- 13.Takatsuna M, Azumi R, Mizusawa T, et al. A case of Helicobacter pylori-negative early gastric adenocarcinoma with gastrointestinal phenotype. Endosc Int Open 9: E863-E866, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watada M, Ueo T, Takahashi H, et al. Qualitative diagnosis of intestinal-type differentiated gastric cancer that originated in Helicobacter pylori-uninfected mucosa based on findings of a light blue crest and white opaque substance: a case report. Gastroenterol Endosc 63: 21922198, 2021. (in Japanese). [Google Scholar]
- 15.Satoi A, Oharazeki T, Toba T, et al. A case of well-differentiated tubular adenocarcinoma with a predominantly intestinal phenotype pattern in a patient without Helicobacter pylori infection. Gastroenterol Endosc 64: 43-49, 2022. (in Japanese). [Google Scholar]
- 16.Sato C, Hirasawa K, Tateishi Y, et al. Clinicopathological features of early gastric cancers arising in Helicobacter pylori uninfected patients. World J Gastroenterol 26: 2618-2631, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takita M, Ohata K, Inamoto R, et al. Endoscopic and histological feature of Helicobacter pylori-negative differentiated gastric adenocarcinoma arising in antrum. JGH Open 5: 4770-4775, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto Y, Fujisaki J, Omae M, et al. Helicobacter pylori-negative gastric cancer: characteristics and endoscopic findings. Dig Endos 27: 551-561, 2015. [DOI] [PubMed] [Google Scholar]
- 19.Wada K, Ueo T, Yonematsu H, et al. Antiacids may increase the appearance of white opaque substance in Helicobacter pylori-eradicated gastric epithelial neoplasia. Endosc Int Open 7: 1144-1149, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tatsugami M, Ito M, Tanaka S, et al. Bile acids promotes intestinal metaplasia and gastric carcinogenesis. Cancer Epidemiol Biomarkers Prev 21: 2101-2107, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Matsuhisa T, Tsukui T. Relation between reflux of bile acids into the stomach and gastric mucosal atrophy, intestinal metaplasia in biopsy specimens. J Clin Biochem Nutr 50: 217-221, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuhisa T, Arakawa T, Watanabe T, et al. Relation between bile acid reflux into the stomach and the risk of atrophic gastritis and intestinal metaplasia: a multicenter study of 2283 cases. Dig Endosc 25: 519-525, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Liu T, Song X, Khan S, et al. The gut microbiota at the intersection of bile acids and intestinal carcinogenesis: an old story, yet mesmerizing. Int J Cancer 146: 1780-1790, 2020. [DOI] [PubMed] [Google Scholar]
- 24.Owen DA. Lamina propia, stomach. In: Histology for the Pathologist. 5th ed. Wolters Kluwer, Philadelphia, 2020: 607. [Google Scholar]
- 25.Yamamoto T, Hamajima E, Kamioka T, et al. A case of mucosal prolapse syndrome complicated by well-differentiated tubular adenocarcinoma and high-grade adenoma after long-term follow-up by colonoscopy. Nihon Shokakibyo Gakkai Zasshi 118: 757-767, 2021. (in Japanese). [DOI] [PubMed] [Google Scholar]
- 26.Yao T, Utsunomiya T, Oya M, et al. Extremely well-differentiated adenocarcinoma of the stomach: clinicopathological and immunohistochemical feature. World J Gastroenterol 12: 2510-2516, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]