Abstract
An 84-year-old woman with IgG4-related disease presented with jaundice and liver dysfunction after coronavirus disease 2019 (COVID-19) vaccination. Serum IgG4 levels were elevated. Diagnostic imaging showed no stenotic lesions in the bile ducts. A liver biopsy was performed because of the enlarged liver. Infiltration of IgG4-positive plasma cells, which accounted for approximately 74% of total plasma cells, was found in the portal area, but there was no evidence of periportal hepatitis, and inflammatory cell infiltration into the lobular space was minimal. IgG4-related hepatopathy was diagnosed. The patient achieved spontaneous remission with no treatment and only follow-up and remains under observation at the time of writing.
Keywords: COVID-19 vaccination, IgG4-related hepatopathy
Introduction
IgG4-related disease has become a well-established disease entity that features high serum IgG4 levels, IgG4-positive plasma cell proliferation and characteristic fibrosis in two or more organs (1,2). Regarding liver involvement, the following disease concepts have been proposed: IgG4-related sclerosing cholangitis, IgG4-related autoimmune hepatitis (AIH) and IgG4-related hepatopathy (3,4).
Recently, activation of various autoimmune diseases has been reported after coronavirus disease 2019 (COVID-19) vaccination. We herein report a case of liver dysfunction after COVID-19 vaccination that was found to be IgG4-related hepatopathy on a liver biopsy.
Case Report
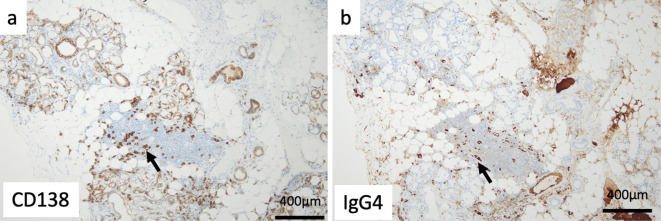
An 80-year-old woman who had a history of left nephrectomy for left kidney cancer at 75 years old with multiple nodules in the lungs presented to our hospital with enlarged generalized lymph nodes and mild enlargement of submandibular glands on computed tomography (Fig. 1a, d, g, h). The serum IgG4 level was high at 2,540 mg/dL. A lip biopsy showed a positive IgG4:CD138 ratio of more than 70% (Fig. 2a, b). IgG4-related disease was diagnosed. However, the patient's symptoms were mild, so she was followed up without treatment.
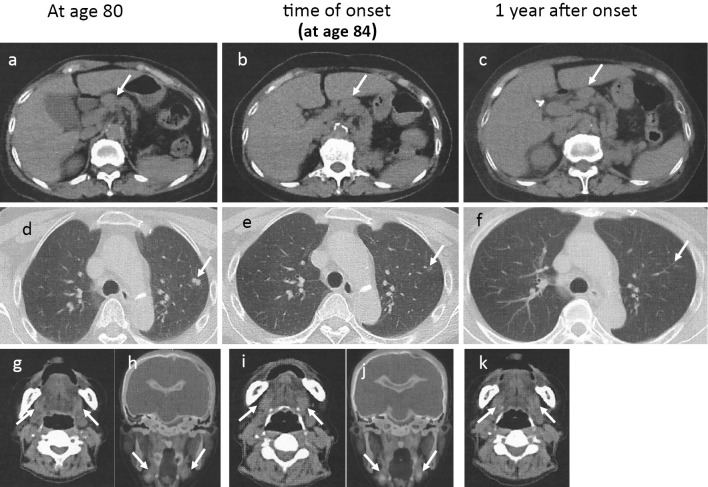
Figure 1.
Computed tomography. At 80 years old: (a) Arrow indicates the hilar lymph node. (d) Arrow indicates the nodule in the lung. (g) Arrow indicates a mildly enlarged submandibular gland. At the onset: (b) Arrow indicates the hilar lymph node. (e) Arrow indicates nodule reduction in the lung. (i) Arrow indicates a mildly enlarged submandibular gland. At one year after the onset: (c) Arrow indicates the hilar lymph node. (f) Arrow indicates the disappearance of the nodule in the lung. (k) Arrow indicates a mildly enlarged submandibular gland. PET-CT findings, at 80 years old: (h) Arrow indicates a mildly enlarged submandibular gland. At the onset: (j) Arrow indicates a mildly enlarged submandibular gland.
Figure 2.
A lip biopsy showed a positive IgG4 (b)-to-CD138 (a) ratio of >70% (original magnification ×400), a: Arrow indicates CD138-positive plasma cell. b: Arrow indicates IgG4-positive plasma cell.
At 84 years old, the patient developed generalized itching, anorexia, and nausea 1 day after receiving the first dose of the COVID-19 vaccine. Her symptoms worsened, and she was hospitalized on day 7 after vaccination.
On admission, the patient was 154 cm tall and weighed 50 kg. Her blood pressure was 126/68 mm Hg; pulse rate, 75 beats per minute; and body temperature, 36.5℃. The bilateral submandibular lymph nodes were swollen. The liver was palpable two fingers' breadth below the costal margin. Bilateral axillary lymph node enlargement was observed. Jaundice was seen all over the body. No abnormalities of the heart, lungs or extremities were noted. There was no history of bronchial asthma, rhinitis or drug allergies.
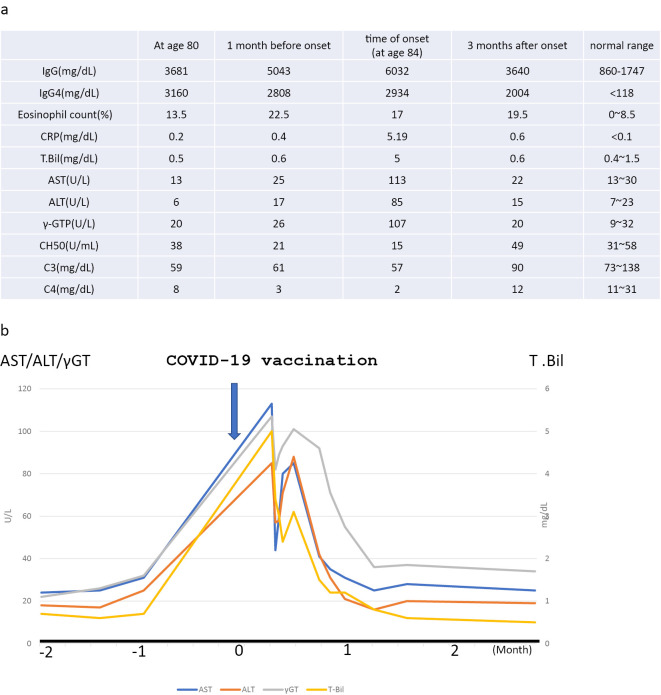
The laboratory findings were as follows: leucocytes, 5,300/μL; eosinophils, 1,272/μL; hemoglobin, 11.2 g/dL; thrombocytes, 106,000/μL; total protein, 10.1 g/dL; albumin, 2.1 g/dL; aspartate aminotransferase (AST), 113 U/L (normal value, 3-38 U/mL); alanine aminotransferase (ALT), 110 U/L (normal value, 4-44 U/mL); γ-glutamyl transpeptidase (GTP), 107 IU/L (normal value, <30 IU/L); total bilirubin, 5.0 mg/dL; creatinine, 0.85 mg/dL; estimated glomerular filtration rate, 38.1 mL/min/1.73 m2; C-reactive protein, 5.16 mg/dL; total complement activity (assessed as CH50), 15 U/mL (normal value, >30 U/mL); complement 3, 57 mg/dL (reference range, 86-160 mg/dL); complement 4, 2 mg/dL (reference range, 17-45 mg/dL); IgG, 6,032 mg/dL (reference range, 861-1,747 mg/dL); IgG4, 2,934 mg/dL (reference range, ≤118 mg/dL); and IgE, 340 mg/dL (reference range, ≤170 IU/ML). Immunological tests were negative for anti-nuclear antibody. Tests for various autoantibodies, including anti-SS-A/Ro antibody, anti-mitochondrial antibody and anti-smooth muscle antibody, were all negative, and tests for hepatitis B and C were also negative. Urinary protein excretion was 0.3 g daily, and the sediment contained <1 erythrocyte per high-power field. The level of urinary N-acetyl-β-d-glucosaminidase was 11.8 IU/gCr, and that of urinary β2-microglobulin was 0.3 mg/gCr (Fig. 3a).
Figure 3.
(a) Findings of four examinations: at 80 years old, 1 month before the onset, at the onset (age 84 years old), 3 months after the onset. (b) Clinical course: the date of COVID-19 vaccination (arrow) is indicated by 0 on the horizontal axis.
Computed tomography showed an enlarged liver and spleen and enlarged bilateral axillary, mediastinal, hilar, periaortic and mesenteric lymph nodes (Fig. 1b, e, i, j). No pancreatic enlargement was seen. Magnetic resonance cholangiopancreatography showed no abnormalities in the bile duct system.
An ultrasound-guided liver biopsy was performed.
Liver biopsy findings
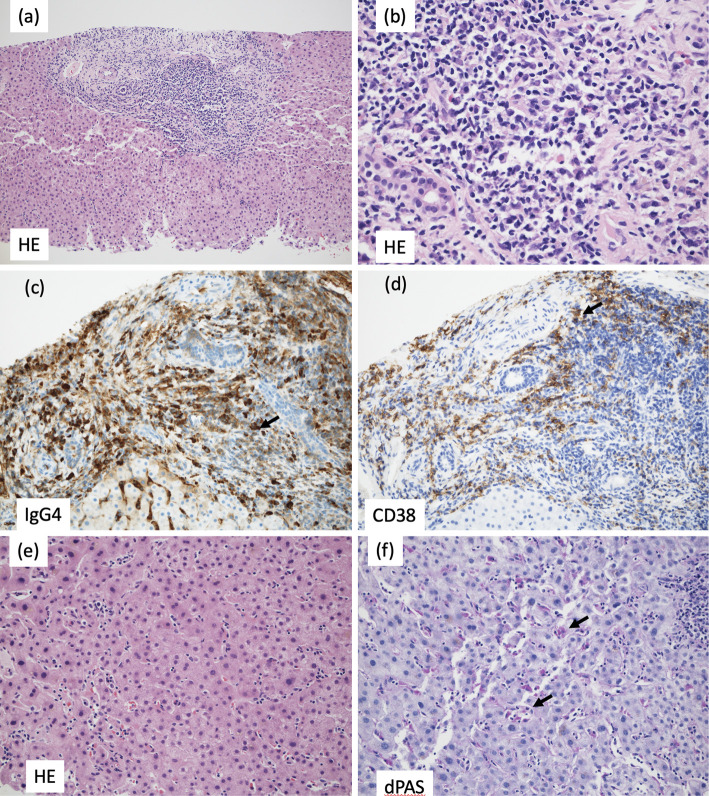
The portal areas were enlarged and showed numerous lymphocytes and plasma cells and a small number of eosinophils. The portal areas were well demarcated, and there was no obvious evidence of periportal hepatitis. The bile ducts were normal and free from inflammation, and no bile duct lesions suggestive of IgG4-related sclerosing cholangitis were identified (Fig. 4a, b). Immunostaining revealed conspicuous infiltration of IgG4-positive cells in the portal areas, and the ratio of IgG4- to CD38-positive cells (indicating the plasma cell ratio) was elevated at 74% (Fig. 4c, d), consistent with IgG4-related hepatopathy. There was an overall poor inflammatory response and some necrosis in the lobules (Fig. 4e). Within the sinusoids, lymphocytes were mildly increased, and aggregates of macrophages positive for periodic acid-Schiff with diastase stain were seen (Fig. 4f), suggesting focal necrosis. This part of the lesion was considered to be related to recovery from mild acute hepatitis. The absence of characteristic centrilobular hepatitis did not support a diagnosis of autoimmune hepatitis.
Figure 4.
Liver biopsy findings. (a) The portal area was enlarged and well demarcated. Hematoxylin and Eosin (H&E) staining; original magnification ×10. (b) The portal area was highly infiltrated by lymphocytes and plasma cells. H&E staining; original magnification ×40. (c) Immunostaining of immunoglobulin G4 (IgG4) showed positive cells (brown color, arrow) in the portal area. Original magnification ×20. (d) Immunostaining of CD38 showed positive cells (brown color, arrow). Original magnification ×20. (e) An overall poor inflammatory response was seen, with necrosis in the lobules. H&E staining; original magnification ×20. (f) Within the sinusoids, lymphocytes were increased, as were macrophages positive for periodic acid-Schiff with diastase (dPAS) stain (arrows indicate representative examples). dPAS stain; original magnification ×20.
The diagnosis
The findings did not meet the criteria for IgG4-related autoimmune hepatitis proposed by Umemura et al. (3,4) or the criteria for IgG4-related sclerosing cholangitis on imaging (5). Therefore, IgG4-related hepatopathy was diagnosed.
Clinical course
After admission, the jaundice resolved without treatment, and liver function tests normalized after four weeks (Fig. 3b). One year and six months later, there was no recurrence of liver function abnormalities and no recurrence of IgG4-related disease (Fig. 1c, f, k).
Discussion
Because a large number of people worldwide have received COVID-19 vaccination, we imagined that there would be many reports of IgG4-related disease diagnosed after COVID-19 vaccination. However, a PubMed search revealed very few reported cases; the identified cases are described below.
Masset et al. reported a patient diagnosed with IgG4-related tubulointerstitial nephritis on a kidney biopsy who was in remission with steroid therapy. However, after COVID-19 vaccination, the patient's renal function deteriorated again, and the patient experienced a relapse of IgG4-related kidney disease (6).
Tasnim et al. presented a case of left pleural effusion after COVID-19 vaccination, with high serum IgG4 levels and increased IgG4-positive plasma cells on a pleural biopsy. They diagnosed the patient with IgG4-related pleural disease (7).
Patel et al. published a case of IgG4-related autoimmune pancreatitis after COVID-19 vaccination. They described the disease as being characterized by hyperglycemia, an abnormal liver function, pancreatic head mass, and high serum IgG4 levels (8).
Efe et al. reviewed 87 patients with liver injury after COVID-19 vaccination, 53% of whom received steroids. The prognosis was generally good, but one patient developed fulminant liver failure and underwent liver transplantation; however, the cases did not include any apparent reports of IgG4-related hepatopathy (9).
The increased immunogenicity produced by COVID-19 vaccination has been hypothesized to cause unexpected and perhaps specific immune activation in patients with autoimmune diseases (10). Of interest in this context is that IgG4-related disease may manifest or recur by the same mechanism as other autoimmune diseases. It is also interesting to note that IgG4-related disease is a multiorgan disorder, but in the cases reported above, including the present case, only one organ was involved.
Among IgG-related diseases, liver involvement is classified into three categories: IgG4-related sclerosing cholangitis, IgG4-related autoimmune hepatitis and IgG4-related hepatopathy. Common findings in these three diseases are high serum IgG4 levels and characteristic histological findings with IgG4-positive plasma cell infiltration and being highly responsive to steroid therapy. IgG4-related sclerosing cholangitis is characterized clinically by obstructive jaundice in addition to imaging findings of bile duct wall thickening and bile duct obstruction, including autoimmune pancreatitis. Because a histological diagnosis of bile duct lesions is usually difficult to make except using surgical specimens, the histological diagnosis is often confirmed by concurrent extrabiliary lesions. IgG4-related AIH is categorized by classical diagnostic criteria for AIH, including characteristic centrilobular hepatitis and periportal or interface hepatitis, as well as histological findings indicating severe infiltration of IgG4+ plasma cells in the liver. IgG4-related hepatopathy is diagnosed after IgG4-related sclerosing cholangitis and IgG4-related AIH are excluded. IgG4-related hepatopathy is usually diagnosed when characteristic histological findings with IgG4-positive plasma cell infiltration are seen in the periportal area only. Centrilobular hepatitis and periportal or interface hepatitis are not observed (3,4).
Treatment of IgG4-related sclerosing cholangitis and IgG4-related liver disease has been reported using corticosteroids, steroid-free immunosuppressive drugs (e.g. mycophenolate mofetil, azathioprine, methotrexate, tacrolimus and cyclophosphamide) and immunosuppressive drugs, such as Rituxan. There are few reports of specific treatments for IgG4-related hepatopathy (11).
Although corticosteroid therapy was initially considered for this patient, no aggressive treatment was eventually given because the liver function improved spontaneously after hospitalization and follow-up observation. If the disease had been induced by drugs, it might have spontaneously resolved if the drugs were discontinued and were not administered again. Based on this idea, a second dose of the COVID-19 vaccine was not administered in this case. Of course, there have been no reports of IgG4-related hepatopathy that resolved without treatment.
In conclusion, we experienced a case of IgG4-related hepatopathy diagnosed by liver biopsy after the development of liver dysfunction with jaundice subsequent to COVID-19 vaccination. Our case represents the first case of proven IgG4-related hepatopathy after COVID-19 vaccination.
The present report was produced in conformity with the Declaration of Helsinki, and the patient gave her written consent for this case report to be published.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Perugino CA, Stone JH. IgG4-related disease: an update on pathophysiology and implications for clinical care. Nat Rev Rheumatol 16: 702-714, 2020. [DOI] [PubMed] [Google Scholar]
- 2.Inoue D, Yoshida K, Yoneda N, et al. IgG4-related disease: dataset of 235 consecutive patients. Medicine (Baltimore) 94: e680, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Umemura T, Zen Y, Hamano H, Ichijo T, Kawa S, Nakanuma Y, Kiyosawa K. IgG4 associated autoimmune hepatitis: a differential diagnosis for classical autoimmune hepatitis. Gut 56: 1471-1472, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umemura T, Zen Y, Hamano H, et al. Immunoglobin G4-hepatopathy: association of immunoglobin G4-bearing plasma cells in liver with autoimmune pancreatitis. Hepatology 46: 463-471, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Naitoh I, Nakazawa T. Classification and diagnostic criteria for IgG4-related sclerosing cholangitis. Gut Liver 16: 28-36, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masset C, Kervella D, Kandel-Aznar C, Fantou A, Blancho G, Hamidou M. Relapse of IgG4-related nephritis following mRNA COVID-19 vaccine. Kidney Int 100: 465-466, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tasnim S, Al-Jobory O, Hallak A, Bharadwaj T, Patel M. IgG4 related pleural disease: recurrent pleural effusion after COVID-19 vaccination. Respirol Case Rep 10: e01026, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel AH, Amin R, Lalos AT. Acute liver injury and IgG4-related autoimmune pancreatitis following mRNA-based COVID-19 vaccination. Hepatol Forum 3: 97-99, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Efe C, Kulkarni AV, Terziroli Beretta-Piccoli B, et al. Liver injury after SARS-CoV-2 vaccination: features of immune-mediated hepatitis, role of corticosteroid therapy and outcome. Hepatology 76: 1576-1586, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klomjit N, Alexander MP, Fervenza FC, et al. COVID-19 vaccination and glomerulonephritis. Kidney Int Rep 6: 2969-2978, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bansal P, Arudra SKC, Sonani B, Gulati D. IgG4-related hepatopathy. Arch Autoimmune Dis 1: 14-16, 2020. [Google Scholar]