Abstract
Background
Dietary protein ingestion augments post (resistance) exercise muscle protein synthesis (MPS) rates. It is thought that the dose of leucine ingested within the protein (leucine threshold hypothesis) and the subsequent plasma leucine variables (leucine trigger hypothesis; peak magnitude, rate of rise, and total availability) determine the magnitude of the postprandial postexercise MPS response.
Methods
A quantitative systematic review was performed extracting data from studies that recruited healthy adults, applied a bout of resistance exercise, ingested a bolus of protein within an hour of exercise, and measured plasma leucine concentrations and MPS rates (delta change from basal).
Results
Ingested leucine dose was associated with the magnitude of the MPS response in older, but not younger, adults over acute (0–2 h, r 2 = 0.64, p = 0.02) and the entire postprandial (>2 h, r 2 = 0.18, p = 0.01) period. However, no single plasma leucine variable possessed substantial predictive capacity over the magnitude of MPS rates in younger or older adults.
Conclusion
Our data provide support that leucine dose provides predictive capacity over postprandial postexercise MPS responses in older adults. However, no threshold in older adults and no plasma leucine variable was correlated with the magnitude of the postexercise anabolic response.
Keywords: amino acids, exercise, leucine, muscle protein synthesis, protein
1. INTRODUCTION
Prolonged resistance exercise training increases skeletal muscle mass and strength, advantageous adaptive responses to support athletic/health goals in a range of individuals (Damas et al., 2016; Farup et al., 2012). Training‐induced increases in muscle mass are mechanistically underpinned by persistent periods of positive net protein balance, and therefore protein accretion, within muscle tissue (Fujita et al., 2007). A single bout of resistance exercise stimulates muscle protein synthesis (MPS) rates, peaking in the immediate hours subsequent (~2–6 h; Moore, Tang, et al., 2009; van Vliet et al., 2019) and remaining elevated for ~24–48 h (Biolo et al., 1995; Burd et al., 2011; Phillips et al., 1997). However, resistance exercise also stimulates muscle protein breakdown (MPB) rates such that, in the postabsorptive state, muscle protein net balance remains negative (Biolo et al., 1995). As a result, nutrition plays a vital role in promoting postexercise net positive protein balance in muscle and, therefore, muscle reconditioning.
Protein ingestion before (Burke et al., 2012; Tipton et al., 2007), immediately after (Brook et al., 2021; Moore, Robinson, et al., 2009; Pennings, Koopman, et al., 2011; Tang et al., 2009), and up to 24‐h (Elliot et al., 2006; Holwerda et al., 2016; Kim et al., 2016) postresistance exercise augments the rise in MPS rates and, albeit less potently, inhibits MPB rates (Biolo et al., 1997) resulting in a positive net muscle protein balance. The magnitude of the (postexercise) MPS response to protein ingestion appears to increase in a dose–response manner plateauing somewhere between 20 and 40 g (Moore, Robinson, et al., 2009; Witard et al., 2014; Yang et al., 2012), a relationship that shifts to the right in more anabolically insensitive older adults (Burd et al., 2013; Cuthbertson et al., 2005; Rennie & Wilkes, 2005; Wall, Gorissen, et al., 2015). However, as opposed to total protein per se, this relationship has been suggested to be more contingent on essential amino acids (Bohé et al., 2003; Cuthbertson et al., 2005; Fujita et al., 2007; Tipton et al., 1999) and, in particular, leucine (Phillips, 2016; Rieu et al., 2006), which has a well‐characterized molecular role in stimulating the mTORC1/P70S6K signaling pathway (the major myocellular anabolic cascade; Dreyer et al., 2008; Drummond & Rasmussen, 2008; Layman, 2002; Norton & Layman, 2006). However, the nature of the relationship between leucine and postprandial MPS (particularly when considered in the postexercise phase) remains to be fully defined.
Some reports imply that simply the amount of leucine contained within the ingested food/meal directly dictates postprandial MPS rates (i.e., “leucine threshold” concept; Breen & Phillips, 2011). This pragmatic dose–response view allows simple recommendations to be made, such as ~2 to ~3 g to be consumed for measurable and optimal postexercise MPS responses, respectively (Phillips et al., 1997; Volpi et al., 2013). Other reports take account of physiological variables such as protein digestion and amino acid absorption which, together, dictate peripheral leucine availability following protein ingestion and, thus, a stimulus actually seen by the muscle (i.e., “leucine trigger” concept; Tang et al., 2009; West et al., 2011). However, even within this more sophisticated view, it is unclear whether the peak concentration (Tang et al., 2009; West et al., 2011), rate of rise (Phillips & Van Loon, 2011; West et al., 2011), or total postprandial availability of plasma (Oikawa et al., 2020; or even intramuscular unbound) leucine is the prime “trigger.” A recent qualitative systematic review (Zaromskyte et al., 2021) supported the utility of the leucine trigger hypothesis within muscle of older individuals and during studies where crystalline amino acid mixtures or isolated proteins were ingested. The predictive value of the hypothesis diminished in younger subjects and/or where protein‐rich whole foods (within their unique matrices; Beals et al., 2018; Burd et al., 2015; Elliot et al., 2006; Van Vliet et al., 2017) and/or mixed meals (Kim et al., 2016; Symons et al., 2011) were ingested. However, the definition of the leucine trigger in this review was binary and defined only as a “greater overall plasma leucine response,” which did not allow for any quantitative dose–response relationship to be established.
We conducted a quantitative systematic review with the primary aim of refining our understanding of the relationship between ingested leucine and the magnitude of postprandial postexercise MPS rates. We compiled data from human studies that applied a study design including bolus ingestion of amino acids or protein (either alone or contained within a food/meal) and the execution of a single resistance exercise bout, combined with the parallel measurements of MPS rates, and with further inclusion criteria applied around postprandial plasma leucine concentrations. We clearly demarcated between the leucine threshold hypothesis (i.e., leucine dose), and the three distinct (sub)variables identified within the leucine trigger hypothesis (i.e., peak magnitude, rate of rise, and total availability of plasma leucine) and evaluated their relationships with the magnitude of postprandial postexercise MPS responses in young and older adults.
2. METHODS
This study was registered on, and the protocol was uploaded to, PROSPERO (CRD42021227295). The review was conducted based on PRISMA guidelines 2020, in line with quantitative systematic reviews (Moher et al., 2009). The primary outcome of this systematic review was to refine our understanding of the relationship between ingested leucine and the magnitude of postprandial postexercise MPS rates. Heterogeneity in the absolute determination of MPS rates and plasma leucine concentrations between studies, methods, and laboratories was accounted for by assessing delta changes on both variables (MPS and plasma leucine concentrations) as our primary depiction of the data.
2.1. Search strategy
A systematic search of the literature was conducted in Medline (https://pubmed.ncbi.nlm.nih.gov/), Cochrane (https://www.cochranelibrary.com/central), and Embase (https://www.embase.com/) databases on the July 12, 2022. The medical subject headings (MeSH) “Leucine”, “Protein Biosynthesis”, “Dietary Supplements”, “Dietary Proteins” and “Muscle Proteins” were utilized. Boolean operators “AND” and “OR” were used to combine search terms. The following search terms were used (protein OR leucine OR amino acid OR supplement* OR diet* OR consume* OR intake* OR ingest* OR powder OR drink* OR shake OR isolate) AND (muscle* OR myofibrillar* OR mixed OR muscular OR protein synthesis) AND (weight* OR resistance* OR strength OR isometric OR train* OR exercise OR lift*) AND (randomized OR randomized control trial).
2.2. Eligibility criteria
All randomized controlled trials (RCT) reporting MPS rates in healthy human adults after bolus ingestion of an amino acid, amino acid mixture, isolated protein source, protein containing whole food, or mixed meal in close temporal proximity (maximally 1 h before or after) to a bout of resistance exercise (exercise against an external load) were considered for inclusion. Resistance exercise protocols were considered broadly, with a variety of exercise protocols included, differing in modalities and mechanics of movement. All these protocols were designed and considered to provide a maximal stimulus, and therefore, we assume that this broad study inclusion allowed the examination of a “maximal” exercise‐induced stimulation of MPS and the further examination of how this is modulated by leucine consumption.
Further inclusion was applied whereby studies had to report plasma leucine concentrations for at least 1.5 h after protein ingestion with time intervals of, at most, 30 min to calculate peak plasma leucine magnitude, rate of rise to peak plasma leucine magnitude, and total postprandial plasma leucine availability. MPS rates needed to be determined by the primed continuous infusion of stable isotopically labeled amino acid(s) (though specific isotope or in which amino acid it was labeled was not required) and plasma leucine concentrations via venous or arterialized‐venous blood sampling methods, with quantification of amino acid concentrations via gas chromatography–mass spectrometry (GC–MS).
2.3. Exclusion criteria
Studies that were excluded were as follows: those where participants were classified as unhealthy; if a source of protein was provided via intravenous infusion or repeated doses (as opposed to a single bolus); if more than one resistance exercise session was performed; where the protein bolus was provided more than 1‐h pre or postexercise; or, if an acute measurement period of MPS was not available.
2.4. Data collection
Two reviewers (K.W. and C.P.K.) screened all titles and abstracts to identify potentially eligible studies, and full papers were obtained and assessed for inclusion independently by these authors. Any disagreement regarding eligibility was resolved through deliberation or referred to a third‐party author (A.J.M.), to resolve the decision, if necessary. All duplicates were identified and removed. Automation tools within the Rayyan software (https://www.rayyan.ai/) were used to filter key words, to detect studies that did not fit the inclusion criteria (i.e., study populations in rats, pigs, and children).
2.5. Data extraction
Predetermined relevant outcome variables from each study were extracted by one reviewer (K.W.) and the other reviewer (C.P.K.) revisited all to check for discrepancies. Relevant variables included: number of participants, participant characteristics (age, sex, and training status, if supplied), protein supplementation protocol (protein dose and leucine dose), exercise intervention, mixed muscle or myofibrillar protein synthesis values and plasma leucine concentrations. If protein or leucine doses were given relative to body weight, this was calculated with the mean body weight of the participants in that group to normalize all data to absolute doses. If the study in question did not provide the leucine dose within the nutritional content provided for the protein source, then manufacturer information was searched (Burd, Andrews, et al., 2012; Burd, Yang, et al., 2012; Chan et al., 2019; Dideriksen et al., 2016; McKendry et al., 2016; Mikkelsen et al., 2015) or corresponding authors were contacted to provide manufacturer information (Agergaard et al., 2017; Areta et al., 2014; McGlory et al., 2016) or nutritional analysis (Holwerda et al., 2016). If the study included more than one protein source (e.g., whey and soy), outcome measures were taken for both and treated as separate study arms. If the study included a placebo or control group that was not a protein source, this was not included in data extraction. Protein sources with additional fortification and co‐ingestion with macronutrients were noted and included. Data were further characterized into whole food sources (mixed macronutrient nonsupplemental protein) and nonwhole food sources. This is represented visually within the presented dataset herein. Data were considered as a whole dataset and then organized into young and older participants. This was based on the descriptive statistics of the participants provided. The mean age of the younger participants within a given study was required to be between 18 and 40 years. The mean age of the older participants within a given study was required to be >55 years. This was to ensure that the threshold for the onset of age‐related sarcopenia had been met (Janssen, 2010). Where numeric data were not reported in tables or text, and authors could not be reached, data were extracted from charts and figures using Web Plot Digitizer (https://automeris.io/WebPlotDigitizer/).
2.6. Risk of bias
The Cochrane Handbook and tools (Higgins et al., 2011, 2019) were used for the risk of bias assessment for each individual study. The quality of each study was assessed by one reviewer (K.W.) and checked by another reviewer (C.K.), and any disagreement was resolved through deliberation between K.W. and C.K. Six main criteria were assessed, and the quality of each study was based on high, low or unclear risk of bias (Supplementary Information—https://doi.org/10.6084/m9.figshare.22203514). Studies with a high risk of bias were due to blinding procedures, usually in the case of whole food protein sources (Beals et al., 2018; Symons et al., 2011), where blinding of the allocated intervention was not possible. Sequence generation was considered a high risk of bias when allocation to the intervention was based on a criterion such as younger or older participants; therefore, assignment to the intervention was nonrandom. Both high risk of bias variables were considered satisfactory for this dataset.
2.7. Synthesis methods
The main outcome variables used in this review have been converted from the data extracted and have been used to visually display the data in the figures. Basal [postabsorptive] fractional synthetic rate (FSR) and postexercise postprandial FSR (%/h) were used to calculate delta change (ΔFSR [%]) for normalization across studies. These were then split into early (0–2‐h postexercise and/or protein ingestion) and the entire postprandial (0–6‐h postexercise and/or protein ingestion) MPS response. Plasma leucine concentrations were displayed as peak plasma leucine concentration (highest single mean value reported), rate of rise to peak plasma leucine magnitude (peak plasma leucine concentration minus basal plasma leucine concentration divided by time in minutes to peak plasma leucine concentration), and total postprandial plasma leucine availability (incremental area under the curve [iAUC/180 min]). Data were analyzed using linear regression; coefficient of determination (r 2), significance (p value), and y‐intercept (b 0) have been presented for interpretation. Subject characteristics are presented as mean ± SD.
3. RESULTS
3.1. Literature search and study inclusion
Figure 1 shows the process of article selection with 38 studies ultimately included. Within these 38 studies, there were 77 study arms (i.e., total number of eligible intervention groups), to determine aspects relating to the leucine threshold hypothesis (Supplementary Information—https://doi.org/10.6084/m9.figshare.22203514, Agergaard et al., 2017; Areta et al., 2014; Atherton et al., 2017; Beals et al., 2018; Borack et al., 2016; Brook et al., 2021; Bukhari et al., 2015; Burd et al., 2010, 2015; Burd, Andrews, et al., 2012; Burd, Yang, et al., 2012; Chan et al., 2019; Churchward‐Venne, Breen, et al., 2014; Churchward‐Venne, Cotie, et al., 2014; Devries et al., 2018a, 2018b; Dickinson et al., 2014; Dideriksen et al., 2016; Dreyer et al., 2008; Fujita et al., 2009; Gwin et al., 2021; Hermans et al., 2021, 2022; Luiking et al., 2014; McGlory et al., 2016; McKendry et al., 2016; Mikkelsen et al., 2015; Monteyne, Coelho, Porter, Abdelrahman, Jameson, Finnigan, et al., 2020; Monteyne, Coelho, Porter, Abdelrahman, Jameson, Jackman, et al., 2020; Moore, Tang, et al., 2009; Oikawa et al., 2020; Pinckaers et al., 2022; Reidy et al., 2013; Reitelseder et al., 2019; Symons et al., 2011; Van Vliet et al., 2017; West et al., 2009; Wilkinson et al., 2018). Studies which met all the inclusion criteria except not taking a basal muscle biopsy (i.e., Dideriksen et al., 2011) were excluded in order to calculate delta change from basal MPS.
FIGURE 1.
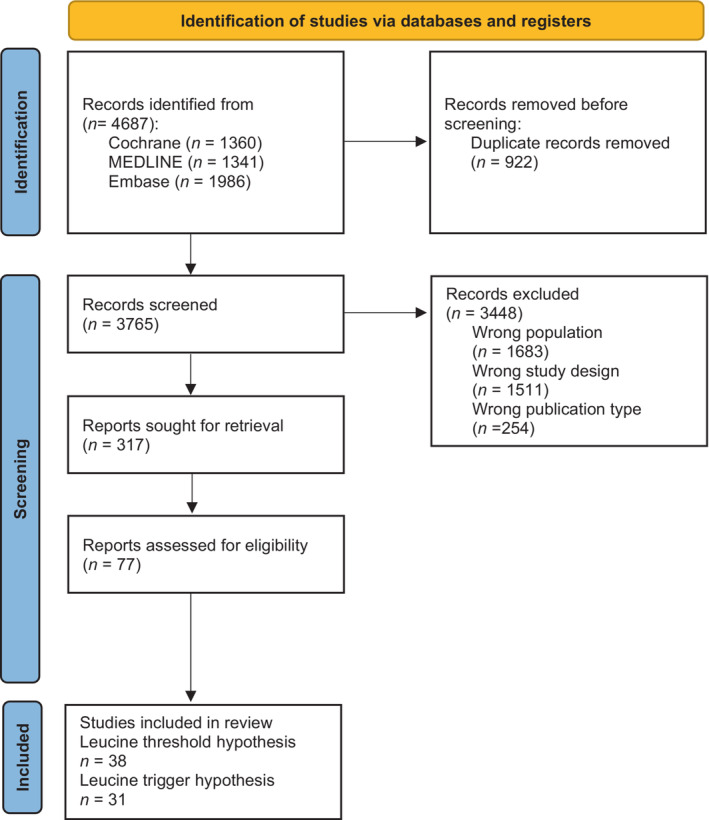
Flow diagram of the screening process in accordance with the PRISMA guidelines.
Of these 38 identified RCTs, six studies were crossover in design (Areta et al., 2014; Brook et al., 2021; Burd et al., 2015; Gwin et al., 2021; Van Vliet et al., 2017; West et al., 2009), whereas the remainder were parallel design. Double‐blinding procedures were used in 11 of the 38 studies, five were single‐blinded, and the remainder were unblinded. Further exclusion was applied, whereby seven studies did not meet the criteria of providing plasma leucine concentrations, leaving 31 studies (61 study arms) included in the further analysis to determine aspects of the leucine trigger hypothesis (Supplementary Information—https://doi.org/10.6084/m9.figshare.22203514).
3.2. Participants' characteristics
Of the total 38 studies, five of the studies recruited females only (Bukhari et al., 2015; Devries et al., 2018a, 2018b; Oikawa et al., 2020; Wilkinson et al., 2018) and six recruited males and females (Areta et al., 2014; Beals et al., 2018; Fujita et al., 2009; Luiking et al., 2014; Reidy et al., 2013; Symons et al., 2011), with the remainder in males only. Of the 77 study arms relating to the leucine threshold analysis, 45 studies were of younger adults and 32 of older adults. The age range of younger participants was 19–29 years (23 ± 2.3 years, 409 male and 40 female participants), and the age range of older adults was 57–74 years (68 ± 3.5 years, 142 male and 106 female participants). Of the 61 study arms relating to the leucine trigger analysis, 35 were studies of younger adults and 26 of older adults. Eleven study arms defined their participants as resistance‐trained individuals, while 19 study arms stated the participants were recreationally active. Furthermore, six study arms recruited low‐to‐moderately active participants with the remainder of the study arms reporting healthy individuals with no specific training status provided.
3.3. Protein sources
Bolus doses of orally administered isolated proteins comprised: whey (33 study arms), casein (three study arms), milk protein concentrate (10 study arms), crystalline essential amino acid mixtures (nine study arms), and isolated protein blends (four study arms). Other sources included protein‐rich foods: pork (two study arms; Beals et al., 2018), beef (three study arms; Burd et al., 2015; Symons et al., 2011), mycoprotein (two study arms; Monteyne, Coelho, Porter, Abdelrahman, Jameson, Finnigan, et al., 2020; Monteyne, Coelho, Porter, Abdelrahman, Jameson, Jackman, et al., 2020), protein‐rich meal replacements (four study arms; Atherton et al., 2017), cheese (Hermans et al., 2022), meal worms (Hermans et al., 2021), collagen protein, potato protein (Pinckaers et al., 2022), egg white (Van Vliet et al., 2017), and egg yolk (Van Vliet et al., 2017), all with one study arm each.
3.4. Resistance exercise interventions
A unilateral exercise model was used for 27 out of the 38 studies, with the remaining 11 studies using a bilateral exercise model. The exercise protocols consisted of a variety of reps and sets ranging from 1 to 10 sets and from 8 to 36 reps or to volitional exhaustion/failure. All studies had a familiarization with the exercise equipment and tested for maximum strength to determine the workload. The intensity ranged from 16% to 90% of one repetition maximum. Within three study arms, maximal leg extension exercise was on a dynamometer (Monteyne, Coelho, Porter, Abdelrahman, Jameson, Finnigan, et al., 2020; Monteyne, Coelho, Porter, Abdelrahman, Jameson, Jackman, et al., 2020). The exercise protocol was either leg press (one study arm; Areta et al., 2014), leg/knee extension (54 study arms), both leg press and leg extension (20 study arms), arm cable curl (elbow flexion; one study arm; West et al., 2009), or combination of upper and lower body resistance exercise session (one study arms; West et al., 2009).
3.5. Postprandial postexercise periods
The measurement of MPS was taken within the mixed muscle protein fraction for four studies (Gwin et al., 2021; Hermans et al., 2021; Monteyne, Coelho, Porter, Abdelrahman, Jameson, Finnigan, et al., 2020; Monteyne, Coelho, Porter, Abdelrahman, Jameson, Jackman, et al., 2020) and the remaining studies measured MPS in myofibrillar proteins. The basal biopsy was conducted preintervention, and the incorporation period for the measurement of MPS ranged from 1.5‐ to 6‐h postprandial, postexercise. All studies collected muscle biopsy tissue from the m. vastus lateralis, except one study with measurements of MPS from tissue collected from the biceps brachii (West et al., 2009).
3.6. Leucine dose
A graphical depiction of the relationship between ingested leucine dose and the delta change in postprandial, postexercise MPS rates is represented in Figure 2, with data illustrated as an early (0–2 h) and entire measurement (>2 h) phase, and presented as an entire dataset (A), and for young (B) and older (C) adults separately. When considering the entire dataset (Figure 2a), leucine dose showed no relationship to delta change in postexercise MPS rates over the early phase (r 2 = 0.03, p = 0.33, b 0 = 78.43), but a significant correlation was observed over the entire measurement period (r 2 = 0.05, p = 0.03, b 0 = 76.2). The latter was mainly driven by data obtained from older adults, given divergent responses between the age categories were observed. Specifically, the relationship between leucine dose and postprandial postexercise MPS change was not present in young adults (Figure 2b) over either the early (r 2 = 0.006, p = 0.74, b 0 = 118.5) or entire (r 2 = 0.01, p = 0.51, b 0 = 108.1) measurement periods, whereas correlations were observed over both periods (r 2 = 0.64, p = 0.02, b 0 = 7.64 and r 2 = 0.18, p = 0.01, b 0 = 42.33 over the early and entire measurements periods, respectively) in older adults (Figure 2c).
FIGURE 2.
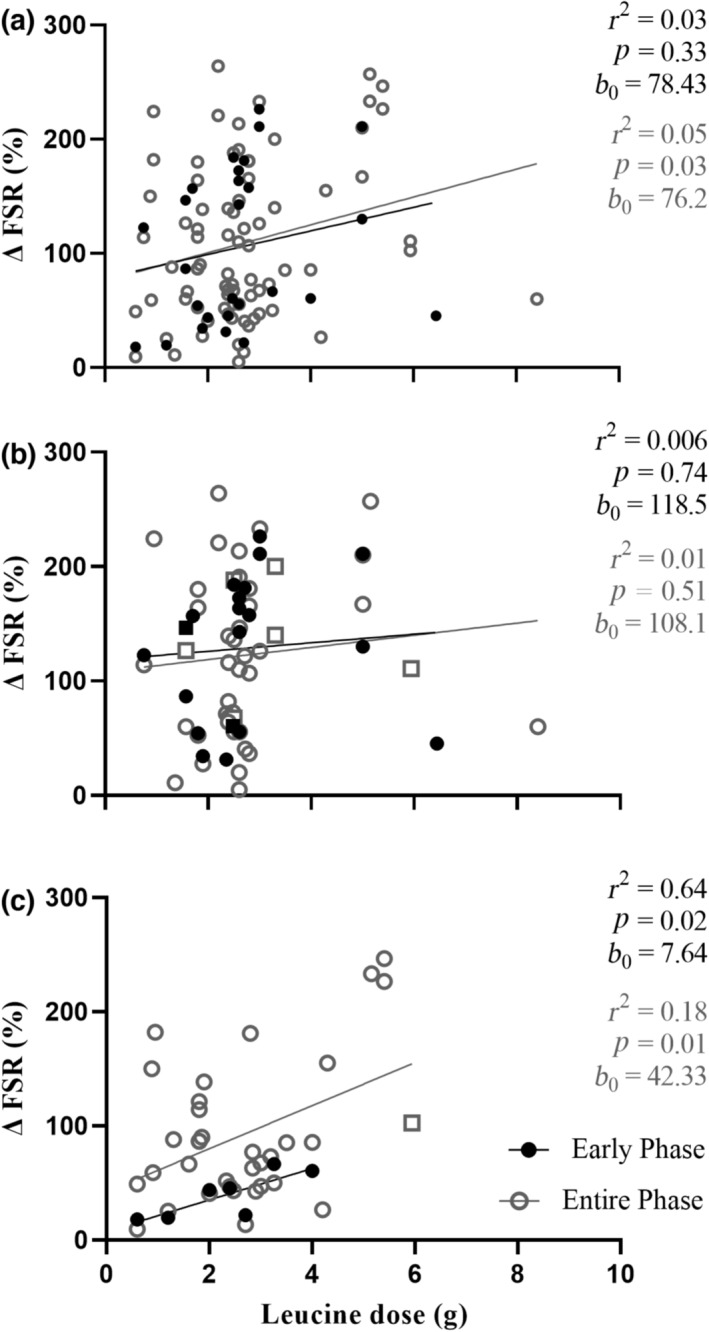
Delta change (postprandial postexercise increase) in muscle protein synthesis rates (MPS), early (0–2 h) and during the entirety of the postprandial period, expressed as fractional synthetic rate (FSR), in response to a leucine dose in all participants (77 study arms) (a), young participants (45 study arms; 19–29 years) (b) and older participants (32 study arms; 57–74 years) (c). Squares represent whole food sources. Data were analyzed by linear regression; coefficient of determination (r 2), p value, and y‐intercept (b 0) are presented.
3.7. Leucine trigger hypothesis
A graphical depiction of the relationship between ingested leucine dose and the peak magnitude, rate of rise, and total postprandial availability of plasma leucine are represented in Figures 3, 4, 5, respectively, with data presented as an entire dataset (A), and for young (B) and older (C) adults separately. Further graphical depiction of the relationship between delta change in postprandial, postexercise MPS and peak magnitude, rate of rise, and total postprandial availability of plasma leucine are represented as an entire dataset (D), and for young (E) and older (F) adults separately.
FIGURE 3.
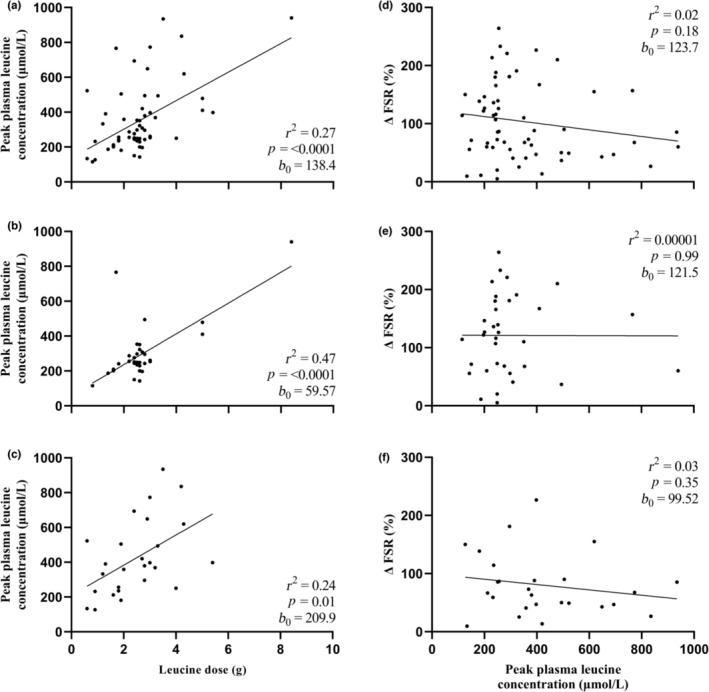
Peak plasma leucine magnitude (highest mean value reported) in response to a leucine dose provided as a bolus protein ingestion within an hour of resistance exercise in all participants (61 study arms) (a), young participants (35 study arms; 19–29 years) (b) and older participants (26 study arms; 57–74 years) (c). Delta change (postprandial postexercise increase) in muscle protein synthesis rates (MPS), expressed as fractional synthetic rate (FSR) in relation to peak plasma leucine magnitude in all participants (d), young participants (e) and older participants (f). Data were analyzed by linear regression; coefficient of determination (r 2), p value, and y‐intercept (b 0) are presented.
FIGURE 4.
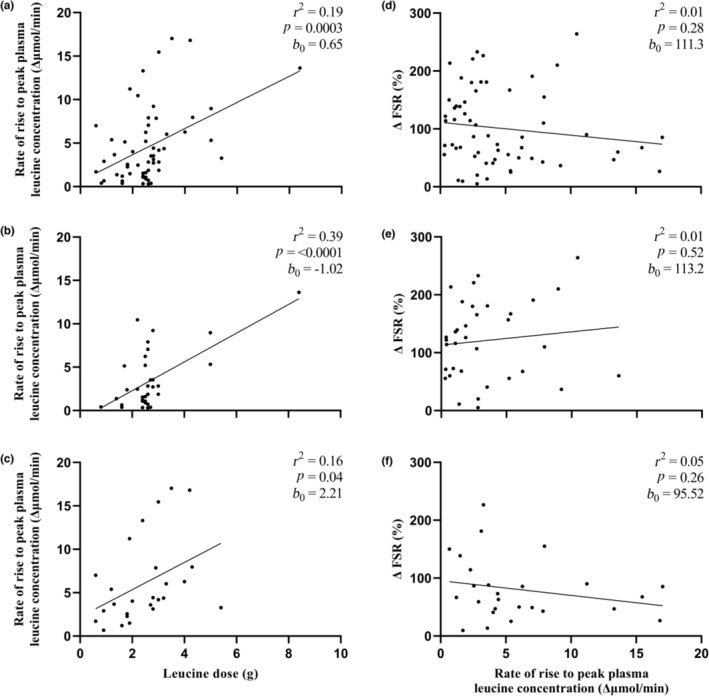
Rate of rise to peak plasma leucine magnitude in response to a leucine dose provided as a bolus protein ingestion within an hour of resistance exercise in all participants (61 study arms) (a), young participants (35 study arms; 19–29 years) (b) and older participants (26 study arms; 57–74 years) (c). Delta change (postprandial postexercise increase) in muscle protein synthesis rates (MPS), expressed as fractional synthetic rate (FSR) in relation to rate of rise to peak plasma leucine magnitude in all participants (d), young participants (e) and older participants (f). Rate of rise to peak plasma leucine magnitude determined from the highest plasma leucine concentration minus basal plasma leucine concentration divided by time in minutes to peak concentration. Data were analyzed by linear regression; coefficient of determination (r 2), p value, and y‐intercept (b 0) are presented.
FIGURE 5.
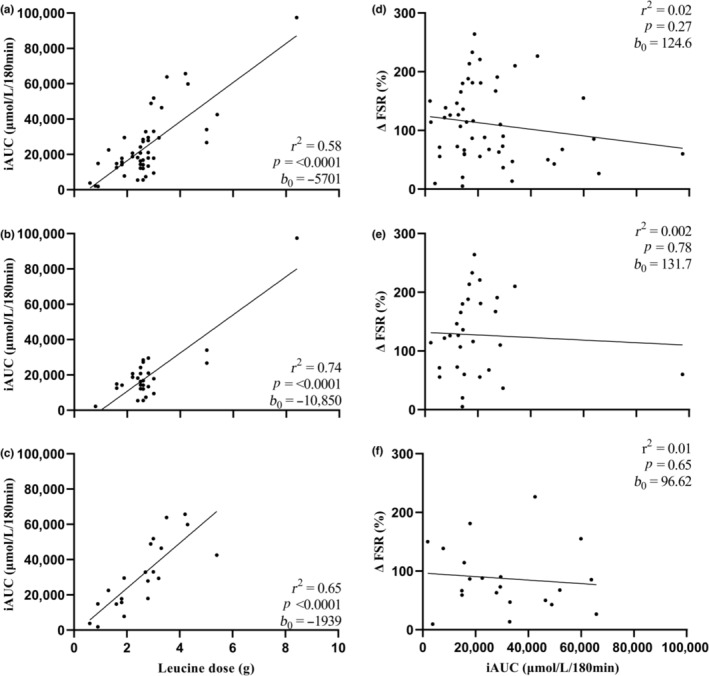
Total postprandial plasma leucine availability, represented as incremental area under the curve over 180 min, in response to a leucine dose provided as a bolus protein ingestion within an hour of resistance exercise in all participants (61 study arms) (a), young participants (35 study arms; 19–29 years) (b) and older participants (26 study arms; 57–74 years) (c). Delta change (postprandial postexercise increase) in muscle protein synthesis rates (MPS), expressed as fractional synthetic rate (FSR) in relation to total postprandial leucine availability in all participants (d), young participants (e) and older participants (f). Data were analyzed by linear regression; coefficient of determination (r 2), p value, and y‐intercept (b 0) are presented.
3.7.1. Peak plasma leucine magnitude
When considering the entire dataset (Figure 3a), the leucine dose showed a relationship with peak plasma leucine magnitude (r 2 = 0.27, p < 0.0001, b 0 = 138.4). A significant relationship was observed between leucine dose and peak plasma leucine magnitude for both younger (Figure 3b; r 2 = 0.47, p < 0.0001, b 0 = 59.57) and older (Figure 3c; r 2 = 0.24, p = 0.01, b 0 = 209.9) adults. However, peak plasma leucine magnitude showed no relationship to delta change in postprandial, postexercise MPS rates when considering the whole dataset (Figure 3d; r 2 = 0.02, p = 0.18, b 0 = 123.7) and this relationship was not altered when split for age; leucine dose showed no relationship to delta change in postexercise MPS rates among the younger (Figure 3e; r 2 = 0.00001, p = 0.99, b 0 = 121.5) or older (Figure 3f; r 2 = 0.03, p = 0.35, b 0 = 99.52) adults.
3.7.2. Rate of rise to peak plasma leucine magnitude
When considering the whole dataset (Figure 4a), there was a relationship between leucine dose and rate of rise to peak plasma leucine magnitude (r 2 = 0.19, p = 0.0003, b 0 = 0.65). When split for age this relationship between leucine dose and rate of rise to peak plasma leucine concentration was present for both younger (Figure 4b; r 2 = 0.39, p < 0.0001, b 0 = −1.02) and older adults (Figure 4c; r 2 = 0.16, p = 0.04, b 0 = 2.21). However, correlations were not observed between peak plasma leucine concentrations and delta change in postprandial, postexercise MPS across the entire dataset (Figure 4d; r 2 = 0.01, p = 0.28, b 0 = 111.3) nor when split into younger and older adults (Figure 4e; r 2 = 0.01, p = 0.52, b 0 = 113.2, Figure 4f; r 2 = 0.05, p = 0.26, b 0 = 95.52 for younger and older adults, respectively).
3.7.3. Total postprandial plasma leucine availability
When considering the entire dataset (Figure 5a), the leucine dose showed a relationship to plasma leucine iAUC (r 2 = 0.58, p < 0.0001, b 0 = −5701). A significant relationship was observed between leucine dose and plasma leucine iAUC for both younger (Figure 5b; r 2 = 0.74, p < 0.0001, b 0 = −10,850) and older (Figure 5c; r 2 = 0.65, p < 0.0001, b 0 = −1939) adults. However, plasma leucine iAUC showed no relationship to delta change in postprandial, postexercise MPS rates when considering the whole dataset (Figure 5d; r 2 = 0.02, p = 0.27, b 0 = 124.6) and this relationship was not altered when split for age; plasma leucine iAUC showed no relationship to delta change in postexercise MPS rates among the younger (Figure 5e; r 2 = 0.002, p = 0.78, b 0 = 131.7) or older (Figure 5f; r 2 = 0.01, p = 0.65, b 0 = 96.62) adults.
4. DISCUSSION
4.1. Principal findings
In the present quantitative systematic review, we provide a detailed examination of the physiological regulation of postexercise MPS rates by leucine ingested within dietary protein in younger and older adults. We first quantified the predictive capacity of leucine dose per se on the magnitude of postexercise MPS rates. We then sequentially examined the relationships between ingested leucine dose and various aspects of its postprandial postexercise availability within the circulation. Finally, we assessed the ability of those aspects of postprandial plasma leucine availability to predict postexercise MPS rates. We report several novel findings that further our understanding of the leucine threshold and trigger concepts. First, ingested leucine dose per se is associated with the magnitude of the postprandial postexercise MPS response, but this relationship exists only in older adults, over both the early and entire measurement periods (Figure 2c). Second, largely irrespective of age, ingested leucine dose is highly predictive of the peak magnitude, rate of rise, and total availability of plasma leucine concentrations during the postprandial postexercise period. Finally, when examining these discreet aspects of postprandial postexercise plasma leucine variables in this reductionist manner, no single variable possessed any association with the magnitude of postprandial postexercise MPS rates in either young or older adults.
4.2. Leucine threshold concept
What is generally referred to as the “leucine threshold” hypothesis posits a simple dose–response relationship between total leucine ingested and the postprandial postexercise MPS response, plateauing at around ~2.5 g (Witard et al., 2014). This is aligned with various applied sports nutrition recommendations to ingest a protein meal containing at least 2–3 g leucine in close temporal proximity to exercise to maximize the postexercise muscle anabolic response (Collins et al., 2021; Dickinson et al., 2013; Katsanos et al., 2006; Morgan et al., 2022; Phillips & Van Loon, 2011; Wall, Morton, et al., 2015). Our present data do not fully support this concept. There was no correlation between ingested leucine dose and the postexercise MPS response over a 6‐h period (Figure 2) in the largest cohort of young individuals studied to date. Indeed, the lines for both the early and entire phase of postexercise MPS intercept (b 0) at around 100% could already be maximal (Figure 2b). Some (Atherton et al., 2017; Dreyer et al., 2008; Gwin et al., 2021), but not all (Churchward‐Venne, Breen, et al., 2014; Fujita et al., 2009), studies demonstrate an increase in MPS with additional leucine over resistance exercise alone, which raises the question of whether leucine increases MPS over and above the stimulus of resistance exercise in younger individuals at all. Of course, there could be a dose–response effect on suppressing MPB and, therefore, hypertrophy, but this has not been investigated to date. This also highlights the difficulty in providing precise prescriptions based on leucine alone, especially given the array of differences across subjects and exercise protocols. In contrast, there was a strong dose–response correlation between ingested leucine dose and postexercise MPS rates in older adults, with overall lower increases observed compared with young. Indeed, in comparison with younger adults where b 0 was around 100%, MPS did not increase to 100% (i.e., double) at all over 2 h, or until around 3–4 g of leucine was ingested during the entire postprandial phase. This is in line with recent similar investigations into the regulation of MPS by leucine in older individuals (Wall et al., 2013) and likely reflects age‐related alterations in digestion and absorption kinetics (Gorissen et al., 2020; Milan et al., 2015), splanchnic extraction (Boirie et al., 1997; Volpi et al., 1999), perfusion (Timmerman et al., 2010), and/or a reduction in sensitivity (and/or delay in response) of muscle to the anabolic properties of dietary protein (all encompassed within the term “anabolic resistance”; Burd et al., 2013; Cuthbertson et al., 2005; Wall, Gorissen, et al., 2015). In line, older adults showed a slower rate of rise to peak, greater variability of peak magnitude and an overall “rightward shift” that was lower, particularly in the early phase, indicating a greater and faster leucine response is required for an equivalent rise in MPS. To a certain extent, this shift to the right could explain the significant correlation in the older adults only, given this provides a greater spread of the data. Irrespective, our findings extend the concept of anabolic resistance to imply that the anabolic sensitivity to leucine becomes of more relevance in terms of governing postexercise postprandial MPS rates in senescent muscle. However, the linear nature of the relationship between leucine dose and postexercise MPS rates in older individuals, and lack of an obvious breakpoint, do not reveal a plateau or “threshold,” unlike previous studies that only compare two or three doses (Moore, Robinson, et al., 2009; Witard et al., 2014; Yang et al., 2012).
4.3. Leucine trigger concept
The utility of comparing leucine dose to postexercise MPS responses does not account for the multitude of mediating physiological factors that could mechanistically modulate this relationship. Attempts have been made to link the two, generally encompassed within the umbrella term “leucine trigger” hypothesis (Phillips & Van Loon, 2011; Witard et al., 2014; Zaromskyte et al., 2021). We show that leucine dose strongly predicts various postprandial candidate “triggers,” such as peak plasma leucine magnitude (Figure 3; Norton et al., 2009; Pennings, Boirie, et al., 2011; Tang et al., 2009; West et al., 2011), the rate of rise to peak plasma leucine magnitude (Figure 4; Burd, Yang, et al., 2012; Wall et al., 2013), and total postprandial plasma leucine availability (iAUC; Figure 5, Mitchell, Phillips, et al., 2015). However, when comparing these variables against postprandial MPS rates, no relationships were observed in the entire cohort, nor when younger and older adults were considered separately (Figures 3, 4, 5). This is surprising given the observed association between leucine dose and postexercise MPS rates, as well as the prevailing wider narrative within the literature where a clear manipulation of postprandial leucinemic variables per se and an association with the consequent muscle anabolic response are seen. West et al. (2011) reported that a more rapid delivery of leucine to the circulation following bolus whey ingestion translated to greater MPS rates compared with the same quantity consumed in a pulse fashion. Similarly, by comparing ingestion of prehydrolyzed casein with intact casein, Pennings, Boirie, et al. (2011) showed greater leucinemia conferred a more potent MPS response in older adults.
The lack of any observed associations, within this systematic review, may be explained by “noise” in the data being too great to pin down one single plasma variable, whereas the leucine dose represented a composite of the total protein dose and all postprandial leucinemic factors thereby revealing the relationship. However, once other variables are introduced, such as comparing different protein sources (Chan et al., 2019; Churchward‐Venne, Breen, et al., 2014; Dideriksen et al., 2011; Reidy et al., 2013), isolated vs whole foods (Burd et al., 2015; Mitchell, McGregor, et al., 2015; Van Vliet et al., 2017), meal ingestion (Kim et al., 2016; Symons et al., 2011) or co‐ingestion with other macronutrients (Gorissen et al., 2014; Hamer et al., 2013; Koopman et al., 2007; Staples et al., 2011), the relationship is far less clear. We (Monteyne, Coelho, Porter, Abdelrahman, Jameson, Finnigan, et al., 2020; Monteyne, Coelho, Porter, Abdelrahman, Jameson, Jackman, et al., 2020; West et al., 2022) and others (Burd et al., 2015; Chan et al., 2019; Van Vliet et al., 2017) have observed a dissociation between circulating leucine concentrations and MPS in a series of recent studies, specifically involving whole food approaches. For example, a 25% greater MPS response was observed with ingestion of skim milk vs. beef despite a significantly lower plasma leucine concentration (Burd et al., 2015). In line, a recent systematic review concluded that the leucine trigger hypothesis was predictive of subsequent MPS responses only if protein isolates were consumed on their own (Zaromskyte et al., 2021). This may also explain why we only observed a relationship between leucine threshold and MPS in older individuals, where all the studies to date have involved protein isolates.
Collectively, therefore, it appears that postprandial plasma leucine responses as the prime determinant of the postexercise MPS response may be of most relevance when all other factors remain the same, and thus, leucine availability is limiting. Once other factors are introduced the influence of leucine diminishes and other regulatory candidates and limiting factors (e.g., total protein dose, other signaling or substrate limiting amino acids, other macro/micronutrients, and hormonal/incretin/neural) must be considered. However, the total protein dose provided did not modulate delta change MPS (Figure S1—https://doi.org/10.6084/m9.figshare.22203514), and as such, any observed relationships between leucine and delta change MPS did not appear to be primarily driven simply by a greater dose of leucine also being associated with a larger dose of total protein. It is important to note that true plasma leucine kinetics, involving multiple pool modeling of exogenous and endogenous leucine rates of appearance and disappearance, as well intramuscular transport, incorporation, oxidation, and efflux, are rarely measured. One might hypothesize that protein sources “other factors” aforementioned could speed the rate of disappearance of leucine into muscle tissue for a greater intracellular stimulatory effect on MPS, while also lowering peak magnitude, rate of rise, and/or total postprandial availability of plasma leucine. Indeed, there has been much debate as to where a potential leucine “sensor” may reside (Wolfson et al., 2016), with an intracellular sensor now considered most likely (Taylor, 2014). Therefore, using plasma leucine variables (only) as proxy markers for MPS triggers may not be an effective tool. We and others may be neglecting key variables such as changes (independently from plasma concentrations) in muscle leucine uptake, intracellular leucine concentration, and intramuscular leucine incorporation into polypeptide chains.
4.4. Conclusions and limitations
This systematic review collated all studies, which have provided a single bolus of protein within 1 h of a single bout of resistance exercise and measured the subsequent MPS response. While there is a clear dose–response of ingested leucine with postexercise MPS rates in older individuals, our data do not identify a precise leucine threshold, as no evident plateau was identified, and a maximal MPS response appears to be achievable in young individuals with protein ingestion per se irrespective of leucine content. Moreover, we report that the postexercise postprandial MPS response cannot be predicted from any single plasma leucine variable and, therefore, we cannot confirm the existence (or at least primacy) of a specific physiological leucine trigger. As such, our results indicate that both leucine dose and plasma leucine concentrations only explain part of the variability in postexercise postprandial MPS responses. Given our data are somewhat at odds with in vitro findings and some individual studies, we leave open several possibilities that our conclusions may be obfuscated by: lack of data across more diverse leucine doses (most studies provided 2–2.5 g leucine) or corrected to total body/lean mass; few reports involving true postprandial leucine kinetics; lack of intramuscular leucine measurements; altered and/or additional regulation by (as yet unidentified) other macro/micronutrients; and the availability of other amino acids required as signal and/or substrate for sustaining optimal MPS rates. Nevertheless, this review has again highlighted anabolic resistance in older individuals and the importance of study design with older individuals needing to encompass a longer postprandial period to ensure that the whole MPS response is captured.
AUTHOR CONTRIBUTIONS
Kiera Wilkinson, Christopher P. Koscien, Alistair J. Monteyne, Benjamin T. Wall, and Francis B. Stephens conceived and designed the study; Kiera Wilkinson, Christopher P. Koscien, and Alistair J. Monteyne conducted the search, study selection, and data extraction; Kiera Wilkinson analyzed the data; Kiera Wilkinson, Alistair J. Monteyne, Benjamin T. Wall, and Francis B. Stephens contributed to the interpretation of the data; Kiera Wilkinson prepared figures; Kiera Wilkinson, Alistair J. Monteyne, Benjamin T. Wall, and F.B.S. drafted the manuscript; Kiera Wilkinson, Benjamin T. Wall, and Francis B. Stephens edited and revised the manuscript; Kiera Wilkinson, Christopher P. Koscien, Alistair J. Monteyne, Benjamin T. Wall, and Francis B. Stephens approved final version of the manuscript.
FUNDING INFORMATION
K.W. PhD studentship is supported by a grant from Beachbody LLC.
CONFLICT OF INTEREST STATEMENT
No conflicts of interest, financial or otherwise, are declared by the authors.
ETHICS APPROVAL
Not applicable.
Supporting information
Data S1.
Data S2.
Figure S1.
Wilkinson, K. , Koscien, C. P. , Monteyne, A. J. , Wall, B. T. , & Stephens, F. B. (2023). Association of postprandial postexercise muscle protein synthesis rates with dietary leucine: A systematic review. Physiological Reports, 11, e15775. 10.14814/phy2.15775
DATA AVAILABILITY STATEMENT
The datasets generated and/or analyzed during the study implementation are available from the corresponding author upon request.
REFERENCES
- Agergaard, J. , Bülow, J. , Jensen, J. K. , Reitelseder, S. , Drummond, M. J. , Schjerling, P. , Scheike, T. , Serena, A. , & Holm, L. (2017). Light‐load resistance exercise increases muscle protein synthesis and hypertrophy signaling in elderly men. American Journal of Physiology. Endocrinology and Metabolism, 312(4), E326–E338. [DOI] [PubMed] [Google Scholar]
- Areta, J. L. , Burke, L. M. , Camera, D. M. , West, D. W. D. , Crawshay, S. , Moore, D. R. , Stellingwerff, T. , Phillips, S. M. , Hawley, J. A. , & Coffey, V. G. (2014). Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short‐term energy deficit. American Journal of Physiology. Endocrinology and Metabolism, 306(8), E989–E997. [DOI] [PubMed] [Google Scholar]
- Atherton, P. J. , Kumar, V. , Selby, A. L. , Rankin, D. , Hildebrandt, W. , Phillips, B. E. , Williams, J. P. , Hiscock, N. , & Smith, K. (2017). Enriching a protein drink with leucine augments muscle protein synthesis after resistance exercise in young and older men. Clinical Nutrition, 36(3), 888–895. [DOI] [PubMed] [Google Scholar]
- Beals, J. W. , Skinner, S. K. , McKenna, C. F. , Poozhikunnel, E. G. , Farooqi, S. A. , van Vliet, S. , Martinez, I. G. , Ulanov, A. V. , Li, Z. , Paluska, S. A. , & Burd, N. A. (2018). Altered anabolic signalling and reduced stimulation of myofibrillar protein synthesis after feeding and resistance exercise in people with obesity. The Journal of Physiology, 596(21), 5119–5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biolo, G. , Maggi, S. P. , Williams, B. D. , Tipton, K. D. , & Wolfe, R. R. (1995). Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. American Journal of Physiology‐Endocrinology and Metabolism, 268(3), E514–E520. [DOI] [PubMed] [Google Scholar]
- Biolo, G. , Tipton, K. D. , Klein, S. , & Wolfe, R. R. (1997). An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. American Journal of Physiology‐Endocrinology and Metabolism, 273(1), E122–E129. [DOI] [PubMed] [Google Scholar]
- Bohé, J. , Low, A. , Wolfe, R. R. , & Rennie, M. J. (2003). Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: A dose‐response study. The Journal of Physiology, 552(1), 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boirie, Y. , Gachon, P. , & Beaufrere, B. (1997). Splanchnic and whole‐body leucine kinetics in young and elderly men. The American Journal of Clinical Nutrition, 65(2), 489–495. [DOI] [PubMed] [Google Scholar]
- Borack, M. S. , Reidy, P. T. , Husaini, S. H. , Markofski, M. M. , Deer, R. R. , Richison, A. B. , Lambert, B. S. , Cope, M. B. , Mukherjea, R. , Jennings, K. , Volpi, E. , & Rasmussen, B. B. (2016). Soy‐dairy protein blend or whey protein isolate ingestion induces similar postexercise muscle mechanistic target of rapamycin complex 1 signaling and protein synthesis responses in older men. The Journal of Nutrition, 146(12), 2468–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen, L. , & Phillips, S. M. (2011). Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutrition & Metabolism, 8(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook, M. , Scaife, P. , Bass, J. J. , Cegielski, J. , Watanabe, S. , Wilkinson, D. J. , Smith, K. , Phillips, B. E. , & Atherton, P. J. (2021). A collagen hydrolysate/milk protein‐blend stimulates muscle anabolism equivalently to an isoenergetic milk protein‐blend containing a greater quantity of essential amino acids in older men. Clinical Nutrition, 40(6), 4456–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari, S. , Phillips, B. E. , Wilkinson, D. J. , Limb, M. C. , Rankin, D. , Mitchell, W. K. , Kobayashi, H. , Greenhaff, P. L. , Smith, K. , & Atherton, P. J. (2015). Intake of low‐dose leucine‐rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. American Journal of Physiology. Endocrinology and Metabolism, 308(12), E1056–E1065. [DOI] [PubMed] [Google Scholar]
- Burd, N. A. , Andrews, R. J. , West, D. W. D. , Little, J. P. , Cochran, A. J. R. , Hector, A. J. , Cashaback, J. G. A. , Gibala, M. J. , Potvin, J. R. , Baker, S. K. , & Phillips, S. M. (2012). Muscle time under tension during resistance exercise stimulates differential muscle protein sub‐fractional synthetic responses in men. The Journal of Physiology, 590(2), 351–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd, N. A. , Gorissen, S. H. , & Van Loon, L. J. (2013). Anabolic resistance of muscle protein synthesis with aging. Exercise and Sport Sciences Reviews, 41(3), 169–173. [DOI] [PubMed] [Google Scholar]
- Burd, N. A. , Gorissen, S. H. , van Vliet, S. , Snijders, T. , & van Loon, L. J. C. (2015). Differences in postprandial protein handling after beef compared with milk ingestion during postexercise recovery: A randomized controlled trial. The American Journal of Clinical Nutrition, 102(4), 828–836. [DOI] [PubMed] [Google Scholar]
- Burd, N. A. , Holwerda, A. M. , Selby, K. C. , West, D. W. , Staples, A. W. , Cain, N. E. , Cashaback, J. G. , Potvin, J. R. , Baker, S. K. , & Phillips, S. M. (2010). Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men. The Journal of Physiology, 588, 3119–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd, N. A. , West, D. W. D. , Moore, D. R. , Atherton, P. J. , Staples, A. W. , Prior, T. , Tang, J. E. , Rennie, M. J. , Baker, S. K. , & Phillips, S. M. (2011). Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. The Journal of Nutrition, 141(4), 568–573. [DOI] [PubMed] [Google Scholar]
- Burd, N. A. , Yang, Y. , Moore, D. R. , Tang, J. E. , Tarnopolsky, M. A. , & Phillips, S. M. (2012). Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolatev.Micellar casein at rest and after resistance exercise in elderly men. British Journal of Nutrition, 108(6), 958–962. [DOI] [PubMed] [Google Scholar]
- Burd, N. A. , Yang, Y. , Moore, D. R. , Tang, J. E. , Tarnopolsky, M. A. , & Phillips, S. M. (2012). Greater stimulation of myofibrillar protein synthesis with ingestion of whey protein isolate v. micellar casein at rest and after resistance exercise in elderly men. British Journal of Nutrition, 108(6), 958–962. [DOI] [PubMed] [Google Scholar]
- Burke, L. M. , Hawley, J. A. , Ross, M. L. , Moore, D. R. , Phillips, S. M. , Slater, G. R. , Stellingwerff, T. , Tipton, K. D. , Garnham, A. P. , & Coffey, V. G. (2012). Preexercise aminoacidemia and muscle protein synthesis after resistance exercise. Medicine and Science in Sports and Exercise, 44(10), 1968–1977. [DOI] [PubMed] [Google Scholar]
- Chan, A. , D'Souza, R. F. , Beals, J. W. , Zeng, N. , Prodhan, U. , Fanning, A. C. , Poppitt, S. D. , Li, Z. , Burd, N. A. , Cameron‐Smith, D. , & Mitchell, C. J. (2019). The degree of aminoacidemia after dairy Protein ingestion does not modulate the postexercise anabolic response in young men: A randomized controlled trial. Journal of Nutrition, 149(9), 1511–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchward‐Venne, T. A. , Breen, L. , di Donato, D. M. , Hector, A. J. , Mitchell, C. J. , Moore, D. R. , Stellingwerff, T. , Breuille, D. , Offord, E. A. , Baker, S. K. , & Phillips, S. M. (2014). Leucine supplementation of a low‐protein mixed macronutrient beverage enhances myofibrillar protein synthesis in young men: A double‐blind, randomized trial. The American Journal of Clinical Nutrition, 99(2), 276–286. [DOI] [PubMed] [Google Scholar]
- Churchward‐Venne, T. A. , Cotie, L. M. , MacDonald, M. J. , Mitchell, C. J. , Prior, T. , Baker, S. K. , & Phillips, S. M. (2014). Citrulline does not enhance blood flow, microvascular circulation, or myofibrillar protein synthesis in elderly men at rest or following exercise. American Journal of Physiology‐Endocrinology and Metabolism, 307(1), E71–E83. [DOI] [PubMed] [Google Scholar]
- Collins, J. , Maughan, R. J. , Gleeson, M. , Bilsborough, J. , Jeukendrup, A. , Morton, J. P. , Phillips, S. M. , Armstrong, L. , Burke, L. M. , Close, G. L. , Duffield, R. , Larson‐Meyer, E. , Louis, J. , Medina, D. , Meyer, F. , Rollo, I. , Sundgot‐Borgen, J. , Wall, B. T. , Boullosa, B. , … McCall, A. (2021). UEFA expert group statement on nutrition in elite football. Current evidence to inform practical recommendations and guide future research. British Journal of Sports Medicine, 55(8), 416. [DOI] [PubMed] [Google Scholar]
- Cuthbertson, D. , Smith, K. , Babraj, J. , Leese, G. , Waddell, T. , Atherton, P. , Wackerhage, H. , Taylor, P. M. , & Rennie, M. J. (2005). Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. American Journal of Physiology. Endocrinology and Metabolism, 19(3), 1–22. [DOI] [PubMed] [Google Scholar]
- Damas, F. , Phillips, S. M. , Libardi, C. A. , Vechin, F. C. , Lixandrão, M. E. , Jannig, P. R. , Costa, L. A. R. , Bacurau, A. V. , Snijders, T. , Parise, G. , Tricoli, V. , Roschel, H. , & Ugrinowitsch, C. (2016). Resistance training‐induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage. The Journal of Physiology, 594(18), 5209–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devries, M. , McGlory, C. , Bolster, D. R. , Kamil, A. , Rahn, M. , Harkness, L. , Baker, S. K. , & Phillips, S. M. (2018a). Protein leucine content is a determinant of shorter‐ and longer‐term muscle protein synthetic responses at rest and following resistance exercise in healthy older women: A randomized, controlled trial. The American Journal of Clinical Nutrition, 107(2), 217–226. [DOI] [PubMed] [Google Scholar]
- Devries, M. , McGlory, C. , Bolster, D. R. , Kamil, A. , Rahn, M. , Harkness, L. , Baker, S. K. , & Phillips, S. M. (2018b). Leucine, not Total protein, content of a supplement is the primary determinant of muscle protein anabolic responses in healthy older women. The Journal of Nutrition, 148(7), 1088–1095. [DOI] [PubMed] [Google Scholar]
- Dickinson, J. , Gundermann, D. M. , Walker, D. K. , Reidy, P. T. , Borack, M. S. , Drummond, M. J. , Arora, M. , Volpi, E. , & Rasmussen, B. B. (2014). Leucine‐enriched amino acid ingestion after resistance exercise prolongs myofibrillar protein synthesis and amino acid transporter expression in older men. The Journal of Nutrition, 144(11), 1694–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson, J. M. , Volpi, E. , & Rasmussen, B. B. (2013). Exercise and nutrition to target protein synthesis impairments in aging skeletal muscle. Exercise and Sport Sciences Reviews, 41(4), 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dideriksen, K. J. , Reitelseder, S. , Malmgaard‐Clausen, N. M. , Bechshoeft, R. , Petersen, R. K. , Mikkelsen, U. R. , & Holm, L. (2016). No effect of anti‐inflammatory medication on postprandial and postexercise muscle protein synthesis in elderly men with slightly elevated systemic inflammation. Experimental Gerontology, 83, 120–129. [DOI] [PubMed] [Google Scholar]
- Dideriksen, K. J. , Reitelseder, S. , Petersen, S. G. , Hjort, M. , Helmark, I. C. , Kjaer, M. , & Holm, L. (2011). Stimulation of muscle protein synthesis by whey and caseinate ingestion after resistance exercise in elderly individuals. Scandinavian Journal of Medicine & Science in Sports, 21(6), e372–e383. [DOI] [PubMed] [Google Scholar]
- Dreyer, H. , Drummond, M. J. , Pennings, B. , Fujita, S. , Glynn, E. L. , Chinkes, D. L. , Dhanani, S. , Volpi, E. , & Rasmussen, B. B. (2008). Leucine‐enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. American Journal of Physiology. Endocrinology and Metabolism, 294(2), E392–E400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond, M. J. , & Rasmussen, B. B. (2008). Leucine‐enriched nutrients and the regulation of mTOR signalling and human skeletal muscle protein synthesis. Current Opinion in Clinical Nutrition and Metabolic Care, 11(3), 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot, T. A. , Cree, M. G. , Sanford, A. P. , Wolfe, R. R. , & Tipton, K. D. (2006). Milk ingestion stimulates net muscle protein synthesis following resistance exercise. Medicine & Science in Sports & Exercise, 38(4), 667–674. [DOI] [PubMed] [Google Scholar]
- Farup, J. , Kjølhede, T. , Sørensen, H. , Dalgas, U. , Møller, A. B. , Vestergaard, P. F. , Ringgaard, S. , Bojsen‐Møller, J. , & Vissing, K. (2012). Muscle morphological and strength adaptations to endurance vs. resistance training. Journal of Strength and Conditioning Research, 26(2), 398–407. [DOI] [PubMed] [Google Scholar]
- Fujita, S. , Dreyer, H. C. , Drummond, M. J. , Glynn, E. L. , Cadenas, J. G. , Yoshizawa, F. , Volpi, E. , & Rasmussen, B. B. (2007). Nutrient signalling in the regulation of human muscle protein synthesis. The Journal of Physiology, 582(2), 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, S. , Dreyer, H. C. , Drummond, M. J. , Glynn, E. L. , Volpi, E. , & Rasmussen, B. B. (2009). Essential amino acid and carbohydrate ingestion before resistance exercise does not enhance postexercise muscle protein synthesis. Journal of Applied Physiology, 106(5), 1730–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorissen, S. H. , Burd, N. A. , Hamer, H. M. , Gijsen, A. P. , Groen, B. B. , & van Loon, L. (2014). Carbohydrate coingestion delays dietary protein digestion and absorption but does not modulate postprandial muscle protein accretion. The Journal of Clinical Endocrinology & Metabolism, 99(6), 2250–2258. [DOI] [PubMed] [Google Scholar]
- Gorissen, S. H. , Trommelen, J. , Kouw, I. W. K. , Kouw, I. W. K. , Pennings, B. , Pennings, B. , Wall, B. T. , Churchward‐Venne, T. A. , Horstman, A. M. H. , Koopman, R. , Burd, N. A. , Fuchs, C. J. , Dirks, M. L. , Res, P. T. , Senden, J. M. G. , Steijns, J. M. J. M. , de Groot, L. C. P. G. M. , Verdijk, L. B. , & van Loon, L. J. C. (2020). Protein type, protein dose, and age modulate dietary protein digestion and phenylalanine absorption kinetics and plasma phenylalanine availability in humans. The Journal of Nutrition, 150(8), 2041–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwin, J. A. , Church, D. D. , Hatch‐McChesney, A. , Howard, E. E. , Carrigan, C. T. , Murphy, N. E. , Wilson, M. A. , Margolis, L. M. , Carbone, J. W. , Wolfe, R. R. , Ferrando, A. A. , & Pasiakos, S. M. (2021). Effects of high versus standard essential amino acid intakes on whole‐body protein turnover and mixed muscle protein synthesis during energy deficit: A randomized, crossover study. Clinical Nutrition, 40(3), 767–777. [DOI] [PubMed] [Google Scholar]
- Hamer, H. M. , Wall, B. T. , Kiskini, A. , de Lange, A. , Groen, B. B. L. , Bakker, J. A. , Gijsen, A. P. , Verdijk, L. B. , & van Loon, L. J. C. (2013). Carbohydrate co‐ingestion with protein does not further augment post‐prandial muscle protein accretion in older men. Nutrition & Metabolism, 10(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans, W. J. H. , Fuchs, C. J. , Hendriks, F. K. , Houben, L. H. P. , Senden, J. M. , Verdijk, L. B. , & van Loon, L. J. C. (2022). Cheese ingestion increases muscle protein synthesis rates both at rest and during recovery from exercise in healthy, young males: A randomized parallel‐group trial. The Journal of Nutrition, 152(4), 1022–1030. [DOI] [PubMed] [Google Scholar]
- Hermans, W. J. H. , Senden, J. M. , Churchward‐Venne, T. A. , Paulussen, K. J. M. , Fuchs, C. J. , Smeets, J. S. J. , van Loon, J. J. A. , Verdijk, L. B. , & van Loon, L. J. C. (2021). Insects are a viable protein source for human consumption: From insect protein digestion to postprandial muscle protein synthesis in vivo in humans: A double‐blind randomized trial. The American Journal of Clinical Nutrition, 114(3), 934–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. , Altman, D. G. , Gøtzsche, P. C. , Jüni, P. , Moher, D. , Oxman, A. D. , Savovic, J. , Schulz, K. F. , Weeks, L. , Sterne, J. A. , Cochrane Bias Methods Group , & Cochrane Statistical Methods Group . (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ, 343, d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. T. , Chandler, J. , Cumpston, M. , Li, T. , Page, M. J. , & Welch, V. A. (2019). Cochrane handbook for systematic reviews of interventions. John Wiley & Sons. [Google Scholar]
- Holwerda, A. , Kouw, I. W. , Trommelen, J. , Halson, S. L. , Wodzig, W. K. , Verdijk, L. B. , & van Loon, L. J. (2016). Physical activity performed in the evening increases the overnight muscle protein synthetic response to Presleep protein ingestion in older men. Journal of Nutrition, 146(7), 1307–1314. [DOI] [PubMed] [Google Scholar]
- Janssen, I. (2010). Evolution of sarcopenia research. Applied Physiology, Nutrition, and Metabolism, 35(5), 707–712. [DOI] [PubMed] [Google Scholar]
- Katsanos, C. S. , Kobayashi, H. , Sheffield‐Moore, M. , Aarsland, A. , & Wolfe, R. R. (2006). A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. American Journal of Physiology‐Endocrinology and Metabolism, 291(2), E381–E387. [DOI] [PubMed] [Google Scholar]
- Kim, I.‐Y. , Schutzler, S. , Schrader, A. , Spencer, H. J. , Azhar, G. , Ferrando, A. A. , & Wolfe, R. R. (2016). The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults. American Journal of Physiology‐Endocrinology and Metabolism, 310(1), E73–E80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman, R. , Beelen, M. , Stellingwerff, T. , Pennings, B. , Saris, W. H. M. , Kies, A. K. , Kuipers, H. , & van Loon, L. J. C. (2007). Coingestion of carbohydrate with protein does not further augment postexercise muscle protein synthesis. American Journal of Physiology‐Endocrinology and Metabolism, 293(3), E833–E842. [DOI] [PubMed] [Google Scholar]
- Layman, D. K. (2002). Role of leucine in protein metabolism during exercise and recovery. Canadian Journal of Applied Physiology, 27(6), 646–662. [DOI] [PubMed] [Google Scholar]
- Luiking, Y. C. , Deutz, N. E. P. , Memelink, R. G. , Verlaan, S. , & Wolfe, R. R. (2014). Postprandial muscle protein synthesis is higher after a high whey protein, leucine‐enriched supplement than after a dairy‐like product in healthy older people: A randomized controlled trial. Nutrition Journal, 13(1), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlory, C. , Wardle, S. L. , Macnaughton, L. S. , Witard, O. C. , Scott, F. , Dick, J. , Bell, J. G. , Phillips, S. M. , Galloway, S. D. R. , Hamilton, D. L. , & Tipton, K. D. (2016). Fish oil supplementation suppresses resistance exercise and feeding‐induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men. Physiological Reports, 4(6), e12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendry, J. , Pérez‐López, A. , McLeod, M. , Luo, D. , Dent, J. R. , Smeuninx, B. , Yu, J. , Taylor, A. E. , Philp, A. , & Breen, L. (2016). Short inter‐set rest blunts resistance exercise‐induced increases in myofibrillar protein synthesis and intracellular signalling in young males. Experimental Physiology, 101(7), 866–882. [DOI] [PubMed] [Google Scholar]
- Mikkelsen, U. R. , Dideriksen, K. , Andersen, M. B. , Boesen, A. , Malmgaard‐Clausen, N. M. , Sørensen, I. J. , Schjerling, P. , Kjær, M. , & Holm, L. (2015). Preserved skeletal muscle protein anabolic response to acute exercise and protein intake in well‐treated rheumatoid arthritis patients. Arthritis Research & Therapy, 17(1), 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milan, A. , D'Souza, R. F. , Pundir, S. , Pileggi, C. A. , Thorstensen, E. B. , Barnett, M. P. , Markworth, J. F. , Cameron‐Smith, D. , & Mitchell, C. J. (2015). Older adults have delayed amino acid absorption after a high protein mixed breakfast meal. The Journal of Nutrition, Health & Aging, 19, 839–845. [DOI] [PubMed] [Google Scholar]
- Mitchell, C. J. , McGregor, R. , D'Souza, R. F. , Thorstensen, E. B. , Markworth, J. F. , Fanning, A. C. , Poppitt, S. D. , & Cameron‐Smith, D. (2015). Consumption of milk protein or whey protein results in a similar increase in muscle protein synthesis in middle aged men. Nutrients, 7(10), 8685–8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, W. K. , Phillips, B. E. , Williams, J. P. , Rankin, D. , Lund, J. N. , Wilkinson, D. J. , Smith, K. , & Atherton, P. J. (2015). The impact of delivery profile of essential amino acids upon skeletal muscle protein synthesis in older men: Clinical efficacy of pulse vs. bolus supply. American Journal of Physiology‐Endocrinology and Metabolism, 309(5), E450–E457. [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , Altman, D. G. , & The PRISMA Group . (2009). Preferred reporting items for systematic reviews and meta‐analyses. PLoS Medicine, 6(7), e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteyne, A. J. , Coelho, M. O. C. , Porter, C. , Abdelrahman, D. R. , Jameson, T. S. O. , Finnigan, T. J. A. , Stephens, F. B. , Dirks, M. L. , & Wall, B. T. (2020). Branched‐chain amino acid fortification does not restore muscle protein synthesis rates following ingestion of lower‐ compared with higher‐dose mycoprotein. The Journal of Nutrition, 150(11), 2931–2941. [DOI] [PubMed] [Google Scholar]
- Monteyne, A. J. , Coelho, M. O. C. , Porter, C. , Abdelrahman, D. R. , Jameson, T. S. O. , Jackman, S. R. , Blackwell, J. R. , Finnigan, T. J. A. , Stephens, F. B. , Dirks, M. L. , & Wall, B. T. (2020). Mycoprotein ingestion stimulates protein synthesis rates to a greater extent than milk protein in rested and exercised skeletal muscle of healthy young men: A randomized controlled trial. The American Journal of Clinical Nutrition, 112(2), 318–333. [DOI] [PubMed] [Google Scholar]
- Moore, D. R. , Robinson, M. J. , Fry, J. L. , Tang, J. E. , Glover, E. I. , Wilkinson, S. B. , Prior, T. , Tarnopolsky, M. A. , & Phillips, S. M. (2009). Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. The American Journal of Clinical Nutrition, 89(1), 161–168. [DOI] [PubMed] [Google Scholar]
- Moore, D. R. , Tang, J. E. , Burd, N. A. , Rerecich, T. , Tarnopolsky, M. A. , & Phillips, S. M. (2009). Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise. The Journal of Physiology, 587, 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, P. T. , Killer, S. , Macnaughton, L. , Dirks, M. , Witard, O. , Wall, B. T. , & Breen, L. (2022). The BASES expert statement on protein recommendations for athletes: Amount, type and timing. The Sport and Exercise Scientist, British Association of Sport and Exercise Sciences, 71, 8–9. [Google Scholar]
- Norton, L. E. , & Layman, D. K. (2006). Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. The Journal of Nutrition, 136(2), 533S–537S. [DOI] [PubMed] [Google Scholar]
- Norton, L. E. , Layman, D. K. , Bunpo, P. , Anthony, T. G. , Brana, D. V. , & Garlick, P. J. (2009). The leucine content of a complete meal directs peak activation but not duration of skeletal muscle protein synthesis and mammalian target of rapamycin signaling in rats. The Journal of Nutrition, 139(6), 1103–1109. [DOI] [PubMed] [Google Scholar]
- Oikawa, S. , Kamal, M. J. , Webb, E. K. , McGlory, C. , Baker, S. K. , & Phillips, S. M. (2020). Whey protein but not collagen peptides stimulate acute and longer‐term muscle protein synthesis with and without resistance exercise in healthy older women: A randomized controlled trial. The American Journal of Clinical Nutrition, 111(3), 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennings, B. , Boirie, Y. , Senden, J. M. G. , Gijsen, A. P. , Kuipers, H. , & van Loon, L. J. C. (2011). Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. The American Journal of Clinical Nutrition, 93(5), 997–1005. [DOI] [PubMed] [Google Scholar]
- Pennings, B. , Koopman, R. , Beelen, M. , Senden, J. M. G. , Saris, W. H. M. , & van Loon, L. J. C. (2011). Exercising before protein intake allows for greater use of dietary protein–derived amino acids for de novo muscle protein synthesis in both young and elderly men. The American Journal of Clinical Nutrition, 93(2), 322–331. [DOI] [PubMed] [Google Scholar]
- Phillips, S. M. (2016). The impact of protein quality on the promotion of resistance exercise‐induced changes in muscle mass. Nutrition & Metabolism, 13(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, S. M. , Tipton, K. D. , Aarsland, A. , Wolf, S. E. , & Wolfe, R. R. (1997). Mixed muscle protein synthesis and breakdown after resistance exercise in humans. American Journal of Physiology, 273(1 Pt 1), E99–E107. [DOI] [PubMed] [Google Scholar]
- Phillips, S. M. , & Van Loon, L. J. (2011). Dietary protein for athletes: From requirements to optimum adaptation. Journal of Sports Sciences, 29(Suppl 1), S29–S38. [DOI] [PubMed] [Google Scholar]
- Pinckaers, P. J. M. , Hendriks, F. K. , Hermans, W. J. H. , Goessens, J. P. B. , Senden, J. M. , Van Kranenburg, J. M. X. , Wodzig, W. K. H. W. , Snijders, T. , & Van Loon, L. J. C. (2022). Potato protein ingestion increases muscle protein synthesis rates at rest and during recovery from exercise in humans. Medicine and Science in Sports and Exercise, 54, 1572–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy, P. , Walker, D. K. , Dickinson, J. M. , Gundermann, D. M. , Drummond, M. J. , Timmerman, K. L. , Fry, C. S. , Borack, M. S. , Cope, M. B. , Mukherjea, R. , Jennings, K. , Volpi, E. , & Rasmussen, B. B. (2013). Protein blend ingestion following resistance exercise promotes human muscle protein synthesis. The Journal of Nutrition, 143(4), 410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitelseder, S. , Dideriksen, K. , Agergaard, J. , Malmgaard‐Clausen, N. M. , Bechshoeft, R. L. , Petersen, R. K. , Serena, A. , Mikkelsen, U. R. , & Holm, L. (2019). Even effect of milk protein and carbohydrate intake but no further effect of heavy resistance exercise on myofibrillar protein synthesis in older men. European Journal of Nutrition, 58(2), 583–595. [DOI] [PubMed] [Google Scholar]
- Rennie, M. J. , & Wilkes, E. A. (2005). Maintenance of the musculoskeletal mass by control of protein turnover: The concept of anabolic resistance and its relevance to the transplant recipient. Annals of Transplantation, 10(4), 31–34. [PubMed] [Google Scholar]
- Rieu, I. , Balage, M. , Sornet, C. , Giraudet, C. , Pujos, E. , Grizard, J. , Mosoni, L. , & Dardevet, D. (2006). Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. The Journal of Physiology, 575(1), 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples, A. W. , Burd, N. A. , West, D. W. , Currie, K. D. , Atherton, P. J. , Moore, D. R. , Rennie, M. J. , Macdonald, M. J. , Baker, S. K. , & Phillips, S. M. (2011). Carbohydrate does not augment exercise‐induced protein accretion versus protein alone. Medicine and Science in Sports and Exercise, 43(7), 1154–1161. [DOI] [PubMed] [Google Scholar]
- Symons, T. B. , Sheffield‐Moore, M. , Mamerow, M. M. , Wolfe, R. R. , & Paddon‐Jones, D. (2011). The anabolic response to resistance exercise and a protein‐rich meal is not diminished by age. Journal of Nutrition, Health, and Aging, 15(5), 376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, J. E. , Moore, D. R. , Kujbida, G. W. , Tarnopolsky, M. A. , & Phillips, S. M. (2009). Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. Journal of Applied Physiology, 107(3), 987–992. [DOI] [PubMed] [Google Scholar]
- Taylor, P. M. (2014). Role of amino acid transporters in amino acid sensing. The American Journal of Clinical Nutrition, 99(1), 223S–230S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman, K. L. , Lee, J. L. , Dreyer, H. C. , Dhanani, S. , Glynn, E. L. , Fry, C. S. , Drummond, M. J. , Sheffield‐Moore, M. , Rasmussen, B. B. , & Volpi, E. (2010). Insulin stimulates human skeletal muscle protein synthesis via an indirect mechanism involving endothelial‐dependent vasodilation and mammalian target of rapamycin complex 1 signaling. The Journal of Clinical Endocrinology & Metabolism, 95(8), 3848–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton, K. D. , Elliott, T. A. , Cree, M. G. , Aarsland, A. A. , Sanford, A. P. , & Wolfe, R. R. (2007). Stimulation of net muscle protein synthesis by whey protein ingestion before and after exercise. American Journal of Physiology—Endocrinology & Metabolism, 292(1), E71–E76. [DOI] [PubMed] [Google Scholar]
- Tipton, K. D. , Gurkin, B. E. , Matin, S. , & Wolfe, R. R. (1999). Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. The Journal of Nutritional Biochemistry, 10(2), 89–95. [DOI] [PubMed] [Google Scholar]
- van Vliet, S. , Beals, J. W. , Holwerda, A. M. , Emmons, R. S. , Goessens, J. P. , Paluska, S. A. , de Lisio, M. , van Loon, L. , & Burd, N. A. (2019). Time‐dependent regulation of postprandial muscle protein synthesis rates after milk protein ingestion in young men. Journal of Applied Physiology, 127(6), 1792–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Vliet, S. , Shy, E. L. , Abou Sawan, S. , Beals, J. W. , West, D. W. , Skinner, S. K. , Ulanov, A. V. , Li, Z. , Paluska, S. A. , Parsons, C. M. , Moore, D. R. , & Burd, N. A. (2017). Consumption of whole eggs promotes greater stimulation of postexercise muscle protein synthesis than consumption of isonitrogenous amounts of egg whites in young men. The American Journal of Clinical Nutrition, 106(6), 1401–1412. [DOI] [PubMed] [Google Scholar]
- Volpi, E. , Campbell, W. W. , Dwyer, J. T. , Johnson, M. A. , Jensen, G. L. , Morley, J. E. , & Wolfe, R. R. (2013). Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 68(6), 677–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi, E. , Mittendorfer, B. , Wolf, S. E. , & Wolfe, R. R. (1999). Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first‐pass splanchnic extraction. American Journal of Physiology‐Endocrinology and Metabolism, 277, E513–E520. [DOI] [PubMed] [Google Scholar]
- Wall, B. T. , Gorissen, S. H. , Pennings, B. , Koopman, R. , Groen, B. B. L. , Verdijk, L. B. , & van Loon, L. J. C. (2015). Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS One, 10(11), e0140903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall, B. T. , Hamer, H. M. , de Lange, A. , Kiskini, A. , Groen, B. B. L. , Senden, J. M. G. , Gijsen, A. P. , Verdijk, L. B. , & van Loon, L. J. C. (2013). Leucine co‐ingestion improves post‐prandial muscle protein accretion in elderly men. Clinical Nutrition, 32(3), 412–419. [DOI] [PubMed] [Google Scholar]
- Wall, B. T. , Morton, J. P. , & Van Loon, L. J. C. (2015). Strategies to maintain skeletal muscle mass in the injured athlete: Nutritional considerations and exercise mimetics. European Journal of Sport Science, 15(1), 53–62. [DOI] [PubMed] [Google Scholar]
- West, D. W. , Burd, N. A. , Coffey, V. G. , Baker, S. K. , Burke, L. M. , Hawley, J. A. , Moore, D. R. , Stellingwerff, T. , & Phillips, S. M. (2011). Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise. American Journal of Clinical Nutrition, 94(3), 795–803. [DOI] [PubMed] [Google Scholar]
- West, D. W. , Kujbida, G. W. , Moore, D. R. , Atherton, P. , Burd, N. A. , Padzik, J. P. , de Lisio, M. , Tang, J. E. , Parise, G. , Rennie, M. J. , Baker, S. K. , & Phillips, S. M. (2009). Resistance exercise‐induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. Journal of Physiology, 587(Pt 21), 5239–5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, S. , Monteyne, A. J. , Whelehan, G. , Abdelrahman, D. R. , Murton, A. J. , Finnigan, T. J. A. , Blackwell, J. R. , Stephens, F. B. , & Wall, B. T. (2022). Mycoprotein ingestion within or without its wholefood matrix results in equivalent stimulation of myofibrillar protein synthesis rates in resting and exercised muscle of young men. British Journal of Nutrition, 130, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson, D. , Bukhari, S. S. I. , Phillips, B. E. , Limb, M. C. , Cegielski, J. , Brook, M. S. , Rankin, D. , Mitchell, W. K. , Kobayashi, H. , Williams, J. P. , Lund, J. , Greenhaff, P. L. , Smith, K. , & Atherton, P. J. (2018). Effects of leucine‐enriched essential amino acid and whey protein bolus dosing upon skeletal muscle protein synthesis at rest and after exercise in older women. Clinical Nutrition, 37(6 Pt A), 2011–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witard, O. C. , Jackman, S. R. , Breen, L. , Smith, K. , Selby, A. , & Tipton, K. D. (2014). Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. American Journal of Clinical Nutrition, 99(1), 86–95. [DOI] [PubMed] [Google Scholar]
- Wolfson, R. L. , Chantranupong, L. , Saxton, R. A. , Shen, K. , Scaria, S. M. , Cantor, J. R. , & Sabatini, D. M. (2016). Sestrin2 is a leucine sensor for the mTORC1 pathway. Science, 351(6268), 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. , Breen, L. , Burd, N. A. , Hector, A. J. , Churchward‐Venne, T. A. , Josse, A. R. , Tarnopolsky, M. A. , & Phillips, S. M. (2012). Resistance exercise enhances myofibrillar protein synthesis with graded intakes of whey protein in older men. British Journal of Nutrition, 108(10), 1780–1788. [DOI] [PubMed] [Google Scholar]
- Zaromskyte, G. , Prokopidis, K. , Ioannidis, T. , Tipton, K. D. , & Witard, O. C. (2021). Evaluating the leucine trigger hypothesis to explain the postprandial regulation of muscle protein synthesis in young and older adults: A systematic review. Frontiers in Nutrition, 8, 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data S2.
Figure S1.
Data Availability Statement
The datasets generated and/or analyzed during the study implementation are available from the corresponding author upon request.


