Abstract
Background
Femoral neck stress fractures are rare fractures traditionally found in athletes and military personnel. There is limited literature on return to activity.
Objectives
To report return to activity rates and times, and long-term outcomes for femoral neck stress fractures reported in the literature. To examine the effects of bone metabolic dysfunction and surgical management on return to activity following FNSF.
Research design & methods
A systematic literature review of case reports and case series on adults with femoral neck stress fracture that were diagnosed by gross fracture line on X-ray or gold-standard diagnosis with MRI was conducted. Initial search was limited to articles published from January 1997 to Jan 2023 listed in Medline, Embase, and Scopus. Additional articles were manually added via search of retained paper sources. Patient demographics, fracture type, return to activity time, and surgical vs non-surgical treatment modality were collected. In addition, long-term outcomes and metabolic effects, if reported, were abstracted.
Results
A total of 40 case reports or case series were retained. 123 stress fractures of the femoral neck from 103 patients were compiled. Of the 103 patients, data on return to activity at least one year following treatment was available for 53 patients. 71% (37/53) of those with long-term follow-up information returned to full pre-injury activity. 24% (13/53) at long-term follow-up had functional recovery but did not return to pre-injury activity due to residual pain. 4% (3/53) had disabling pain. Metabolic workup information was available for 36 patients.
Conclusion
Long-term follow-up and return to activity information following FNSF treatment are not commonly reported. Based on the available data, outcomes appear benign with most returning to full activity. There is a clear need for standardization of follow-up periods and hip function measure after FNSF treatment. Additionally, a sizable proportion of FNSF occurred in a new population of low-activity individuals with abnormal bone metabolism, which warrants further exploration.
Keywords: Femoral neck stress fracture, Femoral neck, Stress fracture, Hip
1. Introduction
Femoral neck stress fractures (FNSF) are uncommon and are traditionally associated with military personnel and more recently endurance athletes who undergo rapid increase in training volume, specifically running or marching.1,2 FNSF are considered high-risk stress fractures due to the risk of malunion and osteonecrosis of the femoral head without treatment.3
Due to the rarity of FNSF, most studies on FNSF consist of small groups, and those investigating larger cohorts focus on military injuries. Indeed, fractures of the femoral neck account for only 2–5% of all stress fractures in athletes but may result in high morbidity and recovery times.4, 5, 6 Return to activity (RTA) and long-term outcomes after FNSF treatment have not been well-studied and a paucity of literature exists.2 Therefore, the aim of this review was to compile existing cases of FNSF in the literature to identify the impact of pre-injury activity level, bone metabolic dysfunction, and surgical management on return to activity time.
An improved understanding of long-term outcomes following FNSF treatment may guide return to activity expectations and patient counseling. In addition, looking at FNSF outcomes in the absence or presence of bone metabolic dysfunction may elucidate opportunities for primary, secondary, or tertiary prevention of femoral neck stress fractures.
2. Methods
2.1. Information sources & search strategy
Articles were collected from the databases Medline, Embase, and Scopus. Database entries published from January 1997–January 2023 were eligible for retention. Due to the low volume of articles on stress fractures of the femoral neck, keyword search yielded more relevant results compared to MeSH search strategy. Three searches were applied to each database. Keywords were A) “femoral neck stress fracture” with case reports filter applied, and B&C) “femoral neck stress fracture” with AND operator for “return” OR “recovery”. The reference lists of retained papers were searched for additional articles in order to find case reports or series that may not have appeared in the databases searched. All publication information of articles retained for this paper were recorded using bibliographic software. The review was registered in PROSPERO, ID: CRD42022368261.
2.2. Selection criteria & data collection process
The following inclusion criteria for yielded studies were applied: adults with femoral neck stress fractures diagnosed by gross fracture line on XR or with MRI, case reports or case series detailing return to activity period following femoral neck stress fracture, one year or greater follow-up after femoral neck stress fracture, and metabolic work-up following diagnosis of femoral neck stress fracture. Articles that met inclusion criteria were excluded if the full text was not available. Exclusion criteria included patients younger than 18 years, reports of femoral neck stress fracture with concomitant fractures not of the femoral neck, and lack of information regarding treatment or return to activity following femoral neck stress fracture. If an individual data point was missing in a case review that overall satisfied the inclusion criteria, the patient was dropped with no assumptions made. Two reviewers applied the selection criteria separately. A consensus was used to resolve any articles over which the reviewers disagreed, and a third reviewer was recruited if an agreement could not be reached.
2.3. Data items
Primary outcomes of interest were the number of patients for whom return to activity information was available, the number of patients who returned to full pre-injury activity, the number of patients who returned to less than pre-injury activity, and the number of patients who were unable to return to activity due to disabling pain. Bone metabolic workup information was desirable, but not required. Based on full text interpretation by 2 authors, long-term outcome was graded as 1) Disabling pain, 2) Return to function but not full pre-injury status, and 3) Return to pre-injury activity.
Additional variables for which data were recorded if available included patient age, sex, and BMI, activity level prior to injury, stress fracture type, initial return to activity time, and number of patients with metabolic workup. Activity level prior to injury was graded as Low, Moderate, or High based on article description. Based on imaging, article grading, and/or description of fracture each FNSF was recorded as either displaced or nondisplaced, complete or incomplete, and if applicable tension or compression sided. Immediate post-treatment return to activity time was recorded in weeks as category 1, 2, or 3 based on commonly reported milestones, defined as 1: Ambulation without walking aids or full weight-bearing, 2: Full weight bearing with loading activities such as stair climbing or marching, or 3: Full pre-injury activity. Metabolic workup information for each patient was recorded as dichotomous yes or no with a separate note detailing pertinent positive or negative findings.
2.4. Synthesis methods
A narrative synthesis was performed to synthesize the findings on femoral neck stress fracture return to activity. The data collected on return to activity from each case report and case series was heterogeneous. While we were able to record weeks, authors often reported different measures of return, necessitating the three different benchmarks of return (unassisted weight bearing, loading, and full return to pre-injury activity). Additionally, long-term recovery benchmarks were difficult to compare as we grouped author descriptions of patient outcomes into 3 categories (disabling pain, moderate limitation of activity, and full pre-injury activity). Even amongst patients with full pre-injury activity recovery, this method of recovery measurement is not adequately homogenous for meta-analysis as there was a wide range of baseline pre-injury activity levels. Furthermore, no validated scoring methods such as the Harris Hip Score were available in any text to objectively compare hip function between subjects. Narrative synthesis framework was guided by Synthesis Without Meta-Analysis reporting guidelines.7
Groups of interest were formed by pre-injury activity level, degree of long-term return to activity, and presence or absence of metabolic dysfunction. The aim was to see if there were any data features correlated with better or worse long-term return to activity. Similarly, we wanted to parse whether pre-injury activity level or presence of metabolic dysfunction exerted protective, inconsequential, or injurious effects on recovery outcomes.
2.5. Risk of bias/quality assessment
The JBI case report and case series checklists, which are widely used for the critical appraisal of case series and reports in systematic reviews, were used to assess bias. Questions posed by the checklists address confounding, selection, and information bias in addition to the importance of clear reporting and statistical analysis. Case report quality assessment parameters included clear reporting of demographic information, complete and linear history, diagnostic tests and differential, interventions, post-intervention state, reporting of harms, and takeaway lessons. Case series quality assessment parameters included clear reporting of demographic information, inclusion criteria, clinical history, standardized method of condition identification and measurement, complete inclusion across specified time periods, outcomes, and follow-up. An overall, study-level risk-of-bias was generated.
3. Results
3.1. Article selection
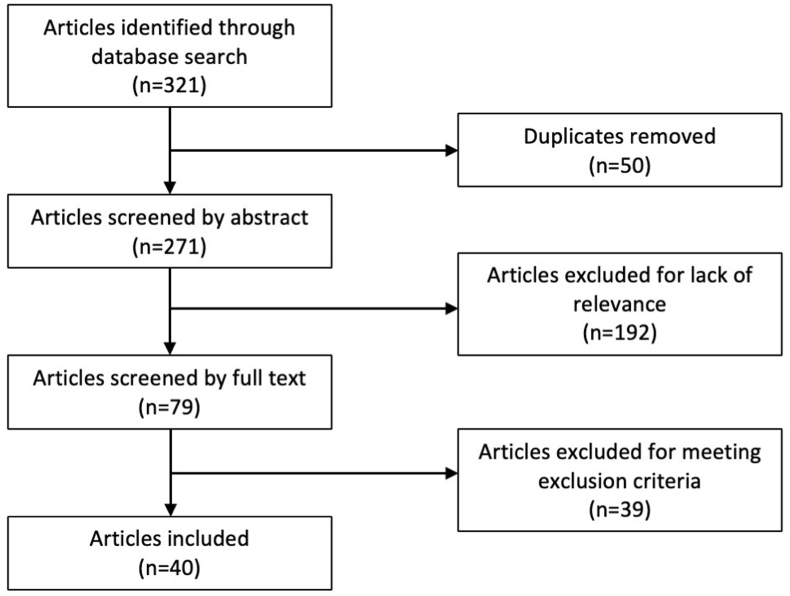
The literature search yielded 321 articles. 50 articles were removed as they were duplicates of other articles already found in the search. 271 articles were screened by title and abstract, upon which 192 were excluded. Full texts of the 79 remaining articles were reviewed. 41 articles were excluded after full text review due to meeting exclusion criteria or the following: the FNSF was iatrogenic, did not detail return to activity, did not report individual FNSFs or return to activity data, or lack of adherence to treatment. Two articles were sourced from reference search (Fig. 1). A total of 40 case reports or case series were retained. 123 stress fractures of the femoral neck from 103 patients were compiled. 74 of the patients were male and 29 of the patients were female.
Fig. 1.
Systematic review flowchart.
3.2. Demographics
The age range was 18–71 years. All 103 patients had information on activity level prior to injury. 82% (84/103) of the patients had high activity levels. Of those with high activity levels, 79% (66/84) were military personnel, 19% (16/84) were recreational endurance athletes with high training volume, and 2% (2/84) worked in demanding manual labor. 14% (14/103) of patients had low activity levels consisting of activities of daily living. Five patients had moderate activity levels consisting of light training.
Information on delay to diagnosis was available for 49.5% (51/103) patients, with an average period between symptom onset and seeking medical attention of 10.6 weeks; however, this period was highly variable and ranged between 0 and 162 weeks, or about 3 years.
14 patients presented with bilateral fractures of the femoral neck at initial evaluation. While most were treated with immediate surgery, one of these patients received conservative therapy. They progressed to displaced fracture with nonunion and ultimately required bilateral THA.8
BMI was available for 42% (43/103) patients. Average BMI was 23.5 ± 1.2 95% CI. Metabolic work-up information was available for 29 patients. 11 of those patients were male, and 18 were female. 13 patients had unremarkable bone metabolism markers. Nine patients had low or deficient levels of Vitamin D. Nine patients had osteopenia or osteoporosis diagnosed by DEXA during workup or as a previous diagnosis. Two female patients had histories of anorexia nervosa with >1 year of clinical recovery prior to injury. Two patients had received oral corticosteroid courses, and two patients were on multi-year antiviral regimens (Table 1).
Table 1.
Metabolic profiles of individual patients in high and low activity groups.
M – Male; F – Female; NR – Not reported.
| Patient # | Male/Female | Article | Activity level | Vit D Deficiency | Hypo-calcemia | High PTH | High ALP | Low PN1P | Osteo-malacia | Osteo-penia | Osteo-porosis | Steroid use | Hx of anorexia | Anti-viral use |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | Cichy 2012 | High | No | No | No | No | NR | NR | NR | NR | No | No | No |
| 2 | M | Jalan 2020 | High | No | No | No | No | NR | No | No | No | Yes | NR | NR |
| 3 | F | Biz 2017 | High | Yes | No | Yes | No | NR | NR | NR | NR | No | NR | NR |
| 4 | F | Biz 2017 | High | No | No | No | No | No | NR | NR | NR | No | NR | NR |
| 5 | F | Biz 2017 | High | Yes | No | Yes | No | NR | NR | NR | NR | No | NR | NR |
| 6 | M | Romero 2008 | High | NR | No | No | No | No | NR | NR | NR | NR | NR | NR |
| 7 | M | Nadwodny 2022 | High | No | No | NR | NR | NR | NR | NR | NR | NR | No | NR |
| 8 | F | Fonte 2018 | High | No | No | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| 9 | M | Santoso 2017 | High | NR | No | NR | No | NR | No | No | No | No | NR | NR |
| 10 | M | Aslam 2004 | High | No | No | NR | NR | NR | No | No | No | NR | NR | NR |
| 11 | F | Cooper 2005 | High | NR | NR | NR | NR | NR | NR | Yes | Yes | NR | No | NR |
| 12 | F | Lombardo 1982 | High | NR | No | NR | NR | NR | No | No | No | NR | NR | NR |
| 13 | F | Lombardo 1982 | High | NR | NR | NR | NR | NR | NR | NR | NR | Yes | NR | NR |
| 14 | F | Adulkasem 2020 | High | Yes | NR | NR | NR | NR | No | Yes | No | NR | NR | NR |
| 15 | F | Kanwat 2019 | Low | No | No | No | NR | NR | No | No | No | NR | NR | NR |
| 16 | F | Vedoya 2020 | Low | Yes | NR | NR | NR | NR | NR | NR | NR | NR | Yes | NR |
| 17 | F | Kim 2020 | Low | Yes | No | No | No | Yes | NR | NR | Yes | NR | NR | NR |
| 18 | F | Terlemez 2020 | Low | No | No | No | No | NR | NR | NR | NR | NR | NR | Yes |
| 19 | M | Chaganty 2019 | Low | No | NR | NR | No | No | NR | Yes | NR | No | NR | Yes |
| 20 | M | Narang 2019 | Low | Yes | Yes | Yes | Yes | No | Yes | NR | NR | Yes | No | NR |
| 21 | M | Lee 2016 | Low | No | No | No | Yes | NR | Yes | NR | NR | NR | NR | Yes |
| 22 | M | Takahara 2004 | Low | NR | NR | NR | NR | NR | No | No | No | NR | NR | NR |
| 23 | M | Nagao 2009 | Low | Yes | No | No | No | No | No | No | No | NR | NR | NR |
| 24 | F | Kalaci 2008 | Low | NR | Yes | NR | Yes | NR | NR | NR | Yes | No | NR | NR |
| 25 | F | Tomar 2020 | Low | Yes | NR | NR | NR | NR | Yes | NR | NR | NR | NR | NR |
| 26 | F | Xiaozuo 2016 | Low | NR | NR | NR | NR | NR | NR | NR | No | No | NR | NR |
3.3. Return to activity & follow-up
71% (37/53) of those with >1 year follow-up information had returned to full pre-injury activity. 24% (13/53) had functional recovery but did not return to full pre-injury activity due to pain or mobility limitations. 4% (3/53) had disabling pain (Table 2).8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46
Table 2.
Full list of FNSFs reviewed with characteristics. Blank cells indicate no data was available.
* = (1 - full weightbearing/2 - loading activities/3- full pre-injury activity); ** = (1 - disabling pain/2 - functional but with residual pain or discomfort/3 - full pre-injury activity).
| Source | Year | Subject # | Sex (M/F) | Age | BMI | Activity level | Symptoms prior to diagnosis (weeks) | Displaced? (D/ND) | Complete/In-complete (C/IC) | Tension/Compression (T/C) | Surgery? (Y/N) | Complications of surgery | Radiographic union evidence (months) | RTA (weeks) | RTA measure (1/2/3)* | Follow-up period (months) | >1 year follow-up outcomes (1/2/3)** | Metabolic work-up, if available |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fullerton | 1988 | 1 | M | 19 | High - military | ND | IC | C | N | 6 | 2 | >24 | 2 | |||||
| Fullerton | 1988 | 2 | M | 23 | High - military | ND | IC | C | N | 6 | 2 | >24 | 3 | |||||
| Fullerton | 1988 | 3 | M | 18 | High - military | ND | IC | C | N | 6 | 2 | >24 | 3 | |||||
| Fullerton | 1988 | 4 | M | 29 | High - military | ND | IC | C | N | 6 | 2 | >24 | 3 | |||||
| Fullerton | 1988 | 5 | M | 18 | High - military | ND | IC | C | N | >24 | ||||||||
| Fullerton | 1988 | 6 | F | 19 | High - military | ND | IC | C | N | 6 | 2 | >24 | 3 | |||||
| Fullerton | 1988 | 7 | M | 18 | High - military | ND | IC | C | N | 6 | 2 | >24 | ||||||
| Fullerton | 1988 | 8 | M | 30 | High - military | ND | IC | C | N | 6 | 2 | >24 | 3 | |||||
| Fullerton | 1988 | 9 | M | 23 | High - military | D | C | – | Y | >24 | ||||||||
| Fullerton | 1988 | 10 | M | 23 | High - military | ND | IC | C | N | 6 | 2 | >24 | 3 | |||||
| Fullerton | 1988 | 11 | M | 23 | High - military | ND | IC | C | N | 6 | 2 | >24 | ||||||
| Fullerton | 1988 | 12 | M | 20 | High - military | ND | IC | C | N | >24 | ||||||||
| Fullerton | 1988 | 13 | M | 18 | High - military | ND | IC | C | N | 6 | 2 | >24 | 2 | |||||
| Fullerton | 1988 | 14 | M | 18 | High - military | ND | IC | C | N | >24 | ||||||||
| Fullerton | 1988 | 15 | M | 20 | High - military | ND | IC | C | N | 6 | 3 | >24 | 2 | |||||
| Fullerton | 1988 | 16 | M | 25 | High - military | ND | IC | C | N | 6 | 3 | >24 | 2 | |||||
| Fullerton | 1988 | 17 | M | 32 | High - military | ND | IC | C | N | 6 | 3 | >24 | 3 | |||||
| Fullerton | 1988 | 18 | M | 18 | High - military | ND | IC | C | N | 6 | 3 | >24 | 3 | |||||
| Fullerton | 1988 | 19 | M | 18 | High - military | D | C | – | Y | Delayed Union | >24 | 2 | ||||||
| Fullerton | 1988 | 20 | M | 25 | High - military | ND | C | – | Y | 6 | 3 | >24 | ||||||
| Fullerton | 1988 | 21 | M | 20 | High - military | ND | IC | C | N | 6 | 3 | >24 | ||||||
| Fullerton | 1988 | 22 | M | 20 | High - military | ND | IC | C | N | >24 | ||||||||
| Fullerton | 1988 | 23 | M | 23 | High - military | ND | IC | C | N | >24 | ||||||||
| Fullerton | 1988 | 24 | M | 20 | High - military | D | C | – | Y | >24 | ||||||||
| Fullerton | 1988 | 25 | M | 19 | High - military | ND | IC | C | N | 6 | 3 | >24 | ||||||
| Fullerton | 1988 | 26 | M | 28 | High - military | ND | C | – | N | 6 | 2 | >24 | 1 | |||||
| Fullerton | 1988 | 27 | M | 28 | High - military | ND | IC | C | N | 6 | 3 | >24 | ||||||
| Fullerton | 1988 | 28 | M | 24 | High - military | ND | IC | C | N | 6 | 2 | >24 | ||||||
| Fullerton | 1988 | 29 | M | 18 | High - military | ND | IC | C | N | 6 | 2 | >24 | 2 | |||||
| Fullerton | 1988 | 30 | M | 33 | High - military | ND | IC | T | N | >24 | 2 | |||||||
| Fullerton | 1988 | 31 | M | 25 | High - military | ND | IC | C | N | >24 | 3 | |||||||
| Fullerton | 1988 | 32 | M | 26 | High - military | ND | IC | C | N | 6 | 2 | >24 | 2 | |||||
| Fullerton | 1988 | 33 | M | 26 | High - military | ND | IC | C | N | 6 | 2 | >24 | ||||||
| Fullerton | 1988 | 34 | F | 24 | High - military | ND | IC | C | N | 6 | 2 | >24 | ||||||
| Fullerton | 1988 | 35 | M | 24 | High - military | ND | IC | C | N | 6 | 2 | >24 | ||||||
| Fullerton | 1988 | 36 | M | 27 | High - military | ND | IC | C | N | 6 | 3 | >24 | ||||||
| Fullerton | 1988 | 37 | M | 29 | High - military | ND | IC | C | N | 6 | 2 | >24 | ||||||
| Fullerton | 1988 | 38 | M | 20 | High - military | ND | IC | C | N | 6 | 2 | >24 | ||||||
| Fullerton | 1988 | 39 | M | 18 | High - military | ND | IC | C | Y | >24 | 3 | |||||||
| Fullerton | 1988 | 40 | M | 19 | High - military | D | IC | T | Y | >24 | 3 | |||||||
| Fullerton | 1988 | 41 | M | 18 | High - military | ND | IC | C | N | 6 | 2 | >24 | ||||||
| Fullerton | 1988 | 42 | M | 18 | High - military | D | C | – | Y | >24 | 1 | |||||||
| Fullerton | 1988 | 43 | M | 19 | High - military | ND | IC | C | N | 6 | 2 | >24 | ||||||
| Fullerton | 1988 | 44 | M | 26 | High - military | ND | IC | C | N | 6 | 2 | >24 | ||||||
| Fullerton | 1988 | 45 | M | 18 | High - military | ND | IC | C | N | 6 | 2 | >24 | ||||||
| Fullerton | 1988 | 46 | M | 22 | High - military | ND | IC | C | N | 6 | 2 | >24 | 3 | |||||
| Fullerton | 1988 | 47 | M | 21 | High - military | D | C | C | Y | >24 | ||||||||
| Weinrich | 2022 | 48 | M | 38 | High - runner | 1 | ND | C | – | Y | 6 | 6 | 1 | 12 | 3 | |||
| Yoon | 2021 | 49 | M | 19 | 22.5 | High - military | 2 | ND | IC | C | Y | 10 | 3 | |||||
| Yoon | 2021 | 50 | M | 20 | 24.5 | High - military | 6 | ND | IC | T | N | 10 | 3 | |||||
| Yoon | 2021 | 51 | M | 21 | 22.2 | High - military | 0 | ND | IC | C | Y | 10 | 3 | |||||
| Yoon | 2021 | 52 | M | 20 | 21.7 | High - military | 1 | ND | C | – | N | 10 | 3 | |||||
| Yoon | 2021 | 53 | M | 20 | 23.9 | High - military | 1 | ND | IC | C | N | 10 | 3 | |||||
| Yoon | 2021 | 54 | M | 20 | 19.9 | High - military | 3 | ND | C | – | Y | 10 | 3 | |||||
| Yoon | 2021 | 55 | M | 19 | 24.9 | High - military | 4 | ND | C | – | Y | 10 | 3 | |||||
| Yoon | 2021 | 56 | M | 20 | 26.1 | High - military | 8 | ND | IC | C | N | 10 | 3 | |||||
| Yoon | 2021 | 56 | M | 26.1 | High - military | 8 | ND | IC | C | N | 10 | 3 | ||||||
| Yoon | 2021 | 57 | M | 19 | 24.9 | High - military | 8 | ND | IC | C | N | 10 | 3 | |||||
| Yoon | 2021 | 57 | M | 24.9 | High - military | 8 | ND | C | – | Y | 10 | 3 | ||||||
| Yoon | 2021 | 58 | M | 20 | 29.3 | High - military | 0 | D | C | – | Y | 10 | 3 | |||||
| Yoon | 2021 | 58 | M | 29.3 | High - military | 0 | ND | IC | C | Y | 10 | 3 | ||||||
| Yoon | 2021 | 59 | M | 18 | 20.2 | High - military | 8 | ND | C | C | Y | 10 | 3 | |||||
| Yoon | 2021 | 59 | M | 20.2 | High - military | 8 | ND | IC | C | N | 10 | 3 | ||||||
| Yoon | 2021 | 60 | M | 20 | 23.7 | High - military | 12 | ND | IC | T | N | 10 | 3 | |||||
| Avrahami | 2012 | 61 | F | 41 | High - endurance athlete | 16 | ND | IC | C | N | 8 | 3 | 4 | |||||
| Gerstmeyer | 2022 | 62 | M | 29 | 24.5 | High - running | 1 | D | C | T | Y | N | 6 | 1 | 18 | 3 | Subclinical hypothyroidism | |
| Cichy | 2012 | 63 | M | 23 | High - running | 1 | D | C | T | Y | N | 6 | 1 | Unremarkable | ||||
| Jalan | 2020 | 64 | M | 36 | High - running | 12 | D | C | T | Y | 12 | 1 | 24 | 3 | 10x IM nandrolone decoanate in 3 months prior to fracture; DEXA normal; unremarkable | |||
| Jalan | 2020 | 64 | M | 36 | High - running | 12 | ND | IC | C | N | 1 | 24 | 3 | 10x IM nandrolone decoanate in 3 months prior to fracture; DEXA normal; unremarkable | ||||
| Gurney | 2006 | 65 | F | 70 | Low - ADLs | 8 | ND | IC | C | N | N | 8 | 1 | 2 | ||||
| Biz | 2017 | 66 | F | 42 | 19.2 | High - running | 4 | ND | IC | C | N | N | 3 | 8 | 1 | 12 | 3 | Vit D deficiency, hyperPTH |
| Biz | 2017 | 67 | F | 28 | 21.2 | High - running | 2 | ND | C | – | N | Progression to complete fracture | 12 | 3 | PCOS, VitD low but not deficient | |||
| Biz | 2017 | 67 | F | 28 | 21.2 | High - running | ND | C | – | Y | N | 12 | 4 | 1 | PCOS, VitD low but not deficient | |||
| Biz | 2017 | 68 | F | 33 | 20.5 | High - running | 4 | ND | C | – | Y | N | 12 | 4 | 1 | 12 | 3 | PCOS, dysmenorrhea, previous hx of anorexia with amenorrhea, Vit D deficiency, hyperPTH |
| Biz | 2017 | 69 | F | 48 | Medium - running | 4 | D | C | – | Y | N | 12 | 7 | 1 | 12 | 3 | Vit D deficiency, hyperPTH | |
| Polacek | 2010 | 70 | F | 38 | High - running | D | C | – | Y | Y | 12 | 1 | 12 | 2 | ||||
| Polacek | 2010 | 71 | M | 39 | High - running | D | C | – | Y | Y | 12 | 1 | 12 | 3 | ||||
| Heig | 2022 | 72 | M | 29 | High - military | ND | IC | T | N | 3 | 6 | 1 | 4 | |||||
| Romero | 2008 | 73 | M | 19 | 22.3 | High - military | ND | IC | C | Y | N | 6 | 3 | 6 | Unremarkable | |||
| 2008 | 73 | M | 19 | 22.3 | High - military | D | C | – | Y | N | 6 | 1 | Unremarkable | |||||
| Nadowdny | 2022 | 74 | M | 38 | 30 | High - running | 1 | ND | IC | C | Y | N | 4 | 2 | 4 | Vit D, Ca normal | ||
| Kanwat | 2019 | 75 | F | 50 | Low - ADLs | 16 | D | C | – | Y | N | 0 | 1 | 12 | 3 | Vit D, Ca, PTH, bone density normal; 2 yrs post-menopausal | ||
| 75 | F | 50 | Low - ADLs | 16 | ND | IC | C | Y | N | |||||||||
| Konetsky | 2013 | 76 | F | 19 | High - military | 2 | ND | IC | C | Y | N | 20 | 3 | 5 | ||||
| Fonte | 2018 | 77 | F | 27 | High - military | 6 | ND | IC | C | N | N | 7 | 12 | 2 | 18 | 3 | Hgb, Fe, Ca WNL; 2 months amenorrhea | |
| Lamothe | 2018 | 78 | F | 23 | Moderate - running | 4 | ND | IC | C | N | 1 | 1 | 6 | |||||
| Vedoya | 2020 | 79 | F | 27 | Low - ADLs | 72 | ND | IC | C | Y | N | 33 | 1 | 13 | 3 | 4 year hx of anorexia nervosa, Vit D low, TP and albumin low | ||
| Vedoya | 2020 | 79 | F | 27 | Low - ADLs | 72 | ND | IC | C | Y | N | 33 | 1 | 13 | 3 | 4 year hx of anorexia nervosa, Vit D low, TP and albumin low | ||
| Kim | 2020 | 80 | F | 71 | Low - ADLs | 12 | ND | IC | T | Y | N | 0 | 1 | 3 | 4 year bisphosphonates; Vit D low, PN1P low | |||
| Terlemez | 2020 | 81 | F | 54 | Low - ADLs | 12 | ND | IC | C | N | 3 | 1 | 2 | 3 year tonofovir therapy, 16 mo ibandronic acid, hypophosphatemia, elevated alkphos, Vit D, PTH, & Ca WNL | ||||
| Santoso | 2017 | 82 | M | 37 | 23.8 | High - manual labor | 3 | ND | IC | C | N | 4 | 2 | 5 | CBC, BMP, PT/PTT, CRP, Ca, P, alkphos WNL | |||
| Santoso | 2017 | 82 | M | 37 | 23.8 | High - manual labor | 3 | ND | IC | C | N | 4 | 2 | 5 | CBC, BMP, PT/PTT, CRP, Ca, P, alkphos WNL | |||
| Aslam | 2004 | 83 | M | 29 | High - manual labor | 12 | D | C | – | Y | N | 3 | 12 | 2 | 12 | 3 | Blood labs normal, no metabolic/endocrine pathologies detected | |
| Hernigou | 2017 | 84 | F | 38 | 19.7 | Moderate - running | ND | IC | C | Y | N | 2 | 3 | 24 | Hx of anorexia nervosa w/1yr clinical recovery, osteopenia (T −1.9), low Ca | |||
| Hernigou | 2017 | 84 | F | 38 | 19.7 | ND | IC | C | Y | N | 2 | 3 | Hx of anorexia nervosa w/1yr clinical recovery, osteopenia (T −1.9), low Ca | |||||
| Petrin | 2016 | 85 | M | 27 | 26 | High | 4 | ND | IC | C | N | 4 | 1 | 4 | ||||
| Pongsamakthai | 2021 | 86 | M | 20 | 23.8 | High - military | 12 | D | C | – | Y | 5 | 6 | 1 | 24 | 2 | ||
| Pongsamakthai | 2021 | 86 | M | 20 | 23.8 | High - military | 12 | D | C | – | Y | N | 24 | 1 | ||||
| Ejnisman | 2013 | 87 | F | 56 | Moderate | 2 | ND | IC | C | N | 12 | 1 | 6 | Alkphos normal, XRs normal | ||||
| Chaganty | 2019 | 88 | M | 35 | 26.4 | Low - ADLs | 12 | D | C | – | Y | 3 | 2 | 1 | 6 | 2 | 2 year hx HIV on zidovudine; Vit D, phos, alkphos, renal, liver WNL, proximal femur osteopenia by XR | |
| Chaganty | 2019 | 88 | M | 35 | 26.4 | Low - ADLs | D | C | – | N - declined | 3 year hx HIV on zidovudine; Vit D, phos, alkphos, renal, liver WNL, proximal femur osteopenia by XR | |||||||
| Narang | 2019 | 89 | M | 56 | Low - ADLs | 36 | ND | IC | C | N | 0 | 1 | 14 | 3 | Vit D deficiency, low Ca, PTH elevated, high P1NP, alkphos elevated, osteomalacia (T −3.5), oral corticosteroid courses | |||
| Diwanje | 2007 | 90 | F | 35 | Moderate - running | 6 | D | C | – | Y | N | 1.5 | 12 | 1 | 31 | 3 | ||
| Diwanje | 2007 | 91 | F | 29 | Moderate -walking | 2 | D | C | – | Y | N | 12 | 1 | 60 | 3 | |||
| Lee | 2016 | 92 | M | 43 | Low - ADLs | 12 | ND | C | – | Y | N | 6 | 1 | 14 | 3 | Hypophosphatemia, hypokalemia, VitD WNL, thyroid WNL, elevated alkphos, elevated osteocalcin, osteoporosis (T −4.69 LS) | ||
| Takahara | 2004 | 93 | M | 30 | 25.8 | Low - ADLs | 1 | ND | IC | C | N | 6 | 1 | 36 | 3 | BMD WNL, no lab abnormalities | ||
| Richardson | 2016 | 94 | F | 43 | High - running | 6 | D | C | – | Y | 6 | 1 | 3 | 2 | FTM on 2 years of testosterone therapy | |||
| Cooper | 2005 | 95 | F | 18 | 20.6 | High - running | ND | IC | C | N | 6 | 1 | 15 | 3 | 8 month amenorrhea, osteoporosis/osteopenia (T −2.8 LS, −1.4 femur neck) | |||
| Nagao | 2009 | 96 | M | 36 | 18.5 | Low - ADLs | 8 | ND | IC | T | N | 16 | 1 | 24 | 2 | alcohol consumption (1.5L/d beer); VitD deficiency, low BMD by DEXA; Ca, Phos, PTH, osteocalcin, P1NP normal | ||
| Nagao | 2009 | 96 | M | 36 | 18.5 | Low - ADLs | D | IC | T | N | 16 | 1 | alcohol consumption (1.5L/d beer); VitD deficiency, low BMD by DEXA; Ca, Phos, PTH, osteocalcin, P1NP normal | |||||
| Nagao | 2009 | 96 | M | 36 | 18.5 | Low - ADLs | D | C | – | Y | Nonunion, focal osteonecrosis | 12 | 1 | alcohol consumption (1.5L/d beer); VitD deficiency, low BMD by DEXA; Ca, Phos, PTH, osteocalcin, P1NP normal | ||||
| Nagao | 2009 | 96 | M | 36 | 18.5 | Low - ADLs | D | C | – | Y | Nonunion, focal osteonecrosis | 12 | 1 | alcohol consumption (1.5L/d beer); VitD deficiency, low BMD by DEXA; Ca, Phos, PTH, osteocalcin, P1NP normal | ||||
| Kalaci | 2008 | 97 | F | 18 | 24.6 | Low - ADLs | 12 | ND | IC | C | Y | 6 | 1 | 6 | low BMD by DEXA (T −3.8), increased alkphos, hypocalcemia | |||
| Kalaci | 2008 | 97 | F | 18 | 24.6 | Low - ADLs | 12 | ND | IC | C | Y | 6 | 1 | 6 | low BMD by DEXA (T −3.8), increased alkphos, hypocalcemia | |||
| Tomar | 2020 | 98 | F | 58 | 36.6 | Low - ADLs | 162 | D | C | – | Y | Nonunion | 6 | 1 | 18 | 1 | osteomalacia, Vit D deficiency | |
| Tomar | 2020 | 98 | F | 58 | 36.6 | Low - ADLs | D | C | C | Y | 12 | 1 | 3 | osteomalacia, Vit D deficiency | ||||
| Tomar | 2020 | 98 | F | 58 | 36.6 | Low - ADLs | D | C | T | Y | 12 | 1 | osteomalacia, Vit D deficiency | |||||
| Lombardo | 1982 | 99 | F | 40 | High - running | 4 | D | C | T | Y | N | 28 | 3 | 36 | 3 | WNL | ||
| Lombardo | 1982 | 100 | F | 24 | High - running | 0 | ND | IC | C | N | N | 6 | 1 | 24 | 3 | 1 year daily dexamethasone use | ||
| Adulkasem | 2020 | 101 | F | 61 | High - running | 1 | D | C | T | Y | N | 3 | 12 | 1 | Vit D deficiency, osteopenia | |||
| Katsougrakis | 2016 | 102 | F | 28 | 19.8 | High - running | 0 | ND | IC | C | N | N | 2 | 8 | 1 | 3 | ||
| Xiaozuo | 2016 | 103 | F | 59 | 26.57 | Low - ADLs | 8 | D | C | – | Y | 12 | 1 | 12 | 3 | Unremarkable | ||
| Xiaozuo | 2016 | 103 | F | 59 | 26.57 | Low - ADLs | 8 | ND | C | – | N | 12 | 1 | Unremarkable |
Mean return to full weight-bearing for conservative versus surgical treatment of FNSF was 7.5 ± 2.4 weeks and 10.3 ± 2.8 weeks (95% CI, p = 0.21), respectively. Mean return to full pre-injury activity for conservative versus surgical treatment was 7.7 and 10.5 weeks (p = 0.002), respectively (Table 3).
Table 3.
Return to activity mean with 95% CI for conservative treatment and surgical treatment patients. N is number of fractures reported.
| Measure | Surgery Mean (weeks) [N] | Conservative Mean (weeks) [N] |
|---|---|---|
| Full weight-bearing | 10.3 ± 2.8 [29] | 7.5 ± 2.4 [15] |
| Loading activities | 8 [2] | 6.1 ± 0.5 [27] |
| Full pre-injury activity | 10.5 ± 0.8 [13] | 7.7 ± 1.0 [15] |
The average length of follow-up including those with <1 year follow up duration extended to a mean of 17.7 months, with the most common follow-up period of 24 months but several as short as 2–3 months. Follow-up of greater than one year was available for 49.5% (51/103) of patients. Of these patients, three patients had disabling pain (Table 4).19,32,43 13 patients had residual pain with activity, while the remaining 37 patients resumed full, pre-injury activity.
Table 4.
Fracture characterization of the 3 patients with disabling pain on >1 year follow-up. Blank cells indicate no data was available.
| Sex | Age | Baseline activity | Displaced/Nondisplaced | Complete/Incomplete | Surgery? | Complications | Follow-up (months) | Metabolic |
|---|---|---|---|---|---|---|---|---|
| F | 58 | Low | Displaced | Complete | Yes | Nonunion | 18 | osteomalacia, Vit D deficiency |
| M | 28 | High | Nondisplaced | Complete | No | >24 | ||
| M | 18 | High | Displaced | Complete | Yes | >24 |
3.4. Study characteristics & risk of bias in studies
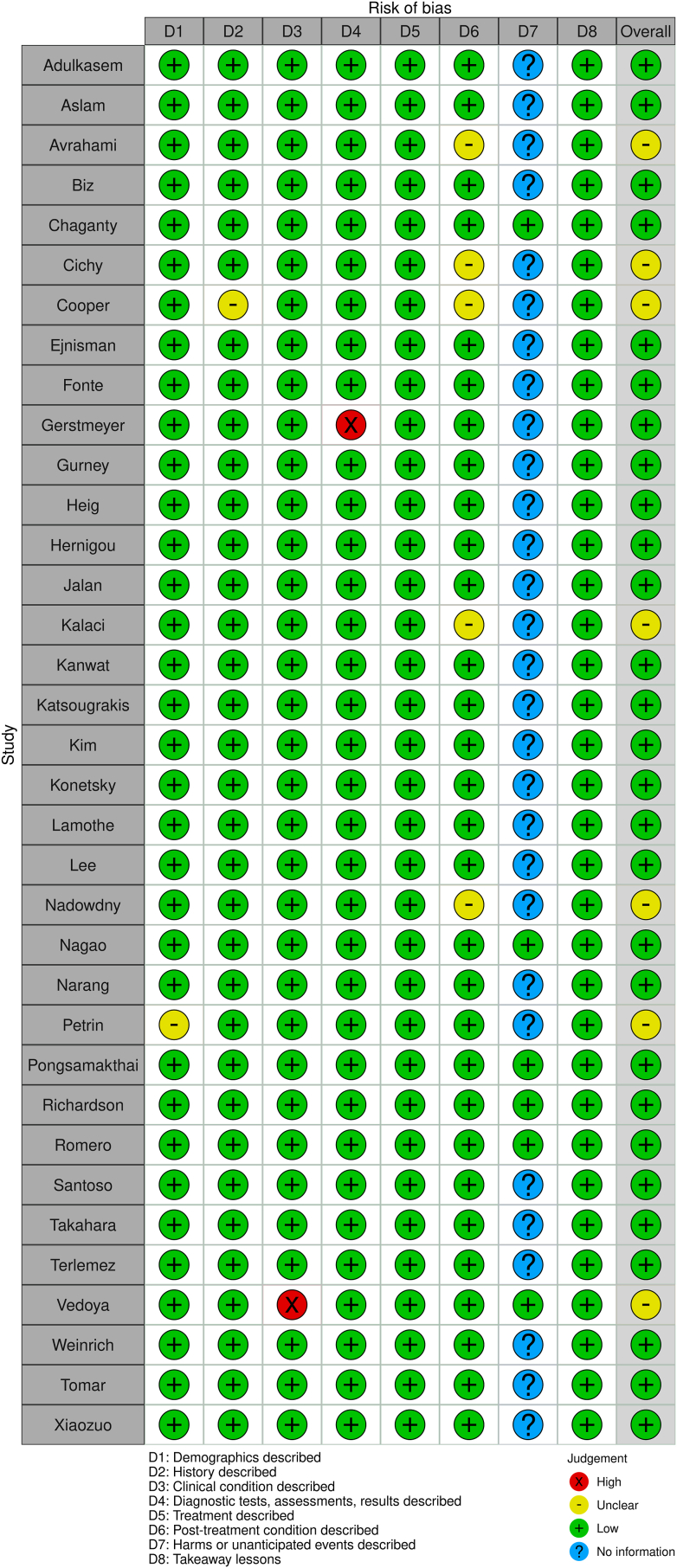
Risk of bias was assessed using the JBI checklist for case reports and case series as applicable. Two master risk tables for case series and case reviews were constructed (Fig. 2, Fig. 3). Overall, the individual case reports satisfied most checklist items suggesting low risk of bias. The case series demonstrated overall higher risk of bias apart from Fullerton and Snowdy, which met all checklist requirements. Robvis tool was used to generate visual representation of risk assessments for both case series and case reports.47 The small sample size, heterogeneity of information included amongst case reports and series, and variable markers of RTA increase the risk of information bias. Additionally, the risk of selection bias remains present in terms of which cases authors decide to report. Considering the higher risk assessments in the case series included, and reasons mentioned above, the overall risk of bias for this review is high.
Fig. 2.
Master risk table for case reports.
Fig. 3.
Master risk table for case series.
4. Discussion
4.1. Risk factors
FNSFs are uncommon and high-risk bone stress injuries commonly associated with a rapid increase in activity such as in new military recruits or athletes increasing training intensity in preparation for a marathon.33,48 Aside from involvement of military personnel and endurance athletes,1 possible risk factors for development of FNSF include female sex, poor physical conditioning prior to exercise,6 low body weight or BMI,49 hip morphology factors,50,51 and decreased bone mineral density.52 Other risk factors that predispose to low bone mineral density such as amenorrhea/oligomenorrhea, calcitonin receptor mutations, and low vitamin D are also presumed to increase risk of FNSF.3,52 Due to the rarity of FNSF, most studies on FNSF consist of small groups, and those investigating larger cohorts focus on military injuries.1,19
Based on our results, the intuitive finding was observed: high-activity patient populations (e.g., military personnel and endurance athletes) represented a large proportion of FNSF cases—64% and 15%, respectively. These fractures are best conceptualized as fatigue fractures, which are the result of abnormal stress on normal bone. However, 14% of FNSFs in the review were observed in low-activity patient populations. These insufficiency fractures result from normal stress on abnormal bone. The similar percentage of FNSF included in this study between high-activity civilian populations and low-activity patients may suggest insufficiency fracture etiology makes up a more sizable proportion of FNSF than is currently represented in the literature.
Given the sizable number of bilateral fracture presentations (n = 14), it is additionally likely that one FNSF may predispose to fracture of the opposing femoral neck, whether from compensatory increased loading of normal bone, or secondary to decreased bone quality of both femoral necks.
4.2. Identification and classification practices
Across the 40 articles reviewed, MRI was the most common diagnostic study of choice for FNSF. Bone scintigraphy and X-ray radiography were also employed. No specific grading scale was preferred to characterize FNSF. Previously established grading scales such as the Arendt,53 Fullerton and Snowdy,19 Provencher,54 and Garden may describe FNSF severity55; however, most clinicians described the fracture in terms of complete or incomplete, displaced or non-displaced, and if applicable, compression or tension sided. Notably, functional hip scores were not used by any author to assess pre- or post-operative hip function.
4.3. Treatment by fracture type
Treatment-wise, compression-sided fractures spanning <50% of the femoral neck were treated conservatively. The conservative protocol generally consisted of 6 weeks strict non-weightbearing followed by gradual return to activity before attainment of full pre-injury activity; this is consistent with previous literature.56,57 In this review, the loading activities benchmark was obtained in a shorter period than both weight-bearing and full return to activity (Table 2). The articles reporting recovery by loading activity benchmark were predominantly military associated and may have required faster return to activity compared to civilians.
Patients with displaced, complete, and/or bilateral fractures received surgical intervention. One patient with bilateral non-displaced fractures who was treated conservatively progressed to displaced fractures, supporting the necessity of fixation for all bilateral FNSFs. Tension-type fractures were generally treated with surgery, but in some cases were treated conservatively. Older literature from Fullerton et al. and Provencher et al. promote conservative treatment,19,54 but more recently practice has moved towards fixation for all lateral tension-type FNSFs.51,57
4.4. Bone metabolism
Bone metabolism workups were often absent. In the FNSFs with bone metabolism workup, a clear difference between the metabolic profiles of high-activity and low-activity FNSF was apparent. Patients with low baseline physical activity who presented with FNSF had more metabolic disturbances, while patients with high baseline physical activity largely had normal laboratory studies.
From a pathophysiological perspective, FNSF in high-activity patients are true fatigue fractures due to excessive, repetitive loading of the femoral neck with normal bone metabolism. On the other hand, the abnormal bone metabolism in low-activity patients predisposes to a FNSF under normal, everyday loads. Specific conditions in the low-activity group included: a history of Vitamin D deficiency, osteopenia/osteoporosis, osteomalacia, anorexia (regardless of clinical recovery status), heavy alcohol consumption, and steroid use.
Interestingly, three out of the 12 low activity patients with metabolic workups were on long-term anti-retroviral therapies (ART). Although associated with loss of bone mass in the hip and femoral neck,58 ART is not a previously identified risk factor for FNSF.
4.5. Return to activity & long term follow-up
While time required for return to full weight-bearing did not show significant difference between the conservative and surgical treatment groups, the conservative treatment group's return to pre-injury activity was significantly shorter than the surgical group's RTA (p = 0.002). The RTA for the two different benchmarks were similar; however, time to weight-bearing was much more variable compared to time to pre-injury activity. This may reflect bias during the grading process by the reviewers, a difference in reporting methods depending on fracture severity, rapid progression from full weight-bearing to pre-injury activity, or other confounding variables which may affect RTA measures differently.
A minimum of 24 months following FNSF treatment is the recommended duration of follow-up to assess for delayed osteonecrosis of the femoral head.56 The average period of follow-up was 17.7 months in this review. While incomplete compression-sided fractures pose low risk of adverse outcomes and may require shorter follow-up duration, several articles report fewer than 12 month follow-up for FNSFs that required surgical treatment.25,28,29,38,39
The most common follow-up modalities were assessment of the patient's ability to walk and XR radiography. No functional hip scores were utilized to assess pre or post-treatment function.
Despite the heterogeneity in assessing long-term FNSF follow-up, our review revealed that most patients reached adequate return to activity. Only three out of 51 patients with >1 year follow-up in our study presented with chronic disabling pain. One of these patients was a military recruit with conservatively-treated tension-sided stress fracture.19 Another was a bilateral stress fracture treated conservatively which progressed.43 These two cases emphasize the importance of early surgical intervention to prevent poor long-term outcomes.
4.6. Limitations
Most limitations of our review can be attributed to the rarity of FNSF, requiring the analysis of case reports and case series. Both types of studies increase risk of selection bias by authors who selectively choose which patient cases to report to the medical community. The small sample size of FNSF cases and the even rarer presentation of poor outcomes also limit our data analysis and conclusions. Additionally, the subjective grading scheme of FNSF severity, return to activity duration, and quality of recovery is strongly liable to the author's interpretation of the patient—increasing case-wide heterogeneity and limiting the scope of our review's induction.
In the context of these limitations, this review supports the need for cohesive and standardized reporting on FNSF characterization and follow-up protocols. A more streamlined process in managing FNSF will enable greater clinical diagnostic accuracy, prevent poor outcomes, and predict patient recovery times.
One possible methodology to answer this standardization gap is utilizing the Harris Hip Score (HHS) to assess pre- and post-operative function after FNSF. The HHS would be a convenient diagnostic tool to accurately report a patient's FNSF morbidity and quantitatively assess the impact of FNSF treatment.
5. Conclusion
Overall, prospect of a full return to activity rates following both surgical and conservative treatment of FNSF appear high, with a low incidence of incomplete or nondisplaced fracture progression, a low incidence of malunion after surgery, and no cases of avascular necrosis in the 103 patients collected during review. Patients with incomplete compression-sided, non-displaced fractures returned to pre-injury activity significantly sooner than patients with fractures that required surgery. Long-term follow-up for these patients was relatively limited and should be conducted to the recommended 24 months following treatment, especially in patients with FNSF that required surgery. In the future, evaluation of recovery after FNSF treatment should include standardized assessment with a validating functional scoring scale, such as the Harris Hip Score.
The current state of the field highlights FNSFs in traditionally high-activity patient populations. This review identifies an unmet need to investigate the prevalence of FNSF in low-activity populations, especially in persons living with HIV + or chronic HBV infections on long-term ART. Our study suggests that low-activity patient populations with bone metabolism disorders are at risk of FNSF and require high clinical suspicion for FNSF upon presentation with hip pain. They may be at risk for poor healing after bone instrumentation and require medical treatment for bone metabolic dysfunction in addition to treatment for FNSF.
Funding
No funding was received to assist with the preparation of this manuscript.
Author contribution
Kristine Yang: Investigation, Data curation, Writing – Original draft preparation, Visualization; Project administration; Senthil Sambandam: Writing - Review and Editing; Michael Huo: Conceptualization, Writing - Review and Editing, Supervision.
Acknowledgements
None.
Footnotes
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jor.2023.07.023.
Contributor Information
Kristine Yang, Email: kristine.yang@utsouthwestern.edu.
Senthil Sambandam, Email: sambandamortho@gmail.com.
Matthew J. Yan, Email: matthewyan@mednet.ucla.edu.
Michael Huo, Email: michael.huo@utsouthwestern.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Rohena-Quinquilla I.R., Rohena-Quinquilla F.J., Scully W.F., Evanson J.R.L. Femoral neck stress injuries: analysis of 156 cases in a U.S. Military population and proposal of a new MRI classification system. AJR Am J Roentgenol. Mar 2018;210(3):601–607. doi: 10.2214/AJR.17.18639. [DOI] [PubMed] [Google Scholar]
- 2.Shaw K.A., Moreland C.M., Hunt T.J., Barkley C., O'Brien F., Jackson K.L. Femoral neck stress fractures in athletes and the military. J Bone Joint Surg Am. Mar 2 2022;104(5):473–482. doi: 10.2106/JBJS.21.00896. [DOI] [PubMed] [Google Scholar]
- 3.Fredericson M., Jennings F., Beaulieu C., Matheson G.O. Stress fractures in athletes. Top Magn Reson Imag. Oct 2006;17(5):309–325. doi: 10.1097/RMR.0b013e3180421c8c. [DOI] [PubMed] [Google Scholar]
- 4.DeFranco M.J., Recht M., Schils J., Parker R.D. Stress fractures of the femur in athletes. Clin Sports Med. Jan 2006;25(1):89–103. doi: 10.1016/j.csm.2005.08.003. ix. [DOI] [PubMed] [Google Scholar]
- 5.Hulkko A., Orava S. Stress fractures in athletes. Int J Sports Med. Jun 1987;8(3):221–226. doi: 10.1055/s-2008-1025659. [DOI] [PubMed] [Google Scholar]
- 6.Kupferer K.R., Bush D.M., Cornell J.E., et al. Femoral neck stress fracture in Air Force basic trainees. Mil Med. Jan 2014;179(1):56–61. doi: 10.7205/MILMED-D-13-00154. [DOI] [PubMed] [Google Scholar]
- 7.Campbell M., McKenzie J.E., Sowden A., et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. Jan 16 2020;368:l6890. doi: 10.1136/bmj.l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagao S., Ito K., Nakamura I. Spontaneous bilateral femoral neck fractures associated with a low serum level of vitamin D in a young adult. J Arthroplasty. Feb 2009;24(2) doi: 10.1016/j.arth.2008.01.309. 322 e1-4. [DOI] [PubMed] [Google Scholar]
- 9.Adulkasem N., Sangsin A., Rojanasthien S., Jingjit W. Femoral neck stress fracture in habitual exercise patient: a case report with literature reviews. Review. J Med Assoc Thail. 2020;103(9):943–947. doi: 10.35755/jmedassocthai.2020.09.11094. [DOI] [Google Scholar]
- 10.Aslam N., Gwilym S., Natarajan R. Femoral neck stress fracture in a sanitary worker. Eur J Emerg Med. Aug 2004;11(4):220–222. doi: 10.1097/01.mej.0000134727.10475.17. [DOI] [PubMed] [Google Scholar]
- 11.Avrahami D., Pajaczkowski J.A. Femoral neck stress fracture in a female athlete: a case report. J Chiropr Med. Dec 2012;11(4):273–279. doi: 10.1016/j.jcm.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biz C., Berizzi A., Crimi A., Marcato C., Trovarelli G., Ruggieri P. Management and treatment of femoral neck stress fractures in recreational runners: a report of four cases and review of the literature. Acta Biomed. Oct 18 2017;88(4S):96–106. doi: 10.23750/abm.v88i4-S.6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaganty S.S., James D. Bilateral sequential femoral neck stress fractures in young adult with HIV infection on antiretroviral therapy: a case report. World J Orthoped. Jun 18 2019;10(6):247–254. doi: 10.5312/wjo.v10.i6.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cichy B., Roche S.J., Wozniak A. Atypical femoral neck stress fracture in a marathon runner: a case report and literature review. Ir J Med Sci. Sep 2012;181(3):427–429. doi: 10.1007/s11845-010-0599-7. [DOI] [PubMed] [Google Scholar]
- 15.Cooper L.A., Joy E.A. Osteoporosis in a female cross-country runner with femoral neck stress fracture. Curr Sports Med Rep. Dec 2005;4(6):321–322. doi: 10.1097/01.csmr.0000306293.64954.93. [DOI] [PubMed] [Google Scholar]
- 16.Diwanji S.R., Kong I.K., Cho S.G., Seon J.K., Yoon T.R. Displaced stress fracture of the femoral neck treated by valgus subtrochanteric osteotomy: 2 case studies. Am J Sports Med. Sep 2007;35(9):1567–1570. doi: 10.1177/0363546507299241. [DOI] [PubMed] [Google Scholar]
- 17.Ejnisman L., Wajnsztejn A., Queiroz R.D., Ejnisman B. Unusual presentation of a femoral stress fracture. BMJ Case Rep. Jan 2 2013 doi: 10.1136/bcr-2012-007828. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fonte H., Rodrigues-Pinto R. Femoral neck stress fracture in a young female recruit: case report. SICOT J. 2018;4:16. doi: 10.1051/sicotj/2018011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fullerton L.R., Jr., Snowdy H.A. Femoral neck stress fractures. Am J Sports Med. 1988;16(4):365–377. doi: 10.1177/036354658801600411. Jul-Aug. [DOI] [PubMed] [Google Scholar]
- 20.Gerstmeyer J.R., Godolias P., Mempel E., Bernstorff M., Schildhauer T.A., Konigshausen M. [Femoral neck stress fracture in a young runner - a rare but severe injury] Sportverletz Sportschaden. Mar 2022;36(1):60–63. doi: 10.1055/a-1554-4309. Laterale Schenkelhalsstressfraktur eines 29-jahrigen Marathonlaufers - eine seltene und risikobehaftete Uberlastungsfraktur des Sportlers. [DOI] [PubMed] [Google Scholar]
- 21.Gurney B., Boissonnault W.G., Andrews R. Differential diagnosis of a femoral neck/head stress fracture. J Orthop Sports Phys Ther. Feb 2006;36(2):80–88. doi: 10.2519/jospt.2006.36.2.80. [DOI] [PubMed] [Google Scholar]
- 22.Heig T., Mombell K., Deafenbaugh B. Bilateral atypical tension-sided femoral neck stress fractures in a military recruit. Mil Med. Mar 28 2022;187(3-4):e527–e529. doi: 10.1093/milmed/usaa343. [DOI] [PubMed] [Google Scholar]
- 23.Hernigou J., Koulischer S., Maes R. Bilateral simultaneous femoral neck stress fracture despite clinical recovery from anorexia nervosa: a case report. JBJS Case Connect. 2017;7(1):e12. doi: 10.2106/JBJS.CC.16.00047. Jan-Mar. [DOI] [PubMed] [Google Scholar]
- 24.Jalan D., Rathore K.S., Elhence A., Kumar Yadav S., Maley D.K. Stress fracture of bilateral neck of femur in a healthy non-athletic young adult - a case report with review of literature. Cureus. Oct 27 2020;12(10) doi: 10.7759/cureus.11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalaci A., Yanat A.N., Sevinc T.T., Dogramaci Y. Insufficiency fractures of both femoral necks in a young adult caused by osteoporosis: a case report. Arch Orthop Trauma Surg. Aug 2008;128(8):865–868. doi: 10.1007/s00402-008-0647-1. [DOI] [PubMed] [Google Scholar]
- 26.Kanwat H., Mittal S., Trikha V., Malhotra R. Unusual bilateral neck of femur stress fracture in a healthy, non-athletic individual - ACase report and literature review. J Orthop Case Rep. 2019;9(2):90–93. doi: 10.13107/jocr.2250-0685.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katsougrakis I., Apostolopoulos A.P., Tross S.Z. Conservative management of a femoral neck stress fracture in a female athlete. A case report and review of the literature. Review. J Long-Term Effect Med Implants. 2016;26(1):7–12. doi: 10.1615/JLongTermEffMedImplants.2016011991. [DOI] [PubMed] [Google Scholar]
- 28.Kim K.K., Park Y.W., Kim T.H., Seo K.D. Atypical femoral neck fracture after prolonged bisphosphonate therapy. J Pathol Transl Med. Jul 2020;54(4):346–350. doi: 10.4132/jptm.2020.05.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konetsky M., Miller J., Tripp C. Femoral neck stress fracture. J Orthop Sports Phys Ther. Apr 2013;43(4):275. doi: 10.2519/jospt.2013.0407. [DOI] [PubMed] [Google Scholar]
- 30.Lamothe M.A., Elliott J.M., Chang A.H. Femoral neck stress fracture in a female runner. J Orthop Sports Phys Ther. Apr 2018;48(4):343. doi: 10.2519/jospt.2018.7479. [DOI] [PubMed] [Google Scholar]
- 31.Lee Y.S., Kim B.K., Lee H.J., Dan J. Pathologic femoral neck fracture due to fanconi syndrome induced by adefovir dipivoxil therapy for hepatitis B. Clin Orthop Surg. Jun 2016;8(2):232–236. doi: 10.4055/cios.2016.8.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lombardo S.J., Benson D.W. Stress fractures of the femur in runners. Am J Sports Med. 1982;10(4):219–227. doi: 10.1177/036354658201000406. Jul-Aug. [DOI] [PubMed] [Google Scholar]
- 33.Nadwodny J.P., Pujalte G., Bertasi T.G.O., Huff T. Intertrochanteric hip stress fracture in a male ultramarathon runner. BMJ Case Rep. Jan 13 2022;15(1) doi: 10.1136/bcr-2020-239594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narang R.K., Reid I. Osteomalacia in subtropical auckland. BMJ Case Rep. Jul 8 2019;12(7) doi: 10.1136/bcr-2019-229657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrin Z., Sinha A., Gupta S., Patel M.K. Young man with sudden severe hip pain secondary to femoral neck stress fracture. BMJ Case Rep. Oct 26 2016 doi: 10.1136/bcr-2016-216820. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polacek M., Smabrekke A. Displaced stress fracture of the femoral neck in young active adults. BMJ Case Rep. Oct 6 2010 doi: 10.1136/bcr.02.2010.2749. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pongsamakthai W., Sangkomkamhang T. Bilateral displaced femoral neck stress fractures treated with valgus subtrochanteric osteotomy: a case report and two-year follow-up. J Clin Orthop Trauma. Nov 2021;22 doi: 10.1016/j.jcot.2021.101575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richardson T., Grant M., Chandran P. A curious case of stress fracture in a transsexual athlete. BMJ Case Rep. Mar 31 2016 doi: 10.1136/bcr-2015-214110. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romero A.N., Kohart S.R. 19-year-old male adolescent with bilateral femoral neck stress fractures: a case report. Mil Med. Jul 2008;173(7):711–713. doi: 10.7205/milmed.173.7.711. [DOI] [PubMed] [Google Scholar]
- 40.Santoso A., Joo S.D., Lee D.H., Seol Y.J., Park K.S., Yoon T.R. Bilateral femoral neck stress fracture presented with unilateral symptoms in a shipman laborer: a case report. Hip Pelvis. Mar 2017;29(1):77–80. doi: 10.5371/hp.2017.29.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takahara K., Nakagawa H., Kamimura M., Hashidate H., Kawaguchi A., Uchiyama S. Unusual stress fracture of the femoral neck in a young adult not caused by excessive stress: a case report. J Orthop Sci. 2004;9(6):650–653. doi: 10.1007/s00776-004-0842-z. [DOI] [PubMed] [Google Scholar]
- 42.Terlemez R., Sonmez M.M., Hamidi A.A., Yilmaz F. Atypical femoral neck stress fracture in a human immunodeficiency virus-infected patient despite anti-osteoporotic treatment: a case report. Turk J Phys Med Rehabil. Sep 2020;66(3):364–367. doi: 10.5606/tftrd.2020.4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomar L., Govil G., Dhawan P. Bilateral femoral neck stress fracture in an obese middle-aged female with osteomalacia and coxa-vara managed by simultaneous bilateral total hip arthroplasty. Cureus. Nov 13 2020;12(11) doi: 10.7759/cureus.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vedoya S.P., Montero A., Del Sel H. Bilateral concomitant femoral neck stress fracture in a sedentary patient with anorexia nervosa. Trauma Case Rep. Jun 2020;27 doi: 10.1016/j.tcr.2020.100302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinrich L., Dahne M., Lindner T., Stockle U., Tsitsilonis S. Femoral neck stress fracture of a male, healthy marathon runner - case report and literature review. Z für Orthop Unfallchirurgie. Oct 2022;160(5):564–571. doi: 10.1055/a-1401-0375. Stressfraktur des Femurhalses eines mannlichen, gesunden Marathonlaufers - Casereport und Literaturreview. [DOI] [PubMed] [Google Scholar]
- 46.Yoon H.K., Ryu Y.K., Song D.G., Yoon B.H. Femoral neck stress fractures in south Korean male military recruits. Clin Orthop Surg. Mar 2021;13(1):24–29. doi: 10.4055/cios20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGuinness L.A., Higgins J.P.T. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. Jan 2021;12(1):55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 48.Neubauer T., Brand J., Lidder S., Krawany M. Stress fractures of the femoral neck in runners: a review. Res Sports Med. Jul-Sep 2016;24(3):185–199. doi: 10.1080/15438627.2016.1191489. [DOI] [PubMed] [Google Scholar]
- 49.Korvala J., Hartikka H., Pihlajamaki H., et al. Genetic predisposition for femoral neck stress fractures in military conscripts. BMC Genet. Oct 21 2010;11:95. doi: 10.1186/1471-2156-11-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuhn K.M., Riccio A.I., Saldua N.S., Cassidy J. Acetabular retroversion in military recruits with femoral neck stress fractures. Clin Orthop Relat Res. Mar 2010;468(3):846–851. doi: 10.1007/s11999-009-0969-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steele C.E., Cochran G., Renninger C., Deafenbaugh B., Kuhn K.M. Femoral neck stress fractures: MRI risk factors for progression. J Bone Joint Surg Am. Sep 5 2018;100(17):1496–1502. doi: 10.2106/JBJS.17.01593. [DOI] [PubMed] [Google Scholar]
- 52.Muldoon M.P., Padgett D.E., Sweet D.E., Deuster P.A., Mack G.R. Femoral neck stress fractures and metabolic bone disease. J Orthop Trauma. 2001;15(3):181–185. doi: 10.1097/00005131-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 53.Arendt E.A., Griffiths H.J. The use of MR imaging in the assessment and clinical management of stress reactions of bone in high-performance athletes. Clin Sports Med. Apr 1997;16(2):291–306. doi: 10.1016/s0278-5919(05)70023-5. [DOI] [PubMed] [Google Scholar]
- 54.Provencher M.T., Baldwin A.J., Gorman J.D., Gould M.T., Shin A.Y. Atypical tensile-sided femoral neck stress fractures: the value of magnetic resonance imaging. Am J Sports Med. Sep 2004;32(6):1528–1534. doi: 10.1177/0363546503262195. [DOI] [PubMed] [Google Scholar]
- 55.Garden R.S. Stability and union in subcapital fractures of the femur. J Bone Joint Surg Br. Nov 1964;46:630–647. [PubMed] [Google Scholar]
- 56.Robertson G.A., Wood A.M. Lower limb stress fractures in sport: optimising their management and outcome. World J Orthoped. Mar 18 2017;8(3):242–255. doi: 10.5312/wjo.v8.i3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robertson G.A., Wood A.M. Femoral neck stress fractures in sport: a current concepts review. Sports Med Int Open. Feb 2017;1(2):E58–E68. doi: 10.1055/s-0043-103946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vlot M.C., Grijsen M.L., Prins J.M., et al. Effect of antiretroviral therapy on bone turnover and bone mineral density in men with primary HIV-1 infection. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0193679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.