Abstract
Hyperventilation (HV) is a voluntary activity that causes changes in the neuronal firing characteristics noticeable in the electroencephalogram (EEG) signals. HV-related changes have been scribed to modulation of pO2/pCO2 blood contents. Therefore, an HV test is routinely used for highlighting brain abnormalities including those depending to neurobiological mechanisms at the basis of neurodegenerative disorders. The main aim of the present paper is to study the effectiveness of HV test in modifying the functional connectivity from the EEG signals that can be typical of a prodromal state of Alzheimer’s disease (AD), the Mild Cognitive Impairment prodromal to Alzheimer condition. MCI subjects and a group of age-matched healthy elderly (Ctrl) were enrolled and subjected to EEG recording during HV, eyes-closed (EC), and eyes-open (EO) conditions. Since the cognitive decline in MCI seems to be a progressive disconnection syndrome, the approach we used in the present study is the graph theory, which allows to describe brain networks with a series of different parameters. Small world (SW), modularity (M), and global efficiency (GE) indexes were computed among the EC, EO, and HV conditions comparing the MCI group to the Ctrl one. All the three graph parameters, computed in the typical EEG frequency bands, showed significant changes among the three conditions, and more interestingly, a significant difference in the GE values between the MCI group and the Ctrl one was obtained, suggesting that the combination of HV test and graph theory parameters should be a powerful tool for the detection of possible cerebral dysfunction and alteration.
Keywords: EEG, Graph theory, Hyperventilation, MCI
Introduction
Hyperventilation (HV) is defined as the condition of voluntary and frequent series of deep respiratory acts prolonged for about 5 minutes that lead to a significant alteration in the partial pressure of gases present in bloodstream [1]. In fact, during the period of HV, the partial pressure of carbon dioxide (pCO2) falls of a mean of 18 mmHg and the partial pressure of oxygen (pO2) rises of about 7 mmHg [2]. Additionally, because of the increase of the air exchange, HV induces respiratory alkalosis, hypocapnia, subsequent vasoconstriction, and changes in blood oxygenation [3]. For all these physiological events, HV causes changes also in the neuronal activity, which can be seen in the electroencephalographic (EEG) signals [3]. In particular, in clinical EEG, the HV test involves the forced breathing from 3 up to 5 minutes [4]. In healthy subjects, this procedure causes a diffuse slowing of the basic rhythm on the EEG [5, 6], although the mechanism at the basis of the origin of these changes in the EEG has not been fully elucidated yet. Consequently, the existing suppositions regarding the influence of HV on the EEG activity of the cerebral cortex are controversial.
According to some authors, the cause of EEG slowing occurring during HV—as revealed by the synchronization of theta power—is an impairment of cerebral circulation due to hypocapnia and associated acute alkalosis, which leads to an inadequate oxygen and glucose supply to the brain [1]. Other authors suppose that HV affects the brain electric signals both in humoral and reflex ways by means of the blood vessel chemoreceptors, which causes reticular brainstem system deactivation [7]. These two possible causes, in turn, lead to the modulation of the activity of the cerebral cortex.
For these reasons, the HV method is commonly used in clinical settings to diagnose dysfunctions of central nervous system in the EEG signals. Due to its “EEG activation procedure” definition, the HV test can be clearly used for highlighting some abnormalities occurring in the EEG signals [7]. This type of research and interpretation of the results will be essential and informative in evaluating and investigating neurobiological mechanisms of neurodevelopmental and neurodegenerative disorders in the worldwide population.
Therefore, it is nowadays crucial to find new experimental procedures and innovative biomarkers to quantify brain functions and alteration across brain areas. Because of this need and for its power to reveal possible cerebral dysfunction, the HV test can be a useful procedure to discover differences between physiological and pathological states.
Recently, one of these neurological conditions on which the scientific community is focusing is the Mild Cognitive Impairment (MCI), a clinical and neuropsychological state characterized by memory and/or other cognitive domain impairment although it does not yet fulfill the definition of dementia [8, 9]. Epidemiological research suggests that MCI could be prodromal to Alzheimer’s Disease (AD) based on the high rate of progression from this state to AD (about 50% of cases after a 3- to 5-year follow-up) [9, 10]. There is a huge need to intercept this prodromal condition to plan optimal and early therapeutic, organizational, and rehabilitative interventions but also to reduce the health and social costs for dementia management.
Among the few existing papers, in 2008, Ponomareva and collaborators studied the EEG alteration in AD patients during 3 minutes of HV, demonstrating as a huge synchronous activity of delta and theta appeared in the AD group compared to healthy subjects, indicating that AD patients can be more sensitive to stress development associated with the HV test [11].
However, most recent researches have focused their attention on the concept that the early identification of the MCI-prodromal-to-dementia condition seems to mainly rely on a progressive disconnection syndrome, and consequently, one of the approaches used to characterize brain networks, its connection, and disconnection is the so-called “graph theory” [12–16]. By means of the graph model, the brain is shaped as a network composed of nodes linked by edges [17]. The characteristics of the graph and thus of the brain networks are measurable through several parameters, such as clustering coefficient, characteristic path length, small world index, modularity, efficiency, and others [13, 18, 19].
Keeping in mind these evidences, in the present study, we employed the graph analysis on EEG data in order to characterize brain networks profiles of subjects diagnosed as MCI compared to the control groups during HV test, eyes-closed, and eyes-open conditions. To the best of our knowledge, this is the first time where the HV experimental procedure is explored in MCI subjects and the first time in which it is characterized by a series of measures deriving by graph theory.
Methods
Participants
Based on the Winblad 2004 and Petersen 2011 criteria [20, 21], a total of 22 MCI subjects (mean age 74.2 years (y) ± 1.5 standard error (SE); mean education 15.1 y ± 0.8 SE; mean Mini Mental State Examination (MMSE) 27.6 ± 0.4 SE) were recruited as volunteers. The inclusion criteria for amnesic MCI subjects were as follows: (i) neuropsychological evaluated objective memory impairment; (ii) normal activities of daily living and evidence of independent living as assessed by a formal questionnaire (IADL); and (iii) a clinical dementia rating score of 0.5. The exclusion criteria for amnesic MCI were as follows: (i) MCI subjects without objective memory deficits; (ii) AD, as diagnosed by NINCDS-ADRDA [22] and DSM IV criteria; (iii) evidence of concomitant extrapyramidal symptoms; (iv) evidence of concomitant dementia such as frontotemporal, vascular dementia, reversible dementias (including dementia of depression), fluctuations in cognitive performance, and/or features of mixed dementias; (v) clinical and indirect evidence of depression; (vi) other psychiatric diseases, epilepsy, drug addiction, alcohol dependence, and use of psychoactive drugs or drugs interfering with brain cognitive functions including acetylcholinesterase inhibitors; and (vii) current or previous uncontrolled systemic diseases or traumatic brain injuries.
11 healthy elderlies (mean age 65.4 y ± 2.9 SE; mean education 14.5 y ± 1.1 SE; mean Mini Mental State Examination (MMSE) 29 ± 0.4 SE) were also enrolled as a control group (Ctrl).
All subjects have been classified as right-handed in the Handedness Questionnaire. Each participant accepted an informed consent in accordance with the World Medical Association Code of Ethics (1997). Experimental procedures were conformed to the Declaration of Helsinki and national guidelines and approved by local Ethics Committee.
Data recordings and preprocessing
Electroencephalographic activity was recorded with a 31-channel system (Easycap, GmbH, Brain products). The electrodes were placed according to the Augmented International 10-20 system with the midfrontal Fpz electrode as reference. Their impedance was kept below 5 kΩ, and the frequency rate was set up at 5000 Hz. To monitor blinking and ocular movement, both vertical and horizontal EOG channels were used.
For each subject, the whole EEG recording session lasted approximately 15 minutes: 5 minutes of eyes-closed (EC), 5 minutes of hyperventilation (HV), and 5 minutes of eyes-open (EO). Instructions for voluntary HV were given according to the standard clinical protocol in EEG studies. More specifically, it was asked to the participants to increase the rate and depth of their breathing keeping their eyes closed. Recordings were performed in an electrically shielded, sound-damped, and dimly lit room with the participant seated on a comfortable armchair.
EEG data were processed using a home-made MATLAB software, based on EEGLAB toolbox (Swartz Center for Computational Neurosciences, La Jolla, CA, USA). EEG records were down sampled with a frequency of 512 Hz, and to extract data in the frequency range of 0.2 to 47 Hz, a band-pass FIR (finite impulse response) filter was applied [23–27]. Epochs of 2 seconds were extracted from the original EEG continuous data. Those with aberrant waveforms or artefactual activity were removed first by an expert visual inspection and then by using the Infomax ICA algorithms [28–31].
Functional connectivity of cortical source analysis
To estimate functional connectivity, time series of cortical electrical neuronal activity was computed using exact Low-Resolution Electromagnetic Tomography (eLORETA) [30] software on 84 Regions of Interest (ROIs) defined according to the Brodmann Areas (BAs), 42 BAs for each of the left and the right hemisphere. In fact, the eLORETA tool has received considerable validation from studies combining LORETA with other more established localization methods, such as functional Magnetic Resonance Imaging (fMRI) [32, 33], structural MRI [34], and Positron Emission Tomography (PET) [35–37]. Moreover, the eLORETA tomography has been validated as a correct source localization also by the 10-20 EEG montage and provided a good reproducibility and stability to study brain networks’ modulations [23, 38] Between all possible pairs of ROIs, for each of the seven independent EEG frequency bands (delta (2–4 Hz), theta (4–8 Hz), alpha 1 (8–10.5 Hz), alpha 2 (10.5–13 Hz), beta 1 (13–20 Hz), beta 2 (20–30 Hz), and gamma (30–45 Hz)) [39] and for each subject, intracortical Lagged Linear Connectivity was computed [40, 41]. In the following graph analysis, these values of connectivity will be used as weight to the brain graph of each subject.
Graph analysis
A graph is a mathematical representation of a complex system; it is represented by a set of nodes (vertices) and links (edges) between couples of nodes. The nodes of a graph could be mean by brain ROIs, while functional, anatomical, or effective connections between brain areas could be the graph edges. When the nodes are linked with connections of variable weights, the graph is a weighted; if the weights are obtained by the magnitudes of temporal correlations in activity, they are quantified by functional connections and those connections may occur also between pairs of regions that are not anatomically connected. In the current study, undirected and weighted networks were built. The nodes of the graph are the 84 BAs as described above while the edges were weighted by the lagged linear connectivity values between each pair of nodes [14].
For each graph, the following parameters were computed: small world (SW) index, modularity (M), and global efficiency (GE) parameters.
The SW index describes the balance between the local connectedness and the global integration of a network [42, 43]. It is defined as the ratio of the normalized clustering coefficient C, representative of local connectedness, and the normalized characteristic path length L, representative of global interconnectedness [38, 44, 45]. Here, the data normalization was obtained by dividing the values of C and L by the mean values obtained by the average measure of each parameter in all frequency bands. M parameter quantifies the tendency of division of a network into modules also called clusters or communities. Networks with high modularity have dense connections between the nodes within modules but sparse connections between nodes in different modules. In other words, M represents the degree to which the nodes of the same modules are correlated with each other but not with other modules. The M parameter also represents the degree to which the network may be subdivided into clearly delineated and nonoverlapping groups. An optimization algorithm is used to estimate the optimal modular structure for the given network. It is computed as
Here, lw is the sum of all weights in the network, estimated as lw = ∑i, j ∈ Nwij, while mi represents the module containing node lw. The term if mi = mj and 0 otherwise. The weights wij are associated to the links (i, j) [46].
The GE parameter represents the efficiency in information transmission between two nodes via multiple parallel paths. It is proportional to the inverse of the average minimum L [47, 48].
It is obtained as
Here, represents the efficiency ∈ij in the communication between vertices i and j defined as the inverse of with shortest distance dij [47].
Statistical evaluation
A statistical ANOVA design was addressed for each graph theory parameters (SW, M, and GE) within the factors Group (MCI, Ctrl), Condition (EO, EC, and HV), and Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, and gamma).
The normality of the data was tested using the Kolmogorov–Smirnov test, and the hypothesis of Gaussianity could not be rejected. Greenhouse and Geisser correction were used for the protection against a possible violation of the sphericity assumption in the repeated measure ANOVA. Besides, post hoc analysis with the Duncan’s test and significance level at 0.05 was performed.
Results
Small world
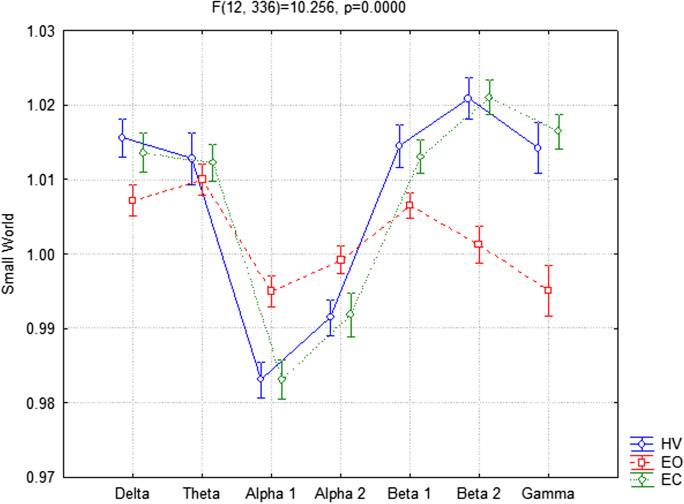
The ANOVA for the evaluation of the SW index showed significant interaction between the factors Condition (EC, EO, and HV) and Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, and gamma) (F(12,336) = 10.256, p < 0.001). In particular, the Duncan post hoc analysis showed that SW index exhibited a significant increase in delta (p = 0.050), beta 1 (p = 0.042), beta 2 (p < 0.001), and gamma (p < 0.001) and a significant decrease in alpha 1 (p < 0.001) and alpha 2 (p = 0.039) in the EC compared to EO condition. The same trends have been obtained for the HV versus the EO, showing an increment in delta (p = 0.007), beta 1 (p = 0.008), beta 2 (p < 0.001), and gamma (p < 0.001) and a decrement in alpha 1 (p < 0.001) and alpha 2 (p=0.018) (Fig. 1). Finally, as we expected from the above results, a post hoc test did not show significant differences between the HV compared to EC condition.
Fig. 1.
Small world (SW) index in EEG frequency bands between hyperventilation (HV), eyes-open (EO), and eyes-closed (EC) conditions considering all participants together
No significant interaction was found among all factors Group (MCI, Ctrl), Condition (EC, EO, and HV), and Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, and gamma) (p = 0.66).
Modularity
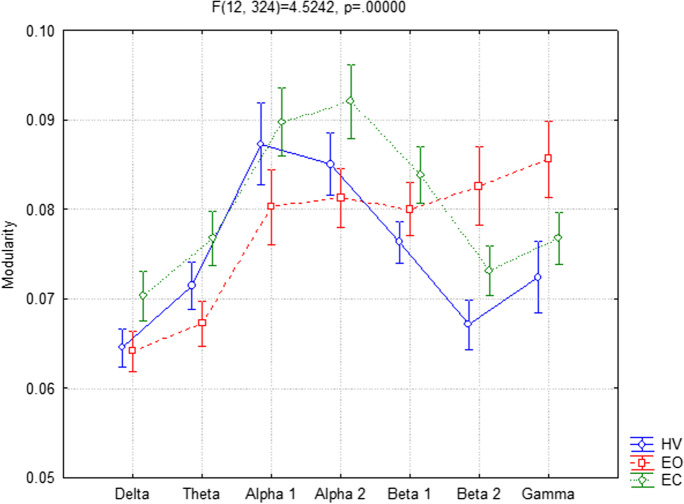
Concerning the M parameter, the ANOVA showed significant interaction (F(12,324) = 4.5242, p < 0.001) between the factors Condition (EC, EO, and HV) and Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, and gamma). In particular, the Duncan post hoc analysis showed that M values are significantly higher in EC conditions in theta (p = 0.012), alpha 1 (p < 0.001), and alpha 2 (p = 0.002) and significantly lower in beta 2 (p = 0.009) and gamma (p = 0.006) compared to EO. Furthermore, in HV versus the EO condition, M exhibited higher values in alpha 1 (p = 0.033) and lower values in beta 2 (p < 0.001) and gamma (p < 0.001). Finally, the post hoc analysis did not show significant differences between HV and EC (Fig. 2).
Fig. 2.
Modularity (M) parameter in EEG frequency bands between hyperventilation (HV), eyes-open (EO), and eyes-closed (EC) conditions considering all participants together
No significant interaction was found among all factors Group, Condition, and Band (p = 0.98).
Global efficiency
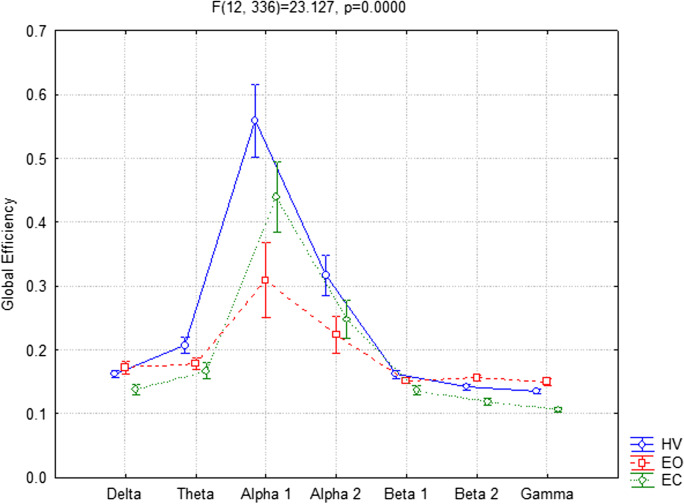
Referring to the GE parameter, the ANOVA showed significant interaction (F(12,336) = 23.137, p < 0.001) between the factors Condition (EC, EO, and HV) and Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, and gamma). More specifically, the Duncan post hoc analysis reveals that the GE values were significantly lower in delta (p = 0.016), beta 2 (p = 0.022), and gamma bands (p = 0.004) and significantly higher in alpha 1 band (p < 0.001) in EC compared to EO condition.
A significant increase of GE parameter in the alpha 1 (p < 0.001) and alpha 2 (p < 0.001) bands was also obtained in the HV compared to the EO.
Interestingly, the post hoc analysis revealed also significantly higher values in theta (p = 0.013), alpha 1 (p < 0.001), alpha 2 (p < 0.001), and gamma (p = 0.045) bands in the HV condition compared to the EC one (Fig. 3).
Fig. 3.
Global efficiency (GE) parameter in EEG frequency bands between hyperventilation (HV), eyes-open (EO), and eyes-closed (EC) conditions considering all participants together
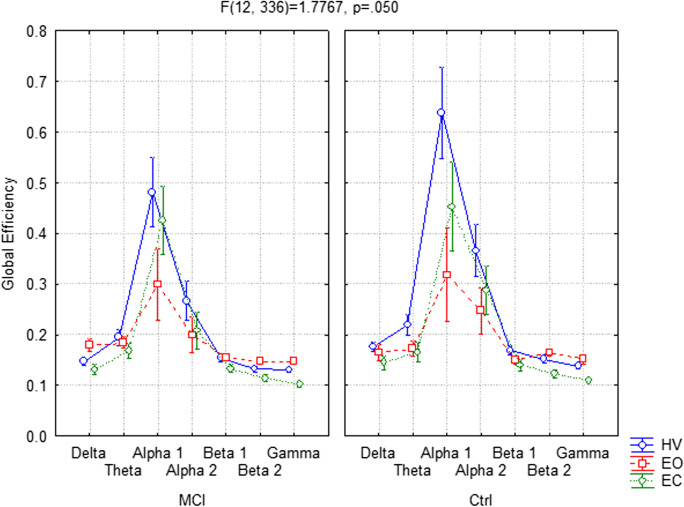
Furthermore, the ANOVA showed significant interaction (F(12,336) = 1.7767, p = 0.050) among all factors Group (MCI, Ctrl), Condition (EC, EO, and HV), and Band (delta, theta, alpha 1, alpha 2, beta 1, beta 2, and gamma) too.
Regarding the MCI group, the Duncan post hoc analysis reveals that the GE parameter exhibited significant lower values in delta (p = 0.050) band and higher values in alpha 1 (p < 0.001) band in the EC condition respect to the EO one. In the HV condition, significantly higher values were obtained in the alpha 1 (p < 0.001) and alpha 2 (p = 0.002) bands compared to the EO condition. The same statistically significant increment was obtained in the HV versus EC (alpha 1 (p = 0.009), alpha 2 (p = 0.006)) (Fig. 4A).
Fig. 4.
Global efficiency (GE) parameter in EEG frequency bands between hyperventilation (HV), eyes open (EO), and eyes closed (EC) conditions, respectively, in Mild Cognitive Impairment (MCI) group (A) and in the control (Ctrl) group (B)
Concerning the Ctrl group, with the Duncan post hoc analysis, significantly higher values in the alpha 1 (p < 0.001) and alpha 2 (p = 0.050) bands were obtained in the EC respect to the EO condition. By comparing HV versus EO, in the HV condition, a significantly higher values were obtained in theta (p = 0.049), alpha 1 (p < 0.001) and alpha 2 (p < 0.001) bands. Higher values of GE during HV in theta (p = 0.026), alpha 1 (p < 0.001), and alpha 2 (p < 0.001) bands were also showed in the respect to EC conditions (Fig. 4B).
Concerning the differences between MCI and Ctrl groups, the Duncan post hoc analysis showed significantly lower values of GE in alpha 1 (p < 0.001) and alpha 2 (p = 0.023) bands for MCI compared to Ctrl subjects.
Discussion
The high rate of conversion of MCI to dementia (usually of the AD type) suggests that the MCI is a possible prodromal condition to AD. So, there is an urgent need to find highly accessible test in order to early intercept this type of neurological disease to reduce the health and social costs for dementia management and to plan early therapeutic and rehabilitative interventions. In this scenario, the EEG and the HV task during EEG are a low-cost, noninvasive techniques and one of the most spread tests in the clinical routine that underlines neural alteration in pathological conditions and that could be used to characterize the MCI condition [2, 49].
Within this theoretical frame, the aim of the present study has been to investigate the networks’ differences between subjects diagnosed as MCI compared to Ctrl groups, on EEG recordings during HV, EC, and EO conditions. The EEG analyses have been conducted throughout the graph analysis, a novel theoretical approach that allows to study how the functionally specialized brain areas mutually interact within the frame of dynamic networks [14, 50]. Actually, hyperventilation might represent assort of “stress test” of neuronal networks as explored with the EEG.
From the obtained results, it has emerged that the SW index, M, and GE parameters showed significant changes among the three analyzed conditions (i.e., EC, EO, and HV), taking the two groups (MCI and Ctrl) together. More specifically, although the SW index and M parameter represent different network features, their values exhibited the same, rather overlapped, trend in HV and EC conditions, whereas significant differences have been observed between HV or EC conditions versus the EO one in all the frequency bands.
Probably the overlapped trends observed in the HV and EC conditions can be due to the modality in which the HV test was performed, namely, with subjects with eyes closed. In fact, several previous studies have demonstrated as the connectivity indexes and more specifically graph theory parameters are modulated by the transition from the open-eye condition to the closed-eye one [51, 52]. Recently, by means of connectivity indexes, Wang and collaborators (2022) demonstrated as the activity of Default Mode Network (DMN) is regulated by eye states; in particular, their results showed that, within this network, the effective connectivity, a measure aimed at accessing direct paths of intracortical causal information flow of oscillatory activity, was enhanced during the eye open state in beta band [53].
With graph parameters, Tan and colleagues (2013) demonstrated that by changing from EC to EO states, the Clustering coefficient decreased in both theta and alpha bands and the Path Lengths reduced in the alpha band [51]. The reduction of local connectivity in theta and alpha has been interpreted by the authors consequent to a sort of suppression induced by the “arousal effect” linked with the visual input, that played a connectivity inhibition role in EO state, whereas the increment of global measures in alpha suggested that the brain would be more “conductive” for promoting information exchanging and processing at global level, when the subject opens his eyes.
In line with the results of our study, Miraglia and collaborators (2016) showed that the EC condition have higher values of SW index in the high-frequency bands (beta 2 and gamma bands) and lower values in alpha bands compared to the EO state in healthy, MCI, and AD patients [52]. Additionally, in EC condition, the healthy group exhibited networks with lower SW index in alpha band compared with the EO condition, suggesting a sort of still preserved brain’s ability to react as rapidly and efficiently as normal when the subject is visually connected with the environment that instead fails in AD subjects.
Last but not least result of the current study regards the GE parameter, which showed a different behaviour compared to the SW index and M parameter. In fact, the GE seems to be sensible not only to the status of eyes but also to the HV tests in which GE exhibited differences compared to the EC and EO conditions. In particular, a significant increment of GE in the alpha bands was obtained in the HV compared to the EO and higher values in theta, alpha, and gamma bands were found in HV compared to the EC one.
Interestingly, a significant difference has emerged comparing the GE values in MCI group versus the Ctrl one. More specifically, during the HV test, the MCI group exhibited significantly lower values in alpha bands compared to the Ctrl group.
In general, the GE parameter is inversely related to topological distance between nodes and is typically interpreted as a measure of the capacity for parallel information transfer and integrated processing [54], providing an indication of how effectively information is integrated across the entirety of the network. Here, the GE, contrary to the other measures, has also shown to have an important role in response to HV, helping to distinguish a pathological state respect to a healthy condition. In particular, within the Ctrl group, the GE parameter was found to considerably increase in the alpha bands, whereas in MCI group, despite the presence of the same increment in alpha, this parameter, which describes the efficiency in the communication flow, seems to be less sensitive to the HV test.
Previous studies investigated changes in the network organization of MCI using advanced graph theoretical approach, including the GE parameter [55, 56]. Specifically, a decrease in GE and therefore in the integration property was found in MCI networks in the entire spectrum [55] and in theta band [56], suggesting a disintegration in functional network during resting state activity. Vecchio and colleagues (2015) suggested as the global functional connectivity reduction in MCI and AD patients was also associated to the reduction of fibers connecting the hemispheres as revealed by diffusion tensor imaging technique [57].
In line with our results, Franciotti and collaborators (2018) showed as both local and global efficiencies were lower in patients (MCI and AD) compared to controls in the EC condition, giving a new piece of evidence in the comprehension of the progression of AD from the prodromal stage to dementia, suggesting that the functional network alteration, evident also in the prodromal stage of AD, begins with the reduction of the number of edges and the loss of local and global efficiency [58].
Furthermore, lower values of GE parameter were found by Stanley and collaborators (2015), and they have been associated with a reduction of working memory performance in healthy elderlies, revealing that high global efficiency of information transfer across the brain is required for successful memory performances [59].
Only Mazzucchi and colleagues (2017) explored the modification of functional connectivity consequent to the HV test in healthy subjects with respect to epileptic patients, revealing that functional connectivity, explained by means of lagged coherence, was increased by HV, in delta, theta, alpha, and beta bands in patients and only in theta in the healthy group respect to a resting state eyes-closed condition [60]. These phenomena can be explained as a modification of functional connectivity in the entire brain probably due to blood gases modifications on receptors during HV [49]. Mainly, HV constitutes a classic activation procedure of the EEG that usually provokes physiologic slowing of the brain rhythms and that therefore can probably lead into changes also at the level of connectivity [1].
In conclusion, the GE parameter has been deeply studied and it has been demonstrated to be sensitive to pathological state due to cognitive decline in both MCI and AD conditions. However, to the best of our knowledge, this is the first study with the aim of analyzing if a classical HV test during EEG recording could be able to characterize patient with mild cognitive decline, eliciting particular alteration in network patterns. Effectively, HV turned out to be a useful test to explore the modification of the global integration property of the brain networks as well as to be underline the difference between healthy and subjects with mild alteration of cognitive function. However, it will be important to deepen these methodologies in a larger sample of participants.
Certainly, this is a pilot study; further evaluations with a larger sample size, more data about scores of clinical scales and new analysis, for example, including correlations between graph parameters and clinical assessment, and classification algorithms from the machine learning theory, are essential to confirm the role of HV test in combination with graph parameters as biomarkers of MCI conditions. However, considering all these results together, the HV test in association to the graph analysis seems to be a promising tool to be taken under consideration for the screening of MCI subjects, opening a new window into the understanding of the pathophysiological mechanism of neurodegeneration.
Funding
This work was partially supported by the Italian Ministry of Health for Institutional Research (Ricerca corrente), for the project “Prediction of conversion from Mild Cognitive Impairment to Alzheimer’s disease based on TMS-EEG biomarkers” (GR-2016-02361802) and by Toto Holding.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
Declarations
Authors’ disclosure statements
None of the authors have potential conflicts of interest to be disclosed.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Van der Worp HB, Kraaier V, Wieneke GH, Van Huffelen AC. Quantitative EEG during progressive hypocarbia and hypoxia. Hyperventilation-induced EEG changes reconsidered. Electroencephalogr Clin Neurophysiol. 1991;79(5):335–341. doi: 10.1016/0013-4694(91)90197-c. [DOI] [PubMed] [Google Scholar]
- 2.Mazzucchi E, et al. Hyperventilation in patients with focal epilepsy: electromagnetic tomography, functional connectivity and graph theory - a possible tool in epilepsy diagnosis? J Clin Neurophysiol. 2017;34(1):92–99. doi: 10.1097/WNP.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 3.Mäkiranta MJ, et al. BOLD-contrast functional MRI signal changes related to intermittent rhythmic delta activity in EEG during voluntary hyperventilation-simultaneous EEG and fMRI study. Neuroimage. 2004;22(1):222–231. doi: 10.1016/j.neuroimage.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Khachidze I, Gugushvili M, Advadze M. EEG characteristics to hyperventilation by age and sex in patients with various neurological disorders. Front Neurol. 2021;12:727297. doi: 10.3389/fneur.2021.727297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Plouin P, Kaminska A, Moutard ML, Soufflet C. Developmental aspects of normal EEG. Handb Clin Neurol. 2013;111:79–85. doi: 10.1016/B978-0-444-52891-9.00007-5. [DOI] [PubMed] [Google Scholar]
- 6.Kennealy JA, Penovich PE, Moore-Nease SE. EEG and spectral analysis in acute hyperventilation. Electroencephalogr Clin Neurophysiol. 1986;63(2):98–106. doi: 10.1016/0013-4694(86)90002-7. [DOI] [PubMed] [Google Scholar]
- 7.Brian JE. Carbon dioxide and the cerebral circulation. Anesthesiology. 1998;88(5):1365–1386. doi: 10.1097/00000542-199805000-00029. [DOI] [PubMed] [Google Scholar]
- 8.Petersen RC, et al. Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory-impaired individuals. JAMA. 1995;273(16):1274–1278. doi: 10.1001/jama.1995.03520400044042. [DOI] [PubMed] [Google Scholar]
- 9.Petersen RC, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 10.Scheltens P, Fox N, Barkhof F, De Carli C. Structural magnetic resonance imaging in the practical assessment of dementia: beyond exclusion. Lancet Neurol. 2002;1(1):13–21. doi: 10.1016/s1474-4422(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 11.Ponomareva NV, Korovaitseva GI, Rogaev EI. EEG alterations in non-demented individuals related to apolipoprotein E genotype and to risk of Alzheimer disease. Neurobiol Aging. 2008;29(6):819–827. doi: 10.1016/j.neurobiolaging.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 12.Vecchio F, Miraglia F, Bramanti P, Rossini PM. Human brain networks in physiological aging: a graph theoretical analysis of cortical connectivity from EEG data. J Alzheimers Dis. 2014;41(4):1239–1249. doi: 10.3233/JAD-140090. [DOI] [PubMed] [Google Scholar]
- 13.Rossini PM, Di Iorio R, Granata G, Miraglia F, Vecchio F. From mild cognitive impairment to Alzheimer's disease: a new perspective in the "Land" of human brain reactivity and connectivity. J Alzheimers Dis. 2016;53(4):1389–1393. doi: 10.3233/jad-160482. [DOI] [PubMed] [Google Scholar]
- 14.Miraglia F, et al. Brain connectivity and graph theory analysis in Alzheimer's and Parkinson's disease: the contribution of electrophysiological techniques. Brain Sci. 2022;12(3):402. doi: 10.3390/brainsci12030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Başar E, Schürmann M. Toward new theories of brain function and brain dynamics. Int J Psychophysiol. 2001;39(2-3):87–89. doi: 10.1016/s0167-8760(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 16.Miller EK, Wilson MA. All my circuits: using multiple electrodes to understand functioning neural networks. Neuron. 2008;60(3):483–488. doi: 10.1016/j.neuron.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 17.Friston KJ, Büchel C. CHAPTER 37 - Functional connectivity: eigenimages and multivariate analyses. In: Friston K, Ashburner J, Kiebel S, Nichols T, Penny W, editors. Statistical parametric mapping. Academic Press; 2007. pp. 492–507. [Google Scholar]
- 18.Vecchio F, Miraglia F, Maria RP. Connectome: graph theory application in functional brain network architecture. Clin Neurophysiol Pract. 2017;2:206–213. doi: 10.1016/j.cnp.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vecchio F, et al. Graph theory on brain cortical sources in Parkinson's disease: the analysis of 'Small World' organization from EEG. Sensors (Basel). 2021;21(21):31. doi: 10.3390/s21217266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winblad B, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 21.Petersen RC. Clinical practice. Mild cognitive impairment. N Engl J Med. 2011;364(23):2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 22.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 23.Miraglia F, et al. Assessing the dependence of the number of EEG channels in the brain networks' modulations. Brain Res Bull. 2021;167:33–36. doi: 10.1016/j.brainresbull.2020.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Pappalettera C, Miraglia F, Cotelli M, Rossini PM, Vecchio F. Analysis of complexity in the EEG activity of Parkinson's disease patients by means of approximate entropy. Geroscience. 2022;44(3):1599–1607. doi: 10.1007/s11357-022-00552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vecchio F, Miraglia F, Judica E, Cotelli M, Alù F, Rossini PM. Human brain networks: a graph theoretical analysis of cortical connectivity normative database from EEG data in healthy elderly subjects. Geroscience. 2020;42(2):575–584. doi: 10.1007/s11357-020-00176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vecchio F, Miraglia F, Alù F, Menna M, Judica E, Cotelli M, Rossini PM. Classification of Alzheimer's disease with respect to physiological aging with innovative EEG biomarkers in a machine learning implementation. J Alzheimers Dis. 2020;75(4):1253–1261. doi: 10.3233/JAD-200171. [DOI] [PubMed] [Google Scholar]
- 27.Pappalettera C, Cacciotti A, Nucci L, Miraglia F, Rossini PM, Vecchio F. Approximate entropy analysis across electroencephalographic rhythmic frequency bands during physiological aging of human brain. Geroscience. 2022. 10.1007/s11357-022-00710-4. [DOI] [PMC free article] [PubMed]
- 28.Hoffmann S, Falkenstein M. The correction of eye blink artefacts in the EEG: a comparison of two prominent methods. PLoS One. 2008;3(8):e3004. doi: 10.1371/journal.pone.0003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iriarte J, et al. Independent component analysis as a tool to eliminate artifacts in EEG: A quantitative study. J Clin Neurophysiol. 2003;20(4):249–257. doi: 10.1097/00004691-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Jung TP, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37(2):163–178. doi: 10.1111/1469-8986.3720163. [DOI] [PubMed] [Google Scholar]
- 31.Vecchio F, Nucci L, Pappalettera C, Miraglia F, Iacoviello D, Rossini PM. Time-frequency analysis of brain activity in response to directional and non-directional visual stimuli: an event related spectral perturbations (ERSP) study. J Neural Eng. 2022;19(6). 10.1088/1741-2552/ac9c96. [DOI] [PubMed]
- 32.Mulert C, et al. Integration of fMRI and simultaneous EEG: towards a comprehensive understanding of localization and time-course of brain activity in target detection. Neuroimage. 2004;22(1):83–94. doi: 10.1016/j.neuroimage.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 33.Vitacco D, Brandeis D, Pascual-Marqui R, Martin E. Correspondence of event-related potential tomography and functional magnetic resonance imaging during language processing. Hum Brain Mapp. 2002;17(1):4–12. doi: 10.1002/hbm.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Worrell GA, et al. Localization of the epileptic focus by low-resolution electromagnetic tomography in patients with a lesion demonstrated by MRI. Brain Topogr. 2000;12(4):273–282. doi: 10.1023/A:1023407521772. [DOI] [PubMed] [Google Scholar]
- 35.Dierks T, et al. Spatial pattern of cerebral glucose metabolism (PET) correlates with localization of intracerebral EEG-generators in Alzheimer's disease. Clin Neurophysiol. 2000;111:1817–1824. doi: 10.1016/S1388-2457(00)00427-2. [DOI] [PubMed] [Google Scholar]
- 36.Pizzagalli DA, et al. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry. 2004;9(4):393–405. doi: 10.1038/sj.mp.4001469. [DOI] [PubMed] [Google Scholar]
- 37.Zumsteg D, Wennberg RA, Treyer V, Buck A, Wieser HG. H2(15) O or 13NH3 PET and electromagnetic tomography (LORETA) during partial status epilepticus. Neurology. 2005;65(10):1657–1660. doi: 10.1212/01.wnl.0000184516.32369.1a. [DOI] [PubMed] [Google Scholar]
- 38.Vecchio F, et al. Human brain networks in physiological and pathological aging: reproducibility of electroencephalogram graph theoretical analysis in cortical connectivity. Brain Connect. 2022;12(1):41–51. doi: 10.1089/brain.2020.0824. [DOI] [PubMed] [Google Scholar]
- 39.Kubicki S, Herrmann WM, Fichte K, Freund G. Reflections on the topics: EEG frequency bands and regulation of vigilance. Pharmakopsychiatr Neuropsychopharmakol. 1979;12(2):237–245. doi: 10.1055/s-0028-1094615. [DOI] [PubMed] [Google Scholar]
- 40.Pascual-Marqui RD. Discrete, 3D distributed, linear imaging methods of electric neuronal activity. Part 1: exact, zero error localization. arXiv:0710.3341 [math-ph]. 2007; http://arxiv.org/pdf/0710.3341.
- 41.Pascual-Marqui RD, et al. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos Trans A Math Phys Eng Sci. 1952;2011(369):3768–3784. doi: 10.1098/rsta.2011.0081. [DOI] [PubMed] [Google Scholar]
- 42.Watts DJ, Strogatz SH. Collective dynamics of 'small-world' networks. Nature. 1998;393(6684):440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- 43.Vecchio F, Pappalettera C, Miraglia F, Deinite G, Manenti R, Judica E, Caliandro P, Rossini PM. Prognostic role of hemispherical functional connectivity in stroke: a study via graph theory versus coherence of electroencephalography rhythms. Stroke. 2022. 10.1161/STROKEAHA.122.040747. [DOI] [PubMed]
- 44.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Vecchio F, et al. Sustainable method for Alzheimer dementia prediction in mild cognitive impairment: electroencephalographic connectivity and graph theory combined with apolipoprotein E. Ann Neurol. 2018;84(2):302–314. doi: 10.1002/ana.25289. [DOI] [PubMed] [Google Scholar]
- 46.Miraglia F, Vecchio F, Rossini PM. Brain electroencephalographic segregation as a biomarker of learning. Neural Netw. 2018;106:168–174. doi: 10.1016/j.neunet.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 47.Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001;87(19):198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- 48.Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12(6):512–523. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- 49.Siddiqui SR, Zafar A, Khan FS, Shaheen M. Effect of hyperventilation on electroencephalographic activity. J Pak Med Assoc. 2011;61(9):850–852. [PubMed] [Google Scholar]
- 50.Hallett M, et al. Human brain connectivity: clinical applications for clinical neurophysiology. Clin Neurophysiol. 2020;131(7):1621–1651. doi: 10.1016/j.clinph.2020.03.031. [DOI] [PubMed] [Google Scholar]
- 51.Tan B, Kong X, Yang P, Jin Z, Li L. The difference of brain functional connectivity between eyes-closed and eyes-open using graph theoretical analysis. Comput Math Methods Med. 2013;2013:976365. doi: 10.1155/2013/976365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miraglia F, Vecchio F, Bramanti P, Rossini PM. EEG characteristics in "eyes-open" versus "eyes-closed" conditions: small-world network architecture in healthy aging and age-related brain degeneration. Clin Neurophysiol. 2016;127(2):1261–1268. doi: 10.1016/j.clinph.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y, et al. Open eyes increase neural oscillation and enhance effective brain connectivity of the default mode network: resting-state electroencephalogram research. Front Neurosci. 2022;16:861247. doi: 10.3389/fnins.2022.861247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bullmore E, Sporns O. The economy of brain network organization. Nat Rev Neurosci. 2012;13(5):336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- 55.Wei C, et al. A comparative study of structural and metabolic brain networks in patients with mild cognitive impairment. Front Aging Neurosci. 2021;13:774607. doi: 10.3389/fnagi.2021.774607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Youssef N, et al. Functional brain networks in mild cognitive impairment based on resting electroencephalography signals. Front Comput Neurosci. 2021;15:698386. doi: 10.3389/fncom.2021.698386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vecchio F, et al. Cortical brain connectivity evaluated by graph theory in dementia: A correlation study between functional and structural data. J Alzheimers Dis. 2015;45(3):745–756. doi: 10.3233/JAD-142484. [DOI] [PubMed] [Google Scholar]
- 58.Franciotti R, et al. Cortical network topology in prodromal and mild dementia due to Alzheimer's disease: Graph theory applied to resting state EEG. Brain Topogr. 2019;32(1):127–141. doi: 10.1007/s10548-018-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stanley ML, Simpson SL, Dagenbach D, Lyday RG, Burdette JH, Laurienti PJ. Changes in brain network efficiency and working memory performance in aging. PLoS One. 2015;10(4):e0123950. doi: 10.1371/journal.pone.0123950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mazzucchi E, et al. 6. Hyperventilation increases brain connectivity in healthy subjects and in focal cryptogenic epileptic patients. Clin Neurophysiol. 2015;126(1):e2. doi: 10.1016/j.clinph.2014.10.025. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.