Abstract
Dietary restriction (DR) increases lifespan in many organisms, but its underlying mechanisms are not fully understood. Mitochondria play a central role in metabolic regulation and are known to undergo changes in structure and function in response to DR. Mitochondrial membrane potential (Δψm) is the driving force for ATP production and mitochondrial outputs that integrate many cellular signals. One such signal regulated by Δψm is nutrient-status sensing. Here, we tested the hypothesis that DR promotes longevity through preserved Δψm during adulthood. Using the nematode Caenorhabditis elegans, we find that Δψm declines with age relatively early in the lifespan, and this decline is attenuated by DR. Pharmacologic depletion of Δψm blocked the longevity and health benefits of DR. Genetic perturbation of Δψm and mitochondrial ATP availability similarly prevented lifespan extension from DR. Taken together, this study provides further evidence that appropriate regulation of Δψm is a critical factor for health and longevity in response to DR.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-023-00766-w.
Keywords: Calorie restriction, Fasting, Aging, Metabolism, Mitochondrial uncoupling, Bioenergetics
Introduction
Dietary restriction (DR) can be defined as decreased nutrient availability in the absence of malnutrition. A variety of different DR regimens have been shown to extend lifespan across taxa, from single-celled budding yeast, to invertebrate models such as nematode worms and fruit flies, to rodents, to primates [1–4]. In the nematode worm, Caenorhabditis elegans, DR can be implemented by complete removal from the bacterial food source during adulthood, referred to as bacterial deprivation [5, 6], by dilution of the bacterial food [7], or by mutations that reduce food consumption, collectively referred to as eat mutants [8]. DR in the worm is known to engage a complex nutrient-sensing network that regulates metabolic function, growth and reproduction, stress resistance, and longevity. Key components of this network include the insulin/IGF-1-like signaling pathway, xenobiotic resistance factors, and energy sensors such as AMP-activated protein kinase (AMPK) and the mechanistic target of rapamycin (mTOR) [2].
Numerous studies have suggested that mitochondrial function plays a central role in longevity determination and the response to DR [9–11], yet the mechanisms underlying this relationship remain unclear [12]. Mitochondrial membrane potential (Δψm) is the voltage potential of the electrochemical proton gradient across the mitochondrial inner membrane that powers ATP production. In yeast, an early indicator of cellular aging is loss of Δψm [13]. Δψm declines with early age similarly in worms [14] and other animals [15–18]. We recently reported that increasing Δψm is sufficient to extend healthy lifespan in C. elegans [14]. This led us to consider how DR may affect Δψm specifically, and if changes in Δψm causally affect longevity from DR in worms.
To test the hypothesis that DR functions through preservation of Δψm with age, we performed DR on animals while perturbing Δψm. Using both genetic and pharmacologic approaches, we observed that DR is sufficient to attenuate the age-associated loss of Δψm and that this is necessary for lifespan extension. These findings support a central role for mitochondrial function in the longevity-promoting effects of DR and emphasize the need for a deeper understanding of in vivo bioenergetics in the context of healthy longevity.
Methods
C. elegans maintenance
Strains used in this study were the N2-Bristol wildtype strain, the DA465 strain harboring an eat-2 mutation (eat-2(ad465) II), the CY121 strain harboring the upc-4 mutation (ucp-4(ok195) V), the VC620 strain harboring the ant-1.2 mutation (ant-1.2(gk294) I), each obtained through the Caenorhabditis Genetics Center (CGC, NIH P40 OD010440). Strain RN70 harboring the mai-2 mutation (mai-2(xm18) IV) was provided by the laboratory of Dr. Rosa E. Navarro González. All strains were maintained at 20˚ C. We used standard nematode culture methods [19] including nematode growth media (NGM) plates and OP50 culture bacteria as a food source. Liquid media was M9 buffer (22 mM KH2PO4, 42 mM Na2HPO4, 86 mM NaCl, 1 mM MgSO4, pH 7).
Fluorescence analysis
TMRE (tetramethylrhodamine, ethyl ester) was dissolved in DMSO and worms were exposed at a final concentration of 1 μM in M9 buffer (0.0001% DMSO final concentration) for 24 h while rotating at 5 rotations per minute at 20˚ C (Life Technologies HulaMixer). Animals stained with TMRE were exposed to FCCP (carbonilcyanide p-triflouromethoxyphenylhydrazone) in 500 μL M9 at a final concentration of 10 μM for 4 h while rotating at 5 rotations per minute at 20˚ C. Perhexiline was used at 30 μM final concentration in the NGM for 24 h at 20˚ C according to previous protocols [20, 21]. MitoTracker Green FM was dissolved in DMSO and worms were exposed at a final concentration of 10 μM in 500 μL M9 buffer for 24 h at 20˚ C. For imaging, worms were anesthetized in 4 mM tetramisole in M9 mounted on 2% agarose pads. Fluorescence was captured on a Zeiss SteREO Lumar.V12 stereoscope (Thornwood, NY, USA) at room temperature. Mean and maximum fluorescence of the pharyngeal tissue was quantified using regions of interest (ROIs) manually selected in ImageJ.
Lifespan measurement
Age-synchronized populations were obtained by timed egg laying. At late L4 stage, all animals were transferred to culture plates seeded with OP50 bacterial food and 50 μM 5-fluorodeoxyuridine (FUDR) to prevent progeny hatching and development. Animals were kept at 20˚ C and were scored at least every other day by gently touching the head. Animals that did not move in response to touch were scored dead and removed from the plate. At day 2 of adulthood, dietary restriction (DR) populations were transferred to culture plates containing 50 μM FUDR without food as previously described [5, 6, 22] according to bacterial deprivation protocol. For FCCP experiments, at day 2 of adulthood, populations were transferred to NGM plates containing 10 μM FCCP. Control populations were simultaneously exposed to vehicle (0.001% ethanol). Fed animals were transferred to new plates as needed to replenish food. Some of the DR animals would display foraging behavior and leave the plate, however, foraging loses living animals, therefore under-representing increase in lifespan in response to DR [5]. All lifespans were from at least 3 biological replicates (separate broods) and pooled for presentation in figures.
Mobility measurement
Body bends were counted for 30 s while worms thrashed in M9 as previously described [23–25]. Resultant body bends per 30 s was multiplied by 2 to present body bends per minute in liquid for all conditions.
Statistics
Survival curves were analyzed by Log Rank (Mantel-Cox) test. For non-lifespan experiments, when two groups were compared, two sample two-tailed unpaired t-tests were used. When more than two groups were compared by ANOVA, Tukey’s post-hoc test or Dunnett’s post hoc test were used to correct for multiple comparisons where indicated in the figure legends. Specific statistical details are outlined in the figure legends for each experiment and in Supplementary Table 1 for lifespans. All tests were carried out in GraphPad Prism 9.3.0.
Results
Dietary restriction (DR) increases mitochondrial membrane potential (Δψm) in vivo
We first confirmed that in vivo Δψm was decreased by day 4 of adulthood [14] by measuring TMRE fluorescence intensity in the mitochondria-rich pharynx (Fig. 1A) [26, 27]. DR by bacterial deprivation (BD) significantly increased Δψm compared to the fed control (Fig. 1B). Mitochondrial mass was unchanged by this DR paradigm (Supplementary Fig. 1A). Similar results were obtained by measuring maximum TMRE fluorescence to avoid potential artifacts from differences in mitochondrial content (Supplementary Fig. 1B) [26–30]. DR is reported to increase mitochondrial mass in some models under some conditions [31, 32], however, in other cases mitochondrial mass remains unchanged by DR [33–35], in line with our results.
Fig. 1.
Dietary restriction (DR) causes increased mitochondrial membrane potential (Δψm). A) Relative TMRE fluorescence in day 1 adult animals versus day 4 adults. Unpaired two-tailed t test, *p < 0.0001. N = 45 animals for day 1, 41 animals for day 2. B) Relative TMRE fluorescence in day 4 adult animals either fully fed or subjected to dietary restriction (DR) by bacterial deprivation. Unpaired two-tailed t test, *p = 0.0003. N = 10 animals each condition. Throughout, dark gray represents fed animals, and light gray represents DR. C) Relative TMRE fluorescence in day 1 adult animals versus day 4 adults in both wildtype (WT) and genetic DR (eat-2) animals. One-way ANOVA with Tukey’s multiple comparisons test, *p < 0.0001. WT day 1 vs eat-2 day 1 p = 0.18. All other comparisons p < 0.0001. WT day 1 N = 47, WT day 4 N = 30, eat-2 day 1 N = 85, eat-2 day 4 N = 43. D) Relative TMRE fluorescence in day 4 adult animals that were fully fed, subjected to DR, and treated with 30 μM perhexiline as indicated. One-way ANOVA with Tukey’s multiple comparisons test, control vs perhexiline *p < 0.0001, perhexiline vs perhexiline DR * p = 0.0409, control vs perhexiline DR p < 0.0001. Control N = 10, perhexiline N = 17, perhexiline DR N = 14. Violin plots are medians ± quartiles (dotted lines)
In order to determine whether preservation of Δψm during aging is unique to the BD method of DR, we repeated these experiments using the eat-2 genetic model of DR. Animals with loss-of-function in the eat-2 gene consume less food than wildtype due to a defect in pharyngeal pumping and have extended lifespan [8]. We again observed increased Δψm in the eat-2 animals compared to wildtype controls at day 4 of adulthood (Fig. 1C). Together these results suggest that DR results in preserved Δψm during aging.
There is emerging evidence that fat metabolism and mitochondrial function play a fundamental role in DR longevity in both C. elegans [21] and mammals [36]. BD upregulates fatty acid oxidation by mitochondria, a process that is required for DR-mediated longevity [21]. Therefore we tested the hypothesis that fatty acid oxidation is what fuels preservation of Δψm by DR by directly inhibiting fat oxidation with the drug perhexiline [20]. Prior work has shown that perhexiline prevents lifespan extension from DR while not affecting fully fed worms [21]. We found that perhexiline prevented preservation of Δψm in response to DR and, indeed, significantly reduced it (Fig. 1D). Perhexiline also significantly reduced Δψm compared to fed control animals, suggesting fatty acid oxidation supports baseline in vivo Δψm.
Preservation of Δψm is required for DR-mediated longevity
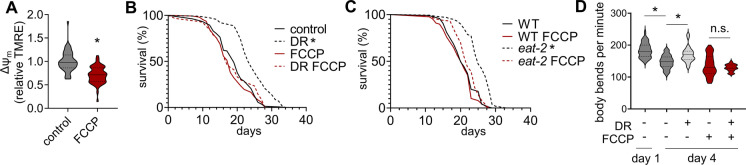
To test the model that preserved Δψm by DR is required for longevity, we exposed animals to the mitochondrial uncoupler, FCCP, and measured the effect on lifespan under control and BD conditions. FCCP exposure was initiated at the same time as BD, day 2 of adulthood. We confirmed that FCCP decreased Δψm in vivo, as expected (Fig. 2A), did not affect mitochondrial mass (Supplementary Fig. 1C), and that FCCP exposure entirely prevented lifespan extension from DR (Fig. 2B). Importantly, we chose a dose of FCCP that did not affect wildtype lifespan (Fig. 2B&C). FCCP treatment similarly prevented lifespan extension by eat-2 mutation (Fig. 2C). However, eat-2 animals treated with FCCP were slightly but significantly longer lived than wildtype animals treated with FCCP. Therefore, we interpret these results to mean that FCCP largely blocks lifespan extension from eat-2 loss of function, but may not totally block lifespan extension.
Fig. 2.
DR-mediated longevity is sensitive to decreased Δψm. A) Relative TMRE fluorescence in day 4 adult animals treated with either vehicle control or 10 μM FCCP. Unpaired two-tailed t test, *p < 0.0001. Control N = 57, FCCP N = 56. Throughout, FCCP treatment is denoted by dark red. B) Survival curves of wildtype animals (WT) subjected to DR and treated with FCCP. Log-rank (Mantel-Cox) test, *p < 0.0001. Detailed statistical information for all lifespans is presented in Supplementary Table 1. C) Survival curves of wildtype (WT) and genetic DR (eat-2) animals treated with FCCP. Log-rank (Mantel-Cox) test, *p < 0.0001. Detailed statistical information for all lifespans is presented in Supplementary Table 1. D) Motility of wildtype day 4 adult animals scored by counting body bends per minute while thrashing in liquid. Animals were subjected to DR and FCCP treatment for 2 days before measurement. One-way ANOVA with Tukey’s multiple comparisons test, WT day 1 vs WT DR day 4 p = 0.0393, WT day 4 vs WT day 4 FCCP p = 0.1270, WT day 4 vs WT day 4 DR FCCP p = 0.0031, WT day 4 vs WT day 4 DR FCCP p = 0.5545, all other comparisons p < 0.0001. WT day 1 N = 40 animals, WT day 4 N = 45, WT day 4 DR N = 49, WT day 4 FCCP N = 70, WT day 4 DR FCCP N = 57. Violin plots are medians ± quartiles (dotted lines)
To assess whether preservation of Δψm also mediated healthspan benefits from DR, we quantified animal motility via thrashing in liquid. We found that by day 4 of adulthood control animals were significantly motility-impaired, and BD was able to reverse the impairment (Fig. 2D). Upon treatment with FCCP we found that BD was no longer able to rescue age-associated loss of motility (Fig. 2D).
DR increases Δψm in ucp-4 mutants, but not in ant-1.2 or mai-2 mutants
To begin to characterize the mechanism of increased Δψm in response to DR, we assessed Δψm of animals with loss-of-function in three genes that endogenously regulate Δψm in different ways (Fig. 3A). We first tested if animals with non-functional uncoupling protein (UCP) responded to DR. UCPs result in decreased Δψm when activated [37, 38]. Therefore, we hypothesized that DR may inhibit UCP and result in increased Δψm. The only known UCP in C. elegans is encoded by the ucp-4 gene [39, 40]. As expected, mutation of ucp-4 results in animals with increased Δψm at day 1 of adulthood (Fig. 3B). Unexpectedly, Δψm in the ucp-4 mutant decreased to the same level as wildtype animals by day 4 of adulthood (Fig. 3B) and ucp-4 mutants showed increased Δψm in response to DR (Fig. 3C).
Fig. 3.
ANT and IF1, but not UCP, mediate the effects of DR in C. elegans. A) Schematic showing the mitochondrial inner membrane and proteins that regulate Δψm. Uncoupling proteins (UCP) allow proton (H.+) reentry to the mitochondrial matrix, dissipating Δψm. C. elegans has a single ucp-4 gene. Adenine Nucleotide Translocase (ANT) transports ADP and ATP into and out of mitochondria, respectively, and allow proton reentry to the matrix. C. elegans ant-1.2 is highly expressed in pharynx. Inhibitory factor 1 (IF1) prevents ATP synthase from working in reverse mode, thereby preventing increased Δψm. C. elegans IF1 is encoded by the mai-2 gene. Loss of function in each of these genes should result in increased Δψm. B) Relative TMRE fluorescence in both day 1 adult and day 4 adult animals for wildtype (WT), UCP loss of function mutants (ucp-4), ANT loss of function mutants (ant-1.2) and IF1 loss of function mutants (mai-2). Within day 1, One-way ANOVA with Dunnett’s multiple comparisons test versus WT, ucp-4 p = 0.0007, ant-1.2 p < 0.0001, mai-2 p = 0.0006. WT N = 40, ucp-4 N = 28, ant-1.2 N = 10, mai-2 N = 16. Within day 4, One-way ANOVA with Dunnett’s multiple comparisons test versus WT, ucp-4 p = 0.8209, ant-1.2 p < 0.0001, mai-2 p < 0.0001. WT N = 63, ucp-4 N = 48, ant-1.2 N = 53, mai-2 N = 56. C) Relative TMRE fluorescence in ucp-4 day 4 adult animals either fully fed or subjected to DR. Unpaired two-tailed t test, *p < 0.0218. Control N = 14, DR N = 19. D) Relative TMRE fluorescence in ant-1.2 day 4 adult animals either fully fed or subjected to DR. Unpaired two-tailed t test, p = 0.3014. Control N = 26, DR N = 23. E) Relative TMRE fluorescence in mai-2 day 4 adult animals either fully fed or subjected to DR. Unpaired two-tailed t test, p = 0.5686. Control N = 23, DR N = 19. F) Survival curves of ucp-4 animals subjected to DR and treated with FCCP. Log-rank (Mantel-Cox) test, *p < 0.0001. Detailed statistical information for all lifespans is presented in Supplementary Table 1. G) Survival curves of ant-1.2 animals subjected to DR and treated with FCCP. Log-rank (Mantel-Cox) test, *p < 0.0001. Detailed statistical information for all lifespans is presented in Supplementary Table 1. H) Survival curves of mai-2 animals subjected to DR and treated with FCCP. Log-rank (Mantel-Cox) test, control vs DR *p = 0.0072, control vs FCCP *p < 0.0001. Detailed statistical information for all lifespans is presented in Supplementary Table 1. Violin plots are medians ± quartiles (dotted lines)
We next tested whether the adenine nucleotide transferase (ANT) plays a role in increased Δψm in response to DR. ANT functions to transport ATP out of mitochondria and cytosolic ADP into mitochondria, and can also result in mitochondrial uncoupling similar to UCPs [41, 42] (Fig. 3A). Therefore we hypothesized that DR may act through ANT activity to modulate Δψm. Worms with loss of function in the ant-1.2 gene showed increased Δψm at day 1 of adulthood (Fig. 3B), as expected [43]. Conversely to ucp-4, however, ant-1.2 mutants had increased Δψm compared to wildtype at day 4 of adulthood (Fig. 3B), indicating that Δψm decline with age was attenuated in these mutants. When subjected to BD, Δψm was not increased in ant-1.2 mutants (Fig. 3D), a departure from the effect of BD in wildtype and in ucp-4 animals.
We last tested the effects of DR on Δψm in animals defective for the mammalian inhibitory factor 1 (IF1), or mai-2 in worms. IF1 normally inhibits ATP synthase reversal, which prevents ATP synthase from hydrolyzing mitochondrial ATP to pump protons out of mitochondria to increase Δψm (Fig. 3A). ATP synthase reversal is well-described under conditions of energetic crisis [44], and IF1 is normally active to prevent this reversal [45]. Therefore, inhibiting IF1 using the mai-2 mutation resulted in increased Δψm at day 1 of adulthood (Fig. 3B), as expected [46]. Similar to animals defective for ant-1.2, mai-2 animals also had increased Δψm at day 4 of adulthood (Fig. 3B), suggesting that Δψm decline with age is attenuated. When subjected to BD, mai-2 mutants did not show increased Δψm (Fig. 3E) similar to the ant-1.2 animals.
DR extends lifespan of ucp-4 mutant worms but not of ant-1.2 or mai-2 mutants
Based on their impact on Δψm, we predicted that the ucp-4 would respond normally to DR, while the ant-1.2 and mai-2 mutant strains may experience reduced lifespan extension following DR. Consistent with this, ucp-4 animals had increased lifespan in response to DR which was reversed by FCCP treatment, similar to wildtype animals (Fig. 3F). Also similar to wildtype, FCCP had no effect on lifespan of fully fed ucp-4 animals. In contrast, both the ant-1.2 and mai-2 strains did not experience full lifespan extension in response to DR (Fig. 3G&H, Supplementary Table 1). When exposed to FCCP, ant-1.2 mutants and mai-2 mutants both experienced decreased lifespan. This sensitivity to mitochondrial uncoupling has been observed previously in the mai-2 mutants [46]. Under control conditions, ucp-4 animals were slightly short lived compared to wildtype, and both ant-1.2 and mai-2 animals were slightly long lived compared to wildtype (Supplementary Fig. 2). Altogether, these experiments show that proper regulation of Δψm is required for the beneficial effects of DR in C. elegans and suggest a model whereby both ANT and IF1 activity play a role in the ability of DR to preserve Δψm during aging.
Discussion
In this study, we show that BD-induced DR in C. elegans attenuates the age-related loss of Δψm, and that this is necessary for lifespan extension and improved motility. The ability of DR to preserve Δψm during early aging does not appear to involve the activity of UCPs, but instead requires both mitochondrial ANT and IF1 activity. We also find that fatty acid metabolism is required for preservation of Δψm by DR, consistent with a recent report in worms that fatty acid metabolism promotes longevity [21], as well as numerous studies in mammals showing that DR increases fatty acid oxidation [36, 47–49]. While it remains difficult to quantify or control Δψm in vivo in mammals, this work should serve to orient future experiments to define mechanisms underlying these observations in worms and other systems.
One limitation of our study is that UCP-4 in C. elegans may also regulate mitochondrial succinate transport in addition to its uncoupling role [40]. UCPs are closely related to metabolite transport proteins of many different varieties [50, 51] and different UCPs may even function differently within a single organism [52]. This is not a major limitation of our study because we found that ucp-4 was not required for preservation of Δψm, and lifespan extension by DR. However, this caveat highlights the need for expanded understanding of UCP activity and DR in both invertebrate and mammalian systems. We do not, therefore, rule out a role for UCP activity in general for DR-mediated longevity in other models. On the contrary, investigating roles for UCPs under conditions of DR will be interesting given that UCPs are a popular target for diseases of aging, such as metabolic syndrome. There is evidence that targeting UCPs will be informative for understanding DR signaling in mammals, especially under conditions of protein restriction [51, 53, 54].
Our data supports a potential direct role for ANT and IF1 downstream of DR. In addition to regulating Δψm, ANT and IF1 also modulate ATP/ADP dynamics in both mitochondria and cytosol, which may contribute to their effects on lifespan. ANT and IF1 seem to interact to modulate ATP synthase reversal under conditions of changing Δψm in vivo [44]. Our data suggest that this three-way interaction centered around Δψm could be important in the biology of DR. Developing tools to precisely control ATP/ADP levels in both the cytosol and in the mitochondrial matrix, as well as control over Δψm, will be necessary first steps in elucidating more detail in experimental models. However, emerging evidence suggests that directly intervening on ANT function is possible to facilitate better understanding of mitochondrial energetics in vivo in the context of aging [18, 55]. Similarly, targeting IF1 in mammalian cells has been informative for probing the mechanisms of nutrient-sensing signaling and Δψm [56, 57].
Our results are also in line with our recent discovery that increased Δψm in itself is sufficient to extend lifespan in C. elegans [14]. We recently proposed an “energetics perspective on geroscience” [58]. The data presented here extend those ideas, showing that increased Δψm may be a fundamental mechanism of lifespan extension, at least in the case of DR for worms. Decreased Δψm has long been hypothesized to cause biological aging [13, 58–61] and the idea that improved Δψm with age is beneficial is not a new one [18, 58]. Now that this idea is established experimentally in worms, new perspectives on biological aging can be tested in vivo. It will be of particular interest to determine whether alternative interventions for increasing lifespan in worms and other organisms similarly impact Δψm. In conclusion, we propose that in vivo Δψm is an important parameter during early phases of aging, and that longevity interventions to target Δψm will be informative for developing mitochondrial therapeutics for healthy aging.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
BJB is supported by the Biological Mechanisms for Healthy Aging (BMHA) Training Grant NIH T32AG066574. This work was supported by P30AG013280 to MK. Some strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). The authors thank the laboratory of Rosa E. Navarro González for providing strain RN70 harboring the mai-2 mutation.
Data Availability
All data are presented in the manuscript and supplementary materials. Raw data files for lifespans are included in the supplementary materials, and other raw data can be made available upon request.
Declarations
Conflicts of interest
All authors declare that they have no conflicts of interest to disclose.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Brandon J. Berry, Email: bberry5@uw.edu
Evan Mjelde, Email: emjelde@uw.edu.
Fatima Carreno, Email: fatimaca@uw.edu.
Kathryn Gilham, Email: kgg4@uw.edu.
Emily J. Hanson, Email: ejhanson@uw.edu
Emily Na, Email: emilyna@uw.edu.
Matt Kaeberlein, Email: kaeber@uw.edu.
References
- 1.Anderson RM, Shanmuganayagam D, Weindruch R. Caloric restriction and aging: studies in mice and monkeys. Toxicol Pathol. 2009;37(1):47–51. doi: 10.1177/0192623308329476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kapahi P, Kaeberlein M, Hansen M. Dietary restriction and lifespan: Lessons from invertebrate models. Ageing Res Rev. 2017;39:3–14. doi: 10.1016/j.arr.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee MB, et al. Antiaging diets: Separating fact from fiction. Science. 2021. 374(6570): p. eabe7365. [DOI] [PMC free article] [PubMed]
- 4.Longo VD, Anderson RM. Nutrition, longevity and disease: From molecular mechanisms to interventions. Cell. 2022;185(9):1455–1470. doi: 10.1016/j.cell.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaeberlein TL, et al. Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell. 2006;5(6):487–494. doi: 10.1111/j.1474-9726.2006.00238.x. [DOI] [PubMed] [Google Scholar]
- 6.Sutphin GL, Kaeberlein M. Dietary restriction by bacterial deprivation increases life span in wild-derived nematodes. Exp Gerontol. 2008;43(3):130–135. doi: 10.1016/j.exger.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8(2): p. 113–27. [DOI] [PMC free article] [PubMed]
- 8.Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1998;95(22):13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brys K, et al. Disruption of insulin signalling preserves bioenergetic competence of mitochondria in ageing Caenorhabditis elegans. BMC Biol. 2010;8:91. doi: 10.1186/1741-7007-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hagopian K, et al. Long-term calorie restriction reduces proton leak and hydrogen peroxide production in liver mitochondria. Am J Physiol Endocrinol Metab. 2005;288(4):E674–E684. doi: 10.1152/ajpendo.00382.2004. [DOI] [PubMed] [Google Scholar]
- 11.Bevilacqua L, et al. Effects of short- and medium-term calorie restriction on muscle mitochondrial proton leak and reactive oxygen species production. Am J Physiol Endocrinol Metab. 2004;286(5):E852–E861. doi: 10.1152/ajpendo.00367.2003. [DOI] [PubMed] [Google Scholar]
- 12.Anderson RM, Weindruch R. Metabolic reprogramming in dietary restriction. Interdiscip Top Gerontol. 2007;35:18–38. doi: 10.1159/000096554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes AL, Gottschling DE. An early age increase in vacuolar pH limits mitochondrial function and lifespan in yeast. Nature. 2012;492(7428):261–265. doi: 10.1038/nature11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry BJ, et al. Optogenetic rejuvenation of mitochondrial membrane potential extends C. elegans lifespan. Nature Aging. 2022. In Press. [DOI] [PMC free article] [PubMed]
- 15.Ames BN, Shigenaga MK, Hagen TM. Mitochondrial decay in aging. Biochim Biophys Acta. 1995;1271(1):165–170. doi: 10.1016/0925-4439(95)00024-X. [DOI] [PubMed] [Google Scholar]
- 16.Sugrue MM, Tatton WG. Mitochondrial membrane potential in aging cells. Biol Signals Recept. 2001;10(3–4):176–188. doi: 10.1159/000046886. [DOI] [PubMed] [Google Scholar]
- 17.Bayliak MM, et al. Middle age as a turning point in mouse cerebral cortex energy and redox metabolism: Modulation by every-other-day fasting. Exp Gerontol. 2021;145:111182. doi: 10.1016/j.exger.2020.111182. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, et al. Reduction of elevated proton leak rejuvenates mitochondria in the aged cardiomyocyte. Elife. 2020;9. [DOI] [PMC free article] [PubMed]
- 19.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weir HJ, et al. Dietary Restriction and AMPK Increase Lifespan via Mitochondrial Network and Peroxisome Remodeling. Cell Metab. 2017;26(6):884–896.e5. doi: 10.1016/j.cmet.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macedo F, et al. Lifespan-extending interventions enhance lipid-supported mitochondrial respiration in Caenorhabditis elegans. FASEB J. 2020;34(8):9972–9981. doi: 10.1096/fj.201901880R. [DOI] [PubMed] [Google Scholar]
- 22.Sutphin GL, Kaeberlein M. Measuring Caenorhabditis elegans life span on solid media. J Vis Exp. 2009(27). [DOI] [PMC free article] [PubMed]
- 23.Berry BJ, et al. Optogenetic control of mitochondrial protonmotive force to impact cellular stress resistance. EMBO Rep. 2020;21(4):e49113. doi: 10.15252/embr.201949113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawin ER, Ranganathan R, Horvitz HR. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron. 2000;26(3): p. 619–31. [DOI] [PubMed]
- 25.Tsalik EL, Hobert O. Functional mapping of neurons that control locomotory behavior in Caenorhabditis elegans. J Neurobiol. 2003;56(2):178–197. doi: 10.1002/neu.10245. [DOI] [PubMed] [Google Scholar]
- 26.Kwon YJ, et al. High-throughput BioSorter quantification of relative mitochondrial content and membrane potential in living Caenorhabditis elegans. Mitochondrion. 2018;40:42–50. doi: 10.1016/j.mito.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dingley S, et al. Mitochondrial respiratory chain dysfunction variably increases oxidant stress in Caenorhabditis elegans. Mitochondrion. 2010;10(2):125–136. doi: 10.1016/j.mito.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aspernig H, et al. Mitochondrial Perturbations Couple mTORC2 to Autophagy in C. elegans. Cell Rep. 2019;29(6): p. 1399–1409.e5. [DOI] [PubMed]
- 29.Shpilka T, et al. UPR mt scales mitochondrial network expansion with protein synthesis via mitochondrial import in Caenorhabditis elegans. Nat Commun. 2021;12(1):479. doi: 10.1038/s41467-020-20784-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perry SW, et al. Mitochondrial membrane potential probes and the proton gradient: a practical usage guide. Biotechniques. 2011;50(2):98–115. doi: 10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nisoli E, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310(5746):314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 32.Guarente L. Mitochondria–a nexus for aging, calorie restriction, and sirtuins? Cell. 2008;132(2):171–176. doi: 10.1016/j.cell.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lanza IR, et al. Chronic caloric restriction preserves mitochondrial function in senescence without increasing mitochondrial biogenesis. Cell Metab. 2012;16(6):777–788. doi: 10.1016/j.cmet.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhoads TW, et al. Molecular and Functional Networks Linked to Sarcopenia Prevention by Caloric Restriction in Rhesus Monkeys. Cell Syst. 2020;10(2):156–168.e5. doi: 10.1016/j.cels.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hancock CR, et al. Does calorie restriction induce mitochondrial biogenesis? A reevaluation FASEB J. 2011;25(2):785–791. doi: 10.1096/fj.10-170415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bruss MD, et al. Calorie restriction increases fatty acid synthesis and whole body fat oxidation rates. Am J Physiol Endocrinol Metab. 2010;298(1):E108–E116. doi: 10.1152/ajpendo.00524.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cho I, Hwang GJ, Cho JH. Uncoupling Protein, UCP-4 May Be Involved in Neuronal Defects During Aging and Resistance to Pathogens in Caenorhabditis elegans. Mol Cells. 2016;39(9):680–686. doi: 10.14348/molcells.2016.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berry BJ, et al. Use the Protonmotive Force: Mitochondrial Uncoupling and Reactive Oxygen Species. J Mol Biol. 2018;430(21):3873–3891. doi: 10.1016/j.jmb.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iser WB, et al. Examination of the requirement for ucp-4, a putative homolog of mammalian uncoupling proteins, for stress tolerance and longevity in C. elegans. Mech Ageing Dev. 2005;126(10): p. 1090–6. [DOI] [PMC free article] [PubMed]
- 40.Pfeiffer M, et al. Caenorhabditis elegans UCP4 protein controls complex II-mediated oxidative phosphorylation through succinate transport. J Biol Chem. 2011;286(43):37712–37720. doi: 10.1074/jbc.M111.271452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertholet AM, et al. H + transport is an integral function of the mitochondrial ADP/ATP carrier. Nature. 2019;571(7766):515–520. doi: 10.1038/s41586-019-1400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chevrollier A, et al. Adenine nucleotide translocase 2 is a key mitochondrial protein in cancer metabolism. Biochim Biophys Acta. 2011;1807(6):562–567. doi: 10.1016/j.bbabio.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 43.Farina F, et al. Differential expression pattern of the four mitochondrial adenine nucleotide transporter ant genes and their roles during the development of Caenorhabditis elegans. Dev Dyn. 2008;237(6):1668–1681. doi: 10.1002/dvdy.21578. [DOI] [PubMed] [Google Scholar]
- 44.Chinopoulos C. Mitochondrial consumption of cytosolic ATP: not so fast. FEBS Lett. 2011;585(9):1255–1259. doi: 10.1016/j.febslet.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 45.García-Aguilar A, Cuezva JM. A Review of the Inhibition of the Mitochondrial ATP Synthase by IF1. Front Physiol. 2018;9:1322. doi: 10.3389/fphys.2018.01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernández-Cárdenas LP, et al. Caenorhabditis elegans ATPase inhibitor factor 1 (IF1) MAI-2 preserves the mitochondrial membrane potential (Δψm) and is important to induce germ cell apoptosis. PLoS ONE. 2017;12(8):e0181984. doi: 10.1371/journal.pone.0181984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teofilović A, et al. Late-Onset Calorie Restriction Improves Lipid Metabolism and Aggravates Inflammation in the Liver of Old Wistar Rats. Front Nutr. 2022;9:899255. doi: 10.3389/fnut.2022.899255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baumeier C, et al. Caloric restriction and intermittent fasting alter hepatic lipid droplet proteome and diacylglycerol species and prevent diabetes in NZO mice. Biochim Biophys Acta. 2015;1851(5):566–576. doi: 10.1016/j.bbalip.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 49.Rhoads TW, et al. Caloric Restriction Engages Hepatic RNA Processing Mechanisms in Rhesus Monkeys. Cell Metab. 2018;27(3):677–688.e5. doi: 10.1016/j.cmet.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ricquier D. Bouillaud F. The uncoupling protein homologues: UCP1, UCP2, UCP3, StUCP and AtUCP. Biochem J. 2000;345 Pt 2(Pt 2): p. 161–79. [PMC free article] [PubMed]
- 51.Fisler JS, Warden CH. Uncoupling proteins, dietary fat and the metabolic syndrome. Nutr Metab (Lond) 2006;3:38. doi: 10.1186/1743-7075-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nedergaard J, Cannon B. The 'novel' 'uncoupling' proteins UCP2 and UCP3: what do they really do? Pros and cons for suggested functions. Exp Physiol. 2003;88(1):65–84. doi: 10.1113/eph8802502. [DOI] [PubMed] [Google Scholar]
- 53.Wanders D, et al. UCP1 is an essential mediator of the effects of methionine restriction on energy balance but not insulin sensitivity. FASEB J. 2015;29(6):2603–2615. doi: 10.1096/fj.14-270348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hill CM, et al. Low protein-induced increases in FGF21 drive UCP1-dependent metabolic but not thermoregulatory endpoints. Sci Rep. 2017;7(1):8209. doi: 10.1038/s41598-017-07498-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roshanravan B, et al. In vivo mitochondrial ATP production is improved in older adult skeletal muscle after a single dose of elamipretide in a randomized trial. PLoS ONE. 2021;16(7):e0253849. doi: 10.1371/journal.pone.0253849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gore E, et al. The Multifaceted ATPase Inhibitory Factor 1 (IF1) in Energy Metabolism Reprogramming and Mitochondrial Dysfunction: A New Player in Age-Associated Disorders? Antioxid Redox Signal. 2022;37(4–6):370–393. doi: 10.1089/ars.2021.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martínez-Reyes I, et al. TCA Cycle and Mitochondrial Membrane Potential Are Necessary for Diverse Biological Functions. Mol Cell. 2016;61(2):199–209. doi: 10.1016/j.molcel.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Berry BJ, Kaeberlein M. An energetics perspective on geroscience: mitochondrial protonmotive force and aging. Geroscience. 2021;43(4):1591–1604. doi: 10.1007/s11357-021-00365-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Angeli S, et al. The mitochondrial permeability transition pore activates the mitochondrial unfolded protein response and promotes aging. Elife. 2021. 10. [DOI] [PMC free article] [PubMed]
- 60.Ye X, et al. A pharmacological network for lifespan extension in Caenorhabditis elegans. Aging Cell. 2014;13(2):206–215. doi: 10.1111/acel.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhou B, et al. Mitochondrial Permeability Uncouples Elevated Autophagy and Lifespan Extension. Cell. 2019;177(2):299–314.e16. doi: 10.1016/j.cell.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are presented in the manuscript and supplementary materials. Raw data files for lifespans are included in the supplementary materials, and other raw data can be made available upon request.