Abstract
Independently, obesity and physical activity (PA) influence cerebral structure in aging, yet their interaction has not been investigated. We examined sex differences in the relationships among PA, obesity, and cerebral structure in aging with 340 participants who completed magnetic resonance imaging (MRI) acquisition to quantify grey matter volume (GMV) and white matter volume (WMV). Height and weight were measured to calculate body mass index (BMI). A PA questionnaire was used to estimate weekly Metabolic Equivalents. The relationships between BMI, PA, and their interaction on GMV Regions of Interest (ROIs) and WMV ROIs were examined. Increased BMI was associated with higher GMV in females, an inverse U relationship was found between PA and GMV in females, and the interaction indicated that regardless of BMI greater PA was associated with enhanced GMV. Males demonstrated an inverse U shape between BMI and GMV, and in males with high PA and had normal weight demonstrated greater GMV than normal weight low PA revealed by the interaction. WMV ROIs had a linear relationship with moderate PA in females, whereas in males, increased BMI was associated with lower WMV as well as a positive relationship with moderate PA and WMV. Males and females have unique relationships among GMV, PA and BMI, suggesting sex-aggregated analyses may lead to biased or non-significant results. These results suggest higher BMI, and PA are associated with increased GMV in females, uniquely different from males, highlighting the importance of sex-disaggregated models. Future work should include other imaging parameters, such as perfusion, to identify if these differences co-occur in the same regions as GMV.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-023-00734-4.
Keywords: Physical activity, Obesity, Gray matter volume, Sexual dimorphisms
Introduction
Obesity has a high incidence in individuals in midlife and into aging, with 39.9% of middle age and older adult populations being overweight and 28.1% considered obese [1]. This has important health implications given that obesity is associated with a higher incidence of diabetes, cardiovascular disease, increased risk of early mortality [2]. A recent Lancet dementia commission paper identified that greater body mass index (BMI), an indirect marker of obesity, is one of the 12 of the most influential modifiable factors in the risk of developing dementia [3]. Notably, increased BMI before the of 65 years, has been associated with a 1.6 increased relative risk of developing dementia [4]. Yet, this relationship is sometimes elusive, as some have found no relationship between BMI and dementia risk [5].
These paradoxical findings also exist in the documented relationship between BMI and brain structure. For example, it has been identified that higher BMI may have deleterious impacts on the brain, including data showing an association between higher BMI and global grey matter (GM) atrophy, as well as atrophy of frontal, temporal, and hippocampal areas [6–9], regions associated with age-related cognitive decline [10, 11]. However, others have identified that there is no relationship between BMI and GM volume (GMV) [12], or that obesity may be protective in some GM regions indicated by a positive relationship between BMI and GMV [13, 14] Given that obesity prevalence is generally higher in females than males [15], and that fat distribution differs between the sexes [16], and over time in females [17, 18], it is possible that some of these contradictory relationships reflect sex-related differences. This could arise due to sex distribution imbalance in some studies, the age range included and how it relates to menopausal status, or the different ways sex is accounted for in studies (i.e., sex as an outcome versus a covariate). For example, Taki and colleagues identified that their results of negative and positive relationships in specific GMV regions with BMI were driven exclusively by males, specifically the medial temporal and frontal regions were negatively associated with GMV, and greater BMI was associated with increased GMV in the anterior cingulate cortex [14]. This was later confirmed in a separate longitudinal study investigating BMI and GMV, specifically increased BMI was associated with decreased global GMV [19]. Finally, Huang and colleagues found that both males and females had positive relationships with BMI and GMV in the cerebellum and postcentral gyrus, and negative relationships were identified in the insula, caudate and medial frontal cortex. Interestingly, only males experienced atrophy of the anterior cingulate cortex with increasing BMI [13], indicating that sex differences with structural brain outcomes could be region dependent.
The evidence on the direction of relationship between BMI and white matter volume (WMV) in aging is mixed, where some find that increased BMI is inversely associated with WMV [8, 20], others found no relationship between BMI and WMV [21, 22] and some report positive relationships [7]. Taken together, the relationship between BMI and WM outcomes is variable, likely sex-dependent with males more negatively affected by greater BMI and potentially region specific.
Another important risk factor for late life disease, and more specifically dementia, is physical inactivity [3, 23]. Furthermore, greater PA is consistently reported to be positively associated with structural outcomes [24–26]. Most research to date has shown a protective effect of PA with GMV within frontal [27, 28, 29] and hippocampal region [28, 30–32]. Recent reviews have extensively covered the relationship between GMV and PA in aging [31, 33, 34].
It has also been shown that the influence of PA on cerebral health is influenced by sex. In some instances, females benefit more from PA in terms of GMV than males [35], whereas Casaletto et al., 2020 identified that males, but not females, have a positive relationship between PA and GMV, particularly within the parahippocampal regions [36]. The effect of sex on the relationship between PA and WMV, has not been extensively investigated, though one study reported that females benefited from higher PA levels but not males [37].
Given the generally negative effect of obesity on brain health in aging, and the positive influence of PA, their interaction, alongside sex differences, could explain some of the contradictory effects observed in the literature. In instances where obesity has a seemingly positive or null effect on GMV, it could partially be due to higher PA levels. It is therefore important to investigate the sex-specific effects of obesity on cerebral macrostructure and how PA might impact these relationships. We examined a group of older cognitively unimpaired females and males from the Pre-symptomatic Evaluation of Novel or Experimental Treatments for Alzheimer's Disease (PREVENT-AD) study across the BMI spectrum (i.e., BMI = 18.6 to 39.9 kg/m2) [38]. The PREVENT-AD dataset includes cognitively normal older adults at baseline, that are at higher risk of developing Alzheimer’s disease due to familial history of Alzheimer’s dementia. Outcomes included GMV, WMV, PA as indicated by energy expenditure per week based on an in-depth adapted version of the Global Physical Activity Questionnaire [39]. Polynomial regressions were employed in males and females separately, to investigate the relationship between GMV and WMV with i) BMI; ii) PA; and iii) the interaction of BMI and PA.
Methods
Participants
A total of 340 participants (247 females, average age of 62.6 years old for the whole sample) from the Pre-symptomatic Evaluation of Novel or Experimental Treatments for Alzheimer’s Disease (PREVENT-AD) study were included here. The data used in preparation of this manuscript were obtained from the PREVENT-AD program data release 6.0. Briefly, the primary goal of PREVENT-AD is to investigate whether serial determination of multi-modal biomarkers of Alzheimer’s disease may be measured and then used in pre-symptomatic individuals with a family history of dementia, to prospectively investigate disease progression and to measure effects of any potentially preventative treatments. The data used here is only from the baseline time-point in cognitively normal participants and is prior to any of the participants beginning the interventional component of this study.
Participants had to be above the age of 60, or above 55 if their own age was within 15 years of symptom onset of their youngest-affected first-degree relative. They all had a parent or a minimum of two siblings diagnosed with Alzheimer’s disease dementia. Other inclusion criteria included having no MRI contraindications, fluent in French and/or English, no evidence of cognitive impairment as determined by cognitive tests. Exclusion criteria included presence of neurological or psychiatric disorders or excessive drinking (more than two drinks per day on a basis). The Montreal Cognitive Assessment (MoCA) [40] and the Clinical Dementia Rating Scale [41] were employed to screen for cognitive impairment, where a score of ≤ 26 out of 30 on the MoCA or greater than 0 on the CDR required further in depth neuropsychological screening assessed by a trained neuropsychologist to rule out mild cognitive impairment. All procedures were completed according to the Declaration of Helsinki, were approved by the McGill Institutional Review Board and the Douglas Mental Health University Institute Research Ethics Board. All participants provided written and informed consent. See [38, 42] for more in-depth information.
Physical health outcomes
For all participants height was measured by asking participants to stand with their back against a wall and feet shoulder width apart, heels touching the wall. They were then asked to take in two large breaths followed by two exhalations and look straight ahead. Height was recorded to the nearest 0.01 m. Weight was measured with a standard clinical scale, and participants were asked to remove excess clothing and shoes for the measurement. Weight was recorded to the nearest 0.01 kg. Body mass index (BMI) was calculated for all participants according to the standard equation of BMI = (body weight [kg]/ height [m2]). BMI Z-Scores were calculated for each sex separately.
Participants with a BMI of 18.5 or lower were removed from the analysis as they were considered underweight and therefore may represent a frail or soon to be frail population (0.39% of original female sample; 1.04% of original male sample). Given the reported relationship between being underweight and increased risk of dementia [43], these participants may not be representative of a normal aging individual. Furthermore, those with class 3 obesity, as defined by BMI of greater than 40 were also excluded. Given the standard bore size of 60 cm used in this study, only females of short stature could have class 3 obesity while still being able to participate in the study, making this obesity sample non-representative of the general population (2% of original female sample removed; 2.1% of original male sample).
The Framingham Risk Factor assessment [44] was employed to take into account cardiovascular risk factors as a potential confound in statistical analyses, given its link with increased risk of dementia [45]. More specifically, sex-specific estimates are utilized, where higher risk is associated with greater points for each of the following: age, high-density lipoprotein cholesterol, total cholesterol, systolic blood pressure (treated vs untreated), smoking status and presence of diabetes.
Cardiovascular fitness and physical activity measures
All participants completed an adapted version of the Global Physical Activity Questionnaire [39], an extensive PA questionnaire which inquired about the type of activities an individual participated in during the previous 12 months (e.g., skiing, running, cycling, walking, etc.), how often each activity was completed for: the number of months in a year (i.e. 1 to 12 months), the number of weeks out of a month (i.e., 1 to 4 weeks), the number of days the activity was completed on a weekly basis (i.e., 1 to 7 days a week), the intensity of each activity (e.g., ranked as light, moderate or intense-specific definitions were provided for each intensity), as well as the hours per session on a weekly basis. Rather than collected on a paper form in person, the questionnaire was completed in an online form, with the exact same questions as the original version. From here, the 2011 Compendium of Physical Activities was referenced for standardized metabolic equivalents (METs) for each type of activity. This allowed for an average of total weekly METs to be calculated for each participant, as well as to segregate activities by overall total METs, referred to as Total ZPA. Moreover, those that are considered moderate to high intensity activities (i.e., > 3 METs), which will be referred to as moderate ZPA from here. It is important to note that as we were aiming to investigate the effects of engaging in PA on brain health, rather than the effects of PA vs no PA, and the interaction between PA and BMI, we excluded individuals within the PA analysis that reported engaging in no PA (27.2% of females and 31% of males did not participate in any PA). Sex disaggregated Z scores for total METs and moderate-high intensity METs were created.
MRI acquisitions
All acquisitions were completed on a 3 T Siemens TIM Trio machine (Siemens Medical Solutions, Erlangen, Germany). A 12-channel head coil was used for acquisitions. An anatomical 1 mm3 magnetization-prepared rapid gradient echo (MPRAGE) sequence (repetition time [TR] = 2300 ms; echo time [TE] = 30 ms; flip angle (FA) = 9°; matrix size = 256 × 256) was employed to quantify grey and white matter volume and for registration purposes. A fluid attenuated inversion recovery (FLAIR) (TR = 5000 ms; TE = 388 ms; inversion time (TI) = 1800 ms; resolution = 1 mm x 1 mm; matrix size = 256 × 256), and T2-weighted sequences were also acquired (TR = 2500 ms; TE = 198 ms; resolution = 0.64 mm × 0.64 mm; matrix size = 320 × 320), alongside the MPRAGE, to estimate the presence and severity of white matter lesions.
Data analysis
GMV and WMV
The T1-weighted MPRAGE images were brain extracted using FSL’s BET (REF) using standard parameters. All MPRAGE images were checked independently by two researchers to ensure the skull and neck were fully removed from all scans. These images were then further preprocessed using SPM’s computational anatomy toolbox (CAT)12 to calculate voxel-based morphometry [46, 47], after the data were segmented into grey and white matter and cerebrospinal fluid (CSF). VBM calculates the difference in grey (GMV) or white matter volume (WMV) of each individual subject compared to the expected volume from a template. Total intracranial volume was adjusted for when investigating WMV and GMV. A statistical map is then created classifying each voxel type into GMV, WMV or CSF according to the highest probability of tissue type.
Registration matrices and warps were calculated to transform GM and WM maps from native T1 space into common MNI space using a non-linear rigid registration with ANTS [48] with b-spline interpolation. The CAT12 internal template was then registered to the common MNI space template and the inverse matrices and warps were applied to each participant’s VBM maps to bring them to the common MNI space. Data were smoothed with a Gaussian filter of 8 mm. We also created an average sex-specific GM and WM mask to restrict voxel-wise analyses to the grey and white matter, respectively.
Region of interest analysis
A hypothesis driven approach was taken to investigate sex-specific influences of ZBMI on regions in GM and WM that are known to decline in aging. More specifically, the LPBA40 atlas [49] was employed to extract GMV and WMV in regions of interest (ROI) in all participants from the following bilateral regions: superior frontal gyri, middle frontal gyri, inferior frontal gyri, superior parietal gyri, superior temporal gyri, medial temporal gyri and hippocampi. Additionally, GMV was extracted from the insular region. Each of these ROIs were chosen because of their well-documented age-related decline [50–52].Prior to ROI extraction, the LPBA atlas was registered using ANTS, as described above, to the same common MNI space utilized throughout. This atlas was then multiplied by each individual’s VBM map to create individual ROI’s that were extracted using the weighted average for each participant with FSLmeants to correct for any potential GM or WM atrophy in GMV and WMV ROI’s, respectively. The GMV and WMV for each participant from each hemispheric region was extracted to a spreadsheet and imported into R/Rstudio (v.24) for further analysis.
Statistical analyses
Statistical analysis of the hypothesis driven data was completed in R and R studio to identify potential relationships between ROIs in GMV and WMV with i) Z-score of BMI; ii) Z-score of physical activity outcomes; iii) and then the interaction of Z-score of BMI with the Z score of total physical activity and Z-score of moderate to intense physical activity. Each of these relationships were investigated separately for males and females. Age (in years), education (in years) and Framingham Risk Factor total score were used as covariates in all analyses. An independent samples t-test was employed to investigated differences between males and females for age, education, MoCA, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), and Framingham total points. Differences in APOE status and smoking status were investigated with a chi-square test. All relationships with each GMV and WMV ROI were investigated as a linear and quadratic relationship with the ‘lm’ package in R. A p-value of 0.05 or less was considered significant. Polynomial regressions were employed, including linear and up to quadratic regressions for all relationships between: i) ZBMI and GMV/WMV ROIs; ii) ZPA and GMV/WMV ROIs; and iii) interaction of ZBMI and ZPA in GMV/WMV ROIs. If both the linear and quadratic regressions were significant for ZBMI, ZPA or the interaction of the two with GMV/WMV ROIs, A Bayesian information criterion was then used to compare the goodness of fit of each type of regression. The regression with the lowest BIC value was the best fit and is presented here[53]. If a significant interaction between ZBMI and ZPA in GMV or WMV ROIs existed, then a Johnson-Neyman test was employed to further investigate the component of the statistical model which was underlying the significant interaction. Briefly, the Johnson-Neyman test is a post-hoc test that allows for the statistically testing of if, and where, a regression interaction goes from non-significant to significant.
Given the large sample size of each sex (n > 50), parametric tests were completed as normality assumptions with this sample should not introduce issues [54–56]. To correct for multiple comparisons in all analyses, a False Discovery Rate was employed using the ‘FDRestimation’ library in Rstudio, to adjust p values for multiple comparisons, only p-values corrected for FDR are presented for the remainder of the manuscript.
Results
A total of 229 older adult females and 93 older adult males participated in this study. Demographics for both sexes can be found in Table 1. Male had lower global cognitive scores as assessed by the MoCA (t = − 2.86; p = 0.004); greater ZBMI (t = 2.86; p = 0.0045); higher SBP (t = 3.13; p = 0.0019); and higher DBP (t = 2.34; p = 0.020). No differences were present between the sexes for age, education, smoking status, total Framingham points or presence of APOE ɛ4 alleles.
Table 1.
Sex-specific demographics
| Females (n = 229) | Males (n = 93) | |
|---|---|---|
| Age (years) | 62.6 ± 4.9 | 62.8 ± 4.9 |
| Education (years) | 15.2 ± 3.3 | 15.9 ± 3.7 |
| MoCA (out of 30) | 28.2 ± 1.4 * | 27.6 ± 1.8 |
| SBP (mmHg) | 125.9 ± 17.4 * | 134.8 ± 34.1 |
| DBP (mmHg) | 73.3 ± 9.3 * | 75.9 ± 8.8 |
| BMI (kg/m2) | 26.1 ± 4.1 * | 27.5 ± 3.8 |
| Smoker (current; ex; never) [%] | 7; 44; 49 | 6; 47; 46 |
| Total Framingham | 11.3 ± 3.5 | 13.5 ± 2.8 |
| APOE Status (0; 1; 2) [%] | 64; 33; 3 | 61; 37;2 |
| BMI status (NW; OW: OB) [%] | 46; 36; 19 | 27; 53; 20 |
| Moderate METs (weekly MET minutes) | 1751.2 ± 1510.8 | 1708.6 ± 1266.6 |
All outcomes reported are in mean ± standard deviation or in percentage; *denotes significant differences between males and females with p < 0.05
Females grey matter volume regions of interest
ZBMI score
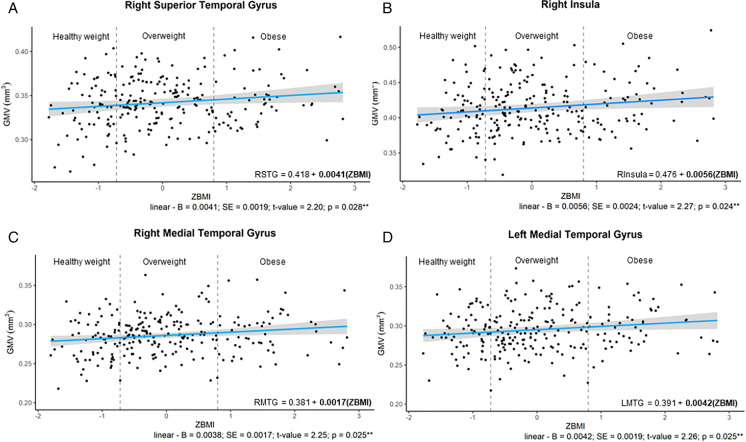
Results of both linear and quadratic regressions demonstrating relationships between ROI-average GMV and ZBMI can be found in Table 1 of the Supplementary Material. GMV in numerous ROIs had linear relationships with ZBMI in females (see Fig. 1). More specifically, linear relationships were identified in the right superior temporal gyrus (adjusted-R2 = 0.153; p = 1.16 × 10−6), the right medial temporal gyrus (adjusted-R2 = 0.151; p = 0.036), the left medial temporal gyrus (adjusted-R2 = 0.162; p = 5.17 × 10−7) and the right insula (adjusted-R2 = 0.060; p = 0.024), where increased BMI was associated with greater GMV in these ROIs.
Fig. 1.
Relationships Between ZBMI in Females and GMV ROIs; A—relationship in right superior temporal gyrus; B – in the right insular gyrus; C – right medial temporal gyrus; D – left medial temporal gyrus. From the y axis to the first dashed vertical grey line are individuals considered healthy weight (BMI = 18.6–24.99 kg/m2; those data points between the two dashed vertical gray line are those considered to be overweight (BMI = 25.0–29.99 kg/m2); individuals to the right of the second vertical dashed line were those with obesity (BMI = 30.0–39.99 kg/m.2). The regression equation present on the graph has bolded the significant component of the polynomial equations. **denotes p-value < 0.05
Moderate ZPA
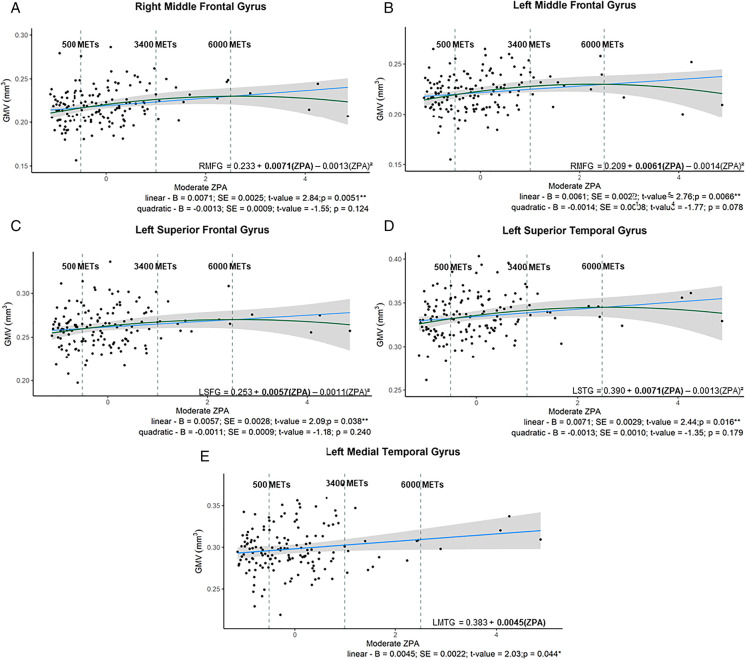
The moderate to vigorous METs physical activity z-score, Moderate ZPA, was associated with a few GMV ROIs (Table 2 in Supplementary Material). More specifically, only the linear component of the quadratic regression was significant in the right middle frontal gyrus (adjusted-R2 = 0.076; p = 0.012), and the bilateral superior frontal gyri (left: adjusted- R2 = 0.045; p = 0.038; right: adjusted-R2 = 0.052; p = 0.049), indicating higher moderate PA amounts corresponded to greater GMV. The left middle frontal gyrus had an inverse quadratic relationship, demonstrating an inverse U shape, (adjusted-R2 = 0.113; p = 0.0027), as did the left superior temporal gyrus (adjusted- R2 = 0.150; p = 0.036. Lastly, greater GMV in the left medial temporal gyrus was linearly associated with increased Moderate ZPA (adjusted- R2 = 0.128; p = 0.044) (see Table 2 in Supplementary Material; Fig. 2A–E).
Fig. 2.
A–E Significant relationships between GMV ROIs and Moderate physical activity (ZPA) in females. A: Quadratic relationship between moderate ZPA and the right middle frontal gyrus; B: quadratic relationship between moderate ZPA and the left middle frontal gyrus; C: quadratic relationship between moderate ZPA and the left superior frontal gyrus; D: quadratic relationship between moderate ZPA and the let superior temporal gyrus; E: linear relationship between moderate ZPA and the left medial temporal gyrus. From the y axis to the first dashed vertical grey line are individuals considered under the recommended PA guidelines (METs minutes per week < 500); those data points between the two dashed vertical grey line are those completing 501 to 3400 METs minutes per week; and individuals to the right of the second vertical dashed line were those completing excess of 6000 METs minutes per week. The regression equation present on the graph has bolded the significant component of the polynomial equations. ** denotes p-value < 0.05
ZBMI and moderate ZPA interactions
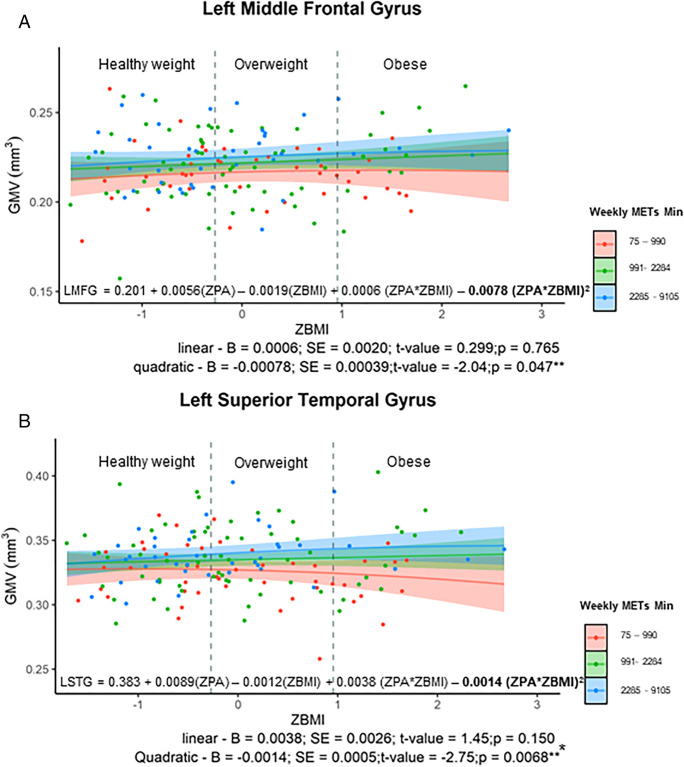
An inverse quadratic relationship was identified for the interaction of ZBMI and moderate ZPA in two regions: the left middle frontal gyrus (adjusted-R2 = 0.098; p = 0.047), and the left superior temporal gyrus (adjusted-R2 = 0.159; p = 0.007) (see Table 3 in Supplementary Material; Fig. 3). A Johnson Neyman test did not reveal a statistically significant difference, so that further inferences on the group driving this interaction of ZBMI and moderate ZPA could not be made using this dataset.
Fig. 3.
(top figure) significant interaction with ZBMI and moderate ZPA in the left middle frontal gyrus. Bottom figure demonstrates the significant interaction of ZBMI with moderate ZPA in the left medial temporal gyrus. For both figures the red line indicates those with the least amount of reported moderate PA; the green were those females reporting the median amount, and finally the blue line indicates those with higher levels of PA. The number of weekly METs minutes are also indicated to provide the actual METs ranges completed for each line. Moreover, the two vertical grey dotted lines provide the healthy weight range (BMI: 18.6–24.99 kg/m2), the overweight range is in between the two vertical lines (BMI: 25–29.99 kg/m2) and those who were considered to be obese to the right of the second vertical line (BMI: 30–39.99 kg/m.2). The regression equation present on the graph has bolded the significant component of the polynomial equations.** denotes significant p-value < 0.05
Males grey matter volume regions of interest
ZBMI score
Several GMV ROIs showed an inverse quadratic relationship (inverse U relationship) between GMV and ZBMI in males, including bilateral superior frontal gyri (right: adjusted-R2 = 0.053; p = 0.0292; left: adjusted-R2 = 0.090; p = 0.022), left superior parietal gyrus (adjusted-R2 = 0.0545; p = 0.040), and the left superior temporal gyrus (adjusted- R2 = 0.150; p = 0.028) (see Table 4 in Supplementary Material and Fig. 4A–D).
Fig. 4.
A–D: Relationships Between ZBMI in Males and GMV ROIs; A – inverse quadratic relationship in right superior frontal gyrus; B – inverse quadratic relationship in the left superior frontal gyrus; C – inverse quadratic relationship in the left superior frontal gyrus; C – inverse quadratic relationship in the left superior parietal gyrus; D – inverse quadratic relationship left superior temporal gyrus. From the y axis to the first dashed vertical grey line are individuals considered healthy weight (BMI = 18.6–24.99 kg/m2; those data points between the two dashed vertical grey line are those considered to be overweight (BMI = 25.0–29.99 kg/m2); individuals to the right of the second vertical dashed line were those with obesity (BMI = 30.0–39.99 kg/m.2). The regression equation present on the graph has bolded the significant component of the polynomial equations.** denotes significant p-value < 0.05
Moderate ZPA
There were no relationships between GMV ROIs and Moderate ZPA for males (p > 0.05).
ZBMI and moderate ZPA interactions
A linear interaction for the interaction of moderate ZPA and ZBMI was identified in one region, the left hippocampus, (adjusted-R2 = 0.261 p = 0.017) (see Table 5 in Supplementary Material; Fig. 5). A Johnson Neyman test revealed that this interaction was driven by the healthy weight males with the highest moderate PA levels having the greatest GMV in the left hippocampus compared to the healthy weight males with the lowest weekly moderate PA (p = 0.01).
Fig. 5.
Significant interaction with ZBMI and total ZPA in the left hippocampus. The red line indicates those with the least amount of reported total PA; the green were those males reporting the median amount, and finally the blue line indicates those with higher levels of PA. The number of weekly METs minutes are also indicated to provide the actual METs ranges completed for each line. Moreover, the two vertical grey dotted lines provide the healthy weight range (BMI: 18.6–24.99 kg/m2), the overweight range is in between the two vertical lines (BMI: 25–29.99 kg/m2) and those who were considered to be obese to the right of the second vertical line (BMI: 30–39.99 kg/m.2). The regression equation present on the graph has bolded the significant component of the polynomial equations. ** denotes significant p-value < 0.05
Females white matter volume regions of interest
ZBMI score
There were no ROI in the WMV that demonstrated a significant relationship with ZBMI in females (p > 0.05).
Moderate ZPA
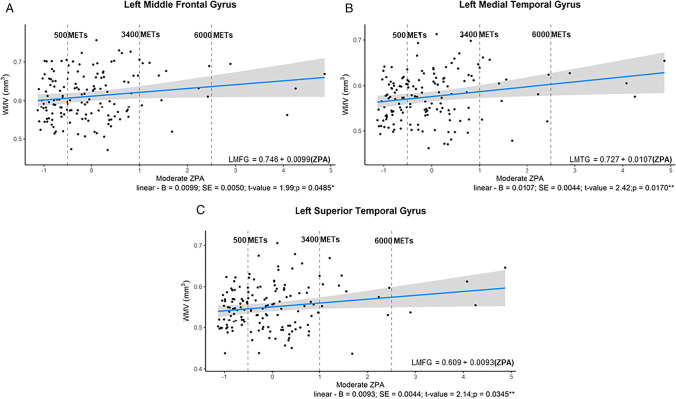
Moderate ZPA demonstrated a positive linear relationship with the left middle frontal gyrus (adjusted- R2 = 0.081; p = 0.049), the left medial temporal gyrus (adjusted- R2 = 0.103; p = 0.0139) and the left superior temporal gyri (adjusted- R2 = 0.049; p = 0.035) (see Table 6 in Supplementary Material; Fig. 6A–C).
Fig. 6.
A–C Significant relationships between WMV ROIs and Moderate ZPA in females. A: linear relationship between moderate ZPA and the left middle frontal gyrus; B: linear relationship between moderate ZPA and the left medial temporal gyrus; C: linear relationship between moderate ZPA and the left superior frontal gyrus. From the y axis to the first dashed vertical gray line are individuals considered under the recommended PA guidelines (METs minutes per week < 500); those data points between the two dashed vertical grey line are those completing 501 to 3400 METs minutes per week; and individuals to the right of the second vertical dashed line were those completing excess of 6000 METs minutes per week. The regression equation present on the graph has bolded the significant component of the polynomial equations. ** denotes p-value < 0.05
ZBMI and moderate ZPA interactions
There were no significant interactions between ZBMI and moderate ZPA in any white matter ROIs (p > 0.05).
Males white matter volume regions of interest
ZBMI score
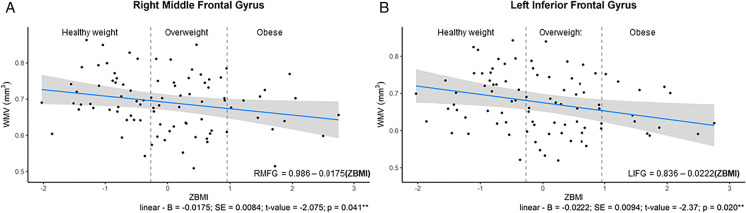
In males there were significantly inverse linear relationships between ZBMI and the right middle frontal gyrus (adjusted- R2 = 0.131; p = 0.041) and the left inferior frontal gyrus (adjusted- R2 = 0.051; p = 0.020) (see Table 7 in Supplementary Material and Fig. 7A–B).
Fig. 7.
A–B Relationships Between ZBMI in Males and WMV ROIs; A – inverse linear relationship in right middle frontal gyrus; B – inverse linear relationship in the left inferior frontal gyrus. From the y axis to the first dashed vertical grey line are individuals considered healthy weight (BMI = 18.6–24.99 kg/m2; those dots between the two dashed vertical gray line are those considered to be overweight (BMI = 25.0–29.99 kg/m2); individuals to the right of the second vertical dashed line were those with obesity (BMI = 30.0–39.99 kg/m.2). The regression equation present on the graph has bolded the significant component of the polynomial equations. ** denotes significant p-value < 0.05
Moderate ZPA
The linear component of the quadratic regression between moderate ZPA and right hippocampal WMV demonstrated a significant relationship (adjusted- R2 = 0.085; p = 0.048) (see Table 8 in Supplementary Material; Fig. 8).
Fig. 8.
Significant relationships between WMV ROIs and moderate ZPA in males the right hippocampus. From the y axis to the first dashed vertical gray line are individuals considered under the recommended PA guidelines (METs minutes per week < 500); those data points between the two dashed vertical grey line are those completing 501 to 1700 METs minutes per week; and individuals to the right of the second vertical dashed line were those completing excess of 3000 METs minutes per week. The regression equation present on the graph has bolded the significant component of the polynomial equations. *denotes significant p-value < 0.05
ZBMI and moderate ZPA interactions
There were no interactions between ZBMI and moderate ZPA in any white matter ROIs for the males (p > 0.05).
Discussion
We examined the sex-specific relationships between cerebral macrostructural outcomes (GMV and WMV), BMI and PA in a generally healthy group of older adults. In females, our data demonstrated that with higher BMI, GMV was increased in several regions showing a benefit of higher BMI. Conversely, in males, an inverse U shape was revealed, indicating those males who were classified as overweight had the largest GMV in ROIs compared to the normal or obese individuals. For moderate ZPA, most regions in females demonstrated an inverse U relationship with GMV suggesting a ceiling effects of PA for females, whereas males had no relationships between moderate ZPA and GMV. The interaction in females between moderate to vigorous PA and ZBMI revealed that those with the highest levels of moderate to vigorous PA across the BMI spectrum had significantly higher GMV in all significant ROIs compared to those with the lowest amounts of PA. In opposition to females, the interaction between ZPA and ZBMI indicates that normal weight males with the highest levels of PA have the greatest GMV compared to those normal weight individuals completing the least amount of moderate PA, though this did not appear to be the case in overweight or obese males. It is worth noting, that a post-hoc power analysis of this interaction revealed a power of 15%, thus, a much larger sample of males across the BMI and PA spectrum, and especially in the obese category, which was sparse in this sample, is necessary to confirm these results.
In females there were no relationships between ZBMI, ZPA and WMV ROIs, except in a few regions where higher moderate ZPA was associated with greater WMV. Conversely males demonstrated a negative relationship between ZBMI and WMV regions, and a positive relationship between moderate ZPA and WMV.
Sex-specific relationships in BMI and structural outcomes
The results presented here in both males and females, separately, are consistent with the existence of the obesity paradox, whereby weight beyond the normal range is found to be protective for brain structure in aging [4, 57]. However, males and females may experience these benefits within a different weight range. In females, our data is consistent with the obesity paradox as observed by [4, 14], with a positive relationship between BMI and GMV so that those with the highest BMI had the largest GMV within temporal regions. On the other hand, data in males indicated an optimal BMI range, with an inverse U relationship where overweight BMI is most protective for GMV. This more closely resembles the original obesity paradox proposed by Gruberg and colleagues [58] as well as work by Pegueroles [57]. It is therefore possible that some of these discrepancies in the literature reflect sex differences and are biased by the distribution of both sex and weight ranges for each sex. For example, while Taki et al. identified a similar linear relationship as ours in their mixed sample [14], only 2% of their sample were considered obese, as opposed to approximately 20%o in ours, making detection of an inverse U relationship more challenging.
To date, there have been very few studies investigating the sex-specific relationships between obesity and brain health, rather introducing sex as a covariate, revealing a significant gap to the current literature. The existing data is mixed, with some work suggesting that females are more likely to experience atrophy or cognitive decline as a consequence of obesity than males[59, 60] whereas others found the opposite, where males were more negatively affected by obesity [61]. Our results of females seemingly being protected by overweight on grey matter atrophy could be related to the fact that obesity in isolation may not be uniquely detrimental to health. It may be that obesity contributes to poor vascular and metabolic health through adipose tissue inflammatory signaling [62], but not when obesity is not associated with chronic low grade inflammation. Therefore, taking these downstream effects into account through the Framingham risk score in statistical analyses isolates the effects of obesity and shows that it is not in fact an independent contributor to decreased structural brain health in this aging sample. Thus, it is possible that as males develop cardiovascular risk factors earlier in life than females (due to protective effects of estrogen in females), males’ levels of inflammation could have been higher here. Indeed, females in our sample had lower Framingham Risk Factor scores than males, indicating a more limited number of cardiovascular risk factors. Future research should more carefully investigate the impact of cardiovascular risk factors in the relationship between BMI and cerebral structure in males and females. In addition, exploring the role of inflammatory markers may help understand further these sex-specific relationships in GMV.
Interestingly, no significant relationships were identified between ZBMI and WMV for females, though males with greater BMI had lower WMV. Previous work has also found this lack of relationship [12, 22], likely due to the unspecific nature of BMI as a marker of obesity. Central adiposity and visceral fat may be more likely to demonstrate a relationship with WMV and WMH lesions [21]. Moreover, a stronger relationship with GMV than WMV, has been somewhat consistently found, and been hypothesized a greater sensitivity of GMV over WMV to the low-grade chronic inflammation associated with obesity [63]. However, obesity is consistently reported to affect white matter microstructure [64, 65], indicating early changes to white matter since it is documented that microstructure declines about two decades before white matter macrostructure [66]. Taken together, it is possible that our sample is too young to capture volume changes in white matter. Thus, future work should not only investigate the sex differences in BMI on microstructure utilizing acquisitions like diffusion weighted imaging or magnetization transfer, but also explore these relationships in a larger age range.
Sex-specific relationships with physical activity and macrostructural outcomes
Females in this sample showed that with increased moderate ZPA and the GMV in the left medial temporal gyrus was greater. Yet, other frontal, temporal and hippocampal regions demonstrated an inverse U shape, indicating that there was likely a ceiling for moderate-vigorous activity possessing beneficial effects. However, it should be noted that few females in our sample completed a high amount of PA and are likely not representative of the overall sample trends. Conversely, total ZPA (see supplementary Fig. 1A-D) demonstrated only linear positive relationships with GMV ROIs. The discrepancy between the two intensities of PA is especially notable in females, as it suggests the presence of a dose response upper limit for the gains of moderate to vigorous activities on overall brain health. In fact, previous work found that, although increasing PA was associated with increased GMV, there was no additional benefit once activity guidelines were met [67]. Here, the ceiling effect of PA occurred above the recommended guidelines of 500 MET minutes/week [68]. Our data suggests this ceiling may occur at around 3000 MET minutes per week, corresponding to about 8.5 h of moderate to vigorous PA. Interestingly, Wood et al. (2016) found that master athletes who were engaged in > 15 h of weekly activity showed no significant differences in WMH, or subcortical gray matter compared to active older adults [67]. Indeed, the data indicated diminishing returns, or a ceiling effect, on cortical decline, as the master athletes had the same decline as the active older adults. Our work and previous studies therefore strongly suggest the presence indicate that there could be diminishing returns of increasing moderate-vigorous PA past a certain dosage, with a ceiling effect in the preservation of GMV.
Males demonstrated a significant positive linear relationship in the left hippocampus for moderate PA. This was not bilaterally evident, though the right hippocampus approached significance, so is likely due to insufficient power. In any case, this finding in the hippocampus confirms previous work by Barha and colleagues (2020) who revealed that only older males who spent a greater amount of time walking in the previous 1 to 10 years had greater hippocampal volume[69]. No other regions had a significant relationship with PA, in stark contrast to a recent paper showing that males are more likely to benefit from the exact same amounts of PA as females for GMV, as a function of PA lowering inflammatory markers more in males [36]. The authors suggested that this may be attributable to differences in immune function between males and females. Males tend to have a lower immune functioning [70] and may therefore benefit more from the immune-enhancing properties of PA than females, who already have a more active immune system [36].
A comparison of the amount of PA reported by each sex can also shed light on potential dose–response effects and how this may interact with sex. In our sample, females participated in a greater amount of total and moderate to vigorous PA than males. Therefore, our sampling of the dose–response relationship is not identical between sexes, potentially explaining the lack of relationship between PA and GMV across most regions in males. It is possible that males might require more PA to see the effects detected in females, perhaps due to the higher number of cardiovascular risk factors and lower immune function in males.
Interaction of BMI and PA on macrostructural health
Early work identified that fit obese males were no more likely to die of a cardiovascular event compared to their lean and equally fit counterparts [71]. This led to the “fat but fit” hypothesis, whereby the positive effects of cardiovascular fitness, or greater PA levels, can attenuate the negative consequences associated with obesity. Other work has observed a similar effect in cognitive health, where those who were obese but fit performed significantly better on cognitive tests compared to their unfit obese counterparts. In fact, high fit obese individuals demonstrated the same cognitive functioning as their non-obese fit counterparts [72]. To our knowledge, only one study to date has investigated the presence of these effects using neuroimaging. Knight and colleagues [73] identified that, regardless of sex, individuals who were overweight to obese had significantly decreased cerebral blood flow, but that this reduction in blood flow was attenuated in those overweight individuals who reported higher levels of PA. Individuals who were obese did not seemingly gain this benefit to cerebral blood flow. These results by Knight et al. are somewhat in contrast to our findings of high PA levels having beneficial effects regardless of BMI for females, but are consistent with our results in males[73]. As Knight et al. did not complete sex-disaggregated analyses, it may be that their results are biased by the relationship between obesity and PA in the male portion of their sample.
Limitations
This study has a few limitations that should be noted when interpreting these results. Firstly, the major limitation to this study is the use of self-reported PA rather than a more objective measure. However, the Global Physical Activity Questionnaire is comprehensive, incorporating seasonality, numerous activities and was completed anonymously online, likely reducing the extent of over reporting.
Secondly, the data utilized here were cross-sectional and thus, cannot be used to investigate the temporality of the relationships among PA, obesity, and macrostructural outcomes. Future work employing longitudinal studies, including additional information about past weight and PA patterns could provide more clarity on these interactions and sex-distinctions.
Thirdly, our sample contained a greater proportion of females and is thus not equivalently powered for looking at these relationships in males. A future study utilizing sex and age matched individuals is warranted.
Moreover, BMI is known to have important limitations as a marker of adiposity since is not overly representative of where adipose tissue is stored or the type of adipose tissue present in individuals. For example, males possess more abdominal visceral fat compared to females [74], whereas females are known to accumulate more subcutaneous fat in the femoral and gluteal regions [75]. Visceral fat is known to have more inflammatory and toxic consequences for the body [76], as well as the brain [21]. Thus, future work should aim to include markers of adiposity that can provide further insight into form of adipose tissue, from something as simple as waist circumference to more advanced measures like dual-energy x-ray absorptiometry or a computed tomography scan to define the amount of subcutaneous versus visceral fat tissue present in each area of the body. Notably, it has recently been proposed it is likely the number of years that an individual has been overweight/obese that is the critical element, ‘obesity-years’, rather than at certain time points [77]. For example, those who maintain a stable weight and BMI into aging have the lowest incidence of dementia [78], and another group demonstrated that those who were overweight and had great cardiovascular risk at midlife, but had significant weight decrease into later life, had the highest risk of poor outcomes in late life [79]. Thus, future studies should aim to collect more data on years of obesity, and lifespan weight changes, to further understand the influence of the timing of obesity.
Moreover, given the role that inflammatory markers have in causing the cascade of vascular changes due to obesity, future work should also investigate the potential modulatory role that inflammatory markers likely have on this interaction between BMI and PA. This is of particular relevance given that there are known differences in inflammatory markers due to sexual dimorphisms [80]; obesity [81] as previously stated, and PA [82].
Finally, the macrostructural outcomes investigated, have been shown to be sensitive but physiologically ambiguous in isolation [83]. Future work should also employ sequences that can investigate combinations of microstructural outcomes, such as diffusion-weighted imaging or magnetic transfer MRI sequences to better understand the underlying changes in brain health related to obesity and PA.
Overall, we identified that males and females demonstrate distinct relationships among BMI,PA, and bulk volumetrics. Overall, females demonstrated that increasing BMIwas related to greater GMV ROIs, whereas males demonstrated that overweight individuals had the most GM Females had an inverse U relationship between moderate to intense PA levels and GMV, indicating saturation effect for the beneficial effects of increasing moderate PA levels, whereas males demonstrated no relationships with GMV ROIs and moderate PA. Finally, across the BMIspectrum, females with the higher levels of PA were found to have more GMV than those with the least amount of PA, with a diminishing effect of PA on GMV at the highest BMIlevels, though larger samples may be necessary to observe these effects in females. Males revealed unique findings as compared to females, where normal weight males participating in the most amount of PA had the highest GMV within the hippocampus compared to those normal weight males completing the least amount of PA, though this should be interpreted with caution due to the small number of obese males. Taken together, this work highlights the need to investigate females and males separately, rather than using sex as a covariate. Future work, in a larger sample, employing more objective measures of PA and more accurate measure of adiposity and body composition are necessary to validate these findings. Additionally, future studies should examine inflammatory markers to further elucidate underlying mechanisms for the relationships identified here, as aging, obesity and PA all have a unique influence on inflammation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
CONSORTIUM – PREVENT-AD Research Group
Anne Labontéf,g, Alexa Pichet Binettef−h, Axel Mathieug, Cynthia Picardf−h, Doris Deaf,g, Claudio Cuelloh, Alan Evansg,h,i, Christine Tardiff,g, Gerhard Mulhauph, Jamie Nearf,h, Jeannie-Marie Leoutsakosk, John CS Bretinerf−h, Judes Poirierf−h, Lisa-Marie Müntermh, Louis Collinsg−i, Mallar Chakravartyf−h, Natasha Rajahf−h, Pedro Rosa-Netof−h, Pierre Bellecc,g,m, Pierre Etiennef−h, Pierre Orbanc,f,g,m, Rick Hogeg−i, Serge Gauthierf−h, Sylvia Villeneuevef−h, Véronique Bohbotf−h, Vladimir Fonovh,i, Yasser Ituria-Medinag−i, Holly Newbold-Foxf, Jacob Vogelf,g, Jennifer Tremblay-Mercierf,g, Justin Katf,g,i, Justin Mirong−i, Masha Dadarh,i, Marie-Elyse Lafaille-Magnanf−h, Pierre-François Meyerf−h, Samir Dash−i, Julie Gonneaudf−h, Gülebru Ayrancif−h, Tharick A Pascoalf−h, Sander CJ Verfaillief,g,n, Sarah Farzinf, Alyssa Salaciakf, Stephanie Tullof,h, Etienne Vachon-Presseauf,o, Leslie-Ann Daousg, Theresa Köbeg,h, Melissa McSweeneyh, Nathalie Nilssonf−h, Morteza Pishnamazif−h, Chirstophe Bedettif, Louise Hudong, Claudia Grecof,g, Frederic St-Ongef−h, Sophie Boutinf,g, Maiya R Geddesf−i, Simon Ducharmef−h, Gabriel Jeanf,g, Elisabeth Sylvainf,g, Marie-Josée Éliseg,h, Gloria Leblond-Baccichetf,g, Julie Baillyf, Bery Mohammediyanf,g, Jordana Remzf, Jean-Paul Soucyh,i
fDouglas Mental Health University Institute, Montreal Canada H4H 1R3.
gSTOP-AD Centre, Montreal Canada, Montreal Canada H4H 1R3.
hMcGill University, Montreal Canada H3A 0G4.
iMcConnell Brain Imaging Centre, Montreal Neurological Institute, Montreal Canada H3A 2B4.
kJohn Hopkins University, Baltimore USA MD 21,218.
c Centre de Recherche de l’Institut Universitaire de Gériatrie de Montréal, 4545 Queen Mary Rd, Montréal Canada H3W 1W6.
mUniversity of Montreal, Montreal Canada H3T 1J4.
oNorthwestern Univeristy, Evanston, USA, IL 60,208.
nAlzheimer’s Center, University of Amsterdam, Amsterdam Netherlands 1081.
Funding
This work was supported by: Data used in the preparation of this article were obtained from the Pre-symptomatic Evaluation of Novel or Experimental Treatments for Alzheimer’s Disease (PREVENT-AD) program data release 6.0. PREVENT-AD was launched in 2011 as a $13.5 million, 7-year public–private partnership using funds provided by McGill University, the Fonds de Recherche du Québec – Santé (FRQ-S), an unrestricted research grant from Pfizer Canada, the Levesque Foundation, the Douglas Hospital Research Centre and Foundation, the Government of Canada, and the Canada Fund for Innovation. Private sector contributions are facilitated by the Development Office of the McGill University Faculty of Medicine and by the Douglas Hospital Research Centre Foundation (http://www.douglas.qc.ca/). Alzheimer’s Association Research grant (SV), project grant through Canadian Institutes of Health Research (SV); Henry J.M. Barnett Heart and Stroke Foundation New Investigator Award (CJG), Michal and Renata Hornstein Chair in Cardiovascular Imaging (CJG); Mirella and Lino Saputo Research Chair in Cardiovascular Health and the Prevention of Cognitive Decline from the Universite de Montreal at the Montreal Heart Institute (LB).
Data availability
All data used in the present study are either publicly available (PREVENT-AD MRIs and demographics: https://openpreventad.loris.ca) or can be shared upon reasonable request and approval by the study scientific committees and/or institutional review boards. Data used in preparation of this article were obtained from the Pre-symptomatic Evaluation of Novel or Experimental Treatments for Alzheimer’s Disease (PREVENT-AD) program (https://douglas.research.mcgill.ca/stop-ad-centre), data release 6.0. A complete listing of PREVENT-AD Research Group can be found in the PREVENT-AD database: https://preventad.loris.ca/acknowledgements/acknowledgements.php?date=%5B2022-08-02%5D.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Brittany Intzandt, Email: brittany.intzandt@sri.utoronto.ca.
Claudine J. Gauthier, Email: claudine.gauthier@concordia.ca
PREVENT-AD Research group:
Anne Labonté, Alexa Pichet Binette, Axel Mathieu, Cynthia Picard, Doris Dea, Claudio Cuello, Alan Evans, Christine Tardif, Gerhard Mulhaup, Jamie Near, Jeannie-Marie Leoutsakos, John C. S. Bretiner, Judes Poirier, Lisa-Marie Münterm, Louis Collins, Mallar Chakravarty, Natasha Rajah, Pedro Rosa-Neto, Pierre Bellec, Pierre Etienne, Pierre Orban, Rick Hoge, Serge Gauthier, Sylvia Villeneueve, Véronique Bohbot, Vladimir Fonov, Yasser Ituria-Medina, Holly Newbold-Fox, Jacob Vogel, Jennifer Tremblay-Mercier, Justin Kat, Justin Miron, Masha Dadar, Marie-Elyse Lafaille-Magnan, Pierre-François Meyer, Samir Das, Julie Gonneaud, Gülebru Ayranci, Tharick A. Pascoal, Sander C. J. Verfaillie, Sarah Farzin, Alyssa Salaciak, Stephanie Tullo, Etienne Vachon-Presseau, Leslie-Ann Daous, Theresa Köbe, Melissa McSweeney, Nathalie Nilsson, Morteza Pishnamazi, Chirstophe Bedetti, Louise Hudon, Claudia Greco, Frederic St-Onge, Sophie Boutin, Maiya R. Geddes, Simon Ducharme, Gabriel Jean, Elisabeth Sylvain, Marie-Josée Élise, Gloria Leblond-Baccichet, Julie Bailly, Bery Mohammediyan, Jordana Remz, and Jean-Paul Soucy
References
- 1.S. C. Government of Canada. Overweight and obese adults, 2018, Jun. 25, 2019. Table 13-10-0096-01 Health characteristics, annual estimates. https://www150.statcan.gc.ca/n1/pub/82-625-x/2019001/article/00005-eng.htm. Accessed 8 Apr 2022. 10.25318/1310009601-eng.
- 2.Hruby A, Hu FB. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics. 2015;33(7):673–689. doi: 10.1007/s40273-014-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingston G, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedditizi E, Peters R, Beckett N. The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing. 2016;45(1):14–21. doi: 10.1093/ageing/afv151. [DOI] [PubMed] [Google Scholar]
- 5.Albanese E, et al. Overweight and Obesity in Midlife and Brain Structure and Dementia 26 Years Later. Am J Epidemiol. 2015;181(9):672–679. doi: 10.1093/aje/kwu331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masouleh SK, et al. Higher body mass index in older adults is associated with lower gray matter volume: implications for memory performance. Neurobiol Aging. 2016;40:1–10. doi: 10.1016/j.neurobiolaging.2015.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Pannacciulli N, Le DSNT, Chen K, Reiman EM, Krakoff J. Relationships between plasma leptin concentrations and human brain structure: A voxel-based morphometric study. Neurosci Lett. 2007;412(3):248–253. doi: 10.1016/j.neulet.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raji CA, et al. Brain structure and obesity. Hum Brain Mapp. 2009;31(3):353–364. doi: 10.1002/hbm.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walther K, Birdsill AC, Glisky EL, Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp. 2010;31(7):1052–1064. doi: 10.1002/hbm.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moscovitch M, Winocur G. “The neuropsychology of memory and aging”, in The handbook of aging and cognition. Hillsdale, NJ, US: Lawrence Erlbaum Associates Inc; 1992. pp. 315–372. [Google Scholar]
- 11.West R. An application of prefrontal cortex theory to cognitive aging. Psychol Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- 12.Debette S, et al. Abdominal obesity and lower gray matter volume: a Mendelian randomization study. Neurobiol Aging. 2014;35(2):378–386. doi: 10.1016/j.neurobiolaging.2013.07.022. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y, et al. Interaction Effect of Sex and Body Mass Index on Gray Matter Volume. Front Hum Neurosci. 2019;13:360. doi: 10.3389/fnhum.2019.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taki Y, et al. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity (Silver Spring) 2008;16(1):119–124. doi: 10.1038/oby.2007.4. [DOI] [PubMed] [Google Scholar]
- 15.Cooper AJ, Gupta SR, Moustafa AF, Chao AM. Sex/Gender Differences in Obesity Prevalence, Comorbidities, and Treatment. Curr Obes Rep. 2021;10(4):458–466. doi: 10.1007/s13679-021-00453-x. [DOI] [PubMed] [Google Scholar]
- 16.Schorr M, et al. Sex differences in body composition and association with cardiometabolic risk. Biol Sex Differ. 2018;9(1):28. doi: 10.1186/s13293-018-0189-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isacco L, Ennequin G, Boisseau N. Influence of the different hormonal status changes during their life on fat mass localisation in women: a narrative review. Arch Physiol Biochem. 2021;10:1–6. 10.1080/13813455.2021.1933045. [DOI] [PubMed]
- 18.Rathnayake N, Rathnayake H, Lekamwasam S. Age-Related Trends in Body Composition among Women Aged 20–80 Years: A Cross-Sectional Study”. Journal of Obesity. 2022;2022:e4767793. doi: 10.1155/2022/4767793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnoldussen IAC, Gustafson DR, Leijsen EMC, de Leeuw F-E, Kiliaan AJ. Adiposity is related to cerebrovascular and brain volumetry outcomes in the RUN DMC study. Neurology. 2019;93(9):e864–e878. doi: 10.1212/WNL.0000000000008002. [DOI] [PubMed] [Google Scholar]
- 20.Ho AJ, et al. The effects of physical activity, education, and body mass index on the aging brain. Hum Brain Mapp. 2011;32(9):1371–1382. doi: 10.1002/hbm.21113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gunstad J, et al. Relationship Between Body Mass Index and Brain Volume in Healthy Adults. Int J Neurosci. 2008;118(11):1582–1593. doi: 10.1080/00207450701392282. [DOI] [PubMed] [Google Scholar]
- 23.Hersi M, Irvine B, Gupta P, Gomes J, Birkett N, Krewski D. Risk factors associated with the onset and progression of Alzheimer’s disease: A systematic review of the evidence. Neurotoxicology. 2017;61:143–187. doi: 10.1016/j.neuro.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Colcombe SJ, et al. Aerobic Exercise Training Increases Brain Volume in Aging Humans. The Journals of Gerontology: Series A. 2006;61(11):1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 25.Hamer M, Sharma N, Batty GD. Association of objectively measured physical activity with brain structure: UK Biobank study. J Intern Med. 2018;284(4):439–443. doi: 10.1111/joim.12772. [DOI] [PubMed] [Google Scholar]
- 26.Raichlen DA, Klimentidis YC, Bharadwaj PK, Alexander GE. Differential associations of engagement in physical activity and estimated cardiorespiratory fitness with brain volume in middle-aged to older adults. Brain Imaging Behav. 2020;14(5):1994–2003. doi: 10.1007/s11682-019-00148-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arenaza-Urquijo EM, et al. Distinct effects of late adulthood cognitive and physical activities on gray matter volume. Brain Imaging Behav. 2017;11(2):346–356. doi: 10.1007/s11682-016-9617-3. [DOI] [PubMed] [Google Scholar]
- 28.Halloway S, Arfanakis K, Wilbur J, Schoeny ME, Pressler SJ. Accelerometer Physical Activity is Associated with Greater Gray Matter Volumes in Older Adults Without Dementia or Mild Cognitive Impairment. J Gerontol B Psychol Sci Soc Sci. 2019;74(7):1142–1151. doi: 10.1093/geronb/gby010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rovio S, et al. Leisure-time physical activity at midlife and the risk of dementia and Alzheimer’s disease. Lancet Neurology. 2005;4(11):705–711. doi: 10.1016/S1474-4422(05)70198-8. [DOI] [PubMed] [Google Scholar]
- 30.Erickson KI, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erickson KI, Leckie RL, Weinstein AM. Physical activity, fitness, and gray matter volume. Neurobiol Aging. 2014;35(Suppl 2):S20–S28. doi: 10.1016/j.neurobiolaging.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steffener J, Habeck C, O’Shea D, Razlighi Q, Bherer L, Stern Y. Differences between chronological and brain age are related to education and self-reported physical activity. Neurobiol Aging. 2016;40:138–144. doi: 10.1016/j.neurobiolaging.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chieffi S, et al. Neuroprotective Effects of Physical Activity: Evidence from Human and Animal Studies. Front Neurol. 2017;8:188. doi: 10.3389/fneur.2017.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Intzandt B, et al. Comparing the effect of cognitive vs. exercise training on brain MRI outcomes in healthy older adults: A systematic review. Neurosci Biobehav Rev. 2021;128:511–533. doi: 10.1016/j.neubiorev.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Varma VR, Chuang Y, Harris GC, Tan EJ, Carlson MC. Low-intensity daily walking activity is associated with hippocampal volume in older adults. Hippocampus. 2015;25(5):605–615. doi: 10.1002/hipo.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casaletto KB, et al. Sexual dimorphism of physical activity on cognitive aging: Role of immune functioning. Brain Behav Immun. 2020;88:699–710. doi: 10.1016/j.bbi.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanders A-M, et al. Linking objective measures of physical activity and capability with brain structure in healthy community dwelling older adults”. Neuroimage Clin. 2021;31:102767. doi: 10.1016/j.nicl.2021.102767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Breitner JCS, Poirier J, Etienne PE, Leoutsakos JM. Rationale and Structure for a New Center for Studies on Prevention of Alzheimer’s Disease (StoP-AD) J Prev Alzheimers Dis. 2016;3(4):236–242. doi: 10.14283/jpad.2016.121. [DOI] [PubMed] [Google Scholar]
- 39.Friedenreich CM, Courneya KS, Bryant HE. The lifetime total physical activity questionnaire: development and reliability. Med Sci Sports Exerc. 1998;30(2):266–274. doi: 10.1097/00005768-199802000-00015. [DOI] [PubMed] [Google Scholar]
- 40.Nasreddine ZS, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 41.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 42.Tremblay-Mercier J, et al. (2021) Open science datasets from PREVENT-AD, a longitudinal cohort of pre-symptomatic Alzheimer’s disease. Neuroimage Clin. 2021;31:102733. doi: 10.1016/j.nicl.2021.102733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albanese E, et al. Body mass index in midlife and dementia: Systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimers Dement (Amst) 2017;8:165–178. doi: 10.1016/j.dadm.2017.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D’Agostino RB, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 45.Song R, et al. Associations Between Cardiovascular Risk, Structural Brain Changes, and Cognitive Decline. J Am Coll Cardiol. 2020;75(20):2525–2534. doi: 10.1016/j.jacc.2020.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 47.Gaser C, Dahnke R, Thompson PM, Kurth F, Luders E. Alzheimer's disease neuroimaging initiative. CAT - A computational anatomy toolbox for the analysis of structural MRI data. 2022. bioRxiv. 2022.06.11.495736. 10.1101/2022.06.11.49573. [DOI] [PMC free article] [PubMed]
- 48.Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shattuck DW, et al. Construction of a 3D probabilistic atlas of human cortical structures. Neuroimage. 2008;39(3):1064–1080. doi: 10.1016/j.neuroimage.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: Evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12(1):95–114. doi: 10.1037/0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- 51.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci. 2003;23(8):3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crivello F, Tzourio-Mazoyer N, Tzourio C, Mazoyer B. Longitudinal Assessment of Global and Regional Rate of Grey Matter Atrophy in 1,172 Healthy Older Adults: Modulation by Sex and Age”. PLOS ONE. 2014;9(12):e114478. doi: 10.1371/journal.pone.0114478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raftery AE. Bayesian Model Selection in Social Research. Sociol Methodol. 1995;25:111–163. doi: 10.2307/271063. [DOI] [Google Scholar]
- 54.Elliott AC, Woodward WA. Statistical analysis quick reference guidebook: With SPSS examples. SAGE; 2007.
- 55.Ghasemi A, Zahediasl S. Normality Tests for Statistical Analysis: A Guide for Non-Statisticians. Int J Endocrinol Metab. 2012;10(2):486–489. doi: 10.5812/ijem.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.J. Pallant, SPSS Survival Manual: A Step by Step Guide to Data Analysis Using IBM SPSS, 7th ed. London: Routledge, 2020. 10.4324/9781003117452.
- 57.Pegueroles J, et al. Obesity and Alzheimer’s disease, does the obesity paradox really exist? A magnetic resonance imaging study. Oncotarget. 2018;9(78):34691–34698. doi: 10.18632/oncotarget.26162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gruberg L, et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39(4):578–584. doi: 10.1016/s0735-1097(01)01802-2. [DOI] [PubMed] [Google Scholar]
- 59.Hayden KM, et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis Assoc Disord. 2006;20(2):93–100. doi: 10.1097/01.wad.0000213814.43047.86. [DOI] [PubMed] [Google Scholar]
- 60.Kim SE, et al. Sex-specific relationship of cardiometabolic syndrome with lower cortical thickness. Neurology. 2019;93(11):e1045–e1057. doi: 10.1212/WNL.0000000000008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Espeland MA, et al. Sex-Related Differences in Brain Volumes and Cerebral Blood Flow Among Overweight and Obese Adults With Type 2 Diabetes: Exploratory Analyses From the Action for Health in Diabetes Brain Magnetic Resonance Imaging Study. J Gerontol A Biol Sci Med Sci. 2020;75(4):771–778. doi: 10.1093/gerona/glz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koenen M, Hill MA, Cohen P, Sowers JR. Obesity, Adipose Tissue and Vascular Dysfunction. Circ Res. 2021;128(7):951–968. doi: 10.1161/CIRCRESAHA.121.318093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vachharajani V, Granger DN. Adipose tissue: a motor for the inflammation associated with obesity. IUBMB Life. 2009;61(4):424–430. doi: 10.1002/iub.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alfaro FJ, Gavrieli A, Saade-Lemus P, Lioutas V-A, Upadhyay J, Novak V. White matter microstructure and cognitive decline in metabolic syndrome: a review of diffusion tensor imaging. Metabolism. 2018;78:52–68. doi: 10.1016/j.metabol.2017.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dekkers IA, Jansen PR, Lamb HJ. Obesity, Brain Volume, and White Matter Microstructure at MRI: A Cross-sectional UK Biobank Study. Radiology. 2019;291(3):763–771. doi: 10.1148/radiol.2019181012. [DOI] [PubMed] [Google Scholar]
- 66.Irimia A. Cross-Sectional Volumes and Trajectories of the Human Brain, Gray Matter, White Matter and Cerebrospinal Fluid in 9473 Typically Aging Adults. Neuroinform. 2021;19(2):347–366. doi: 10.1007/s12021-020-09480-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wood KN, Nikolov R, Shoemaker JK. Impact of Long-Term Endurance Training vs. Guideline-Based Physical Activity on Brain Structure in Healthy Aging. Front Aging Neurosci. 2016;8:155. doi: 10.3389/fnagi.2016.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lauer EE, Jackson AW, Martin SB, Morrow JR. Meeting USDHHS Physical Activity Guidelines and Health Outcomes. Int J Exerc Sci. 2017;10(1):121–127. doi: 10.70252/AZKZ3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barha CK, Best JR, Rosano C, Yaffe K, Catov JM, Liu-Ambrose T. Sex-Specific Relationship Between Long-Term Maintenance of Physical Activity and Cognition in the Health ABC Study: Potential Role of Hippocampal and Dorsolateral Prefrontal Cortex Volume. J Gerontol A Biol Sci Med Sci. 2020;75(4):764–770. doi: 10.1093/gerona/glz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–38. 10.1038/nri.2016.90. [DOI] [PubMed]
- 71.Wei M, et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA. 1999;282(16):1547–1553. doi: 10.1001/jama.282.16.1547. [DOI] [PubMed] [Google Scholar]
- 72.Boidin M, et al. Obese but Fit: The Benefits of Fitness on Cognition in Obese Older Adults. Can J Cardiol. 2020;36(11):1747–1753. doi: 10.1016/j.cjca.2020.01.005. [DOI] [PubMed] [Google Scholar]
- 73.Knight SP, et al. Obesity is associated with reduced cerebral blood flow – modified by physical activity. Neurobiol Aging. 2021;105:35–47. doi: 10.1016/j.neurobiolaging.2021.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Grauer WO, Moss AA, Cann CE, Goldberg HI. Quantification of body fat distribution in the abdomen using computed tomography. Am J Clin Nutr. 1984;39(4):631–637. doi: 10.1093/ajcn/39.4.631. [DOI] [PubMed] [Google Scholar]
- 75.Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. 2012;3(1):13. doi: 10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chait A, den Hartigh LJ. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front Cardiovasc Med. 2020;7:22. doi: 10.3389/fcvm.2020.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abdullah A, et al. The number of years lived with obesity and the risk of all-cause and cause-specific mortality. Int J Epidemiol. 2011;40(4):985–996. doi: 10.1093/ije/dyr018. [DOI] [PubMed] [Google Scholar]
- 78.Power BD, Alfonso H, Flicker L, Hankey GJ, Yeap BB, Almeida OP. Changes in body mass in later life and incident dementia. Int Psychogeriatr. 2013;25(3):467–478. doi: 10.1017/S1041610212001834. [DOI] [PubMed] [Google Scholar]
- 79.Strandberg TE, et al. Explaining the obesity paradox: cardiovascular risk, weight change, and mortality during long-term follow-up in men. Eur Heart J. 2009;30(14):1720–1727. doi: 10.1093/eurheartj/ehp162. [DOI] [PubMed] [Google Scholar]
- 80.Rathod K, et al. Sex differences in the inflammatory response and inflammation-induced vascular dysfunction. The Lancet. 2017;389:S20. doi: 10.1016/S0140-6736(17)30416-6. [DOI] [Google Scholar]
- 81.Cohen E, Margalit I, Shochat T, Goldberg E, Krause I. <p>Markers of Chronic Inflammation in Overweight and Obese Individuals and the Role of Gender: A Cross-Sectional Study of a Large Cohort</p>. JIR. 2021;14:567–573. doi: 10.2147/JIR.S294368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hamer M, et al. Physical activity and inflammatory markers over 10 years follow up in men and women from the Whitehall II cohort study. Circulation. 2012;126(8):928–933. doi: 10.1161/CIRCULATIONAHA.112.103879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tardif CL, et al. Investigation of the confounding effects of vasculature and metabolism on computational anatomy studies. Neuroimage. 2017;149:233–243. doi: 10.1016/j.neuroimage.2017.01.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in the present study are either publicly available (PREVENT-AD MRIs and demographics: https://openpreventad.loris.ca) or can be shared upon reasonable request and approval by the study scientific committees and/or institutional review boards. Data used in preparation of this article were obtained from the Pre-symptomatic Evaluation of Novel or Experimental Treatments for Alzheimer’s Disease (PREVENT-AD) program (https://douglas.research.mcgill.ca/stop-ad-centre), data release 6.0. A complete listing of PREVENT-AD Research Group can be found in the PREVENT-AD database: https://preventad.loris.ca/acknowledgements/acknowledgements.php?date=%5B2022-08-02%5D.