Abstract
Aims
Gamma oscillations (≈25–100 Hz) are believed to play an essential role in cognition, and aberrant gamma oscillations occur in brain aging and neurodegeneration. This study examined age-related changes in visually evoked gamma oscillations at two different time points 5 years apart and tested the hypothesis that the power of gamma oscillations correlated to cognitive skills.
Methods
The cohort consists of elderly males belonging to the Metropolit 1953 Danish Male Birth Cohort (first visit, N=124; second visit, N=88) over a 5-year period from 63 to 68 years of age. Cognitive functions were assessed using a neuropsychological test battery measuring global cognition, intelligence, memory, and processing speed. The power of steady-state visual evoked potentials (SSVEP) was measured at 8 Hz (alpha) and 36 Hz (gamma) frequencies using EEG scalp electrodes.
Results
Over the 5-year period cognitive performance remained relatively stable while the power of visually evoked gamma oscillations shifted from posterior to anterior brain regions with increasing age. A higher-than-average cognitive score was correlated with higher gamma power in parieto-occipital areas and lower in frontocentral areas, i.e., preserved distribution of the evoked activity.
Conclusions
Our data reveal that the distribution of visually evoked gamma activity becomes distributed with age. Preserved posterior-occipital gamma power in participants with a high level of cognitive performance is consistent with a close association between the ability to produce gamma oscillations and cognition. The data may contribute to our understanding of the mechanisms that link evoked gamma activity and cognition in the aging brain.
Keywords: Aging, Cognition, Gamma oscillations, Steady-state evoked potential, Electrophysiology
Introduction
Healthy aging is associated with changes in cerebral network function, which may be assessed by electrophysiological methods. Typically, age-related brain changes are associated with an amplitude decrease of alpha activity (8–13.9 Hz), slowing of background activity, and an increase in delta (0.5–3.9 Hz) and theta power (4–7.9 Hz) [1, 2]. The literature also often reports a posterior-to-anterior shift in oscillatory activity, where older adults exhibit increased frontal and decreased occipital activity [3]. In addition, weakened spontaneous gamma oscillations have been observed in both healthy aging [4] and patients diagnosed with mild cognitive impairment (MCI) and Alzheimer’s disease (AD) [5]. Although an exact correlation between electroencephalography (EEG) oscillations and cognitive processes cannot be established, it is postulated that distinct cognitive functions may be influenced by EEG oscillations depending on where in the brain they occur and their parameters (i.e., amplitude, frequency, phase, and coherence) [6].
Alpha oscillations are synchronized in the resting state and when the eyes are closed. Moreover, the alpha power follows a downward gradient from the posterior region to anterior regions, which may reflect alpha oscillation’s role in modulating the perceptual load, perception, and attention [7] by suppressing the processing of irrelevant visual stimuli [8, 9]. Gamma band oscillations (30–80 Hz) are believed to underlie both bottom-up (e.g., perceptual feature binding) and top-down information processing, including attention and memory [10]. For example, studies have found increased gamma band activity during selective attention towards sensory information and observed increased gamma activity during encoding to predict successful memory formation [10, 11, 6].
Steady-state visual evoked potentials (SSVEP) may be elicited by a flickering stimulus at a fixed rate. A high stimulation rate prevents the neural activity from returning to baseline, resulting in a semi-sinusoidal signal [12–15]. Transformation of the signal from the time to the frequency domain generates a power peak corresponding to the stimulus frequency and its harmonics. Because the SSVEP power peak is at the stimulus frequency, the signal is less affected by noise components, such as blink, muscle, and electrical artifacts [16]. These properties make SSVEP useful for studying neural processes related to fast rhythmic brain activity, and the technique has been applied to a range of cognitive and clinical neuroscience studies [15].
The exact neurophysiological underpinnings of the steady state evoked potential (SSEP) have yet to be entirely understood. It has been suggested that the SSEP response results from the superimposition of repeated transient responses to the stimulation stream [17]. However, the SSEP may also be influenced by endogenous rhythmic neural generators, which may alter the firing frequency of neurons targeted by the synaptic input and lead to synchronization to the exogenous stimulation [18, 17]. As such, SSEPs represent neural entrainment, which may reflect how well an ensemble of neurons transition from spontaneous oscillations to stimulation-induced rhythmic activity [17, 15]. Previous cross-sectional studies from our group of the Metropolit 1953 Danish Male Birth Cohort have reported that alpha and gamma power and phase were correlated with intelligence [19], memory [14], and cognitive decline [20]. The current study used a longitudinal design to examine whether the spectral power of alpha (8 Hz) and gamma (36 Hz) oscillations declined with age and whether the distribution of gamma power correlated with cognitive performance. The gamma band was chosen because of its essential role in upholding higher-order cognitive functions and neurodegenerative disease, while the alpha band power was used as a benchmark for the brain’s ability to produce rhythmic activity, where we expect little or no age-related changes. These findings will provide insight into electrophysiological changes occurring during healthy aging, which can further elaborate our understanding of pathological aging, and aid in finding electrophysiological markers for cognitive decline and AD.
Methods
Participants were recruited from the Metropolit 1953 Danish Male Birth Cohort, which was established in 1965 and defined as all boys born in 1953 in the Copenhagen Metropolitan area [21]. Participants of this cohort have been examined multiple times previously with regard to physiological state and cognitive skills, and the cohort is optimal for assessing age-related changes in brain function from a life course perspective. Our published work has reported subtle age-related changes in brain structure [22], electrical activity [14, 23, 19, 20], and cerebrovascular function [24] brought on by aging. For the current study, we examined participants who underwent repeated examinations at two time points five years apart, the first in 2015–2016 (T1) and the second in 2021–2022 (T2).
Participant characteristics, neuropsychological results and SSVEP power are presented in Table 1.
Table 1.
Participant demographic and characteristics at both timepoints
| T1 | T2 | |
|---|---|---|
| Participants, N | 124 | 88 |
| Age, mean [sd] | 63 [0.66] | 67.25 [0.58] |
| [Range] | 62–64 | 66–68 |
| ACE (# correct) mean [sd] | 93.41 (5.1) | 95.63 (3.7) |
| IST (# correct) mean [sd] | 31.56 [10.47] | 33.15 [9.1] |
| VPA Wordpair (# errors), mean [sd] | 12.39 [9.1] | 10.48 [7.6] |
| VPA Retention (# errors), mean [sd] | 5.01 [3.6] | 4 [2.79] |
| PAL socre (# correct), mean [sd] | 18.5 [3.2] | 17.65 [3.9] |
| TMT A (seconds to completion), mean [sd] | 30.72 [7.94] | 32.77 [12.1] |
| TMT B (seconds to completion), mean [sd] | 70.41 [20.87] | 78.75 [25.24] |
| SDMT (# correct), (mean) [sd] | 47.10 [8.73] | 46.06 [9.12] |
| Alpha power | ||
| Occipital, mean [sd] | 69.8 [110] | 53.5 [78] |
| Log+1, mean [sd] | 3.45 [1.33] | 3.31 [1.27] |
| Parietal, mean [sd] | 9.78 [12] | 12.7 [14.8] |
| Log+1, mean [sd] | 1.86 [1.07] | 2.06 [1.10] |
| Central, mean [sd] | 9.06 [14.7] | 7.05 [10.1] |
| Log+1, mean [sd] | 1.59 [1.20] | 1.55 [1.02] |
| Temporal, mean [sd] | 6.3 [8.9] | 5.31 [7.53] |
| Log+1, mean [sd] | 1.51 [0.96] | 1.46 [0.83] |
| Frontal, mean [sd] | 17.8 [26.7] | 16.1 [25.9] |
| Log+1, mean [sd] | 2.10 [1.32] | 2.06 [1.24] |
| Gamma power | ||
| Occipital, mean [sd] | 24.6 [43.4] | 16.9 [20.7] |
| Log+1, mean [sd] | 2.44 [1.26] | 2.28 [1.15] |
| Parietal, mean [sd] | 3.85 [6.5] | 12.3 [18.6] |
| Log+1, mean [sd] | 1.04 [0.98] | 1.88 [1.25] |
| Central, mean [sd] | 3.08 [6.17] | 17.1 [27.7] |
| Log+1, mean [sd] | 0.84 [0.99] | 2.03 [1.43] |
| Temporal, mean [sd] | 2.72 [4.55] | 15.3 [20.9] |
| Log+1, mean [sd] | 0.91 [0.82] | 2.08 [1.26] |
| Frontal, mean [sd] | 6.39 [12.8] | 18 [27] |
| Log+1, mean [sd] | 1.37 [1.01] | 2.16 [1.33] |
ACE, Addenbrooke's Cognitive Examination; IST, Intelligenz Struktur Test; VPA, Verbal Paired Associates; TMT A, Trail Making A; TMT B, Trail Making B; SDMT, Symbol Digits Modalities Test
Neuropsychological examination
A comprehensive neuropsychological test battery assessed cognitive functions at both time points. The included tests were: Addenbrooke’s Cognitive Examination (ACE) for measuring global cognition and ruling out dementia; Verbal Paired Associates (VPA) (word pairs and retention) to measure verbal memory functions; Symbol Digits Modalities Test (SDMT) and Trail Making A/B to measure processing speed; and Subtests from Intelligenz Struktur Test (IST) to estimate intelligence. The Paired Associates Learning (PAL) task from Cambridge Neuropsychological Test Automated Battery (CANTAB) was administered to measure visual associative memory. All neuropsychological tests were administered by certified hospital staff.
Signal acquisition
EEG was recorded with a 64-channel elastic Quick-Cap connected to a Neuroscan bio-amplifier (SynAmps, Compumedics, (http://compumedicsneuroscan.com/). Electrodes were placed according to the international 10-20 system. Neuroscan (Curry version X) was used to record EEG signals, with a sampling frequency of 2 kHz. All EEG electrodes were online referenced between Cz and Cpz. The ground electrode was between Fz and Fpz. Two horizontal eye electrodes (EOG) were placed parallel to the right and left eye, and two vertical EOG electrodes were placed above and below the left eye. The electrocardiogram (ECG) and electromyography (EMG) electrodes were included to detect and remove ECG and muscle artifacts from the EEG signal during signal processing. For the ECG, one electrode was placed just under the right clavicle and the other at the left lower chest. In addition, two electrodes were placed under the chin lateral to the midline for the EMG.
Experimental setup and SSVEP stimulation
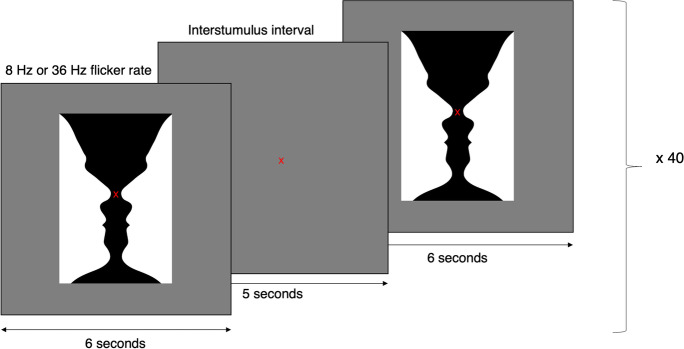
The experimental setup was described previously [20, 14, 23, 19]. Participants were seated comfortably in a dark room, with a 60 cm distance to a computer screen, which acted as the visual stimulator. To elicit an SSVEP response, participants were first presented with a fixation cross in the center of the screen to maintain focus. After 5 s of fixation, a flickering image of Rubin’s vase appeared in the center fixation area of the stimulator screen (Fig. 1). The image flickered for 6 s, followed by 5 s of rest, with only the fixation cross shown on the screen. This was repeated 40 times for each stimulus frequency while simultaneously recording EEG, EOG, ECG, and EMG. The participants were presented with visual stimulation at 8 Hz to assess the SSVEP power in the alpha band and at 36 Hz to assess SSVEP power in the gamma band. Rubin’s vase was chosen as the stimulus target because it is a complex high-contrast picture requiring cognitive processing but does not elicit an emotional response. In addition, the nature of the figure makes it more engaging to look at, which aids in improving the participant’s attention. These are factors previously observed to be crucial to produce potentials in the gamma band [25–27], also in our laboratory [20, 23].
Fig. 1.
The steady-state visual evoked paradigm. The participants viewed the image of Rubin’s vase for 6 s, followed by a 5-s interstimulus interval. The stimulation was presented with an 8 Hz and 36 Hz flicker rate
Preprocessing and artifact reduction
Signal processing was carried out using the EEGLAB v.2021 [28] toolbox in MATLAB (R2021b, MathWorks, Natick, MA, USA). All EEG electrodes were re-referenced to the common average and downsampled to 250 Hz. An anti-aliasing filter (linear phase FIR filter using a Kaiser window) and a high-pass filter with a 0.5 Hz cut-off were applied. All EEG recordings were visually inspected and bad EEG segments and channels were removed. Independent component analysis (ICA) was implemented to detect and remove components containing eye blinks and muscle artifacts with 90% and 95% accuracy, respectively, using the ICLabel plugin in EEGlab, which automatically classifies the source of the independent components [29]. Previously removed bad channels were interpolated using the spherical spline method of EEGLAB [30]. Data were extracted in epochs, where signal epochs were defined as 0.500 to 6000 ms relative to the stimulus onset to exclude the initial transient response. Baseline epochs were defined as −2000 to 0 ms relative to stimulus onset.
Power calculation of SSVEP peak
We applied power spectrum analysis using Welch’s method [31] to compute the peak power for each stimulation frequency, i.e., 8 Hz or 36 Hz. The spectral values were multiplied by their frequency to compensate for the 1/f shape of the EEG power spectrum. The electrodes were divided into regions of interest (ROI) based on their scalp placement as per the following:
Occipital region (O): PO7, PO5, PO3, POZ, PO4, PO6, PO8, O1, OZ, O2,
Parietal region (P): CP3, CP1, CPZ, CP2, CP4, P3, P1, PZ, P2, P4
Central region (C): FC3, FC1, FCZ, FC2, FC4, C3, C1, CZ, C2, C4
Temporal region (T): FT7, FC5, T7, C5, TP7, CP5, FC6, FT8, C6, T8, CP6, TP8
Frontal region (F): FP1, FPZ, FP2, AF3, AF4, F7, F5, F3, F1, FZ, F2, F4, F6, F8
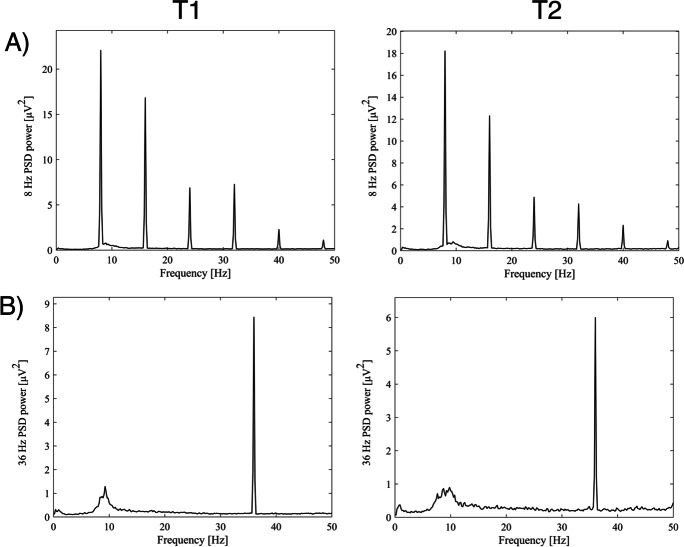
To extract the signal, we calculated an average across all signal epochs, where all 40 epochs of 6 s were combined into a 1×6 s averaged epoch. The power spectrum of the averaged epochs was estimated using Welch’s method. The observed peak at the stimulation frequency (8 or 36 Hz) was defined as the peak power estimate for each electrode. SSVEP power was calculated as the average power estimate for each ROI at the stimulation frequency (8 Hz or 36 Hz) (Fig. 2).
Fig. 2.
Visualization of the occipital power of the 8 Hz and 36 Hz peaks, and their harmonics, averaged across all participants for each time point. PSD, power spectral density
To extract the baseline activity, we first calculated the average for all baseline periods, defined as the −2000 to 0 pre-stimulus interval. Welch’s method was applied to estimate the power spectrum of the averaged baseline periods. We extracted two values from the baseline parameter: the mean and the standard deviation of the power estimate across the frequency band (0.5–40 Hz) over the entire scalp. To normalize the SSVEP power values and reduce the confounding effects of background activity, the SSVEP power is expressed as a Z score, reflecting the number of standard deviations from the baseline activity to the SSVEP peak. The Z score is calculated using the following formula:
where the subscript i denotes the ith ROI, xi is the average power estimate at the stimulation frequency from the ith ROI and and SD are the mean and the standard deviation of the baseline activity as described above. This provides an SSVEP power value for each ROI. A higher SSVEP power value corresponds to a more prominent signal in a specific ROI relative to the baseline activity across the entire scalp. The same procedure was repeated for all ROIs and both frequency paradigms, yielding ten SSVEP power values.
where O, P, C, T or F refers to the region of interest (occipital, parietal, central, temporal, frontal) and 8 refers to 36 the alpha or gamma frequency paradigm, respectively.
Handling of bad data and missing data points
In total, 136 individuals participated in the study at T1 and 115 at T2. From this, we obtained EEG recordings from 124 for alpha SSVEP at T1, 123 for gamma SSVEP at T1, 88 for alpha SSVEP at T2, and 83 for gamma SSVEP at T2. The missing participants were excluded from further analysis due to extensive artifacts in the EEG recordings, as detected under the visual inspection of the EEG recording. Furthermore, because of technical difficulties with the EEG stimulator, some participants from T2 were excluded from the EEG dataset. VPA retention data were missing for 1 participant at T1 due to insufficient time because of delays. PAL score was missing for 7 participants in relation to the V8 data frame and for 5 in relation to the V36 data frame, due to technical errors or task incompletion.
Statistical analysis
All statistical analyses were calculated with RStudio Version 1.3.1093 (R Core Team, 2013. R: A language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/).
Linear mixed-effects models
Linear mixed-effects models (LMM) were applied to assess longitudinal changes in occipital alpha and gamma SSVEP power. Time (T1, T2) was set as the fixed effect, participant as the repeated effect, and an unstructured covariance pattern to account for the correlation between the repeated measurements. T1 was set as the reference in both the alpha and gamma power. SSVEP power values were log-transformed (constant of 1 added to the SSVEP power value because of < 0 values) to reduce heteroscedasticity and obtain a normal distribution in line with the model assumptions.
Multiple linear regression
Multiple linear regression was applied to assess the association between SSVEP power and cognitive functions. Cognition was set as the dependent variable in the model. We made two composite variables for the independent variable from the SSVEP power values. The first was defined as parieto-occipital (PO) power, which was calculated as the average of the occipital and posterior SSVEP power. The second was defined as frontocentral power and was calculated as the average of the frontal and central SSVEP power.
The composite variables were constructed for three reasons. Firstly, since the ROI SSVEP power values are highly correlated, all five of them could not be set as regressors in the multivariate regression model. This would cause multicollinearity and would make the model estimates unreliable. This problem was solved by making composite variables. This was confirmed by calculating the variance inflation factor for each model, which was never observed to be above 3.5. Secondly, the defined composite variables enable a more intuitive interpretation of the data. Finally, two composite variables based on posterior and frontal gamma power allow us to assess more directly how posterior vs. anterior oscillatory activity affects cognition. The multiple linear regression models were only run for SSVEP power and cognition at T2 to limit the number of statistical tests.
Cognition was assessed in four cognitive domains, namely global cognition (ACE), intelligence (IST), memory (VPA wordpair, VPA retention), and processing speed (TMT A, TMT B, SDMT). This constitutes seven different multiple linear models per frequency band. Power values (independent variable) were log-transformed. In cases where linear regression diagnostics revealed large deviations from normality, the dependent variable was also log-transformed (y+1). This was only necessary for TMT A and TMT B. p values were adjusted for multiple testing using the Benjamini-Hochberg procedure.
Analysis of variance
Within-groups two-way ANOVA was performed to compare differences in spectral distribution between regions of interest in the alpha and gamma band occurring with time. Spectral power was set as the dependent variable, while time (T1 and T2) and ROI (O, P, C, T and F) were set as predictors. Post hoc Bonferroni adjusted pairwise t test was used to compare mean alpha/gamma power values between the ROI. All power values were log transformed to comply with the ANOVA model’s assumption of normally distributed data.
Other statistical tests
The Pearson correlation coefficient was used to assess the correlation between power variables.
Results
Repeated cognitive testing on two occasions 5 years apart revealed that cognition was relatively preserved for participants who participated in both examinations, except for processing speed which had a decline. Thus, the results reported in the following are related to healthy brain aging and not cognitive decline. Hence, the data analysis focuses on the effect of aging and the interplay of the general level of cognition with alpha and gamma power.
No change in longitudinal occipital alpha or gamma power with increasing age
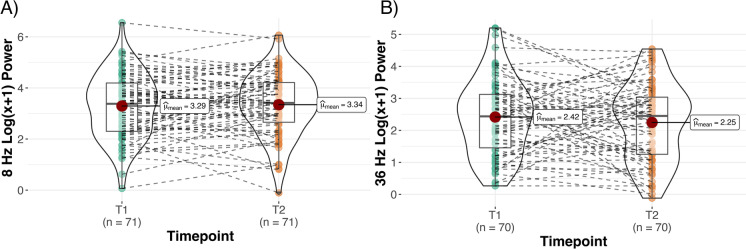
First, we assessed whether there were any longitudinal changes in occipital alpha and gamma power. We observed no significant differences in PO8 and PO36 between T1 and T2, indicating preservation of occipital alpha and gamma power for the two measurements taken 5 years apart (Fig. 3).
Fig. 3.
Trajectory of A alpha and B gamma occipital SSVEP power from T1 to T2
No change in distribution of regional alpha power across the scalp with age
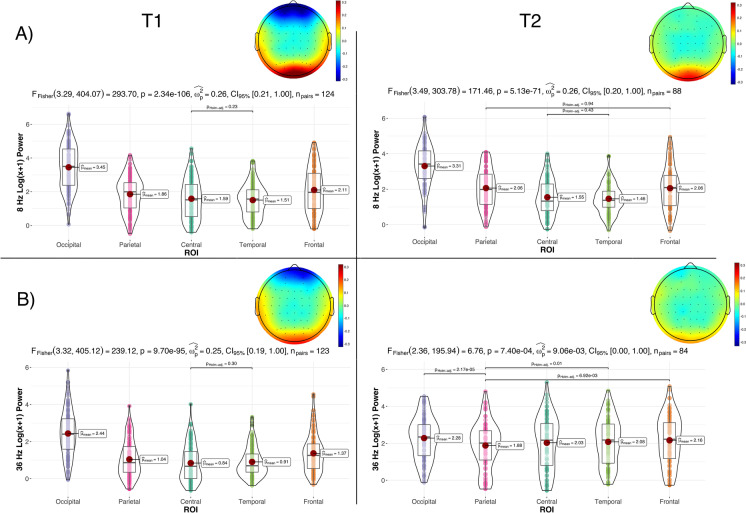
We observed a significant main of ROI (F(3.29, 230.25)=202.57, p<0.05, eta2[g]=0.28) and of the ROI*time interaction (F(3.45, 241.24)=4.72, p<0.05, eta2[g]=0.003) when assessing regional differences in spectral power in the alpha band. Post hoc multiple comparisons pairwise t test showed a statistically significant difference between all the comparisons (p <0.001), except between PC8 and PT8 at T1. PO8 had the highest reported power, as expected, due to the visual nature of the stimulus. At T2, the post hoc multiple comparison pairwise t tests revealed statistically significant differences between all comparisons (p<0.001), except between PC8 and PT8 and PP8 and PF8. PO8 still had the highest reported alpha power at T2. Thus, there was little or no change in the distribution of regional alpha power across the scalp with increasing age, suggesting that the ability to generate fundamental brain oscillations related to thalamocortical connections was preserved with age (Fig. 4A). The effect of time was only significant for PP8.
Fig. 4.
Regional differences in power distribution in A alpha and B gamma SSVEP power at T1 and T2, with corresponding topographic plots averaged across all participants. Note that the alpha plot for T1 and T2 and the gamma plot for T1 show the non-significant differences, because the remaining pairwise comparisons are statistically significant. In contrast, the gamma plot for T2 shows the only significant pairwise comparisons
SSVEP gamma response was more evenly distributed across the scalp with age
The same analysis as above was conducted concerning SSVEP gamma power. We observed a significant main effect of ROI (F(3.22, 222.05)=74.35, p<0.05, eta2[g]=0.085), time (F(1,69)=21.32, p<0.05, eta2[g]=0.086), and the ROI*time interaction (F(2.93, 202.10)=49.75, p<0.05, eta2[g]=0.038). Post hoc multiple comparisons pairwise t test showed a statistically significant difference between all comparisons (p<0.001), except between PC36 and PT36 at T1. PO36 had the highest reported power compared to the remaining ROIs. At T2, post hoc multiple comparison pairwise t tests showed a statistically significant difference between (1) PF36 and PP36, (2) PO36 and PP36, and (3) PP36 and PT36. The effect of time was significant for all ROI (p<0.05), except for PO36. Thus, the gamma power difference between regions was less distinguishable at T2 compared to T1 in EEG recordings from the same individuals taken 5 years apart, which may reflect brain aging (Fig. 4B).
Change in correlation pattern between ROI in SSVEP gamma power between T1 and T2
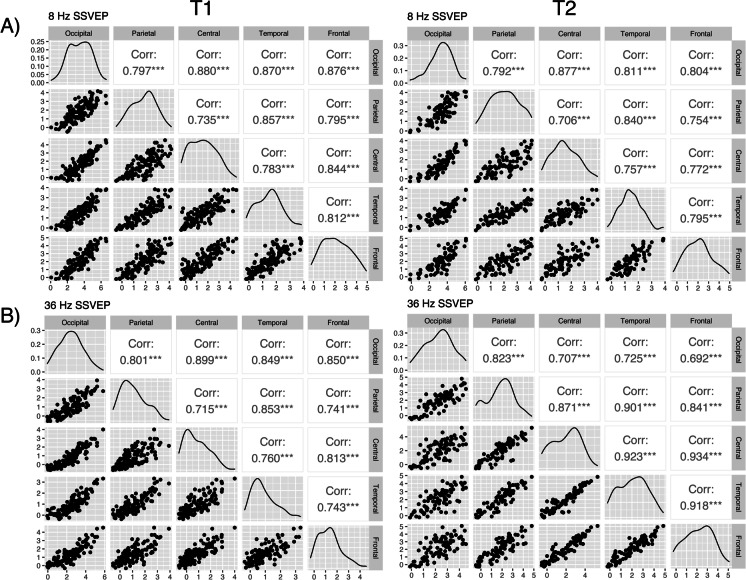
To further explore the relationship of the steady-state power response between the ROIs at T1 and T2, we calculated the correlation coefficient between all ROIs (i.e., observed power in all regions were compared with each other). For the alpha power, we observed significant positive correlations between all ROI at T1 (r=0.81, p≤0.001) and T2 (r=0.82, p≤0.001), where the strongest correlation coefficients were between the occipital lobe and all remaining regions. The correlation strength and pattern did not differ significantly between T1 and T2 (Fig. 5A).
Fig. 5.
Correlation matrices showing correlation patterns between regional SSVEP power at the A alpha and B gamma stimulation frequencies at both timepoints. The scatterplots to the left display the linearity between the variables. The distribution of the variables can be seen along the diagonal. The Pearson correlation coefficient is displayed to the right
Similarly, for the gamma SSVEP power at T1, we observed a significant positive correlation between all ROIs. The correlation coefficients were the strongest between the occipital gamma power (PO36) and all the remaining regions (r=0.90, p≤0.001) and slightly weaker between the remaining ROIs compared to each other (Fig. 5B). This indicates that SSVEP activation in the occipital lobe synchronizes across the brain. However, at T2, there was a shift in the correlation pattern. The correlation coefficients between PO36 and the remaining ROIs, which were the strongest at T1, were now the weakest (r=0.70, p≤0.001). Conversely, the correlations between the extra-occipital regions (e.g., between PT36 and PF36), which were previously the weakest, were now one of the strongest (r=0.93, p≤0.001) (Fig. 5B). We believe this may reflect a loss of synchrony due to brain aging.
Gamma SSVEP is associated with intelligence, memory performance, and processing speed
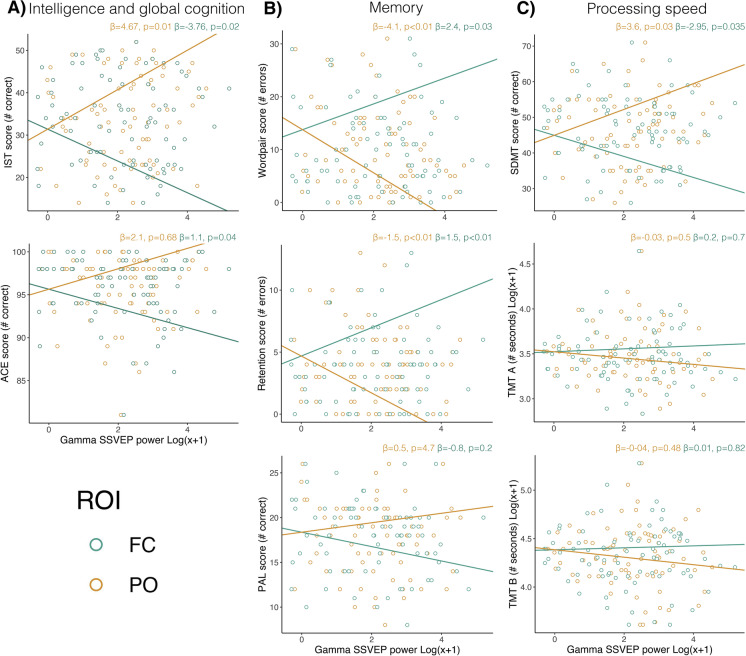
Seven multiple linear regression models assessed the association between gamma SSVEP and cognition. The ACE score was significantly associated with lower frontocentral power (p=0.04). The model explained 2.7% of the variability of the dependent variable (r2=0.027). IST was associated with parieto-occipital (p=0.01) and frontocentral (p=0.02) SSVEP gamma power, where increased parieto-occipital SSVEP gamma power and decreased frontocentral SSVEP gamma power predicted higher scores. The model accounted for 6% of variability in the dependent variable (Fig. 6A). VPA wordpair results were significantly associated with parieto-occipital (p=0.003) and frontocentral (p=0.031) SSVEP gamma power. More errors were predicted by decreased parieto-occipital SSVEP gamma power and increased frontocentral SSVEP gamma power. The same pattern was observed for the association between VPA retention score with parieto-occipital (p=0.003) and frontocentral (p=0.008) SSVEP gamma power. The models accounted for 9% and 8.1% of the variability of the dependent variable, respectively (Fig. 6B). Finally, higher parieto-occipital (p=0.03) and lower frontocentral (p=0.04) SSVEP gamma power were associated with higher SDMT scores. The model accounted for 3.7% of the total variability of the dependent variable (Fig. 6C). However, this did not remain significant after correcting for multiple testing. These results indicate that better performance in several cognitive domains is associated with higher parieto-occipital and lower frontocentral gamma SSVEP power.
Fig. 6.
A Association between gamma SSVEP power and intelligence and global cognition. B Association between gamma SSVEP power and memory. C Association between gamma SSVEP and processing speed
Discussion
The SSVEP power reflects how well an ensemble of neurons may reorganize spontaneous oscillations to a synchronized signal in response to an external stimulus. The current study aimed to investigate whether this neurophysiological mechanism changes with age and whether the ability to generate fast cortical oscillations is related to cognitive performance in an aging cohort. To do this, we measured the power of the SSVEP in the alpha (8 Hz) and gamma (36 Hz) bands at the respective frequency peaks in the same group of elderly participants from a Danish Birth Cohort 5 years apart.
Overall, alpha power seems stable with age and unrelated to cognitive performance. We observed no longitudinal change in PO8 (i.e., occipital alpha SSVEP power) with age, no significant alteration in correlation pattern, and no associations with cognitive performance. In comparison, gamma power was less concentrated in the occipital lobe and more distributed across the scalp with increasing age. We also observed a change in the correlation pattern between the ROI from T1 to T2, where PO36 went from being most strongly correlated to the other regions to being the least correlated. Finally, gamma SSVEP power correlated with global cognition, intelligence, memory, and processing speed, where higher posterior-occipital and lower frontocentral evoked power was associated with better cognitive performance.
Alterations in gamma oscillations with aging
Gamma oscillations are affected by aging. Murty et al. [4] observed reduced 32 Hz SSVEP responses with increasing age, while the power of alpha oscillations was preserved. Gaetz et al. [32] assessed changes in gamma band frequency and amplitude in a life-span study assessing 8- to 35-year-olds using magnetoencephalography (MEG). They observed decreases in gamma responses with age and reduced occipital lobe volumetry. Our own previous study of the Metropolit cohort reported reduced SSVEP gamma power in a group of elderly participants as compared to a young control group [14]. The present study reported preserved alpha and gamma power in the occipital region over a 5-year time period, but a wider distribution of visually evoked gamma power with age. The discrepancies in findings from previous studies may be explained by differences in study design. Previous assessments of age-related changes in gamma oscillations used a cross-sectional/life-span approach [4, 32, 14]. Here, we used a longitudinal approach and studied the same subjects at two time points 5 years apart, i.e., a short age range for assessing changes of brain EEG activity. However, whereas cross-sectional studies may point to differences between age groups, longitudinal studies investigate actual changes in the variable of interest [33] and are crucial for understanding age-related changes and their underlying mechanisms. Because of their inherently different study designs, cross-sectional and longitudinal studies often have divergent results [33, 34]. Therefore, comparisons should always be conducted cautiously and with this aspect in mind. Nevertheless, we posit that gamma power decreases with age and that the distribution of gamma power becomes more widespread with age, and look forward to re-examine the participants in the Metropolit Birth Cohort 5 years from now to elucidate this topic in more detail.
Regional changes in gamma power
The more distributed activation pattern in T2 than in T1 reflects that the gamma power evoked by visual stimulation is less concentrated in the occipital lobe with age. The finding is in line with the posterior-to-anterior shift of oscillatory activity, commonly observed in the aging population [3, 35]. Thus, although we did not observe a significant decline in occipital gamma SSVEP with time, we see a noticeable increase in SSVEP power in the frontocentral ROIs. We propose that this change in global SSVEP power and the altered correlation pattern observed between the ROIs at T2 reflects alterations in neural network synchronization. This may be caused by alterations in age-related coherence of the cerebral white matter integrity, which occurs in the genu of the corpus callosum, the centrum semiovale, and frontal and parietal pericallosal white matter despite the absence of significant white matter volume reductions [36]. As these white matter changes affect the communication and synchrony between neurons, they are also likely to affect the SSVEP gamma power, which reflects the number of neurons synchronized to the frequency of the visual stimulus.
Alterations and disruption of the myelin sheaths negatively affect the efficiency of neural firing, disrupting the timing of sequential events in neural circuits — ultimately leading to the cognitive tardiness observed with increasing age [37]. It has been proposed that for the aging brain to compensate for the loss of connectivity, it will accelerate brain activity [38]. The reasoning behind this idea is as follows: brain regions communicate by transmitting neural information via anatomically connected neurons. It is further postulated that coherence (or synchrony) between neuronal groups is necessary for the flexible and effective communication required for upholding cognitive functions [39]. Due to naturally occurring delays in neuronal activity, there will always be some phase discrepancy between the signals from different neuronal groups. However, as brain connectivity is reduced in aging, neural communication becomes slower, resulting in potentials not arriving at the point of peak excitability synchronously. Once the phase difference between two signals exceeds an entire cycle, the brain may respond with compensatory speeding, i.e., by increasing the firing rate between two neuronal groups. Consequently, the signal will again concur at peak excitability. However, since connectivity strength between neuronal groups is not uniform, an increase in neural firing will be widely distributed (especially at high frequencies), resulting in a broad power spectrum instead of distinct frequency peaks [38].
Another contributing factor to changes in oscillatory activity may be linked to altered inhibition-excitation balance in the aging brain. Proper functioning of the cerebral cortex is dependent on a precise balance between excitatory and inhibitory neurotransmitters [40]. For example, the γ-aminobutyric acidergic (GABAergic) system plays an important role in gamma synchronization and in supporting cognitive functions [41, 42]. Furthermore, studies have observed changes in GABA signaling in aging and AD [42, 43]. Therefore, age-related alterations in the GABAergic inhibitory system may affect the synchronization of neural activity, thus contributing to changes in distribution of gamma activity on a regional basis from posterior to frontal regions [43].
Gamma oscillations and cognition
Gamma power is correlated with global cognition, intelligence, memory, and processing speed. Moreover, the association between gamma power and cognitive performance followed the same pattern across all cognitive domains, showing that better cognitive performance was correlated with increased posterior and decreased frontocentral gamma power. This finding may support the hypothesis that gamma oscillations maintain and uphold cognitive functions. Specifically, it illustrates how higher posterior SSVEP gamma activation may reflect successful gamma synchrony in response to the visual flickers. In comparison, more widespread activation may reflect the inability of brain networks to synchronize gamma activity regionally, which is associated with a low level of cognitive performance.
Gamma rhythms have been examined in both cortical and subcortical regions. They are commonly observed in the visual system and in the hippocampus, where they are believed to be involved in memory and attentional processes [44–46, 11, 10, 47]. Specifically, it is suggested that an essential mechanism for upholding cognitive functions relies on gamma synchronization [48–50]. In line with this, abnormal gamma oscillations have also been linked to neurodegenerative diseases such as MCI and AD, which are characterized by memory impairments and functional network alterations [44, 46, 51, 50]. Further underlining the critical role of gamma oscillations in upholding brain and cognition, an increasing body of research indicates possible neuroprotective effects of entraining gamma rhythms [52]. These findings include preserved functional connectivity, improved cognition, and reduced atrophy in AD [53, 54] and MCI [55] patients.
Compensation or dedifferentiation
The idea of compensatory brain activation in response to age-related loss of structural integrity is pertinent when examining age-related changes in the brain and cognition. Neural compensation is defined as the increased recruitment of neural resources for upholding cognitive functions [56]. In neuroimaging studies, this is typically demonstrated as older adults exhibiting increased and more widespread activation than their younger counterparts, and for this increase in activation to be associated with better cognitive outcome [56, 38]. Conversely, a counter hypothesis suggests that increased and widespread activation in older populations results from dedifferentiation. That is, rather than reflecting additional recruitment of neural resources to uphold task demand, the increased activity reflects impairment in allocating neural resources [57]. Whether the widespread activation pattern observed in our participants for gamma activity at T2 as compared to T1 reflects compensation or dedifferentiation is unknown. To elucidate this topic, it is necessary to examine this association in the participants as they continue to age.
Limitations of study
The current study’s generalizability is limited due to its consisting of an all-male cohort. When the Metropolit cohort was established in 1965, it was with the main purpose of studying social mobility. Since social mobility at the time was primarily related to the occupation of the male breadwinner, only men were included in the study [21]. Despite the apparent limitations of assessing a male-only cohort, we deem the data gathered over the years valuable for research on aging and believe they should be used. Additionally, it is possible that the participants in the best physical and mental shape have been more likely to return for re-examinations. This is a common limitation for longitudinal studies and may contribute to explaining the lack of cognitive decline in most of the domains.
Another possible limitation is using sensor space instead of source space analysis. Sensor space analysis is believed to be more susceptible to volume conduction, which can lead to overlapping signals from different sources [58]. However, the problem of sensor space analysis is most critical when working with a small number of electrodes. Since we have employed a set-up including a large number of electrodes (n=64), which have been grouped into relatively large regions of interest, we do not believe volume conduction poses a significant problem. However, suppose one was to compare specific cortical regions in proximity to one another (e.g., primary visual cortex vs. secondary visual cortex). In that case, volume conduction is a concern, and conducting a source space analysis would be critical. Additionally, the overarching goal of this study is to assess the usefulness of the SSVEP method in a clinical setting on large populations of patients, where there is unlikely to be time and resources readily available to conduct source space analysis [4].
We note that we have not directly measured network formation or functional integration across brain regions, as we have not assessed coherence or phase synchrony. Instead, we have conducted a spectral analysis, assessing the power of the EEG signal. However, a common assumption of the SSVEP responses is that they represent neural entrainment and synchronization to a rhythmic stimulus [59, 17, 15]. Since power represents the activity in a given frequency band, increased/higher power response is interpreted as representing a stronger response to the visual stimuli, indicating a more successful synchronization.
Conclusion
Our findings indicate age-related changes in gamma oscillations, including a posterior-to-anterior shift in oscillatory activity and loss of synchrony in the gamma band. Changes in gamma power occur before potential changes in alpha band power. Additionally, our data support the importance of gamma synchrony for upholding cognitive functions. We interpret these findings as indicating that a high posterior gamma response to visually evoked stimulations reflects better neuronal functioning and correlates with better global cognition, memory, intelligence, and processing speed. Viewed with other evidence highlighting the importance of gamma oscillations in cognition, in addition to novel findings indicating the neuroprotective effects of gamma oscillations, we see future potential for gamma SSVEPs as a clinical biomarker for detecting subtle brain changes in individuals at risk for cognitive decline.
Acknowledgements
We want to thank Sine Kongsbak Arvedsen and Natalia Christina Brandstrup for their work with coordinating the project and the extensive data collection. We also thank Keng Wah Niels Pang and Miki Nikolic for all technical assistance and their valuable input on processing of the EEG data.
Funding
The study was supported by a Nordea Foundation Grant to the Center for Healthy Ageing at the University of Copenhagen. The Copenhagen Ageing and Midlife Biobank is supported by grants from the Velux Foundation (VELUX26145 and 31539).
Declarations
Ethical approval and consent to participate
The study was approved by the Capital Region of Denmark’s Committee on Health Research Ethics (H-1–2014032). All participants provided written consent regarding their participation and publication of the current data.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rossini PM, Rossi S, Babiloni C, Polich J. Clinical neurophysiology of aging brain: from normal aging to neurodegeneration. Prog Neurobiol. 2007;83(6):375–400. doi: 10.1016/j.pneurobio.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Ishii R, Canuet L, Aoki Y, Hata M, Iwase M, Ikeda S, Nishida K, Ikeda M. Healthy and pathological brain aging: from the perspective of oscillations, functional connectivity, and signal complexity. Neuropsychobiology. 2018;75(4):151–161. doi: 10.1159/000486870. [DOI] [PubMed] [Google Scholar]
- 3.Perinelli A, Assecondi S, Tagliabue CF, Mazza V. Power shift and connectivity changes in healthy aging during resting-state EEG. NeuroImage. 2022;256:119247. doi: 10.1016/j.neuroimage.2022.119247. [DOI] [PubMed] [Google Scholar]
- 4.Murty DV, Manikandan K, Kumar WS, Ramesh RG, Purokayastha S, Javali M, Rao NP, Ray S. Gamma oscillations weaken with age in healthy elderly in human EEG. NeuroImage. 2020;215:116826. doi: 10.1016/j.neuroimage.2020.116826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murty DV, Manikandan K, Kumar WS, Ramesh RG, Purokayastha S, Nagendra B, Abhishek ML, Balakrishnan A, Javali M, Rao NP, Ray S. Stimulus-induced gamma rhythms are weaker in human elderly with mild cognitive impairment and alzheimer’s disease. eLife. 2021;10:1–22. doi: 10.7554/eLife.61666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herrmann CS, Struber D, Helfrich RF, Engel AK. EEG oscillations: from correlation to causal- ity. Int J Psychophysiol. 2016;103:12–21. doi: 10.1016/j.ijpsycho.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Klimesch W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn Sci. 2012;16(12):606–617. doi: 10.1016/j.tics.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clayton FJ, Sears C, Davis A, Hulme C. Verbal task demands are key in explaining the relationship between paired-associate learning and reading ability. J Exp Child Psychol. 2018;171:46–54. doi: 10.1016/j.jecp.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Gutteling TP, Sillekens L, Lavie N, Jensen O. Alpha oscillations reflect suppression of distractors with increased perceptual load. Prog Neurobiol. 2022;214:102285. doi: 10.1016/j.pneurobio.2022.102285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrmann CS, Munk MH, Engel AK. Cognitive functions of gamma-band activity: memory match and utilization. Trends Cogn Sci. 2004;8(8):347–355. doi: 10.1016/j.tics.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Jensen O, Kaiser J, Lachaux JP. Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci. 2007;30(7):317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Muller-Putz GR, Scherer R, Brauneis C, Pfurtscheller G. Steady-state visual evoked potential (SSVEP)-based communication: impact of harmonic frequency components. J Neural Eng. 2005;2(4):123–130. doi: 10.1088/1741-2560/2/4/008. [DOI] [PubMed] [Google Scholar]
- 13.Sharma K, Kar S. Extracting multiple commands from a single SSVEP flicker using eye accommodation. Biocybernetics Biomed Eng. 2019;39(3):914–922. doi: 10.1016/j.bbe.2019.08.002. [DOI] [Google Scholar]
- 14.Horwitz A, Thomsen MD, Wiegand I, Horwitz H, Klemp M, Nikolic M, Rask L, Lauritzen M, Benedek K. Visual steady state in relation to age and cognitive function. PLoS One. 2017;12(2):1–23. doi: 10.1371/journal.pone.0171859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vialatte FB, Maurice M, Dauwels J, Cichocki A. Steady-state visually evoked potentials: focus on essential paradigms and future perspectives. Prog Neurobiol. 2010;90(4):418–438. doi: 10.1016/j.pneurobio.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Silberstein RB, Nunez PL, Pipingas A, Harris P, Danieli F. Steady state visually evoked potential (SSVEP) topography in a graded working memory task. Int J Psychophysiol. 2001;42(2):219–232. doi: 10.1016/S0167-8760(01)00167-2. [DOI] [PubMed] [Google Scholar]
- 17.Kabdebon C, Flo A, de Heering A, Aslin R. The power of rhythms: how steady-state evoked responses reveal early neurocognitive development. NeuroImage. 2022;254:119150. [DOI] [PMC free article] [PubMed]
- 18.Calderone DJ, Lakatos P, Butler PD, Castellanos FX. Entrainment of neural oscillations as a modifiable substrate of attention. Trends Cogn Sci. 2014;18(6):300–309. doi: 10.1016/j.tics.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horwitz A, Klemp M, Horwitz H, Thomsen MD, Rostrup E, Mortensen EL, Osler M, Lauritzen M, Benedek K. Brain responses to passive sensory stimulation correlate with intelligence. Front Aging Neurosci. 2019;10:1–17. doi: 10.3389/fnagi.2019.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richard N, Nikolic M, Mortensen EL, Osler M, Lauritzen M, Benedek K. Steady-state visual evoked potential temporal dynamics reveal correlates of cognitive decline. Clin Neurophysiol. 2020;131(4):836–846. doi: 10.1016/j.clinph.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Osler M, Lund R, Kriegbaum M, Christensen U, Andersen AMN. Cohort profile: the Metropolit 1953 Danish male birth cohort. Int J Epidemiol. 2006;35(3):541–545. doi: 10.1093/ije/dyi300. [DOI] [PubMed] [Google Scholar]
- 22.Zarnani K, Nichols TE, Alfaro-Almagro F, Fagerlund B, Lauritzen M, Rostrup E, Smith SM. Discovering markers of healthy aging: a prospective study in a Danish male birth cohort. Aging. 2019;11(16):5943–5974. doi: 10.18632/aging.102151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horwitz A, Mortensen EL, Osler M, Fagerlund B, Lauritzen M, Benedek K. Passive double sensory evoked coherence correlates with long-term memory capacity. Front Hum Neurosci. 2017;11:1–21. doi: 10.3389/fnhum.2017.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vestergaard MB, Lindberg U, Knudsen MH, Urdanibia-Centelles O, Bakhtiari A, Mortensen EL, Osler M, Fagerlund B, Benedek K, Lauritzen M, Larsson HBW. Subclinical cognitive deficits are associated with reduced cerebrovascular response to visual stimulation in mid-sixties men. GeroScience. 2022;0123456789. [DOI] [PMC free article] [PubMed]
- 25.Keil A, Muller MM, Ray WJ, Gruber T, Elbert T. Human gamma band activity and perception of a gestalt. J Neurosci. 1999;19(16):7152–7161. doi: 10.1523/JNEUROSCI.19-16-07152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schadow J, Lenz D, Thaerig S, Busch NA, Frund I, Rieger JW, Herrmann CS. Stimulus intensity affects early sensory processing: visual contrast modulates evoked gamma-band activity in human EEG. Int J Psychophysiol. 2007;66(1):28–36. doi: 10.1016/j.ijpsycho.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Joon Kim Y, Grabowecky M, Paller KA, Muthu K, Suzuki S. Attention induces synchronization based response gain in steady-state visual evoked potentials. Nat Neurosci. 2007;10(1):117–125. doi: 10.1038/nn1821. [DOI] [PubMed] [Google Scholar]
- 28.Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Pion-Tonachini L, Kreutz-Delgado K, Makeig S. ICLabel: An automated electroencephalographic independent component classifier, dataset, and website. NeuroImage. 2019;198(April):181–197. doi: 10.1016/j.neuroimage.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrin F, Pernier J, Bertrand O. Spherical splines for scalp potential and current density mapping 10.1016/0013-4694(89)90180-6: electroencephalography and clinical neurophysiology — ScienceDirect.com. ElectroencephaJography and clinical Neurophysiology. 1989;72(2):184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- 31.Welch PD. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans Audio Electroacoust. 1967;15(2):70–73. doi: 10.1109/TAU.1967.1161901. [DOI] [Google Scholar]
- 32.Gaetz W, Roberts TP, Singh KD, Muthukumaraswamy SD. Functional and structural correlates of the aging brain: relating visual cortex (V1) gamma band responses to age-related structural change. Hum Brain Mapp. 2012;33(9):2035–2046. doi: 10.1002/hbm.21339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Raz N, Dale AM, Walhovd KB. Accelerating cortical thinning: unique to dementia or universal in aging? Cereb Cortex. 2014;24(4):919–934. doi: 10.1093/cercor/bhs379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5(2):87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 35.Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18(5):1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan EV, Adalsteinsson E, Hedehus M, Ju C, Moseley M, Lim KO, Pfefferbaum A. Equivalent disruption of regional white matter microstructure in ageing healthy men and women. NeuroReport. 2001;12(1):99–104. doi: 10.1097/00001756-200101220-00027. [DOI] [PubMed] [Google Scholar]
- 37.Peters A. The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol. 2002;31(8-9):581–593. doi: 10.1023/A:1025731309829. [DOI] [PubMed] [Google Scholar]
- 38.Hong SL, Rebec GV. A new perspective on behavioral inconsistency and neural noise in aging: compensatory speeding of neural communication. Front Aging Neurosci. 2012;4:1–6. [DOI] [PMC free article] [PubMed]
- 39.Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 40.Lehmann K, Steinecke A, Bolz J. GABA through the ages: regulation of cortical function and plasticity by inhibitory interneurons. Neural Plasticity. 2012;2012. [DOI] [PMC free article] [PubMed]
- 41.Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophr Bull. 2008;34(5):944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prevot T, Sibille E. Altered GABA-mediated information processing and cognitive dysfunctions in´ depression and other brain disorders. Mol Psychiatry. 2021;26(1):151–167. doi: 10.1038/s41380-020-0727-3. [DOI] [PubMed] [Google Scholar]
- 43.Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464(7288):529–535. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mably AJ, Colgin LL. Gamma oscillations in cognitive disorders. Curr Opin Neurobiol. 2018;52:182–187. doi: 10.1016/j.conb.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han C, Shapley R, Xing D. Gamma rhythms in the visual cortex: functions and mechanisms. Cogn Neurodyn. 2022;16(4):745–756. doi: 10.1007/s11571-021-09767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Traikapi A, Konstantinou N. Gamma oscillations in Alzheimer’s disease and their potential therapeutic role. Front Syst Neurosci. 2021;15. [DOI] [PMC free article] [PubMed]
- 47.Lundqvist M, Rose J, Herman P, Brincat SLL, Buschman TJJ, Miller EKK. Gamma and beta bursts underlie working memory. Neuron. 2016;90(1):152–164. doi: 10.1016/j.neuron.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamamoto J, Suh J, Takeuchi D, Tonegawa S. Successful execution of working memory linked to synchronized high-frequency gamma oscillations. Cell. 2014;157(4):845–857. doi: 10.1016/j.cell.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 49.Fries P. Rhythms for cognition: communication through coherence. Neuron. 2015;88(1):220–235. doi: 10.1016/j.neuron.2015.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferretti A, Rogers-Healion K, Fotros A. The therapeutic potential of restoring gamma oscillations in Alzheimer’s disease. Adv Psychiatry Behav Health. 2022;2(1):47–55. doi: 10.1016/j.ypsc.2022.05.002. [DOI] [Google Scholar]
- 51.Puttaert D, Coquelet N, Wens V, Peigneux P, Fery P, Rovai A, Trotta N, Sadeghi N, Coolen T, Bier JC, Goldman S, De Tiege X. Alterations in resting-state network dynamics along the Alzheimer’s` disease continuum. Sci Rep. 2020;10(1):1–13. doi: 10.1038/s41598-020-76201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adaikkan C, Tsai LH. Gamma entrainment: impact on neurocircuits, glia, and therapeutic opportunities. Trends Neurosci. 2020;43(1):24–41. doi: 10.1016/j.tins.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Chan D, Suk H-J, Jackson BL, Milman NP, Stark D, Klerman EB, Kitchener E, Fernandez Avalos VS, Banerjee A, Beach SD, Blanchard J, Stearns C, Boes A, Uitermarkt B, Gander P, Howard M III, Sternberg EJ, Nieto-Castanon A, Anteraper S, et al. Gamma frequency sensory stimulation in probable mild Alzheimer’s dementia patients: results of a preliminary clinical trial. SSRN Electron J. 2021.
- 54.Clements-Cortes A, Ahonen H, Evans M, Freedman M, Bartel L. Short-term effects of rhythmic sensory stimulation in Alzheimer’s disease: an exploratory pilot study. J Alzheimers Dis. 2016;52(2):651–660. doi: 10.3233/JAD-160081. [DOI] [PubMed] [Google Scholar]
- 55.He Q, Colon-Motas KM, Pybus AF, Piendel L, Seppa JK, Walker ML, Manzanares CM, Qiu D, Miocinovic S, Wood LB, Levey AI, Lah JJ, Singer AC. A feasibility trial of gamma sensory flicker for patients with prodromal Alzheimer’s disease. Alzheimer’s Dementia. 2021;7(1):1–11. doi: 10.1002/trc2.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cabeza R, Albert M, Belleville S, Craik FI, Duarte A, Grady CL, Lindenberger U, Nyberg L, Park DC, Reuter-Lorenz PA, Rugg MD, Steffener J, Rajah MN. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci. 2018;19(11):701–710. doi: 10.1038/s41583-018-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koen JD, Rugg MD. Neural dedifferentiation in the aging brain. Trends Cogn Sci. 2019;23(7):547–559. doi: 10.1016/j.tics.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schaworonkow N, Nikulin VV. Is sensor space analysis good enough? Spatial patterns as a tool for assessing spatial mixing of EEG/MEG rhythms. NeuroImage. 2022;253:119093. doi: 10.1016/j.neuroimage.2022.119093. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Xu P, Huang Y, Cheng K, Yao D. SSVEP response is related to functional brain network topology entrained by the flickering stimulus. PLoS One. 2013;8(9). [DOI] [PMC free article] [PubMed]