Abstract
A growing number of pharmaceutical and small molecule interventions are reported to extend the lifespan of laboratory animals including Caenorhabditis, Drosophila, and mouse. However, the degree to which these pro-longevity interventions are conserved across species is unclear. Here, we took two approaches to ask the question: to what extent do longevity intervention studies in Caenorhabditis and Drosophila recapitulate effects on mouse lifespan? The first approach analyzes all published reports on longevity in the literature collated by the DrugAge database, and the second approach focused on results designed for reproducibility as reported from the NIA-supported Interventions Testing Program (ITP) and the Caenorhabditis Interventions Testing Program (CITP). Using published data sources, we identify only modest sensitivity and specificity of Drosophila interventional studies for identifying pro-longevity compounds in mouse lifespan studies. Surprisingly, reported studies in C. elegans show little predictive value for identifying drugs that extend lifespan in mice. The results therefore suggest caution should be used when making assumptions about the translatability of lifespan-extending compounds across species, including human intervention.
Keywords: Aging, Longevity, Comparative biology, Interventions
The fields of aging research and geroscience have devoted significant resources toward understanding interventions that extend longevity and health span and thus may improve the health of the growing aging community [1]. A growing number of promising drugs and small compound interventions have been shown to extend lifespan in laboratory animal models. There are significant advantages to using laboratory animals to discover novel regulators of longevity foremost being the relatively short lifespans of these species. Multiple positive interventions have been discovered within each species tested; however, the extent to which an effect of an intervention in one species translates to a similar outcome in another is still unknown [2, 3]. This is an important clinical question for moving drugs from laboratory animals to human populations. But, this is also of great importance during pre-clinical studies as the translatability of pro-longevity drugs and compounds between lab animal models would assist in better understanding the mechanisms by which they might affect outcomes clinically.
Many factors have driven the selection of Caenorhabditis (primarily C. elegans), Drosophila melangoster, and mice being the most commonly used for interventional aging studies. For longevity studies, the short lifespans of Caenorhabditis and Drosophila provide means to screen compounds quickly; however, these species lack many of the same anatomical structures of humans and are genetically distantly related. Mice have advantages in that they have common mammalian physiological function and anatomy and, in some ways, phenocopy the pathologies and diseases of humans; however, the lifespan of mice is manyfold greater than that of common invertebrate models. It would then make intuitive sense to use the strengths of each model to drive longevity intervention discovery. That is, screening potential compounds using invertebrate models for those that extend longevity might streamline further testing on the aging physiology, health span, and longevity in mice [4–6]. However, it is unclear whether compounds that affect longevity in Caenorhabditis or Drosophila provide any predictive value for the effectiveness of compounds on mouse lifespan.
Here, we addressed this question using two methods: (1) an analysis using data exclusively from the DrugAge database to test the degree of predictability of results among drugs tested among all three species and (2) an analysis of results from drugs tested in mice by the Interventions Testing Program (ITP), in Caenorhabditis by the Caenorhabditis Interventions Testing Program (CITP), and in the literature for Drosophila. With the first approach, we sought to capture the largest breadth of published data possible and provide a practical view of the translatability of studies in the general literature. With the second, our goal was to test data sets designed to be reproducible and with a high degree of reliability rather than with single reports. Overall, we identify modest sensitivity and specificity in predicting compounds that affect mouse lifespan from Drosophila and little predictive value for identifying similar compounds from Caenorhabditis studies.
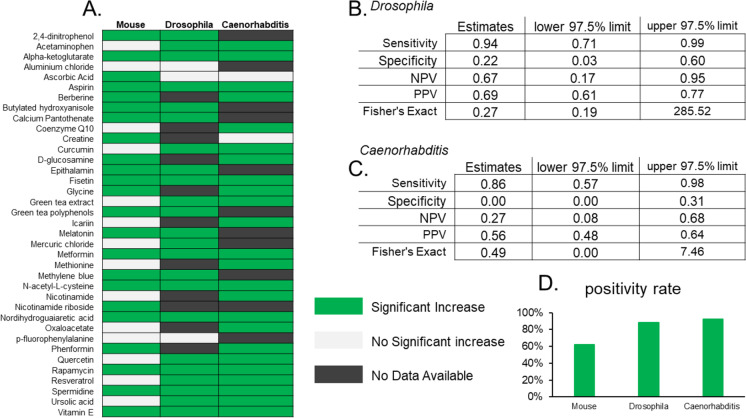
Using data from the DrugAge database, we initially found 37 compounds for which lifespan data was reported in mice and also for either Drosophila or Caenorhabditis (Fig. 1a). Using a binary diagnostic framework, we then asked to what degree are drug interventional longevity studies using invertebrates predictive of similar outcomes from longevity studies using mice. We find that studies testing compounds on Drosophila longevity have modest predictive value for outcomes in mice, with a high sensitivity of 0.94, indicating 94% of compounds which extend lifespan in mice were also positive in Drosophila. However, the same data set also indicates a specificity of only 0.22, suggesting only 22% of compounds with no effect in mice were also reported negative in Drosophila (Fig. 1b). Using these values, we calculated the positive predictive value (PPV) of Drosophila is 0.69, indicating a 69% chance compounds which extend lifespan in Drosophila also extend lifespan in a mouse. While these results seem to suggest a good correlation between these species in predictive value, it is important to consider that in this dataset, 62% of all compounds tested in mice extend lifespan (Fig. 1d). Or in other words, there is nearly the same chance in picking out a positive result in mice from any random compound in these published data set as there is in guiding choices by those that are positive in Drosophila. Based on the nature of the data sets, i.e., those that have been published in the existing literature, many of these compounds are likely to be positive. That is, it is far more likely that “positive” effects are published than are “negative” or “no” effects of interventions. However, these analyses then also show that looking at data from data generated in Drosophila provides little additional value in determining the potential positive effects of interventions in mice. In addition, these results suggest that while Drosophila lifespan studies provide good sensitivity for lifespan studies in mice, they have a poor ability to detect “true negatives” as indicated by the low specificity and modest PPV and negative predictive value (NPV). These results may also be indicative of reverse causality, as often drugs which are reported to extend lifespan in invertebrates are subsequently tested in mice, making it more likely that a compound tested in mice has positive effects in invertebrates.
Fig. 1.
A Heatmap of lifespan effects of compounds based on the DrugAge database. B Binary diagnostic test using Drosophila to predict mouse lifespan effects. C Binary diagnostic test using Caenorhabditis to predict mouse lifespan effects. D Percentage of compounds with significant positive lifespan effect per species
Somewhat surprisingly, the analysis of drug interventional longevity studies using Caenorhabditis from the DrugAge database shows an even poorer predictive value for mouse lifespan effects with a sensitivity of 0.86 and specificity of 0.0 (Fig. 1c). That is, there was no relationship between the compounds reported negative in mice with those in Caenorhabditis. Moreover, we calculate the PPV of studies in Caenorhabditis to predict mouse effects as 0.56, meaning that this approach is worse than the method of predicting the beneficial effect on mouse lifespan than randomly selecting any drug from the pool of those tested in a DrugAge subset. This suggests that Caenorhabditis has an even poorer ability than Drosophila to detect lifespan effects in mice and notably that negative findings in Caenorhabditis actually appear to inverse findings in mice with a NPV of 0.27. Taken together, while positive effects of interventions in both Drosophila and Caenorhabditis lifespan studies may have relatively high sensitivity for similar positive effects in mouse lifespan studies, their utility for predicting outcomes in mice for any screening purposes is severely limited due to their overwhelming lack of specificity.
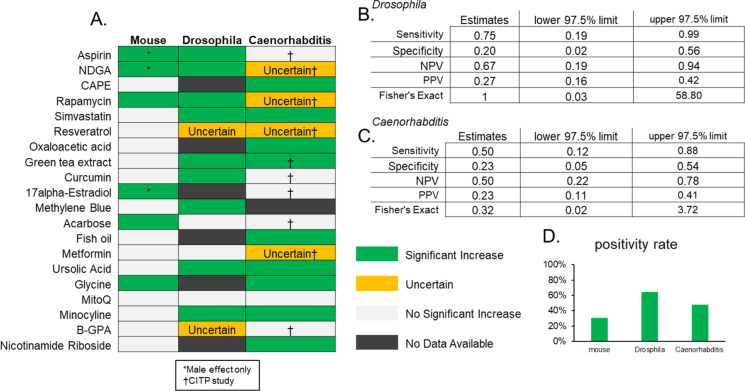
While initially somewhat surprising, we suspect a significant factor in the low specificity of Drosophila and Caenorhabditis studies using this method might be the overwhelmingly high positivity rate of the analyzed published studies with 88% for Drosophila and 92% for Caenorhabditis compared to only 62% in mouse studies (Fig. 1d). In addition, the DrugAge database (and our own subsequent analysis) does not screen the included reports for reproducibility or rigor likely further complicating the interpretation of these outcomes. Therefore, we took a separate approach focused first on mouse longevity interventional studies from the ITP which has been designed with high degrees of rigor and reproducibility from its beginnings [7]. In the published literature at the time of searching, there were 20 compounds for which mouse lifespan studies have been conducted by the ITP that have also been tested (and reported) for lifespan studies in Drosophila or Caenorhabditis (Fig. 2a). As described in “Methods,” we gave preference for Caenorhabditis studies reported from the CITP which, like the ITP, has been designed for high reproducibility and rigor, and Drosophila studies were from our own curated searches of the literature. Similar to results from our first analysis, we found that Drosophila lifespan studies on drugs and compounds provided a modest predictive value of mouse lifespan effects with a sensitivity of 0.75 and specificity of 0.20 (Fig. 2b). Again, like our first study, we found even lower predictive value for Caenorhabditis lifespan studies for identifying drugs and compounds with effects in mice with a sensitivity of 0.5 and specificity of 0.23 (Fig. 2c). The PPV for both species in predicting benefits on mouse lifespan was dramatically lower than those calculated from the DrugAge data sets; the PPV for Drosophila in these selected data was calculated as 0.27 and that for Caenorhabditis at 0.23. Notably, the positivity rate of drugs in this ITP-focused analysis was remarkably lower than with our first broad analysis of all published literature; only 30% of mice, 64% of Drosophila, and 47% of Caenorhabditis drug studies showed positive lifespan effects in this collated data set (Fig. 2d). Again, these data suggest that selecting interventions based on existing invertebrate studies provides little additional value. The particularly large reduction in the positivity rate of mouse and Caenorhabditis may be an indicator of the large degree of rigor and reproducibility of the ITP and CITP studies, respectively, and provides further support for their utility.
Fig. 2.
A Heatmap of lifespan effects of compounds based on ITP, CITP, and literature review. B Binary diagnostic test using Drosophila to predict mouse lifespan effects. C Binary diagnostic test using Caenorhabditis to predict mouse lifespan effects. D Percentage of compounds with significant positive lifespan effect per species
We show here that in the published literature, there is surprisingly a relatively low translatability of drugs shown to extend lifespan in either Drosophila or Caenorhabditis to lifespan outcomes in studies using mice. We used two different approaches: one using all reports of any effects collated by DrugAge and a second focused on studies from sources designed with a high degree of rigor. We found no specificity from Caenorhabditis studies using the first method but higher (though still low) using the second method. One possibility may be an inherent bias in these studies in that some compounds were tested by the CITP because of their use in the ITP. On the other hand, studies from the DrugAge database are also biased with high positive rates (Fig. 1d) possibly due to selection pressure for positive outcomes for studies to be published. However, it is noteworthy that even when results in Drosophila and Caenorhabditis are in the same direction in our analysis, the same effect in mice is seen only 9/16 times in our first analysis and only 1/6 times in our second. These outcomes suggest that even lifespan effects in multiple species do not make it exceptionally likely any given compound will extend the lifespan in another species. Overall, these results indicate that lifespan extension by a compound in Drosophila, and particularly Caenorhabditis, is not a strong predictor of its effect in mice.
While there may be species-specific drivers of aging, it is commonly thought that the biology of aging has similar hallmarks and potential mediators across animals [8]. Rather than our study arguing against this idea, these outcomes might at least partially represent differences in anatomy, physiology, or function in these disparate species. For example, drug metabolism and pharmacology differ among different species, and thus dosing of a given compound across species may not be equivalent. Physical differences in the administration of drugs, i.e., ingestion vs direct application, among animal models may also present a confound that is hard to control. Modes of action of different compounds may require the presence of specific organs present in mammals that are absent in invertebrate models. Lastly, the most common causes of death and pathologies that drive morbidity differ drastically between these three species. The cause of death analysis of mice indicates cancer is overwhelmingly the most common, ranging from 80 to 90% in some strains, while the cause of death in Drosophila is most commonly connected to disruption of intestinal barrier function, and only 18.2 and 1.3% of flies have been found to present with testis and gut tumors respectively upon death. The cause of death in Caenorhabditis is also uniquely associated with the pathology of distinct “atrophied” or “swollen” pharynx, indicating end-of-life pathology in these all of these model organisms is highly distinct and notably different from humans where heart diseases are most common followed by cancers [9–15]. It is therefore likely that some compounds which extend lifespan in organisms may do so through the prevention or delaying of particular conditions and not general geroprotective effects, therefore decreasing their likelihood of extending lifespan in other organism with different end-of-life pathology. The use of lifespan studies in model organisms is a foundational component of aging research, due in part to the difficulty of conducting clinical survival studies over decades in humans. However, for these reasons, greater acceptance of healthspan or healthy aging outcomes may have increased value in light of the challenges in translating across species [16, 17].
There are limitations and caveats to both the data used for this meta-analysis and this study itself. First, we identified reported compounds tested for lifespan effects without discussion of dose, dosing regimen, age at treatment, etc. All are potential factors in the outcome of compounds, and thus a single “null” outcome may not reflect the complete findings of that compound. Second, we used data in our analysis with no specific discussion for rigor only that an effect had been reported (which at least provides some level of rigor in peer review), and generally, these data have not been repeated. We chose relatively rigorous studies for our second analysis but are specifically limited to the published literature. While our goal for the first analysis was to present the largest most practical interpretation of the literature possible while minimizing our ability to bias selection, we acknowledge that the presence of one positive study should not necessarily be taken over the rest of the reported literature. It may be of value to perform secondary analysis, such as classifying compounds used by action or target, though we suspect based on our studies here, the results would not be particularly different. Lastly, our sole reliance on ITP mouse data for our second study comes with the caveat that the studies were performed exclusively in the genetically heterogenous HET-3 mouse model and may not be indicative of effects among all mouse strains.
Despite these limitations, we believe the data presented provide an important novel insight into the translatability of lifespan-extending compounds between species. Our data suggest that interventional studies testing effects on longevity in model organisms such as Drosophila and Caenorhabditis may be poor predictors of similar lifespan effects in mice. On the other hand, there are significant benefits, short lifespan and low cost are two significant ones, to using these models to screen and identify new potential compounds that may be beneficial for aging. However, such approaches might benefit from alternative outcomes in addition to a lifespan that might inform on the likelihood of potential effects in other species. Given the low translatability of lifespan-extending compounds between laboratory model species, it is questionable how well such interventions may translate long-term to the general human population. This may also be the case in reverse; human populations may benefit from drug interventions that have limited impact on model organism lifespan. An example of such may be particular effects on all-cause mortality in healthy elderly taking aspirin and pre-diabetics taking metformin reporting modestly decreased and no effects respectively, while some model organisms would indicate an increase in lifespan expected [18, 19]. This further reinforces the results from our analysis which suggest great caution should be applied when extrapolating lifespan results between species and in particular from lab models to humans.
Methods
DrugAge analysis
The DrugAge database (https://genomics.senescence.info/drugs/), as reported by Barardo et al., is a curated database of lifespan-extending compounds reported in the scientific literation with data for over 1000 distinct compounds [20]. We performed an initial search to identify studies testing the effect of compounds on lifespan in mice with all potential effects (positive, neutral, negative). We then used this initial list to query for similar tests in Drosophila and Caenorhabditis. Reported lifespan data for each species were categorized into either (1) significant lifespan increase with drug or compound or (2) no significant lifespan increase, to minimize potential bias. All those in group 1 reported at least one entry in DrugAge for that given compound and organism with a statistically significant increase in lifespan. Those in group 2 then included those with no statistically significant increase in lifespan, those with no effect, and those with a decrease in lifespan. This method was used to reduce potential bias from experiments in which some doses of a given compound appear toxic and others may improve lifespan thereby obfuscating results. Additionally, in prioritizing positive findings, we believe this method provides a “realistic” outlook on results given the presence of publication bias and the tendency for researchers to be more likely to attempt future lifespan studies based on a first publication of positive findings in another species.
ITP analysis
Data from our first method include any report on lifespan effect regardless of sample or effect size, reproduction in the literature, or further study. In our second analysis, we sought to focus on highly rigorous and reproducible data as the targets for at least 2 species by using published data from the ITP (mice) and CITP (Caenorhabditis) [21–32]. Similar to the method above, we first identified all compounds tested in mice by the ITP and in the freely available literature as of 1/20/22. Using these compounds, we then conducted a literature search using both PubMed and Google Scholar searching for lifespan studies with the same compound in different species with the query of [drug name][species name]lifespan. Using this method, we identified 20 out of our original list of 36 ITP-tested compounds for which there was a lifespan study reported in Drosophila or Caenorhabditis [21–66]. For Caenorhabditis, if CITP results were available for a given compound, we prioritized these as the most rigorous and dismissed any other lifespan reports for that compound in Caenorhabditis. Furthermore, to increase the rigor and reliability of the lifespan studies selected for this analysis, we separated results into 3 categories: (1) a significant increase result refers to a reported statistically significant effect which is either uncontradicted by any other literature, supported by the overwhelming majority of reports, or, for Caenorhabditis CITP results, produces a lifespan increase in the majority of strains tested; (2) “Uncertain” for compounds which have directly contradicting results or when compounds have been demonstrated to have a lifespan effect dependent on diet or strain, or for CITP show significant effects in less than half of strains tested; and (3) no significant increase results encapsulate drugs which either have no reports of a significant increase in lifespan or, for CITP results, have an effect in one or fewer of the tested strains.
Statistics
Statistical analysis of data was performed using a binary diagnostic framework. For the DrugAge analysis, the classification of each drug in an organism led to its categorization as positive or negative. For Drosophila, Caenorhabditis, or Saccharomyces, a 2 × 2 table was generated with the given organism results as the rows and mouse results as the columns. This data matrix was then entered into Rstudio, and the BDtest function was used to calculate sensitivity, specificity, positive predictive value, and negative predictive value with a 95% confidence interval. Additionally, Fisher’s exact test was performed with the “fisher.test” function. For the ITP analysis, data was also interpreted as a matrix; however, multiple analyses were performed with differing allocation of the “Uncertain” category, in the primary analysis whose results are displayed in Fig. 2 “Uncertain” was interpreted as a positive; however, calculation with “Uncertain” interpreted as a negative or eliminated from analysis entirely was also performed with minimal impact on the interpretation of results. Finally, to calculate the positivity rates for each species, the sum of positive findings was divided by the number of compounds tested in each species.
Funding
This research was funded in part by R01 AG050797, R01 AG057431, T32 AG021890, I01 BX004167, P30 AG013319, P30 AG044271.
Data Availability
Raw data were generated from cited publications and databases. Full data analyses used to generate figures will be provided upon reasonable request.
Declarations
Conflicts of interests
The authors declare no conflicts of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kennedy BK, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–13. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berkel C, Cacan E. A collective analysis of lifespan-extending compounds in diverse model organisms, and of species whose lifespan can be extended the most by the application of compounds. Biogerontology. 2021;22(6):639–653. doi: 10.1007/s10522-021-09941-y. [DOI] [PubMed] [Google Scholar]
- 3.Moskalev A, et al. Targeting aging mechanisms: pharmacological perspectives”. Trends Endocrinol Metab: TEM. 2022;33(4):266–280. doi: 10.1016/j.tem.2022.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Pitt JN, et al. WormBot, an open-source robotics platform for survival and behavior analysis in C. elegans. GeroScience. 2019;41(6):961–973. doi: 10.1007/s11357-019-00124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulterijs S, Braeckman BP. Phenotypic Screening in C elegans as a Tool for the Discovery of New Geroprotective Drugs. Pharmaceuticals (Basel, Switzerland) 2020;13(8):164. doi: 10.3390/ph13080164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S, Min K. Drosophila melanogaster as a model system in the study of pharmacological interventions in aging. Transl Med Aging. 2019;3:98–103. doi: 10.1016/j.tma.2019.09.004. [DOI] [Google Scholar]
- 7.Nadon NL, et al. Design of aging intervention studies: the NIA interventions testing program. Age (Dordrecht, Netherlands) 2008;30(4):187–199. doi: 10.1007/s11357-008-9048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heron M. Deaths: Leading Causes for 2019. Natl Vital Stat Rep: Centers Dis Control Prev Natl Center Health Stat Natl Vital Stat Syst. 2021;70(9):1–114. [PubMed] [Google Scholar]
- 10.Snyder JM, et al. Cause-of-death analysis in rodent aging studies. Vet Pathol. 2016;53(2):233–243. doi: 10.1177/0300985815610391. [DOI] [PubMed] [Google Scholar]
- 11.Lipman R, et al. Genetic loci that influence cause of death in a heterogeneous mouse stock. J Gerontol Ser A, Biol Sci Med Sci. 2004;59(10):977–83. doi: 10.1093/gerona/59.10.b977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y, et al. Two forms of death in ageing Caenorhabditis elegans. Nat Commun. 2017;8:15458. doi: 10.1038/ncomms15458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galimov ER, et al. Coupling of rigor mortis and intestinal necrosis during C. elegans organismal death”. Cell Rep. 2018;22(10):2730–2741. doi: 10.1016/j.celrep.2018.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rera M, et al. Intestinal barrier dysfunction links metabolic and inflammatory markers of aging to death in Drosophila. Proc Natl Acad Sci USA. 2012;109(52):21528–33. doi: 10.1073/pnas.1215849110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salomon RN, Jackson FR. Tumors of testis and midgut in aging flies. Fly. 2008;2(6):265–8. doi: 10.4161/fly.7396. [DOI] [PubMed] [Google Scholar]
- 16.Seals DR, et al. Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J Physiol. 2016;594(8):2001–24. doi: 10.1113/jphysiol.2014.282665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palliyaguru DL, et al. Frailty index as a biomarker of lifespan and healthspan: Focus on pharmacological interventions. Mech Ageing Dev. 2019;180:42–48. doi: 10.1016/j.mad.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNeil JJ, et al. Effect of Aspirin on All-Cause Mortality in the Healthy Elderly. N Engl J Med. 2018;379(16):1519–1528. doi: 10.1056/NEJMoa1803955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee CG, et al. Effect of metformin and lifestyle interventions on mortality in the diabetes prevention program and diabetes prevention program outcomes study. Diabetes Care. 2021;44(12):2775–2782. doi: 10.2337/dc21-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barardo D, et al. The DrugAge database of aging-related drugs. Aging cell. 2017;16(3):594–597. doi: 10.1111/acel.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strong R, Miller RA, Astle CM, Floyd RA, Flurkey K, Hensley KL, Javors MA, Leeuwenburgh C, Nelson JF, Ongini E, Nadon NL, Warner HR, Harrison DE. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7(5):641–650. doi: 10.1111/j.1474-9726.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66(2):191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strong R, Miller RA, Astle CM, Baur JA, de Cabo R, Fernandez E, Guo W, Javors M, Kirkland JL, Nelson JF, Sinclair DA, Teter B, Williams D, Zaveri N, Nadon NL, Harrison DE. Evaluation of resveratrol, green tea extract, curcumin, oxaloacetic acid, and medium-chain triglyceride oil on life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2013;68(1):6–16. doi: 10.1093/gerona/gls070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrison DE, Strong R, Allison DB, Ames BN, Astle CM, Atamna H, Fernandez E, Flurkey K, Javors MA, Nadon NL, Nelson JF, Pletcher S, Simpkins JW, Smith D, Wilkinson JE, Miller RA. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13(2):273–282. doi: 10.1111/acel.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strong R, Miller RA, Antebi A, Astle CM, Bogue M, Denzel MS, Fernandez E, Flurkey K, Hamilton KL, Lamming DW, Javors MA, de Magalhães JP, Martinez PA, McCord JM, Miller BF, Müller M, Nelson JF, Ndukum J, Rainger GE, Richardson A, Sabatini DM, Salmon AB, Simpkins JW, Steegenga WT, Nadon NL, Harrison DE. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15(5):872–884. doi: 10.1111/acel.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller RA, Harrison DE, Astle CM, Bogue MA, Brind J, Fernandez E, Flurkey K, Javors M, Ladiges W, Leeuwenburgh C, Macchiarini F, Nelson J, Ryazanov AG, Snyder J, Stearns TM, Vaughan DE, Strong R. Glycine supplementation extends lifespan of male and female mice. Aging Cell. 2019;18(3):e12953. doi: 10.1111/acel.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller RA, Harrison DE, Allison DB, Bogue M, Debarba L, Diaz V, Fernandez E, Galecki A, Garvey WT, Jayarathne H, Kumar N, Javors MA, Ladiges WC, Macchiarini F, Nelson J, Reifsnyder P, Rosenthal NA, Sadagurski M, Salmon AB, Smith DL, Jr, Snyder JM, Lombard DB, Strong R. Canagliflozin extends life span in genetically heterogeneous male but not female mice. JCI Insight. 2020;5(21):e140019. doi: 10.1172/jci.insight.140019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lucanic M, Plummer W, Chen E, et al. Impact of genetic background and experimental reproducibility on identifying chemical compounds with robust longevity effects. Nat Commun. 2017;8:14256. doi: 10.1038/ncomms14256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banse SA, Sedore CA, Johnson E, Coleman-Hulbert AL, Onken B, Hall D, Jackson EG, Huynh P, Foulger AC, Guo S, Garrett T, Xue J, Inman D, Morshead ML, Plummer WT, Chen E, Bhaumik D, Chen MK, Harinath G, Chamoli M, Quinn RP, Falkowski R, Edgar D, Schmidt MO, Lucanic M, Guo M, Driscoll M, Lithgow GJ, Phillips PC. Antioxidants green tea extract and nordihydroguaiaretic acid confer species and strain specific lifespan and health effects in Caenorhabditis nematodes. bioRxiv 10.1101/2021.11.09.464847
- 31.Onken B, Sedore CA, Coleman-Hulbert AL, Hall D, Johnson E, Jones EG, Banse SA, Huynh P, Guo S, Xue J, Chen E, Harinath G, Foulger AC, Chao EA, Hope J, Bhaumik D, Plummer T, Inman D, Morshead M, Guo M, Lithgow GJ, Phillips PC, Driscoll M. Metformin treatment of diverse Caenorhabditis species reveals the importance of genetic background in longevity and healthspan extension outcomes. Aging Cell. 2022;21(1):e13488. doi: 10.1111/acel.13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coleman-Hulbert AL, Johnson E, Sedore CA, Banse SA, Guo M, Driscoll M, Lithgow GJ, Phillips PC. Caenorhabditis Intervention Testing Program: the creatine analog β-guanidinopropionic acid does not extend lifespan in nematodes. MicroPubl Biol. 2020;2020. 10.17912/micropub.biology.000207. [DOI] [PMC free article] [PubMed]
- 33.Yang S, Long LH, Li D, Zhang JK, Jin S, Wang F, Chen JG. β-Guanidinopropionic acid extends the lifespan of Drosophila melanogaster via an AMP-activated protein kinase-dependent increase in autophagy. Aging Cell. 2015;14(6):1024–1033. doi: 10.1111/acel.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dorigatti JD, Thyne KM, Ginsburg BC, Salmon AB. Beta-guanidinopropionic acid does not extend Drosophila lifespan. Biochem Biophys Rep. 2021;3(27):101040. doi: 10.1016/j.bbrep.2021.101040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Havermann S, Chovolou Y, Humpf HU, Wätjen W. Caffeic acid phenethylester increases stress resistance and enhances lifespan in Caenorhabditis elegans by modulation of the insulin-like DAF-16 signalling pathway. PLoS ONE. 2014;9(6):e100256. doi: 10.1371/journal.pone.0100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jahn A, Scherer B, Fritz G, Honnen S. Statins Induce a DAF-16/Foxo-dependent longevity phenotype via JNK-1 through mevalonate depletion in C. elegans. Aging Dis. 2020;11(1):60–72. doi: 10.14336/AD.2019.0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Williams DS, Cash A, Hamadani L, Diemer T. Oxaloacetate supplementation increases lifespan in Caenorhabditis elegans through an AMPK/FOXO-dependent pathway. Aging Cell. 2009;8(6):765–768. doi: 10.1111/j.1474-9726.2009.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugawara S, Honma T, Ito J, Kijima R, Tsuduki T. Fish oil changes the lifespan of Caenorhabditis elegans via lipid peroxidation. J Clin Biochem Nutr. 2013;52(2):139–145. doi: 10.3164/jcbn.12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Negi H, Shukla A, Khan F, Pandey R. 3β-Hydroxy-urs-12-en-28-oic acid prolongs lifespan in C. elegans by modulating JNK-1. Biochem Biophys Res Commun. 2016;480(4):539–543. doi: 10.1016/j.bbrc.2016.10.073. [DOI] [PubMed] [Google Scholar]
- 40.Negi H, Saikia SK, Pandey R. 3β-Hydroxy-urs-12-en-28-oic Acid Modulates Dietary Restriction Mediated Longevity and Ameliorates Toxic Protein Aggregation in C. elegans. J Gerontol A Biol Sci Med Sci. 2017;72(12):1614–1619. doi: 10.1093/gerona/glx118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Edwards C, Canfield J, Copes N, Brito A, Rehan M, Lipps D, Brunquell J, Westerheide SD, Bradshaw PC. Mechanisms of amino acid-mediated lifespan extension in Caenorhabditis elegans. BMC Genet. 2015;16(1):8. doi: 10.1186/s12863-015-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu YJ, Janssens GE, McIntyre RL, Molenaars M, Kamble R, Gao AW, Jongejan A, Weeghel MV, MacInnes AW, Houtkooper RH. Glycine promotes longevity in Caenorhabditis elegans in a methionine cycle-dependent fashion. PLoS Genet. 2019;15(3):e1007633. doi: 10.1371/journal.pgen.1007633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng LF, Gruber J, Cheah IK, Goo CK, Cheong WF, Shui G, Sit KP, Wenk MR, Halliwell B. The mitochondria-targeted antioxidant MitoQ extends lifespan and improves healthspan of a transgenic Caenorhabditis elegans model of Alzheimer disease. Free Radic Biol Med. 2014;71:390–401. doi: 10.1016/j.freeradbiomed.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Solis GM, Kardakaris R, Valentine ER, Bar-Peled L, Chen AL, Blewett MM, McCormick MA, Williamson JR, Kennedy B, Cravatt BF, Petrascheck M. Translation attenuation by minocycline enhances longevity and proteostasis in old post-stress-responsive organisms. Elife. 2018;27(7):e40314. doi: 10.7554/eLife.40314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Cantó C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, Guarente L, Auwerx J. The NAD(+)/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154(2):430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danilov A, Shaposhnikov M, Shevchenko O, Zemskaya N, Zhavoronkov A, Moskalev A. Influence of non-steroidal anti-inflammatory drugs on Drosophila melanogaster longevity. Oncotarget. 2015;6(23):19428–44. doi: 10.18632/oncotarget.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miquel J, Fleming J, Economos AC. Antioxidants, metabolic rate and aging in Drosophila. Arch Gerontol Geriatr. 1982;1(2):159–165. doi: 10.1016/0167-4943(82)90016-4. [DOI] [PubMed] [Google Scholar]
- 48.Spindler SR, Mote PL, Lublin AL, Flegal JM, Dhahbi JM, Li R. Nordihydroguaiaretic acid extends the lifespan of drosophila and mice, increases mortality-related tumors and hemorrhagic diathesis, and alters energy homeostasis in mice. J Gerontol A Biol Sci Med Sci. 2015;70(12):1479–1489. doi: 10.1093/gerona/glu190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bjedov I, Toivonen JM, Kerr F, Slack C, Jacobson J, Foley A, Partridge L. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11(1):35–46. doi: 10.1016/j.cmet.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fan X, Liang Q, Lian T, Wu Q, Gaur U, Li D, Yang D, Mao X, Jin Z, Li Y, Yang M. Rapamycin preserves gut homeostasis during Drosophila aging. Oncotarget. 2015;6(34):35274–83. doi: 10.18632/oncotarget.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang A, Mouser J, Pitt J, Promislow D, Kaeberlein M. Rapamycin enhances survival in a Drosophila model of mitochondrial disease. Oncotarget. 2016;7(49):80131–80139. doi: 10.18632/oncotarget.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schinaman JM, Rana A, Ja WW, Clark RI, Walker DW. Rapamycin modulates tissue aging and lifespan independently of the gut microbiota in Drosophila. Sci Rep. 2019;9(1):7824. doi: 10.1038/s41598-019-44106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spindler SR, Li R, Dhahbi JM, Yamakawa A, Mote P, Bodmer R, Ocorr K, Williams RT, Wang Y, Ablao KP. Statin treatment increases lifespan and improves cardiac health in Drosophila by decreasing specific protein prenylation. PLoS ONE. 2012;7(6):e39581. doi: 10.1371/journal.pone.0039581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C, Wheeler CT, Alberico T, Sun X, Seeberger J, Laslo M, Spangler E, Kern B, de Cabo R, Zou S. The effect of resveratrol on lifespan depends on both gender and dietary nutrient composition in Drosophila melanogaster. Age (Dordr) 2013;35(1):69–81. doi: 10.1007/s11357-011-9332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li YM, Chan HY, Huang Y, Chen ZY. Green tea catechins upregulate superoxide dismutase and catalase in fruit flies. Mol Nutr Food Res. 2007;51(5):546–554. doi: 10.1002/mnfr.200600238. [DOI] [PubMed] [Google Scholar]
- 56.Wagner AE, Piegholdt S, Rabe D, Baenas N, Schloesser A, Eggersdorfer M, Stocker A, Rimbach G. Epigallocatechin gallate affects glucose metabolism and increases fitness and lifespan in Drosophila melanogaster. Oncotarget. 2015;6(31):30568–78. doi: 10.18632/oncotarget.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suckow BK, Suckow MA. Lifespan extension by the antioxidant curcumin in Drosophila melanogaster. Int J Biomed Sci. 2006;2(4):402–405. [PMC free article] [PubMed] [Google Scholar]
- 58.Shen LR, Xiao F, Yuan P, Chen Y, Gao QK, Parnell LD, Meydani M, Ordovas JM, Li D, Lai CQ. Curcumin-supplemented diets increase superoxide dismutase activity and mean lifespan in Drosophila. Age (Dordr) 2013;35(4):1133–1142. doi: 10.1007/s11357-012-9438-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Massie HR, Aiello VR, Williams TR. Influence of photosensitizers and light on the life span of Drosophila. Mech Ageing Dev. 1993;68(1–3):175–182. doi: 10.1016/0047-6374(93)90149-l. [DOI] [PubMed] [Google Scholar]
- 60.Thiel AL, Ragab M, Wagner AE, Divanovic S, Derer S, Sina C. Purification and functional characterization of the chloroform/methanol-soluble protein 3 (CM3) from Triticum aestivum in Drosophila melanogaster. Front Nutr. 2020;23(7):607937. doi: 10.3389/fnut.2020.607937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Slack C, Foley A, Partridge L. Activation of AMPK by the putative dietary restriction mimetic metformin is insufficient to extend lifespan in Drosophila. PLoS ONE. 2012;7(10):e47699. doi: 10.1371/journal.pone.0047699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Staats S, Wagner AE, Lüersen K, Künstner A, Meyer T, Kahns AK, Derer S, Graspeuntner S, Rupp J, Busch H, Sina C, Ipharraguerre IR, Rimbach G. Dietary ursolic acid improves health span and life span in male Drosophila melanogaster. BioFactors. 2019;45(2):169–186. doi: 10.1002/biof.1467. [DOI] [PubMed] [Google Scholar]
- 63.Magwere T, West M, Riyahi K, Murphy MP, Smith RA, Partridge L. The effects of exogenous antioxidants on lifespan and oxidative stress resistance in Drosophila melanogaster. Mech Ageing Dev. 2006;127(4):356–370. doi: 10.1016/j.mad.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 64.Oxenkrug G, Navrotskaya V, Vorobyova L, Summergrad P. Minocycline effect on life and health span of Drosophila melanogaster. Aging Dis. 2012;3(5):352–359. [PMC free article] [PubMed] [Google Scholar]
- 65.Lee GJ, Lim JJ, Hyun S. Minocycline treatment increases resistance to oxidative stress and extends lifespan in Drosophila via FOXO. Oncotarget. 2017;8(50):87878–87890. doi: 10.18632/oncotarget.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lucanic M, et al. Impact of genetic background and experimental reproducibility on identifying chemical compounds with robust longevity effects. Nat Commun. 2017;8:14256. doi: 10.1038/ncomms14256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data were generated from cited publications and databases. Full data analyses used to generate figures will be provided upon reasonable request.