Abstract
Locus of control (LOC) describes whether an individual thinks that they themselves (internal LOC) or external factors (external LOC) have more influence on their lives. LOC varies by domain, and a person’s LOC for their intellectual capacities (LOC-Cognition) may be a marker of resilience in older adults at risk for dementia, with internal LOC-Cognition relating to better outcomes and improved treatment adherence. Vagal control, a key component of parasympathetic autonomic nervous system (ANS) regulation, may reflect a neurophysiological biomarker of internal LOC-Cognition. We used canonical correlation analysis (CCA) to identify a shared neurophysiological marker of ANS regulation from electrocardiogram (during auditory working memory) and functional connectivity (FC) data. A canonical variable from root mean square of successive differences (RMSSD) time series and between-network FC was significantly related to internal LOC-Cognition (β = 0.266, SE = 0.971, CI = [0.190, 4.073], p = 0.031) in 65 participants (mean age = 74.7, 32 female) with amnestic mild cognitive impairment (aMCI). Follow-up data from 55 of these individuals (mean age = 73.6, 22 females) was used to show reliability of this relationship (β = 0.271, SE = 0.971, CI = [0.033, 2.630], p = 0.047), and a second sample (40 participants with aMCI/healthy cognition, mean age = 72.7, 24 females) showed that the canonical vector biomarker generalized to visual working memory (β = 0.36, SE = 0.136, CI = [0.023, 0.574], p = 0.037), but not inhibition task RMSSD data (β = 0.08, SE = 1.486, CI = [− 0.354, 0.657], p = 0.685). This canonical vector may represent a biomarker of autonomic regulation that explains how some older adults maintain internal LOC-Cognition as dementia progresses. Future work should further test the causality of this relationship and the modifiability of this biomarker.
Keywords: Locus of control, Dementia, LOC-Cognition, Autonomic flexibility
Introduction
Locus of control (LOC) describes a personality trait based on whether an individual thinks that they themselves (internal LOC) or external factors (external LOC) have more influence on their lives. Internal LOC has been theorized to enable better coping mechanisms and adjustment to chronic diseases [34]. In addition to reflecting a personality trait, LOC has been shown to vary by domain, for example, the extent to which someone believes they are in control of their health (LOC-Health) or intellectual capacities (LOC-Cognition). Lachman [16] found that these domain-specific LOC measures show age-related differences and are stronger predictors of behavioral outcomes in older adults than overall LOC. Critically, our recent studies suggest that the early stages of dementia (specifically, mild cognitive impairment,MCI) are associated with a shift in LOC-Cognition, with a decrease in internal LOC-Cognition and an increase in external LOC-Cognition [32]. This is in line with research from other disorders showing that worsening depression symptoms predict a shift from internal to external LOC [15]. Additionally, one study suggested that internal LOC might be protective against cognitive decline and brain aging in older adults [31], and maintaining a belief that you are in control of your own cognition may be an essential driver of adherence to interventions in older adults at risk for dementia [44]. Not only do these findings imply that LOC-Cognition may be an indicator of dementia progression, but LOC-Cognition may also act as a feed-forward mechanism for the progression of dementia, leading to less resilience as individuals’ cognition declines alongside their belief that they are in control of their cognition.
Identifying potentially modifiable mechanisms that underpin internal LOC-Cognition among older adults at risk for dementia is fundamental for developing effective interventions that can promote internal LOC-Cognition to potentially improve both adherence to interventions and general cognitive outcomes. Research has shown that individuals with strong internal LOC show altered stress response or better adaptation capacity [22, 31]. Of note, autonomic nervous system (ANS) regulation serves as the primary indicator of stress response or adaptation capacity [40]. Cumulative work, including ours, has shown that ANS regulation, and selected brain networks that are tightly interwoven via bidirectional communication, support “autonomic flexibility”, regulating physiological and behavioral responses to threatening or challenging stimuli and influencing health [29]. Particularly, impaired vagal control capacity (an aspect of autonomic flexibility involving control over the parasympathetic, or “rest-and-digest” branch, of the ANS) at rest and during selected cognitively challenging tasks (e.g., working memory tasks) is related to neurodegeneration and cognitive deficits in individuals at risk for or with dementia [17, 20, 25]. Together, these findings suggest that the capacity to control autonomic flexibility via vagal nerve systems may be a mechanism explaining differences in internal LOC-Cognition in older adults at risk for dementia.
In this study, we tested a novel hypothesis that patterns of autonomic regulation captured via neural and peripheral physiological measures would reflect an older person’s internal LOC-Cognition. We assessed the vagal control of ANS activities (via root mean square of successive differences; RMSSD) of older adults with amnestic mild cognitive impairment (aMCI) during working memory tasks. Working memory is a critical component of cognitive decline during dementia that can be assessed in a single, short session, and has been shown to elicit reliable autonomic responses in older adults [28]. While resting-state HRV measures can also reflect parasympathetic adaptation capacity, there is evidence that there may be dissociable autonomic responses to specific environmental/cognitive stressors that relate to individual differences in participant traits [21, 39]. Given our focus on a population at risk for cognitive decline, particularly in executive functions such as working memory, and resilience markers related specifically to their cognition (LOC-Cognition), we chose to measure HRV data during a cognitive stressor to focus on autonomic signals that may specifically reflect vagal adaptation capacity related to at risk cognitive processes. We used canonical correlation analysis (CCA), which maximizes the linear correspondence between two sets of variables, to obtain correlated features from RMSSD time series and brain resting-state functional connectivity (FC) derived from fMRI data (see Fig. 1). Resting-state FC is a trait measure of brain function that has shown associations with HRV measures [7, 8]. By constraining the CCA using resting-state FC, we aimed to identify linear correspondences between FC and HRV measures to isolate aspects of autonomic regulation that are shared between the peripheral and central nervous systems. Given that dementia pathology predominantly affects the brain, we believe that these shared signals are critical for understanding individual differences in autonomic regulation in dementia. The identified canonical vector reflected a potential biomarker reflecting autonomic regulation, and was significantly associated with internal LOC-Cognition at two separate time points within an aging sample at risk for dementia, as well as in a separate validation sample consisting both cognitively healthy older adults and those with aMCI.
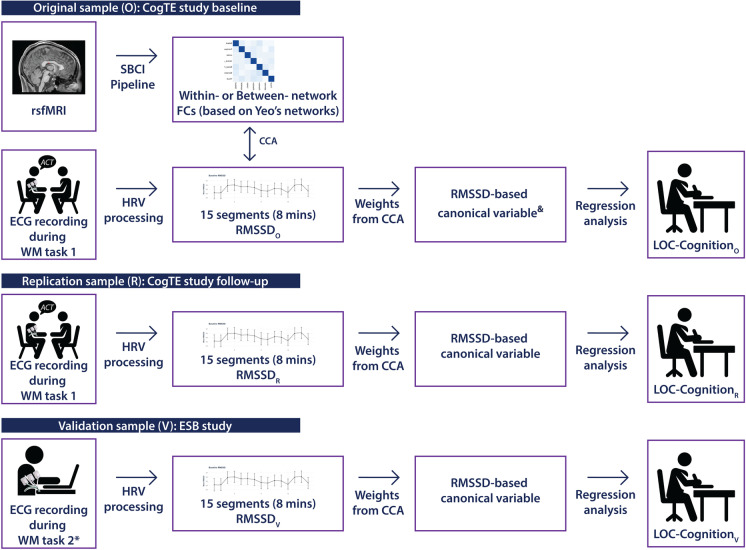
Fig. 1.
Overview of the design and analytical flow throughout the original, replication and validation samples. We generated two sets of RMSSD based on CCA of within-networks FCs, as well as CCA of between-network FCs. Note: *we also included an inhibition task as comparison. “Weights from CCA” were all from CCA in SampleO
Methods
Design overview
Figure 1 displays an overview of the study procedure. Briefly, we used a sample of 65 older adults (mean age = 74.72 7.64) from the “CogTE” study [19] as the original sample set (SampleO) where we collected measures of resting-state fMRI (rsfMRI), working memory-based task electrocardiogram (ECG), and LOC-Cognition. In SampleO, we extracted RMSSD-based canonical variables (RMSSD-betweenFC-varO and RMSSD-withinFC-varO) based on CCA of RMSSD and FC (separating averages of all within-network connections for withinFC and all between-network connections for between FC) and analyzed the relationship between autonomic flexibility and LOC-Cognition (see below for more details). As part of this analysis, CCA on SampleO also generates canonical vectors (RMSSD-betweenFC-vecO and RMSSD-withinFC-vecO), which reflect a series of weights that can be applied to other RMSSD data. We used two samples—the 6-week follow-up data of the original sample set (SampleR) and a sample set of 40 older adults (mean age = 72.83 10.10) (SampleV, ESB study [32])—for replication and validation of canonical vectors that showed a significant relationship to LOC-Cognition in SampleO. We directly developed RMSSD canonical variables (RMSSD-betweenFC-varR and RMSSD-betweenFC-varV) using the canonical vector (RMSSD-betweenFC-vecO) identified as significantly related to LOC-Cognition in in SampleO (rsfMRI data were not included in the replication samples). These variables were used to replicate and validate the relationship between RMSSD-betweenFC-varO and LOC-CognitionO using RMSSD canonical variables (RMSSD-betweenFC-varR and RMSSD-betweenFC-varV) and LOC-Cognition scores (LOC-CognitionR and LOC-CognitionV) from each sample.
Participants
Sample from CogTE study
Older adults with aMCI were recruited from university affiliated clinics. Description of the inclusion and exclusion criteria is available from the original report [19]. Out of the original 84 participants, 19 were excluded from the analyses due to the incomplete ECG recordings, leaving 65 participants as SampleO. The original CogTE study included intervention and control groups. SampleO was baseline data from both groups. Replication sampleR was from the 6-week follow-up data (post-intervention) from both groups, further excluding 10 participants missing valid RMSSD data.
Sample from ESB study
Older adults with aMCI or age-, sex-, and education-matched healthy cognition (HC) were recruited from local community or university affiliated clinics. Description of the inclusion and exclusion criteria is available from the original report [32]. We will refer to this validation sample as SampleV. Description of all samples is available in Table 1.
Table 1.
Characteristics of original, replication, and validation samples
| CogTE study | ESB study (SampleV) | ||||
|---|---|---|---|---|---|
| SampleO aMCI (n = 65) | SampleR aMCI (n = 55) | Total (n = 40) | HC (n = 22) | aMCI (n = 18) | |
| Age, mean (SD) | 74.72 (7.64) | 73.65 (7.08) | 72.67 (10.06) | 71.23 (9.61) | 74.44 (10.60) |
| Years of education, mean (SD) | 16.44 (2.44) | 16.30 (2.50) | 15.52 (2.64) | 15.64 (2.50) | 15.39 (2.87) |
| Male, n (%) | 33 (50.7) | 33 (60) | 16 (40) | 8 (36.4) | 8 (44.4) |
| MoCA, mean (SD) | 23.92 (2.59) | 24.21 (2.88) | 25.23 (2.76) | 26.14 (2.67) | 24.17 (2.55) |
| Internal LOC, mean (SD) | 5.09 (0.65) | 5.19 (0.58) | 5.01 (0.81) | 5.33 (0.52) | 4.64 (0.96) |
| External LOC, mean (SD) | 2.42 (0.63) | 2.35 (0.61) | 2.47 (0.87) | 2.09 (0.72) | 2.95 (0.82) |
aMCI amnesic mild cognitive impairment, SD standard deviation, n number of participants, MoCA Montreal cognitive assessment, LOC locus of control (cognition)
Measures
LOC-Cognition
As in previous studies in our lab [32, 33], LOC-Cognition was assessed with the Personality in Intellectual Aging Contexts (PIC) Inventory Control Scales-Short Form [16]. The scale includes three 12-item subscales: internal (reflecting internal LOC-Cognition), chance, and powerful others. Internal LOC-Cognition assesses perceived control over one's intellectual competence. The other two subscales assess the perception that environment (chance) or other individuals (powerful others) are responsible for one's cognitive capabilities. Responses were made on a 6-point scale, from 1 (strongly agree) to 6 (strongly disagree). Items of a subscale were averaged so that higher scores in all subscales indicated higher levels of LOC-Cognition. In the following analyses, we focused on internal LOC,Cronbach’s alpha was 0.75 for SampleO, 0.67 for SampleR, and 0.83 for SampleV. As a comparison to internal LOC-Cognition, we used averaged scores of chance and powerful others to reflect external LOC-Cognition [32, 47].
Brain network FC measures
Data acquisition
Imaging data were collected using a research-dedicated 3 T Siemens TrioTIM scanner (Erlangen, Germany) with a 32-channel head coil in SampleO. Each magnetic resonance session began with a scout image, followed by an MPRAGE scan (TI = 1100 ms, TE/TR = 3.44 ms/2530 ms, 1-mm isotropic resolution, 256 × 256 matrix, FA = 7, 1-mm slice thickness, 192 slices), which provides high-resolution structural-weighted anatomical images for image-registration purposes. A 2D axial fast gradient-recalled echo pulse sequence was used to generate field maps to correct for field inhomogeneity distortions in echo-planar imaging sequences. Blood-oxygen-level-dependent (BOLD) functional data were collected using a gradient echo-planar imaging sequence (TE/TR = 30 ms/2500 ms, 4-mm isotropic resolution, 64 × 64 matrix, FA = 90, 4-mm slice thickness, 37 contiguous axial slices). Participants were instructed to relax with their eyes open without falling asleep. An in-scanner camera was used to ensure compliance.
Data preprocessing
Resting-state fMRI data were preprocessed using scripts from a pipeline developed in our lab [6]. The script used for preprocessing is available at: https://github.com/sbci-brain/SBCI_Pipeline/blob/master/integrated_pipeline/preproc_step5_fmri.sh, and was carried out using FreeSurfer. Preprocessing included these steps: motion correction, slice-time correction, intensity normalization, and co-registration and normalization to the cortical surface (fsaverage). Additionally, motion, the first five components of the signal from the ventricular cerebrospinal fluid and white matter, the global signal, and a linear trend were regressed out alongside temporal filtering (0.009–0.08 Hz). Images were spatially smoothed using a kernel with 5 mm FWHM. Images were projected onto the gray-white matter boundary, and time series were extracted and correlated to generate a functional connectivity matrix (https://github.com/sbci-brain/SBCI_Pipeline/blob/master/integrated_pipeline/sbci_step6_functional.sh). Edges were averaged both within and between the 7 large-scale cortical networks (visual, somatomotor, dorsal attention, ventral attention, limbic, frontoparietal, and default mode) and identified by Yeo and Krienen et al. [46] to give 7 average within-network functional connectivity and 21 between-network functional connectivity values per participant.
RMSSD measures
Data acquisition
ECG data in both CogTE and ESB studies were acquired with Mindware and BioLab software. Heart rate variability (HRV) was monitored continuously using a standard lead-II electrode configuration, while participants performed an auditory working memory (i.e., auditory consonant trigrams) task in CogTE, or Stroop (inhibition) and Dual 1-back (visual working memory) tasks in a random order across participants in ESB. The same protocol was used to collect ECG data for all three samples. Across both studies and subjects, the same environmental control was applied, including a fixed time window and room temperature for conducting ECG recording, and restricting from beta-blocker and caffeine intake on the day before ECG recording.
Data preprocessing
We filtered the ECG raw signal using a bandpass Butterworth filter of 4th order in the range [0.1, 100] Hz and preprocessed RMSSD data using the Heartpy library of Python [43]. The frequency range is recommended by the American Heart Association [1]. RMSSD, a vagal regulation capacity measure from HRV, was used as the outcome measure. The advantage of using RMSSD, compared to HF-HRV, is that RMSSD is less affected by the respiration frequency. Across all ECG data, we used the first 8-min data. The ECG data was processed in segments of 60 s with a 30-s sliding window. From 15 segments generated, we extracted the RMSSD and calculated the natural log. If there were missing values in four segments or less, we used interpolation (mean of the previous and the following variables). Of note, we only included participants with at least 11/15 segments across samples.
Data analysis
CCA is a statistical method that identifies and quantifies the associations between two linearly combined sets of variables X and Y (without assuming any particular form of precedence or directionality) [14]. The main goal is to find coefficient vectors (or canonical vectors) a and b, such that the correlation () between the sets is as large as possible:
| 1 |
where X and Y come from the same number of observations n and have dimensions n x p and n x q, respectively, a ϵ Rp, and b ϵ Rq. With this, we obtained the canonical weights for the canonical variable explaining the highest correlation between the two sets of variables.
We examined CCA between RMSSD segments and FC measures in SampleO. To understand whether within- or between-network FC played different roles in adaptation capacity related to internal LOC-Cognition, we separated FC into within-network features (n = 7) and between-network features (n = 21) and ran two sets of CCA. CCA was only performed on the data from SampleO; to generate the RMSSD canonical variables for the replication and validation data (RMSSD-betweenFC-varR and RMSSD-betweenFC-varV), we used SampleO’s CCA weights/canonical vector (RMSSD-betweenFC-vecO, representing a series of weights for each RMSSD segment). We did not use RMSSD-withinFC-vecO in later canonical variable calculation due to the insignificant findings between RMSSD-withinFC-varO and internal LOC-cognition (see results). To obtain values of the corresponding canonical variable (cvn), which is RMSSD-betweenFC-varR for SampleR and RMSSD-betweenFC-varV for SampleV, respectively), we used the following formula described earlier [45]:
| 2 |
where a is RMSSD-betweenFC-vecO, X is the matrix containing RMSSD segment values, n is the number of participants according to the sample used (65, 55, or 40), and s is the number of RMSSD segments (15). CCA was conducted in R with the cancor() function of CCA package.
Linear regression
To determine the correlation between LOC-Cognition and RMSSD canonical variables, we used linear regression (stats package) in R.
Using SampleO, we calculated the correlation between RMSSD canonical variables (RMSSD-betweenFC-varO based on RMSSD-betweenFC-vecO and RMSSD-withinFC-varO based on RMSSD-withinFC-vecO, respectively) and internal LOC-CognitionO, with alpha set at 0.05. These analyses were also performed with external LOC-Cognition, for comparison purposes.
To assess the reproducibility of our findings, we performed two additional sets of linear regression: (1) using SampleR, we examined the relationship between RMSSD-betweenFC-varR and internal LOC-CognitionR. SampleR consisted both groups’ post-intervention data (from CogTE study); therefore, we controlled for the group to remove intervention effect confounds; (2) using SampleV, we examined the relationship between RMSSD-betweenFC-varV and internal LOC-CognitionV. For SampleV, we used RMSSD data during a visual working memory task and an inhibition task to test the generalizability of RMSSD-betweenFC-vecO. We controlled for MOCA as SampleV included both HC and aMCI.
Results
Canonical correlation analysis
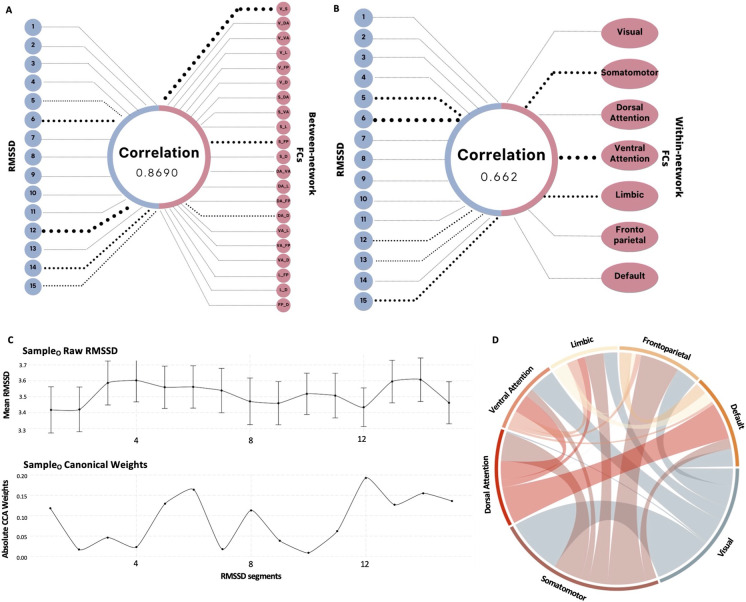
First, we attempted to extract an HRV biomarker reflecting adaptation capacity during an auditory working memory task by performing a CCA on the RMSSD time series constrained by either within- or between-network FC. CCA on SampleO identified a shared component characterized by a correlation of 86.9% and 66.23% between features from the RMSSD time series and between- and within-Yeo network FC, respectively. Figure 2A and B display the weights of RMSSD segments and brain networks contributing to the correlation. The CCA weights in both RMSSD canonical vectors (RMSSD-betweenFC-vecO and RMSSD-withinFC-vecO) are highest for segments 5, 6, 12, 13, 14, and 15 in both scenarios, meaning these segments contribute the most to the canonical variables (RMSSD-betweenFC-varO and RMSSD-withinFC-varO). A detailed behavior of the raw RMSSD at each segment is presented in Fig. 2C, along with the absolute value of CCA weights. The network contributing most to the withinFC CCA weights in canonical covariate 1 (CC1) is the ventral attention network, and the betweenFC CC1 weights are highest in for connections between the somatomotor and visual networks, followed by somatomotor-frontoparietal and dorsal attention-default mode network FC (see Fig. 2D). Note that we are considering the absolute value to determine the contribution of the segment or brain network; nevertheless, we use the original weight for calculating the canonical variable of each feature.
Fig. 2.
CCA results of RMSSD and brain network FC measures from SampleO. A Canonical correlation structure of RMSSD segments and between-network FCs for CC1-RMSSD-betweenFC-varO. B Canonical correlation structure of RMSSD and within-network FCs for CC1-RMSSD-withinFC-varO. C Raw values (Mean for SampleO RMSSD segments, and absolute CCA weights/canonical vector for SampleO RMSSD segment contributions to CC1-RMSSD-betweenFC-varO, bottom. D Absolute CCA weights/canonical vectors for between-network FCs (the colors are arbitrary) contributions to CC1-RMSSD-betweenFC-varO. Note. In A and B, the top 3 FC and top 5 RMSSD values in terms of contribution are shown with dotted lines, and the width of the line in A, B, and D represents the contribution of that particular connection or segment to CC1-RMSSD-betweenFC-varO or CC1-RMSSD-withinFC-varO. ln RMSSD, natural log-transformed root mean square of successive differences. V, visual; S, somatomotor; DA, dorsal attention; VA, ventral attention; L, limbic; FP, frontoparietal; D, default.
Linear regression
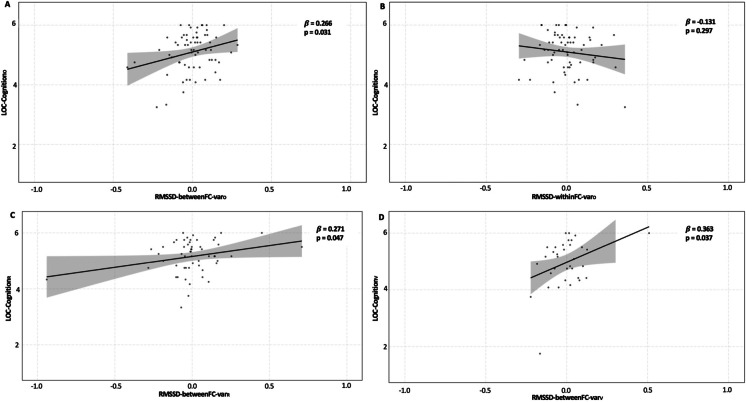
To assess the relationship between the potential autonomic flexibility biomarkers and participants’ internal LOC-Cognition, we performed linear regression analyses. RMSSD-betweenFC-varO (β = 0.266, SE = 0.971, CI = [0.190, 4.073], p = 0.031, Fig. 3A), but not RMSSD-witihnFC-varO (β = − 0.131, SE = 0.999, CI = [− 3.046, 0.946], p = 0.297, Fig. 3B), was significantly associated with the extent to which a participant believed they were in control of their own cognition. For comparison, we also performed linear regression analyses to test for the relationship between the canonical variables and external LOC and found no significant relationships for either RMSSD-betweenFC-varO (β = 0.095, SE = 1.003, CI = [− 1.237, 2.772], p = 0.447) or RMSSD-withinFC-varO (β = − 0.004, SE = 1.008, CI = [− 2.047, 1.980], p = 0.973). Given the significant relationship between RMSSD-betweenFC-varO and internal LOC-CognitionO, we performed follow-up analyses to test the reliability and validity of RMSSD-betweenFC-vecO by applying it to RMSSD data from replication and validation samples.
Fig. 3.
Relationship between RMSSD canonical variables and participants’ belief in their own control of their own intellectual abilities. A Linear regression analysis showed that RMSSD-betweenFC-varO was significantly related to internal LOC-Cognition in the original sample, when HRV data was collected during an ACT (auditory working memory) task. B Linear regression analysis showed that RMSSD-withinFC-varO was not significantly related to internal LOC-Cognition in the original sample, when HRV data was collected during an ACT (auditory working memory) task. C Linear regression analysis on the replication sample, which was the post-intervention data of the original sample, controlling for intervention effects, identified the same significant association between RMSSD-betweenFC-varR and internal LOC-Cognition. D Linear regression analysis in the validation sample, consisting both healthy older adults and individuals with aMCI (controlling for MoCA score), identified the same significant association between RMSSD-betweenFC-varV and internal LOC-Cognition, when HRV data was collected during an n-back (visual working memory) task
Reliability
We applied RMSSD-betweenFC-vecO to RMSSD data collected during the same auditory working memory task post-intervention to generate a canonical variable: RMSSD-betweenFC-varR, and performed the same linear regression as above with internal LOC-CognitionR, controlling for intervention effects. We found that, similarly to that in SampleO, RMSSD-betweenFC-varR had a significant association with LOC-CognitionR (β = 0.271, SE = 0.971, CI = [0.033, 2.630], p = 0.047), suggesting this relationship is reliable over a 1 month period (see Fig. 3C).
Validity
To additionally test the validity of RMSSD-betweenFC-vecO, we used RMSSD data from another project collected while participants completed a similar working memory task while having HRV data collected, as well as completing the same measure of internal LOC-Cognition. The working memory task in the replication sample was a visual task, while the original sample used an auditory working memory task. We also used HRV data collected during the Stroop task (measuring inhibition), allowing us to assess the generalizability of RMSSD-betweenFC-vecO. To control for the fact that this sample included both healthy older adults and individuals with aMCI, we also included MoCA score as a covariate in these regressions. We found that RMSSD-betweenFC-varV generated using visual working memory RMSSD data (β = 0.36, SE = 0.136, CI = [0.023, 0.574], p = 0.037, Fig. 3D), but not RMSSD-betweenFC-varV generated using Stroop task RMSSD data (β = 0.08, SE = 1.486, CI = [− 0.354, 0.657], p = 0.685), was significantly associated with LOC-CognitionV. This demonstrates the validity of our original finding, and suggests that RMSSD-betweenFC-vecO generalizes across working memory tasks using different sensory modalities, but not to RMSSD data collected during executive function tasks more broadly.
Discussion
Using CCA, we identified a canonical variable reflecting autonomic regulation, using both neural and peripheral physiological signals. The RMSSD canonical variable generated using CCA constrained by between-network FC (RMSSD-betweenFC-varO) was significantly associated with older adults’ belief that they were in control of their intellectual abilities (internal LOC-Cognition). No similar relationships were found using a similar RMSSD canonical variable that was constrained by within-network FC or when associating either canonical variable with external LOC-Cognition. A replication analysis suggested that the RMSSD canonical vector generated by CCA with RMSSD and between-network FC data (RMSSD-betweenFC-vecO) could be used to generate a canonical variable (RMSSD-betweenFC-varR) that showed the same relationship using RMSSD data collected during the same auditory working memory task 6 weeks later. Validation of RMSSD-betweenFC-vecO in a second dataset suggested that the relationship between canonical variables generated using this vector and internal LOC-Cognition generalizes to RMSSD data collected during working memory tasks presented in a different format (visual stimuli), but not tasks measuring inhibition (Stroop task). Overall, these findings suggest that this marker of autonomic regulation relates to internal LOC-Cognition in older adults at risk for dementia and may provide a potential means of intervening in internal LOC-Cognition to improve resilience and adherence to interventions in this at-risk population. Previous studies have generally focused on identifying autonomic markers that may be important in dementia using mean HRV (F. [17] or specific dynamic patterns of HRV (Q. [5]. By using a data-driven approach that maximized the association between HRV time series and brain networks, we hoped to find a marker of adaptation capacity that reflects integrated peripheral and neural signals underlying autonomic regulation and is sensitive to processes seen in brain aging. Replicating and validating the relationship between this marker and a behavioral trait reflecting resilience provide confidence that this approach has identified a meaningful marker of adaptation capacity that may implicate future therapeutics development for slowing/preventing dementia. Particularly, interventions aimed at modifying adaptation capacity may benefit from the ability to use this marker as a validated measure to assess whether autonomic regulation is being adjusted in a meaningful way. One example is research that aims to modulate adaptation capacity during cognitive training (F. V. [18] to put participants in the optimal state to show broad cognitive improvements that may rely on autonomic regulation during training (Quanjing [5].
These findings have several additional implications. Firstly, the finding of constraining the HRV data using between-network, but not within-network, FC suggests that identifying specific neural signals reflecting autonomic function is a critical aspect of understanding adaptation capacity and how it relates to internal LOC-Cognition. CCA weights were characterized by high influence from connections between the somatomotor network and visual, frontoparietal, and dorsal attention networks, as well as by connections between the dorsal attention and default mode network. Previous work has often highlighted cingulate, insular, and subcortical regions as critical elements of the central regulation of ANS [37]. Our results suggest that connections between sensory regions may have been overlooked, in line with recent work highlighting the hierarchical involvement of the whole brain in processing ANS signals [38]. Tight coupling between autonomic and sensorimotor functions is a critical aspect of adaptive behavior, and our results add to the literature suggesting a critical relationship between sensorimotor brain regions and the ANS [23, 35]. This is particularly important in individuals at risk for dementia because sensory regions are affected later by dementia pathology [2, 3] and may therefore be more easily modifiable by interventions aimed at causing brain plasticity to slow or prevent cognitive decline. Additionally, the involvement of connections between the dorsal attention and default mode networks is also of note. These connections are known to be critical for dynamic changes between internal and external states [41] and are also known to change during aging and dementia [9]. Our findings suggest that these task-related dynamics may also relate to autonomic regulation. The findings that between-network connections were more functionally relevant are also in line with findings showing that these connections are essential for explaining individual differences in behavior (van den [42]. Why these connections are more strongly related to the patterns of autonomic regulation seen in the working memory HRV data is a question for future research,however, the neurovisceral integration model [38] suggests that communication between different levels of neural hierarchies (at which these different networks exist: Margulies et al. [24] is critical for autonomic regulation, allowing for both bottom-up and top-down communication between brain and peripheral signals. These between-network connections may be more involved in this type of hierarchical integration of signals during autonomic regulation.
The finding that these results generalize across working memory tasks is also particularly interesting. We used a sliding window approach to generate time series of HRV data in response to cognitive stress to build on previous work suggesting that these conditions are essential for identifying signals reflecting adaptation capacity [5]. The results further support this approach and suggest that HRV responses at specific time points to cognitive stress seem to be particularly important for identifying markers of adaptation capacity that relate to resilience. Our findings go further to suggest that markers of adaptation capacity identified using different cognitive stressors may reflect measures of resilience to different extents. The results of this study do not imply that autonomic responses during, for example, the Stroop task do not contain important information about adaptation capacity and resilience. However, they do suggest that markers of adaptation capacity developed during specific cognitive stressors contain unique information and cannot necessarily be assumed to produce the same autonomic responses: the canonical vector generated during working memory could not be applied to HRV data during the Stroop task to produce a canonical variable that similarly reflected internal LOC-Cognition. It is less clear from this study exactly what this unique information is, except that it occurs more at specific time points and that the specific temporal pattern is unique to working memory tasks. Given that the important time points are relatively early on (5 and 6) and late into task performance (12–15) and appear to occur in runs, they may reflect a person encountering a challenge in the task and adapting to it or a response to increased boredom/fatigue. We do not know from these findings whether these responses do not occur in the Stroop task, if they occur differently, or at different time points, given we are validating a specific biomarker developed in the working memory task and did not conduct an exploratory CCA in the Stroop task. However, it is interesting that they do appear to occur in the same timeframe in a different working memory task, suggesting they may reflect a general response to this type of task. It is also unknown if this pattern appears at rest or during recovery, but given the lack of generalization to the Stroop task and the specific temporal nature of the response, we believe it seems unlikely.
An important question we are unable to answer in this study is the extent to which the state of participants during fMRI data collection would alter this biomarker. Although participants were cognitively challenged during HRV data collection, they were at rest for fMRI data collection. Resting-state data is the most commonly used fMRI modality when assessing trait relationships with behavior, as we have done in this study. Some research suggests that trait FC is robust to task-related changes, reflecting a “fingerprint” [11] of the person that changes little during tasks [13] and is governed by similar principles to task-related activity [26]. Alternatively, some studies suggest that FC measures obtained from task-based data may be better able to predict behavioral traits [10], suggesting important changes in response to task demands. Answering this question is also not straightforward methodologically,there is disagreement in the field over how to generate FC from task-based data: should task-related signals be regressed out to generate true baseline FC (e.g., Gratton et al. [13] or do task-related changes reflect traits in a similar way to how we have conceptualized HRV responses to cognitive demands in this study? Should you analyze task-related changes specifically using a Psychophysiological Interaction (PPI) analysis [30] or collapse across the entire time series to generate FC data [12]? Although beyond the scope of the current study, these are important questions for future research,given that the state of participants during HRV data collection is critical for the identification of this biomarker, it may also be true that the state of participants during fMRI data collection is equally important. However, it is important to note that using resting-state fMRI data to constrain the CCA produced significant findings, suggesting this specific biomarker has value, whether or not it can be improved upon by using task-based fMRI data.
There are some limitations associated with this study. To create a common correlated space between the functional networks (Y) and HRV (X), we use CCA. CCA builds a simple linear model in X space and one in Y space to create a joint correlated space. The linear models generate positive and negative weights. For interpretability, we only focused on the magnitude of the weights when attempting to understand which connections were contributing most strongly to the canonical vector. We have avoided interpreting the direction due to some of the controversies over how to explain negative FC and its physiological meaning [4]. Future studies may choose to use non-negative CCA [36]; instead, however, we chose to use regular CCA to try and maximize the correlation between HRV and FC data. In addition, for simplicity, we only used RMSSD measures from the HRV time series and Yeo and Krienen’s [46] cortical networks for resting-state FC. More advanced data-driven extraction of whole brain (including subcortical regions) FC and raw HRV signals (using time series signal processing or a more advanced deep learning approach) could potentially reveal additional shared signals reflecting adaptation capacity. Additionally, although we aimed to replicate and validate our findings, all samples used were relatively small and demographically homogenous. Larger samples will be needed to understand the true generalizability and effect sizes related to the RMSSD-betweenFC-vecO biomarker. In general, our sample size is on the smaller side for CCA analyses, although critically we reduced the dimensions of the brain and HRV data prior to CCA to ensure the number of variables did not exceed the number of samples [27]. Importantly, we replicated and validated our results in an independent sample, suggesting that the sample size was sufficient to identify meaningful signal in the data. It is a challenge to collect large datasets in this critical at-risk population, particularly due to the noisy HRV data seen in older adults at risk for dementia that often leads to considerable data loss (as seen in our study). CCA in small samples can lead to noisy estimates, which is why it is particularly important in these studies to validate using out-of-sample predictions, as in this study. Finally, all our analyses were cross-sectional in nature. To understand whether there are any causal relationships between internal LOC-Cognition and RMSSD-betweenFC-vecO, future studies will need to use designs with an experimental manipulation or longitudinal data as individuals progress into dementia and potentially shift to a more external LOC-Cognition. These studies will be essential if RMSSD-betweenFC-vecO is to help identify any means of intervening to improve/maintain internal LOC-Cognition in older adults at risk for dementia.
Acknowledgements
This work is funded by NIH R01NR015452 and U24 AG072701, and Alzheimer’s Association AARG-22-926139 awarded to F.V. Lin
Data and code availability
Data and code used for this project can be accessed at https://github.com/CogTLab/CCA_LOC.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lizbeth Peralta-Malváez and Adam Turnbull share equal contribution.
References
- 1.Bailey JJ, Berson AS, Garson Jr A, Horan LG, Macfarlane PW, Mortara DW, Zywietz C. Recommendations for standardization and specifications in automated electrocardiography: bandwidth and digital signal processing. A report for health professionals by an ad hoc writing group of the Committee on Electrocardiography and Cardiac Electrophysiology of the Council on Clinical Cardiology, American Heart Association. Circulation. 1990;81(2):730–739. [DOI] [PubMed]
- 2.Braak H, Del Tredici K, Schultz C, Braak E. Vulnerability of select neuronal types to Alzheimer's disease. Ann N Y Acad Sci. 2000;924(1):53–61. doi: 10.1111/j.1749-6632.2000.tb05560.x. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. 2011;70(11):960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 4.Buckner RL, Krienen FM, Yeo BT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat Neurosci. 2013;16(7):832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- 5.Chen Q, Yang H, Rooks B, Anthony M, Zhang Z, Tadin D, … Lin FV. Autonomic flexibility reflects learning and associated neuroplasticity in old age. Hum Brain Mapp. 2020;41(13):3608–3619. 10.1002/hbm.25034. [DOI] [PMC free article] [PubMed]
- 6.Cole M, Murray K, St‐Onge E, Risk B, Zhong J, Schifitto G, Descoteaux M, Zhang Z. Surface‐Based Connectivity Integration: An atlas‐free approach to jointly study functional and structural connectivity. Human Brain Mapping. 2021;42(11):3481–99. [DOI] [PMC free article] [PubMed]
- 7.de la Cruz F, Schumann A, Köhler S, Reichenbach JR, Wagner G, Bär K-J. The relationship between heart rate and functional connectivity of brain regions involved in autonomic control. Neuroimage. 2019;196:318–328. doi: 10.1016/j.neuroimage.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Ding K, Tarumi T, Wang C, Vernino S, Zhang R, Zhu DC. Central autonomic network functional connectivity: correlation with baroreflex function and cardiovascular variability in older adults. Brain Struct Funct. 2020;225(5):1575–1585. doi: 10.1007/s00429-020-02075-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esposito R, Cieri F, Chiacchiaretta P, Cera N, Lauriola M, Di Giannantonio M, … Ferretti A. Modifications in resting state functional anticorrelation between default mode network and dorsal attention network: comparison among young adults, healthy elders and mild cognitive impairment patients. Brain Imaging Behav. 2018;12(1):127–141. [DOI] [PubMed]
- 10.Finn ES, Scheinost D, Finn DM, Shen X, Papademetris X, Constable RT. Can brain state be manipulated to emphasize individual differences in functional connectivity? Neuroimage. 2017;160:140–151. doi: 10.1016/j.neuroimage.2017.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finn ES, Shen X, Scheinost D, Rosenberg MD, Huang J, Chun MM, … Constable RT. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci. 2015;18(11):1664. [DOI] [PMC free article] [PubMed]
- 12.Gonzalez-Castillo J, Bandettini PA. Task-based dynamic functional connectivity: Recent findings and open questions. Neuroimage. 2018;180:526–533. doi: 10.1016/j.neuroimage.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gratton C, Laumann TO, Nielsen AN, Greene DJ, Gordon EM, Gilmore AW, … Schlaggar BL. Functional brain networks are dominated by stable group and individual factors, not cognitive or daily variation. Neuron. 2018;98(2):439–452. e435. [DOI] [PMC free article] [PubMed]
- 14.Hotelling H. Relations between two sets of variates. InBreakthroughs in statistics 1992 (pp. 162-190). Springer, New York, NY.
- 15.Hovenkamp-Hermelink JH, Jeronimus BF, Spinhoven P, Penninx BW, Schoevers RA, Riese H. Differential associations of locus of control with anxiety, depression and life-events: a five-wave, nine-year study to test stability and change. J Affect Disord. 2019;253:26–34. doi: 10.1016/j.jad.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Lachman ME. Locus of control in aging research: a case for multidimensional and domain-specific assessment. Psychol Aging. 1986;1(1):34. doi: 10.1037/0882-7974.1.1.34. [DOI] [PubMed] [Google Scholar]
- 17.Lin F, Ren P, Wang X, Anthony M, Tadin D, Heffner KL. Cortical thickness is associated with altered autonomic function in cognitively impaired and non-impaired older adults. J Physiol. 2017;595(22):6969–6978. doi: 10.1113/JP274714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin FV, Heffner K, Gevirtz R, Zhang Z, Tadin D, Porsteinsson A. Targeting autonomic flexibility to enhance cognitive training outcomes in older adults with mild cognitive impairment: study protocol for a randomized controlled trial. Trials. 2021;22(1):1–15. doi: 10.1186/s13063-021-05530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin FV, Tao Y, Chen Q, Anthony M, Zhang Z, Tadin D, Heffner KL. Processing speed and attention training modifies autonomic flexibility: a mechanistic intervention study. Neuroimage. 2020;213:116730. doi: 10.1016/j.neuroimage.2020.116730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu KY, Elliott T, Knowles M, Howard R. Heart rate variability in relation to cognition and behavior in neurodegenerative diseases: a systematic review and meta-analysis. Ageing Res Rev 2022;73:101539. 10.1016/j.arr.2021.101539. [DOI] [PMC free article] [PubMed]
- 21.Lorenz TK. Autonomic, endocrine, and psychological stress responses to different forms of blood draw. PLoS One. 2021;16(9):e0257110. doi: 10.1371/journal.pone.0257110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynam I, Catley D, Goggin K, Rabinowitz JL, Gerkovich MM, Williams K, … MOTIV8. Autonomous regulation and locus of control as predictors of antiretroviral medication adherence. J Health Psychol. 2009;14(4):578–586. [DOI] [PMC free article] [PubMed]
- 23.M'hamed SB, Sequeira H, Poulain P, Bennis M, Roy J. Sensorimotor cortex projections to the ventrolateral and the dorsomedial medulla oblongata in the rat. Neurosci Lett 1993;164(1-2):195-198. [DOI] [PubMed]
- 24.Margulies DS, Ghosh SS, Goulas A, Falkiewicz M, Huntenburg JM, Langs G, … Petrides M. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc Natl Acad Sci. 2016;113(44):12574–12579. [DOI] [PMC free article] [PubMed]
- 25.McDermott K, Ren P, Lin F. The mediating role of hippocampal networks on stress regulation in amnestic mild cognitive impairment. Neurobiol Stress. 2019;10:100162. 10.1016/j.ynstr.2019.100162. [DOI] [PMC free article] [PubMed]
- 26.Mennes M, Kelly C, Zuo X-N, Di Martino A, Biswal BB, Castellanos FX, Milham MP. Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. Neuroimage. 2010;50(4):1690–1701. doi: 10.1016/j.neuroimage.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mihalik A, Chapman J, Adams RA, Winter NR, Ferreira FS, Shawe-Taylor J, Mourão-Miranda J, Alzheimer’s Disease Neuroimaging Initiative. Canonical correlation analysis and partial least squares for identifying brain-behaviour associations: a tutorial and a comparative study. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2022;7(11):1055–67. [DOI] [PubMed]
- 28.Mukherjee S, Yadav R, Yung I, Zajdel DP, Oken BS. Sensitivity to mental effort and test–retest reliability of heart rate variability measures in healthy seniors. Clin Neurophysiol. 2011;122(10):2059–2066. doi: 10.1016/j.clinph.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulcahy JS, Larsson DEO, Garfinkel SN, Critchley HD. Heart rate variability as a biomarker in health and affective disorders: a perspective on neuroimaging studies. NeuroImage. 2019;202:116072. 10.1016/j.neuroimage.2019.116072. [DOI] [PubMed]
- 30.O’Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H. Tools of the trade: psychophysiological interactions and functional connectivity. Soc Cogn Affect Neurosci. 2012;7(5):604–609. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pruessner JC, Baldwin MW, Dedovic K, Renwick R, Mahani NK, Lord C, … Lupien S. Self-esteem, locus of control, hippocampal volume, and cortisol regulation in young and old adulthood. Neuroimage. 2005;28(4):815–826. [DOI] [PubMed]
- 32.Ren P, Anthony M, Chapman BP, Heffner K, Lin F. Amygdala functional connectivity is associated with locus of control in the context of cognitive aging. Neuropsychologia. 2017;99:199–206. doi: 10.1016/j.neuropsychologia.2017.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ren P, Chapman B, Zhang Z, Schifitto G, Lin F. Functional and structural connectivity of the amygdala underpins locus of control in mild cognitive impairment. NeuroImage Clin. 2018;20:297–304. [DOI] [PMC free article] [PubMed]
- 34.Rotter JB. Generalized expectancies for internal versus external control of reinforcement. Psychol Monogr Gen Appl. 1966;80(1):1. doi: 10.1037/h0092976. [DOI] [PubMed] [Google Scholar]
- 35.Sequeira H, Viltart O, Ba-M’Hamed S, Poulain P. Cortical control of somato-cardiovascular integration: neuroanatomical studies. Brain Res Bull. 2000;53(1):87–93. doi: 10.1016/S0361-9230(00)00312-9. [DOI] [PubMed] [Google Scholar]
- 36.Sigg C, Fischer B, Ommer B, Roth V, Buhmann J. Nonnegative CCA for audiovisual source separation. In2007 IEEE Workshop on Machine Learning for Signal Processing 2007 Aug 27 (pp. 253–258). IEEE.
- 37.Sklerov M, Dayan E, Browner N. Functional neuroimaging of the central autonomic network: recent developments and clinical implications. Clin Auton Res. 2019;29(6):555–566. doi: 10.1007/s10286-018-0577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith R, Thayer JF, Khalsa SS, Lane RD. The hierarchical basis of neurovisceral integration. Neurosci Biobehav Rev. 2017;75:274–296. doi: 10.1016/j.neubiorev.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Stemmler G, Wacker J. Personality, emotion, and individual differences in physiological responses. Biol Psychol. 2010;84(3):541–551. doi: 10.1016/j.biopsycho.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61(3):201–216. doi: 10.1016/S0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 41.Van Calster L, D'Argembeau A, Salmon E, Peters F, Majerus S. Fluctuations of attentional networks and default mode network during the resting state reflect variations in cognitive states: evidence from a novel resting-state experience sampling method. J Cogn Neurosci. 2017;29(1):95–113. doi: 10.1162/jocn_a_01025. [DOI] [PubMed] [Google Scholar]
- 42.van den Heuvel MP, Sporns O. A cross-disorder connectome landscape of brain dysconnectivity. Nat Rev Neurosci. 2019;20(7):435–446. doi: 10.1038/s41583-019-0177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Gent P, Farah H, van Nes N, van Arem B. Analysing noisy driver physiology real-time using off-the-shelf sensors: heart rate analysis software from the taking the fast lane project. J. Open Res Softw. 2019;7(1):32.
- 44.Voils CI, Steffens DC, Bosworth HB, Flint EP. Social support and locus of control as predictors of adherence to antidepressant medication in an elderly population. Am J Geriatr Psychiatry. 2005;13(2):157–165. doi: 10.1097/00019442-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Wang H-T, Smallwood J, Mourao-Miranda J, Xia CH, Satterthwaite TD, Bassett DS, Bzdok D. Finding the needle in a high-dimensional haystack: canonical correlation analysis for neuroscientists. Neuroimage. 2020;216:116745. doi: 10.1016/j.neuroimage.2020.116745. [DOI] [PubMed] [Google Scholar]
- 46.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, … Polimeni JR. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125-65. [DOI] [PMC free article] [PubMed]
- 47.Zahodne LB, Meyer OL, Choi E, Thomas ML, Willis SL, Marsiske M, … Parisi JM. External locus of control contributes to racial disparities in memory and reasoning training gains in ACTIVE. Psychol Aging. 2015;30(3):561. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data and code used for this project can be accessed at https://github.com/CogTLab/CCA_LOC.