Abstract
Cohort studies report inconsistent associations between omega-3 polyunsaturated fatty acids (n-3 PUFA) or fish oil and dementia risk. Furthermore, evidence relating omega-6 polyunsaturated fatty acids (n-6 PUFA) with dementia is scarce. Here, we included 440,750 dementia-free participants from UK Biobank to comprehensively investigate the associations between plasma levels of different types of PUFA, fish oil supplementation, and dementia risk. During a median follow-up of 9.25 years, 7768 incident dementia events occurred. Higher plasma levels of five PUFA measures showed consistent associations with lower dementia risk (hazard ratios [95% confidence intervals] for per standard deviation increment of plasma concentrations 0.85 [0.81–0.89] for total PUFAs; 0.90 [0.86–0.95] for omega-3 PUFAs; 0.92 [0.87–0.96] for docosahexaenoic acid (DHA); 0.86 [0.82–0.90] for omega-6 PUFAs; 0.86 [0.82–0.90] for linoleic acid (LA); all p < 0.001). Compared with non-users, fish oil supplement users had a 7% decreased risk of developing all-cause dementia (0.93 [0.89–0.97], p = 0.002), and the relationship was partially mediated by plasma n-3 PUFA levels (omega-3 PUFAs: proportion of mediation = 57.99%; DHA: proportion of mediation = 56.95%). Furthermore, we observed significant associations of plasma n-3 PUFA levels and fish oil supplementation with peripheral immune markers that were related to dementia risk, as well as the positive associations of plasma PUFA levels with brain gray matter volumes and white matter microstructural integrity, suggesting they may affect dementia risk by affecting peripheral immunity and brain structure. Taken together, higher plasma PUFA levels and fish oil supplementation were associated with lower risk of incident dementia. This study may support the value of interventions to target PUFAs (specifically n-3 PUFAs) to prevent dementia.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-023-00778-6.
Keywords: Dementia, Polyunsaturated fatty acids, Omega-3, Omega-6, Fish oil, UK Biobank
Introduction
Dementia is a leading cause of disability and death affecting around 50 million people worldwide, which brings a huge socioeconomic burden and becomes a growing public health concern [1]. In recent years, more attention has been paid to potentially modifiable risk factors for dementia; the role of diet and nutrition in dementia prevention has also raised great interest.
Polyunsaturated fatty acids (PUFA), of which omega-3 (n-3) and omega-6 (n-6) fatty acids are two main types, are mainly derived from diet. The importance of PUFA in neural development, aging, and neurodegeneration has been shown in both animal and clinical studies [2]. Previously, several longitudinal studies have reported associations between higher blood levels of n-3 PUFA and lower risk of incident dementia, mainly the docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) [3–7], while limited research has failed to find the evidence relating n-6 PUFA with dementia [4, 7]. Besides, an increased number of neuroimaging studies have been conducted over the years to assess the impact of PUFA on human brain structure. The majority specifically investigated associations between n-3 PUFA biostatus/intake and neuroimaging outcomes [8], while studies on n-6 PUFA are scarce. Taken together, a systemic study based on a large-scale cohort is needed to further clarify the associations of different types of PUFAs with dementia and brain structure.
Fish oil supplements are rich source of pure marine omega-3 PUFAs and have been widely used in the UK and other developed countries. Although evidence for a beneficial role of n-3 PUFA in cognitive aging was supported by previous studies [9], whether using fish oil supplements is a potentially effective way in dementia prevention raise considerable controversy. Previous randomized controlled trials have yielded mixed results [10–12]. Of note, two recent longitudinal studies provided population-based evidence for linking fish oil supplementation with decreased risk of dementia, and the correlation may be partially mediated by several blood biomarkers [13, 14]. However, the role of peripheral immune markers in fish oil supplementation-dementia association was not assessed. Recent research provided clear evidence of the correlation between peripheral immunity and dementia, revealing that increased adaptive immune markers (i.e., lymphocytes and lymphocyte-to-monocyte ratio (LMR)) were associated with lower dementia risk, while increased innate immune markers (i.e., neutrophils, neutrophil-to-lymphocyte ratio (NLR), and systemic immune-inflammation index (SII)) were associated with higher dementia risk [15]. Considering the potent immunomodulatory effects of omega-3 PUFAs [16], fish oil supplementation may impact dementia risks through its potential effect on peripheral immunity. Therefore, the presented study aimed to evaluate these interesting issues further.
In this study, we took advantage of the large sample size of the UK Biobank (UKB) cohort with a long-term follow-up, to investigate the associations of plasma PUFA levels and fish oil supplementation with risk of incident dementia. Furthermore, we explored the associations of plasma PUFA levels and fish oil supplementation with peripheral immune markers and brain structural measures. Considering the strong relationship between fish oil and circulating PUFAs, we also assessed whether plasma PUFA levels mediated the fish oil supplementation-dementia association.
Methods
Data source and study population
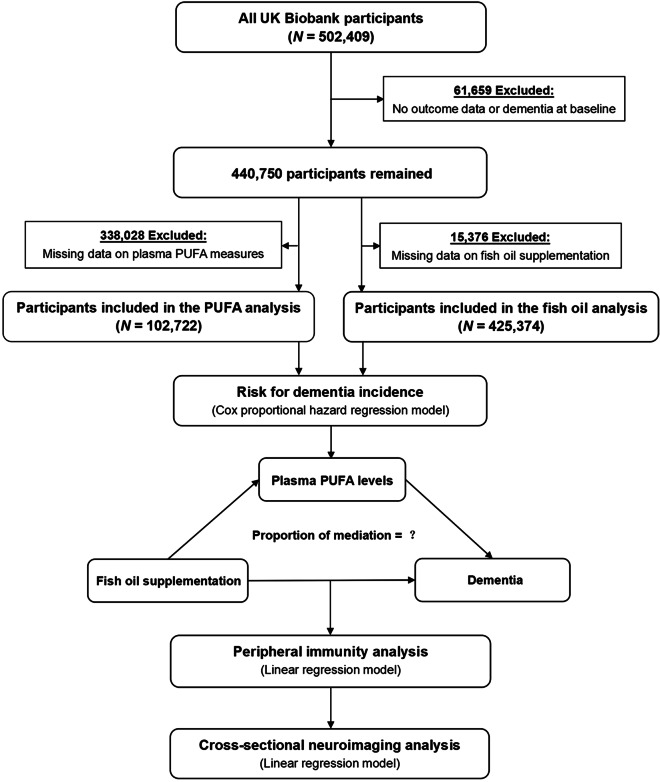
This study is based on data from the UKB cohort that received ethical approval from the National Health Service North West Multicenter Research Ethics Committee. All participants provided electronic signed consent for their data to be used in health-related research. The UK Biobank is a very large and detailed prospective cohort study that recruited over 500,000 participants aged 37–73 years from 2006 to 2010 [17]. All participants attended one of 22 assessment centers across England, Scotland, and Wales, where they completed a touch screen questionnaire and a face-to-face interview, provided biological samples, and took a set of physical measurements at baseline. In the present study, after excluding participants with dementia at baseline or without follow-up data (N = 61,659), 440,750 participants remained for the main analysis (Fig. 1). All analyses were conducted under UKB application number 19542.
Fig. 1.
Flow chart of study design. PUFA, polyunsaturated fatty acid
Plasma PUFA levels and fish oil supplementation
Plasma PUFA levels (absolute concentrations and percentages of total fatty acids) were measured by nuclear magnetic resonance (NMR) in blood samples collected between 2007 and 2010, including total PUFAs, omega-3 PUFAs, docosahexaenoic acid (DHA), omega-6 PUFAs, and linoleic acid (LA) [17–19]. Information on fish oil supplementation was collected through a touchscreen questionnaire. Participants were asked, “Do you regularly take any of the following?” and could select more than one answer from a list of supplements, including fish oil supplements. We scored fish oil supplementation as “1 = yes” or “0 = no.”
Dementia diagnosis
Dementia diagnoses were ascertained according to the three-character International Classification of Diseases (ICD) codes, extracted from UK Biobank health outcome datasets first occurrences of health outcomes (Category 1712) and algorithmically defined outcomes (Category 42). All-cause dementia was defined as ICD-10 codes F00 and G30 (Alzheimer’s disease), F01 (vascular dementia) and F02-03. Follow-up visits began from the date of attending the assessment center (Field 53) to the earliest date of dementia diagnosis, date of death (Field 40,000), or the last available date from the hospital inpatient data (Field 41,280–41,281) or primary care data (Field 42,040), whichever occurred first.
Covariates
We selected several possible confounding variables, including age at baseline; sex; education, categorized as higher (college/university degree or other professional qualifications) or lower; APOE ɛ4 status (carrier or non-carrier), defined by genetic information; self-reported ethnicity (white or non-white); Townsend deprivation index, a composite measure of material deprivation based on unemployment, non-car ownership, non-home ownership, and household overcrowding, the higher the index was, the lower the degree of socioeconomic status the person had; body mass index (BMI); smoking status (never, former or current); alcohol drinking, categorized as non-drinker (never or special occasions only), low to moderate drinker (once a month to four times a week) or heavy drinker (daily or almost daily); physical activity; cardiovascular diseases (CVD) diagnosed by doctor (yes or no); fruit intake (< 2.0, 2.0–3.9, or ≥ 4.0 servings/day); vegetable intake (< 2.0, 2.0–3.9, or ≥ 4.0 servings/day); oily fish intake (< 1, 1, or ≥ 2 times/week); processed meat intake (< 2 or ≥ 2 times/week); red meat intake (< 2 or ≥ 2 times/week); vitamin supplementation (yes or no); and mineral and other dietary supplementation (yes or no). Details of these assessments can be found on the UK Biobank website (www.ukbiobank.ac.uk).
Peripheral immune markers
Blood samples collected from UK Biobank participants were analyzed at the central laboratory within 24 h of blood draw, and differential leukocyte counts were acquired from the automated quantitative analyzer. Detailed information can be found in the online document (https://biobank.ndph.ox.ac.uk/showcase/ukb/docs/haematology.pdf). We extracted baseline counts of neutrophils, monocytes, platelets, and lymphocytes and further calculated four ratios including NLR (neutrophils/lymphocytes), PLR (platelets/lymphocytes), SII (neutrophils*platelets/lymphocytes), and LMR (lymphocytes/monocytes). The increased levels of neutrophils, monocytes, NLR, PLR, and SII reflect the relatively higher peripheral innate immunity, while the increased levels of lymphocytes and LMR reflect the relatively higher peripheral adaptive immunity [15].
Brain imaging data
The large-scale quality-controlled imaging-derived phenotypes (IDPs) used in this study included 68 cortical regions, 45 subcortical regions, and tract-specific fractional anisotropy (FA) and mean diffusivity (MD) values of 48 white matter tracts. Detailed information is described in eMethods in the Supplement.
Statistical analysis
Baseline characteristics of participants were displayed in the form of mean (standard deviation [SD]) for continuous variables and number (percentage) for categorical variables. To minimize the potential for attrition bias and maximize the statistical power, multiple imputation with chained equations was used to assign any missing covariate values [20]. The number and percentage of missing covariates are shown in eTable 1.
The flowchart of study design is presented in Fig. 1. Firstly, multivariable Cox proportional hazard regression models were used to investigate the independent association of plasma PUFA levels (N = 102,722) and fish oil supplementation (N = 425,374) with risk of incident dementia after confirming whether the proportional hazard assumption was met. Prior to analysis, plasma PUFA concentrations and proportions (percentages of total fatty acids) were standardized to Z scores (Z = (value–mean) / SD) such that the hazard ratio represents the predicted effect of per SD increment of plasma PUFA levels. Cox analyses started with a minimally adjusted model including age, sex, education, and APOE ɛ4 status as covariates (model 1). Model 2 was additionally adjusted for ethnicity, Townsend deprivation index, body mass index, smoking status, alcohol drinking, physical activity, and cardiovascular diseases. Model 3 was further adjusted for intake of oily fish, fruits, vegetables, processed meat and red meat, vitamin, and mineral supplementation in addition to the covariates in model 2. Secondly, mediation analyses (N = 102,343) were conducted to investigate whether plasma PUFA levels mediated the association between fish oil supplementation and dementia, adjusting for covariates in model 1.
We also performed several subgroup and sensitivity analyses. In the PUFA analysis, we utilized interaction terms for age, sex, and APOE ε4 status to estimate whether the strata effect existed (p < 0.05) in different subgroups. After that, three subgroup analyses were performed through stratification of age (< 65 or ≥ 65 years), sex (male or female), and APOE ε4 status (carrier or non-carrier). To minimize the influence of reverse causation, we also conducted a sensitivity analysis by excluding incident dementia cases during the first 2 years of follow-up. In the fish oil analysis, subgroup analyses were performed to estimate potential modification effects according to age, sex, APOE ε4 status, education (low or high), obesity (yes (BMI ≥ 30) or no (BMI < 30)), current smoking status (yes or no), CVD diagnosed by doctor (yes or no), and oily fish intake (< 1 or ≥ 1 times/week). The p value for interaction was tested by modelling the cross-product term of the stratifying variable with fish oil supplement use. Furthermore, two sensitivity analyses were conducted by excluding participants occurred dementia in the first 2 years of follow-up and by excluding participants who took any other supplements. Subsequently, we investigated the associations of plasma PUFA levels and fish oil supplementation with two main dementia subtypes, Alzheimer’s disease (AD) and vascular dementia (VD).
Next, we applied a linear regression model to investigate the associations of plasma PUFA levels (N = 99,974) and fish oil supplementation (N = 412,641) with several peripheral immune markers that were related to dementia risk [15]. Moreover, cross-sectional neuroimaging analyses were conducted to examine the associations of plasma PUFA levels (N = 8761) and fish oil supplementation (N = 35,415) with brain structure, adjusting for age, sex, education, and APOE ε4 status. Bonferroni corrections were conducted for multiple comparisons.
All analyses were performed using R software, version 4.1.2. A two-sided p < 0.05 was considered as statistical significance.
Results
Baseline characteristics
Table 1 shows the baseline characteristics of study participants by incident dementia status. Among the 440,750 individuals (mean [SD] age, 56.96 [8.03] years; 54.2% female), during a median follow-up of 9.25 years, 7768 incident dementia events occurred. As for those who converted into dementia, they had lower plasma levels of total PUFAs, omega-6 PUFAs, and LA at baseline. Participant characteristics in the PUFA and fish oil analysis are presented in eTables 2 and 3, respectively. Compared with non-users, fish oil users had higher plasma levels of omega-3 PUFAs and DHA at baseline.
Table 1.
Baseline characteristics of study participants by incident dementia status
| Variables | Total (N = 440,750) | Non-dementia (N = 432,982) | Dementia (N = 7768) | p value |
|---|---|---|---|---|
| Age at baseline, years, mean (SD) | 56.96 (8.03) | 56.83 (8.01) | 64.23 (4.81) | < 0.001 |
| Sex, N (%) | < 0.001 | |||
| Female | 239,064 (54.2%) | 235,370 (54.4%) | 3694 (47.6%) | |
| Male | 201,686 (45.8%) | 197,612 (45.6%) | 4074 (52.4%) | |
| Education, N (%) a | < 0.001 | |||
| Low | 231,523 (53.6%) | 226,643 (53.4%) | 4880 (65.5%) | |
| High | 200,259 (46.4%) | 197,687 (46.6%) | 2572 (34.5%) | |
| APOE ε4 status, N (%) | < 0.001 | |||
| Non-carrier | 306,010 (71.6%) | 302,482 (72.0%) | 3528 (47.5%) | |
| Carrier | 121,444 (28.4%) | 117,539 (28.0%) | 3905 (52.5%) | |
| Ethnicity, N (%) | < 0.001 | |||
| Non-white | 22,846 (5.2%) | 22,531 (5.2%) | 315 (4.1%) | |
| White | 415,508 (94.8%) | 408,115 (94.8%) | 7393 (95.9%) | |
| Townsend deprivation index, mean (SD) | − 1.28 (3.10) | − 1.28 (3.10) | − 0.90 (3.33) | < 0.001 |
| BMI (kg/m2), mean (SD) | 27.56 (4.85) | 27.55 (4.85) | 27.80 (4.94) | < 0.001 |
| Smoking status, N (%) | 0.001 | |||
| Never | 236,077 (53.9%) | 232,508 (54.0%) | 3569 (46.4%) | |
| Previous | 154,658 (35.3%) | 151,373 (35.2%) | 3285 (42.8%) | |
| Current | 47,389 (10.8%) | 46,562 (10.8%) | 827 (10.8%) | |
| Alcohol drinking, N (%)b | < 0.001 | |||
| Non-drinker | 88,390 (20.1%) | 86,204 (20.0%) | 2186 (28.3%) | |
| Low to moderate drinker | 262,056 (59.6%) | 258,095 (59.8%) | 3961 (51.3%) | |
| Heavy drinker | 89,003 (20.3%) | 87,425 (20.2%) | 1578 (20.4%) | |
| Physical activity, MET-min/week, mean (SD) c | 2662 (2733) | 2662 (2730) | 2692 (2908) | 0.443 |
| CVD diagnosed by doctor, N(%)d | 13,7372 (31.3%) | 133,496 (31.0%) | 3876 (50.3%) | < 0.001 |
| Fatty acid concentrations (mmol/L), mean (SD) | ||||
| Total fatty acids | 11.86 (2.40) | 11.86 (2.40) | 11.68 (2.44) | 0.003 |
| Total PUFAs | 4.97 (0.80) | 4.98 (0.80) | 4.85 (0.84) | < 0.001 |
| Omega-3 PUFAs | 0.53 (0.22) | 0.53 (0.22) | 0.53 (0.23) | 0.158 |
| DHA | 0.23 (0.08) | 0.23 (0.08) | 0.23 (0.09) | 0.968 |
| Omega-6 PUFAs | 4.45 (0.68) | 4.45 (0.68) | 4.32 (0.72) | < 0.001 |
| LA | 3.41 (0.69) | 3.41 (0.69) | 3.26 (0.72) | < 0.001 |
| Fatty acid composition (% of total fatty acids), mean (SD) | ||||
| Total PUFAs | 42.42 (3.79) | 42.43 (3.79) | 41.97 (3.90) | < 0.001 |
| Omega-3 PUFAs | 4.42 (1.56) | 4.41 (1.56) | 4.56 (1.62) | < 0.001 |
| DHA | 2.00 (0.68) | 2.00 (0.68) | 2.03 (0.70) | 0.085 |
| Omega-6 PUFAs | 38.00 (3.65) | 38.01 (3.65) | 37.41 (3.63) | < 0.001 |
| LA | 28.94 (3.46) | 28.96 (3.45) | 28.00 (3.46) | < 0.001 |
| Fish oil supplementation, N (%) | < 0.001 | |||
| No | 289,706 (68.1%) | 285,098 (68.2%) | 4608 (62.6%) | |
| Yes | 135,668 (31.9%) | 132,919 (31.8%) | 2749 (37.4%) | |
| Oily fish intake, times/week, N (%) | < 0.001 | |||
| < 1 | 191,911 (43.9%) | 189,037 (44.0%) | 2874 (37.6%) | |
| 1 | 165,197 (37.8%) | 162,340 (37.8%) | 2857 (37.3%) | |
| ≥ 2 | 80,020 (18.3%) | 78,099 (18.2%) | 1921 (25.1%) | |
| Fruit intake, servings/day, N (%)e | < 0.001 | |||
| < 2.0 | 151,939 (35.0%) | 149,561 (35.0%) | 2378 (31.4%) | |
| 2.0–3.9 | 206,276 (47.5%) | 202,748 (47.5%) | 3528 (46.6%) | |
| ≥ 4.0 | 76,175 (17.5%) | 74,507 (17.5%) | 1668 (22.0%) | |
| Vegetable intake, servings/day, N (%)f | < 0.001 | |||
| < 2.0 | 294,224 (68.3%) | 289,431 (68.3%) | 4793 (64.5%) | |
| 2.0–3.9 | 122,667 (28.4%) | 120,355 (28.4%) | 2312 (31.1%) | |
| ≥ 4.0 | 14,094 (3.3%) | 13,766 (3.3%) | 328 (4.4%) | |
| Processed meat intake, times/week, N (%) | < 0.001 | |||
| < 2 | 302,424 (68.9%) | 297,346 (69.0%) | 5078 (65.9%) | |
| ≥ 2 | 136,386 (31.1%) | 133,764 (31.0%) | 2622 (34.1%) | |
| Red meat intake, times/week, N (%) g | 0.001 | |||
| < 2 | 214,327 (49.2%) | 210,732 (49.2%) | 3595 (47.3%) | |
| ≥ 2 | 221,525 (50.8%) | 217,521 (50.8%) | 4004 (52.7%) | |
| Vitamin supplementation, N(%) | 139,035 (32.0%) | 136,513 (32.0%) | 2522 (33.5%) | 0.005 |
| Mineral supplementation, N (%) | 119,887 (27.5%) | 117,599 (27.5%) | 2288 (30.3%) | < 0.001 |
p values are derived using Student’s t test, Mann–Whitney U test, or chi-squared test
SD standard deviation, APOE apolipoprotein E, BMI body mass index, MET metabolic equivalent task, CVD cardiovascular disease, PUFA polyunsaturated fatty acids, DHA docosahexaenoic acid, LA linoleic acid
aEducation level is categorized as higher (college/university degree or other professional qualification) or lower
bAlcohol drinking is categorized as non-drinker (never or special occasions only), low to moderate drinker (once a month to four times a week), or heavy drinker (daily or almost daily)
cPhysical activity includes walking, moderate, and vigorous activity
dCVD includes heart attack, angina, stroke, and high blood pressure
eFruit intake includes fresh fruit intake and dried fruit intake. Amount per serving refers to 1 piece fresh fruit or 5 pieces dried fruit
fVegetable intake includes cooked vegetable intake and salad/raw vegetable intake. Amount per serving refers to 3 heaped tablespoons
gRed meat includes beef, lamb/mutton, and pork
Higher plasma PUFA levels were associated with lower risk of incident dementia
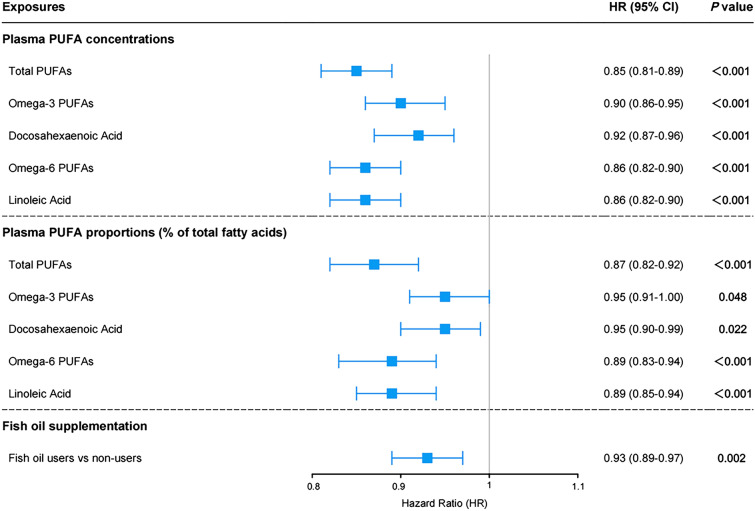
After adjustment for age, sex, education, and APOE ε4 status (model 1), higher plasma concentrations of five PUFA measures were significantly associated with lower risk of all-cause dementia (total PUFAs: HR [95% CI] = 0.85 [0.81–0.89]; omega-3 PUFAs 0.90 [0.86–0.95]; DHA 0.92 [0.87–0.96]; omega-6 PUFAs 0.86 [0.82–0.90]; LA 0.86 [0.82–0.90]; all p < 0.001) (Fig. 2). When analyzing percentages of total fatty acids, five PUFA measures also showed significant inverse associations with incident dementia after additional adjustment for plasma concentration of total fatty acids (total PUFAs: HR [95% CI] = 0.87 [0.82–0.92], p < 0.001; omega-3 PUFAs 0.95 [0.91–1.00], p = 0.048; DHA 0.95 [0.90–0.99], p = 0.022; omega-6 PUFAs 0.89 [0.83–0.94], p < 0.001; LA 0.89 [0.85–0.94], p < 0.001). These results were statistically robust after applying models 2 and 3 (eTable 4) or excluding participants who occurred dementia in the first 2 years of follow-up (eTable 5). In stratified analyses according to baseline age, higher plasma concentrations of omega-3 PUFAs and DHA were associated with decreased risk of all-cause dementia among older participants (age ≥ 65 years) but not among younger individuals (eTable 6). When stratifying participants by sex and APOE ε4 status, no significant interactions were found (eTables 7–9). As for the relations with dementia subtypes, higher plasma levels of total PUFAs, omega-6 PUFAs, and LA were associated with lower risks for both AD and VD, while no significant associations of omega-3 PUFAs and DHA with dementia subtypes were observed (eTable 10).
Fig. 2.
Associations of plasma PUFA levels and fish oil supplementation with dementia risk. Plasma PUFA concentrations and proportions were standardized to Z values. Results were adjusted for age, sex, education, and APOE ε4 status (model 1). For plasma PUFA proportions, results were further adjusted for absolute concentration of total fatty acids. PUFA, polyunsaturated fatty acids; CI, confidence interval
Fish oil supplementation was associated with lower risk of incident dementia
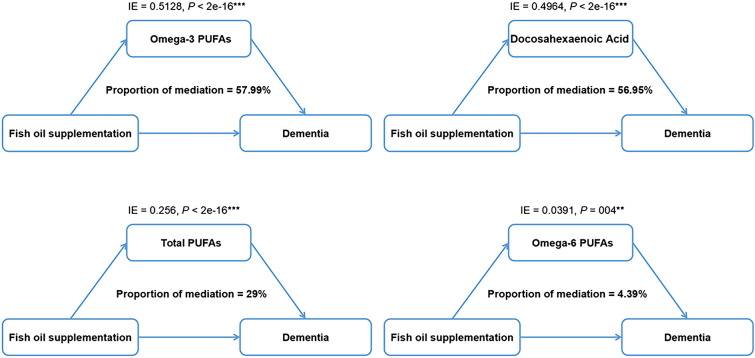
Basically adjusted for covariates in model 1, fish oil supplement users had a 7% lower risk of developing all-cause dementia compared with non-users (Fig. 2). In the fully adjusted model (model 3), the correlation between fish oil supplementation and decreased risk of incident dementia remained significant (HR [95% CI] = 0.94 [0.89–0.99], p = 0.014) (eTable 11). The trends of results were not changed largely in sensitivity analyses (eTable 12). In mediation analyses, the relationship between fish oil supplementation and dementia was partially mediated by plasma n-3 PUFA levels (omega-3 PUFAs: proportion of mediation = 57.99%; DHA: proportion of mediation = 56.95%, Fig. 3).
Fig. 3.
Mediation analysis of the relationship between fish oil supplementation and dementia. The relationship between fish oil supplementation and dementia was partially mediated by plasma concentrations of omega-3 PUFAs, docosahexaenoic acid, total PUFAs, and omega-6 PUFAs. Results were adjusted for age, sex, education, and APOE ε4 status. PUFA, polyunsaturated fatty acids; IE, indirect effect
We also conducted subgroup analyses according to several potential risk factors (eFigure 1). The results suggest that relationships between fish oil supplementation and decreased risk of all-cause dementia were stronger among older participants (p for interaction < 0.001), men (p for interaction = 0.033), APOE ε4 non-carriers (p for interaction = 0.013), and those who intake oily fish less frequently (p for interaction = 0.012). For the associations with dementia subtypes, fish oil supplementation was significantly associated with lower risk of VD, but not AD (eTable 13).
Associations of plasma PUFA levels and fish oil supplementation with peripheral immunity
After full adjustment, plasma n-3 PUFA levels and fish oil supplementation showed significant inverse associations with neutrophils, the main components of innate immunity. Similar findings were observed in the correlation with ratios of innate immunity over adaptive immunity, including NLR and SII. On the contrary, we found that plasma n-3 PUFA levels and fish oil supplementation were positively related to lymphocytes, the core adaptive immunity components. Similar results were observed for LMR (eTables 14–18).
Associations of plasma PUFA levels and fish oil supplementation with brain structure
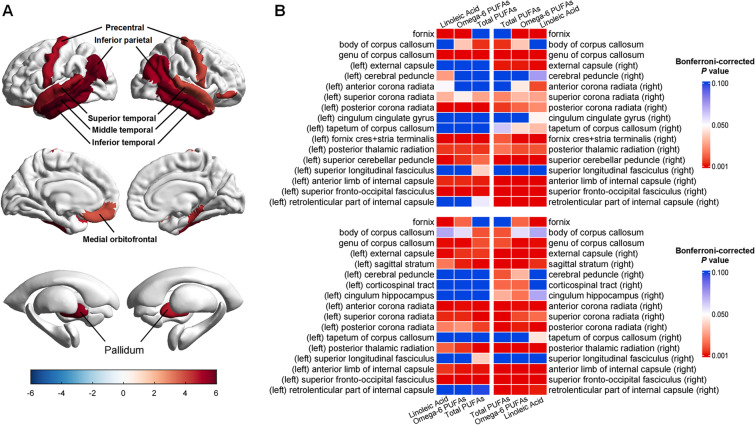
After multi-correction, plasma LA concentration was positively related to volume of right entorhinal cortex. Higher proportions of total PUFAs, omega-6 PUFAs, and LA were associated with larger cortical volumes in superior, middle and inferior temporal gyrus, inferior parietal cortex, bankssts (banks of superior temporal sulcus), precentral gyrus, parahippocampal gyrus, pars orbitalis and medial orbitofrontal cortex, as well as larger subcortical volumes in pallidum (Fig. 4A and eTables 19–20). In addition, our diffusion tensor imaging (DTI) results showed significant associations between plasma PUFA levels and white matter microstructural integrity. Higher plasma levels of total PUFAs, omega-6 PUFAs, and LA were related to higher FA and lower MD values in the majority of white matter tracts, including fornix, genu, body and tapetum of corpus callosum, external capsule, cerebral peduncle, anterior, superior and posterior corona radiata, posterior thalamic radiation, superior longitudinal fasciculus, superior fronto-occipital fasciculus, anterior limb, and retrolenticular part of internal capsule (Fig. 4B and eTables 21–22). No significant association was observed between fish oil supplementation and brain structure (eTables 23–24).
Fig. 4.
Associations between plasma PUFA levels and brain structure. A The volumes of cortical and subcortical regions were positively correlated with plasma proportions of total PUFAs, omega-6 PUFAs. and linoleic acid. B Top panel, the heatmap shows the Bonferroni-corrected p values for the associations between plasma PUFA levels and FA values of white matter tracts. Bottom panel, the heatmap shows the Bonferroni-corrected p values for the associations between plasma PUFA levels and MD values of white matter tracts. PUFA, polyunsaturated fatty acids; FA, fractional anisotropy; MD, mean diffusivity
Discussion
To our knowledge, this is the first prospective cohort study concurrently investigating the associations of PUFA biostatus (i.e., plasma concentrations and proportions) and intake (i.e., fish oil supplementation) with risk of incident dementia. Our results suggest that higher plasma PUFA levels and habitual use of fish oil supplements were associated with lower dementia risk and the relationship between fish oil supplementation and dementia may be partially mediated by plasma n-3 PUFA levels. We also observed significant associations of plasma PUFA levels and fish oil supplementation with peripheral immune markers that were related to dementia risk [15], as well as the positive associations of plasma PUFA levels with brain gray matter volumes and white matter microstructural integrity, suggesting they may affect dementia risk by affecting peripheral immunity and brain structure.
Our findings on plasma PUFAs and fish oil supplementation are in agreement with accumulating evidence for a beneficial role of long-chain n-3 PUFA in cognitive aging [9]. A recent longitudinal study reported that higher levels of plasma omega-3 index (EPA + DHA) were associated with lower risks for dementia and related outcomes [6]. The Framingham Heart study, during a mean follow-up of 9.1 years, observed that participants in the top quartile of plasma DHA level had half the risk of developing dementia compared with those in the lower 3 quartiles [3]. A previous meta-analysis of circulating metabolites associated with general cognitive ability and dementia also supported the evidence that high DHA did have cognitive benefits [21]. Similarly, Liu et al. also examined the use of fish oil supplements in the UK Biobank cohort, although with a smaller sample size, and showed a potentially beneficial association of fish oil supplementation with risk of all-cause dementia among the elderly population [13]. Several potential mechanisms may explain the investigated results, including the important role of n-3 PUFA in clearing amyloid-β (Aβ) peptide, increasing neurotrophic and neuroprotective factors, and counteracting neuro-inflammation in cognitive aging and dementia, as well as DHA in synaptic plasticity and hippocampal neurogenesis [22–24].
Previously, only few studies have investigated the relationships between n-6 PUFA and dementia and reported inconsistent results [4, 7, 25]. The failed finding of the correlation may be due to the limited sample size and insufficient length of follow-up [4, 7]. In the present study, we first reported that higher plasma levels of omega-6 PUFAs and LA were significantly associated with lower risks of incident all-cause and AD dementia. Similar to our findings, the Chicago Health and Aging Project observed the relationship between high dietary intake of n-6 PUFAs and a significantly reduced risk for AD dementia [25]. Since studies on the relationship between n-6 PUFA and dementia are scarce and discrepancies may result from sample heterogeneity and limited statistical power, future research are needed for confirmation of our results.
The impact of dietary PUFAs on the immune system has been explored for decades, with special focus on the n-3 PUFAs. Several investigations have shown that n-3 PUFAs exert immunomodulatory activity, either by targeting immune and non-immune cells directly or by targeting the gut microbiome indirectly [26]. This family of polyunsaturated fatty acids exerts major alterations on the activation of cells from both the innate and the adaptive immune system, although the mechanisms for such regulation are diverse [27]. Moreover, our recent longitudinal research provided clear evidence of the correlation between peripheral immunity and dementia, revealing that increased adaptive immune markers were associated with lower dementia risk, while increased innate immune markers were associated with higher dementia risk [15]. In the present study, plasma PUFA levels and fish oil supplementation were positively correlated with adaptive immune markers (i.e., lymphocytes and LMR), which were protective factors against dementia. On the contrary, higher plasma n-3 PUFA levels and habitual use of fish oil supplements were significantly associated with lower levels of innate immune markers (i.e., neutrophils, NLR, and SII) that were detrimental to dementia. Taken together, we postulate that high plasma PUFA levels (specifically n-3 PUFA) and fish oil supplementation may protect against dementia partially through the potential impact on peripheral immunity.
In neuroimaging analyses, we observed that the increased plasma levels of total PUFAs, omega-6 PUFAs, and LA were significantly associated with larger gray matter volumes in several dementia-related brain regions [28], such as the temporal cortex, inferior parietal cortex, medial orbitofrontal cortex, and entorhinal cortex. Moreover, our DTI findings are in accordance with the evidence from previous studies, which suggest a positive correlation between PUFA and white matter microstructural integrity [8, 29–33]. The mechanisms underlying the correlation may be explained by the biophysiological roles of PUFA in myelination and human brain white matter development [34]. Previously, several studies have reported the relationships between DTI-derived white matter indices and cognitive function [29, 35–37]. White matter microstructural integrity was positively associated with cognitive performance [29], while deficits in white matter microstructure predicted cognitive decline [36]. Collectively, higher plasma PUFA levels might be related with decreased dementia risk via protective effect on brain macrostructure and microstructure.
Our study has several major strengths. Firstly, all analyses were based on a longitudinal cohort with large sample size and long-term follow-up, which provided enough incident dementia cases and adequate support from statistical power. Secondly, we comprehensively assessed the effect of PUFA on dementia in terms of both biostatus and intake. We also acknowledge some limitations. First, as plasma PUFA levels can vary depending on recent dietary intake and other factors, PUFA profiles of other tissues (e.g., adipose tissue) or blood cells (e.g., erythrocytes) may better reflect long-term PUFA status. Second, fish oil supplementation habits can change considerably over the months and years. However, the stability and reliability of fish oil use among UKB participants has been established before [13]. Moreover, the lack of detailed information on fish oil use, such as specific dose, frequency, and duration, prevented us from assessing dose–response relationships and appropriate duration of supplementation in the present study. Finally, the majority of UKB participants are British, which may limit the generalizability of our results to other ethnicities. Therefore, confirmatory research in other populations is recommended.
In conclusion, our findings suggest that higher plasma PUFA levels and habitual use of fish oil supplements were associated with lower risk of incident dementia, and that plasma n-3 PUFA levels partially mediated the fish oil supplementation-dementia relationship. Furthermore, peripheral immunity and brain structure may be involved in pathways linking PUFAs/fish oil to dementia. This study provides population-based evidence to support the value of interventions to target PUFAs (specifically n-3 PUFAs) to prevent dementia.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors gratefully thank all the participants and professionals contributing to the UK Biobank.
Author contribution
JTY and WC had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: JTY. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: YH, SYH, HFW, and WZ. Critical revision of the manuscript for important intellectual content: YH, SYH, HFW, WZ, YTD, QD, JFF, WC, and JTY. Statistical analysis: YH, HFW, WZ, and WC. Obtained funding: JFF, WC, and JTY. Administrative, technical, or material support: QD, JFF, WC, and JTY. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the Science and Technology Innovation 2030 Major Projects (2022ZD0211600), National Natural Science Foundation of China (82,071,201, 82,071,997), Shanghai Municipal Science and Technology Major Project (2018SHZDZX01), Research Start-Up Fund of Huashan Hospital (2022QD002), Excellence 2025 Talent Cultivation Program at Fudan University (3,030,277,001), Shanghai Talent Development Funding for The Project (2,019,074), Shanghai Rising-Star Program (21QA1408700), 111 Project (B18015), and ZHANGJIANG LAB, Tianqiao and Chrissy Chen Institute, the State Key Laboratory of Neurobiology and Frontiers Center for Brain Science of Ministry of Education, and Shanghai Center for Brain Science and Brain-Inspired Technology, Fudan University. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.\
Data Availability
The UK Biobank data used for this article requires a data user agreement approval. Please see www.ukbiobank.ac.uk for access.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yu He and Shu-Yi Huang contributed equally to the present work.
References
- 1.Patterson C. World Alzheimer Report 2018. London: Alzheimer’s Disease International; 2018. [Google Scholar]
- 2.Janssen CI, Kiliaan AJ. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Prog Lipid Res. 2014;53:1–17. doi: 10.1016/j.plipres.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Schaefer EJ, Bongard V, Beiser AS, et al. Plasma phosphatidylcholine docosahexaenoic acid content and risk of dementia and Alzheimer disease: the Framingham Heart Study. Arch Neurol. 2006;63(11):1545–1550. doi: 10.1001/archneur.63.11.1545. [DOI] [PubMed] [Google Scholar]
- 4.Samieri C, Féart C, Letenneur L, et al. Low plasma eicosapentaenoic acid and depressive symptomatology are independent predictors of dementia risk. Am J Clin Nutr. 2008;88(3):714–721. doi: 10.1093/ajcn/88.3.714. [DOI] [PubMed] [Google Scholar]
- 5.Ammann EM, Pottala JV, Robinson JG, Espeland MA, Harris WS. Erythrocyte omega-3 fatty acids are inversely associated with incident dementia: Secondary analyses of longitudinal data from the Women’s Health Initiative Memory Study (WHIMS) Prostaglandins Leukot Essent Fatty Acids. 2017;121:68–75. doi: 10.1016/j.plefa.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas A, Baillet M, Proust-Lima C, et al. Blood polyunsaturated omega-3 fatty acids, brain atrophy, cognitive decline, and dementia risk. Alzheimers Dement. 2020 doi: 10.1002/alz.12195. [DOI] [PubMed] [Google Scholar]
- 7.Melo van Lent D, Egert S, Wolfsgruber S, et al. Eicosapentaenoic acid is associated with decreased incidence of Alzheimer’s dementia in the oldest old. Nutrients. 2021;13(2):461. doi: 10.3390/nu13020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McNamara RK, Asch RH, Lindquist DM, Krikorian R. Role of polyunsaturated fatty acids in human brain structure and function across the lifespan: an update on neuroimaging findings. Prostaglandins Leukot Essent Fatty Acids. 2018;136:23–34. doi: 10.1016/j.plefa.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cederholm T, Salem N, Jr, Palmblad J. ω-3 fatty acids in the prevention of cognitive decline in humans. Adv Nutr. 2013;4(6):672–676. doi: 10.3945/an.113.004556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quinn JF, Raman R, Thomas RG, et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA. 2010;304(17):1903–1911. doi: 10.1001/jama.2010.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freund-Levi Y, Eriksdotter-Jönhagen M, Cederholm T, et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol. 2006;63(10):1402–1408. doi: 10.1001/archneur.63.10.1402. [DOI] [PubMed] [Google Scholar]
- 12.van de Rest O, Geleijnse JM, Kok FJ, et al. Effect of fish oil on cognitive performance in older subjects: a randomized, controlled trial. Neurology. 2008;71(6):430–438. doi: 10.1212/01.wnl.0000324268.45138.86. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Zhuang P, Li Y, et al. Association of fish oil supplementation with risk of incident dementia: a prospective study of 215,083 older adults. Clin Nutr. 2022;41(3):589–598. doi: 10.1016/j.clnu.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Ma H, Zhou T, Li X, Heianza Y, Qi L. Use of fish oil supplements is differently related to incidence of all-cause and vascular dementia among people with the distinct APOE ε4 dosage. Clin Nutr. 2022;41(3):731–736. doi: 10.1016/j.clnu.2022.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang YR, Wang JJ, Chen SF, et al. Peripheral immunity is associated with the risk of incident dementia. Mol Psychiatry. 2022;27(4):1956–1962. doi: 10.1038/s41380-022-01446-5. [DOI] [PubMed] [Google Scholar]
- 16.Torrinhas RS, Calder PC, Lemos GO, Waitzberg DL. Parenteral fish oil: an adjuvant pharmacotherapy for coronavirus disease 2019? Nutrition. 2021;81:110900. doi: 10.1016/j.nut.2020.110900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Würtz P, Kangas AJ, Soininen P, Lawlor DA, Davey Smith G, Ala-Korpela M. Quantitative serum nuclear magnetic resonance metabolomics in large-scale epidemiology: a primer on -omic technologies. Am J Epidemiol. 2017;186(9):1084–1096. doi: 10.1093/aje/kwx016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Y, Chatterjee R, Ronanki A, Ye K. Circulating polyunsaturated fatty acids and COVID-19: a prospective cohort study and Mendelian randomization analysis. Front Med (Lausanne) 2022;9:923746. doi: 10.3389/fmed.2022.923746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Lee SJ, Teunissen CE, Pool R, et al. Circulating metabolites and general cognitive ability and dementia: evidence from 11 cohort studies. Alzheimers Dement. 2018;14(6):707–722. doi: 10.1016/j.jalz.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Cunnane SC, Plourde M, Pifferi F, Bégin M, Féart C, Barberger-Gateau P. Fish, docosahexaenoic acid and Alzheimer’s disease. Prog Lipid Res. 2009;48(5):239–256. doi: 10.1016/j.plipres.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Yanai H. Effects of N-3 Polyunsaturated Fatty Acids on Dementia. J Clin Med Res. 2017;9(1):1–9. doi: 10.14740/jocmr2815w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parletta N, Milte CM, Meyer BJ. Nutritional modulation of cognitive function and mental health. J Nutr Biochem. 2013;24(5):725–743. doi: 10.1016/j.jnutbio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Morris MC, Evans DA, Bienias JL, et al. Dietary fats and the risk of incident Alzheimer disease. Arch Neurol. 2003;60(2):194–200. doi: 10.1001/archneur.60.2.194. [DOI] [PubMed] [Google Scholar]
- 26.Velotti F, Costantini L, Merendino N. Omega-3 polyunsaturated fatty acids (n-3 PUFAs) for Immunomodulation in COVID-19 related acute respiratory distress syndrome (ARDS) J Clin Med. 2022;12(1):304. doi: 10.3390/jcm12010304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutiérrez S, Svahn SL, Johansson ME. Effects of omega-3 fatty acids on immune cells. Int J Mol Sci. 2019;20(20):5028. doi: 10.3390/ijms20205028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pini L, Pievani M, Bocchetta M, et al. Brain atrophy in Alzheimer’s disease and aging. Ageing Res Rev. 2016;30:25–48. doi: 10.1016/j.arr.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Gu Y, Vorburger RS, Gazes Y, et al. White matter integrity as a mediator in the relationship between dietary nutrients and cognition in the elderly. Ann Neurol. 2016;79(6):1014–1025. doi: 10.1002/ana.24674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters BD, Duran M, Vlieger EJ, et al. Polyunsaturated fatty acids and brain white matter anisotropy in recent-onset schizophrenia: a preliminary study. Prostaglandins Leukot Essent Fatty Acids. 2009;81(1):61–63. doi: 10.1016/j.plefa.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Peters BD, Machielsen MW, Hoen WP, et al. Polyunsaturated fatty acid concentration predicts myelin integrity in early-phase psychosis. Schizophr Bull. 2013;39(4):830–838. doi: 10.1093/schbul/sbs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chhetry BT, Hezghia A, Miller JM, et al. Omega-3 polyunsaturated fatty acid supplementation and white matter changes in major depression. J Psychiatr Res. 2016;75:65–74. doi: 10.1016/j.jpsychires.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Witte AV, Kerti L, Hermannstädter HM, et al. Long-chain omega-3 fatty acids improve brain function and structure in older adults. Cereb Cortex. 2014;24(11):3059–3068. doi: 10.1093/cercor/bht163. [DOI] [PubMed] [Google Scholar]
- 34.Peters BD, Voineskos AN, Szeszko PR, et al. Brain white matter development is associated with a human-specific haplotype increasing the synthesis of long chain fatty acids. J Neurosci. 2014;34(18):6367–6376. doi: 10.1523/JNEUROSCI.2818-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosano C, Aizenstein HJ, Newman AB, et al. Neuroimaging differences between older adults with maintained versus declining cognition over a 10-year period. Neuroimage. 2012;62(1):307–313. doi: 10.1016/j.neuroimage.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selnes P, Aarsland D, Bjørnerud A, et al. Diffusion tensor imaging surpasses cerebrospinal fluid as predictor of cognitive decline and medial temporal lobe atrophy in subjective cognitive impairment and mild cognitive impairment. J Alzheimers Dis. 2013;33(3):723–736. doi: 10.3233/JAD-2012-121603. [DOI] [PubMed] [Google Scholar]
- 37.Amlien IK, Fjell AM. Diffusion tensor imaging of white matter degeneration in Alzheimer’s disease and mild cognitive impairment. Neuroscience. 2014;276:206–215. doi: 10.1016/j.neuroscience.2014.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The UK Biobank data used for this article requires a data user agreement approval. Please see www.ukbiobank.ac.uk for access.