Abstract
Dementia refers to a particular group of symptoms characterized by difficulties with memory, language, problem-solving, and other thinking skills that affect a person’s ability to perform everyday activities. Alzheimer’s disease (AD) is the most common form of dementia, affecting about 6.2 million Americans aged 65 years and older. Likewise, cardiovascular diseases (CVDs) are a major cause of disability and premature death, impacting 126.9 million adults in the USA, a number that increases with age. Consequently, CVDs and cardiovascular risk factors are associated with an increased risk of AD and cognitive impairment. They share important age-related cardiometabolic and lifestyle risk factors, that make them among the leading causes of death. Additionally, there are several premises and hypotheses about the mechanisms underlying the association between AD and CVD. Although AD and CVD may be considered deleterious to health, the study of their combination constitutes a clinical challenge, and investigations to understand the mechanistic pathways for the cause-effect and/or shared pathology between these two disease constellations remains an active area of research. AD pathology is propagated by the amyloid β (Aβ) peptides. These peptides give rise to small, toxic, and soluble Aβ oligomers (SPOs) that are nonfibrillar, and it is their levels that show a robust correlation with the extent of cognitive impairment. This review will elucidate the interplay between the effects of accumulating SPOs in AD and CVDs, the resulting ER stress response, and their role in vascular dysfunction. We will also address the potential underlying mechanisms, including the possibility that SPOs are among the causes of vascular injury in CVD associated with cognitive decline. By revealing common mechanistic underpinnings of AD and CVD, we hope that novel experimental therapeutics can be designed to reduce the burden of these devastating diseases.
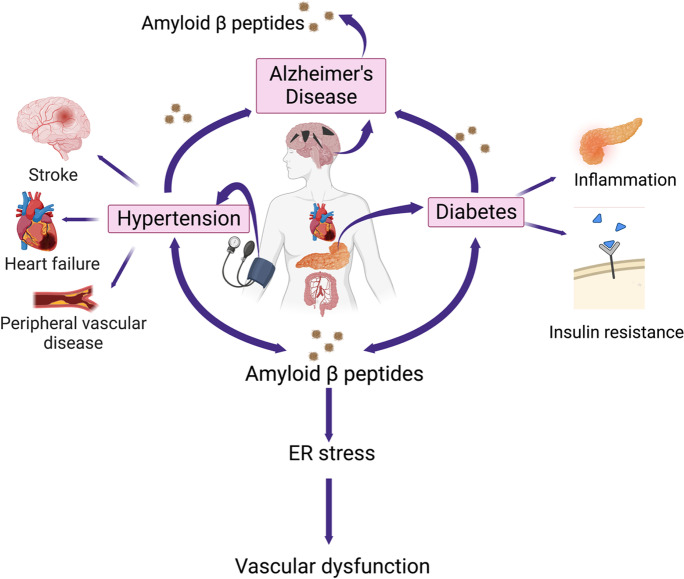
Graphical abstract.
Alzheimer’s disease (AD) pathology leads to the release of Aβ peptides, and their accumulation in the peripheral organs has varying effects on various components of the cardiovascular system including endoplasmic reticulum (ER) stress and vascular damage. Image created with BioRender.com
Keywords: Hypertension, Vascular dysfunction, Aging, Dementia
Introduction
Alzheimer’s disease (AD) is the most common form of dementia with 6.2 million Americans aged 65 years and older living with AD in 2021 [1]. Additionally, the U.S Census and the Chicago Health and Aging Project (CHAP) observed that 1 in 10 people are living with AD in 2020 [2], and it is the sixth-leading cause of death in USA [3]. Furthermore, epidemiological studies suggest that cardiovascular diseases (CVDs) and cardiovascular risk factors are associated with an increased risk of AD and mild cognitive impairment, which is its clinical stage precursor [4]. They are a major cause of disability and premature death worldwide [5], and in the USA, they affected 126.9 million adults in 2018, a number that increases with age in both males and females [6]. It is thus evident that AD and CVD pose a major global burden of disease with high-cost implications on the government and the health sector.
There are several premises and hypotheses about the mechanisms underlying the association between AD and CVD, the main ones being hypoperfusion, emboli, atherosclerosis, and the fact that in both the heart and brain of AD patients, amyloid deposits may be present, thus causing damage to these organs [7]. The “amyloid cascade hypothesis” implies that the two major hallmarks of the pathology of AD are the extracellular accumulation of Aβ peptides into senile plaques, and the intracellular hyperphosphorylation of tau proteins into neurofibrillary tangles, resulting in vascular damage, cell loss, dementia, and the progression of AD pathology [8, 9]. However, the presence of senile plaques in a particular region of the AD brain shows a relatively weak correlation with the severity of dementia [10]. To this end, recent studies have shown that the Aβ peptides give rise to small, toxic, and soluble Aβ oligomers (SPOs) that are nonfibrillar, and it is their levels that show a strong correlation with the extent of cognitive impairment rather than the amyloid plaques containing insoluble Aβ fibrils [11, 12]. The main isoforms of the neurotoxic Aβ peptides include Aβ1-40 and Aβ1-42, and they are byproducts of the metabolism of the parental amyloid precursor protein (APP) [11, 13] found in the endoplasmic reticulum (ER) and Golgi/trans-Golgi network (TGN) and endosomal, lysosomal, and mitochondrial membranes [11, 14]. Since the ER is responsible for the proper folding and processing of nascent proteins and Ca2+ homeostasis [15], the accumulation of these toxic insoluble and SPOs induces ER stress, leading to cell death in several diseases, such as AD [16], diabetes, sepsis, and hypertension [9, 17]. ER stress in turn activates the unfolded protein response (UPR) in a bid to reduce protein synthesis and increase their degradation. However, failure of this response to restore homeostasis can result in oxidative stress due to increased reactive oxygen species (ROS) and eventually cell death [18].
The accumulation of Aβ peptides in vascular disease
Evidence that Aβ peptides have powerful vascular effects suggests a link between AD, vascular diseases [19], and endothelial dysfunction as found in hypertension and diabetes [20]. Aβ peptides are toxic to the brain and peripheral endothelial cells and cause cellular damage, enhance vasoconstriction, and impair endothelium-dependent vasodilation hence promoting atherosclerosis and vascular disease [21]. In addition, the inflammatory nature of both CVD and AD involves multiple common cellular and molecular mechanisms [22]. Aβ1-42, being more hydrophobic and fibrillogenic, is the main peptide found in parenchymal lesions of AD while Aβ1-40 is involved in the pathogenesis of cerebral amyloid angiopathy [23]. Normally, an equilibrium exists between Aβ peptides production and removal in different compartments in the central nervous system [24]. However, any imbalance results in the accumulation of Aβ1-40 in the blood, vascular wall, and heart tissue as seen in CVDs [22]. Activation of the amyloidogenic pathway impairs the vasodilatory nature of arterioles by enhancing endothelin-1 expression [25], reduces endothelium-dependent vasodilation by reducing endothelial nitric oxide synthase (eNOS) activity, induces oxidative stress [26], and increases responsiveness to vasoconstrictors [27]. In addition, Aβ peptides inhibit telomerase activity leading to vascular aging as a result of telomere shortening [28]. Amyloid deposition in the vessels [29] can cause arterial stiffness by structural or cellular changes [30], and this is a frequent finding in patients with CVD and a risk factor for cognitive decline later in life. It causes structural changes in the brain such as white matter lesions, cortical infarcts, and cortical brain atrophy [31]. Aβ peptides deposition in arterial vessel walls impedes perivascular drainage of Aβ peptides due to AD pathology leading to intracerebral hemorrhage and increased Aβ peptides [32]. These morphological changes are easily observed in patients with AD who show changes in retinal microvasculature [33], which have been postulated to precede the majority of neurodegeneration that characterizes AD progression [34]. When inferring to the causal nature of the occurrence of AD and CVD, literature shows a synergistic interaction between vascular and neurodegenerative processes early in disease pathogenesis [35]. There is a reciprocal relationship between Aβ accumulation and cerebrovascular insult, such that Aβ peptides deposition provokes vascular changes [32]. Furthermore, in a study using mice fed a high-salt diet (HSD) by Faraco and colleagues, a causal link was observed between the HSD, endothelial dysfunction, and tau pathology. The cognitive impairment produced by HSD was also linked to an altered immune response originating in the gut [36, 37]. HSD leads to a gut-initiated adaptive immune response mediated by Th17 lymphocytes and an increase in circulating IL17. This leads to the inhibition of eNOS and reduced vascular nitric oxide (NO) production, which, in turn, impairs endothelial vasoactivity and lowers cerebral blood flow [36]. However, it is not just the cerebral hypoperfusion that contributes to the cognitive impairment induced by HSD, but rather the excessive phosphorylation and accumulation of insoluble tau aggregates [38] which mediate neuronal and endothelial dysfunction hence contributing to neurodegenerative pathologies like dementia and AD [37, 39, 40].
By the same token, in the peripheral vasculature, the abnormal deposition of free cholesterol in coronary arteries is toxic to many different vascular cell types, including macrophages, endothelial cells, and smooth muscle cells [41]. This toxicity directs these vascular cells to apoptosis and eventually vascular dysfunction [41, 42]. Although there is evidence that peripheral vascular and cerebrovascular disease pathology can accelerate the progression of AD, the relationship between them is complex, and further research is necessary to discern the exact nature of these relationships at different stages of disease progression [35].
Evidence of the association between AD and CVD
In addition to the information above, it is important to understand the consequences of pathology to the brain since it is an important target for hypertension and other CVDs that subsequently contribute to cognitive impairment [43]. The vasculature in the brain controls blood flow via autoregulation through myogenic, metabolic and neurogenic mechanisms, which maintain relatively constant blood flow during both increases and decreases in blood pressure [44]. There are large intracranial and extracranial arteries that provide significant vascular resistance in the brain compared to peripheral organs which obtain vascular resistance from small arteries and arterioles [44]. The cerebral endothelium blood-brain barrier (BBB), has specialized tight junctions that do not allow ions to pass freely and ensure low hydraulic conductivity and transcellular transport. This compares more to the epithelium than the endothelium in the periphery. It is therefore crucial for the brain to regulate water movement tightly to prevent increased intracranial pressure that would otherwise cause severe neurologic complications and death [44]. Normal brain function depends on receiving 20% of the cardiac output of oxygenated blood [45], and impaired cardiac function can lead to reduced intracranial blood flow and ischemia as observed in AD patients [46]. This leads to hypoperfusion and microvascular damage and contributes to aggregation of blood products, impaired Aβ peptides clearance, and endothelial dysfunction [47]. Reduced cerebral blood flow can also be a result of decreased endothelial NO synthesis in AD [48], which otherwise prevents cerebrovascular disease (CBVD) by preserving cerebral blood flow and preventing inflammation, thrombosis and apoptosis [49]. However, accumulated Aβ peptides induce endothelial NO dysfunction through the reduction of eNOS activity. They alter the pattern of eNOS phosphorylation at Ser1177, Ser116, and Thr495 in cerebral vessels [50, 51], making it a prominent component of not only CVD, but also neurodegenerative pathologies like AD [43]. Additionally, Aβ accumulation can contribute to the leakage of the BBB [52], contributing to higher oxidative damage and protease activity [32]. When found in the neutrophils and vessel walls, this accumulation leads to the activation of neuroinflammatory responses that disrupt the BBB [53]. Moreover, the resultant arterial stiffness causes uncoupling of the neurovascular unit leading to brain dysfunction [54].
This is also corroborated by studies showing that atrial fibrillation increases the risk of AD by 1.5–2.5-fold [55, 56]. Atrial fibrillation is a major risk factor for stroke, which is a major risk factor for vascular dementia. It causes embolism through thrombus formation in the heart, and this can cause cerebral infarction hence vascular dementia [55]. Through cerebral hypoperfusion, atrial fibrillation can cause hypoperfusive vascular dementia and accelerate the formation of senile plaques, amyloid angiopathy, and neurofibrillary tangles as seen in AD. As a result, the role that atrial fibrillation plays in inducing dementia in cerebrovascular diseases and AD in the elderly shows that its management can be crucial in the prevention of these dementias [55].
Inflammatory markers have been established as important predictors of CVD, and there is extensive evidence that inflammation may play a role in the development of AD pathology [4]. Studies show that measuring C-reactive protein (CRP) and IL-6 could predict myocardial infarction and stroke, and they are strong independent predictors of all causes of CVD and related mortalities [57]. Furthermore, CRP and other systemic inflammatory markers are associated with the onset of AD, and through formation of white matter lesions, they can accelerate the progression of AD [58]. Microglia in neurodegenerative disorders and macrophages in CVDs play a pivotal role in inflammation [59]. Activated microglia express pattern recognition receptors (PRRs) including Toll-like receptors (TLRs), particularly TLR-4 and TLR-6 which are co-expressed with cell surface co-receptors CD36 and CD14 [60, 61]. They trigger multiple innate immune signaling pathways including IL-1β, IL-2, IL-6, IL-8, tumor necrosis factor (TNFα), and chemokines which activate nuclear factor κB (NF-κB) signaling [62–65]. The TLR4-TLR6-CD36 complex has different ligands including fibrillar or soluble Aβ, cholesterol crystals, and oxidized low-density lipoproteins (oxLDL) which increase the production of pro-inflammatory mediators [66, 67]. Further, these molecules activate the NOD-LRR- and pyrin domain-containing 3 (NLRP3) inflammasome [68], and through phosphorylated caspase-1, they stimulate the secretion of active IL-1 β or IL-18 [62]. Similarly, in atherosclerotic CVD, the inflammasome is activated by directly binding oxLDL with CD36, TLR or through NOD-like receptors (NLR) [65, 69]. Studies with NLRP3 inflammasome and caspase-1 knockout mice show that their inhibition can be beneficial to attenuate vascular and brain inflammation and may be a good target for therapeutic interventions [59].
Although a majority of heart failure cases are known to result from ischemia, those without causative events are termed as idiopathic dilated cardiomyopathy (iDCM), and recent evidence has classified them as misfolding diseases, similar to neurodegenerative diseases like AD [70]. A study demonstrated that in humans, protein misfolding is implicated in the etiology and pathogenesis of iDCM [70]. Intermediate oligomers similar to those observed in the brains of patients with AD were observed in the myocardium of the patients with iDCM. In addition, missense mutations in the Presenilin (PSEN1 and PSEN2) genes alter presenilin expression and its interaction with proteins involved in excitation-contraction coupling, and they are also known to catalyze the cleavage and release of Aβ peptides from the amyloid precursor protein (APP). Furthermore, oligomers increase cytosolic Ca2+ which in failing hearts is coupled with Ca2+ depletion that favors protein misfolding [70]. These new pathogenic mechanisms, if well exploited, can be an important basis for the advancement of novel strategies for early diagnosis and treatment of iDCM.
Sex specificity during AD and CVD pathology
Over and above, there is a significant role played by sex differences in the initiation, progression, and clinical manifestation of AD [71]. This has been illustrated in studies showing that in most incidences of AD, almost 66% are women [72]. Further, reports show that after the age of 65, the cases of AD are 16.7% (1 in 6) for women and 9.1% (1 in 11) for men [73]. One major explanation for this difference can be inferred to the sex hormones, whereby in women with menopause, estrogen deficiency has been tightly linked with AD pathology [74]. Estrogen is regulated by the nuclear receptors, estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) [75], which are broadly distributed in the CNS [76] and bind to the estrogen response element thus imparting neuroprotection [77]. ERα acts like an agonist and is more effective in the induction of transcription coupled to the hormone response element compared to ERβ, which acts like an antagonist in some cases [78]. However, 17β-estradiol also mediates rapid signaling events via pathways that involve transmembrane ERs, such as G-protein-coupled ER 1, (GPER, formerly known as GPR30) [79]. In the past 20 years, dysregulation of GPER signaling has been implicated in several cardiovascular diseases. However, its role in dementia and AD is still unclear.
In AD, estrogen plays several neuroprotective roles, among them decreasing Aβ toxicity by inducing the breakdown of APP into the non-amyloidogenic pathway [80], improving synaptic plasticity, diminishing brain inflammation [81], and reducing tau protein hyperphosphorylation [81, 82]. This clarifies why reduced expression of ERα has been observed in hippocampal neurons of AD patients, and the reduced expression of ERβ in female AD patients has been associated with abnormal mitochondrial function and elevated markers of oxidative stress [81]. Conversely, in postmenopausal women, hormone replacement therapy may attenuate AD onset, demonstrating the importance of estrogen signaling in the progression of AD [81]. For this reason, therapeutic strategies can focus on developing drugs that have the potential to target different estrogen effects, estrogen-metabolizing enzyme expression, or estrogen receptors expression to treat neurodegenerative conditions [81].
In the same breath, CVDs have also been shown to contribute to cognitive decline in a sex-dependent manner. A recent study demonstrated that women with CVD were 1.5 times more likely to experience AD than men [83]. Women with heart failure and coronary heart disease were 1.3 and 1.6 times more likely to develop AD than men with the same condition [83]. In another study, cognitive performance was shown to be worse in hypertensive menopausal women compared to normotensive and postmenopausal women, and this had a negative impact on cortical functions or semantic memory [84]. This is further supported by findings that show blood pressure is typically lower in women before menopause than in age-matched men, but after menopause the incidence of hypertension in women increases dramatically [85].
Another study demonstrated how psychological stress leads to the development of hypertension by showing the interplay between the CNS and the neuroendocrine system [85]. In postmenopausal women, psychological stress causes greater pressor responses than in premenopausal women [86], and this effect is attenuated by estrogen hormone replacement [87, 88]. In reference to psychological stress, the amygdala, which is part of the limbic system, is functionally implicated in memory and sensory integration, reaction to stress, heart rate and blood pressure control, reproduction, and social behavior [89]. To this end, it has recently been shown that increased neural activities in the medial amygdala (MeA) mediate stress-induced pressor responses in animals and humans [90], and the MeA neurons express abundant ERα [91]. Using a knockout model for ERα in female mice, the study demonstrated that estrogen acts on the ERα expressed by MeA neurons to prevent the stress-induced pressor responses through inhibition of MeA neuron firing during stress [85]. Furthermore, treatment of control mice with 17β-estradiol prevented the stress induced increases in both blood pressure and heart rate, and this effect was blunted in ER knockout mice indicating that ERα expressed by MeA neurons is a key site for the antihypertensive effects of 17β-estradiol during stress. Consequently, ERα expressed by MeA can be a potential therapeutic target for hypertension, particularly in postmenopausal women [85].
Moreover, a sex-specific association between diabetes and brain structure abnormalities and function has been shown whereby diabetes increases the risk of having lacunes and brain atrophy in women than in men and is further associated with decreased executive function, processing speed and language showing increased and impaired cognitive function in women but not in men [92].
Although there have been many research studies on AD, its root cause remains unknown, and preventive treatment through the study of its risk factors may be the best strategy for clinicians to slow down its progression [59]. Furthermore, the well-known cardiovascular risk factors for AD pathology include hypertension, diabetes mellitus, smoking, apolipoprotein E (ApoE) e4 allele, hypercholesterolemia, homocysteinemia, and age [43, 93, 94]. Indeed, studies show a strong and likely causal association between CVD and their risk factors with the incidence of cognitive decline and AD [7] through their shared genetic and biochemical profiles and common triggers [95]. This therefore reveals a potential opportunity to prevent dementia through the management and treatment of CVDs and their risk factors either by pharmacological therapy or lifestyle modification [96]. Although CVDs include a diverse set of diseases, this review will focus on the association of AD and two major CVDs, hypertension [35] and diabetes [97] (Table 1).
Table 1.
Association between AD pathology and CVDs
| Hypertension | Contribution to cognitive decline/dementia | [98–101] |
| Promotes development of microhemorrhages | [102–104] | |
| Promotes accumulation of Aβ peptides | [99, 100, 103, 105–107] | |
| Contributes to white matter lesions | [101, 108, 109] | |
| Diabetes | Reduced expression of insulin receptors | [110, 111] |
| Hyperglycemia and ROS generation | [97, 112–116] | |
| Insulin-induced production of pro-inflammatory cytokines | [117–121] | |
| Inflammation | Production of CRP and IL-6 | [57] |
| Formation of white matter lesions | [58] | |
| Activation of the innate immune system | [60–65] |
Association between AD and hypertension
Epidemiology of hypertension and cognitive impairment
Although there has been tremendous progress in the prevention and treatment of hypertension, it still remains a major cause of morbidity and mortality worldwide [122]. Based on the 2017 guideline from the American College of Cardiology (ACC) and American Heart Association (AHA), blood pressure categories are (1) normal (< 120 systolic and < 80 mmHg diastolic), (2) elevated (120–129 systolic and < 80 mmHg diastolic), (3) stage 1 hypertension (130–139 systolic or 80–89 mmHg diastolic), and (4) stage 2 hypertension (≥ 140 systolic or ≥ 90 mmHg diastolic) [123]. Due to these changes, almost half of American adults have high blood pressure, and this scenario worsens with aging. Hypertension is thus categorized as a disease of aging [124] and is a risk factor for stroke, ischemic white matter lesions, silent infarcts, general atherosclerosis, myocardial infarction, and cardiovascular morbidity and mortality [125]. In addition, studies done at the end of the nineteenth century (1960s and 1970s) by Spieth [126] and Wilkie and Eisdorfer [98] show evidence that high blood pressure imparts deleterious effects on cognitive function by impairing various domains of cognition [127, 128]. In fact, many epidemiological studies demonstrate a causal relationship between blood pressure and the incidence of vascular cognitive impairment (VCI) and AD [129–131], most of which will not be focused on in this review, but we will highlight two major longitudinal studies that were carried out in the 1960s to illustrate this phenomenon. Prospective longitudinal studies offer the best methodology for examining a causal relationship between blood pressure and the incidence of dementia [98, 131]. This is potentially due to the long lag phase between the presence of hypertension and the onset of dementia in the longitudinal studies compared to the short-term cross-sectional studies, which miss this association [98, 131].
Be that as it may, hypertension’s impact on late-life cognitive outcomes appears the greatest when considered in middle age [131]. This was demonstrated in the Honolulu-Asia aging study (HAAS), a longitudinal study where 3735 participants were followed up from 1968 to 1970, 1971 to 1974 and 1991 to 1993. The study showed that in subjects who were never treated for hypertension, higher blood pressure was associated with a significantly increased risk of dementia, owing to VCI and AD [129]. Compared with normotensive individuals, patients with hypertension (Systolic blood pressure, SBP ≥ 160 mmHg) had a 4.8-fold higher risk of dementia and an odds ratios of 3.8 for diastolic blood pressure (DBP 90–94 mmHg and 4.3 for DBP ≥ 95 mmHg compared with DBP 80–89 mmHg) [124, 129]. Autopsy analyses of these patients revealed that those with SBP ≥ 160 mmHg had lower brain weights and greater numbers of Aβ plaques in both the neocortex and hippocampus, while those with DBP ≥ 95 mmHg had greater numbers of neurofibrillary tangles in the hippocampus [99, 130]. This demonstrated that Mid-life SBP is a significant predictor of reduced cognitive function in later life, and early control of SBP levels may reduce the risk for cognitive impairment in old age [129].
In the same breath, the Swedish Göteborg H-70 study, which started in 1971 [107], analyzed the relation between blood pressure and the development of dementia in those non-demented at age 70. The participants were followed up for 15 years at age intervals 70–75, 75–79, and 79–85 years. They demonstrated that participants who developed dementia at age 79–85 years had significantly higher SBP (mean 178 vs. 164 mmHg) and higher DBP (mean 101 vs. 92 mmHg) at age 70 than those who did not develop dementia [107, 124]. Since hypertension is known to induce white matter lesions, which are also common in dementias of old age [100, 132, 133], the study postulated that the lesions arise from hypertension, which causes hyalinization of the vessel walls [101], resulting in hypoperfusion and ischemia in the deep white matter supplied by long penetrating end-arteries [101]. The resulting demyelination leads to dementia through disconnection of subcortical-cortical association pathways [107].
Although these studies show the effect of high blood pressure on cognitive decline in elderly adults, there are several studies that suggest worse outcomes in older individuals with low BP [131]. For instance, a study that combined data from the Rotterdam study and the Swedish Göteborg H-70 study with a 2.1 years follow up [100] documented that hypertension in the elderly adults appeared protective but only among those who were taking antihypertensive medication. They showed an inverse association between BP and dementia, with a reduced relative risk for dementia of 0.93 (95%, CI 0.88–0.99) per 10 mmHg higher SBP and 0.89 (95%, CI 0.79–1.00) per 10 mmHg DBP [100, 128]. This study demonstrates that in these elderly adults, either higher blood pressure levels are needed to maintain an adequate cerebral perfusion, or the lower blood pressure are secondary to brain lesions in preclinical stages of dementia [100].
Cognitive domains targeted by hypertension
The majority of early studies assessed cognitive function in individuals with hypertension by using tests like the Mini-Mental Status Examination (MMSE), which measures global cognitive outcomes, or composite measures of several composite tests [131]. However, subsequent studies show that hypertension impairs cognitive function by targeting specific cognitive domains [131]. On this account, the specific cognitive domains that are negatively affected by hypertension include executive function [108], memory, and attention/speed of processing [109]. They are considered to be involved with subcortical diseases, such as typical vascular disease or pure vascular dementia [134], and have been used to prove that increased blood pressure is associated with cognitive impairment.
For instance, the Tromso study [135] used the more specific Digital Symbol Substitution Test, which tests for speed of processing. This study demonstrated that in adult men (45–55 years), higher SBP and DBP was associated with poorer cognition, while at older ages (65 years), they had better cognition. The women, however, showed a weaker and opposite correlation than the men whereby a higher SBP was associated with better cognition at a younger age and higher SBP poorer cognition at older ages [135].
Another 10-year follow-up study used the Digit Symbol Substitution Test and MMSE to investigate the association between midlife SBP and late-life cognitive decline in elderly men (68–79 years) [136]. Participants with high SBP in midlife experienced a greater decline in cognitive performance and had larger white matter hyperintensity (WMH) volumes than those with low SBP in midlife [136]. Overall, the declining speed of processing and executive function are the most common cognitive changes associated with hypertension [131]. Furthermore, the pattern of cognitive impairment associated with hypertension is often distinguished from the pattern associated with neurodegenerative dementias such as AD by the lack of consistent findings for an impact of hypertension on memory function, a defining characteristic of clinically diagnosed AD [131].
Association between hypertension and cognitive decline
Hypertension-related cognitive decline is a consequence of the interplay between the reorganization of functional blood flow and vascular damage in the brain [137]. In both humans and animal models, hypertension causes pathological alterations to the cerebral microvessels (small vessel disease) through endothelial damage [138], phenotypic changes of the vascular smooth muscle cells, fibrinoid necrosis, pericyte injury, pathological remodeling of the extracellular matrix and activation of matrix metalloproteinases [139], enlargement of perivascular spaces, perivascular edema [140], and inflammation [138].
As a result of these disturbances in blood flow, there is microvascular rupture, rarefaction, impaired vasodilation and BBB dysfunction, which results in brain ischemia and neuroinflammation [124]. Subsequently, the damage is observed as lesions affecting both the gray and white matter, which manifest as complete and incomplete microinfarcts, microhemorrhages and white matter hyperintensities (WMHs) [124, 137]. These detrimental effects are important determinants of cognitive impairment [128]. For instance, microinfarcts and infarcts are strategically placed in the brain regions involved in cognition including the hippocampus, medial thalamus, and frontal lobe [39, 141] where they cause cognitive dysfunction. In addition, microinfarcts induce lasting functional impairments that extend well beyond their core [142]. White matter lesions affect cognitive function by impairing the connectivity between the anterior thalamus to the frontal cortex [39]. These correlate with a reduction in processing speed, which is a typical feature of cognitive impairment [143].
Direct link between hypertension and AD
Although the impact of hypertension on cognitive function is well-established, a few studies have shown that hypertension-induced lesions and AD may have an additive or synergistic effect and produce a more severe cognitive impairment than either process alone [144].
Hypertension promotes atherosclerosis in both the extracranial and intracranial arteries feeding the brain [145] whereby it leads to the thickening of vessel walls, reduced vessel elasticity, and the narrowing of the lumen in small vessels [146]. This reduces cerebral blood flow, which is a prominent step in the pathophysiology of both AD and CVD. In addition, arterial stenosis causes hypoperfusion, which activates β-secretase activity and increases Aβ peptides production [147] thus promoting atherosclerosis by inducing inflammation, endothelial dysfunction, and oxidative stress [148]. In addition, more atherosclerosis in cerebral vessels has been reported in the brains of AD and hypertensive patients due to increased amyloid plaques and neurofibrillary tangles [149, 150]. Further evidence shows that hypertension and aging exacerbate the progression of AD through oxidative microvascular damage and brain inflammation [151, 152] whereby they promote the activation of NADPH oxidase in the cerebral vasculature and in turn promote AD pathogenesis [153, 154]. Furthermore, an interaction between vascular and genetic factors has been shown whereby hypertension interacts with Apoε4 to promote amyloid deposition in healthy individuals, increasing the susceptibility to AD [150].
Most studies use animal models that mimic the early onset genetic AD, but these constitute only a small fraction of the majority of AD cases and beg the question whether they represent what happens in the natural evolution of the human AD pathology [155]. To this end, a study developed an animal model of hypertension-related “Alzheimer-like” pathology to determine the influence of hypertension on late-onset AD. They showed that hypertension itself triggers neuroinflammation before Aβ peptides deposition, suggesting that stimulating inflammation in the appropriate time window may represent a promising strategy to limit vascular-triggered AD pathology [155].
Furthermore, hypertension induced by angiotensin II administration showed increased β-secretase activity and the rate of cleavage of the APP protein in mice [156], while the genetic deletion of angiotensin 1 receptor (AT1R) or administration of the renin-angiotensin system blockers ameliorates amyloid deposition and behavioral dysfunction in APP-overexpressing mice [157, 158]. Another study induced hypertension in the Tg2576 mouse model of AD to determine if hypertension exacerbates the progression of cognitive decline in AD patients by promoting the development of cerebral microhemorrhages (CMHs) [102]. The majority of AD patients have cerebral amyloid angiopathy (CAA) which is a common age-related cerebrovascular pathology caused by the deposition of Aβ peptides in the cerebral arteries, arterioles, and capillaries [102]. This makes them fragile and vulnerable to pressure-induced rupture both in humans and mice [103, 104]. Consequently, this study showed that amyloid pathologies exacerbate the effects of hypertension, thus promoting the development of CMHs, which contribute to their deleterious effects on cognitive function [102]. Therefore, therapeutic strategies that prevent CMHs, reduce blood pressure and preserve microvascular integrity can exert neuroprotective effects in high-risk elderly AD patients [102].
Several studies have shown that anti-hypertensive drugs can delay and possibly prevent the pathogenesis of AD [159–161]. Clinical trials and experimental studies suggest that antihypertensive medications, including ACE inhibitors, AT1R blockers, and diuretics may improve AD biomarkers such as Aβ neuropathology, cerebral blood flow, and inflammatory markers, and reduce the incidence of AD [162]. Although there are previous controlled clinical trials that failed to show the outcome benefits of antihypertensive drugs on cognitive performance in AD patients [59], it has been shown that methods that effectively lower blood pressure in midlife can retain or improve cognitive function by reducing the risk of AD and/or CVD [35, 163]. In addition, there are various protective factors that have been demonstrated to have positive effects on cognition and may play a role in AD prevention. Among them, physical activity [164], having a higher education/occupation, and greater engagement in cognitive activities have been shown to provide a higher reserve against AD [165].
Association between AD and diabetes
The link between diabetes and AD goes beyond the epidemiological association whereby the brains of AD patients show evidence of reduced expression of insulin and neuronal insulin receptors [110]. This leads to insulin resistance and the breakdown of the entire insulin signaling pathway, brain metabolism and cognitive function, making it one of the best documented abnormalities in AD [111]. AD is therefore described as a neuroendocrine disorder resembling type 2 diabetes mellitus (T2DM) and some studies have coined the term “type 3 diabetes” to account for the abnormalities that are specifically associated with the concurrent AD-type neurodegeneration and diabetes [166]. Insulin resistance reduces the ability of cells to take up glucose resulting in hyperglycemia [97] which can increase neuronal susceptibility to stress-induced effects [167]. This leads to accumulation of advanced glycation end products (AGEs) [112] hence excessive production of ROS [113, 114], which stimulate downstream pathways related to APP processing and markedly accelerate Aβ peptides production and aggregation [115, 116]. Further, insulin can independently contribute to peripheral inflammatory responses thus promoting AD pathogenesis [168]. For instance, proinflammatory cytokines (IL-6, TNF-α) are shown to correlate with decreased levels of the insulin degrading enzyme (IDE) which is responsible for the degradation of intracellular insulin [117], and proinflammatory cytokines are also able to cross the BBB and induce central nervous system inflammation [118]. Neuronal insulin homeostasis is an important factor in amyloid-related neurodegeneration [169, 170], since it mediates the regulation of Aβ metabolism [171]. Insulin and Aβ are both degraded by IDE [172]. Since insulin regulates IDE expression through the insulin-PI3K-Akt mechanism in the brain [119], failure of this mechanism leads to down-regulation of IDE [120]. However, since it has higher affinity for insulin than for Aβ, the increased amounts of insulin deprives Aβ of its principal degradation mechanism leading to its accumulation [121]. Finally, as with other CVD risk factors, treatment for diabetes has been shown to alter the risk of AD, and several drugs have been studied in clinical trials for this purpose including metformin [173], pioglitazone [174], and the use of intranasal insulin to enhance memory performance in AD patients [175].
There are many other lifestyle, environmental, and behavioral risk factors of CVDs including obesity, dyslipidemia, and metabolic syndrome [176, 177] that have been shown to increase inflammation and the risk for AD [177]. However, they have been discussed in detail in other review articles [4, 35, 59, 97], hence this review will not delve into their mechanisms.
Aβ peptide generation during AD pathology
As described above, Aβ peptides accumulation and subsequent formation of senile plaques as well as the intracellular hyperphosphorylation of tau proteins into neurofibrillary tangles are signatures of AD [8, 9]. Aβ peptides are byproducts of the metabolism of the parental APP, which is a larger precursor molecule widely produced by brain neurons, vascular and blood cells (including platelets), and, to a lesser extent, astrocytes [178]. The APP can be processed through two enzymatic pathways; the non-amyloidogenic pathway which depends on its location on the plasma membrane, the site of its processing (membrane or endosome) and the environmental pH [179], and the amyloidogenic pathway [180] (Fig. 1).
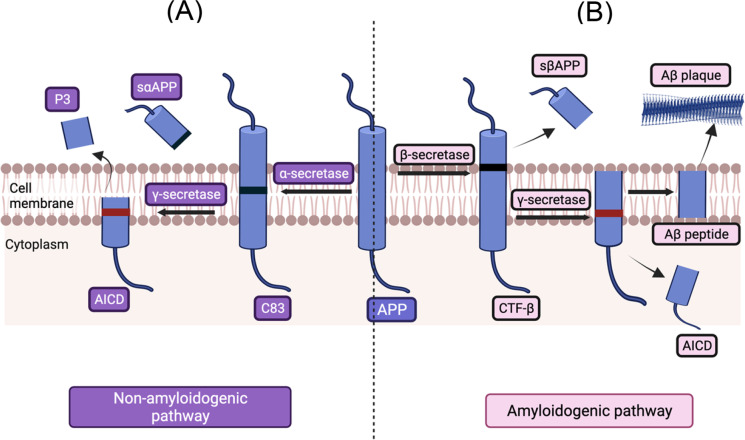
Fig. 1.
The amyloid precursor protein (APP) is processed through the (A) non-amyloidogenic enzymatic pathway; α-secretase cleaves the APP producing a large soluble fragment (sαAPP), and the C-terminal fragment of APP that remains anchored to the membrane is proteolyzed by the γ-secretase enzymatic pathway, releasing the APP intracellular domain into the cell. In the (B) amyloidogenic enzymatic pathway, β-secretase cleaves the APP producing sAPPβ and a carboxyterminal fragment β (CTFβ) that remains attached to the membrane and is proteolyzed by the γ-secretase to produce the Aβ peptide. Image created with BioRender.com
In the non-amyloidogenic pathway, the α-secretase cleaves the APP within the ectodomain, which corresponds to the Aβ fragment, thus producing bigger soluble fragments and avoiding the formation of the Aβ peptides [180]. Subsequently, this process releases the secreted/soluble APP (sαAPP), which possesses different neurotrophic and neuroprotective properties. In addition, the C-terminal fragment of APP that remains anchored to the membrane is once again proteolyzed by the γ-secretase producing fragments which have low-potency cellular toxic properties. Simultaneously, the APP intracellular domain (AICD), which has neuroprotective properties is released inside the cell [180, 181]. In the amyloidogenic pathway, the APP is first proteolyzed by the β-secretase, or BACE1, which generates a soluble fragment from the N-terminal domain called sAPPβ and a carboxyterminal fragment β (CTFβ) that remains attached to the membrane and is proteolyzed by the γ-secretase to produce the Aβ peptide [182]. The cleavage site by γ-secretase depends on the location of processing (endosomes or Golgi network) and generates either Aβ1-40, which is mostly found in vascular lesions, and Aβ1-42, which is mainly found in AD-associated brain lesions [22]. Although their processing is a normal physiological process, genetic studies of AD have shown that mutations in the APP gene results in the excessive production of Aβ peptides [183], which initially accumulate in the cerebral regions with neuronal populations at high metabolic bio-energetic activity rates, spread to brainstem, and eventually reach the cerebellum [184]. The imbalance between neuronal production of Aβ peptides and their extracellular clearance enhances their accumulation extracellularly or intracellularly [185]. It represents the upstream event of Aβ peptide dyshomeostasis associated with protein misfolding, aggregation, and incipient extracellular accumulation in plaques [186, 187]. Further, Aβ peptides can be detected in plasma, in cerebrospinal fluid (CSF), and in cell culture media [188, 189]. They impart their toxicity through several mechanisms including oxidative stress through generation of free radicals; membrane permeabilization through pore formation; excitotoxicity through interaction with some neurotransmitter receptors; mitochondrial dysfunction and alteration of cell signaling pathways [11, 181, 185].
Genetic linkage analysis followed by positional cloning in humans identified two major forms of AD [190]. The first is the autosomal dominant type also called the familial AD (FAD) that is commonly linked with an early onset AD (EOAD) pathology (< 65 years). It is estimated that pathogenic mutations in the genes encoding APP [191] and the γ-secretase-complex components, PSEN1 and PSEN2 [192, 193], are responsible for up to 71% of early-onset AD cases, but can only explain 1% of all AD cases [181]. APP mutations can affect the total amount of Aβ peptides produced; if they occur at the β-secretase cleavage site, they increase Aβ peptides production, whereas if they occur at the γ-secretase cleavage site, they influence the relative amount of Aβ1-42:Aβ1-40 peptides ratio [194, 195] and/or the amyloidogenic potential of the Aβ peptides [196]. Mutations in PSEN1 or PSEN2 that form the active core of γ-secretase complex affect the catalytic activity of γ-secretase (endopeptidase- or carboxy-peptidase-like) activity. They shift the production of Aβ1-42 and Aβ1-40 to a longer and more neurotoxic species (Aβ1-43), and a genetically driven loss of function of γ-secretase [197] shows a dramatic reduction in Aβ peptides production [198]. Therefore, the toxic dysfunction mechanism is used to describe AD-related genetic changes in γ-secretase [196, 198]. Despite this, all mutations associated with familial type of AD (APP, PSEN1, and PSEN2) in one way or another increase Aβ peptides production or modify its production rate [181, 182]. The other form of AD is the sporadic form, characterized by a late onset (80–90 years of age), also called Late-Onset AD (LOAD) [189]. At present, no causal (autosomal dominant or recessive) genetic mutations are known in association with LOAD [199]. However, it has been hypothesized to be a multifactorial disease with more than 50 susceptibility genes/loci associated with its risk [199] and linked to Aβ peptides homeostasis through its expression, trafficking, and degradation [200]. In addition, several genes related to LOAD play a role in the regulation of inflammatory and immune response pathways, endocytosis and cellular trafficking, cholesterol transport and lipid metabolism, and post-translational modification including ubiquitination, which is a crucial mechanism of cellular protein clearance [199]. LOAD therefore presents with the failure of the mechanisms of quality control [24] to clear the Aβ peptides from the interstices of the brain [201] representing the key event in Aβ aggregation [24]. Individuals homozygous for the apolipoprotein E, specifically the APOe4 allele have an increased risk of developing the sporadic form of AD compared to those who do not carry the Apo e4 allele [181, 196]. Clinical and neuropathologic studies show a significant association between ApoE genotype and Aβ metabolism and homeostasis [202]. ApoE e4 is correlated with increased intraneuronal accumulation of misfolded Aβ peptides, formation of neurotoxic Aβ species, and plaque accumulation in brain tissues from AD patients [203, 204]. The effect of APOe4 on Aβ metabolism, accumulation and aggregation appears to be most pronounced during the initial phase of Aβ dyshomeostasis [205], and increasing age exacerbates this effect. This indicates a potential synergistic interaction between ApoE and aging-related metabolic changes [206]. A study showed that female carriers of the Apoe4 allele had significantly higher levels of Aβ peptides compared to the males [202], suggesting that changes in expression of the key secretases implicated in APP cleavage and the regions with an inferred influence by the e4 allele display distinct patterns of expression between sexes [202]. ApoE can physically interact with Aβ peptides, affect their physical/conformational properties, and enhance plaque formation [207]. For instance, in a mouse model of aging, ApoE e4 facilitates the formation of Aβ fibrils by accelerating the initial seeding or nucleation of Aβ peptide deposition thus increasing the brain Aβ peptides half-life [208]. Since the major receptors of ApoE are low-density lipoproteins (LDL) receptors (LDLRs) and LDL receptor-related protein 1 (LRP1) [209], it affects cellular uptake and the efficiency of Aβ peptides clearance through the BBB [210]. Through its association with circulating levels of cholesterol as well as atherosclerosis, it can also affect the risk of AD indirectly through the vascular component [189].
Formation of soluble Aβ aggregates and their mechanisms of toxicity
After being generated as soluble monomers, Aβ peptides form different intermediate aggregation states including dimers and trimers, soluble oligomers, protofibrils, and eventually fibrils that accumulate in plaques, the neuropathological hallmark of AD [187]. It is the longer, more hydrophobic Aβ1–42 and not the physiological Aβ1–40 [211] that is more likely to form toxic, soluble protein oligomeric intermediates (SPOs) before progressing to the insoluble plaque in AD brains [212]. The term “soluble” describes any form of Aβ assemblies that are soluble in aqueous buffer [11] and are not pelleted from physiological fluids by high-speed centrifugation [11, 213].
Understanding the process for SPO aggregation is exceedingly complex and involves a dynamic equilibrium including a diversity of sizes and conformations of aggregated Aβ peptides. The formation of these oligomeric aggregates, some of which are nuclei, is the rate-limiting step in the aggregation process. This rate limitation is evidenced by the presence of a lag during the aggregation process as well as the cessation of this lag upon the introduction of preformed seeding nuclei [214]. Additionally, the nucleation-limited nature of the aggregation process indicates that the formation of a certain SPO species may be the necessary event triggering exponential growth of aggregates. For these reasons, recent work toward developing AD therapeutics has focused on identifying molecules that can inhibit the formation of Aβ oligomers [215]. This endeavor necessitates an understanding of the atomic structure of SPOs as well as the physics of their formation both in general and in the presence of inhibitors.
Current understanding of SPOs stems primarily from experimental techniques, molecular dynamics and Monte Carlo simulations, and the molecular theory [216]. These studies aim to clarify oligomer structures and kinetic pathways of SPO formation. Collectively, these works report a multitude of prospective structural motifs for each mass of oligomer species and numerous potential kinetic schemes for oligomer formation. However, despite this increased interest in oligomers, the exact structures and physics of the aggregation process of these species have remained ambiguous [194]. Moreover, information regarding the relative propensity of various SPO species to form and their importance in the aggregation process is also still unclear and requires further study.
The toxic potential of SPOs is shown when low-number oligomers (small, low-n Aβ oligomers) of naturally secreted trimeric Aβ from human cells injected into wild-type mice hippocampus hinder modulation of synaptic plasticity by inhibiting long-term hippocampal potentiation (LTP) and enhancing long-term depression (LTD) leading to synaptic loss [217, 218]. This inhibition occurs at picomolar to nanomolar Aβ peptide concentrations in cell-derived Aβ oligomers, which is similar to the concentration found in human CSF [218, 219]. This differs from the synthetic Aβ peptides which have a single defined length and are applied to neurons at 2–4 orders of magnitude higher concentrations to achieve similar biological effects [218]. This shows that pure synthetic Aβ peptide may not closely mimic the natural aggregation states of the heterogenous Aβ peptides in vivo compared to the cell derived Aβ which contains multiple forms of various Aβ species [220]. Furthermore, it is the smaller and readily diffusible Aβ oligomers [221] and larger protofibril aggregates [222] that impart neurotoxicity but not the monomers and the highly insoluble amyloid plaque cores [221, 223]. Upon formation of SPOs, they are released extracellularly and impart their toxicity through different mechanisms [11]. They bind to different receptors including N-methyl-d-aspartate NMDA-type glutamate receptor (NMDAR) which causes Ca2+ dyshomeostasis, and synaptic loss [224]; the insulin receptor leading to its loss and impairment of LTP-associated kinase function [225]; the frizzled receptor (Fz) which results in tau phosphorylation and formation of neurofibrillary tangles[226]; the N-formyl peptide receptor-like 1 (FPRL1) [227–229] leading to the clearance of amyloid-β peptides and the activation of inflammation, NADPH oxidase, and superoxide radical production [230]; and the nerve growth factor (NGF) receptor which activates the apoptotic signals [231]. Further, they can form membrane pores which allow abnormal flow of Ca2+ ions hence cellular dysfunction [232], and they can also enter the cytosolic compartment and inhibit the proteasome [233]. SPOs have also been shown to accumulate intracellularly [234] since APP is localized in the membranes of the ER, Golgi network, endosomes, lysosomes, and mitochondria [235]. Aβ peptides are also produced in the secretory pathway of the ER and the trans-Golgi network and bind to the ER binding protein (ERAB) [236]. In addition, extracellular Aβ peptides can be internalized and accumulated by cells into their intracellular pools through various receptors including α7 nicotinic acetylcholine receptor (α7nAChR) which binds Aβ1-42 with high affinity [237, 238], low-density lipoprotein receptor, FPRL1, and the scavenger receptor for advanced glycation end-products (RAGE) [11, 234].
The ER and protein quality control
The ER lumen constitutes a specialized environment for protein folding [239]. When misfolded proteins accumulate in the cytosol, they expose their buried hydrophobic amino acid residues, which are recognized by different quality control chaperone systems that either repair or degrade them [240]. The repair process can be futile in cases where the proteins are irreversibly damaged, in which case they are directed toward rapid degradation. However, the accumulation of misfolded proteins can overwhelm the cellular quality control system resulting in protein aggregates that are potentially toxic as is the case in AD. They inhibit the 26S proteasome activity [241] and instead get degraded by UPR activated-autophagy, which is a controlled self-degradation process that can promote cell survival by eliminating damaged cellular components [242]. The ER lacks the UPS, hence luminal and membrane proteins are retro translocated to the cytosol in an ATP-dependent process to the 26S proteasome. As the protein aggregates keep accumulating, they overwhelm the cells resulting in ER stress [241] which in turn activates the unfolded protein response (UPR) in a bid to reduce protein synthesis and increase their degradation. The UPR achieves this through activating the transcription of genes encoding chaperones, folding enzymes, and other proteins involved in ER-associated degradation (ERAD); attenuating translation processes hence eliminating the synthesis of new proteins into the ER; and activating the apoptotic pathways if ER homeostasis cannot be restored [243]. The ER stress sensors IRE1, PERK, and ATF6 normally remain bound to BiP/Grp78, preventing their activation. However, during ER stress, the unfolded proteins sequester BiP/Grp78 from the membrane, a process that initiates signals within the ER to reduce global protein synthesis, promote protein folding, and increase the degradation of misfolded proteins [244]. However, failure of this response to restore homeostasis can result in oxidative stress and eventually apoptotic cell death [18].
ER stress, Ca2+ homeostasis, and the unfolded protein response
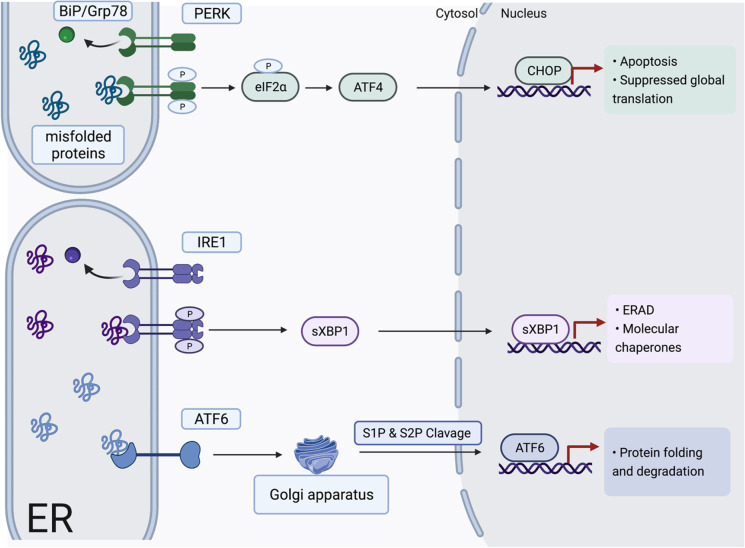
During the UPR, BiP/Grp78 dissociates from the three ER transmembrane protein sensors and promotes their activation via oligomerization. The first sensor is PERK, which upon activation through oligomerization and trans-phosphorylation [245] phosphorylates the α-subunit of eukaryotic translation initiation factor 2 (eIF2α), which in turn reduces the ER folding load by suppressing global translation [246]. It also increases the translation of select messenger ribonucleic acids (mRNAs) including activating transcription factor-4 (ATF4) which targets CCAAT/enhancer-binding protein homologous protein (CHOP), the master regulator of UPR-mediated apoptosis [247]. Deletion of the CHOP gene protects against cell death induced by pharmacological ER stressors, mechanical stretching [248] and pressure overload [249]. The second sensor is IRE1 which is activated through dimerization and transphosphorylation [245], resulting in endoribonuclease activity that splices the mRNA encoding the transcription factor X-box binding protein (Xbp1). Translation of the spliced Xbp1 transcript produces a transcription factor that induces the expression of genes encoding molecular chaperones, protein folding enzymes, and components of ERAD [250]. The third UPR effector, ATF6, acts as a transcription factor, which upon activation is cleaved by site-1 and site-2 proteases (S1P and S2P) in the Golgi apparatus. It then translocates to the nucleus and upregulates the expression of genes involved in protein folding and degradation [251].
After prolonged and severe activation of the UPR, the loss of cellular nutrients and energy leads to the loss of ER homeostasis, and Ca2+ signaling plays a role in recognizing the disrupted reticular homeostasis [252]. The ER senses and integrates many of its incoming signals, particularly changes in free and bound Ca2+ concentrations inside and outside of the ER compartment and through its membrane, it modulates its own luminal Ca2+ dynamics, and generates appropriate signals to maintain balanced homeostasis [252]. In addition to the ER membrane, the Ca2+-binding protein BiP/Grp78 is important in sensing the accumulation of mis-folded proteins in the ER [253]. Furthermore, depletion of the ER Ca2+ stores activates store-operated calcium entry (SOCE) which affects the availability of cytoplasmic Ca2+ for intercellular signaling, leading to a rapid accumulation of misfolded proteins [254]. Other ER luminal Ca2+ buffers also play a role in regulating the UPR. Calreticulin associates with ATF6 and, together with BiP, maintains ATF6 in an inactive state [255]. Upon ER stress, both BiP and calreticulin dissociate from ATF6, promoting its trafficking to the Golgi apparatus where it is proteolytically processed [255]. Overall, these premises show the disruption of Ca2+ homeostasis in the ER leads to activation of ER stress coping responses including the UPR and its importance in providing the appropriate coping responses through integrated signaling mechanisms in response to cellular stresses [252].
Although the canonical UPR pathway uses several short-term mechanisms to improve ER function, there is also longer-lasting enhancement of the ER folding environment through gene regulation [256]. These processes involve some non-canonical mechanisms emanating from the UPR signaling pathway which regulate genes involved in other cellular processes like metabolism, inflammation [257], transcription, and mRNA expression. However, these pathways go beyond the scope of this review and have been discussed at length in other reviews [256]. Moreover, there is an integrated stress response (ISR) that occurs with intracellular accumulation of misfolded proteins in the ER. At the core of the stress stimuli that activates the ISR is the phosphorylation of the alpha subunit of eIF2α on serine 51 by PERK and other kinases [258]. This phosphorylation causes a reduction in global protein synthesis and allows for cell survival and recovery by activating the translation of ISR-specific mRNAs, such as ATF4 [259]. Once ER stress has been restored, the ISR is terminated through the dephosphorylation of eIF2α by growth arrest and DNA damage-inducible protein (GADD34) phosphatase, thus restoring protein synthesis and normal cell functioning [259]. However, when cellular homeostasis cannot be restored, eIF2α can allow the synthesis of death-inducing proteins, as well as those that accumulate in the cell and aggravate the proteotoxicity and oxidative stress [260] (Fig. 2).
Fig. 2.
Accumulation of misfolded proteins sequester Bip/Grp78 from the ER membrane during ER stress leading to the activation of the unfolded protein response; ER stress sensor PERK is activated, and it phosphorylates eIF2α and ATF4, which targets CHOP resulting in apoptosis. IRE1 activation leads to splicing of Xbp1 whose translation activates components of ERAD. ATF6 activation leads to its cleavage in the Golgi apparatus by S1P and S2P and its translocation into the nucleus to upregulate the expression of proteins involved in protein folding and degradation. Image created with BioRender.com
ER stress in AD and CVD
ER stress in AD
The diseases that are caused by misfolded proteins are called “conformational or folding diseases” [261]. These include AD, Parkinson’s disease [262] and Huntington’s disease [263], and CVDs [261, 264]. In AD, an accumulation of Aβ peptides results in chronic activation of ER stress [265] with the proapoptotic phase of ER stress likely to predominate and contribute to neurodegeneration [266]. Further, in AD patients, the ER stress response is activated [265], and proteins involved in ER stress-induced apoptosis are upregulated [267, 268]. This shows a high possibility that ER stress invoked by the accumulation of Aβ peptides is one of the key mechanisms involved in the onset of AD pathology [261]. To elaborate this further, the relationship between the accumulation of Aβ peptides and ER stress has been explored through many experimental models based on the culture of neuronal cells, cell lines, and brain slices [269, 270] to elucidate the connection between extracellular Aβ peptides accumulation and their intracellular ER effects.
The Ca2+ hypothesis of AD is the most widely suggested mechanism which attempts to explain how amyloid formation and accumulation might account for both the progressive decline in memory and increase in neuronal cell apoptosis [271]. It shows that the release and formation of Aβ1-42 peptides may enhance intracellular Ca2+ entry by binding to the cellular prion protein (PrPC), which functions as an Aβ peptides receptor [272]. Aβ1-42 peptides also function as channels or activate channels in the plasma membrane [273]. This leads to the altered expression of components such as the inositol 1,4,5-trisphosphate (IP3)-receptor (IP3R) [274], ryanodine receptor (RyR), the APP intracellular domain [275, 276], and the buffer calbindin which functions in learning and memory formation [277]. Eventually, this remodeling results in the upregulation of Ca2+ signaling and the disruption of synaptic mechanisms responsible for learning and memory and stimulates the mitochondria to release cytochrome C, further triggering ER stress, increased ROS production, and resulting in cell death by activation of caspases [273].
Recent evidence purports the increased regulation of gene expression of ATF4 in the cortex of AD brains, whereby its protein levels are 1.9 times higher than in age-matched controls [278]. In the APP/PS1 mice, which have synaptic and memory deficits due to Aβ overload, ATF4 is increased in the hippocampus [278]. It binds the regulatory region of PS1 gene which stimulates γ-secretase activation, thus promoting Aβ production [279]. Furthermore, it acts as an inhibitor of synaptic plasticity and long-term memory, showing that in AD, ATF4 not only acts as a downstream effector of Aβ peptides, but also as an upstream initiator for memory decline, and targeting it particularly through the eIF2α kinases can form a basis for novel therapeutic interventions [280].
The neuronal ER is a very specialized organ with different functional sub-compartments because its tubules and cisternae extend from the nuclear envelope into dendrites and dendritic spines and also along axons as far as presynaptic terminals. For this reason, ER stress in neurons plays a crucial role in the pathogenesis of AD [281]. Further evidence is shown in a study of brains from AD patients that describes the changes in neuronal UPR [282]. They observed that in AD, pPERK, peIF2α, and pIRE1α were increased in the hippocampal neurons associated with granulovacuolar degeneration bodies (GVD) and pPERK was particularly abundant in the hippocampus and subiculum regions. GVD are large cytoplasmic vacuoles that are reminiscent of the autophagic vacuoles commonly observed in AD [283]. pPERK-positive neurons were also abundant in neurons that stained for phosphorylated tau protein and also had abundant staining for a tau kinase called glycogen synthase kinase-3β (GSK-3β), which shows it can enhance neurofibrillary tangle formation [284]. Another study in AD patients [285] also observed that activation of PERK, eIF2α, and p38 MAPK correlates with abnormal tau levels. These studies demonstrate the role that prolonged neuron-specific activation of the UPR plays in both tau phosphorylation and neurodegeneration in AD pathogenesis [281].
There is emerging evidence that several pathways associated with the UPR can trigger inflammatory responses and apoptotic cell death in AD pathology [281]. The innate immune response of the inflammasome and the cytokines and chemokines released in AD has been discussed above (evidence of the association between AD and CVD). Notwithstanding, ER stress induces several pathways which can activate NF-κB signaling, hence eliciting a myriad of immune responses [281]. In AD, NF-κB regulation of BACE1 transcription may be altered due to chronic stress, and the functional NF-κB site in the BACE1 promoter is stimulatory in activated astrocytic and Aβ-exposed neuronal cells, and repressive in neuronal and non-activated astrocytic cells [286]. This inefficient transcriptional regulation of BACE1 by NF-κB accounts for increased BACE1 transcription and subsequent amyloidogenic APP processing in a cell type-specific manner [286]. In addition, the NF-κB signaling pathway has been shown to be one of the major neuroprotective pathways in AD [287] whereby neuronal cells treated with low concentrations of non-toxic Aβ peptide induce NF-κB activation [288], which leads to neuroprotection against subsequent treatment with highly toxic Aβ peptide concentrations. This explains the increased BACE1 expression and neurodegeneration due to the reduced NF-κB immunoreactivity around mature amyloid plaque stages in AD [289].
Inflammatory responses and apoptosis associated with ER stress can also be triggered by ER-resident inflammatory caspases, including caspase-4 in humans and caspase-12 [281]. In the brains of AD mice, caspase-12 levels are strongly upregulated, and the enzyme colocalizes with Hip-2 protein, which modulates its activity through the ubiquitin/proteasome system [290]. Cells exposed to Aβ peptides increase the expression of Hip-2, which not only stabilizes caspase-12 but also induces its proteolytic activation showing that Hip-2 is an essential upstream regulator of the expression and activation of caspase-12 in ER stress-mediated Aβ neurotoxicity and cell death [290]. In postmortem samples from AD patients, increasing levels of caspase-4 were observed with progressive cognitive decline, and its increased expression is associated with neuritic plaque changes in AD [291]. Furthermore, there seems to be negative feedback for caspase-4 activation in AD whereby neurons prevent the prolonged Aβ-induced activation of caspase-4 and subsequent inflammatory and pathological responses in AD [281].
Be that as it may, there are some therapeutic strategies proposed to prevent neuronal degeneration. The ER-UPR could be a suitable target, whereby manipulating ER-associated quality control mechanisms or the inhibition of the apoptotic pathways associated with the UPR can be beneficial to treat or prevent AD [292]. Alternatively, the selective activation of PERK pathway is an early event of Aβ peptides to induce ER stress. Therefore, inhibiting the PERK-eIF2α pathway promotes the induction of ER chaperones and in turn confers resistance to aggregated protein toxicity in neuronal cells [293]. Therefore, the selection of compounds that act in the multiple branches of the UPR could represent a good strategy to prevent the abnormal processing of APP as well as the deleterious downstream events that characterize AD pathology [293, 294].
ER stress in hypertension
One of the links that have been shown to exist between ER stress and hypertension is their shared cellular perturbations including oxidative stress and alterations in intracellular Ca2+ [295]. Ang II is one of the major circulating factors that signal the central effector systems to restore cardiovascular balance. Despite many regions of the brain being sensitive to Ang II, there are some unique BBB-deficient regions, called circumventricular organs (CVOs) that are primary sensors for this blood-borne signal [295]. Specifically, one study focused on the circumventricular subfornical organ (SFO) [296], which is crucial in Ang II-dependent hypertension and is influenced by alterations in Ca2+ and redox signaling which are pivotal ER modulators [295]. This study demonstrated that ER stress in the brain is functionally linked to elevations in arterial pressure and renal sympathetic nerve activity. Further, an increase in circulating Ang II induces ER stress in the SFO, indicating the causative role it plays in the pathogenesis of hypertension [296].
Another study showed that in aorta from normotensive rats, induction of ER stress elevates their blood pressure and activates fibrosis and collagen deposition. It also activates the apoptotic cellular signaling pathways thus contributing to aortic stiffening and vascular dysfunction. However, inhibition of the ER stress in Ang II rat models of hypertension attenuates this effect [297], showing that the identification of a signaling pathway linked to aortic apoptosis and fibrosis can be a potential therapeutic target to resolve CVDs [298]. In addition, ER stress contributes to hypertension in spontaneously hypertensive rats, and treatment with the molecular chaperone 4-phenylbutyric acid (4-PBA) decreases the blood pressure in these rats, and also improves their nitric oxide-dependent resistance vessel vasodilation [18].
Further evidence emanates from a study that identified ATF4 as a hypertension-specific biomarker and a target gene for miR-1283, which regulates the PERK-eIF2α-ATF4 signaling pathway by downregulating ATF4 mRNA [299]. The regulatory relationship between miR-1283 and ATF4 is shown in human aortic endothelial cells (HAECs) where miR-1283 overexpression downregulates ATF4 and miR-1283 inhibition upregulates ATF4 [299]. In addition, miR-1283 is associated with essential hypertension and gestational hypertension [300, 301]. Wild-type mice on a HSD became hypertensive and had elevated levels of vasoactive substances and clotting factors that cause vascular injury and functional disturbances. However, knockout of the mouse miR-1283 target gene ATF4 stabilized the blood pressure and elevated NO levels, which is a protective factor in cells [302]. Furthermore, in the knockout HSD, the pro-apoptotic genes and proteins of ATF4, CHOP, BID, BIM, and caspase-3 were downregulated, while the anti-apoptotic genes and proteins of BCL-X and BCL-2 were upregulated. This study elaborates the contribution of the miR-1283/ATF4 axis to ER stress and apoptosis by regulating the ATF4/CHOP signaling pathway in the development of hypertension, and poses as a potential target for the prevention and treatment of hypertension [302].
Furthermore, Nox, a family of enzymes that generate ROS [303], is increased during hypertension, leading to oxidative stress, alterations in vascular structure, and eventual vascular dysfunction. The imbalance caused by ROS resulting in vascular dysfunction converges with ER stress through the activation of NF-κB and transforming growth factor beta 1 (TGFβ-1), and both processes contribute to hypertension development [17, 304].
ER stress in diabetes
ER stress may also contribute to diabetes. In a population of Pima Indians, mutations in ATF6 correlate with increased susceptibility to T2DM [305], since its reduced efficacy decreases the induction of ER chaperones and protein disulfide isomerases, eventually impairing insulin folding [266]. In addition, mutation of PERK increases pancreatic β cell apoptosis leading to type 1 diabetes as is observed in Wolcott–Rallison syndrome [306]. Oxidative stress also acts upstream or downstream of the ER to induce ER stress, which results in pancreatic β cell dysfunction and eventually insulin resistance and diabetes [17].
Conclusion and future perspectives
Although there are many epidemiological studies that demonstrate the association of neurodegenerative diseases and CVDs, not much has been shown on how the toxic effects of circulating SPOs impact peripheral vascular damage directly. Further studies are therefore necessary to build on the existing knowledge and bridge the gap in understanding the complex mechanisms involved during AD progression and the effects on peripheral cardiovascular diseases. This will further inform the development of novel therapeutic approaches to control the overall vascular function.
Funding
This work was supported by the National Institutes of Health (R00GM118885 and R01HL149762 to CFW; DK132948, P01HL134604 to RCW; R00HL151889 and P20GM103641-Pilot Project to CGM).
Declaration
Conflict of interest
None.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Report AA. Report 2021. Alzheimer’s Dement. 2021;17(3):327–406. doi: 10.1002/alz.12328. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimer’s Association. Alzheimer ’ s Disease Facts and Figures On the Front Lines : Primary Care Physicians and Alzheimer’s care in America;2020. [Online]. Available: https://www.alz.org/news/2020/primary-care-physicians-on-the-front-lines-of-diag. Accessed 1 Dec 2020.
- 3.Xu J, Murphy SL, Kockanek KD, Arias E. Mortality in the United States, 2018. NCHS Data Brief. 2020;355:1–8. [PubMed] [Google Scholar]
- 4.Stampfer MJ. Cardiovascular disease and Alzheimer’s disease: Common links. J. Intern. Med. 2006;260(3):211–223. doi: 10.1111/j.1365-2796.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO). Global burden solutions exist; 2016. p. 8. [Online]. Available: https://www.who.int/cardiovascular_diseases/global-hearts/GHI_Brochure.pdf. Accessed 20 Apr 2021.
- 6.Aparicio HJ, et al. Heart Disease and Stroke Statistics-2021 Update A Report from the American Heart Association. 2021. [DOI] [PubMed] [Google Scholar]
- 7.Tini G, Scagliola R, Monacelli F, La Malfa G, Porto I, Brunelli C, Rosa GM. Alzheimer’s disease and cardiovascular disease: a particular association. Cardiol. Res. Pract. 2020;2020. 10.1155/2020/2617970. [DOI] [PMC free article] [PubMed]
- 8.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 9.Sengupta U, Nilson AN, Kayed R. The role of amyloid-β oligomers in toxicity, propagation, and immunotherapy. EBioMedicine. 2016;6:42–49. doi: 10.1016/j.ebiom.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh DM, Klyubin I, Fadeeva JV, Rowan MJ, Selkoe DJ. Amyloid-β oligomers: their production, toxicity and therapeutic inhibition. Biochem. Soc. Trans. 2002;30(4):552–557. doi: 10.1042/BST0300552. [DOI] [PubMed] [Google Scholar]
- 11.Sakono M, Zako T. Amyloid oligomers: formation and toxicity of Aβ oligomers. FEBS J. 2010;277(6):1348–1358. doi: 10.1111/j.1742-4658.2010.07568.x. [DOI] [PubMed] [Google Scholar]
- 12.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P. Diffusible, nonfibrillar ligands derived from Aβ1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. U. S. A. 1998;95(11):6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011;1(1):1–23. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung ES, Hong H, Kim C, Inhee MJ. Acute ER stress regulates amyloid precursor protein processing through ubiquitin-dependent degradation. Sci. Rep. 2015;5:1–9. doi: 10.1038/srep08805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fonseca ACRG, Ferreiro E, Oliveira CR, Cardoso SM, Pereira CF. Activation of the endoplasmic reticulum stress response by the amyloid-beta 1-40 peptide in brain endothelial cells. Biochim. Biophys. Acta - Mol. Basis Dis. 2013;1832(12):2191–2203. doi: 10.1016/j.bbadis.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto S, Saido TC. Critical review: involvement of endoplasmic reticulum stress in the aetiology of Alzheimer’s disease. Open Biol. 2018;8(4). 10.1098/rsob.180024. [DOI] [PMC free article] [PubMed]
- 17.Santos CXC, Nabeebaccus AA, Shah AM, Camargo LL, Filho SV, Lopes LR. Endoplasmic reticulum stress and nox-mediated reactive oxygen species signaling in the peripheral vasculature: potential role in hypertension. Antioxidants Redox Signal. 2014;20(1):121–134. doi: 10.1089/ars.2013.5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlisle RE, Werner KE, Yum V, Lu C, Tat V, Memon M, No Y, Ask K, Dickhout JG. Endoplasmic reticulum stress inhibition reduces hypertension through the preservation of resistance blood vessel structure and function. J. Hypertens. 2016;34(8):1556–1569. doi: 10.1097/HJH.0000000000000943. [DOI] [PubMed] [Google Scholar]
- 19.Niwa K, Kazama K, Younkin SG, Carlson GA, Iadecola C. Alterations in cerebral blood flow and glucose utilization in mice overexpressing the amyloid precursor protein. Neurobiol. Dis. 2002;9(1):61–68. doi: 10.1006/nbdi.2001.0460. [DOI] [PubMed] [Google Scholar]
- 20.de Bruijn RFAG, Ikram MA. Cardiovascular risk factors and future risk of Alzheimer’s disease. BMC Med. 2014;12(1):1–9. doi: 10.1186/s12916-014-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thomas T, Thomas G, McLendon C, Sutton T, Mullan M. β-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380(6570):168–171. doi: 10.1038/380168a0. [DOI] [PubMed] [Google Scholar]
- 22.Stakos SK, Dimitrios A, Kimon S, Dimitrios B, Marco S, Eleftherios Z, Vlachogiannis NI, Simon T-C. The Alzheimer’s disease amyloid-beta hypothesis in cardiovascular aging and disease: JACC focus seminar. J. Am. Coll. Cardiol. 2020;75(8):952–967. doi: 10.1016/j.jacc.2019.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9(7):689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 24.Mawuenyega BRJ, Kwasi G, Wendy S, Vitaliy O, Ling M, Tom K, Morris JC, Yarasheski KE. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330(6012):1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deane R, Wu Z, Zlokovic BV. RAGE mediates amyloid-β peptide transport across the blood-brain barrier and accumulation in brain. Nat. Med. 2003;9(7):907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- 26.Park MBS, Laibaik ZP, Rose P, Carmen C, Josef A, Norris Erin H, Linda Y, Steven Y, George C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc. Natl. Acad. Sci. 2008;105(4):1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niwa K, Porter VA, Kazama KEN, Cornfield D, Carlson GA, Iadecola C. Aβ-peptides enhance vasoconstriction in cerebral circulation. Am. J. Physiol. Circ. Physiol. 2001;281(6):H2417–H2424. doi: 10.1152/ajpheart.2001.281.6.H2417. [DOI] [PubMed] [Google Scholar]
- 28.Wang J, Zhao C, Zhao A, Li M, Ren J, Qu X. New insights in amyloid beta interactions with human telomerase. J. Am. Chem. Soc. 2015;137(3):1213–1219. doi: 10.1021/ja511030s. [DOI] [PubMed] [Google Scholar]
- 29.Hughes TM, Craft S, Lopez OL. Review of ‘the potential role of arterial stiffness in the pathogenesis of Alzheimer’s disease. Neurodegener. Dis. Manag. 2015;5(2):121–135. doi: 10.2217/nmt.14.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes MR, Timothy KL, Emma B-M, Eric MD, Mathis Chester SB, Steven DK. IC-P-018: arterial stiffness is associated with amyloid deposition in the brain independent of blood pressure. Alzheimer’s Dement. 2013;9:P19–P20. doi: 10.1016/j.jalz.2013.05.019. [DOI] [Google Scholar]
- 31.Henry-Feugeas M-C, Roy C, Baron G, Schouman-Claeys E. Leukoaraiosis and pulse-wave encephalopathy: observations with phase-contrast MRI in mild cognitive impairment. J. Neuroradiol. 2009;36(4):212–218. doi: 10.1016/j.neurad.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 32.Kalaria RN, Akinyemi R, Ihara M. Does vascular pathology contribute to Alzheimer changes? J. Neurol. Sci. 2012;322(1):141–147. doi: 10.1016/j.jns.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 33.Williams MGJ, Michael A, McGowa AJ, Cardwell CR, Cheung CY, David C, Peter P, Giuliana S, Maxwell AP. Retinal microvascular network attenuation in Alzheimer’s disease. Alzheimer’s Dement. Diagnosis, Assess. Dis. Monit. 2015;1(2):229–235. doi: 10.1016/j.dadm.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feke GT, Hyman BT, Stern RA, Pasquale LR. Retinal blood flow in mild cognitive impairment and Alzheimer’s disease. Alzheimer’s Dement. Diagnosis, Assess. Dis. Monit. 2015;1(2):144–151. doi: 10.1016/j.dadm.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santos CY, Snyder PJ, Wu WC, Zhang M, Echeverria A, Alber J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: a review and synthesis. Alzheimer’s Dement. Diagnosis, Assess. Dis. Monit. 2017;7:69–87. doi: 10.1016/j.dadm.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faraco KK, Giuseppe BD, Lidia G-B, Gang W, Gianfranco R, Haejoo C, Izaskun B, Santisteban MM, Segarra SG. Dietary salt promotes neurovascular and cognitive dysfunction through a gut-initiated TH17 response. Nat. Neurosci. 2018;21(2):240–249. doi: 10.1038/s41593-017-0059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kendig MD, Morris MJ. Reviewing the effects of dietary salt on cognition:mechanisms and future directions. Asia Pac. J. Clin. Nutr. 2019;28(1):6–14. doi: 10.6133/apjcn.201903_28(1).0002. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Mandelkow E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016;17(1):22–35. doi: 10.1038/nrn.2015.1. [DOI] [PubMed] [Google Scholar]
- 39.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80(4):844–66. [DOI] [PMC free article] [PubMed]
- 40.Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, Harrington MG, Pa J, Law M, Wang DJ, Jacobs RE. Vascular dysfunction—the disregarded partner of Alzheimer’s disease. Alzheimer’s Dement. 2019;15(1):158–167. doi: 10.1016/j.jalz.2018.07.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Libby P. Atherosclerosis: disease biology affecting the coronary vasculature. Am. J. Cardiol. 2006;98(12):S3–S9. doi: 10.1016/j.amjcard.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 42.Li LJ, Yankun GM, Laura C, George K, Westover EJ, Miroslav D, Covey DF, Freed JH, Maxfield FR. Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids: implications for depletion of endoplasmic reticulum calcium stores and apoptos. J. Biol. Chem. 2004;279(35):37030–37039. doi: 10.1074/jbc.M405195200. [DOI] [PubMed] [Google Scholar]
- 43.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(9):2672–2713. doi: 10.1161/STR.0b013e3182299496.Vascular. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.M. J. Cipolla, “The Cerebral Circulation,” Colloq. Ser. Integr. Syst. Physiol. From Mol. to Funct., vol. 1, no. 1, pp. 1–59, Jan. 2009. 10.4199/C00005ED1V01Y200912ISP002.
- 45.Stukas S, Robert J, Wellington CL. High-density lipoproteins and cerebrovascular integrity in Alzheimer’s disease. Cell Metab. 2014;19(4):574–591. doi: 10.1016/j.cmet.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 46.Sara E Berman, Leonardo A Rivera-Rivera, Lindsay R Clark, Annie M Racine, Jon G Keevil, Lisa C Bratzke, Cynthia M Carlsson, Barbara B Bendlin, Howard A Rowley, Kaj Blennow, Henrik Zetterberg, Sanjay Asthana, Patrick Turski, Sterling C Johnson, Oliver Wieben, “Intracranial arterial 4D-flow is associated with metrics of brain health and Alzheimer’s disease,” Alzheimer’s Dement. Amst. vol. 1, no. 4, pp. 420–428, 2015. 10.1016/j.dadm.2015.09.005. [DOI] [PMC free article] [PubMed]
- 47.Suri Sana EM, Mackay CE, Kelly ME, Germuska MT, Frisoni N, Matthews GB, Ebmeier PM, Bulte KP, Filippini DP. Reduced cerebrovascular reactivity in young adults carrying the APOE ε4 allele. Alzheimers Dement. 2015;11(6):648–657.e1. doi: 10.1016/j.jalz.2014.05.1755. [DOI] [PubMed] [Google Scholar]
- 48.S. A. Austin, A. V Santhanam, D. J. Hinton, D.-S. Choi, and Z. S. Katusic, “Endothelial nitric oxide deficiency promotes Alzheimer’s disease pathology,” J. Neurochem., vol. 127, no. 5, pp. 691–700, Dec. 2013. 10.1111/jnc.12334. [DOI] [PMC free article] [PubMed]
- 49.D. N. Atochin and P. L. Huang, “Endothelial nitric oxide synthase transgenic models of endothelial dysfunction,” Pflugers Arch., vol. 460, no. 6, pp. 965–974, Nov. 2010. 10.1007/s00424-010-0867-4. [DOI] [PMC free article] [PubMed]
- 50.Teresa LG, Maria G, Carmine V, Angelo M, Alessandra A, Gennaro M, Roberta P, Loredana C, Giulio S. Mechanisms of soluble β-amyloid impairment of endothelial function. J. Biol. Chem. 2004;279(46):48135–48142. doi: 10.1074/jbc.M407358200. [DOI] [PubMed] [Google Scholar]
- 51.Meakin PJ, Coull BM, Tuharska Z, McCaffery C, Akoumianakis I, Antoniades C, Brown J, Griffin KJ, Platt F, Ozber CH, Yuldasheva NY, Makava N, Skromna A, Prescott A, McNeilly AD, Siddiqui M, Palmer CN, Khan F, Ashford ML. Elevated circulating amyloid concentrations in obesity and diabetes promote vascular dysfunction. J. Clin. Invest. 2020;140(8):4104–4117. doi: 10.1172/JCI122237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hartz AMS, Bauer B, Soldner ELB, Wolf A, Boy S, Backhaus R, Mihaljevic I, Bogdahn U, Klünemann HH, Schuierer G, Schlachetzki F. Amyloid-β contributes to blood-brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke. 2012;43(2):514–523. doi: 10.1161/STROKEAHA.111.627562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vijay CWCG, Wells JS, Jennings S, Mathis M, Minagar JM, Alexander A. Metabolic modulation of cytokine-induced brain endothelial adhesion molecule expression. Microcirculation. 2012;19(2):155–165. doi: 10.1111/j.1549-8719.2011.00141.x. [DOI] [PubMed] [Google Scholar]
- 54.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J. Appl. Physiol. 2006;100(1):328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 55.Ihara M, Washida K. Linking atrial fibrillation with Alzheimer’s disease: Epidemiological, pathological, and mechanistic evidence. J. Alzheimer’s Dis. 2018;62(1):61–72. doi: 10.3233/JAD-170970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vlachopoulos C, O'Rourke M, Nichols WW. McDonald’s blood flow in arteries, Sixth edition: theoretical, experimental an clinical principles. Boca Raton, United States: CRC Press; 2011. [Google Scholar]
- 57.A. Singh-Manoux, M. J. Shipley, J. A. Bell, M. Canonico, A. Elbaz, and M. Kivimäki, “Association between inflammatory biomarkers and all-cause, cardiovascular and cancer-related mortality,” CMAJ, vol. 189, no. 10, pp. E384–E390, Mar. 2017. 10.1503/cmaj.160313. [DOI] [PMC free article] [PubMed]
- 58.van Dijk EJ, Prins ND, Vermeer SE, Vrooman HA, Hofman A, Koudstaal PJ, Breteler MMB. C-reactive protein and cerebral small-vessel disease: the Rotterdam Scan Study. Circulation. 2005;112(6):900–905. doi: 10.1161/CIRCULATIONAHA.104.506337. [DOI] [PubMed] [Google Scholar]
- 59.Leszek J, Mikhaylenko EV, Belousov DM, Koutsouraki E, Szczechowiak K, Kobusiak-Prokopowicz M, Mysiak A, Diniz BS, Somasundaram SG, Kirkland CE, Aliev G. The links between cardiovascular diseases and Alzheimer’s disease. Curr. Neuropharmacol. 2021;19(2):152–169. doi: 10.2174/1570159x18666200729093724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amarante-Mendes GP, Adjemian S, Branco LM, Zanetti LC, Weinlich R, Bortoluci KR. Pattern recognition receptors and the host cell death molecular machinery. Front. Immunol. 2018;9:2379. doi: 10.3389/fimmu.2018.02379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heneka MT, Kummer MP, Latz E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014;14(7):463–477. doi: 10.1038/nri3705. [DOI] [PubMed] [Google Scholar]
- 63.Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020;20(2):95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 64.Stewart Cameron R, Stuart Lynda M, Kim W, Gils V, Deng JM, Halle J, Rayner A, Boyer KJ, Zhong L. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010;11(2):155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frederick S, Alena G, Rayner KJ, Parisa K, Bhama R, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 2013;14(8):812–820. doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Labzin LI, Heneka MT, Latz E. Innate immunity and neurodegeneration. Annu. Rev. Med. 2018;69:437–449. doi: 10.1146/annurev-med-050715-104343. [DOI] [PubMed] [Google Scholar]
- 67.Decourt B, Lahiri DK, Sabbagh MN. Targeting tumor necrosis factor alpha for Alzheimer’s disease. Curr. Alzheimer Res. 2017;14(4):412–425. doi: 10.2174/1567205013666160930110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elliott EI, Sutterwala FS. Initiation and perpetuation of NLRP 3 inflammasome activation and assembly. Immunol. Rev. 2015;265(1):35–52. doi: 10.1111/imr.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karasawa T, Takahashi M. Role of NLRP3 inflammasomes in atherosclerosis. J. Atheroscler. Thromb. 2017:RV17001. [DOI] [PMC free article] [PubMed]
- 70.Gianni D, et al. Protein aggregates and novel presenilin gene variants in idiopathic dilated cardiomyopathy. Circulation. 2010;121(10):1216–1226. doi: 10.1161/CIRCULATIONAHA.109.879510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch. Gen. Psychiatry. 2005;62(6):685–691. doi: 10.1001/archpsyc.62.6.685. [DOI] [PubMed] [Google Scholar]
- 72.Brookmeyer R, et al. National estimates of the prevalence of Alzheimer’s disease in the United States. Alzheimer’s Dement. 2011;7(1):61–73. doi: 10.1016/j.jalz.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.A. Association. Thies W, Bleiler L. 2013 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2013;9(2):208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 74.Nebel RA, et al. Understanding the impact of sex and gender in Alzheimer’s disease: a call to action. Alzheimer’s Dement. 2018;14(9):1171–1183. doi: 10.1016/j.jalz.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katzenellenbogen BS. Estrogen receptors: bioactivities and interactions with cell signaling pathways. Biol. Reprod. 1996;54(2):287–293. doi: 10.1095/biolreprod54.2.287. [DOI] [PubMed] [Google Scholar]
- 76.Pérez SE, Chen E-Y, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Dev. brain Res. 2003;145(1):117–139. doi: 10.1016/S0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- 77.Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol. Med. 2013;19(3):197–209. doi: 10.1016/j.molmed.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cowley SM, Parker MG. A comparison of transcriptional activation by ERα and ERβ. J. Steroid Biochem. Mol. Biol. 1999;69(1–6):165–175. doi: 10.1016/S0960-0760(99)00055-2. [DOI] [PubMed] [Google Scholar]
- 79.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011;7(12):715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jaffe AB, Toran-Allerand CD, Greengard P, Gandy SE. Estrogen regulates metabolism of Alzheimer amyloid beta precursor protein. J. Biol. Chem. 1994;269(18):13065–13068. doi: 10.1016/S0021-9258(17)36796-0. [DOI] [PubMed] [Google Scholar]
- 81.Uddin MS, et al. Estrogen signaling in Alzheimer’s disease: molecular insights and therapeutic targets for Alzheimer’s dementia. Mol. Neurobiol. 2020;57(6):2654–2670. doi: 10.1007/s12035-020-01911-8. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Q-G, Wang R, Khan M, Mahesh V, Brann DW. Role of Dickkopf-1, an antagonist of the Wnt/β-catenin signaling pathway, in estrogen-induced neuroprotection and attenuation of tau phosphorylation. J. Neurosci. 2008;28(34):8430–8441. doi: 10.1523/JNEUROSCI.2752-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dong C, et al. Sex differences in the association between cardiovascular diseases and dementia subtypes: a prospective analysis of 464,616 UK Biobank participants. Biol. Sex Differ. 2022;13(1):21. doi: 10.1186/s13293-022-00431-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.J. M. Zilberman, G. H. Cerezo, M. Del Sueldo, C. Fernandez-Pérez, N. Martell-Claros, and A. Vicario, “Association between hypertension, menopause, and cognition in women,” J. Clin. Hypertens., vol. 17, no. 12, pp. 970–976, Dec. 2015. 10.1111/jch.12643. [DOI] [PMC free article] [PubMed]
- 85.Hinton AO, et al. Estrogen receptor-α in the medial amygdala prevents stress-induced elevations in blood pressure in females. Hypertension. 2016;67(6):1321–1330. doi: 10.1161/HYPERTENSIONAHA.116.07175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lindheim SR, et al. Behavioral stress responses in premenopausal and postmenopausal women and the effects of estrogen. Am. J. Obstet. Gynecol. 1992;167(6):1831–1836. doi: 10.1016/0002-9378(92)91783-7. [DOI] [PubMed] [Google Scholar]
- 87.Manhem K, Ahlm H, Milsom I, Svensson A. Transdermal oestrogen reduces daytime blood pressure in hypertensive women. J. Hum. Hypertens. 1998;12(5):323–327. doi: 10.1038/sj.jhh.1000563. [DOI] [PubMed] [Google Scholar]
- 88.Reckelhoff JF, Fortepiani LA. Novel mechanisms responsible for postmenopausal hypertension. Hypertension. 2004;43(5):918–923. doi: 10.1161/01.HYP.0000124670.03674.15. [DOI] [PubMed] [Google Scholar]
- 89.B. S. Chozick, “The behavioral effects of lesions of the amygdala: a review,” Int. J. Neurosci., vol. 29, no. 3–4, pp. 205–221, Jan. 1986. 10.3109/00207458608986152. [DOI] [PubMed]
- 90.Gianaros PJ, Sheu LK, Matthews KA, Jennings JR, Manuck SB, Hariri AR. Individual differences in stressor-evoked blood pressure reactivity vary with activation, volume, and functional connectivity of the amygdala. J. Neurosci. 2008;28(4):990–999. doi: 10.1523/JNEUROSCI.3606-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor α and β in the mouse central nervous system: In vivo autoradiographic and immunocytochemical analyses. J. Comp. Neurol. 2004;473(2):270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- 92.Thomas EG et al. Sex-specific associations of diabetes with brain structure and function in a geriatric population. Frontiers in Aging Neuroscience. 2022;14, [Online]. Available: https://www.frontiersin.org/articles/10.3389/fnagi.2022.885787. Accessed 11 Oct 2022. [DOI] [PMC free article] [PubMed]
- 93.Casserly I, Topol EJ. Convergence of atherosclerosis and Alzheimer’s disease: inflammation, cholesterol, and misfolded proteins. Lancet. 2004;363(9415):1139–1146. doi: 10.1016/S0140-6736(04)15900-X. [DOI] [PubMed] [Google Scholar]
- 94.K. A. Jellinger, “Morphologic diagnosis of ‘vascular dementia’; A critical update,” J. Neurol. Sci., vol. 270, no. 1, pp. 1–12, Jul. 2008. 10.1016/j.jns.2008.03.006. [DOI] [PubMed]
- 95.Lee T, Lee H. Identification of disease-related genes that are common between alzheimer’s and cardiovascular disease using blood genome-wide transcriptome analysis. Biomedicines. 2021;9(11). 10.3390/biomedicines9111525. [DOI] [PMC free article] [PubMed]
- 96.O’Brien JT, Markus HS. Vascular risk factors and Alzheimer’s disease. BMC Med. 2014;12(1):218. doi: 10.1186/s12916-014-0218-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nday CM, Eleftheriadou D, Jackson G. Shared pathological pathways of Alzheimer’s disease with specific comorbidities: current perspectives and interventions. J. Neurochem. 2018;144(4):360–389. doi: 10.1111/jnc.14256. [DOI] [PubMed] [Google Scholar]
- 98.Wilkie F, Eisdorfer C. Intelligence and blood pressure in the aged. Science. 1971;172(3986):959–962. doi: 10.1126/science.172.3986.959. [DOI] [PubMed] [Google Scholar]
- 99.Petrovitch H, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS☆. Neurobiol. Aging. 2000;21(1):57–62. doi: 10.1016/S0197-4580(00)00106-8. [DOI] [PubMed] [Google Scholar]
- 100.Ruitenberg A, et al. Blood pressure and risk of dementia: results from the Rotterdam study and the Gothenburg H-70 study. Dement. Geriatr. Cogn. Disord. 2001;12(1):33–39. doi: 10.1159/000051233. [DOI] [PubMed] [Google Scholar]
- 101.Scheinberg P. Dementia due to vascular disease—a multifactorial disorder. Stroke. 1988;19(10):1291–1299. doi: 10.1161/01.STR.19.10.1291. [DOI] [PubMed] [Google Scholar]
- 102.Nyúl-Tóth Ádám UZ, Stefano T, Tamas K, Peter T, Veronica G, Amber T, Andriy Y, Anna C. Increases in hypertension-induced cerebral microhemorrhages exacerbate gait dysfunction in a mouse model of Alzheimer’s disease. GeroScience. 2020;42(6):1685–1698. doi: 10.1007/s11357-020-00256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Justine LI, Hur MV, Nicolas A, Mégane B, Gilbert B, Claire K-T, Thierry C, Régis B. Cerebrovascular β-amyloid deposition and associated microhemorrhages in a Tg2576 Alzheimer mouse model are reduced with a DHA-enriched diet. FASEB J. 2018;32(9):4972–4983. doi: 10.1096/fj.201800200R. [DOI] [PubMed] [Google Scholar]
- 104.Michael Neethu FMJ, Mahoney GM, Kelley K, Sumbria RK, Vasilevko V, Joanne VR, Cribbs DH, Annlia P-H. Effects of dabigatran in mouse models of aging and cerebral amyloid angiopathy. Front. Neurol. 2019:966. [DOI] [PMC free article] [PubMed]
- 105.Liu J, Liu S, Matsumoto Y, Murakami S, Sugakawa Y, Kami A, Tanabe C, Maeda T, Michikawa M, Komano H, Zou K. Angiotensin type 1a receptor deficiency decreases amyloid β-protein generation and ameliorates brain amyloid pathology. Sci. Rep. 2015;5(1):1–10. doi: 10.1038/srep12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Faraco IC, Giuseppe PL, Ping Z, Wenjie L, Paul SM, Josef A. Hypertension enhances Aβ-induced neurovascular dysfunction, promotes β-secretase activity, and leads to amyloidogenic processing of APP. J. Cereb. Blood Flow Metab. 2016;36(1):241–252. doi: 10.1038/jcbfm.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Skoog I, et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–1145. doi: 10.1016/S0140-6736(96)90608-X. [DOI] [PubMed] [Google Scholar]
- 108.R. F. Gottesman et al., “Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study,” JAMA Neurol., vol. 71, no. 10, pp. 1218–1227, Oct. 2014. 10.1001/jamaneurol.2014.1646. [DOI] [PMC free article] [PubMed]
- 109.F. C. Goldstein, I. M. Hajjar, C. B. Dunn, A. I. Levey, and W. Wharton, “The relationship between cognitive functioning and the JNC-8 guidelines for hypertension in older adults,” Journals Gerontol. Ser. A, vol. 72, no. 1, pp. 121–126, Jan. 2017. 10.1093/gerona/glw181. [DOI] [PMC free article] [PubMed]
- 110.Luchsinger JA, Tang M-X, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology. 2004;63(7):1187–1192. doi: 10.1212/01.WNL.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 111.de la Monte SM, Tong M, Daiello LA, Ott BR. Early-stage Alzheimer’s disease is associated with simultaneous systemic and central nervous system dysregulation of insulin-linked metabolic pathways. J. Alzheimer’s Dis. 2019;68(2):657–668. doi: 10.3233/JAD-180906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aragno BG, Manuela MR, Claudio M, Francesca R, Catalano MG, Nicoletta P, Oliviero D. Up-regulation of advanced glycated products receptors in the brain of diabetic rats is prevented by antioxidant treatment. Endocrinology. 2005;146(12):5561–5567. doi: 10.1210/en.2005-0712. [DOI] [PubMed] [Google Scholar]
- 113.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 114.Shah GN, Morofuji Y, Banks WA, Price TO. High glucose-induced mitochondrial respiration and reactive oxygen species in mouse cerebral pericytes is reversed by pharmacological inhibition of mitochondrial carbonic anhydrases: implications for cerebral microvascular disease in diabetes. Biochem. Biophys. Res. Commun. 2013;440(2):354–358. doi: 10.1016/j.bbrc.2013.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ko S-Y, Lin Y-P, Lin Y-S, Chang S-S. Advanced glycation end products enhance amyloid precursor protein expression by inducing reactive oxygen species. Free Radic. Biol. Med. 2010;49(3):474–480. doi: 10.1016/j.freeradbiomed.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 116.Iannuzzi C, Maritato R, Irace G, Sirangelo I. Glycation accelerates fibrillization of the amyloidogenic W7FW14F apomyoglobin. PLoS One. 2013;8(12):e80768. doi: 10.1371/journal.pone.0080768. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 117.Yamamoto M, Kiyota T, Walsh SM, Liu J, Kipnis J, Ikezu T. Cytokine-mediated inhibition of fibrillar amyloid-β peptide degradation by human mononuclear phagocytes. J. Immunol. 2008;181(6):3877–3886. doi: 10.4049/jimmunol.181.6.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fishel GD, Mark A, Stennis WG, Montine TJ, Qin W, Green PS, Jacob KJ, Cook DG, Peskind ER, Baker LD. Hyperinsulinemia provokes synchronous increases in central inflammation and β-amyloid in normal adults. Arch. Neurol. 2005;62(10):1539–1544. doi: 10.1001/archneur.62.10.noc50112. [DOI] [PubMed] [Google Scholar]
- 119.Zhao W-Q, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. Eur. J. Pharmacol. 2004;490(1):71–81. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]
- 120.Williamson R, McNeilly A, Sutherland C. Insulin resistance in the brain: an old-age or new-age problem? Biochem. Pharmacol. 2012;84(6):737–745. doi: 10.1016/j.bcp.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 121.Caccamo A, Oddo S, Sugarman MC, Akbari Y, LaFerla FM. Age-and region-dependent alterations in Aβ-degrading enzymes: implications for Aβ-induced disorders. Neurobiol. Aging. 2005;26(5):645–654. doi: 10.1016/j.neurobiolaging.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 122.Moser M, Roccella EJ. The treatment of hypertension: a remarkable success story. J. Clin. Hypertens. 2013;15(2):88–91. doi: 10.1111/jch.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Whelton PK et al. “2017 ACC / AHA / AAPA / ABC / ACPM / AGS / APhA / ASH / ASPC / NMA / PCNA Guideline for the prevention , detection , evaluation , and management of high blood pressure in adults. Hypertension. 2018;71(6):1269–1324. [Online]. Available: http://www.onlinejacc.org/content/accj/71/19/e127.full.pdf?_ga=2.23738239.413968245.1589686952-2022385710.1589686952. Accessed 1 Nov 2022. [DOI] [PubMed]
- 124.Ungvari Z, et al. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat. Rev. Nephrol. 2021;17(10):639–654. doi: 10.1038/s41581-021-00430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kannel WB. Risk stratification in hypertension: new insights from the Framingham Study. Am. J. Hypertens. 2000;13:3–10. doi: 10.1016/s0895-7061(99)00252-6. [DOI] [PubMed] [Google Scholar]
- 126.Spieth W. Cardiovascular health status, age and psychological performance. J. Gerontol. 1964;19(3):277–284. doi: 10.1093/geronj/19.3.277. [DOI] [PubMed] [Google Scholar]
- 127.Wilkie PL, Eisdorfer C, Nowlin JB. Memory and blood pressure in the aged. Exp. Aging Res. 1976;2(1):3–16. doi: 10.1080/03610737608257972. [DOI] [PubMed] [Google Scholar]
- 128.Iadecola C, Gottesman RF. Neurovascular and cognitive dysfunction in hypertension: epidemiology, pathobiology, and treatment. Circ. Res. 2019;124(7):1025–1044. doi: 10.1161/CIRCRESAHA.118.313260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late-life cognitive function: the Honolulu-Asia Aging Study. JAMA J. Am. Med. Assoc. 1995;274(23):1846–1851. doi: 10.1001/jama.1995.03530230032026. [DOI] [PubMed] [Google Scholar]
- 130.Skoog I, Gutafson D. Hypertension and related factors in the etiology of Alzhemier’s disease. Alzheimer’s Dis. Relat. Disord. Etiol. Pathog. Ther. Chichester John Wiley Sons Ltd. 1999:523–30.
- 131.Iadecola C, et al. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension. 2016;68(6):e67–e94. doi: 10.1161/HYP.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.de la Monte SM. Quantitation of cerebral atrophy in preclinical and end-stage alzheimer’s disease. Ann. Neurol. 1989;25(5):450–459. doi: 10.1002/ana.410250506. [DOI] [PubMed] [Google Scholar]
- 133.Skoog I, Palmertz B, Andreasson L-A. The prevalence of white-matter lesions on computed tomography of the brain in demented and nondemented 85-year-olds. J. Geriatr. Psychiatry Neurol. 1994;7(3):169–175. doi: 10.1177/089198879400700308. [DOI] [PubMed] [Google Scholar]
- 134.Smits LL, et al. Trajectories of cognitive decline in different types of dementia. Psychol. Med. 2015;45(5):1051–1059. doi: 10.1017/S0033291714002153. [DOI] [PubMed] [Google Scholar]
- 135.Hestad K, Engedal K, Schirmer H, Strand BH. The effect of blood pressure on cognitive performance. An 8-year follow-up of the Tromsø Study, Comprising People Aged 45–74 Years. Frontiers in Psychology. 2020;11. [Online]. Available: https://www.frontiersin.org/articles/10.3389/fpsyg.2020.00607. Accessed 25 Sept 2022. [DOI] [PMC free article] [PubMed]
- 136.Swan GE, et al. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51(4):986–993. doi: 10.1212/WNL.51.4.986. [DOI] [PubMed] [Google Scholar]
- 137.Gąsecki D, Kwarciany M, Nyka W, Narkiewicz K. Hypertension, brain damage and cognitive decline. Curr. Hypertens. Rep. 2013;15(6):547–558. doi: 10.1007/s11906-013-0398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler. Thromb. Vasc. Biol. 2007;27(2):303–309. doi: 10.1161/01.ATV.0000253885.41509.25. [DOI] [PubMed] [Google Scholar]
- 139.van Dinther M, et al. Assessment of microvascular rarefaction in human brain disorders using physiological magnetic resonance imaging. J. Cereb. Blood Flow Metab. 2022;42(5):718–737. doi: 10.1177/0271678X221076557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jiménez-Balado J, et al. Prevalence of hippocampal enlarged perivascular spaces in a sample of patients with hypertension and their relation with vascular risk factors and cognitive function. J Neurol Neurosurg Psychiatry. 2018;89(6):651–656. doi: 10.1136/jnnp-2017-316724. [DOI] [PubMed] [Google Scholar]
- 141.Biesbroek JM, Weaver NA, Biessels GJ. Lesion location and cognitive impact of cerebral small vessel disease. Clin. Sci. 2017;131(8):715–728. doi: 10.1042/CS20160452. [DOI] [PubMed] [Google Scholar]
- 142.Summers PM, et al. Functional deficits induced by cortical microinfarcts. J. Cereb. Blood Flow Metab. 2017;37(11):3599–3614. doi: 10.1177/0271678X16685573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Carnevale L, et al. Brain MRI fiber-tracking reveals white matter alterations in hypertensive patients without damage at conventional neuroimaging. Cardiovasc. Res. 2018;114(11):1536–1546. doi: 10.1093/cvr/cvy104. [DOI] [PubMed] [Google Scholar]
- 144.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120(3):287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ritz K, Denswil NP, Stam OCG, van Lieshout JJ, Daemen MJAP. Cause and mechanisms of intracranial atherosclerosis. Circulation. 2014;130(16):1407–1414. doi: 10.1161/CIRCULATIONAHA.114.011147. [DOI] [PubMed] [Google Scholar]
- 146.De Jong GI, De Vos RAI, Steur ENHJ, Luiten PGM. Cerebrovascular hypoperfusion: a risk factor for Alzheimer’s disease? Animal model and postmortem human studies. Ann. N. Y. Acad. Sci. 1997;826(1):56–74. doi: 10.1111/j.1749-6632.1997.tb48461.x. [DOI] [PubMed] [Google Scholar]
- 147.Jun HJ, Aono SJ, Masaru I, Masatsugu H, Takayuki N, Kazuhisa N, Katsuji I, Akiyoshi O, Hideki O. Deletion of the angiotensin II type 1a receptor prevents atherosclerotic plaque rupture in apolipoprotein E -/- mice. Arterioscler. Thromb. Vasc. Biol. 2012;32(6):1453–1459. doi: 10.1161/ATVBAHA.112.249516. [DOI] [PubMed] [Google Scholar]
- 148.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 2004;5(5):347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- 149.Yarchoan M, et al. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain. 2012;135(12):3749–3756. doi: 10.1093/brain/aws271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Sr, Diaz-Arrastia R, Park DC. Risk factors for β-amyloid deposition in healthy aging: vascular and genetic effects. JAMA Neurol. 2013;70(5):600–606. doi: 10.1001/jamaneurol.2013.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Carnevale D, et al. Hypertension induces brain β-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension. 2012;60(1):188–197. doi: 10.1161/HYPERTENSIONAHA.112.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Carnevale D, et al. Role of neuroinflammation in hypertension-induced brain amyloid pathology. Neurobiol. Aging. 2012;33(1):205.e19–205.e29. doi: 10.1016/j.neurobiolaging.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 153.Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J. Cereb. Blood Flow Metab. 2007;27(12):1908–1918. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- 154.Kazama K, et al. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase–derived radicals. Circ. Res. 2004;95(10):1019–1026. doi: 10.1161/01.RES.0000148637.85595.c5. [DOI] [PubMed] [Google Scholar]
- 155.Carnevale LG, Daniela MG, Andrea ID, Valentina F, Bell RD, Igor B, Fabio P, Berislav Z, Shidu YS. ‘Alzheimer-like’ pathology in a murine model of arterial hypertension. Biochem. Soc. Trans. 2011;39(4):939–944. doi: 10.1042/BST0390939. [DOI] [PubMed] [Google Scholar]
- 156.Faraco G, et al. Hypertension enhances Aβ-induced neurovascular dysfunction, promotes β-secretase activity, and leads to amyloidogenic processing of APP. J. Cereb. Blood Flow Metab. 2015;36(1):241–252. doi: 10.1038/jcbfm.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang J, et al. Valsartan lowers brain β-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J. Clin. Invest. 2007;117(11):3393–3402. doi: 10.1172/JCI31547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Royea J, Zhang L, Tong X-K, Hamel E. Angiotensin IV receptors mediate the cognitive and cerebrovascular benefits of Losartan in a mouse model of Alzheimer's disease. J. Neurosci. 2017;37(22):5562–5573. doi: 10.1523/JNEUROSCI.0329-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Khachaturian BJCS, Ara S, Zandi PP, Lyketsos CG, Hayden KM, Ingmar S, Norton MC, Tschanz JAT, Mayer LS, Welsh-Bohmer KA. Antihypertensive medication use and incident Alzheimer disease: the Cache County Study. Arch. Neurol. 2006;63(5):686–692. doi: 10.1001/archneur.63.5.noc60013. [DOI] [PubMed] [Google Scholar]
- 160.Gelber RP, Ross GW, Petrovitch H, Masaki KH, Launer LJ, White LR. Antihypertensive medication use and risk of cognitive impairment: the Honolulu-Asia Aging Study. Neurology. 2013;81(10):888–895. doi: 10.1212/WNL.0b013e3182a351d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Soto G, Maria E, Abellan van Kan RY, Fati N, Sophie G-G, Matteo C, Christelle C, Vellas B. Angiotensin-converting enzyme inhibitors and Alzheimer’s disease progression in older adults: results from the réseau sur la maladie d’Alzheimer français cohort. J. Am. Geriatr. Soc. 2013;61(9):1482–1488. doi: 10.1111/jgs.12415. [DOI] [PubMed] [Google Scholar]
- 162.Barthold D, Joyce G, Wharton W, Kehoe P, Zissimopoulos J. The association of multiple anti-hypertensive medication classes with Alzheimer’s disease incidence across sex, race, and ethnicity. PLoS One. 2018;13(11):e0206705. doi: 10.1371/journal.pone.0206705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.G. S. Murray, Michael D., Hendrie Hugh C., Lane Kathleen A., Zheng Mengjie, Ambuehl Roberta, Li Shanshan, Unverzagt Frederick W., Callahan Christopher M., “Antihypertensive medication and dementia risk in older adult African Americans with hypertension: a prospective cohort study,” J. Gen. Intern. Med., vol. 33, no. 4, pp. 455–462, Apr. 2018. 10.1007/s11606-017-4281-x. [DOI] [PMC free article] [PubMed]
- 164.Karp A, Paillard-Borg S, Wang H-X, Silverstein M, Winblad B, Fratiglioni L. Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dement. Geriatr. Cogn. Disord. 2006;21(2):65–73. doi: 10.1159/000089919. [DOI] [PubMed] [Google Scholar]
- 165.Daffner KR. Promoting successful cognitive aging: a comprehensive review. J. Alzheimer’s Dis. 2010;19:1101–1122. doi: 10.3233/JAD-2010-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.De la Monte SM. Type 3 diabetes is sporadic Alzheimer-s disease: mini-review. Eur. Neuropsychopharmacol. 2014;24(12):1954–1960. doi: 10.1016/j.euroneuro.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cardoso S, Santos MS, Seiça R, Moreira PI. Cortical and hippocampal mitochondria bioenergetics and oxidative status during hyperglycemia and/or insulin-induced hypoglycemia. Biochim. Biophys. Acta (BBA)-Molecular Basis Dis. 2010;1802(11):942–951. doi: 10.1016/j.bbadis.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 168.Dandona P. Endothelium, inflammation, and diabetes. Curr. Diab. Rep. 2002;2(4):311–315. doi: 10.1007/s11892-002-0019-0. [DOI] [PubMed] [Google Scholar]
- 169.Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch. Neurol. 2011;68(1):51–57. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bedse G, Di Domenico F, Serviddio G, Cassano T. Aberrant insulin signaling in Alzheimer’s disease: current knowledge. Front. Neurosci. 2015;9:204. doi: 10.3389/fnins.2015.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rensink KB, Annemieke AM, Irene O-H, Roelie DB, Bosch RR, Hans J, De Waal RMW, Verbeek MM. Insulin inhibits amyloid β-induced cell death in cultured human brain pericytes. Neurobiol. Aging. 2004;25(1):93–103. doi: 10.1016/S0197-4580(03)00039-3. [DOI] [PubMed] [Google Scholar]
- 172.Farris GS, Wesley MS, Yang C, Loren L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ. Insulin-degrading enzyme regulates the levels of insulin, amyloid β-protein, and the β-amyloid precursor protein intracellular domain in vivo. Proc. Natl. Acad. Sci. 2003;100(7):4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Luchsinger BE, José A, Thania P, Helena C, Pankaj M, Jason S, Gnanavalli P, Masanori I, Jennifer M, Devanand DP. Metformin in amnestic mild cognitive impairment: results of a pilot randomized placebo controlled clinical trial. J. Alzheimer’s Dis. 2016;51(2):501–514. doi: 10.3233/JAD-150493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Roses BSK, Allen D, Saunders AM, Lutz MW, Nanyin Z, Hariri AR, Asin KE, Crenshaw DG, Kumar B, Burns DK. New applications of disease genetics and pharmacogenetics to drug development. Curr. Opin. Pharmacol. 2014;14:81–89. doi: 10.1016/j.coph.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 175.Reger DW, Mark A, Stennis WG, Green PS, Wilkinson CW, Baker LD, Brenna C, Fishel MA, Plymate SR, Breitner JCS. Intranasal insulin improves cognition and modulates β-amyloid in early AD. Neurology. 2008;70(6):440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 176.Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, Stern Y. Physical activity, diet, and risk of Alzheimer disease. Jama. 2009;302(6):627–637. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Morris MC, Tangney CC, Wang Y, Sacks FM, Bennett DA, Aggarwal NT. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimer’s Dement. 2015;11(9):1007–1014. doi: 10.1016/j.jalz.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Scheltens P, Blennow K, Breteler MM, de Strooper B, Frisoni GB. Alzheimer‘s disease. Lancet. 2016:505–17. [DOI] [PubMed]
- 179.Obregon D, Hou H, Deng J, Giunta B, Tian J, Darlington D, Shahaduzzaman M, Zhu Y, Mori T, Mattson MP, Tan J. Soluble amyloid precursor protein-α modulates β-secretase activity and amyloid-β generation. Nat. Commun. 2012;3(1):1–9. doi: 10.1038/ncomms1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Postina R. A Closer Look at gamma-Secretase. Current Alzheimer Research. 2008;5(2):179–186. doi: 10.2174/156720508783954668. [DOI] [PubMed] [Google Scholar]
- 181.Carrillo-Mora P, Luna R, Colín-Barenque L. Amyloid beta: multiple mechanisms of toxicity and only some protective effects? Oxid. Med. Cell. Longev. 2014;2014. 10.1155/2014/795375. [DOI] [PMC free article] [PubMed]
- 182.Pierrot N, Jean-Noël O. Processing of amyloid precursor protein and amyloid peptide neurotoxicity. Curr. Alzheimer Res. 2008;5:92–99. doi: 10.2174/156720508783954721. [DOI] [PubMed] [Google Scholar]
- 183.Walsh BDBJ, Dominic M, Hartley DM, Yoko K, Youcef F, Condron MM, Aleksey L, Benedek GB, Selkoe DJ, Teplow DB. Amyloid β-protein fibrillogenesis: structure and biological activity of protofibrillar intermediates. J Bio Chem. 1999;274(36):25945–25952. doi: 10.1074/jbc.274.36.25945. [DOI] [PubMed] [Google Scholar]
- 184.Thal DR, Rüb U, Orantes M, Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58(12):1791–1800. doi: 10.1212/WNL.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 185.Guerrero-Muñoz MJ, Castillo-Carranza DL, Kayed R. Therapeutic approaches against common structural features of toxic oligomers shared by multiple amyloidogenic proteins. Biochem. Pharmacol. 2014;88(4):468–478. doi: 10.1016/j.bcp.2013.12.023. [DOI] [PubMed] [Google Scholar]
- 186.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 187.Hampel H, Hardy J, Blennow K, Chen C, Perry G, Kim SH, Villemagne VL, Aisen P, Vendruscolo M, Iwatsubo T, Masters CL. The amyloid- β pathway in Alzheimer ’ s disease. Mol. Psychiatry. 2021;26(10):5481–5503. doi: 10.1038/s41380-021-01249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM, Cai XD, DM MK, Tintner R, Frangione B, Younkin SG. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- 189.Martins IJ, Hone E, Foster JK, Sünram-Lea SI, Gnjec A, Fuller SJ, Nolan D, Gandy SE, Martins RN. Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer’s disease and cardiovascular disease. Mol. Psychiatry. 2006;11(8):721–736. doi: 10.1038/sj.mp.4001854. [DOI] [PubMed] [Google Scholar]
- 190.Bertram L, Lill CM, Tanzi RE. The genetics of alzheimer disease: Back to the future. Neuron. 2010;68(2):270–281. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 191.Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, Mant R, Phillippa N, Karen R, Penelope R, Chris T, Marg P-V. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 192.Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, Crowley AC. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269(5226):973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 193.Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin J-F, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, Da Silva HAR, Haines JL, Pericak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, George-Hyslop PHS. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375(6534):754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 194.Benilova I, Karran E, De Strooper B. The toxic Aβ oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat. Neurosci. 2012;15(3):349–357. doi: 10.1038/nn.3028. [DOI] [PubMed] [Google Scholar]
- 195.Pimenova AA, Raj T, Goate AM. Untangling genetic risk for Alzheimer’s disease. Biol. Psychiatry. 2018;83(4):300–310. doi: 10.1016/j.biopsych.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sleegers K, Lambert J-C, Bertram L, Cruts M, Amouyel P, Van Broeckhoven C. The pursuit of susceptibility genes for Alzheimer’s disease: progress and prospects. Trends Genet. 2010;26(2):84–93. doi: 10.1016/j.tig.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 197.Bentahir WJ, Mostafa NO, Jan V, Alexandra T, Katrien H, Esselmann Hermann DSB. Presenilin clinical mutations can affect γ-secretase activity by different mechanisms. J. Neurochem. 2006;96(3):732–742. doi: 10.1111/j.1471-4159.2005.03578.x. [DOI] [PubMed] [Google Scholar]
- 198.Chávez-Gutiérrez VCS, Lucía BL, Iryna B, Annelies V, Manasi B, Marianne B, Sam L, Lujia Z, Esselmann Hermann DSB, Jens W, Lutgarde S, Eric K, Harrie G, Joost S, Frederic R, Kerensa B. The mechanism of γ-Secretase dysfunction in familial Alzheimer disease. EMBO J. 2012;31(10):2261–2274. doi: 10.1038/emboj.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sims R, Hill M, Williams J. The multiplex model of the genetics of Alzheimer’s disease. Nat. Neurosci. 2020;23(3):311–322. doi: 10.1038/s41593-020-0599-5. [DOI] [PubMed] [Google Scholar]
- 200.Lambert, J.C., Ibrahim-Verbaas, C.A., Harold, D., Naj, A.C., Sims, R., Bellenguez, C., Jun, G., DeStefano, A.L., Bis, J.C., Beecham, G.W. and Grenier-Boley, B., Russo G., Thorton-Wells T. A., Jones N., Smith A. V., Chouraki V., Thomas C., Ikram M. A., “Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease,” Nat. Genet., vol. 45, no. 12, pp. 1452–1458, Dec. 2013. 10.1038/ng.2802. [DOI] [PMC free article] [PubMed]
- 201.Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL. Alzheimer’s disease. Nat. Rev. Dis. Prim. 2015;1(1):15056. doi: 10.1038/nrdp.2015.56. [DOI] [PubMed] [Google Scholar]
- 202.Nyarko JNK, Quartey MO, Pennington PR, Heistad RM, Dea D, Poirier J, Baker GB, Mousseau DD. Profiles of β-amyloid peptides and key secretases in brain autopsy samples differ with sex and APOE ε4 status: impact for risk and progression of Alzheimer disease. Neuroscience. 2018;373:20–36. doi: 10.1016/j.neuroscience.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 203.Polvikoski HM, Tuomo SR, Kainulainen Katariina KK, Alpo V, Auli V, Leena N, Pirjo H. Apolipoprotein E, dementia, and cortical deposition of β-amyloid protein. N. Engl. J. Med. 1995;333(19):1242–1248. doi: 10.1056/NEJM199511093331902. [DOI] [PubMed] [Google Scholar]
- 204.Schmechel DE, Saunders AM, Strittmatter WJ, Joo SH, Hulette C, Crain B, Goldgaber D, Roses AD. “Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease,” Proc. Natl. Acad. Sci. U. S. A., vol. 90, no. 20, pp. 9649–9653, Oct. 1993. 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed]
- 205.Lim YY, Mormino EC, Initiative ADN. APOE genotype and early β-amyloid accumulation in older adults without dementia. Neurology. 2017;89(10):1028–1034. doi: 10.1212/WNL.0000000000004336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lim YY, Kalinowski P, Pietrzak RH, Laws SM, Burnham SC, Ames D, Villemagne VL, Fowler CJ, Rainey-Smith SR, Martins RN, Rowe CC, Masters CL, Maruff PT. Association of β-amyloid and apolipoprotein E ε4 with memory decline in preclinical Alzheimer disease. JAMA Neurol. 2018;75(4):488–494. doi: 10.1001/jamaneurol.2017.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bales BM, Kelly R, Tatyana V, Dodel RC, Yansheng D, Altstiel Larry PP, Paul H, Johnstone EM, Little SP, Cummins DJ, Ghetti Bernardino PSM. Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nat. Genet. 1997;17(3):263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- 208.Yamazaki Y, Zhao N, Caulfield TR, Liu C-C, Bu G. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat. Rev. Neurol. 2019;15(9):501–518. doi: 10.1038/s41582-019-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kanekiyo T, Xu H, Bu G. ApoE and Aβ in Alzheimer’s disease: accidental encounters or partners? Neuron. 2014;81(4):740–754. doi: 10.1016/j.neuron.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Z. B. V. Bell, Robert D., Sagare Abhay P., Friedman Alan E., Bedi Gurrinder S., Holtzman David M., Deane Rashid., “Transport pathways for clearance of human Alzheimer’s amyloid β-peptide and apolipoproteins E and J in the mouse central nervous system,” J. Cereb. Blood Flow Metab., vol. 27, no. 5, pp. 909–918, Nov. 2006. 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed]
- 211.Placanica L, Zhu L, Yue-Ming L. Gender- and age-dependent γ-secretase activity in mouse brain and its implication in sporadic Alzheimer disease. PLoS One. 2009;4(4). 10.1371/journal.pone.0005088. [DOI] [PMC free article] [PubMed]
- 212.C. A. Lemere, J. K. Blusztajn, H. Yamaguchi, T. Wisniewski, T. C. Saido, and D. J. Selkoe, “Sequence of deposition of heterogeneous amyloid beta-peptides and APO E in Down syndrome: implications for initial events in amyloid plaque formation.,” Neurobiol. Dis., vol. 3, no. 1, pp. 16–32, Feb. 1996, [Online]. Available: https://neuro.unboundmedicine.com/medline/citation/9173910/Sequence_of_deposition_of_heterogeneous_amyloid_beta_peptides_and_APO_E_in_Down_syndrome:_implications_for_initial_events_in_amyloid_plaque_formation_. [DOI] [PubMed]
- 213.Kulenkampff K, Wolf Perez A-M, Sormanni P, Habchi J, Vendruscolo M. Quantifying misfolded protein oligomers as drug targets and biomarkers in Alzheimer and Parkinson diseases. Nat. Rev. Chem. 2021;5(4):277–294. doi: 10.1038/s41570-021-00254-9. [DOI] [PubMed] [Google Scholar]
- 214.Hortschansky P, Schroeckh V, Christopeit T, Zandomeneghi G, Fändrich M. The aggregation kinetics of Alzheimer’s β-amyloid peptide is controlled by stochastic nucleation. Protein Sci. 2005;14(7):1753–1759. doi: 10.1110/ps.041266605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Van Der Munnik NP, Moss MA, Uline MJ. Obstacles to translating the promise of nanoparticles into viable amyloid disease therapeutics. Phys. Biol. 2019;16(2). 10.1088/1478-3975/aafc66. [DOI] [PubMed]
- 216.van der Munnik NP, Sajib MSJ, Moss MA, Wei T, Uline MJ. Determining the potential of mean force for amyloid-β dimerization: combining self-consistent field theory with molecular dynamics simulation. J. Chem. Theory Comput. 2018;14(5):2696–2704. doi: 10.1021/acs.jctc.7b01057. [DOI] [PubMed] [Google Scholar]
- 217.Li S, Selkoe DJ. A mechanistic hypothesis for the impairment of synaptic plasticity by soluble Aβ oligomers from Alzheimer’s brain. J. Neurochem. 2020;154(6):583–597. doi: 10.1111/jnc.15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid β-protein on hippocampal synaptic plasticity: a potent role for trimers. J. Physiol. 2006;572(2):477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Walsh SDJ, Dominic M, Igor K, Fadeeva JV, Cullen WK, Roger A, Wolfe MS, Rowan MJ. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 220.Brinkmalm WDM, Gunnar HW, Zemin W, Wen L, O’Malley TT, Xin S, Frosch MP, Selkoe DJ, Erik P, Henrik Z, Kaj B. Identification of neurotoxic cross-linked amyloid-β dimers in the Alzheimer’s brain. Brain. 2019;142(5):1441–1457. doi: 10.1093/brain/awz066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Shankar SNE, Ganesh M, Shaomin L, Mehta TH, Amaya G-M, Smith Imelda SDJ, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008;14(8):837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Klyubin I, Cullen WK, Hu N-W, Rowan MJ. Alzheimer’s disease Aβ assemblies mediating rapid disruption of synaptic plasticity and memory. Mol. Brain. 2012;5:25. doi: 10.1186/1756-6606-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li S, Jin M, Liu L, Dang Y, Ostaszewski BL, Selkoe DJ. Decoding the synaptic dysfunction of bioactive human AD brain soluble Aβ to inspire novel therapeutic avenues for Alzheimer’s disease. Acta Neuropathol. Commun. 2018;6(1):121. doi: 10.1186/s40478-018-0626-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.De Felice KWL, Fernanda G, Velasco PT, Lambert MP, Kirsten V, Fernandez SJ, Ferreira ST. Aβ oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J. Biol. Chem. 2007;282(15):11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 225.Townsend M, Mehta T, Selkoe DJ. Soluble Aβ inhibits specific signal transduction cascades common to the insulin receptor pathway. J. Biol. Chem. 2007;282(46):33305–33312. doi: 10.1074/jbc.M610390200. [DOI] [PubMed] [Google Scholar]
- 226.Magdesian MH, Carvalho MMVF, Mendes FA, Saraiva LM, Juliano MA, Juliano L, Garcia-Abreu J, Ferreira ST. Amyloid-β binds to the extracellular cysteine-rich domain of Frizzled and inhibits Wnt/β-catenin signaling. J. Biol. Chem. 2008;283(14):9359–9368. doi: 10.1074/jbc.M707108200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Salminen A, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Inflammation in Alzheimer’s disease: amyloid-β oligomers trigger innate immunity defence via pattern recognition receptors. Prog. Neurobiol. 2009;87(3):181–194. doi: 10.1016/j.pneurobio.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 228.Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends Immunol. 2002;23(11):541–548. doi: 10.1016/S1471-4906(02)02316-5. [DOI] [PubMed] [Google Scholar]
- 229.Le Y, Gong W, Tiffany HL, Tumanov A, Nedospasov S, Shen W, Dunlop NM, Gao J-L, Murphy PM, Oppenheim JJ, Wang JM. Amyloid (beta)42 activates a G-protein-coupled chemoattractant receptor, FPR-like-1. J. Neurosci. 2001;21(2):2–6. doi: 10.1523/jneurosci.21-02-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Rabiet MJ, Huet E, Boulay F. The N-formyl peptide receptors and the anaphylatoxin C5a receptors: an overview. Biochimie. 2007;89(9):1089–1106. doi: 10.1016/j.biochi.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Coulson EJ. Does the p75 neurotrophin receptor mediate Aβ-induced toxicity in Alzheimer’s disease? J. Neurochem. 2006;98(3):654–660. doi: 10.1111/j.1471-4159.2006.03905.x. [DOI] [PubMed] [Google Scholar]
- 232.Kawahara M, Kuroda Y. Molecular mechanism of neurodegeneration induced by Alzheimer’s β-amyloid protein: channel formation and disruption of calcium homeostasis. Brain Res. Bull. 2000;53(4):389–397. doi: 10.1016/S0361-9230(00)00370-1. [DOI] [PubMed] [Google Scholar]
- 233.Oh S, Hong HS, Hwang E, Sim HJ, Lee W, Shin SJ, Mook-Jung I. Amyloid peptide attenuates the proteasome activity in neuronal cells. Mech. Ageing Dev. 2005;126(12):1292–1299. doi: 10.1016/j.mad.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 234.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-β in Alzheimer’s disease. Nat. Rev. Neurosci. 2007;8(7):499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 235.Kinoshita A, Fukumoto H, Shah T, Whelan CM, Irizarry MC, Hyman BT. Demonstration by FRET of BACE interaction with the amyloid precursor protein at the cell surface and in early endosomes. J. Cell Sci. 2003;116(16):3339–3346. doi: 10.1242/jcs.00643. [DOI] [PubMed] [Google Scholar]
- 236.Du Yan S, Fu J, Soto C, Chen X, Zhu H, Al-Mohanna F, Collison K, Zhu A, Stern E, Saido T, Tohyama M, Ogawa S, Roher A, Stern D. An intracellular protein that binds amyloid-β peptide and mediates neurotoxicity in Alzheimer’s disease. Nature. 1997;389(6652):689–695. doi: 10.1038/39522. [DOI] [PubMed] [Google Scholar]
- 237.Wang H-Y, Lee DHS, D’Andrea MR, Peterson PA, Shank RP, Reitz AB. β-Amyloid1–42 binds to α7 nicotinic acetylcholine receptor with high affinity: implications for Alzheimer’s disease pathology. J. Biol. Chem. 2000;275(8):5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- 238.Nagele RG, D’andrea MR, Anderson WJ, Wang H-Y. Intracellular accumulation of β-amyloid1–42 in neurons is facilitated by the α7 nicotinic acetylcholine receptor in Alzheimer’s disease. Neuroscience. 2002;110(2):199–211. doi: 10.1016/S0306-4522(01)00460-2. [DOI] [PubMed] [Google Scholar]
- 239.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2008;7(12):1013–1030. doi: 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- 240.Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005;62(6):670–684. doi: 10.1007/s00018-004-4464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Buchberger A, Bukau B, Sommer T. Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol. Cell. 2010;40(2):238–252. doi: 10.1016/j.molcel.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 242.Bernales S, Schuck S, Walter P. ER-Phagy: selective autophagy of the endoplasmic reticulum. Autophagy. 2007;3(3):285–287. doi: 10.4161/auto.3930. [DOI] [PubMed] [Google Scholar]
- 243.Groenendyk J, Sreenivasaiah PK, Kim DH, Agellon LB, Michalak M. Biology of endoplasmic reticulum stress in the heart. Circ. Res. 2010;107(10):1185–1197. doi: 10.1161/CIRCRESAHA.110.227033. [DOI] [PubMed] [Google Scholar]
- 244.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 245.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000;2(6):326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 246.Battson ML, Lee DM, Gentile CL. Endoplasmic reticulum stress and the development of endothelial dysfunction. Am. J. Physiol. - Hear. Circ. Physiol. 2017;312(3):H355–H367. doi: 10.1152/ajpheart.00437.2016. [DOI] [PubMed] [Google Scholar]
- 247.Cao SS, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 2014;21(3):396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Cheng WP, Wang BW, Shyu KG. Regulation of GADD153 induced by mechanical stress in cardiomyocytes. Eur. J. Clin. Invest. 2009;39(11):960–971. doi: 10.1111/j.1365-2362.2009.02193.x. [DOI] [PubMed] [Google Scholar]
- 249.Fu HY, Okada KI, Liao Y, Tsukamoto O, Isomura T, Asai M, Sawada T, Okuda K, Asano Y, Sanada S, Asanuma H. Ablation of C/EBP homologous protein attenuates endoplasmic reticulum–mediated apoptosis and cardiac dysfunction induced by pressure overload. Circulation. 2010;122(4):361–369. doi: 10.1161/CIRCULATIONAHA.109.917914. [DOI] [PubMed] [Google Scholar]
- 250.Urra H, Dufey E, Lisbona F, Rojas-Rivera D, Hetz C. When ER stress reaches a dead end. Biochim. Biophys. Acta - Mol. Cell Res. 2013;1833(12):3507–3517. doi: 10.1016/j.bbamcr.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 251.Yamazaki KM, Hiroaki HN, Kunihiro H, Yasuhiro T, Maro O, Ryouji O, Tao H, Shotaro N, Jian Y, Paton AW, Patos JC. Activation of the Akt-NF-kappaB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J. Immunol. 2009;183(2):1480–1487. doi: 10.4049/jimmunol.0900017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Krebs J, Agellon LB, Michalak M. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 2015;460(1):114–121. doi: 10.1016/j.bbrc.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 253.Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin. Cell Dev. Biol. 2007;18(6):716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Soboloff J, Rothberg BS, Madesh M, Gill DL. STIM proteins: dynamic calcium signal transducers. Nat. Rev. Mol. Cell Biol. 2012;13(9):549–565. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Hong BP, Min LS, Huang Jen-Ming LAS, Gogia RK, Mingqing L. Underglycosylation of ATF6 as a novel sensing mechanism for activation of the unfolded protein response*. J. Biol. Chem. 2004;279(12):11354–11363. doi: 10.1074/jbc.M309804200. [DOI] [PubMed] [Google Scholar]
- 256.Arensdorf A, Diedrichs D, Rutkowski T. Regulation of the transcriptome by ER stress: non-canonical mechanisms and physiological consequences. Frontiers in Genetics. 2013;4. [Online]. Available: https://www.frontiersin.org/articles/10.3389/fgene.2013.00256. Accessed 10 Oct 2022. [DOI] [PMC free article] [PubMed]
- 257.Garg AD, Kaczmarek A, Krysko O, Vandenabeele P, Krysko DV, Agostinis P. ER stress-induced inflammation: does it aid or impede disease progression? Trends Mol. Med. 2012;18(10):589–598. doi: 10.1016/j.molmed.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 258.Ron D. Translational control in the endoplasmic reticulum stress response. J. Clin. Invest. 2002;110(10):1383–1388. doi: 10.1172/JCI0216784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Pakos-Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, Gorman AM. The integrated stress response. EMBO Rep. 2016;17(10):1374–1395. doi: 10.15252/embr.201642195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Liu L, et al. GADD34 facilitates cell death resulting from proteasome inhibition. Anticancer Res. 2015;35(10):5317–5324. [PubMed] [Google Scholar]
- 261.Yoshida H. ER stress and diseases. FEBS J. 2007;274(3):630–658. doi: 10.1111/j.1742-4658.2007.05639.x. [DOI] [PubMed] [Google Scholar]
- 262.Niwa M, Sidrauski C, Kaufman RJ, Walter P. A role for presenilin-1 in nuclear accumulation of Ire1 fragments and induction of the mammalian unfolded protein response. Cell. 1999;99(7):691–702. doi: 10.1016/S0092-8674(00)81667-0. [DOI] [PubMed] [Google Scholar]
- 263.Kakizuka A. Protein precipitation: a common etiology in neurodegenerative disorders? Trends Genet. 1998;14(10):396–402. doi: 10.1016/S0168-9525(98)01559-5. [DOI] [PubMed] [Google Scholar]
- 264.Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ. Res. 2010;107(9):1071–1082. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- 265.Hoozemans JJM, et al. The unfolded protein response is activated in Alzheimer’s disease. Acta Neuropathol. 2005;110(2):165–172. doi: 10.1007/s00401-005-1038-0. [DOI] [PubMed] [Google Scholar]
- 266.Glembotski CC. Endoplasmic reticulum stress in the heart. Circ. Res. 2007;101(10):975–984. doi: 10.1161/CIRCRESAHA.107.161273. [DOI] [PubMed] [Google Scholar]
- 267.Hitomi Yutaka IK, Katayama J, Eguchi T, Takashi K, Manabu T, Yoshihisa K, Takayuki M, Satoru Y, Yoshio B. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Aβ-induced cell death. J. Cell Biol. 2004;165(3):347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Onuki SE, Reiko BY, Katayama Taiichi TK, Hiroaki K, Tadashi B, Masaya T. An RNA-dependent protein kinase is involved in tunicamycin-induced apoptosis and Alzheimer’s disease. EMBO J. 2004;23(4):959–968. doi: 10.1038/sj.emboj.7600049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Nishitsuji MH, Kazuchika TT, Kenichi I, Kazuhiro I, Rie T, Lambert MP, Klein WL. The E693 Delta mutation in amyloid precursor protein increases intracellular accumulation of amyloid β oligomers and causes endoplasmic reticulum stress-induced apoptosis in cultured cells. Am. J. Pathol. 2009;174(3):957–969. doi: 10.2353/ajpath.2009.080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Alberdi MC, Elena WA, María A, Sánchez-Gómez Ma V, Cavaliere F, Rodríguez JJ, Alexei V. Ca2+-dependent endoplasmic reticulum stress correlates with astrogliosis in oligomeric amyloid β-treated astrocytes and in a model of A lzheimer’s disease. Aging Cell. 2013;12(2):292–302. doi: 10.1111/acel.12054. [DOI] [PubMed] [Google Scholar]
- 271.LaFerla FM. Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat. Rev. Neurosci. 2002;3(11):862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- 272.Laurén J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature. 2009;457(7233):1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Berridge MJ. Calcium hypothesis of Alzheimer’s disease. Pflugers Arch. Eur. J. Physiol. 2010;459(3):441–449. doi: 10.1007/s00424-009-0736-1. [DOI] [PubMed] [Google Scholar]
- 274.Ferreiro E, Resende R, Costa R, Oliveira CR, Pereira CMF. An endoplasmic-reticulum-specific apoptotic pathway is involved in prion and amyloid-beta peptides neurotoxicity. Neurobiol. Dis. 2006;23(3):669–678. doi: 10.1016/j.nbd.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 275.Stutzmann GE, Smith I, Caccamo A, Oddo S, LaFerla FM, Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice. J. Neurosci. 2006;26(19):5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Smith IF, Hitt B, Green KN, Oddo S, LaFerla FM. Enhanced caffeine-induced Ca2+ release in the 3xTg-AD mouse model of Alzheimer’s disease. J. Neurochem. 2005;94(6):1711–1718. doi: 10.1111/j.1471-4159.2005.03332.x. [DOI] [PubMed] [Google Scholar]
- 277.Palop ML, Jorge J, Brian J, Lisa K, Chin Jeannie Y, Gui-Qiu RJ, Eliezer M. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer’s disease-related cognitive deficits. Proc. Natl. Acad. Sci. 2003;100(16):9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Ohno M. Roles of eIF2α kinases in the pathogenesis of Alzheimer’s disease. Front. Mol. Neurosci. 2014;7:22. doi: 10.3389/fnmol.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Mitsuda T, Hayakawa Y, Itoh M, Ohta K, Nakagawa T. ATF4 regulates γ-secretase activity during amino acid imbalance. Biochem. Biophys. Res. Commun. 2007;352(3):722–727. doi: 10.1016/j.bbrc.2006.11.075. [DOI] [PubMed] [Google Scholar]
- 280.Wei N, Zhu L-Q, Liu D. ATF4: a novel potential therapeutic target for Alzheimer’s disease. Mol. Neurobiol. 2015;52(3):1765–1770. doi: 10.1007/s12035-014-8970-8. [DOI] [PubMed] [Google Scholar]
- 281.Salminen A, Kauppinen A, Suuronen T, Kaarniranta K, Ojala J. ER stress in Alzheimer’s disease: A novel neuronal trigger for inflammation and Alzheimer’s pathology. J. Neuroinflammation. 2009;6:1–13. doi: 10.1186/1742-2094-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Hoozemans JJM, van Haastert ES, Nijholt DAT, Rozemuller AJM, Eikelenboom P, Scheper W. The UNFOLDED protein response is activated in pretangle neurons in Alzheimer’s disease hippocampus. Am. J. Pathol. 2009;174(4):1241–1251. doi: 10.2353/ajpath.2009.080814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Lagalwar S, Berry RW, Binder LI. Relation of hippocampal phospho-SAPK/JNK granules in Alzheimer’s disease and tauopathies to granulovacuolar degeneration bodies. Acta Neuropathol. 2007;113(1):63–73. doi: 10.1007/s00401-006-0159-4. [DOI] [PubMed] [Google Scholar]
- 284.Resende R, Ferreiro E, Pereira C, Oliveira CR. ER stress is involved in Aβ-induced GSK-3β activation and tau phosphorylation. J. Neurosci. Res. 2008;86(9):2091–2099. doi: 10.1002/jnr.21648. [DOI] [PubMed] [Google Scholar]
- 285.Unterberger U, Höftberger R, Gelpi E, Flicker H, Budka H, Voigtländer T. Endoplasmic reticulum stress features are prominent in Alzheimer disease but not in prion diseases in vivo. J. Neuropathol. Exp. Neurol. 2006;65(4):348–357. doi: 10.1097/01.jnen.0000218445.30535.6f. [DOI] [PubMed] [Google Scholar]
- 286.Roßner S, Sastre M, Bourne K, Lichtenthaler SF. Transcriptional and translational regulation of BACE1 expression—implications for Alzheimer’s disease. Prog. Neurobiol. 2006;79(2):95–111. doi: 10.1016/j.pneurobio.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 287.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430(7000):631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Kaltschmidt B, Uherek M, Volk B, Baeuerle PA, Kaltschmidt C. Transcription factor NF-κB is activated in primary neurons by amyloid β peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc. Natl. Acad. Sci. 1997;94(6):2642–2647. doi: 10.1073/pnas.94.6.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Kaltschmidt B, Uherek M, Wellmann H, Volk B, Kaltschmidt C. Inhibition of NF-κB potentiates amyloid β-mediated neuronal apoptosis. Proc. Natl. Acad. Sci. 1999;96(16):9409–9414. doi: 10.1073/pnas.96.16.9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Song S, et al. E2-25K/Hip-2 regulates caspase-12 in ER stress–mediated Aβ neurotoxicity. J. Cell Biol. 2008;182(4):675–684. doi: 10.1083/jcb.200711066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Kajiwara Y, et al. FE65 binds teashirt, inhibiting expression of the primate-specific caspase-4. PLoS One. 2009;4(4):e5071. doi: 10.1371/journal.pone.0005071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kraskiewicz H, FitzGerald U. InterfERing with endoplasmic reticulum stress. Trends Pharmacol. Sci. 2012;33(2):53–63. doi: 10.1016/j.tips.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 293.Lee LHJ, Yeon D, Kyu-Sun L, Kim YYC, Hee D, Hun NY, Kweon Y, Hee-Yeon J, Hyung LS, Young LJ. Activation of PERK signaling attenuates Aβ-mediated ER stress. PLoS One. 2010;5(5):e10489. doi: 10.1371/journal.pone.0010489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Sokka A-L, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, Belluardo N, Lindholm D, Korhonen L. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J. Neurosci. 2007;27(4):901–908. doi: 10.1523/JNEUROSCI.4289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zimmerman MC, Davisson RL. Redox signaling in central neural regulation of cardiovascular function. Prog. Biophys. Mol. Biol. 2004;84(2–3):125–149. doi: 10.1016/j.pbiomolbio.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 296.Young CN, et al. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J. Clin. Invest. 2012;122(11):3960–3964. doi: 10.1172/JCI64583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Spitler KM, Webb RC. Endoplasmic reticulum stress contributes to aortic stiffening via pro-apoptotic and fibrotic signaling mechanisms. Hypertension. 2015;63(3):1–15. doi: 10.1161/HypertensionAHA.113.02558.CHBPR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Spitler KM, Webb RC. Endoplasmic reticulum stress contributes to aortic stiffening via proapoptotic and fibrotic signaling mechanisms. Hypertension. 2014;63(3):40–45. doi: 10.1161/HYPERTENSIONAHA.113.02558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.He L, Yuan J, Xu Q, Chen R, Chen L, Fang M. miRNA-1283 regulates the PERK/ATF4 pathway in vascular injury by targeting ATF4. PLoS One. 2016;11(8):1–17. doi: 10.1371/journal.pone.0159171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Yang S, et al. The human ATF1 rs11169571 polymorphism increases essential hypertension risk through modifying miRNA binding. FEBS Lett. 2015;589(16):2087–2093. doi: 10.1016/j.febslet.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 301.Wang D, Song W, Na Q. The emerging roles of placenta-specific microRNAs in regulating trophoblast proliferation during the first trimester. Aust. New Zeal. J. Obstet. Gynaecol. 2012;52(6):565–570. doi: 10.1111/j.1479-828X.2012.01481.x. [DOI] [PubMed] [Google Scholar]
- 302.Chen W, et al. Mir-1283 contributes to endoplasmic reticulum stress in the development of hypertension through the activating transcription factor-4 (Atf4)/C/Ebp-homologous protein (Chop) signaling pathway. Med. Sci. Monit. 2021;27:1–18. doi: 10.12659/MSM.930552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Brandes RP, Weissmann N, Schröder K. NADPH oxidases in cardiovascular disease. Free Radic. Biol. Med. 2010;49(5):687–706. doi: 10.1016/j.freeradbiomed.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 304.Popovic N, Bridenbaugh EA, Neiger JD, Hu J-J, Vannucci M, Mo Q, Trzeciakowski J, Miller MW, Fossum TW, Humphrey JD, Wilson E. Transforming growth factor-β signaling in hypertensive remodeling of porcine aorta. Am. J. Physiol. Circ. Physiol. 2009;297(6):H2044–H2053. doi: 10.1152/ajpheart.01015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Thameem F, Farook VS, Bogardus C, Prochazka M. Association of amino acid variants in the activating transcription factor 6 gene (ATF6) on 1q21-q23 with type 2 diabetes in Pima Indians. Diabetes. 2006;55(3):839–842. doi: 10.2337/diabetes.55.03.06.db05-1002. [DOI] [PubMed] [Google Scholar]
- 306.Araki E, Oyadomari S, Mori M. Endoplasmic reticulum stress and diabetes mellitus. Intern. Med. 2003;42(1):7–14. doi: 10.2169/internalmedicine.42.7. [DOI] [PubMed] [Google Scholar]