Abstract
Obesity and type 2 diabetes (T2D) are associated with increased risk of pancreatic cancer. Here we assessed the relationship between pancreatic cancer and two distinct measures of obesity, namely total adiposity, using BMI, versus abdominal adiposity, using BMI adjusted waist-to-hip ratio (WHRadjBMI) by utilising polygenic scores (PGS) and Mendelian randomisation (MR) analyses. We constructed z-score weighted PGS for BMI and WHRadjBMI using publicly available data and tested for their association with pancreatic cancer defined in UK biobank (UKBB). Using publicly available summary statistics, we then performed bi-directional MR analyses between the two obesity traits and pancreatic cancer. PGSBMI was significantly (multiple testing-corrected) associated with pancreatic cancer (OR[95%CI] = 1.0804[1.025–1.14], P = 0.0037). The significance of association declined after T2D adjustment (OR[95%CI] = 1.073[1.018–1.13], P = 0.00904). PGSWHRadjBMI association with pancreatic cancer was at the margin of statistical significance (OR[95%CI] = 1.047[0.99–1.104], P = 0.086). T2D adjustment effectively lost any suggestive association of PGSWHRadjBMI with pancreatic cancer (OR[95%CI] = 1.039[0.99–1.097], P = 0.14). MR analyses showed a nominally significant causal effect of WHRadjBMI on pancreatic cancer (OR[95%CI] = 1.00095[1.00011–1.0018], P = 0.027) but not for BMI on pancreatic cancer. Overall, we show that abdominal adiposity measured using WHRadjBMI, may be a more important causal risk factor for pancreatic cancer compared to total adiposity, with T2D being a potential driver of this relationship.
Subject terms: Obesity, Genome-wide association studies
Introduction
Pancreatic cancer is a rare form of cancer, associated with poor prognosis and low survival rates [1]. Furthermore, epidemiological evidence from observational studies suggests obesity and type 2 diabetes (T2D) are major risk factors for pancreatic cancer [2, 3]. Body mass index (BMI) and waist-to-hip ratio (WHR) are two common metrics used to assess total and abdominal adiposity. However, despite being a routine measure of adiposity in clinical and research settings, BMI is an imperfect measure of metabolic health. Alternatively, WHR represents abdominal adiposity which has a stronger correlation to the metabolic syndrome compared to total adiposity [4]. To date, only 22 genome-wide significant signals are established in genome-wide association studies (GWAS) for pancreatic cancer [5]. In contrast, more than 600 and 300 signals have been reported for BMI and WHR, respectively [6, 7]. These individual associations from GWAS, however, do not explain the shared co-morbidity between obesity and pancreatic cancer. Nevertheless, genomic loci identified in GWAS could be implemented in methods such as polygenic scores (PGS) [8] and Mendelian randomization (MR) [9]. PGS can be used to define the shared genetic component between epidemiologically related phenotypes, while MR uses genetic variants as instruments to assess causality in relationships between phenotypes. In the present study, the impact of total and abdominal adiposity on pancreatic cancer risk was examined through PGS analyses, using publicly available GWAS of obesity traits data and information about pancreatic cancer within UK biobank. Moreover, using established genetic variants, we conducted a bi-directional MR between two adiposity traits and pancreatic cancer to assess the causal relationships between them.
Materials and methods
UK Biobank
The UK Biobank (UKBB) resource (www.ukbiobank.ac.uk) was used to define adiposity and cancer phenotypes for this study. We used the BMI data collected at the time of recruitment (UKBB field 21001). WHR data were computed by dividing waist circumference (UKBB field 48) by hip circumference (UKBB field 49) measured at baseline. BMI and WHR data were available for 457,270 individuals (Supplementary Fig. 1). For pancreatic cancer, we used a combination of hospital admissions data, the tenth revision of the International Classification of Disease (ICD-10) codes and self-report data. Individuals with an ICD-10 code (code C25) and who self-reported to have a pancreatic cancer diagnosis (code 1026) were set as cases, while individuals with no cancer diagnosis were set as controls. In total, there were 1416 cases and 455,854 controls (n = 457,270) for pancreatic cancer. To limit confounding by ancestry, only individuals of European ancestry were included in our analyses (Supplementary Methods, Supplementary Fig. 1).
UKBB GWAS
We performed single phenotype GWAS in UKBB using the BOLT-LMM software [10]. BOLT-LMM applies a linear mixed model while age, sex, genotyping array and six principal components (PCs) were used as covariates for pancreatic cancer and BMI. BMI was an extra covariate in WHR GWAS to obtain WHRadjBMI analyses. The statistical threshold for genome-wide significant SNPs used was P < 5 × 10−8.
Genetic correlation estimation
To estimate the genetic correlation (rG) between adiposity phenotypes (BMI/WHRadjBMI), T2D (Supplementary Methods), and pancreatic cancer in UKBB, we used the linkage disequilibrium (LD) score (LDSC) regression approach and tool [11].
Polygenic scores
To construct BMI and WHRadjBMI PGS, we used risk-increasing alleles at 567 and 274 SNPs, respectively. The SNP list was obtained from recent large-scale GWAS meta-analyses by GIANT consortium [6, 7]. However, as the target data for PGS analysis was the UKBB, which was part of the GIANT meta-analyses, we used weights from the study which did not include UKBB in the meta-analyses [12, 13] (Supplementary Fig. 2). We used the PLINK software [14] to generate the PGS. We used sex, age, genotyping array and six PCs as covariates in the regression model. As a sensitivity analysis, we ran a regression model with T2D as an extra covariate.
Mendelian randomization
To assess causality between the two adiposity measures and pancreatic cancer, we performed bi-directional MR using the TwoSampleMR R package [15]. We obtained the genetic instrument for BMI (566 SNPs) and WHRadjBMI (278 SNPs) from the GIANT consortium [6, 7]. The genetic instruments for pancreatic cancer (16 SNPs) were obtained from Klein et al [5]. The causal effect estimate was derived from the inverse-variance weighted (IVW) method [16]. The MR-Egger, simple mode, weighted mode and weighted median tests were used as sensitivity analyses [17]. We excluded palindromic SNPs from the exposure-outcome pairs and matched alleles between summary statistics as part of the TwoSampleMR pipeline. Outliers were removed after inspection of scatter plots and leave-one-out results. Heterogeneity among the genetic instruments was evaluated using Cochran’s Q test.
Results
UKBB GWAS and genetic correlation estimates
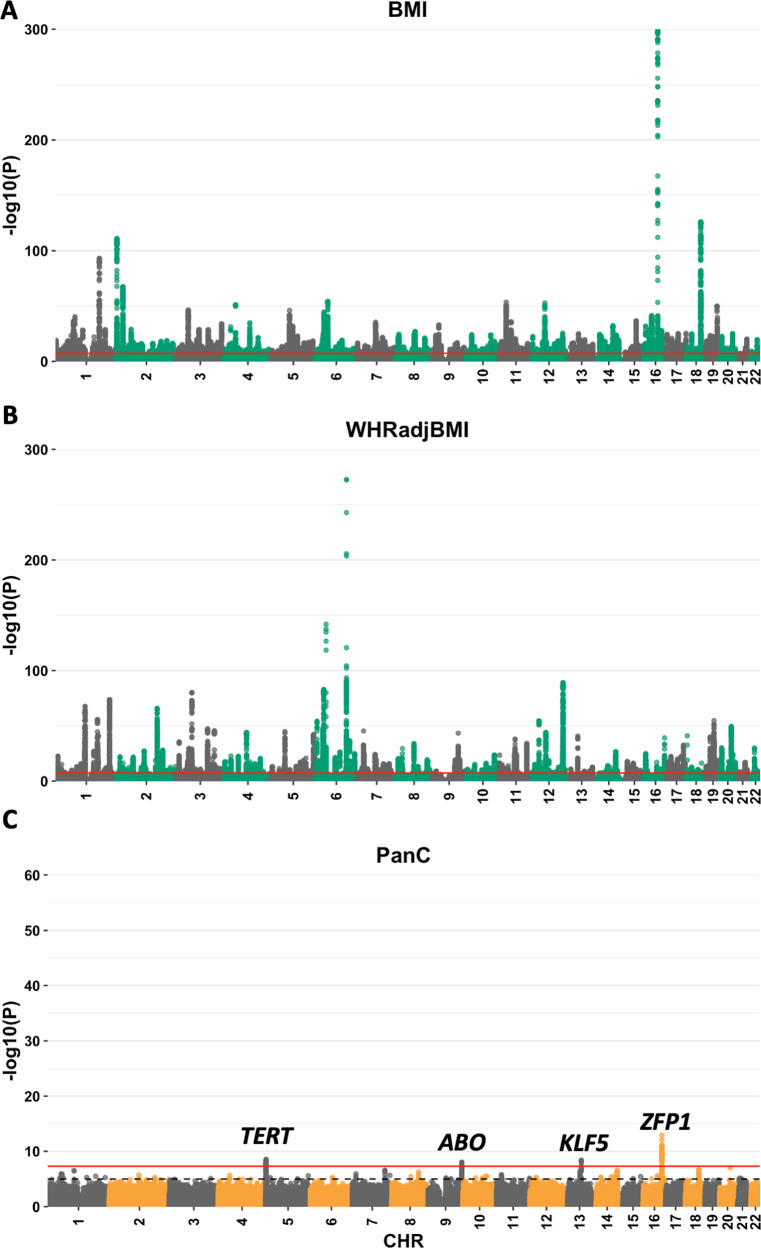
In UKBB GWAS, we identified 998, 1014 and 4 significant independent SNPs at 901, 718, 4 loci for BMI, WHRadjBMI and pancreatic cancer respectively (Fig. 1). The four loci identified for pancreatic cancer were TERT, ABO, KLF and ZFP1 (Fig. 1C) in line with recently published GWAS of pancreatic cancer [5]. None of the obesity signals were shared with pancreatic cancer in the UKBB. However, 3 of the 22 established pancreatic cancer loci by Klein et al. [5] were shared with WHRadjBMI in UKBB and had same direction of effect. These were NR5A2, ETAA1 and ZNRF3. Conversely, only ETAA1 from Klein et al. [5] was shared with BMI in the UKBB. Additionally, there was positive genetic correlation between both obesity measures and pancreatic cancer, but the estimates did not meet statistical significance (rGBMI = 0.472, P = 0.479, rGWHRadjBMI = 0.098, P = 0.671) (Supplementary Table 1). Similarly, the genetic correlation between T2D and pancreatic cancer in the UKBB was underpowered and did not meet statistical significance (rG = −0.0139, P = 0.961) (Supplementary Table 1).
Fig. 1. UK Biobank GWAS results.
Manhattan plots of A BMI, B WHRadjBMI, and C pancreatic cancer GWAS in UK Biobank. The red horizontal line shows genome-wide significance threshold (P < 5 × 10−8). The dashed grey line shows suggestive significance threshold (P < 1 × 10−5).
Effects of obesity variants on pancreatic cancer via polygenic scores
We identified a significant (Bonferroni multiple testing corrected P = 0.05/2 tests = 0.025) direct association between BMI PGS and pancreatic cancer (OR[95%CI] = 1.0804[1.025–1.14], P = 0.0037). We also identified a direct association between WHRadjBMI PGS and pancreatic cancer, however, this association was not statistically significant (OR[95%CI] = 1.047[0.99–1.104], P = 0.086) (Table 1). To determine if the association between adiposity PGS and pancreatic cancer was driven by T2D, we adjusted for T2D in the association tests. After T2D adjustment, the significance of the association for both BMI and WHRadjBMI PGS declined to suggest that T2D could be acting via adiposity in pancreatic cancer risk (ORBMI_PGS[95%CI] = 1.073[1.018–1.13], P = 0.00904); ORWHRadjBMI_PGS[95%CI] = 1.039[0.99–1.097], P = 0.14). Notably, the decline in association after T2D adjustment was more for WHRadjBMI PGS than BMI PGS (Table 1).
Table 1.
Association between adiposity polygenic scores and pancreatic cancer.
| Unadjusted model | T2D adjusted model | |||
|---|---|---|---|---|
| Adiposity trait | OR (95%CI) | P | OR (95%CI) | P |
| BMI | 1.0804 (1.025–1.14) | 0.0037 | 1.073 (1.018–1.13) | 0.00904 |
| WHRadjBMI | 1.047 (0.99–1.104) | 0.086 | 1.039 (0.99–1.097) | 0.14 |
OR(95%CI) = Odds ratio of association and the lower and upper 95% confidence intervals (CI).
Causality results using Mendelian randomization
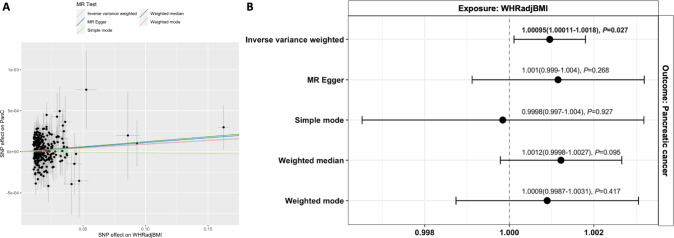
We report a causal effect of WHRadjBMI on pancreatic cancer at nominal significance (OR[95%CI] = 1.00095[1.00011–1.0018], P = 0.027) based on the IVW method, indicating a weak but positive causal effect estimate (Fig. 2). However, none of the other MR tests for this direction were significant. The Cochran’s Q test indicated the absence of heterogeneity among the genetic instruments (QIVW = 258.08, P = 0.787). On the contrary, we have not identified any causal effect (Bonferroni P = 0.05/4 tests = 0.0125) of BMI on pancreatic cancer in either of the MR tests performed (Supplementary Table 2). There was no evidence of a causal effect from pancreatic cancer to WHRadjBMI (ORIVW(P) = 0.143(0.604). The results from pancreatic cancer to BMI were less informative with large standard errors despite nominal significance in some of the sensitivity MR tests (ORWeightedMedian[95%CI] = 58.105[3.997–844.69], P = 0.003) (Supplementary Table 2).
Fig. 2. Abdominal obesity MR results.
A Scatter and B forest plots for the WHRadjBMI to pancreatic cancer MR test. The scatter plot includes the intercepts of the various MR methods used while the odds ratio plot shows the MR effect estimate for each MR method used.
Discussion
In this study, using large-scale datasets and a multi-method approach, we show that abdominal obesity assessed using WHRadjBMI is a causal risk factor for pancreatic cancer, in line with epidemiological evidence [18].
The mechanisms underlying the obesity-pancreatic cancer co-morbidity remain unclear. However, several factors such as inflammation, insulin resistance and hyperinsulinemia are potential mechanisms linking obesity to cancers including that of the pancreas [3, 19]. Notably, majority of these factors are hallmarks of metabolic syndrome which correlate with abdominal obesity [20]. Therefore, it is not surprising that our Mendelian randomisation results show that WHRadjBMI rather than BMI is a more important causal risk factor for pancreatic cancer. Furthermore, the metabolic syndrome is considered a predictor of T2D [21]. In our polygenic score analyses, we show that after adjusting for T2D status, the significance of the association declined modestly for PGSBMI while any evidence of association in PGSWHRadjBMI on pancreatic cancer risk was effectively lost. Taken together, our polygenic analyses and Mendelian randomization suggest that the metabolic syndrome proxied by abdominal obesity may be a causal risk factor for pancreatic cancer. Additionally, obesity-associated T2D [22] may be a potential cause driver of the metabolic syndrome underlying pancreatic cancer progression in obesity [3].
Several limitations in our present studies should be considered. Pancreatic cancer is a rare form of cancer characterised by low sample sizes as compared to other more common cancers. Consequently, there is less power in GWAS to identify genetic loci amenable for statistical analyses. Additionally, the causal effect identified in MR is only nominally significant and therefore interpretation of our findings should consider this. Future work will focus on validating our results in larger datasets, especially for pancreatic cancer to improve statistical power of the analyses. Moreover, further analyses to properly control for T2D would be needed due to the complex relationship between obesity and T2D, more so in Mendelian randomization. Additional analyses to include components of the metabolic dysfunction such as fasting glucose levels will be part of future direction of this effort.
In conclusion, we show that abdominal adiposity measured through WHRadjBMI, may be a more important risk factor for pancreatic cancer, compared to total adiposity. Our results highlight the relationship between the metabolic syndrome component and a higher risk for pancreatic cancer, with T2D being a potential driver of this association. Furthermore, we demonstrate the importance and therefore encourage the assessment of diverse measures of obesity in clinical practice and research in the context of pancreatic cancer risk. Additionally, healthcare providers should emphasise the need for patients to monitor their visceral weight gain and not just overall weight gain to minimise the risk for pancreatic cancer.
Supplementary information
Acknowledgements
We would like to thank the Genetic Investigation of Anthropometric Traits (GIANT) consortium for enabling access to their GWAS summary statistics which we have used in our PGS and MR analyses. We would also like to appreciate the participants and staff of the UKBB for their valuable contributions. This work was conducted under the UKBB application number 37685.
Author contributions
JDM, MK, PF and IP designed the study and interpreted the results. JGM performed the analyses, wrote the original draft of the manuscript, and edited the manuscript. VP, LD, AU, IP, AB, ZB and MK performed the polygenic score and MR analyses and contributed to the interpretation of the results. All authors revised the manuscript.
Funding
This research has been conducted using the UK Biobank resource application number 37685. This research was in part funded by the World Cancer Research Fund (WCRF UK) and World Cancer Research Fund International (2017/1641), the Diabetes UK (BDA number: 20/0006307), the European Union’s Horizon 2020 research and innovation programme (LONGITOOLS, H2020-SCI-2019-874739), Agence Nationale de la Recherche (PreciDIAB, ANR-18-IBHU-0001), by the European Union through the “Fonds Européen de Dévelopment Regional” (FEDER), by the “Conseil Régional des Hauts-de-France” (Hauts-de-France Regional Council) and by the “Métropole Européenne de Lille” (MEL, European Metropolis of Lille).
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Philippe Froguel, Email: p.froguel@ic.ac.uk.
Inga Prokopenko, Email: i.prokopenko@surrey.ac.uk.
Supplementary information
The online version contains supplementary material available at 10.1038/s41431-023-01301-3.
References
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res [Internet] 2014;74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 2.Xu M, Jung X, Hines OJ, Eibl G, Chen Y. Obesity and pancreatic cancer: overview of epidemiology and potential prevention by weight loss. Pancreas [Internet] 2018;47:158–62. doi: 10.1097/MPA.0000000000000974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Froguel P, Bonnefond A. Does Type 2 diabetes increase the risk of developing cancer? Diabetes Manag [Internet] 2013;3:439–41. doi: 10.2217/dmt.13.49. [DOI] [Google Scholar]
- 4.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome. Circulation [Internet]. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 5.Klein AP, Wolpin BM, Risch HA, Stolzenberg-Solomon RZ, Mocci E, Zhang M, et al. Genome-wide meta-analysis identifies five new susceptibility loci for pancreatic cancer. Nat Commun. 2018;9:1–11. doi: 10.1038/s41467-018-02942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet [Internet] 2018;27:3641–9. doi: 10.1093/hmg/ddy271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulit SL, Stoneman C, Morris AP, Wood AR, Glastonbury CA, Tyrrell J, et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum Mol Genet [Internet] 2019;28:166–74. doi: 10.1093/hmg/ddy327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis CM, Vassos E. Polygenic risk scores: from research tools to clinical instruments. Genome Med [Internet] 2020;12:1–11. doi: 10.1186/s13073-020-00742-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Smith GD. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat Med [Internet] 2008;27:1133–63. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 10.Loh PR, Tucker G, Bulik-Sullivan BK, Vilhjálmsson BJ, Finucane HK, Salem RM, et al. Efficient Bayesian mixed-model analysis increases association power in large cohorts. Nat Genet. 2015;47:284–90. doi: 10.1038/ng.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulik-Sullivan B, Loh PR, Finucane HK, Ripke S, Yang J, Patterson N, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–5. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nat [Internet] 2015;518:197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shungin D, Winkler T, Croteau-Chonka DC, Ferreira T, Locke AE, Mägi R, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518:187–96. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-base platform supports systematic causal inference across the human phenome. Elife. 2018;7:e34408. doi: 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol [Internet] 2013;37:658–65. doi: 10.1002/gepi.21758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowden J, Holmes MV. Meta-analysis and Mendelian randomization: a review. Res Synth Methods [Internet] 2019;10:486–96. doi: 10.1002/jrsm.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia B, He Q, Pan Y, Gao F, Liu A, Tang Y, et al. Metabolic syndrome and risk of pancreatic cancer: a population-based prospective cohort study. Int J Cancer [Internet] 2020;147:3384–93. doi: 10.1002/ijc.33172. [DOI] [PubMed] [Google Scholar]
- 19.Scully T, Ettela A, LeRoith D, Gallagher EJ. Obesity, type 2 diabetes, and cancer risk. Front Oncol. 2021;10:3196. doi: 10.3389/fonc.2020.615375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lalia AZ, Dasari S, Johnson ML, Robinson MM, Konopka AR, DIstelmaier K, et al. Predictors of whole-body insulin sensitivity across ages and adiposity in adult humans. J Clin Endocrinol Metab [Internet] 2016;101:626–34. doi: 10.1210/jc.2015-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin JA, Lee JH, Lim SY, Ha HS, Kwon HS, Park YM, et al. Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J Diabetes Investig [Internet] 2013;4:334–43. doi: 10.1111/jdi.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fall T, Hägg S, Mägi R, Ploner A, Fischer K, Horikoshi M, et al. The role of adiposity in cardiometabolic traits: a mendelian randomization analysis. PLoS Med [Internet] 2013;10:e1001474. doi: 10.1371/journal.pmed.1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.