Abstract
The challenges and ambiguities in providing an accurate diagnosis for patients with neurodevelopmental disorders have led researchers to apply epigenetics as a technique to validate the diagnosis provided based on the clinical examination and genetic testing results. Genome-wide DNA methylation analysis has recently been adapted for clinical testing of patients with genetic neurodevelopmental disorders. In this paper, preliminary data demonstrating a DNA methylation signature for Renpenning syndrome (RENS1 – OMIM 309500), which is an X-linked recessive neurodevelopmental disorder caused by variants in polyglutamine-binding protein 1 (PQBP1) is reported. The identified episignature was then utilized to construct a highly sensitive and specific binary classification model. Besides providing evidence for the existence of a DNA methylation episignature for Renpenning syndrome, this study increases the knowledge of the molecular mechanisms related to the disease. Moreover, the availability of more subjects in future may facilitate the establishment of an episignature that can be utilized for diagnosis in a clinical setting and for reclassification of variants of unknown clinical significance.
Subject terms: DNA methylation, Neurodevelopmental disorders, Diagnostic markers
Introduction
Mendelian neurodevelopmental disorders (NDs) and congenital anomalies (CAs) occur at an approximate rate of 40–82 per 1000 live births [1]. Traditional genetic testing leaves a notable proportion of patients with rare genetic disorders unresolved. In many occurrences, the causal variant is not known or the detected variant is of unknown clinical significance. Many NDs/CAs are associated with variants in genes that regulate epigenetic mechanisms, such as DNA methylation and acetylation, largely affecting histone modification and chromatin remodeling [2]. Such disorders were hence called “Mendelian Disorders of the Epigenetic Machinery” by Bjornsson et al. [3, 4]. One of the most studied epigenetic mechanisms is DNA methylation of cytosine-guanine dinucleotides (CpGs). It has been shown that for many NDs, variants in the disorder-causing gene alter the DNA methylation pattern throughout the genome, a phenomenon referred to as an episignature. These alterations are hypothesized to occur at the embryonic stage or very early in development, and are thus present in numerous tissues including peripheral blood, which is easily accessible and the most common source of DNA samples in clinical setting [5]. Genome-wide DNA methylation analysis has been utilized in the clinical setting for the molecular diagnosis of neurodevelopmental disorders for a few years [6] and currently, there are 65 Mendelian congenital anomalies associated with more than 70 genes, for which an episignature has been identified [7].
Renpenning syndrome (RENS1 – OMIM 309500) was first described by Renpenning et al., (1962) who reported a Canadian Mennonite family in which 20 males manifested features including intellectual disability, microcephaly, short stature, and small testes [8, 9]. Stevenson et al. attributed the condition to a locus in Xp11.2-p11.4 using linkage analysis [10]. The causal variant was later reported to be the duplication of one cytosine residue in exon 6 (c.640dup) in the polyglutamine-binding protein 1 gene, PQBP1 [9]. Several X-linked intellectual disabilities with similar clinical manifestations have been reported to be associated with variants in PQBP1, including Sutherland-Haan, Golabi-Ito-Hall, Porteous, and Hamel syndromes, and nonsyndromic XLID 55 (IDX55) [11–15]. Due to the similarity in clinical manifestation and genetic etiology, Stevenson et al suggested these disorders be grouped under the name of Renpenning syndrome [16, 17]. Indeed, OMIM 309500 does list these XLID syndromes under Renpenning syndrome. Since then, several studies and case reports describing patients with RENS1, harboring PQBP1 variants have been reported, further adding to the phenotypic and molecular profile [18–20]. Overall, the clinical presentation of patients with RENS1 is heterogenous, but is clinically recognizable. Affected individuals present with subtle but distinct craniofacial appearance, microcephaly, short stature and lean body profile. Cardiac, renal, and anorectal malformations also add to the phenotypic presentation in some cases. Clinical variability is noted in patients, with the same variant from different families and also within the members of the same family. The reason for this notable inter- and intra-familial variability is not known but the differences may occur due to genetic variability in other genes coding for proteins interacting with PQBP1. RENS1 includes many X-linked recessive disorders and carrier females are usually asymptomatic due to the skewed X-chromosome inactivation in favor of the wild-type variant; however, there are reports in the literature on females with characteristic features of the disorder [21].
The PQBP1 protein has a WW domain, a polar amino-acid-rich domain (PRD) followed by aspartate-arginine or glutamate-arginine repeats (DR/ER repeat), and a C-terminal domain (CTD) [22]. The protein has a significant role in transcription activation in the early stages of development and pre-mRNA splicing [21, 23]. In vitro studies revealed that the spliceosomal protein U5-15kD interacts with a YxxPxxVL motif in the C-terminal of PQBP1, and variants in that motif result in the loss-of-function phenotype of PQBP1 by the disruption of its splicing function [24]. Most of the variants reported as the causal variants for RENS1 are truncating variants that result in the partial or total loss of the C-terminal domain [24, 25]. A missense variant in the WW domain has also been identified (c.194 A > G) [12, 26]. The WW domain functions in transcription regulation by interacting with the pre-mRNA splicing factor WW domain-binding protein 11 (WBP11) and RNA polymerase II [26]. The c.194 A > G variant alters the highly conserved tyrosine residue to a cytosine, impairing the transcription promoting activity of RNA polymerase II [12]. The abundance of functional evidence in literature on the role of PQBP1 in RNA splicing and translation and the fact that the majority of PQBP1 variants responsible for RENS1 impair the splicing function of the gene, suggest that Renpenning syndrome is a spliceopathy [25–28].
This study provides preliminary evidence that RENS1 is associated with a DNA methylation signature. Using genome-wide DNA methylation analysis, probes that are differentially methylated between individuals with a definitive diagnosis of RENS1 and a group of controls are identified. The selected probes are shown to differentiate between the two groups by applying machine learning models. Based on the identified probes, a binary support vector machine is constructed that separates individuals with RENS1 from controls with full accuracy. The specificity of the model is also examined by supplying a large number of samples from other NDs/CAs to the classifier.
Materials and methods
Study cohorts
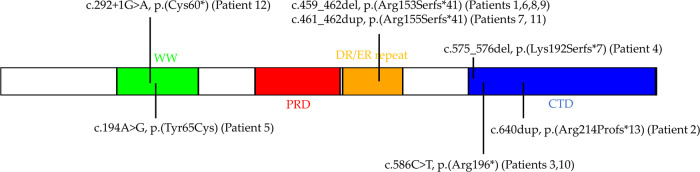
This study included 12 male individuals with genetic variants in PQBP1 and clinical features consistent with RENS1. The detailed list of genetic variants classified as pathogenic or likely pathogenic according to the American College of Medical Genetics guidelines is presented in Table 1. The variants have been published on ClinVar, with the accession numbers and URLs in Table S1. The location of the variants and the corresponding protein changes are depicted in Fig. 1. All of the samples and records were de-identified. The research was conducted in accordance with all relevant ethical regulations. The study protocol has been approved by the Western University Research Ethics Board (REB 106302).
Table 1.
Genetic and summarized clinical information of the patients.
| Patient | Sex (real/predicted by methylation) | Age (real/predicted by methylation) | PQBP1 Variant | Variant Identification Method | Clinical Features |
|---|---|---|---|---|---|
| 1 | m/m | 58/57.2 | c.459_462del,p.(Arg153Serfs*41) [15, 16, 50] | Linkage followed by specific gene testing based on physical characteristics. | From Kindred 8600 with Renpenning syndrome. Global developmental delay (GDD), microcephaly, and short stature. Phenotypic variability was observed within the family [15]. |
| 2 | m/m | 55.5/56.8 | c.640dup,p.(Arg214Profs*13) [8] | Linkage followed by specific gene testing based on physical characteristics. | From the original Canadian Mennonite family with Renpenning syndrome (Kindred 8110). GDD, impaired speech, recurrent seizures at childhood, and facial dysmorphisms. Phenotypic variability was observed within the family [9]. |
| 3 | m/m | 40.7/44.6 | c.586 C > T,p.(Arg196*) [16] | Not available. | Intellectual disability (ID), microcephaly, and vision problems are the only features available. |
| 4 | m/m | 40/52.5 | c.575_576del,p.(Lys192Serfs*7) [8] | Linkage followed by specific gene testing based on physical characteristics. | From Kindred 9008 with microcephaly and ID [8]. Severe ID, GDD, microcephaly, spasticity, kyphoscoliosis, seizures in childhood, and impaired speech. |
| 5 | m/m | 21/25.3 | c.194 A > G,p.(Tyr65Cys) [11, 15, 25, 46] | Specific gene testing based on physical characteristics. | From Kindred 8275 with Golabi-Ito-Hall syndrome. ID, seizures, brain abnormalities, skeletal abnormalities, impaired speech, overall extremely small, microcephaly, and facial dysmorphisms. Phenotypic variability was observed within the family [11]. |
| 6 | m/m | 1.5/4 | c.459_462del,p.(Arg153Serfs*41) | Specific gene testing based on physical characteristics. | Mild developmental delay, microcephaly, and facial dysmorphisms. |
| 7 | m/m | Unknown/43.3 | c.461_462dup,p.(Arg155Serfs*41) [50] | Linkage followed by specific gene testing based on physical characteristics. | Facial dysmorphisms are the only features available. |
| 8 | m/m | 34.9/37.9 | c.459_462del,p.(Arg153Serfs*41) | Specific gene testing based on physical characteristics. | Short stature, microcephaly with brachycephaly, prominent ears, relatively bulbous nose. |
| 9 | m/m | 35.4/43.1 | c.459_462del,p.(Arg153Serfs*41) | Not available. | Microcephaly, limited speech and daily activity. |
| 10 | m/m | 4.9/8.2 | c.586 C > T,p.(Arg196*) | Whole X-chromosome exome sequencing. | Microcephaly, developmental delay, and facial dysmorphisms. |
| 11 | m/m | 29.7/37.7 | c.461_462dup,p.(Asp156Alafs*12) | Linkage followed by specific gene testing based on physical characteristics. | From the Sutherland-Haan syndrome family. Strabismus, developmental delay, brachycephaly, and mild dysphasia. Phenotypic variability was observed within the family [10]. |
| 12 | m/m | 4.5/7.7 | c.292 + 1 G > A | Linkage followed by specific gene testing. | Moderate to severe ID, microcephaly, brain abnormalities, and facial dysmorphisms. |
Fig. 1. PQBP1 Protein domains.
The location of variants on the PQBP1 protein are indicated.
DNA methylation analysis
DNA methylation analysis was performed using the Illumina Infinium MethylationEPIC BeadChip microarrays (San Diego, CA) as previously described [5, 29]. Following bisulfite conversion, DNA samples extracted from peripheral blood of the case subjects were supplied to the arrays, according to the manufacturer’s protocol. These arrays include over 860,000 CpG sites in the human genome. Details of DNA methylation protocol, methylation data analysis and episignature discovery are as previously described [5, 30–32]. In summary, the resulting methylated and unmethylated signal intensities were imported into R 4.0.2 for analysis. Normalization was performed by the Illumina normalization method with background correction using the minfi package [33]. Those probes located on X and Y chromosomes, known to contain SNPs at or near the CpG interrogation or single nucleotide extension sites, known to cross-react with chromosomal locations other than their target regions, and suggested by Illumina to be problematic were excluded, resulting in 776,314 probes remaining for the analysis. Arrays having more than 5% probe failure rate and those that were previously identified in our database to impose batch effect were excluded from the analysis. The age of one sample (Patient 7) was predicted using the wateRmelon package [34]. All of the samples were examined for genome-wide methylation density, and those deviating from a bimodal distribution are excluded (all samples passed). All 12 samples had pathogenic or likely pathogenic PQBP1 variants. Factor analysis using a principal component analysis (PCA) was performed to observe the overall data structure of the batches and to identify the outlier samples. All 12 patients were used for probe selection and model construction.
Selection of matched controls for methylation profiling
For mapping the episignature (probe selection), MatchIt package [35] was used to randomly select controls, matched for age, sex, and array type, from the EpiSign Knowledge Database (EKD) at the London Health Sciences Centre (LHSC) [5]. The control sample size was increased until both the matching quality and sample size were optimized and consistent across all analyses. This led to the determination of a control sample size five times larger than that of the cases. Increasing the sample size beyond this value impaired the matching quality. After every matching trial, PCA was performed to detect outliers and examine the data structure. Outlier samples and those with aberrant data structures were removed at each trial, and the process was repeated until no outlier sample was observed in the PCA’s first two components.
DNA methylation profiling of RENS1
Methylation level of each probe was calculated as the ratio of methylated signal intensity over the sum of methylated and unmethylated signal intensities, called the β-values, ranging between zero (no methylation) and one (full methylation). β-values were then converted to M-values by logit transformation using the formula log2(β/(1-β)) to obtain homoscedasticity for linear regression modeling, which was used for identifying the differentially methylated probes, by the limma package [36]. The analysis was adjusted for blood cell type compositions, estimated using the algorithm developed by Houseman et al. [37]. The estimated blood cell proportions were added to the model matrix of the linear models as confounding variables. The generated p values were moderated using the eBayes function in the limma package and were corrected for multiple testing using the Benjamini and Hochberg (BH) method. Then, the probe selection process was performed in three steps. Firstly, 800 probes were selected which had the highest product of methylation difference means between case and control samples and the negative of the logarithm of multiple-testing corrected p values derived from the linear modeling. Secondly, a receiver’s operating characteristic (ROC) curve analysis was performed and 160 probes with the highest area under the ROC curve (AUC) were retained. Lastly, those probes having a pair-wise Pearson’s correlation coefficients greater than 0.85 within case and control samples separately, were removed. This resulted in identification of 116 differentially methylated probes. These probes were used for the construction of a hierarchical clustering model using Ward’s method on Euclidean distance, as well as a multidimensional scaling (MDS) model by scaling of the pair-wise Euclidean distances between samples.
Construction of a classification model
A binary support vector machine (SVM) classification model was constructed based on the 116 selected probes, using the e1071 package as described previously [5, 31, 32]. For each sample, the classifier creates a methylation variant pathogenicity (MVP) score ranging from 0 to 1. A sample is identified as having a methylation pattern similar to the signature detected for the syndrome if the MVP score is near 1, and it is indicated as having a methylation behavior similar to controls if the score is near 0. Two MVP plots were generated to confirm the specificity of the classification model. In the first plot, the 12 samples with pathogenic or likely pathogenic variants in PQBP1, as well as the 60 control samples were used for training the model. The specificity of the model was then assessed with additional control subjects and cases of 57 other constitutional disorders with confirmed episignatures from EKD (https://episign.lhsc.on.ca/index.html). Next, another model was trained using the 12 samples with pathogenic or likely pathogenic variants in PQBP1, the 60 matched control samples, and 75% of other control subjects and patients with 57 other NDs/CAs from the clinical EKD housed at the LHSC (https://episign.lhsc.on.ca/index.html) as the training set. This step allows the preferential selection of probes that are not overlapping with other genetic disorders and improve the specificity of the classifier.
Identification of the differentially methylated regions of RENS1
In order to detect the differentially methylated regions (DMRs), the DMRcate package [38] was used, and regions containing at least 5 different CpGs within 1 kb with a minimum methylation difference of 5% between the case and the control samples and a Fisher’s multiple comparison p value < 0.01 were selected. Using the R package missMethyl version 1.28.0 [39], gene ontology (GO) enrichment analysis was performed for the detected DMRs.
Results
Identification and validation of an episignature for RENS1
The case cohort consisted of 12 males with a clinical diagnosis of RENS1 and a pathogenic or likely pathogenic variant in PQBP1. All patients had inherited PQBP1 variants from their asymptomatic carrier mothers and to the best of our knowledge, only the variant for Patient 12 was novel. Ages spanned from 1.5 to 58 years, with a median of 35.2 years and a mean of 30.8 years. Eleven patients had a truncating variant, including patient 12 with a splice site variant, and patient 5 had a missense variant, all of which were classified as pathogenic or likely pathogenic according to the American College of Medical Genetics guideline. The detailed genetic information and summarized clinical information for these patients are included in Table 1. All 12 patients were used for probe selection and model construction.
We selected 60 age, sex, and array type-matched control samples from EKD (https://episign.lhsc.on.ca/index.html), with a case to control ratio of 1:5. The 116 probes identified as differentially methylated between the case and control groups were used to construct unsupervised (hierarchical clustering and multidimensional scaling) and supervised (support vector machine) models. The identifying episignature of the syndrome consists of the methylation levels at the 116 probes selected as differentially methylated between the case and control groups.
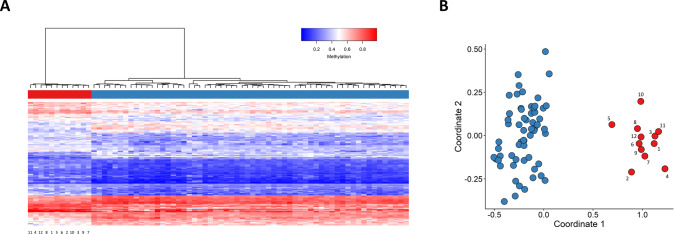
The robustness of the episignature in distinguishing between cases and controls was assessed by performing hierarchical clustering (Fig. 2A) as well as multidimensional scaling (MDS) (Fig. 2B). Clear separation was observed between the two groups in both plots.
Fig. 2. Assessing the robustness of the identified episignature.
A Hierarchical clustering using the selected probes was performed. Rows represent the selected probes and columns indicate samples. On the heatmap pane, case samples are illustrated with red, while control samples are demonstrated with blue. Methylation levels range from blue (no methylation or 0) to red (full methylation or 1) on the heatmap color scale. It is observed that the identified probes are capable of separating RENS1 samples from control samples. B Multidimensional scaling (MDS) using the selected CpG sites, demonstrating the strength of the episignature in differentiating between case and control samples. Red and blue circles represent case and control samples, respectively.
In order to further validate the detected epi-signature, 12 rounds of cross-validation were performed, using 11 case samples for training and 1 for testing at each round. A multidimensional scaling plot was created at each step. Testing samples with truncating variants always clustered with the remaining case samples, while the sample with a missense variant (Patient 5) demonstrated an intermediate methylation profile (Fig. 3).
Fig. 3. 12 rounds of cross-validation.
At each round, one of the 12 samples are retained for testing, and it is observed that the testing sample (black) clusters with the training case samples (red) for all trials, with the exception of Patient 5 which falls closer to control samples (Blue).
Binary prediction model construction
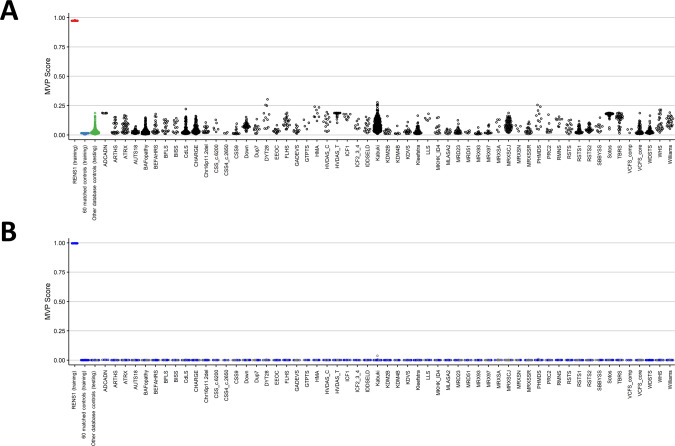
Using the identified CpG sites, a binary support vector machine (SVM) classifier is trained in order to differentiate RENS1 samples from control samples more accurately. This binary model creates a methylation variant pathogenicity (MVP) score for each sample, ranging from 0 to 1, where scores near 1 indicate high similarity between the methylation profile of the sample and the identified episignature, while lower scores imply dissimilarity to the methylation pattern specific to RENS1. First, the model is constructed using only the 12 RENS1 samples and the 60 control samples for training, and subsequently, the model is supplied with control samples and case samples from 57 other NDs/CAs from our database (https://episign.lhsc.on.ca/index.html) in order to assess specificity. All the testing samples from controls and other syndromes received MVP scores below 0.5, indicating a specificity above 99% (Fig. 4A).
Fig. 4. Methylation variant pathogenicity (MVP) scores generated by the support vector machine (SVM) classifier.
A The model was trained using only the 12 RENS1 samples and the 60 matched control samples. This plot demonstrates the high specificity of the detected RENS1 episignature. B The classifier was constructed using the 12 RENS1 samples, the 60 matched control samples, and 75% of other control samples and case samples from other NDs/CAs for training (blue) and the remaining 25% for testing (gray). It is observed that the model’s specificity has significantly increased.
For the purpose of achieving higher specificity (decreasing the MVP scores for control samples and samples from other NDs/CAs), another SVM classifier was trained, using the 12 RENS1 samples, the 60 matched control samples, and 75% of other control samples and case samples from other syndromes for training, and the remaining 25% for testing. This approach ensures RENS1 episignature probes are trained against other EpiSign syndromes methylation profiles further improving the specificity of the classifier. As demonstrated in Fig. 4B, all testing samples from controls and other disorders received MVP scores near 0, indicating full specificity of the classifier.
Detection of the regions of differential methylation
By assessing all differentially methylated probes genome wide, 119 DMRs were identified (Table S2), where a region was considered differentially methylated between the case and control groups if it contained a minimum of 5 different CpGs within 1 kb with a minimum methylation difference of 5% and a Fisher’s multiple comparison p value < 0.01. 75 CpG sites were common between the 1000 probes with the highest product of the mean methylation difference between the case and control groups and the negative of the logarithm of the adjusted p values, and probes within the DMR regions. The DMRs contained several genes known to be involved in neurodevelopmental disorders. For instance, one region on chromosome 6 overlapped ATG5, a gene with a crucial role in the central nervous system and causal for spinocerebellar ataxia, autosomal recessive 25 (SCAR25), which is a neurodevelopmental disorder with characteristics such as developmental delay [40, 41]. Another interesting region was on chromosome 7, overlapping a number of homeobox genes. In order to identify gene categories and molecular pathways associated with the DMRs, GO enrichment analysis was performed using the 119 DMRs and 7 GO terms were found enriched in the identified regions (p value < 0.001) (Table S3). The majority of the enriched terms were related to membrane functions.
Discussion
The diagnostic utility of genome-wide DNA methylation for Mendelian neurodevelopmental disorders has been established previously by our group and others [5, 42–44]. This method has proved successful in providing a definitive diagnosis for patients with a variant of unknown significance or those with no detected genetic variants with a clinical diagnosis, as well as for reclassifying patients with an incorrect initial diagnosis [32]. Here, using a relatively small cohort size, promising results for the existence of a DNA methylation signature for Renpenning syndrome are provided.
RENS1, first described in 1962 [8], is caused by variants in PQBP1. The term Renpenning syndrome refers to several X-linked intellectual disabilities caused by PQBP1 variants, including Renpenning syndrome, Sutherland-Haan, Golabi-Ito-Hall, Porteous, and Hamel syndromes, and IDX55 [11–17]. The clinical manifestations of these syndromes are generally common; however, patients reported with Hamel syndrome are the most severely affected. For instance, malformations are typically absent in RENS1 patients, but some Hamel syndrome patients have presented with cardiac malformations [16]. On the other hand, individuals reported with IDX55 were among those with the least severe abnormalities [16]. Two clinical differences have been observed between Golabi-Ito-Hall syndrome and the other associated syndromes: more severely affected growth, and the absence of small testes in the two Golabi-Ito-Hall syndrome case subjects that were reported in 1984 [12, 45]. The typical characteristic features of RENS1 include intellectual disability, short stature, microcephaly, and small testes. Besides microcephaly, there are other craniofacial abnormalities associated with this syndrome; such as, aberrant nasal configuration, short philtrum, cupped ears, and narrow and tall with upslanting palpebral fissures [16]. It is worth mentioning that the clinical features observed in RENS1 patients are variable within and among families [16].
PQBP1 is known to influence alternative mRNA splicing of multiple mRNA targets [28]. Pre-mRNA splicing can affect the structure and function of membrane proteins [46] and membrane functions are known to be critical in the regulation of cellular survival pathways [47]. This suggests that variants in PQBP1 may result in RENS1 by impairing membrane function through defective pre-mRNA splicing. Here, GO enrichment analysis reveals enrichment in several terms related to membrane functions, providing evidence for this hypothesis. Two voltage-dependent calcium channels, CACNA1C and CACNB2, in particular, contribute to the enrichment of several biological categories (Table S3). The relationship between defects in RNA splicing and altered DNA methylation across the genome still remains unclear and functional studies are required to reveal any contributing mechanisms.
The robustness of episignatures or the level of DNA methylation differences observed between patients with a disorder and the control group varies in different syndromes. The high specificity of the SVM classifier constructed on the basis of the detected episignature, is illustrated in Fig. 4B, where controls and samples from patients with other EpiSign disorders receive low MVP scores. The unsupervised models, hierarchical clustering and MDS (Fig. 2) are of particular interest. Although in both plots the case and control groups form two clearly distinct and distant clusters, Patient 5 manifests a slightly different methylation profile on the heatmap, and is located closer to the control samples compared to the remaining RENS1 samples on the MDS. Also, in Fig. 3, Patient 5 clusters closer to control samples. In spite of a slightly different methylation pattern, Patient 5, with a missense PQBP1 mutation, was included for probe selection and model construction, since the patient had a confirmed diagnosis of Renpenning syndrome [12]. This also ensures that the final classifier is as sensitive as possible to a wide range of variant types. The different methylation pattern observed for Patient 5 may indicate that the missense variant in the WW domain may more adversely affect its function than its absence. Some studies suggest that this variant might result in both loss and gain of function alterations that impact mRNA splicing in conjunction with each other [48]. Therefore, it is possible that missense variants result in a different episignature; however, additional cases with missense variants will be required to investigate this hypothesis. The different methylation pattern of Patient 5 is also consistent with our knowledge of the phenotypic differences between Golabi-Ito-Hall syndrome, which is associated with the missense variant, and the other RENS1 disorders [12]. Patient 5 is a member of the original Golabi-Ito-Hall family (his testes were of normal size and his growth was extremely affected) [12]. Based on the clinical features, Golabi-Ito-Hall syndrome is a unique disorder, but since it had many characteristics in common with the other RENS1 subtypes, the family was screened and found to have a PQBP1 variant. Thus, as stated in the paper by Lubs et al. [11], it is considered as a subtype of RENS1.
One limitation of this study is that there was only one individual with a missense variant (Patient 5). The fact that Patient 5 fell between the case and control samples in the leave-one-out cross-validation (Fig. 3), provides limited evidence the episignature may be sensitive to samples with missense variants and probably other in-frame variants as well to some degree. However, more validation samples with these variant types are required for confirmation. Another limitation of this study is the small size of the study cohort and unavailability of an independent validation set. The enlargement of the cohort size and the availability of subjects with different variant types in the foreseeable future will facilitate the use of a larger training cohort and independent validation set, potentially resulting in a more sensitive episignature and enabling its application for diagnosis in a clinical setting.
To date, the majority of syndromes for which a DNA methylation signature has been reported have been “Mendelian Disorders of Epigenetic Machinery” or disorders caused by variants in a gene with transcriptional and chromatin remodeling activity [3, 5]. In this paper, preliminary evidence for the existence of a DNA methylation episignature for RENS1 is provided. RENS1 is proposed here to be a spliceopathy due to the function of PQBP1 in pre-mRNA splicing. Armfield X-linked intellectual disability syndrome (MRXSA) is a spliceosomopathy with a known DNA methylation signature [49, 50]. The discovery of episignatures for these two “opathies” expands episignatures beyond “Mendelian Disorders of Epigenetic Machinery” and prompt the idea that both spliceopathies and spliceosomopathies can potentially be another category of syndromes associated with a specific DNA methylation profile.
Supplementary information
Acknowledgements
We would like to thank the families for their contribution to Greenwood Genetic Center’s (GGC’s) study of X-linked intellectual disabilities. We also appreciate Anna Crockett’s assistance in preparing revisions to the revising of the paper. Dedicated to the memory of Ethan Francis Schwartz, 1996–1998.
Author contributions
Conceptualization: SH, BS, CES; Data curation: SH, JK; Formal analysis: SH, MAL, RR; Funding acquisition: BS, CES; Investigation: SH, MAL, JK, CDS, RCC; Methodology: SH, MAL, RR; Project administration: HM, BS, CES; Resources: JK, CDS, MLT, RES, BS, CES; Software: SH, MAL, RR; Supervision: BS, CES; Validation: SH, AF, PB; Visualization: SH; Writing-original draft: SH, AF; Writing-review and editing: PB, HM, RES, BS, CES.
Funding
This work was funded in part by the government of Canada through Genome Canada and the Ontario Genomics Institute (OGI-188) awarded to BS, NIH grant HD-026202 to CES, the South Carolina Department of Disabilities and Special Needs, and the Greenwood Genetic Center Foundation.
Data availability
Some of the datasets used in this study are publicly available and may be obtained from gene expression omnibus (GEO) using the following accession numbers. GEO: GSE116992, GSE66552, GSE74432, GSE97362, GSE116300, GSE95040, GSE 104451, GSE125367, GSE55491, GSE108423, GSE116300, GSE 89353, GSE52588, GSE42861, GSE85210, GSE87571, GSE87648, GSE99863, and GSE35069. These include DNA methylation data from patients with Kabuki syndrome, Sotos syndrome, CHARGE syndrome, immunodeficiency-centromeric instability-facial anomalies (ICF) syndrome, Williams-Beuren syndrome, Chr7q11.23 duplication syndrome, BAFopathies, Down syndrome, a large cohort of unresolved subjects with developmental delays and congenital abnormalities, and also several large cohorts of DNA methylation data from the general population. The rest of the data including the RENS1 samples are not available due to the restrictions of the ethics approval. The variants are publicly available on ClinVar, with accession numbers SCV002525441.1- SCV002525447.1.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interest. CES was previously affiliated with GGC, which provides commercial diagnostic services using EpiSign. This study however, was initiated after his retirement from GGC in collaboration with BS. Some of the genome-wide data was generated at GGC on a research basis at the request of CES. Thus, it is not linked to the commercial diagnostic services at GGC.
Ethics approval
All of the samples and records were de-identified. The study protocol has been approved by the Western University Research Ethics Board (REB 106302), and the IRB of Self Regional Healthcare. Informed consent was obtained by physicians for use of the clinical information of the described patients.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bekim Sadikovic, Email: bekim.sadikovic@lhsc.on.ca.
Charles E. Schwartz, Email: charles.schwartz224@gmail.com
Supplementary information
The online version contains supplementary material available at 10.1038/s41431-023-01313-z.
References
- 1.Christianson A, Howson CP, Modell B. March of Dimes. Global report on birth defect. The hidden toll of dying and disabled children. New York: White Plains; 2006.
- 2.Sadikovic B, Aref-Eshghi E, Levy MA, Rodenhiser D. DNA methylation signatures in mendelian developmental disorders as a diagnostic bridge between genotype and phenotype. Epigenomics. 2019;11:563–75. doi: 10.2217/epi-2018-0192. [DOI] [PubMed] [Google Scholar]
- 3.Bjornsson HT. The Mendelian disorders of the epigenetic machinery. Genome Res. 2015;25:1473–81. doi: 10.1101/gr.190629.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fahrner J, Bjornsson H. Mendelian disorders of the epigenetic machinery: Tipping the balance of chromatin states. Ann Rev Genom Hum Genet. 2014;15:269–93. doi: 10.1146/annurev-genom-090613-094245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aref-Eshghi E, Kerkhof J, Pedro VP, Barat-Houari M, Ruiz-Pallares N, Andrau JC, et al. Evaluation of DNA methylation episignatures for diagnosis and phenotype correlations in 42 Mendelian neurodevelopmental disorders. Am J Hum Genet. 2020;106:356–70. doi: 10.1016/j.ajhg.2020.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadikovic B, Levy MA, Kerkhof J, Aref-Eshghi E, Schenkel L, Stuart A, et al. Clinical epigenomics: genome-wide DNA methylation analysis for the diagnosis of Mendelian disorders. Genet Med. 2021;23:1–10. doi: 10.1038/s41436-020-01096-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy MA, McConkey H, Kerkhof J, Barat-Houari M, Bargiacchi S, Biamino E, et al. Novel diagnostic DNA methylation episignatures expand and refine the epigenetic landscapes of Mendelian disorders. Hum Genet Genom Adv. 2021;3:100075. doi: 10.1016/j.xhgg.2021.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renpenning H, Gerrard JW, Zaleski WA, Tabata T. Familial sex-linked mental retardation. Can Med Assoc J. 1962;87:954–6. [PMC free article] [PubMed] [Google Scholar]
- 9.Lenski C, Abidi F, Meindl A, Gibson A, Platzer M, Kooy RF, et al. Novel truncating mutations in the polyglutamine tract binding protein 1 gene (PQBP1) cause Renpenning syndrome and X-linked mental retardation in another family with microcephaly. Am J Hum Genet. 2004;74:777–80. doi: 10.1086/383205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stevenson RE, Arena JF, Ouzts E, Gibson A, Shokeir MHK, Vnencak-Jones C, et al. Renpenning syndrome maps to Xp11. Am J Hum Genet. 1998;62:1092–101. doi: 10.1086/301835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutherland GR, Gedeon AK, Haan EA, Woodroffe P, Mulley JC. Linkage studies with the gene for an X-linked syndrome of mental retardation, microcephaly and spastic diplegia (MRX2) Am J Med Genet. 1988;30:493–508. doi: 10.1002/ajmg.1320300152. [DOI] [PubMed] [Google Scholar]
- 12.Lubs H, Abidi FE, Echeverri R, Holloway L, Meindl A, Stevenson RE, et al. Golabi-Ito-Hall syndrome results from a missense mutation in the WW domain of the PQBP1 gene. J Med Genet. 2006;43:1–4. doi: 10.1136/jmg.2005.037556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Porteous M, Johnson H, Burn J, Curtis A, Lindsay S, Bhattacharya S, et al. A new mental retardation syndrome mapping to the pericentromeric region of the X-chromosome. Am J Hum Genet. 1992;51:A106.
- 14.Hamel BCJ, Mariman ECM, Van Beersum SEC, Schoonbrood-Lenssen AMJ, Ropers HH. Mental retardation, congenital heart defect, cleft palate, short stature, and facial anomalies: A new X-linked multiple congenital anomalies/mental retardation syndrome: Clinical description and molecular studies. Am J Med Genet. 1994;51:591–7. doi: 10.1002/ajmg.1320510459. [DOI] [PubMed] [Google Scholar]
- 15.Deqaqi SC, N’Guessan M, Forner J, Sbiti A, Beldjord C, Chelly J, et al. A gene for non-specific X-linked mental retardation (MRX55) is located in Xp11. Ann Genet. 1998;41:11–6. [PubMed] [Google Scholar]
- 16.Stevenson RE, Bennett CW, Abidi F, Kleefstra T, Porteous M, Simensen RJ, et al. Renpenning syndrome comes into focus. Am J Med Genet. 2005;134 A:415–21. doi: 10.1002/ajmg.a.30664. [DOI] [PubMed] [Google Scholar]
- 17.Germanaud D, Rossi M, Bussy G, Gérard D, Hertz-Pannier L, Blanchet P, et al. The Renpenning syndrome spectrum: New clinical insights supported by 13 new PQBP1-mutated males. Clin Genet. 2011;79:225–35. doi: 10.1111/j.1399-0004.2010.01551.x. [DOI] [PubMed] [Google Scholar]
- 18.Masih S, Moirangthem A, Phadke SR. Renpenning syndrome in an Indian patient. Am J Med Genet Part A. 2020;182:293–5. doi: 10.1002/ajmg.a.61457. [DOI] [PubMed] [Google Scholar]
- 19.Kaymakçalan H, Ercan-Şençiçek AG, Cebeci AN, Dong W, Yalçın ASY. A rare etiology of tetralogy of Fallot with pulmonary atresia: Renpenning syndrome. Anatol J Cardiol. 2022;26:149–50. doi: 10.5152/AnatolJCardiol.2021.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeong H-I, Yang A, Kim J, Jang J-H, Cho SY, Jin D-K. First Korean case of Renpenning syndrome with novel mutation in PQBP1 diagnosed by targeted exome sequencing, and literature review. Ann Clin Lab Sci. 2018;48:522–7. [PubMed] [Google Scholar]
- 21.Cho RY, Peñaherrera MS, Du Souich C, Huang L, Mwenifumbo J, Nelson TN, et al. Renpenning syndrome in a female. Am J Med Genet Part A. 2020;182:498–503. doi: 10.1002/ajmg.a.61451. [DOI] [PubMed] [Google Scholar]
- 22.Rees M, Gorba C, De Chiara C, Bui TTT, Garcia-Maya M, Drake AF, et al. Solution model of the intrinsically disordered polyglutamine tract-binding protein-1. Biophys J. 2012;102:1608–16. doi: 10.1016/j.bpj.2012.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahman SK, Okazawa H, Chen YW. Frameshift PQBP-1 mutants K192Sfs*7 and R153Sfs*41 implicated in X-linked intellectual disability form stable dimers. J Struct Biol. 2019;206:305–13. doi: 10.1016/j.jsb.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 24.Mizuguchi M, Obita T, Serita T, Kojima R, Nabeshima Y, Okazawa H. Mutations in the PQBP1 gene prevent its interaction with the spliceosomal protein U5-15kD. Nat Commun. 2014;5:1–5. doi: 10.1038/ncomms4822. [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki Y, Thomsen GH. The splicing factor PQBP1 regulates mesodermal and neural development through FGF signaling. Dev. 2014;141:3740–51. doi: 10.1242/dev.106658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tapia VE, Nicolaescu E, McDonald CB, Musi V, Oka T, Inayoshi Y, et al. Y65C missense mutation in the WW domain of the Golabi-Ito-Hall syndrome protein PQBP1 affects its binding activity and deregulates pre-mRNA splicing. J Biol Chem. 2010;285:19391–401. doi: 10.1074/jbc.M109.084525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan D, Zhang ZC, Zhang X, Li Q, Han J. X chromosome-linked intellectual disability protein PQBP1 associates with and regulates the translation of specific mRNAs. Hum Mol Genet. 2015;24:4599–614. doi: 10.1093/hmg/ddv191. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Moore MJ, Adelmant G, Marto JA, Silver PA. PQBP1, a factor linked to intellectual disability, affects alternative splicing associated with neurite outgrowth. Genes Dev. 2013;27:615–26. doi: 10.1101/gad.212308.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sadikovic B, Levy MA, Aref-Eshghi E. Functional annotation of genomic variation: DNA methylation episignatures in neurodevelopmental Mendelian disorders. Hum Mol Genet. 2020;29:R27–32. doi: 10.1093/hmg/ddaa144. [DOI] [PubMed] [Google Scholar]
- 30.Aref-Eshghi E, Bend EG, Hood RL, Schenkel LC, Carere DA, Chakrabarti R, et al. BAFopathies’ DNA methylation epi-signatures demonstrate diagnostic utility and functional continuum of Coffin–Siris and Nicolaides–Baraitser syndromes. Nat Commun. 2018;9:4885. doi: 10.1038/s41467-018-07193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aref-Eshghi E, Rodenhiser DI, Schenkel LC, Lin H, Skinner C, Ainsworth P, et al. Genomic DNA methylation signatures enable concurrent diagnosis and clinical genetic variant classification in neurodevelopmental syndromes. Am J Hum Genet. 2018;102:156–74. doi: 10.1016/j.ajhg.2017.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aref-Eshghi E, Bend EG, Colaiacovo S, Caudle M, Chakrabarti R, Napier M, et al. Diagnostic utility of genome-wide DNA methylation testing in genetically unsolved individuals with suspected hereditary conditions. Am J Hum Genet. 2019;104:685–700. doi: 10.1016/j.ajhg.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aryee MJ, Jaffe AE, Corrada-Bravo H, Ladd-Acosta C, Feinberg AP, Hansen KD, et al. Minfi: A flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;30:1363–9. doi: 10.1093/bioinformatics/btu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pidsley R, Wong CCY, Volta M, Lunnon K, Mill J, Schalkwyk LC. A data-driven approach to preprocessing Illumina 450K methylation array data. BMC Genom. 2013;14:1–10. doi: 10.1186/1471-2164-14-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho DE, Imai K, King G, Stuart EA. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal. 2007;15:199–236. doi: 10.1093/pan/mpl013. [DOI] [Google Scholar]
- 36.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinforma. 2012;13:1–16. doi: 10.1186/1471-2105-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters TJ, Buckley MJ, Statham AL. De novo identification of differentially methylated regions in the human genome. Epigenet Chromatin. 2015;8:1–16. doi: 10.1186/1756-8935-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phipson B, Maksimovic J, Oshlack A. MissMethyl: An R package for analyzing data from Illumina’s HumanMethylation450 platform. Bioinformatics. 2016;32:286–8. doi: 10.1093/bioinformatics/btv560. [DOI] [PubMed] [Google Scholar]
- 40.Lv X, Jiang H, Li B, Liang Q, Wang S, Zhao Q, et al. The crucial role of Atg5 in cortical neurogenesis during early brain development. Sci Rep. 2014;4:1–9. doi: 10.1038/srep06010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim M, Sandford E, Gatica D, Qiu Y, Liu X, Zheng Y, et al. Mutation in ATG5 reduces autophagy and leads to ataxia with developmental delay. Elife. 2016;5:e12245. doi: 10.7554/eLife.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choufani S, Cytrynbaum C, Chung BHY, Turinsky AL, Grafodatskaya D, Chen YA, et al. NSD1 mutations generate a genome-wide DNA methylation signature. Nat Commun. 2015;6:1–7. doi: 10.1038/ncomms10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aref-Eshghi E, Schenkel LC, Lin H, Skinner C, Ainsworth P, Paré G, et al. The defining DNA methylation signature of Kabuki syndrome enables functional assessment of genetic variants of unknown clinical significance. Epigenetics. 2017;12:923–33. doi: 10.1080/15592294.2017.1381807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schenkel LC, Kernohan KD, McBride A, Reina D, Hodge A, Ainsworth PJ, et al. Identification of epigenetic signature associated with alpha thalassemia/mental retardation X-linked syndrome. Epigenet Chromatin. 2017;10:1–11. doi: 10.1186/s13072-017-0118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golabi M, Ito M, Hall BD. A new X-linked multiple congenital anomalies/mental retardation syndrome. Am J Med Genet. 1984;17:367–74. doi: 10.1002/ajmg.1320170130. [DOI] [PubMed] [Google Scholar]
- 46.Mittendorf K, Deatherage C, Ohi M, Sanders C. Tailoring of membrane proteins by alternative splicing of pre-mRNA. Biochemistry. 2012;51:5541–56. doi: 10.1021/bi3007065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sural-Fehr T, Bongarzone E. How membrane dysfunction influences neuronal survival pathways in sphingolipid storage disorders. J Neurosci Res. 2016;94:1042–8. doi: 10.1002/jnr.23763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sudol M, McDonald CB, Farooq A. Molecular Insights into the WW domain of the Golabi-Ito-Hall Syndrome Protein PQBP1. FEBS Lett. 2012;586:2795–9. doi: 10.1016/j.febslet.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haghshenas S, Levy MA, Kerkhof J, Aref-Eshghi E, McConkey H, Balci T, et al. Detection of a dna methylation signature for the intellectual developmental disorder, x-linked, syndromic, armfield type. Int J Mol Sci. 2021;22:1–13. doi: 10.3390/ijms22031111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee YR, Khan K, Armfield-Uhas K, Srikanth S, Thompson NA, Pardo M, et al. Mutations in FAM50A suggest that Armfield XLID syndrome is a spliceosomopathy. Nat Commun. 2020;11:3698. doi: 10.1038/s41467-020-17452-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Some of the datasets used in this study are publicly available and may be obtained from gene expression omnibus (GEO) using the following accession numbers. GEO: GSE116992, GSE66552, GSE74432, GSE97362, GSE116300, GSE95040, GSE 104451, GSE125367, GSE55491, GSE108423, GSE116300, GSE 89353, GSE52588, GSE42861, GSE85210, GSE87571, GSE87648, GSE99863, and GSE35069. These include DNA methylation data from patients with Kabuki syndrome, Sotos syndrome, CHARGE syndrome, immunodeficiency-centromeric instability-facial anomalies (ICF) syndrome, Williams-Beuren syndrome, Chr7q11.23 duplication syndrome, BAFopathies, Down syndrome, a large cohort of unresolved subjects with developmental delays and congenital abnormalities, and also several large cohorts of DNA methylation data from the general population. The rest of the data including the RENS1 samples are not available due to the restrictions of the ethics approval. The variants are publicly available on ClinVar, with accession numbers SCV002525441.1- SCV002525447.1.