Abstract
Background
Endurance and frequent exercise are associated with earlier onset of arrhythmogenic right ventricular cardiomyopathy (ARVC) and ventricular arrhythmias (VA) in desmosomal gene variant carriers. Individuals with the pathogenic c.40_42del; p.(Arg14del) variant in the PLN gene are frequently diagnosed with ARVC or dilated cardiomyopathy (DCM). The aim of this study was to evaluate the effect of exercise in PLN p.(Arg14del) carriers.
Methods
In total, 207 adult PLN p.(Arg14del) carriers (39.1% male; mean age 53 ± 15 years) were interviewed on their regular physical activity since the age of 10 years. The association of exercise with diagnosis of ARVC, DCM, sustained VA and hospitalisation for heart failure (HF) was studied.
Results
Individuals participated in regular physical activities with a median of 1661 metabolic equivalent of task (MET) hours per year (31.9 MET-hours per week) until clinical presentation. The 50% most and least active individuals had a similar frequency of sustained VA (18.3% vs 18.4%; p = 0.974) and hospitalisation for HF (9.6% vs 8.7%; p = 0.827). There was no relationship between exercise and survival free from (incident) sustained VA (p = 0.65), hospitalisation for HF (p = 0.81), diagnosis of ARVC (p = 0.67) or DCM (p = 0.39) during follow-up. In multivariate analyses, exercise was not associated with sustained VA or HF hospitalisation during follow-up in this relatively not-active cohort.
Conclusion
There was no association between the amount of exercise and the susceptibility to develop ARVC, DCM, VA or HF in PLN p.(Arg14del) carriers. This suggested unaffected PLN p.(Arg14del) carriers can safely perform mild-moderate exercise, in contrast to desmosomal variant carriers and ARVC patients.
Supplementary Information
The online version of this article (10.1007/s12471-023-01800-4) contains supplementary material, which is available to authorized users.
Keywords: Arrhythmogenic right ventricular cardiomyopathy, Dilated cardiomyopathy, Genetics, Exercise, Penetrance
What’s new?
In carriers of PLN p.(Arg14del), an exercise history of mild-moderate exercise prior to clinical presentation does not predict: (1) development of ventricular arrhythmias or heart failure (time/severity) or (2) a diagnosis of cardiomyopathy.
There is no reason to limit mild-moderate exercise in PLN p.(Arg14del) individuals without signs or symptoms.
As malignant ventricular arrhythmias occur in nearly 50% of these individuals while being active, a clinical cardiomyopathy diagnosis or the presence of arrhythmia-related risk factors (decreased left ventricular ejection fraction, prior sustained or non-sustained ventricular tachycardia, low-voltage electrocardiogram, number of leads with negative T waves or premature ventricular contraction count) warrant discussion of exercise restrictions.
Introduction
Inherited cardiomyopathies, such as arrhythmogenic cardiomyopathy (ACM) and dilated cardiomyopathy (DCM), may present with arrhythmias, heart failure (HF), or even sudden cardiac death (SCD). ACM can present predominantly in the right ventricle (arrhythmogenic right ventricular cardiomyopathy (ARVC)), the left ventricle, or both. In ARVC patients, (likely) pathogenic variants are mainly identified in genes encoding desmosomal proteins [1]. In DCM patients, variants can be identified in a broad spectrum of genes, mainly encoding proteins of the cytoskeleton, sarcomere or nucleus. The non-desmosomal variant p.(Arg14del) in the gene encoding phospholamban (PLN) is common in the Netherlands and can be found both in patients diagnosed with ARVC or DCM [2–4]. PLN is important for Ca2+ homeostasis in cardiac muscle by regulating the sarcoplasmic reticulum Ca2+ pump [5]. Individuals with PLN p.(Arg14del) generally present from adulthood onwards with a low-voltage electrocardiogram (ECG), negative T waves, a high count of premature ventricular contractions (PVCs), malignant ventricular arrhythmias (VA) and HF [6]. Factors that promote penetrance and influence expression in this autosomal dominantly inherited disorder remain unknown [7].
Because several studies have confirmed a role for vigorous exercise in penetrance and arrhythmic risk in ARVC, guidelines recommend that individuals with ARVC should not participate in competitive or frequent high-intensity exercise [8]. High duration and intensive exercise have been demonstrated to increase penetrance among carriers of desmosomal variants and risk of developing a ventricular tachycardia (VT), structural dysfunction, and HF [9–13]. Even in genotype-positive family members exercise restriction was found to be protective [14]. However, exercise also gives physical and mental advantages [15], and restrictions prevent individuals from benefitting from these. As PLN p.(Arg14del) may manifest as ARVC [4], we evaluated the influence of exercise on age-related penetrance, arrhythmic risk, and progression to HF.
Methods
Study population
The study population was recruited from 3 Dutch university medical centres (Amsterdam, Groningen and Utrecht). Subjects who were a carrier of PLN p.(Arg14del) and ≥ 18 years of age were invited by a letter to participate in a detailed interview to document exercise history or could self-apply via the website of the PLN Patient Foundation (www.plnheartdiseasefoundation.org). The study protocol was exempted from approval by the Medical Ethics Committee of the Amsterdam University Medical Centres because the Act of Medical Research involving Human Subjects did not apply (W17_076 #17.094).
Genotype and phenotype
Genotype was derived from sequencing of PLN in a diagnostic setting [2]. Clinical and demographic data were drawn from the ACM registry [16].
Clinical variables
Clinical presentation was defined as a first visit for PLN p.(Arg14del) related complaints or family screening. We used 3 clinical endpoints: a clinical diagnosis of ARVC or DCM, sustained VA and hospitalisation for HF [17]. Sustained VA was defined as ventricular tachycardia of > 30 s or < 30 s when terminated electrically (including appropriate implantable cardioverter-defibrillator (ICD) therapy) or pharmacologically; ventricular fibrillation (VF); and/or resuscitated or aborted cardiac arrest, as described previously [7]. When an individual reached one of these clinical endpoints, he/she was defined as having a cardiac phenotype and considered penetrant for disease. ARVC diagnosis was assigned according to the 2010 Task Force Criteria [18]. DCM diagnosis was based on decreased left ventricular ejection fraction (LVEF) and increased left ventricular volume (LVEDD > 112% of predicted corrected for age and body surface area, and a reduced LV function [EF < 45% or fractional shortening < 35% on echo] or cardiac MR with LVEF < 45% and left ventricular end-diastolic volume > 2 SD above reference value) [17, 19]. Risk factors for malignant ventricular arrhythmia or HF, previously established in PLN p.(Arg14del), were also collected: LVEF < 45%, (non‑)sustained ventricular tachycardia (VT), > 500 PVC count/24 h, number of leads showing negative T leads and presence of low-voltage ECG [6, 7].
Exercise interviews
Exercise history was obtained by structured telephone interviews. Participants were prompted to list regular exercise done since the age of 10 years for leisure including sports, for work, and for transportation (by bicycle). Details of collection of intensity and duration of each activity have previously been described [9]. All responses were transcribed on pre-prepared data collection sheets.
Analysis of exercise history
For each participant, type and intensity of the activities were expressed in metabolic equivalent (MET) using the Ainsworth compendium [20] (FvL). This MET-score reflects the energy expenditure in comparison to a resting state [21]. By multiplying the frequency and duration of each activity with the MET-values, an estimation of annual MET-hours was obtained. We calculated exercise dose (MET-hours/year) preceding and following clinical presentation.
Statistical analysis
Categorical variables are reported as frequency (%) and group differences were calculated by using the chi-square or Fisher’s exact test. Continuous variables are reported as mean ± standard deviation (SD) or median (interquartile range) and were compared across groups using a t-test or Mann-Whitney U test. MET-hours/year before and after clinical presentation were determined and compared using the Wilcoxon signed rank test. The cumulative probability of survival free from clinical endpoints was calculated with the Kaplan-Meier method. For individuals without a clinical outcome, end of follow-up (censoring) was defined by last clinical follow-up date.
Differences in survival between individuals when split by median or quartiles of annual MET-hours of activity were evaluated with the log-rank test. Cox proportional hazard models with a Firth correction for rare outcomes were used to test the association of exercise prior to clinical presentation and outcomes. Known risk factors for PLN p.(Arg14del) carriers were selected as covariates [6, 7]. These covariates at clinical presentation, were controlled for using different models based on the hypothetical relations between variables. Model 1 adjusts only for age and sex, whereas model 2 consists of all covariates. Missing values were accounted for by multiple imputation (R-package MICE; 20 imputation sets with each 10 iterations). Results were pooled over the imputation sets using Rubin’s rules. Restricted cubic spline analyses were performed to further assess the association between mean MET-hours/year until presentation as a continuous variable and risk of outcomes. A two-sided p-value of < 0.05 was considered significant. Statistical analyses were performed using SPSS (version 25.0; IBM Corp., Armonk, NY, USA), and RStudio (version 1.1.383; RStudio, Boston, MA, USA).
Results
Study population
Out of 590 adults from the PLN Registry (April 2017), 346 (58.6%) agreed to be interviewed, of whom 75 were self-referred via the PLN Foundation’s website. Of them, 299 (86.4%) were interviewed, of whom 207 (69.2%) had an adequate amount of clinical information available to determine phenotype and clinical outcomes with a median follow-up duration of 5.9 years.
Tab. 1 summarises the clinical characteristics. A minority were male (39.1%) and most presented because of family history (65.7%). At last follow-up 18% had experienced a sustained VA and more than 9% had been hospitalised for HF. Nine participants experienced both a sustained VA and hospitalisation for HF at last follow-up.
Table 1.
Clinical characteristics of study population
| Characteristic | Total (N = 207) |
Active (n = 104)a |
Inactive (n = 103)b |
P-value |
|---|---|---|---|---|
| Male | 81 (39.1) | 47 (45.2) | 34 (33.0) | 0.064 |
| Proband | 48 (23.2) | 24 (23.1) | 24 (23.3) | 0.608 |
| Caucasian | 207 (100) | 104 (100) | 103 (100) | 1 |
| Age at interview, years | 53 ± 15 | 53 ± 13 | 52 ± 16 | 0.707 |
| Mean MET-hours per year until presentation | 1661 (852–3199) | 3199 (1662–5623) | 852 (381–1190) | < 0.001 |
| Presentation | ||||
| Age at presentation, years | 45 ± 15 | 45 ± 14 | 44 ± 16 | 0.576 |
| Type of presentation: | ||||
| – Resuscitated SCD | 4 (1.9) | 4 (3.8) | 0 | 0.121 |
| – Symptomatic | 48 (23.2) | 23 (22.1) | 25 (24.3) | 0.713 |
| – Sustained VA | 13 (6.3) | 8 (7.7) | 5 (4.9) | 0.241 |
| – Positive family history | 136 (65.7) | 66 (63.5) | 70 (68.0) | 0.495 |
| At last follow-up visit | ||||
| Age at last clinical follow-up, years | 52 ± 15 | 52 ± 13 | 51 ± 16 | 0.716 |
| Task Force Criteria met | 19 (9.2) | 10 (9.6) | 9 (8.7) | 0.259 |
| Fulfilment DCM diagnosis | 52 (25.1) | 28 (26.9) | 24 (23.3) | 0.193 |
| ICD implantation | 72 (34.8) | 38 (36.5) | 34 (33.0) | 0.594 |
| Sustained VA: | 38 (18.4) | 19 (18.3) | 19 (18.4) | 0.974 |
| – Sustained VT/VF | 18 (8.7) | 11 (10.6) | 7 (6.8) | 0.334 |
| – Appropriate ICD shock | 15 (7.2) | 7 (6.7) | 8 (7.8) | 0.774 |
| Hospitalisation for heart failure | 19 (9.2) | 10 (9.6) | 9 (8.7) | 0.827 |
| RVEF < 45% | 39 (30.2) | 24 (34.3) | 15 (25.4) | 0.275 |
| LVEF < 45% | 49 (23.7) | 28 (35) | 21 (29.2) | 0.442 |
| Cardiac transplantation | 11 (5.3) | 7 (6.7) | 4 (3.9) | 0.834 |
| Death | 2 (1) | 0 | 2 (1.9) | 0.224 |
| Follow-up duration, years | 5.9 (2.8–9.1) | 6 (3.1–8.8) | 5.6 (2.3–9) | 0.692 |
SCD sudden cardiac death, VA ventricular arrhythmias, DCM dilated cardiomyopathy, VT ventricular tachycardia, VF ventricular fibrillation, ICD implantable cardioverter-defibrillator, RVEF right ventricular ejection fraction, LVEF left ventricular ejection fraction
Data are n (%), mean ± standard deviation, or median (interquartile range)
aActive group consisted of individuals who participated in 50% highest mean metabolic equivalent of task (MET) hours per year until presentation
bInactive group consisted of individuals who participated in 50% lowest mean MET-hours per year until presentation
Exercise history
Exercise history before and after clinical presentation are shown in Tab. 2. As can be appreciated, this was not a particularly athletic cohort. Prior to presentation, the study population participated in activities with a median of 1661 MET-hours/year, which included leisure activities, such as sports, with a median of 490 MET-hours/year (9.4 MET-hours/week). This equals walking for pleasure for 2.5 h per week. The Dutch Health Council advises adults to exercise at a moderate intensity for half an hour 5 days per week at a minimum [22], which is met by half of the Dutch population [23].
Table 2.
Exercise history of study population before and after clinical presentation (N = 207)
| Time spent on activity | Before presentation | After presentation | P-value |
|---|---|---|---|
| Mean MET-hours/year spent on any activitya | 1661 (852–3200) | 1152 (199–2880) | < 0.001 |
| – Leisure activity | 490 (168–1148) | 282 (0–720) | < 0.001 |
| – Work activity | 607 (0–2239) | 0 (0–2095) | 0.004 |
| – Transportation activity | 0 (0–203) | 0 | < 0.001 |
| Mean hours/year spent on any activity | 367 (147–1038) | 180 (36–840) | < 0.001 |
| – Leisure activity | 79 (28–162) | 45 (0–137) | 0.002 |
| – Work activity | 178 (0–850) | 0 (0–720) | 0.028 |
| – Transportation activity | 0 (0–49) | 0 | < 0.001 |
| Assigned METs of activities performed | 4.1 (3.1–5.2) | 3.4 (2.6–5.0) | < 0.001 |
Data median (interquartile range)
aMetabolic equivalent of task (MET) represents expenditure of energy on activity compared with resting state
Association of exercise prior to presentation with clinical outcomes
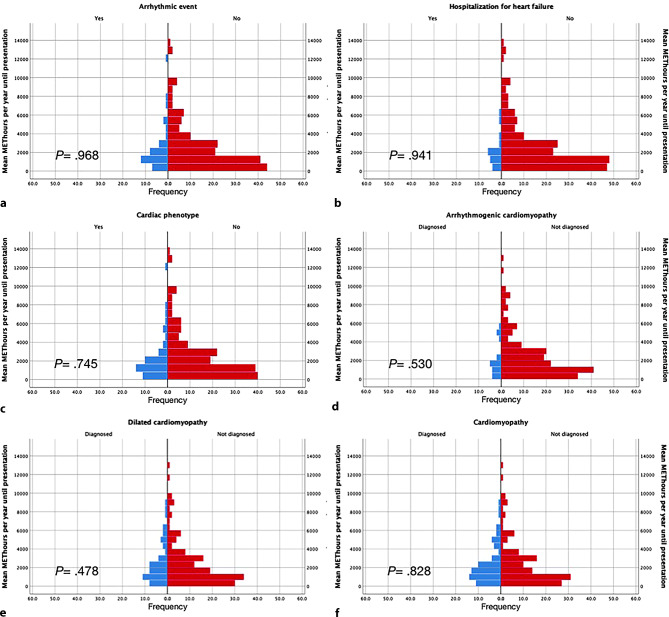
Figure 1 shows median MET-hours/year before presentation in individuals with and without: (a) a sustained VA, (b) HF hospitalisation, (c) any cardiac phenotype during follow-up (including sustained VA, HF, ARVC or DCM diagnosis), (d) ARVC diagnosis, (e) DCM diagnosis and (f) any cardiomyopathy diagnosis at last follow-up. For all outcomes, there was no significant difference in amount of exercise (MET-hours/year) prior to presentation. Tab. 1 shows that there were no differences in clinical outcome when comparing individuals participating in the 50% higher mean MET-hours/year with those in the lower 50% mean MET-hours/year until presentation. This includes a similar frequency of sustained VA (18.3% vs 18.4%; p = 0.974) and hospitalisation for HF at last follow-up (9.6% vs 8.7%; p = 0.827). There were also no differences in clinical outcome characteristics when comparing individuals from the 25% highest mean MET-hours/year with those in the 25% lowest mean MET-hours/year until presentation (data not shown). Figure S1 in the Electronic Supplementary Material shows similar results when looking only at the median MET-hours/year spent on leisure activities, including sports.
Fig. 1.
Mean MET-hours per year until presentation in individuals with and without a clinical outcome. Individuals who reached a clinical outcome are depicted in blue, whereas individuals who did not reach a clinical outcome are depicted in red. a Arrhythmic event (sustained VA), b hospitalisation for heart failure, c any cardiac phenotype, d diagnosis of arrhythmogenic right ventricular cardiomyopathy, e diagnosis of dilated cardiomyopathy and f diagnosis of any cardiomyopathy
Thirty-eight individuals (18.4%) had experienced a sustained VA at last follow-up, including 11.6% (n = 24) with a sustained VT/VF and 6.8% (n = 14) with appropriate ICD therapy. We also asked participants on the circumstances of their first arrhythmia. Almost half (n = 12/25; 48%) were physically active (e.g. bicycling, gardening).
Association of exercise prior to presentation with clinical outcomes during follow-up
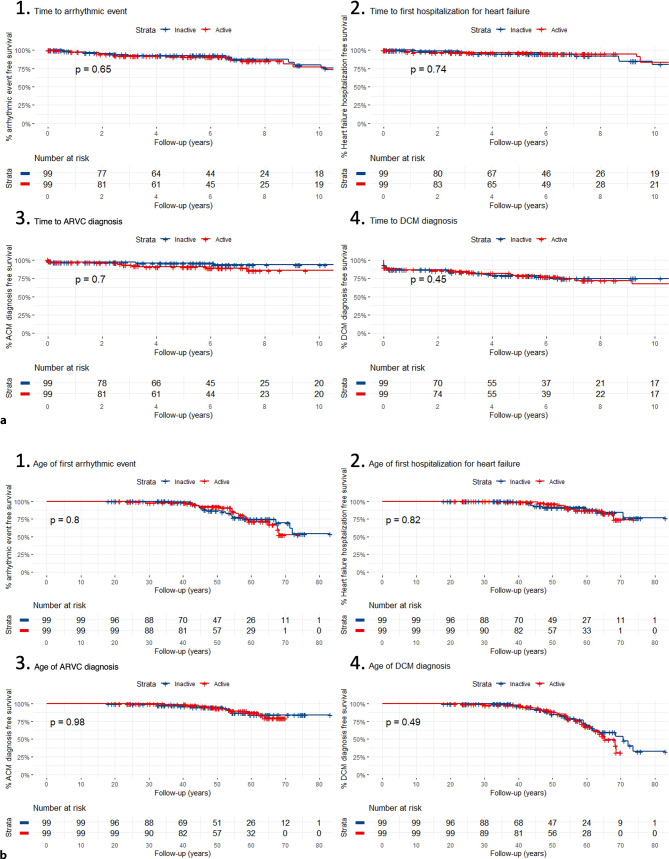
We then looked at survival free from clinical outcomes during follow-up. Nine patients were excluded due to insufficient duration of follow-up. Tab. 3 shows the baseline characteristics of this subpopulation, and Table S1 in the Electronic Supplementary Material lists the univariate and multivariate analyses of survival free from VA and HF hospitalisation. There was no difference between the active and inactive groups in survival free from new sustained VA or HF hospitalisation during follow-up (Fig. 2a, panels 1 and 2) or from diagnosis of ARVC or DCM (baseline or follow-up) (Fig. 2a, panels 3 and 4). There was also no difference between the quartiles of activity in survival free from diagnosis of ARVC or DCM (baseline or follow-up) or new sustained VA or hospitalisation for HF during follow-up (see Figures S2 and S3 in Electronic Supplementary Material).
Table 3.
Baseline characteristics of 198 carriers with additional clinical follow-up
| Characteristic | Total (N = 198) |
Active (n = 99) |
Inactive (n = 99) |
P-value |
|---|---|---|---|---|
| Male | 79 (39.9) | 46 (46.5) | 33 (33.3) | 0.059 |
| Age at presentation, years | 44 | 43 | 45 | 0.228 |
| Non-sustained VT | 33 (16.7) | 17 (17.2) | 16 (16.2) | 0.849 |
| Negative T wave in precordial leads (V1–V6) | 33/159 (20.8) | 17/79 (21.5) | 16/80 (22.2) | 0.813 |
| Negative T wave in inferior leads (II, III, aVF) | 18/159 (11.3) | 11/79 (13.9) | 7/80 (8.8) | 0.303 |
| Microvoltages on ECG | 34/161 (21.1) | 16/80 (20) | 18/81 (22.2) | 0.730 |
| RVEF < 45% | 23/138 (16.7) | 16/69 (27.1) | 7/69 (10.1) | 0.07 |
| LVEF < 45% | 33/140 (23.6) | 19/70 (27.1) | 14/70 (20) | 0.412 |
| PVC count > 500 | 27/76 (35.5) | 14/35 (40) | 13/41 (31.7) | 0.446 |
Data are n (%) or mean
VT ventricular tachycardia, ECG electrocardiogram, RVEF right ventricular ejection fraction, LVEF left ventricular ejection fraction, PVC premature ventricular contraction
aActive group consisted of individuals who participated in 50% highest mean metabolic equivalent of task (MET) hours per year until presentation
bInactive group consisted of individuals who participated in 50% lowest mean MET-hours per year until presentation
Fig. 2.
Kaplan-Meier curves of a clinical outcomes during follow-up and b age of clinical outcomes, stratified by level of activeness. Red line depicts active individuals: those who participated in 50% highest mean MET-hours per year until presentation. Blue line reflects the inactive group: individuals who participated in 50% lowest mean MET-hours per year until presentation. With (1) arrhythmic event (sustained ventricular arrhythmias), (2) hospitalisation for heart failure, (3) diagnosis of arrhythmogenic right ventricular cardiomyopathy (ARVC), and (4) diagnosis of dilated cardiomyopathy (DCM)
Next, we used Cox regression with a Firth correction for rare outcomes to assess the independent effect of exercise (MET-hours/year) controlling for clinical risk factors on survival free from sustained VA or HF hospitalisation (see Table S1 in Electronic Supplementary Material). Model 1 showed that when controlling for sex and age of presentation, there was no association with the amount of exercise (MET-hours/year) and either sustained VA or HF hospitalisation. Model 2, which includes clinical covariates, likewise showed that sustained VA and HF hospitalisation were not associated with the amount of exercise. Restricted cubic splines showed non-linearity, with model 1 showing p = 0.242 for HF hospitalisation and p = 0.177 for VA and model 2 showing p = 0.308 for HF hospitalisation and p = 0.391 for VA (see Figure S4 in Electronic Supplementary Material). The relationship between mean MET-hours/year until presentation and all outcomes showed in all curves a bell-shaped relationship with the top right after the median of 1660 mean MET-hours/year until presentation.
Penetrance
Figure 2b and S3 (in Electronic Supplementary Material) show the association between exercise and life-time penetrance when split by median MET-hours/year and quartiles of MET-hours/year respectively. These figures highlight that the amount of exercise prior to presentation had no effect on the penetrance of an arrhythmic phenotype, hospitalisation for HF or ARVC/DCM diagnosis.
Exercise after presentation
Most participants (64.7%) decreased their annual exercise duration and intensity after first presentation (Tab. 2). This was significant for both MET-hours and for total hours spent on any activity (p < 0.001). More than half (n = 131; 63.2%) of the study population decreased their MET-hours after presentation, and most also decreased their time spent on being active (n = 127; 61.4%). In contrast, 63 individuals (30.4%) increased their level of activity in MET-hours/year and 68 (32.9%) individuals increased their time spent on activity.
Exercise restriction is advised for ARVC patients and those with a (likely) pathogenic desmosomal gene variant and this advice is generally also given to PLN p.(Arg14del) carriers with ARVC. To assess the independent effect of decreasing exercise after presentation controlling for the same risk factors as previously, we used Cox regression. The univariable and multivariable analyses (see Table S2 in Electronic Supplementary Material) show similar results as presented in supplementary Tab. 1. After controlling for covariates, both model 1 and 2 showed no association with decreasing amount of exercise after presentation and either sustained VA or HF.
Discussion
This study assessed the effect of the amount of exercise as measured by MET-hours/year on penetrance and clinical outcomes in individuals with PLN p.(Arg14del). Our results suggest exercise history prior to clinical presentation does not predict development of an arrhythmic phenotype or HF in this cohort that was only moderately active. These results are in contrast with studies of ARVC patients who generally have (likely) pathogenic desmosomal gene variants.
Prior studies on influence of exercise on development of ARVC
The relationship between athlete status and the ARVC-phenotype has been studied extensively. Currently, both definite ARVC patients and those with a (likely) pathogenic variant in a desmosomal gene, are discouraged from competitive or frequent high-intensity endurance exercise [8].
An association between exercise and the risk of VA, HF and diagnosis of ARVC in desmosomal variant carriers has been established by multiple studies [9, 24]. In addition, for TMEM43 p.(Ser358Leu) related ARVC, ≥ 9.0 MET-hours/day in the year prior to ICD implantation was associated with an 9.1-fold increased hazard of first appropriate ICD discharge [25]. Moreover, even in gene-elusive ARVC patients exercise has been shown to have a negative effect by modifying cardiac structure and promoting arrhythmias [26]. However, exercise in Dsp haploinsufficient mice partially restored dysregulated gene expression without changing cardiac function, showing that the detrimental effect of exercise is not that straightforward [27].
Impact of exercise on clinical course of PLN variant carriers
For a yet unaffected, asymptomatic, PLN p.(Arg14del) carrier, data from the current study strongly suggest there is no reason to limit exercise if this is of limited-moderate intensity (i.e. 6 METs or less) [8]. This contrasts with desmosomal gene carriers and might be explained by the fact that this PLN cohort was not particularly active or that different pathophysiological mechanisms eventually lead to disease. For unaffected asymptomatic PLN p.(Arg14del) carriers that want to exercise more intensively, and thereby fulfil the definition of an athlete (≥ 18 MET-hours/week), the uncertainty of an effect of more intensive exercise has to be discussed and participation should be a shared decision.
For an affected individual, the data also suggest that mild-moderate exercise as reflected by the number of MET-hours does not negatively influence disease course. However, it has been observed that incident malignant arrhythmias in PLN p.(Arg14del) are often associated with exercise. Previously, it was shown that 74% of malignant VA in PLN p.(Arg14del) variant carriers occurred during or shortly after exercise, independent of the intensity [7]. Additionally, out of 19 individuals of whom the circumstances of SCD were known, 13 (68%) were exercising. Similarly in our cohort, those who experienced a VA and could recall their activity at their first arrhythmia, almost half (n = 12/25; 48%) said they were active. Thus, for the patient already displaying risk factors related to the development of an arrhythmia (decreased LVEF, a prior sustained or non-sustained VT, low-voltage ECG, the number of leads with negative T waves or PVC count), and for those fulfilling 2010 Task Force Criteria for ARVC [18], it is reasonable to discuss exercise restrictions and make a shared decision on the intensity of participation [8]. In short, it appears that exercise can be an arrhythmic trigger in PLN cardiomyopathy patients but is likely not associated with disease development or progression.
Study limitations
Recall bias and social desirability bias were limiting factors, as exercise history was assessed retrospectively. A potential future prospective study on exercise in a large number of phenotype negative PLN p.(Arg14del) carriers would be ideal to study the effect of exercise on disease penetrance. By using the same methods as previous studies of exercise in ARVC patients, we tried to minimise the aforementioned limiting factors. Mortality was not available as an outcome measure, as patients had to be alive to participate in the interview, which could lead to underrepresentation of patients with worse outcomes. On the other hand, we recruited patients from tertiary care centres, who commonly have more severe disease and worse outcomes. Last, even though we had a response rate of 58.6%, voluntary response bias could have played a role.
In addition, the relatively small study population that was not particularly active may hamper generalisation of these findings to more intensive levels of activity and providing advice to high level athletes with this PLN variant.
Conclusion
There was no association between exercise (MET-hours/year) and lifetime disease penetrance, sustained VA or HF hospitalisation during follow-up in mild-moderately active PLN p.(Arg14del) carriers. This seems to contrast with prior studies in ARVC patients and desmosomal variant carriers. In addition, diagnosis of ARVC or DCM was not associated with mild-moderate exercise. Exercise restrictions are not advised for mild-moderately active (those not participating in frequent or competitive or endurance exercise (> 6 METs)) PLN p.(Arg14del) carriers without any known risk factors for arrhythmias. However, PLN p.(Arg14del) carriers with risk-factors may benefit from exercise restriction because arrhythmias are associated with exercise in nearly half of the events.
Supplementary Information
Table S1 Hazard ratios for sustained ventricular arrhythmias or heart failure according to exercise history and clinical characteristics for 198 individuals
Table S2 Hazard ratios for arrhythmic event or heart failure according to increasing activity after presentation and clinical characteristics
Figure S1 Mean MET-hours per year for leisure activities until presentation in individuals with and without an event or diagnosis
Figure S2 Kaplan-Meier curves of event or diagnosis during follow-up stratified by level of activeness (quartiles)
Figure S3 Kaplan-Meier curves of age of event or diagnosis stratified by level of activeness (quartiles)
Figure S4 Restricted cubic splines showing non-linearity. Mean MET-hours/year until presentation and all outcomes show a bell-shaped relationship with the top right after the median of 1660 mean MET-hours/year until presentation. Model 1 showing a p = 0.242 for HF hospitalisation and b p = 0.177 for VA. Model 2 showing c p = 0.308 for HF hospitalisation and d p = 0.391 for VA
Acknowledgments
Funding
This work was supported by the Netherlands Cardiovascular Research Initiative, an initiative supported by the Dutch Heart Foundation (CardioVasculair Onderzoek Nederland (CVON) projects: PREDICT2 2018-30, eDETECT 2015-12, DOSIS 2014-40, DOUBLE-DOSE 2020B005). Additional financial support was received from CURE-PLaN, a network funded by the Leducq Foundation, and the Dutch PLN Patient Foundation. Support was also provided by the Netherlands Organisation for Scientific Research (NWO)—travel grant 040.11.586 to C. A. James.
Conflict of interest
C.A. James receives salary support form an investigator-initiated research grant from Boston Scientific Corp. and serves as a consultant for Pfizer Inc. and StrideBio, Inc. F.H. M. van Lint, F. Hassanzada,T.E. Verstraelen, W. Wang, L.P. Bosman, P.A. van der Zwaag, T. Oomen, H. Calkins, B. Murray, C. Tichnell, T.M.A. Beuren, F.W. Asselbergs, A. Houweling, M.P. van den Berg, A.A.M. Wilde and J. P. van Tintelen declare that they have no competing interests.
Footnotes
F. H. M. van Lint, F. Hassanzada, C. A. James and J. P. van Tintelen contributed equally to this work.
References
- 1.Groeneweg JA, Bhonsale A, James CA, et al. Clinical presentation, long-term follow-up, and outcomes of 1001 Arrhythmogenic right ventricular Dysplasia/Cardiomyopathy patients and family members. Circ Cardiovasc Genet. 2015;8:437–446. doi: 10.1161/CIRCGENETICS.114.001003. [DOI] [PubMed] [Google Scholar]
- 2.Van der Zwaag PA, van Rijsingen IA, al Asimaki AJ. Phospholamban R14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: evidence supporting the concept of arrhythmogenic cardiomyopathy. Eur J Heart Fail. 2012;14:1199–1207. doi: 10.1093/eurjhf/hfs119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan E, Peterson L, Ai T, et al. Evidence-Based Assessment of Genes in Dilated Cardiomyopathy. Circulation. 2021;144:7–19. doi: 10.1161/CIRCULATIONAHA.120.053033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James CA, Jongbloed JDH, Hershberger RE, et al. International Evidence Based Reappraisal of Genes Associated With Arrhythmogenic Right Ventricular Cardiomyopathy Using the Clinical Genome Resource Framework. Circ Genom Precis Med. 2021;14:e003273. doi: 10.1161/CIRCGEN.120.003273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang HS, Arvanitis DA, Dong M, et al. SERCA2a superinhibition by human phospholamban triggers electrical and structural remodeling in mouse hearts. Physiol Genomics. 2011;43:357–364. doi: 10.1152/physiolgenomics.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verstraelen TE, van Lint FHM, Bosman LP, et al. Prediction of ventricular arrhythmia in phospholamban p.Arg14del mutation carriers-reaching the frontiers of individual risk prediction. Eur Heart J. 2021;42:2842–2850. doi: 10.1093/eurheartj/ehab294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Rijsingen IA, van der Zwaag PA, Groeneweg JA, et al. Outcome in phospholamban R14del carriers: results of a large multicentre cohort study. Circ Cardiovasc Genet. 2014;7:455–465. doi: 10.1161/CIRCGENETICS.113.000374. [DOI] [PubMed] [Google Scholar]
- 8.Towbin JA, McKenna WJ, Abrams DJ, et al. HRS Expert Consensus Statement on Evaluation, Risk Stratification, and Management of Arrhythmogenic Cardiomyopathy. Heart Rhythm. 2019;2019:e301–e72. doi: 10.1016/j.hrthm.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 9.James CA, Bhonsale A, Tichnell C, et al. Exercise increases age-related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated desmosomal mutation carriers. J Am Coll Cardiol. 2013;62:1290–1297. doi: 10.1016/j.jacc.2013.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saberniak J, Hasselberg NE, Borgquist R, et al. Vigorous physical activity impairs myocardial function in patients with arrhythmogenic right ventricular cardiomyopathy and in mutation positive family members. Eur J Heart Fail. 2014;16:1337–1344. doi: 10.1002/ejhf.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lie OH, Dejgaard LA, Saberniak J, et al. Harmful Effects of Exercise Intensity and Exercise Duration in Patients With Arrhythmogenic Cardiomyopathy. JACC Clin Electrophysiol. 2018;4:744–753. doi: 10.1016/j.jacep.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 12.La Gerche A, Robberecht C, Kuiperi C, et al. Lower than expected desmosomal gene mutation prevalence in endurance athletes with complex ventricular arrhythmias of right ventricular origin. Heart. 2010;96:1268–1274. doi: 10.1136/hrt.2009.189621. [DOI] [PubMed] [Google Scholar]
- 13.Mazzanti A, Ng K, Faragli A, et al. Arrhythmogenic Right Ventricular Cardiomyopathy: Clinical Course and Predictors of Arrhythmic Risk. J Am Coll Cardiol. 2016;68:2540–2550. doi: 10.1016/j.jacc.2016.09.951. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Tichnell C, Murray BA, et al. Exercise restriction is protective for genotype-positive family members of arrhythmogenic right ventricular cardiomyopathy patients. Europace. 2020;22:1270–1278. doi: 10.1093/europace/euaa105. [DOI] [PubMed] [Google Scholar]
- 15.Okely AD, Salmon J, Vella S, et al. A systematic review to update the Australian physical activity guidelines for children and young people. Faculty of Social Sciences—Papers. 2012. https://ro.uow.edu.au/sspapers/1246/. Accessed December 2022.
- 16.Bosman LP, Verstraelen TE, van Lint FHM, et al. The Netherlands Arrhythmogenic Cardiomyopathy Registry: design and status update. Neth Heart J. 2019;27:480–486. doi: 10.1007/s12471-019-1270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ponikowski P, Voors AA, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;2016(18):891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 18.Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, et al. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson. 2015;17:29. doi: 10.1186/s12968-015-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ainsworth BE, Haskell WL, Herrmann SD, et al. Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;2011:1575–81. [DOI] [PubMed]
- 21.Franklin BA, Brinks J, Berra K, et al. Using Metabolic Equivalents in Clinical Practice. Am J Cardiol. 2018;121:382–387. doi: 10.1016/j.amjcard.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 22. Beweegrichtlijnen. Gezondheidsraad, Vol. 2017. (Dutch). The Hague: Gezondheidsraad; 2017.
- 23.Gezondheidsenquête/Leefstijlmonitor, CBS i.s.m. RIVM, Trimbos Instituut, VeiligheidNL, Rutgers, SOA Aids Nederland en Voedingscentrum [Internet]. 2019. Available from: https://statline.rivm.nl/#/RIVM/nl/dataset/50080NED/table?dl=4C860.
- 24.Sawant AC, Te Riele AS, Tichnell C, et al. Safety of American Heart Association-recommended minimum exercise for desmosomal mutation carriers. Heart Rhythm. 2016;13:199–207. doi: 10.1016/j.hrthm.2015.08.035. [DOI] [PubMed] [Google Scholar]
- 25.Paulin FL, Hodgkinson KA, MacLaughlan S, et al. Exercise and arrhythmic risk in TMEM43 p.S358L arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2020;17:1159–1166. doi: 10.1016/j.hrthm.2020.02.028. [DOI] [PubMed] [Google Scholar]
- 26.Sawant AC, Bhonsale A, te Riele AS, et al. Exercise has a disproportionate role in the pathogenesis of arrhythmogenic right ventricular dysplasia/cardiomyopathy in patients without desmosomal mutations. J Am Heart Assoc. 2014;3:e001471. doi: 10.1161/JAHA.114.001471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheedipudi SM, Hu J, Fan S, et al. Exercise Restores Dysregulated Gene Expression in a Mouse Model of Arrhythmogenic Cardiomyopathy. Cardiovasc Res. 2020;116:1199–1213. doi: 10.1093/cvr/cvz199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Hazard ratios for sustained ventricular arrhythmias or heart failure according to exercise history and clinical characteristics for 198 individuals
Table S2 Hazard ratios for arrhythmic event or heart failure according to increasing activity after presentation and clinical characteristics
Figure S1 Mean MET-hours per year for leisure activities until presentation in individuals with and without an event or diagnosis
Figure S2 Kaplan-Meier curves of event or diagnosis during follow-up stratified by level of activeness (quartiles)
Figure S3 Kaplan-Meier curves of age of event or diagnosis stratified by level of activeness (quartiles)
Figure S4 Restricted cubic splines showing non-linearity. Mean MET-hours/year until presentation and all outcomes show a bell-shaped relationship with the top right after the median of 1660 mean MET-hours/year until presentation. Model 1 showing a p = 0.242 for HF hospitalisation and b p = 0.177 for VA. Model 2 showing c p = 0.308 for HF hospitalisation and d p = 0.391 for VA