Abstract
Peripheral artery disease (PAD), defined as reduced blood flow to the lower limbs, is a serious disorder that can lead to loss of function in the lower extremities and even loss of limbs. One of the main risk factors for PAD is age, with up to 25% of adults over the age of 55 and up to 40% over the age of 80 presenting with some form of the disease. While age is the largest risk factor for PAD, other risk factors include atherosclerosis, smoking, hypertension, and diabetes. Furthermore, previous studies have suggested that the incidence of PAD is significantly increased in patients with Alzheimer’s disease (AD). Attenuation of mTOR with rapamycin significantly improves cerebral blood flow and heart function in aged rodents as well as in mouse models of atherosclerosis, atherosclerosis-driven cognitive impairment, and AD. In this study, we show that rapamycin treatment improves peripheral blood flow in aged mice and in mouse models of atherosclerosis and AD. Inhibition of mTOR with rapamycin ameliorates deficits in baseline hind paw perfusion in aged mice and restores levels of blood flow to levels indistinguishable from those of young controls. Furthermore, rapamycin treatment ameliorates peripheral blood flow deficits in mouse models of atherosclerosis and AD. These data indicate that mTOR is causally involved in the reduction of blood flow to lower limbs associated with aging, atherosclerosis, and AD-like progression in model mice. Rapamycin or other mTOR inhibitors may have potential as interventions to treat peripheral artery disease and other peripheral circulation-related conditions.
Keywords: Peripheral blood flow, mTOR, Aging, Rapamycin, Atherosclerosis, Alzheimer’s disease
Introduction
Peripheral artery disease (PAD) is defined as a decrease in blood flow to the lower limbs caused by the narrowing or blockage of arteries [1]. Age is one of the main risk factors for PAD that is exacerbated by age-related diseases such as atherosclerosis, diabetes, and hypertension [2]. If left untreated, PAD can lead to functional decline in the lower limbs that in severe cases may require amputation [1]. PAD is common in elderly adults with approximately 10–25% of individuals over the age of 55 showing some form of the disease [3]. The prevalence of PAD increases with age such that approximately 40% of individuals over the age of 80 are affected [4]. Although PAD is often undiagnosed, some studies have suggested that the prevalence of PAD may increase in AD patients [5].
Rapamycin inhibits the mechanistic/mammalian target of rapamycin complex 1 (mTORC1), a central regulator of aging [6–9]. Consistent with the central role of mTOR as a driver of aging, attenuation of mTOR activity with rapamycin blocks or retards progression of both aging and age-related disease processes [10–16]. Notably, rapamycin restores cerebrovascular integrity and function in models of AD [11, 12, 17], negates basal cerebral blood flow (CBF) and neurovascular coupling deficits associated with normative aging in rats [16], and ameliorates heart function deficits in aged mice [13, 15]. In addition, mTORC1 attenuation with rapamycin restores brain vascular density, CBF, spatial learning, and memory and reduces aortic plaques in a mouse model of atherosclerosis and vascular cognitive impairment while decreasing body weight and fat mass [18]. Atherosclerosis is one of the major underlying causes of PAD, since atherosclerotic plaques forming in arteries of the lower limbs reduce blood flow and lead to PAD as well as critical limb ischemia (CLI), a more severe form of PAD.
We had previously demonstrated that mTORC1 drives age-associated brain vascular disintegration and decreased basal and evoked cerebral blood flow in aged rats [16], in various mouse models of AD [11, 12], and in a model of atherosclerosis and vascular cognitive impairment [18]. Studies of other groups showed that mTORC1 drives heart aging [13, 15]. We thus hypothesized that mTORC1 may also be causally involved in the etiology of age-related loss of peripheral perfusion similar to PAD in mice. The present study tested this hypothesis through the attenuation of mTORC1 by systemic rapamycin treatment. Our data show that systemic mTORC1 attenuation negates decreases in peripheral blood flow associated with aging, atherosclerosis, and progression of AD-like disease in mice. Taken together, our data suggest that inhibition of mTORC1 with rapamycin or other mTORC1 inhibitors may delay or treat reduced blood flow to the lower limbs associated with normative aging or with cardiovascular and neurological diseases of aging.
Methods
Animals
All studies were performed under the approval of the UT Health San Antonio Institutional Animal Care and Use Committee (Animal Welfare Assurance Number: A3345-01) and in compliance with the ARRIVE guidelines (Animal Research: Reporting In Vivo Experiments) for reporting animal experiments. All mice were housed ≤5 animals per cage and maintained on a 12-h light/12-h dark cycle with ad libitum access to food and water.
Male LDLR−/− (B6.129S7-LdldrtmlHer/J, Jackson Laboratories, Bar Harbor, ME) mice were used as a model of atherosclerosis [18]. Only males were included in the LDLR−/− cohort because studies of this cohort that we reported previously [18] included measures of spatial learning and memory, where female performance in the Morris water maze could potentially have increased the variability of the data. To induce atherosclerosis, LDLR−/− mice were fed a high-fat diet (21% saturated milk fat, 0.2% cholesterol, supplemented into AIN-76A, BioServ) that was supplemented with either rapamycin (rapa, 14ppm) or vehicle (eudragit), that were incorporated into the special diet starting at 7 months. Because LDLR−/− mice are bred in homozygosity, WT animals for this experiment were bred independently as age-matched C57Bl/6J mice (controls) and were fed standard mouse chow. Peripheral cutaneous blood flow in the hind paw was measured between 14 and 15 months of age of LDLR−/− mice and age-matched controls.
Male and female hAPP(J20) mice were bred in our colony and maintained through heterozygous crosses with C57BL/6J mice as previously reported [11, 17, 19]. Non-transgenic littermates were used as wildtype (WT) controls. Microencapsulated rapamycin (14ppm) or eudragit (vehicle) was supplemented into the chow of transgenic hAPP(J20) mice and non-transgenic (WT) littermates beginning at 4 months of age. Peripheral cutaneous blood flow in the left hind paw was measured between 20 and 24 months of age, with no difference in mean age (22 months) among the treatment groups.
To investigate age-related decline in peripheral cutaneous blood flow, we used male and female 22-month-old non-transgenic (WT) littermates of the hAPP(J20) mice fed either eudragit (vehicle) or rapamycin diets, with an additional group of 4-month-old C57BL/6J WT male and female mice as young adult controls for this study. Data from male and female 22-month-old WT+Vehicle mice used in the hAPP J20 comparisons were used to define the impact of age in peripheral cutaneous blood flow through comparisons with 4-month-old C57BL/6J WT mice as young adult controls.
Cutaneous blood flow monitoring
Mice were anesthetized and maintained under ~1.5–2% isoflurane in oxygen and were placed on a mouse heating pad to maintain body temperature. Blood flow in the left hind paw (foot pad facing up) was monitored by laser speckle contrast imaging using the PeriCam PSI high-resolution imager (Perimed, Sweden). Animals’ foot pads were uniformly placed at a constant distance from the detector in all measurements; thus, potential variability associated with differences in the distance (i.e., as in measures of cerebral blood flow) was ruled out. We also ensured that the instrument would report zero perfusion in tissues from dead animals and used signals associated with reperfusion to verify the instrument’s ability to record increases in blood flow. Further, all animals were measured under the same conditions and settings and we report baseline foot pad blood flow values in relative units as measures normalized to control groups. Our data have very small variance, delineating differences between experimental groups and providing additional circumstantial evidence that supports our measurements as not arising from pervasive measurement error. A stable baseline was established and recorded for 2 min. To measure evoked cutaneous vascular perfusion changes, a thin layer of topical cream containing 10% menthol and 30% methyl salicylate (Icy Hot®) was applied to the foot pad of the left hind paw and elicited blood flow was monitored for five minutes.
Data and statistical analysis
The recorded data was analyzed using the manufacturer’s software, PIMSoft (Perimed, Sweden). A region of interest (ROI) was selected around the left hind paw to include the tarsus and metatarsus, excluding the calcaneus and phalanges. The average perfusion intensity per second for the entire ROI was calculated automatically by the software. Baseline data are expressed as percentage of wildtype or young control perfusion and these data were analyzed with a one-way ANOVAs followed by Tukey’s multiple comparison post hoc tests. Time course data assessing evoked perfusion was measured ± 5 s at each 1-min interval after topical menthol/methyl salicylate application. The evoked perfusion was analyzed with a two-way repeated measures ANOVA with Tukey’s multiple comparison post hoc tests. P<0.05 was considered significant.
Results
Peripheral blood flow is restored with mTOR inhibition by rapamycin in aged mice
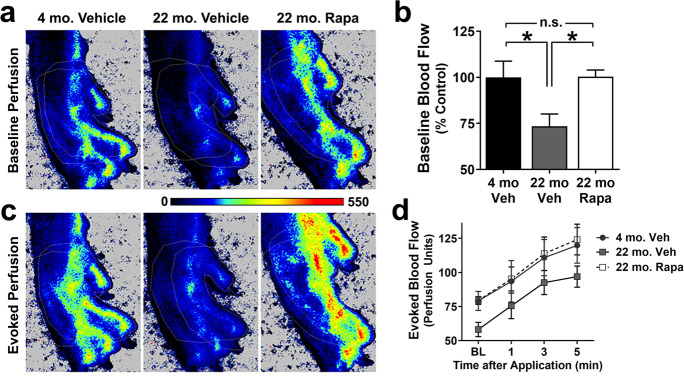
Numerous reports have shown that peripheral blood flow declines with age in humans [1, 20–22]. To determine a role of mTOR in the etiology of age-related declines in peripheral perfusion, skin blood flow was measured using laser speckle contrast imaging in the left hind paw of young (4 mo.) and aged (22 mo.) mice as well as in aged (22 mo.) mice systemically treated with the mTORC1 inhibitor, rapamycin. We found a significant reduction in baseline cutaneous blood flow in aged WT mice (p=0.034, Fig. 1A, B) that were negated by chronic mTOR inhibition as peripheral blood flow in aged WT mice treated with rapamycin was indistinguishable from that of young mice (Fig. 1A, B). No significant difference was observed with sex (p=0.28, main effect by 2-way ANOVA). These data indicate that mTOR is causally involved in the decline in peripheral vascular function associated with normative aging.
Fig. 1.
mTOR contributes to age-related peripheral blood flow deficits. A Representative images of baseline hind paw perfusion in 4- and 22-month-old animals captured with laser speckle contrast imaging. B Baseline blood flow is significantly reduced in 22-month-old animals treated with vehicle (22 mo. Veh) relative to 4-month-old animals (4 mo. Veh.) (*, Tukey’s q(17)=3.91, p=0.034). The age-dependent deficit is abolished with mTOR inhibition in 22-month-old mice treated with rapamycin (22 mo. Rapa) (*, Tukey’s q(17)=3.81, p=0.039). C Representative images of evoked hind paw perfusion after 5 min of the application of a menthol/methyl salicylate cream. These images correspond to the same animals shown in respective images of panel A. D All groups showed increased blood flow with time (F(3, 51)=33.2, p<0.0001), but no significant group differences were detected (F(2, 17)=1.86, p=0.18) using two-way repeated measures ANOVA. Data are mean ± SEM. n= 6–7 per group
To define the impact of age and the role of mTORC1 on evoked peripheral vascular responses, we stimulated skin blood flow in the left hind paw with topically applied vasodilators. As expected, cutaneous perfusion of the hind paw significantly increased with time in all groups after topical application of 10% menthol and 30% methyl salicylate (Fig. 1C, D), which have been shown to acutely increase skin blood flow [23]. Menthol increases blood flow through endothelial NO and hyperpolarization factor/s (EDHF) release and through sensory nerve responses [24] and also acts on transient receptor potential melastatin-related 8 (TRPM8) calcium channels in smooth muscle cells to elicit vasoconstriction [25]. Methyl salicylate is a transient receptor potential voltage 1 (TRPV1) agonist [26]; thus, it is expected to have a distinct impact on different types of vascular cells.
Although baseline cutaneous blood flow was significantly decreased in vehicle- but not in rapamycin-treated 22-month-old mice, neither the rate of evoked blood flow increase nor its magnitude was affected by age or treatment (Fig. 1C, D). Taken together, these data indicate that baseline peripheral cutaneous perfusion is impaired in 22-month-old mice and that this deficit is largely driven by mTORC1. In contrast, responses to pleiotropic vasodilators menthol and methyl salicylate are not impaired in 22-month-old mice.
Rapamycin ameliorates peripheral vascular deficits in the LDLR−/− mouse model of atherosclerosis
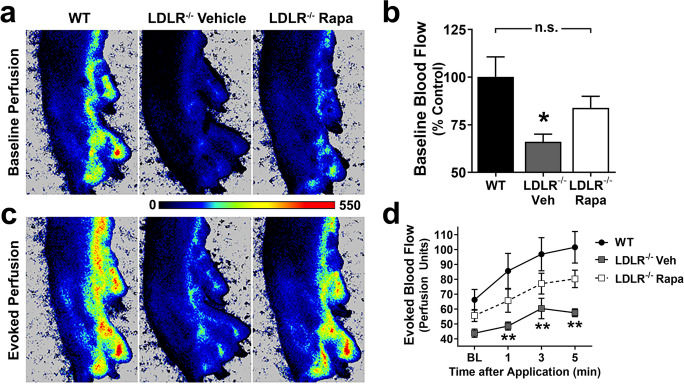
Atherosclerosis is frequently accompanied by peripheral vascular disease in humans [27]. To determine if mTOR attenuation with rapamycin can ameliorate peripheral blood flow deficits in atherosclerosis, we measured basal blood flow in the left hind paw of LDLR−/− mice [18, 28] fed with a high-fat diet to induce atherosclerosis and treated with rapamycin or with eudragit (vehicle) using laser speckle contrast imaging. Baseline flood flow in the left hind paw was significantly decreased in LDLR−/− mice fed a high-fat diet and treated with vehicle as compared to WT controls (p = 0.028, Fig. 2A, B). In contrast, the baseline left hind paw perfusion of LDLR−/− mice fed with a high-fat diet and treated with rapamycin was indistinguishable from that of age-matched WT control mice, suggesting that mTORC1 drives peripheral vascular impairment associated with atherosclerosis in the LDLR−/− mouse model.
Fig. 2.
mTOR drives peripheral blood flow deficits in a mouse model of atherosclerosis. A Representative images of baseline perfusion of the left hind paw captured with laser speckle contrast imaging. B LDLR−/− mice with vehicle (LDLR−/− Veh) have significantly reduced baseline perfusion relative to WT controls (*, Tukey’s q(21)=3.97, p=0.028). Inhibition of mTOR improves perfusion in LDLR−/− mice on a high-fat diet with rapamycin (LDLR−/− Rapa) to a level that is not significantly different from WT controls (Tukey’s q(21)=2.11, p=0.31, n.s.). C Representative images of evoked hind paw perfusion after 5 min of the application of menthol/methyl salicylate cream. These images are from the same animals shown in the respective images of panel A. D LDLR−/− Veh mice on high-fat diet have consistently reduced blood flow at all time points relative to WT (**, Tukey’s q(84)>4.33, p<0.009, applied to significant main effects of group (F(2,21)=4.98, p=0.02) and time (F(3,63)=30.32, p<0.0001) via two-way repeated measures ANOVA). Data are mean ± SEM. n= 6–9 per group
While topical application of vasodilators increased microperfusion in all groups, evoked blood flow was significantly lower in vehicle-treated, high-fat diet-fed LDLR−/− group (p<0.009), but not in rapamycin-treated, high-fat diet-fed LDLR−/− animals as compared to WT controls at all times after stimulation (Fig. 2)C, D. These data indicate that decreased basal cutaneous peripheral blood flow as well as deficits in evoked blood flow in LDLR−/− mice modeling atherosclerosis can be mitigated by mTOR inhibition. Furthermore, these data suggest that mTOR drives peripheral vascular impairment in the LDLR−/− model of atherosclerosis which recapitulates reduced blood flow to the lower limbs of peripheral artery disease (PAD) patients.
Inhibition of mTOR restores peripheral blood flow in a mouse model of Alzheimer’s disease
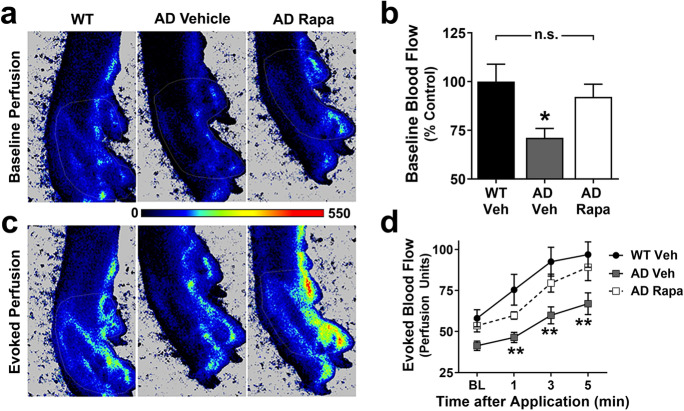
Previous studies have suggested that the incidence of PAD is increased in patients with Alzheimer’s disease (AD) [5] and our prior work [11, 12, 17, 29] demonstrated an involvement of mTORC1 in the etiology of brain vascular dysfunction in models of AD. Because our studies of Figs. 1 and 2 suggested a role for mTOR in the etiology of reduced peripheral blood flow both in aging and in cardiovascular disease, we next sought to determine whether decreased peripheral blood flow would be present in a model of AD and, if so, whether mTORC1 would be causally involved. To this aim, next we examined peripheral cutaneous circulation as both baseline perfusion and evoked blood flow in the left hind paw of mice modeling Alzheimer’s disease (AD, hAPP(J20) mice, [30, 31]) with laser speckle contrast imaging. Similar to our observations in aged mice (Fig. 1) and in mice modeling atherosclerosis (Fig. 2), we found profound deficits in peripheral circulation in hAPP(J20) as compared to WT littermates at 22 months of age (p=0.044, Fig. 3A, B). Attenuation of mTOR with systemic rapamycin, however, restored baseline blood flow in the hind paw of hAPP(J20) mice to levels indistinguishable from littermate controls. No significant difference was observed with sex (p=0.85, main effect by 2-way ANOVA). These data indicate that hAPP(J20) mice recapitulate impaired peripheral blood flow of AD and that these deficits are driven by mTORC1 (Fig. 3A, B).
Fig. 3.
Cutaneous vascular impairments due to Alzheimer’s disease are driven by mTOR. A Representative images of baseline hind paw perfusion captured with laser speckle contrast imaging as measured in 22-month-old animals. B Baseline blood flow is significantly reduced in hAPP(J20) mice treated with vehicle (AD Veh) compared to WT littermates (*, Tukey’s q(15)=3.77, p=0.044). Inhibition of mTOR with rapamycin in hAPP(J20) mice (AD Rapa) mice restored baseline perfusion to levels not significantly different from those of WT mice (Tukey’s q(15)=1.07, p=0.73, n.s.). C Representative images of evoked blood flow 5 min after topical application of a 10% menthol and 30% methyl salicylate cream. These images correspond to the same animals shown in panel A, respectively. D The time course of evoked blood flow demonstrates that 22-month-old AD Veh mice have consistently reduced blood flow at all time points relative to age-matched WT littermate controls (** indicates Tukey’s q>4.32, p<0.009, applied to significant main effects of time (F(3, 45)=43.74, p<0.0001) and group (F(2,15)=5.55, p=0.016) via two-way repeated measures ANOVA. AD Rapa peripheral blood flow was not significantly different than WT littermate controls (p>0.30 at each time point). Data are mean ± SEM. n= 5–7 per group
Furthermore, topical application of vasodilators to the hind paw increased cutaneous microvascular perfusion in all groups (Fig. 3C, D) but the magnitude of this increase was significantly decreased in hAPP(J20) mice as compared with WT littermates. This deficit, however, was negated by rapamycin treatment (Fig. 1D), suggesting that, in addition to its role in regulating basal peripheral perfusion, mTOR mediates reduced peripheral circulation responses to vasodilators in late stages of AD-like disease.
Discussion
Our studies show that attenuation of mTORC1 by rapamycin ameliorates profound age-dependent (Fig. 1), atherosclerosis-associated (Fig. 2), and AD-like disease-related (Fig. 3) deficits in basal peripheral blood flow. These data provide strong evidence that mTOR is a central mediator of peripheral vascular dysfunction associated with normative aging as well as with two distinct disease processes, atherosclerosis and AD, modeled in mice. These observations are consistent with previous studies showing that mTOR attenuation with rapamycin improves heart function in aged mice [13, 15, 32, 33]. Conversely, hyperactivation of mTOR in tuberous sclerosis complex (TSC) patients may lead to premature vascular aging [34].
A recent study showed that the severity of age-related peripheral vascular dysfunction correlates with cognitive dysfunction in older adults [35]. In agreement with these observations, our laboratory previously showed profound brain vascular dysfunction and damage associated with cognitive impairment in LDLR−/− mice, where hypercholesterolemia and pro-atherosclerotic vascular lesions are proportional to the severity of cerebromicrovascular dysfunction and BBB breakdown [18, 29].
Our data are also consistent with prior studies from our laboratory that showed that attenuation of mTOR with rapamycin relieves profound deficits in cerebrovascular function and restores brain vascular integrity in normative aging [16, 36], in mice modeling atherosclerosis [18], and in several different mouse models of AD [11, 12, 17, 29]. These data indicate that mTOR mediates brain vascular disintegration and dysfunction leading to cognitive decline in normative aging and also cognitive dysfunction arising from distinct brain disease processes. Together, these observations suggest the existence of mTOR-mediated mechanisms that are common to brain dysfunction in aging and brain vascular dysfunction associated with atherosclerosis and AD. We further propose that mTOR-dependent mechanisms underlying brain aging, brain vascular dysfunction associated with atherosclerosis, and AD may overlap with mechanisms by which mTOR regulates aging itself [6, 8–10, 16, 36]. Whether the mechanisms by which mTOR drives vascular aging in the brain and periphery are the same, however, remains unclear.
Cutaneous menthol increases skin blood flow through endothelial NO and hyperpolarization factor/s (EDHF) release, as well as sensory nerve responses [24]. Because attenuation of mTOR activity is sufficient to negate these deficits, our data suggest that mTOR mediates age- or disease-associated reduced NO or EDHF bioavailability. In agreement with this notion, previous studies have shown that mTOR represses eNOS activation in cerebral microvasculature during aging [16], during progression of AD [11, 12, 17], and in cognitive dysfunction associated with atherosclerosis [18] such that attenuation of mTOR with rapamycin restores cerebral blood in all these conditions. The impact of mTOR attenuation is abolished if nitric oxide synthase (NOS) activity is blocked pharmacologically, demonstrating that relief of mTOR inhibition of NOS is essential for the restoration of brain vascular integrity and function by rapamycin [11]. Further studies are needed to determine the cellular mechanisms by which systemic mTOR inhibition restores baseline peripheral blood flow in aging, atherosclerosis, and AD-like disease. Consistent with our observations, a negative association between AD and cutaneous vasodilation has been suggested [21, 37].
Reduced bioavailability of NO has been associated with cerebral, extracranial, and peripheral vascular dysfunction in AD [38], suggesting that vascular dysfunction associated with AD may also involve deficits in peripheral circulation. Our previous studies showed that mTOR inhibits eNOS activation and thus decreases NO bioavailability in brain microvasculature and restores brain vascular function in aging and in AD [11, 12, 17]. Consistent with this notion, it was recently reported that exercise can improve peripheral vascular function in AD patients [39]. Taken together, these studies suggest that, similar to our observations in cerebrovasculature, mTOR attenuation alleviates mTOR-mediated inhibition of eNOS activity, restoring NO bioavailability and peripheral blood flow.
mTOR is a signaling hub for various cellular processes that could be involved in the regulation of peripheral circulation, including increased autophagy and improved proteostasis [40, 41]. Indeed, reduction of mTORC1 by either rapamycin treatment, caloric restriction, or intermittent fasting increases autophagy [41, 42]. In addition to rapamycin, caloric restriction, dietary restriction, and intermittent fasting (time-restricted feeding) are also known to reduce mTOR activity either chronically or transiently [43, 44] and have been shown to improve vascular health in humans, non-human primates, and rodent models [43, 45]. Moreover, attenuation of mTOR signaling by rapamycin increases endothelial nitric oxide synthase (eNOS) activity [11], and an increase in nitric oxide availability has been observed with caloric restriction [45] and in association with intermittent fasting [46]. Increased nitric oxide bioavailability in the peripheral vasculature as a result of mTOR attenuation with rapamycin could thus underlie the observed improvement in baseline peripheral blood flow in aged mice and in mouse models of atherosclerosis and AD. Thus, rapamycin may improve all NO bioavailability, thus negating one of the central impairments in endothelial cell dysfunction [47], leading to restored vascular function in both brain and the periphery. Caloric and dietary restriction, intermittent fasting, and treatment with rapamycin could thus be used as potential interventions to improve peripheral blood flow and prevent peripheral artery disease [48].
In conclusion, our studies suggest that, in addition to central vascular impairment in aging and age-associated neurological diseases such as AD and vascular dementia [11, 12, 17, 18, 29], peripheral vascular decline is mediated by mTOR. Interventions that reduce mTOR activity in the vasculature thus have significant promise to prevent or treat PAD. Furthermore, the primary way that humans regulate body temperature is via skin blood flow which is significantly reduced in aged individuals [20, 21]. Thus, in addition to the potential translational impact of pharmacological mTOR attenuation on the incidence of peripheral artery disease (PAD), our studies may also have implications for the prevention of hyperthermia in the elderly.
Funding
US Department of Veterans Affairs Biomedical Laboratory Research and Development Service (VA-BLRDS) Merit Award 2I0 1BX002211-06A1 (VG), IK2 BX003798-01A1 (SAH), NIH/NIA R01AG057964-01 (VG), 1RF1 AG068283-01 (VG), the Robert L. Bailey and daughter Lisa K. Bailey Alzheimer’s Fund in memory of Jo Nell Bailey (VG), William & Ella Owens Medical Research Foundation Grant (VG), the San Antonio Medical Foundation (VG), the JMR Barker Foundation (VG), NCATS/NIH UL1 TR002645 Pilot Award (VG), Alzheimer’s Association AARF-17-504221 (CEV), NIH Biology of Aging Training Grant T32 AG-021890 (CEV, SAH).
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Krishna SM, Moxon JV, Golledge J. A review of the pathophysiology and potential biomarkers for peripheral artery disease. Int. J. Mol. Sci. 2015;16(5):11294–11322. doi: 10.3390/ijms160511294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meijer WT, Grobbee DE, Hunink MG, Hofman A, Hoes AW. Determinants of peripheral arterial disease in the elderly: the Rotterdam study. Arch. Intern. Med. 2000;160(19):2934–2938. doi: 10.1001/archinte.160.19.2934. [DOI] [PubMed] [Google Scholar]
- 3.Norman PE, Eikelboom JW, Hankey GJ. Peripheral arterial disease: prognostic significance and prevention of atherothrombotic complications. Med. J. Aust. 2004;181(3):150–154. doi: 10.5694/j.1326-5377.2004.tb06206.x. [DOI] [PubMed] [Google Scholar]
- 4.Bergiers S, Vaes B, Degryse J. To screen or not to screen for peripheral arterial disease in subjects aged 80 and over in primary health care: a cross-sectional analysis from the BELFRAIL study. BMC Fam. Pract. 2011;12:39. doi: 10.1186/1471-2296-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tasci I, Safer U, Naharci MI, Gezer M, Demir O, Bozoglu E, et al. Undetected peripheral arterial disease among older adults with Alzheimer’s disease and other dementias. Am. J. Alzheimers Dis. Other Dement. 2018;33(1):5–11. doi: 10.1177/1533317517724000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nat. 2009;460(7253):392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strong R, Miller RA, Bogue M, Fernandez E, Javors MA, Libert S, et al. Rapamycin-mediated mouse lifespan extension: late-life dosage regimes with sex-specific effects. Aging Cell. 2020;19(11):e13269. doi: 10.1111/acel.13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66(2):191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller RA, Harrison DE, Astle CM, Fernandez E, Flurkey K, Han M, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13(3):468–477. doi: 10.1111/acel.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11(4):675–682. doi: 10.1111/j.1474-9726.2012.00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin AL, Zheng W, Halloran JJ, Burbank RR, Hussong SA, Hart MJ, et al. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2013;33(9):1412–1421. doi: 10.1038/jcbfm.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin AL, Jahrling JB, Zhang W, DeRosa N, Bakshi V, Romero P, et al. Rapamycin rescues vascular, metabolic and learning deficits in apolipoprotein E4 transgenic mice with pre-symptomatic Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2017;37(1):217–226. doi: 10.1177/0271678X15621575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai DF, Karunadharma PP, Chiao YA, Basisty N, Crispin D, Hsieh EJ, et al. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell. 2014;13(3):529–539. doi: 10.1111/acel.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Bokov A, Gelfond J, Soto V, Ikeno Y, Hubbard G, et al. Rapamycin extends life and health in C57BL/6 mice. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69(2):119–130. doi: 10.1093/gerona/glt056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flynn JM, O'Leary MN, Zambataro CA, Academia EC, Presley MP, Garrett BJ, et al. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell. 2013;12(5):851–862. doi: 10.1111/acel.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Skike CE, Lin AL, Roberts Burbank R, Halloran JJ, Hernandez SF, Cuvillier J, et al. mTOR drives cerebrovascular, synaptic, and cognitive dysfunction in normative aging. Aging Cell. 2020;19(1):e13057. doi: 10.1111/acel.13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Skike CE, Hussong SA, Hernandez SF, Banh AQ, DeRosa N, Galvan V. mTOR attenuation with rapamycin reverses neurovascular uncoupling and memory deficits in mice modeling Alzheimer’s disease. J. Neurosci. 2021;41(19):4305–4320. doi: 10.1523/JNEUROSCI.2144-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jahrling JB, Lin AL, DeRosa N, Hussong SA, Van Skike CE, Girotti M, et al. mTOR drives cerebral blood flow and memory deficits in LDLR(-/-) mice modeling atherosclerosis and vascular cognitive impairment. J Cereb Blood Flow Metab. 2018;38(1):58–74. doi: 10.1177/0271678X17705973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cremers CM, Knoefler D, Gates S, Martin N, Dahl JU, Lempart J, et al. Polyphosphate: a conserved modifier of amyloidogenic processes. Mol. Cell. 2016;63(5):768–780. doi: 10.1016/j.molcel.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J. Am. Coll. Cardiol. 1994;24(2):471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 21.Algotsson A, Nordberg A, Almkvist O, Winblad B. Skin vessel reactivity is impaired in Alzheimer’s disease. Neurobiol. Aging. 1995;16(4):577–582. doi: 10.1016/0197-4580(95)00077-r. [DOI] [PubMed] [Google Scholar]
- 22.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 23.Petrofsky JS, Laymon M, Berk L, Bains G. Effect of ThermaCare HeatWraps and Icy Hot cream/patches on skin and quadriceps muscle temperature and blood flow. J. Chiropr. Med. 2016;15(1):9–18. doi: 10.1016/j.jcm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craighead DH, McCartney NB, Tumlinson JH, Alexander LM. Mechanisms and time course of menthol-induced cutaneous vasodilation. Microvasc. Res. 2017;110:43–47. doi: 10.1016/j.mvr.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson CD, Melanaphy D, Purse A, Stokesberry SA, Dickson P, Zholos AV. Transient receptor potential melastatin 8 channel involvement in the regulation of vascular tone. Am. J. Physiol. Heart Circ. Physiol. 2009;296(6):H1868–H1877. doi: 10.1152/ajpheart.01112.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohta T, Imagawa T, Ito S. Involvement of transient receptor potential vanilloid subtype 1 in analgesic action of methylsalicylate. Mol. Pharmacol. 2009;75(2):307–317. doi: 10.1124/mol.108.051292. [DOI] [PubMed] [Google Scholar]
- 27.Campia U, Gerhard-Herman M, Piazza G, Goldhaber SZ. Peripheral artery disease: past, present, and future. Am. J. Med. 2019;132(10):1133–1141. doi: 10.1016/j.amjmed.2019.04.043. [DOI] [PubMed] [Google Scholar]
- 28.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Invest. 1993;92(2):883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Skike CE, Jahrling JB, Olson AB, Sayre NL, Hussong SA, Ungvari Z, et al. Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer’s disease and vascular cognitive impairment. Am. J. Physiol. Heart Circ. Physiol. 2018;314(4):H693–H703. doi: 10.1152/ajpheart.00570.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, et al. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc. Natl. Acad. Sci. U. S. A. 1999;96(6):3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, et al. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 2000;20(11):4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiao YA, Kolwicz SC, Basisty N, Gagnidze A, Zhang J, Gu H, et al. Rapamycin transiently induces mitochondrial remodeling to reprogram energy metabolism in old hearts. Aging (Albany NY) 2016;8(2):314–327. doi: 10.18632/aging.100881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quarles E, Basisty N, Chiao YA, Merrihew G, Gu H, Sweetwyne MT, et al. Rapamycin persistently improves cardiac function in aged, male and female mice, even following cessation of treatment. Aging Cell. 2020;19(2):e13086. doi: 10.1111/acel.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skrzypczyk P, Wabik AM, Szyszka M, Jozwiak S, Bombinski P, Jakimow-Kostrzewa A, et al. Early vascular aging in children with tuberous sclerosis complex. Front. Pediatr. 2021;9:767394. doi: 10.3389/fped.2021.767394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Csipo T, Lipecz A, Fulop GA, Hand RA, Ngo BN, Dzialendzik M, et al. Age-related decline in peripheral vascular health predicts cognitive impairment. Geroscience. 2019;41(2):125–136. doi: 10.1007/s11357-019-00063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Halloran J, Hussong SA, Burbank R, Podlutskaya N, Fischer KE, Sloane LB, et al. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience. 2012;223:102–113. doi: 10.1016/j.neuroscience.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalman J, Szakacs R, Torok T, Rozsa Z, Barzo P, Rudas L, et al. Decreased cutaneous vasodilatation to isometric handgrip exercise in Alzheimer’s disease. Int. J. Geriatr. Psychiatry. 2002;17(4):371–374. doi: 10.1002/gps.609. [DOI] [PubMed] [Google Scholar]
- 38.Venturelli M, Pedrinolla A, Boscolo Galazzo I, Fonte C, Smania N, Tamburin S, et al. Impact of nitric oxide bioavailability on the progressive cerebral and peripheral circulatory impairments during aging and Alzheimer’s disease. Front. Physiol. 2018;9:169. doi: 10.3389/fphys.2018.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pedrinolla A, Venturelli M, Fonte C, Tamburin S, Di Baldassarre A, Naro F, et al. Exercise training improves vascular function in patients with Alzheimer’s disease. Eur. J. Appl. Physiol. 2020;120(10):2233–2245. doi: 10.1007/s00421-020-04447-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su KH, Dai C. mTORC1 senses stresses: coupling stress to proteostasis. Bioessays. 2017;39(5) 10.1002/bies.201600268. [DOI] [PMC free article] [PubMed]
- 41.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;169(2):361–371. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 42.Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019;11(6) 10.3390/nu11061234. [DOI] [PMC free article] [PubMed]
- 43.Green CL, Lamming DW, Fontana L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell. Biol. 2022;23(1):56–73. doi: 10.1038/s41580-021-00411-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gnoni M, Beas R, Vasquez-Garagatti R. Is there any role of intermittent fasting in the prevention and improving clinical outcomes of COVID-19?: intersection between inflammation, mTOR pathway, autophagy and calorie restriction. Virusdisease. 2021;32(4):625–634. doi: 10.1007/s13337-021-00703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Balasubramanian P, Del Favero J, Ungvari A, Papp M, Tarantini A, Price N, et al. Time-restricted feeding (TRF) for prevention of age-related vascular cognitive impairment and dementia. Ageing Res. Rev. 2020;64:101189. doi: 10.1016/j.arr.2020.101189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui J, Lee S, Sun Y, Zhang C, Hill MA, Li Y, et al. Alternate day fasting improves endothelial function in type 2 diabetic mice: role of adipose-derived hormones. Front. Cardiovasc. Med. 2022;9:925080. doi: 10.3389/fcvm.2022.925080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cyr AR, Huckaby LV, Shiva SS, Zuckerbraun BS. Nitric oxide and endothelial dysfunction. Crit. Care Clin. 2020;36(2):307–321. doi: 10.1016/j.ccc.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cecchini AL, Biscetti F, Rando MM, Nardella E, Pecorini G, Eraso LH, et al. Dietary risk factors and eating behaviors in peripheral arterial disease (PAD). Int. J. Mol. Sci. 2022;23(18) 10.3390/ijms231810814. [DOI] [PMC free article] [PubMed]