Abstract
Whole-brain irradiation (WBI, also known as whole-brain radiation therapy) is a mainstay treatment modality for patients with multiple brain metastases. It is also used as a prophylactic treatment for microscopic tumors that cannot be detected by magnetic resonance imaging. WBI induces a progressive cognitive decline in ~ 50% of the patients surviving over 6 months, significantly compromising the quality of life. There is increasing preclinical evidence that radiation-induced injury to the cerebral microvasculature and accelerated neurovascular senescence plays a central role in this side effect of WBI. To better understand this side effect, male C57BL/6 mice were first subjected to a clinically relevant protocol of fractionated WBI (5 Gy, two doses per week, for 4 weeks). Nine months post the WBI treatment, we applied two-photon microscopy and Doppler optical coherence tomography to measure capillary red-blood-cell (RBC) flux, capillary morphology, and microvascular oxygen partial pressure (PO2) in the cerebral somatosensory cortex in the awake, head-restrained, WPI-treated mice and their age-matched controls, through a cover-glass-sealed chronic cranial window. Thanks to the extended penetration depth with the fluorophore — Alexa680, measurements of capillary blood flow properties (e.g., RBC flux, speed, and linear density) in the cerebral subcortical white matter were enabled. We found that the WBI-treated mice exhibited a significantly decreased capillary RBC flux in the white matter. WBI also caused a significant reduction in capillary diameter, as well as a large (although insignificant) reduction in segment density at the deeper cortical layers (e.g., 600–700 μm), while the other morphological properties (e.g., segment length and tortuosity) were not obviously affected. In addition, we found that PO2 measured in the arterioles and venules, as well as the calculated oxygen saturation and oxygen extraction fraction, were not obviously affected by WBI. Lastly, WBI was associated with a significant increase in the erythrocyte-associated transients of PO2, while the changes of other cerebral capillary PO2 properties (e.g., capillary mean-PO2, RBC-PO2, and InterRBC-PO2) were not significant. Collectively, our findings support the notion that WBI results in persistent cerebral white matter microvascular impairment, which likely contributes to the WBI-induced brain injury and cognitive decline. Further studies are warranted to assess the WBI-induced changes in brain tissue oxygenation and malfunction of the white matter microvasculature as well.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-023-00735-3.
Keywords: Whole-brain radiation, Vascular cognitive impairment, White matter, Optical microscopy, Microvascular blood flow
Introduction
Approximately 10–30% of the adult patients with systemic malignancies (e.g., lung cancer, breast cancer, and malignant melanoma) and 6–10% of the children with systemic primary tumors (e.g., osteogenic sarcoma, rhabdomyosarcoma, and testicular germ cell tumor) develop brain metastases [1, 2]. This represents a significant challenge, as the efficiency of systemic chemotherapy is very limited for brain metastases. Despite the development of new therapeutic interventions such as stereotactic radiosurgery and fractionated stereotactic radiosurgery, whole-brain irradiation (WBI, a.k.a., whole-brain radiation therapy or WBRT) remains a mainstay treatment modality for patients with multiple brain metastases and poor prognosis and is also used as a prophylaxis for microscopic tumors that cannot be detected by magnetic resonance imaging.[3–6] Over 200,000 tumor patients are treated with either WBI or partial large field radiation every year in the USA [7]. Radiotherapy improves overall survival, preserves functional independence, and results in less development of new brain relapses [6, 8]. Although, clinically, WBI exerts significant anti-tumor effects, it also leads to serious unwanted side effects by promoting radiation-induced damage to the healthy brain tissue [9–12]. Because cancer survival rate continues to improve due to technological advancements (including earlier detection of tumors and advances in cancer treatment protocols), understanding the side effects of WBI that affect the quality of life has become a priority. While the early and delayed WBI-induced side effects were transient, 50–90% of the patients who survived for over 6 months after the WBI treatment experienced progressive deterioration of cognitive functions (including impairments of memory, attention, and executive function), which affected the financial, professional, and social activities and severely compromised the quality of life and autonomy [7, 13–15]. Preclinical studies confirmed that similar WBI regimens would also promote progressive impairment of cognitive functions in rodent models [11, 12, 16–21] and non-human primates[22, 23], mimicking several aspects of cognitive decline manifested in the WBI-treated patients. These mechanistic studies suggest that the primary mechanism underlying the WBI-induced cognitive decline is likely independent of the effects of cancer per se on the brain and is not primarily dependent on the radiation-induced neurodegeneration [15, 24]. Instead, the WBI-induced cognitive impairment, at least in part, reflected the damages to the neurovascular unit (NVU) as well as to the cerebral white matter [16, 24–26].
Normal cognitive function is critically dependent on the adequate supply of oxygen and nutrients to neurons through cerebral blood flow (CBF). The NVU, consisting of vascular endothelial cells, astrocytes, pericytes, and smooth muscle cells that interact with neurons, has multifaceted functions in regulating CBF and oxygen delivery to the brain parenchyma [27]. The cells of NVU are in control of angiogenesis and are responsible for the dynamic remodeling of the cerebral microvasculature. In addition, the NVU is also responsible for neurovascular coupling (a.k.a., functional hyperemia), which adjusts the local blood flow to meet the increased metabolic needs of the firing neurons in a moment-to-moment manner. Importantly, it has been shown that WBI could lead to pathologic cerebral vascular remodeling that might result in vascular rarefaction[24, 28–31] and impairment of neurovascular coupling[16, 21]. These neurovascular alterations have been causally linked to the genesis of WBI-induced cognitive decline [16, 31]. Besides, it was reported in human studies that WBI would induce white matter abnormalities, which were strongly correlated with neurocognitive dysfunction [32–36]. The WBI-associated major pathological changes involved damages to the vascular endothelial cells and demyelination in the white matter [37]. In addition, a study in non-human primates showed that the animals receiving WBI treatments were susceptible to the neurological injuries, including the deteriorated glutamatergic neurotransmission and signal transduction within the white matter, which might persist for years [38]. Another study in a rodent model reported that WBI caused damages to oligodendrocytes and oligodendrocyte precursors, eventually leading to white matter demyelination [39]. Despite these advances, no data on the effects of WBI on the cerebral microcirculation and oxygenation are available.
In the present study, we investigated the WBI-induced effects on the cerebral microvascular blood flow, oxygenation, and microvascular morphological changes in a clinically relevant irradiation mouse model. We employed a home-built two-photon laser scanning microscope to assess the effects of WBI on the cerebral capillary red-blood-cell (RBC) flow parameters, e.g., RBC flux, speed, and linear density. The deep imaging penetration with a near-infrared fluorophore – Alexa680 enabled the capillary RBC flow measurements in the cerebral cortical gray matter and subcortical white matter [40]. Along with these functional measurements, three-dimensional two-photon microscopic angiograms were also acquired to assess the microvascular morphological changes. We further applied Doppler optical coherence tomography (Doppler-OCT) to corroborate our findings on the effect of WBI on CBF [41]. Besides, we applied two-photon phosphorescence lifetime microscopy (2PLM) with a phosphorescent oxygen probe — Oxyphor2P to measure the cerebral cortical intravascular oxygen partial pressure (PO2)[42, 43], allowing us to investigate the changes of oxygen delivery in the microvascular networks at the sub-capillary level. These measurements were performed in the control and WBI-treated awake mice at rest, via a chronic cranial window. With these multifaceted measurements, we found that WBI resulted in a significant decrease in RBC flux in the subcortical white matter capillaries, a significant decrease in microvascular diameter, and a trend for reduction in the microvascular segment density at the deep cortical layers (e.g., 600–700 μm). We also found in the WBI mice a significant increase in the erythrocyte-associated transients of PO2 (EATs). Other parameters, such as RBC flux in the cortical gray matter capillaries, RBC speed, linear density, vascular oxygenation, and oxygen extraction fraction (OEF), were not obviously affected. Overall, our data support the notion that WBI induces persistent cerebral white matter microvascular impairment, which likely contributes to the WBI-induced brain injury and cognitive decline.
Materials and methods
Experimental animals and the fractionated WBI protocol
The effect of WBI on neurovascular senescence in the p16-3MR transgenic mouse model has recently been characterized [16, 44]. The p16-3MR mouse carries a trimodal fusion protein (3MR) under the control of the p16Ink4a promoter that enables the identification and elimination of the senescent cells [44]. With this model, we have demonstrated that WBI would significantly increase the prevalence of senescent astrocytes and microvascular endothelial cells in the NVU [44]. In the present study, we used the same mouse model and the same WBI protocol to assess the effects of WBI on the cerebral microvascular blood flow, oxygenation, and morphological properties.
Briefly, 3-month-old male, p16-3MR mice were housed in the specific pathogen-free animal facility at the University of Oklahoma Health Sciences Center (OUHSC) and fed standard rodent chow and water ad libitum, following the standard husbandry techniques. One week before irradiation, mice were transferred to the conventional animal facility at OUHSC and housed under similar conditions. Following our published protocol [16], after acclimating to the conventional facility for 1 week, the mice were randomly assigned to either irradiated or sham-irradiated control groups. Animals were first anesthetized via intraperitoneal injection of ketamine/xylazine (100/15 mg/kg). The mice in the irradiated group were subjected to a clinically relevant WBI (5 Gy, twice per week, for a total cumulative dose of 40 Gy). Radiation was administered using a 137Cesium gamma irradiator (GammaCell 40, Nordion International). A Cerrobend® shield was utilized to minimize the exposure to radiation outside the brain. The dose of radiation received by the mice was verified with film dosimetry, as previously described [20, 21, 31]. The mice were then left to recover before being shipped to Massachusetts General Hospital for imaging. All experimental protocols were approved by the participating institutions’ Institutional Animal Care and Use Committees.
Animal preparation for imaging
The control and WBI-treated mice (12–15 months old) were shipped to Massachusetts General Hospital. To prepare for imaging, a custom-made head-post was glued on the skull over the right hemisphere to enable head immobilization. A cranial window (round shape, 3 mm in diameter) was implanted over the somatosensory cortex in the left hemisphere (AP: − 2.0 mm, ML: − 3.0 mm relative to bregma), following our previously established protocols [43, 45]. The dura was kept intact. The cranial window was sealed with dental acrylic [46]. After surgery, each mouse was given 5 days to recover before starting the habituation training. The training was conducted under the microscope while the mouse was resting on a suspended soft fabric bed on a home-built platform. Each mouse was gradually habituated to the progressively more extended periods (e.g., from 10 min to 2 h) of head restraint. Mice were rewarded with sweetened milk every 15 min during the training and experiments. While head-restrained, the animals were free to readjust their body position and, from time to time, displayed natural grooming behavior. The imaging experiments were conducted on these mice at the age of 15–18 months.
Two-photon microscope
We employed a home-built two-photon microscope in this work [40, 43, 47, 48]. Briefly, a tunable ultrafast laser (InSight DeepSee, Spectra-Physics; tuning range: 680 to 1300 nm, ~ 120-fs pulse width, 80-MHz repetition rate) was used for two-photon excitation. Laser power was controlled by an electro-optic modulator (EOM; 350–105-02-BK EO, ConOptics). The laser beam was focused with a water-immersion objective lens (XLUMPLFLN20XW, Olympus) and scanned in the X–Y plane by a pair of galvanometer scanners (6210H, Cambridge Technology). The objective was moved along the Z-axis by a motorized stage (M-112.1DG, Physik Instrumente) for probing different cortical depths. The emitted fluorescence or phosphorescence was filtered by the band-pass filters and subsequently detected by a photon-counting photomultiplier tube (PMT; H10770PA-50, Hamamatsu). The output from the PMT was discriminated by a discriminator (C9744, Hamamatsu) and then digitized by a 50-MHz acquisition card (NI PCle-6537, National Instruments).
Measurements of capillary RBC flow parameters
The measurements were enabled by two-photon microscopic imaging of Alexa680-labeled blood plasma [40]. Here, Alexa680 molecules (2P-λexc = 1280 nm, λem = 700 nm) were conjugated with 70 KDa dextran. Before imaging, the dextran-Alexa680 solution (0.1 mL at 5% W/V in PBS) was injected retro-orbitally into the bloodstream under brief anesthesia (1.5–2% isoflurane, during ~ 2 min). Next, the animal was recovered from anesthesia and then head-fixed under the microscope. The imaging session was started ~ 30 min after injection.
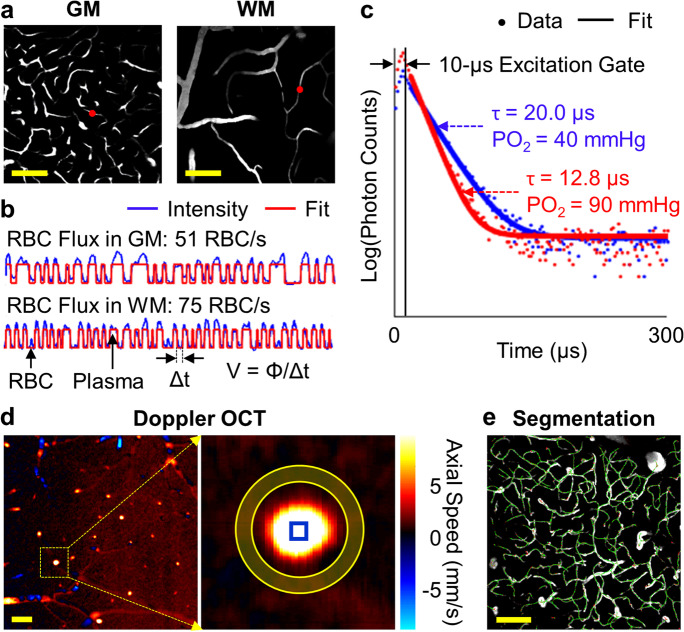
At each depth in the mouse brain, we first acquired a fluorescence survey image revealing the microvasculature over a 0.5 × 0.5 mm2 field of view (FOV) by raster-scanning the focused excitation beam (Fig. 1a). Then, we manually selected the measurement locations within the capillary segments that could be visually identified in the survey image. Here, capillaries were identified empirically based on vascular caliber. While imaging, the focused laser beam was parked at each measurement location for 0.5 s. The emitted fluorescence was detected by a photon counter and then binned with 250-μs-wide bins for post-processing. The amplitude of fluorescence intensity integrated over a 250-μs-wide bin could be adjusted by laser power and the duration of the laser “ON” phase in the corresponding 250-μs-long excitation, and both parameters were controlled by EOM and kept constant for imaging at the same depth. As the Alexa680 molecules label blood plasma but not RBCs, the changes in fluorescence intensity encoded the passing of RBCs (i.e., “valleys” in the experimental time course) and blood plasma flowing through the optical focus (i.e., “peaks” in the experimental time course; Fig. 1b). The fluorescence intensity time courses (each time course consisted of 2000 points with 250-μs temporal resolution) were segmented with a binary thresholding approach [40]. The segmentation was evaluated by the coefficient of determination (R2) between the experimental and fitted time courses, and the data with R2 < 0.5 were excluded from the analysis. This criterion helps select the measurements with a single-file RBC-flowing pattern, which are mostly capillaries. Thus, RBC flux was explicitly calculated by counting the number of detected RBCs (i.e., “valleys” in the segmented curve; Fig. 1b) normalized by the acquisition time (i.e., 0.5 s in this work). In addition, RBC speed was estimated as v = ø/Δt, where Δt was the “valley” width in time (i.e., the time duration for an RBC passing through the focal zone; Fig. 1b), and ø is the RBC diameter, assumed to be constant as 6 μm in this work [49]. RBC linear density was estimated as the percentage of the number of points (or pixels) identified as RBCs to the total number of points in the fluorescence intensity time course. RBC linear density can also be calculated as the ratio of RBC flux to RBC speed. The relative changes of capillary RBC flow parameters (e.g., RBC flux, speed, or linear density) were calculated as the ratio of the difference between the mean values of the control and WBI groups to the mean value of the control group.
Fig. 1.
Imaging methods. (a) Representative two-photon angiographic images acquired in the cerebral cortical gray matter (GM; Z = 0.5 mm) and white matter (WM; Z = 1.0 mm). (b) Two 0.5-s-long fluorescence intensity time courses acquired at the locations denoted by the red dots in the panel (a). (c) Two examples of phosphorescence decays (venule in blue and arteriole in red) for PO2 recording. A 300-μs-long cycle includes a 10-μs-long EOM-gated excitation, followed by a 290-μs-long detection of phosphorescence. (d) A Doppler-OCT image acquired at the cortical surface (left), and the zoomed-in view of a surfacing venule (right) enclosed by the inner circle of the yellow ring. The offset axial flow speed was calculated by averaging over the pixels in the shaded area between the yellow circles. The maximum axial flow speed was calculated by averaging over the pixels enclosed by the blue square in the zoomed-in image. (e) Segmentation of an angiogram (maximum intensity projection of the angiogram across the depth range of 100-200 µm under the brain surface). The segmented centerlines are in green. Scale bars: 100 μm
The measurements in the gray matter were performed typically at the cortical depths from 0.2 to 0.6 mm with 0.2-mm depth intervals. The measurements in the subcortical white matter were performed at the depth range defined by an OCT-based method (Fig. 8), with 0.02-mm depth intervals. The order of measurements in the gray matter and white matter was alternated between animals to reduce the influence of animal physiology, which might vary along with the experiment, on comparing the measurements performed in the gray matter and white matter. During the experiment, the water-immersion objective lens (XLUMPLFLN20XW, Olympus) was heated by an electric heater (TC-HLS-05, Bioscience Tools) to maintain the water temperature between the cranial window and the objective lens at 36–37 °C. The duration of the imaging session in each mouse was ~ 2 h.
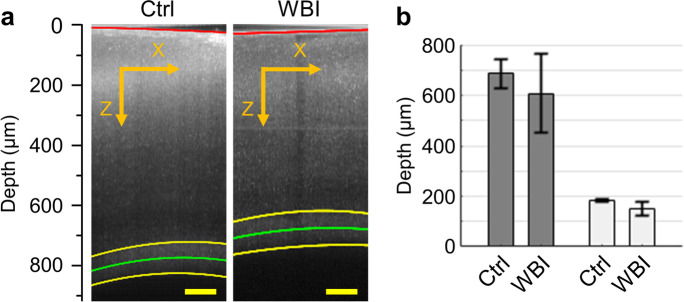
Fig. 8.
Cerebral gray matter and white matter thickness. (a) Two representative OCT B-scan images acquired in a control and a WBI-treated mouse. The red curve in the image delineates the cortical surface, and the two yellow curves delineate the upper and lower boundaries of subcortical white matter, with the green curve representing the midline of the white matter. Scale bars: 100 µm. (b) Comparison of the cerebral gray matter and white matter thickness with the OCT measurements performed in n = 3 control (Ctrl) and n = 3 WBI-treated (WBI) mice. The data were first averaged over the images in each mouse and then over mice. Data are expressed as mean ± STD. No significant difference was observed in this analysis (Student’s t-test)
Measurements of cerebral blood flow
A spectral-domain optical coherence tomography (SD-OCT) system was employed to measure blood flow in the surfacing venules within the top 0.1-mm depth under the cortical surface. The SD-OCT system was equipped with a light source with a central wavelength of 1310 nm and a bandwidth of 170 nm (LS2000B, Thorlabs). The resolution, pixel size, and imaging range along the axial direction (Z) were estimated as 3.5 µm, 2.8 µm, and 1.6 mm in biological tissue, respectively. The transverse resolution with a 10 × objective lens (Plan Apo NIR, Mitutoyo) was estimated as 3.5 µm. More details about the OCT system can be found elsewhere [50, 51].
Volumetric Doppler-OCT imaging was performed in a 1 × 1 mm2 lateral FOV that enclosed the region of interest (ROI) for two-photon imaging. The scan steps along the fast (X) and slow (Y) scanning directions were 0.26 μm and 1.95 μm, respectively. The axial flow speed (Vz; Fig. 1d) was computed with a Kasai autocorrelation algorithm [52]. However, imperfect telecentricity of the optics might induce phase shift, resulting in an “offset” flow speed even in static tissue [52]. To eliminate this effect, we first estimated this “offset” Vz value with the nearby pixels for each identified vessel (e.g., averaging over the pixels in the yellow shaded area between the two yellow circles in the zoomed-in Doppler-OCT image in Fig. 1d) and then subtracted this offset value from the original Vz image. Blood flow (in µL/min) was thus calculated by integrating the corrected Vz values over a manually drawn geometrical shape (e.g., the inner yellow circle in the zoomed-in Doppler-OCT image in Fig. 1d) that fully enclosed the cross-section of the vessel. The maximum Vz was calculated by averaging the Vz values in the central part of the selected vessel (e.g., the pixels enclosed by the blue square in the zoomed-in Doppler image in Fig. 1d). In this work, only venules were selected for analysis, as the measurements in arterioles might be affected by the aliasing effect caused by high flow speed [41]. Forty volumes were repeated for the ROI in each mouse. The duration of the OCT experiment in each mouse was ~ 1.5 h.
Measurements of intravascular PO2
We employed 2PLIM with a phosphorescent oxygen probe — Oxyphor2P (2P-λexc = 950 nm, λem = 757 nm) for PO2 imaging [42]. Oxyphor2P exhibits red-shifted excitation and emission, higher quantum yield, much larger two-photon absorption cross-section, and better-defined single-exponential kinetics comparing with its predecessor — PtP-C343 [53]. Before imaging, the Oxyphor2P solution (0.1 mL at ~ 34 μM in PBS) was injected retro-orbitally into the bloodstream under brief anesthesia (1.5–2% isoflurane, during ~ 2 min) [43]. Next, the animal was recovered from anesthesia and then head-fixed under the microscope. The imaging session was started ~ 30 min after injection.
At each depth in the mouse brain, we first acquired a phosphorescence survey image revealing the microvasculature over a 0.5 × 0.5 mm2 FOV by raster-scanning the focused excitation beam. Next, similar to the capillary RBC flow measurements in Fig. 1a, we manually selected the measurement locations within the vascular segments (e.g., arterioles, venules, and capillaries), guided by the survey image. At each selected location, Oxyphor2P molecules in the focal volume were excited with a 10-μs-long laser excitation at 950 nm gated by the EOM, followed by a 290-μs-long detection of the emitted phosphorescence. Such 300-μs-long excitation/detection cycle was typically repeated 2000 times (0.6 s) to obtain an average phosphorescence decay with sufficient signal-to-noise ratio (SNR). We rejected the initial 5-μs of data from the 290-μs-long decay to match the Oxyphor2P calibration procedure. The phosphorescence lifetime (τ) was calculated by fitting the remaining 285-μs-long decay to a single-exponential function, using a standard non-linear least square minimization algorithm (Fig. 1c) [47, 53]. The lifetime was then converted to absolute PO2 using a Stern–Volmer type calibration. The PO2 measurements were performed typically at the depths from the cortical surface down to 0.3 mm under the cortical depth, with 0.05-mm depth intervals. During the experiment, the water-immersion objective lens (XLUMPLFLN20XW, Olympus) was heated by an electric heater (TC-HLS-05, Bioscience Tools) to maintain the water temperature between the cranial window and the objective lens at 36–37 °C. The duration of PO2 measurements in each mouse was ~ 2 h.
Calculation of SO2 and OEF
Oxygen saturation of hemoglobin (SO2) was computed using the Hill equation with the parameters of h = 2.59 and P50 = 40.2 mmHg [54], where h is the Hill coefficient, and P50 is the PO2 for which hemoglobin is half-saturated. OEF was calculated as (SO2,A–SO2,V)/SO2,A, where SO2,A and SO2,V represent the SO2 in the arteriole and venule, respectively. In this work, OEF was calculated with the PO2 measurements performed within the top 100-μm depth under the cortical surface.
Calculation of capillary Mean-PO2, RBC-PO2, InterRBC-PO2, and EATs
The detailed procedures could be found in the previous works [43, 55, 56]. Capillaries were identified empirically based on vascular caliber. Similarly, the phosphorescence intensity time courses (each time course consisted of 2000 points with 300-μs temporal resolution) were segmented with a binary thresholding approach. The data with R2 ≥ 0.5 were included into the analysis. This criterion helps select the measurements with a single-file RBC-flowing pattern, which are mostly capillaries. In the present work, we applied a simplified approach for calculating the capillary PO2 properties. The repeated 2000 phosphorescence decay curves obtained in each capillary were separated into two groups: the RBC group and the plasma group. The RBC group consisted of the decays acquired when the RBCs were passing through the optical focus, corresponding to the “valleys” in the segmented curve; and the plasma group consisted of the decays acquired when plasma was passing through the optical focus, corresponding to the “peaks” in the segmented curve (Fig. 1b). For each capillary, the PO2 calculated with the decay averaging over the 2000 repetitions is termed Mean-PO2; the PO2 calculated with the decay averaging over the RBC group is termed RBC-PO2; the PO2 calculated with the decay averaging over the plasma group is termed InterRBC-PO2. EATs were calculated as RBC-PO2 − InterRBC-PO2.
Acquisition of microvascular angiograms and morphological analysis
The cerebral microvascular angiograms were acquired by two-photon microscopic imaging of the Alexa680-labeled blood plasma. Before imaging, the dextran-Alexa680 solution (0.1 mL at 5% W/V in PBS) was injected retro-orbitally into the bloodstream under brief anesthesia (1.5–2% isoflurane, during ~ 2 min). Next, the animal was recovered from anesthesia and then head-fixed under the microscope. The imaging session was started ~ 30 min after injection. The angiographic image stacks were acquired within a 0.7 × 0.7 mm2 FOV (512 × 512 pixels, 1.37-μm pixel size), centered over the same ROI as for PO2 imaging. The imaging was typically performed from the cortical surface down to ~ 0.9 mm under the cortical surface with 2-μm intervals. The average imaging penetration depth was ~ 896 ± 28 µm in the control mice and ~ 761 ± 35 µm in the WBI mice. The duration of the two-photon angiographic imaging in each mouse was ~ 1 h.
For the morphological analysis, the two-photon angiograms were first segmented into binary images (e.g., the values of one represent vessels, and the values of zero represent extravascular tissue; Fig. 1e) using a deep-learning-based algorithm [57]. Next, anatomical models (i.e., graph-based representations of the vascular networks consisting of nodes and edges) were generated with a Laplacian optimization framework [58]. Thus, the microvascular morphological properties (e.g., diameter, vascular segment length, segment density, and tortuosity) were extracted. Specifically, vascular diameter (in µm) was defined as the FWHM of the profile along the radial direction of a vessel; segment length (in µm) was defined as the physical length of a vascular segment between the two connecting bifurcations; segment density was defined as the number of vascular segments in a unit volume of tissue (e.g., number of segments per mm3); tortuosity was defined as the ratio of the segment length (typically curved) to the length of the straight line connecting the end-points of that segment. In this analysis, we focused only on capillaries and the smallest arterioles and venules, so the vessels with a diameter of > 10 µm were rejected. The images acquired at the depths of < 100 μm or > 700 μm were excluded, as the former included many large vessels, and the latter might be too noisy, affecting the analysis.
Estimation of the cerebral gray matter and white matter thickness
We used the SD-OCT system to estimate the cerebral gray matter and white matter thickness. Due to the strong backscattered light originating from the highly scattering myelinated axons [40], intensity of an OCT image appears typically higher in the white matter than in the gray matter. In each mouse, volumetric OCT imaging was performed in a lateral FOV of 1 × 1 mm2 (pixel size 1.95 μm), overlaying the same ROI as for the RBC flux measurements. During imaging, the optical focus was positioned in the deeper part of the cortex to facilitate visualizing the interface between gray matter and white matter. We segmented the OCT B-scan (X–Z) images with a local intensity thresholding algorithm to retrieve the coordinates of the pixels along the cortical surface (the red curve) and the upper and lower white matter boundaries (the two yellow curves; Fig. 8a). However, the automatic segmentation might be incorrect where the SNR was low. In that case, we manually corrected the incorrectly segmented pixels at the boundaries. We fitted the coordinates of the remaining pixels with a second-order polynomial model to smoothly delineate the cortical surface and the white matter boundaries. Finally, the gray matter and white matter thickness of each mouse was calculated as the distance between the corresponding fitted boundaries in each B-scan image and then averaged over several B-scan images evenly spaced along the Y-axis. The estimated gray matter and white matter thickness was used to separate the capillary RBC flow parameters into the gray matter and white matter sub-groups for analysis too.
Statistical analysis
Study design and reporting followed ARRIVE guidelines. All data are presented as mean ± STD, where applicable. Statistical comparisons were conducted using Student’s t-test (MATLAB, MathWorks Inc.). A P-value of less than 0.05 was considered statistically significant. Sample sizes were chosen to detect 30% difference between the mean values (coefficient of variance = 0.2, power = 0.8, α = 0.05), and indicated in the text and figure legends, where relevant.
Results
WBI impairs cerebral microvascular blood flow
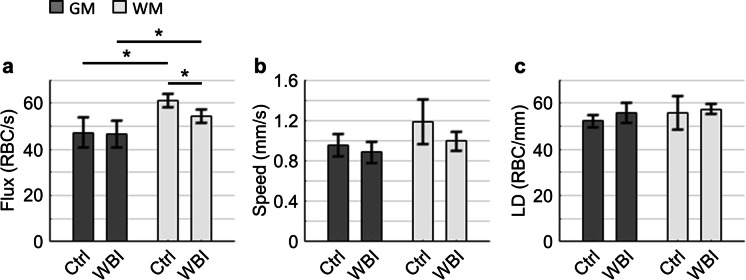
We applied two-photon fluorescence laser scanning microscopy to investigate the WBI-induced impact on the cerebral capillary RBC flow (Fig. 1). We found that in both the control and WBI-treated mice, the mean RBC flux in the white matter capillaries (Control: ~ 61 ± 3 RBC/s, WBI: ~ 54 ± 3 RBC/s) was significantly higher than in the gray matter capillaries (Control: ~ 47 ± 6 RBC/s, WBI: ~ 46 ± 6 RBC/s; Fig. 2a). Interestingly, we found that WBI induced a significant decrease in RBC flux in the white matter capillaries, while the change in the gray matter counterpart was negligible (Fig. 2a). A similar trend was observed in RBC speed, but without reaching statistical significance (Fig. 2b). No obvious trend in RBC linear density was found (Fig. 2c). Histograms with the same data are presented in Supplementary Figs. 1–3.
Fig. 2.
Capillary RBC flow parameters. (a–c) Comparisons of capillary RBC flux (a), speed (b), and linear density (LD; c). This analysis was made with the measurements performed in n = 3 control (Ctrl; in total 291 Gy matter capillaries and 163 white matter capillaries) and n = 4 WBI-treated mice (WBI; in total 534 Gy matter capillaries and 160 white matter capillaries). The data were first averaged over the capillaries in each mouse and then over mice. GM and WM stand for gray matter and white matter, respectively. Data are expressed as mean ± STD. The asterisk symbols indicate statistical significance (Student’s t-test, P < 0.05)
We employed a SD-OCT system to assess the blood flow changes in the larger surfacing venules (15–25 µm in diameter), located within the top 0.1 mm under the cortical surface. As shown in Fig. 3, no significant difference in blood flow (Fig. 3a) or maximum Vz (Fig. 3b) between the two groups was found.
Fig. 3.
Venular blood flow measured with Doppler-OCT. (a, b) Blood flow (a) and maximum axial flow speed (Max. Vz; b) measured in the surfacing venules within the top 100-µm depth under the cortical surface. This analysis was made with the measurements performed in n = 4 control mice (Ctrl; in total 20 vessels) and n = 5 WBI-treated mice (WBI; in total 27 vessels). The data were first averaged over the vessels in each mouse and then over mice. Data are expressed as mean ± STD. No significant difference was observed in this analysis (Student’s t-test)
Effect of WBI on cerebral oxygenation
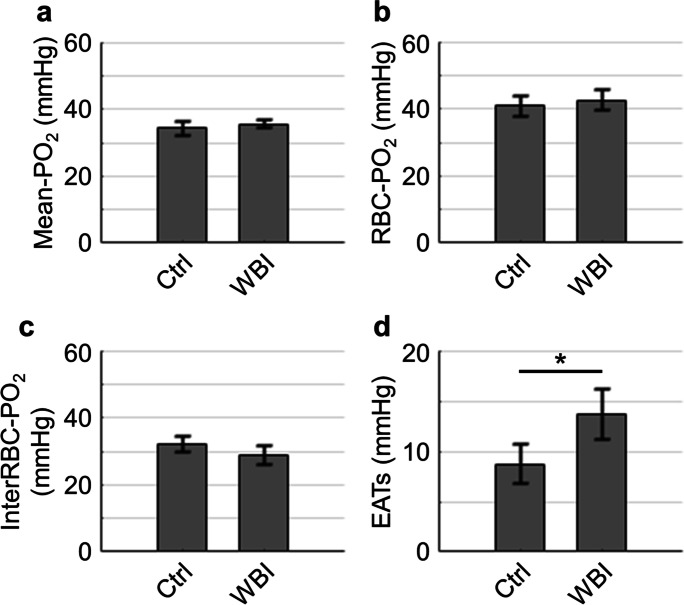
To determine whether WBI imposed an impact on cerebral oxygenation, we applied 2PLM with a phosphorescent oxygen probe — Oxyphor2P to measure intravascular PO2 in the arterioles, venules, and capillaries. Our results show that WBI did not significantly affect the arteriolar and venular PO2 (Fig. 4a) and SO2 (Fig. 4b), while a trend of larger OEF was found in the WBI-treated mice, but it was not statistically significant (Fig. 4c). WBI also did not induce any significant changes in capillary Mean-PO2 (Fig. 5a and Supplementary Fig. 4), RBC-PO2 (Fig. 5b), and InterRBC-PO2 (Fig. 5c). Interestingly, we found that EATs were significantly higher in the WBI-treated mice as compared to the control mice (Fig. 5d).
Fig. 4.
Cerebral vascular oxygenation. (a) Comparison of the PO2 measurements (a) performed in the arterioles (A) and venules (V) selected within the top 100-µm depth under the cortical surface. This analysis was made with the measurements performed in n = 4 control mice (Ctrl; in total 90 and 166 samples acquired in the arterioles and venules, respectively), and n = 6 WBI-treated mice (WBI; in total 153 and 252 samples acquired in the arterioles and venules, respectively). (b) Comparison of SO2 calculated based on the PO2 data from the panel (a). (c) Comparison of OEF calculated based on the SO2 data from the panel (b). The data were first averaged over the vessels in each mouse and then over mice. Data are expressed as mean ± STD. No significant difference was observed in this analysis (Student’s t-test)
Fig. 5.
Cerebral capillary PO2 properties. (a–d). Comparisons of capillary Mean-PO2 (a), RBC-PO2 (b), InterRBC-PO2 (c), and EATs (d). This analysis was made with the measurements performed in n = 4 control (Ctrl; in total 366 capillaries) and n = 5 WBI-treated mice (WBI; in total 511 capillaries). The measurement depth ranged from the cortical surface down to 300 µm under the cortical surface. The data were first averaged over the vessels in each mouse and then over mice. Data are expressed as mean ± STD. The asterisk symbol indicates statistical significance (Student’s t-test, P < 0.05)
Evidence of cerebral structural changes associated with WBI
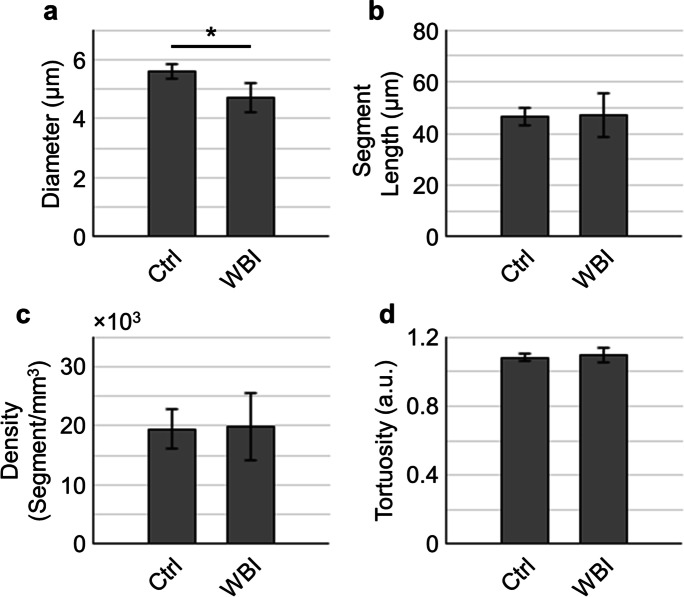
In addition to the assessments on the functional parameters, we also investigated the WBI-induced cerebral microvascular morphological changes with the two-photon angiograms. The results in Fig. 6 show that microvascular diameter (Fig. 6a) was significantly reduced in the WBI group, while the segment length (Fig. 6b), segment density (Fig. 6c), and tortuosity (Fig. 6d), averaged over all depths, did not differ significantly between the two groups. We further conducted a depth-dependent analysis (Fig. 7), revealing a variation of the morphological properties across different depth ranges. More interestingly, we found that segment density at 600–700 μm, potentially mixing the deep cortex and subcortical white matter, exhibited a strong trend of lower values in the WBI-treated mice as compared to that in the control mice (Fig. 7c). This finding indicates a WBI-induced alteration of the microvasculature in the cerebral white matter.
Fig. 6.
Cerebral microvascular morphological properties. (a–d) Comparisons of the cerebral microvascular diameter (a), segment length (b), segment density (c), and tortuosity (d) extracted from the two-photon angiograms. The angiograms were acquired in n = 3 control mice (Ctrl; in total 7876 capillaries) and n = 3 WBI-treated mice (WBI: in total 4798 capillaries). The data were first averaged over the angiogram in each mouse and then over mice. Data are expressed as mean ± STD. The asterisk symbol indicates statistical significance (Student’s t-test, P < 0.05)
Fig. 7.
Depth-dependent changes of the cerebral microvascular morphological properties. (a–d) Comparisons of the cerebral microvascular diameter (a), segment length (b), segment density (c), and tortuosity (d) in the depth ranges of 100–200 µm, 200–300 µm, 300–400 µm, 400–500 µm, 500–600 µm, and 600–700 µm, with the same data as in Fig. 6. The data were first averaged over the angiogram in each mouse and then over mice. Data are expressed as mean ± STD. No significant difference was observed in this analysis (Student’s t-test)
Last, we applied OCT to estimate the cerebral gray matter and white matter thickness to provide a descriptive indicator for the WBI-associated brain atrophy, which has been observed in patients [59–61]. For this analysis, transition of the OCT signal amplitude, from outside the brain to the gray matter and then to the white matter, was utilized to estimate the gray matter and white matter thickness (Fig. 8a) in the interrogated brain regions. Our result in Fig. 8b revealed a noticeable, though insignificant, WBI-induced decrease in both the gray matter and white matter thickness (Fig. 8b).
Discussion
We have performed the multifaceted measurements with two-photon microscopy and optical coherence tomography to comprehensively characterize the effects of WBI on the cerebral microvascular blood flow, oxygenation, and morphology in mice with the irradiation-induced, accelerated neurovascular senescence. All the parameters were measured in awake mice at rest, free of the confounding effects of anesthesia on neuronal activity, CBF, and brain metabolism.
We first assessed the WBI-induced changes of capillary RBC flow parameters. The mean gray matter RBC flux values reported in this work (47 ± 6 RBC/s and 46 ± 6 RBC/s in the control and WBI mice, respectively) are in good agreement with the previous data acquired in awake mice, e.g., as reported by Lyons et al. (~ 42 RBC/s in 3–6-month-old C57BL/6 mice)[62], Moeini et al. (~ 42 RBC/s in the 13–16-month-old C57BL/6 mice)[63], and Li et al. (~ 41 RBC/s in 3–5-month-old C57BL/6 mice)[43], using the similar imaging approaches. We found a higher capillary RBC flux in the white matter than in the gray matter in both groups: 61 ± 3 RBC/s in the white matter vs. 47 ± 6 RBC/s in the gray matter in the control group and 54 ± 3 RBC/s in the white matter vs. 46 ± 6 RBC/s in the gray matter in the WBI group. The observation of capillary RBC flux being higher in the white matter than in the gray matter has also been made in our previous work with 3–5-month-old, isoflurane-anesthetized C57BL/6 mice (gray matter RBC flux: 48 ± 10 RBC/s, white matter RBC flux: 67 ± 12 RBC/s) [40]. This could likely be explained by the much lower capillary resistance to blood flow in the white matter than in the gray matter [64–66], resulting in higher RBC flux and speed in the white matter capillary segments in spite of lower blood perfusion due to much lower capillary density in the white matter. The animal age, sex, and anesthesia condition during experiment may also differentially affect the distributions of capillary blood flow in the cerebral gray matter and white matter as well. The relative changes of RBC flux in the gray matter and white matter were calculated as ~ 1.9% and ~ 10.9%, respectively. This finding is consistent with our previous work, demonstrating that cerebral white matter was more vulnerable to pathological hemodynamic perturbations [40]. Microvascular impairments in the cerebral white matter have been regarded as a leading cause of cognitive decline and even vascular dementia in elders [67, 68]. The higher vulnerability of cerebral white matter to global hypoperfusion or hemodynamic perturbation compared to the cerebral gray matter is likely partially due to its distal location in the arterial blood supplying network [40, 69, 70]. The consequences of prolonged insufficient blood supply to the white matter include the development of demyelination and/or myelin repair failure, which can be diagnosed by the appearance of MRI hyperintensities [71]. In addition, our Doppler-OCT measurements of venular blood flow appeared slightly larger than that was reported by Moeini et al. (~ 0.05 μL/min in the 13–16-month-old C57BL/6 mice)[63]. Here, different mouse model and/or vessel size chosen for analysis might contribute to this discrepancy. Our measurements indicate that the venular blood flow at the cortical surface may not be very sensitive to the blood flow in the subcortical white matter. Better assessment of the blood flow could be obtained by localized measurements directly in the white matter region.
Next, we investigated the WBI-induced changes of cerebral oxygenation with the 2PLM imaging of vascular PO2. The arteriolar PO2 and SO2, as well as capillary Mean-PO2, RBC-PO2, and InterRBC-PO2 reported in this work, were lower, as compared to our previous work with younger female mice (3–5-month-old, C57BL/6) [43]. This might be associated with mouse sex and/or age, as shown in a previous work that cerebral vascular oxygenation deteriorated in aged animals [63]. Decrease in arterial oxygenation was typically associated with increase in OEF, as seen in Fig. 4c as compared to a previous study with younger wild-type mice [43], to maintain the normal brain metabolism in the condition of compromised CBF. The EAT amplitude shown in Fig. 5d was comparable with that was reported in the previous works [43, 62]. This observed higher EATs in the WBI-treated mice suggests a potentially greater intravascular resistance to the diffusive oxygen transport from RBCs to extravascular tissue [55, 72–74], thus, a compromised cerebral tissue oxygenation.
Furthermore, the microvascular morphological properties shown in the present work (Figs. 5 and 7) are reasonably comparable with a previous study, in which the same measures averaging over all the cortical regions in mouse brain were reported [75]. While, in that work, brain tissue preparation associated with ex vivo imaging would potentially cause deformation of the sample, confounding the comparisons with our data. The estimated cerebral gray matter and white matter thickness shown in Fig. 8 were slightly smaller than our previously reported values obtained in younger animals [40].
The key findings of this study are that a clinical regimen of WBI leads to a significant decrease in capillary RBC flux in the subcortical white matter, significant reduction of microvascular diameter, and a strong trend of reduced microvascular segment density at the depth range of 600–700 μm, likely covering the bottom part of the cortex and the sub-cortical white matter. Our findings extend the results of the previously reported pre-clinical[38, 76–80] and clinical[33, 81–96] investigations, demonstrating that cerebral white matter was sensitive to radiation-induced injury. We posit that the observed microvascular changes, combined with impairments of astrocyte- and endothelium-dependent local vasoregulatory mechanisms[16] after WBI result in ischemic injury to the white matter, which in turn contributes to cognitive dysfunction [78, 97]. There has been strong evidence that white matter was particularly sensitive to hypoperfusion and ischemic injury[98, 99] and that microvascular rarefaction was causally linked to the white matter damage and cognitive decline in a wide range of pathophysiological conditions [100, 101]. The pathophysiological role of irradiation-induced white matter ischemia could be supported by the demonstrations of white matter hyperintensities and other radiological signs of small vessel pathologies and consequential ischemia in the brains of the WBI-treated cancer survivors [87, 97, 102]. In addition to promoting microvascular rarefaction and dysfunction, radiation was also shown to induce blood–brain-barrier disruption in the white matter,104 which may also contribute to the white matter damage. There are also experimental and clinical evidences that the irradiation-induced microvascular damage is associated with blood–brain-barrier disruption, which is manifested as increased microvascular leakiness [104–107]. Recent studies demonstrate that the senolytic treatments improve blood–brain-barrier integrity in the WBI-treated mice (Ungvari et al. 2022, manuscript in preparation). Several preclinical studies demonstrated that vascular risk factors, including hypertension, obesity, metabolic disease, and advanced aging would promote both microvascular structural injury and CBF dysregulation, accelerating the progress of cognitive decline [108–111]. It was reported in other works that these vascular risk factors would also exacerbate the γ-irradiation-induced microvascular injury and dysfunction, as well as white matter damage [94, 112].
Presently, there is no cure for white matter injury clinically. Experimental studies demonstrate that the WBI-induced microcirculatory injury can be alleviated by senolytic treatments [16] (Ungvari et al. 2022, manuscript in preparation). Further studies needed to determine whether senolytic treatment regiments can prevent WM injury as well. Potential therapeutic approaches in animal studies also involve mesenchymal stem cells, which shows unique effects on facilitating producing growth factors and cytokines, immunomodulation, and neuroprotection against oxidative stress [113]. Thyroxin also shows a protection effect against preoligodendrocyte apoptosis and white matter injury via the upregulation of neurotrophic factor in the immature rat brain [114].
One of the primary mechanisms mediating the long-lasting radiation effects is thought to be the damage to vascular endothelial cells, leading to a cascade of pathological events including microvascular endothelial and astrocyte senescence, impairing angiogenic processes and endothelial apoptosis, promoting microvascular rerafaction [30, 115–117].
The mechanisms by which γ-irradiation leads to microvascular rarefaction and neurovascular injury include induction of DNA-damage-mediated senescence of the cells in NVU, impairing endothelial angiogenic capacity and altering the pathways of regulating local CBF [16, 118, 119]. Previous studies showed that WBI also induced neurovascular senescence and microvascular rarefaction in hippocampus via similar mechanisms [16, 24, 28–31]. Previously, we have shown that pharmacological or genetic depletion of the senescent cells facilitated improving cognitive function in the WBI-treated mice [16]. Thus, further studies are warranted to determine whether such interventions can benefit angiogenesis and increase in the blood flow to the white matter.
A growing body of evidence supports the hypothesis that the radiation-induced side effects involve an acute and a long-lasting oxidative stress [120]. Irradiation induces ischemia and tissue hypoxia, likely primarily mediated through the damages to vascular endothelial cells [121]. Tissue hypoxia is typically associated with an increased immune response and exacerbation of oxidative stress [120, 122]. Normally, increased oxidative stress induces a robust upregulation of antioxidant systems (e.g., through activation of the Nrf2-ARE pathway). However, there is evidence that this homeostatic antioxidative response may be impaired in the irradiated brain [123]. The brain and the cerebral microcirculation are particularly vulnerable to the oxidative stress-mediated macromolecular damage, partially because of the high level of oxygen metabolism and low energy store in the neural tissue[124], the relatively low levels of antioxidant defenses [125], and the susceptibility of microvascular endothelial cells and astrocytes to the irradiation-induced, ROS-mediated DNA damage and consequential cellular functional impairment[16, 118, 126].
The present work has limitations. First, reductions of capillary RBC flux and microvascular segment density in the white matter are expected to define the brain regions prone to ischemia. In this study, we provided only intravascular PO2 measurements. Additional measurements for assessing tissue oxygenation would be informative to determine the area at risk of ischemia in the white matter. Second, as reported, WBI would induce progressive cognitive decline in human patients. Thus, it will be important to understand the time course of pathological microvascular remodeling and CBF dysregulation induced by the WBI treatment. Besides, Doppler-OCT blood flow measurements were only provided in the venules, as the calculation of arteriolar blood flow might be subjected to an aliasing effect caused by high flow speed. However, we expect that the venular blood flow measurements might be sufficient to study the CBF changes across different groups because the input of blood from the arterioles into the cortex should approximately be equal to the output from the venules given the size of our interrogated brain regions [127]. Lastly, capillaries and the smallest arterioles and venules (diameter of ≤ 10 µm) were not strictly distinguished for the measurements of capillary RBC flux and capillary PO2. Data selection based on vascular caliber and R2 ≥ 0.5 ensures that most of the selected vessels were capillaries.
In conclusion, our findings support the notion that WBI leads to persistent structural and functional microvascular impairment in the cerebral subcortical white matter, which likely contributes to the WBI-induced white matter injury and cognitive decline.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank Waleed Tahir and Lei Tian from Boston University, and Rafat Damseh and Frédéric Lesage from École Polytechnique de Montréal, Université de Montréal, for their technical supports on the microvascular morphological analysis.
Author contribution
B.L., A.Y., S.S., and Z.U. designed the study; A.Y. prepared the WBI model with the guidance from S.U., S.T., W.E.S., and A.C.; B.L., I.S., and S.E.E. performed the experiments; B.L., J.E.P., M.A.H.A., and J.L. analyzed the data with the guidance from S.S.; B.L. and S.S. interpreted the results with the help from Z.U. and D.A.B.; S.V. developed the phosphorescence nanoprobe Oxyphor-2P and helped with the PO2 measurements; S.R.A. synthetized Oxyphor-2P with the guidance from S.V.; B.L. synthetized the dextran-Alexa680 dye with the guidance from C.R.; B.L. and Z.U. wrote the manuscript with help from S.S. and all other authors; S.S. and B.L. developed the imaging and data processing methods; B.F. performed the animal surgeries.
Funding
This work was supported by the grants from the American Heart Association, the Oklahoma Center for the Advancement of Science and Technology, the National Institute on Aging (RF1AG072295, R01AG055395, R01AG068295, R01AG070915, and K01AG073614), the National Institute of Neurological Disorders and Stroke (R01NS100782, R01NS091230, R01NS115401, U19NS123717, and RF1NS121095), the National Cancer Center (R01CA255840), the National Institute of Biomedical Imaging and Bioengineering (U24EB028941), the National Heart, Lung, and Blood Institute (U01HL133362), the National Institute of Mental Health (R00MH120053), the Oklahoma Shared Clinical and Translational Resources (U54GM104938) with an Institutional Development Award (IDeA) from NIGMS, the Presbyterian Health Foundation, the Reynolds Foundation, the NIA-supported Geroscience Training Program in Oklahoma (T32AG052363), the Oklahoma Nathan Shock Center (P30AG050911), the Cellular and Molecular GeroScience CoBRE (P20GM125528), and the Science and Technology Innovation Committee of Shenzhen Municipality (JSGG20210420091601003).
Declarations
Disclaimer
The funding sources had no role in the study design, in the collection, analysis and interpretation of data, in the writing of the report, or in the decision to submit the article for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the American Heart Association, or the Presbyterian Health Foundation.
Conflict of interest
Dr. Anna Csiszar serves as Associate Editor for the Journal of Gerontology, Series A: Biological Sciences and Medical Sciences and GeroScience. Dr. Andriy Yabluchanskiy serves as Guest Editor for the American Journal of Physiology-Heart and Circulatory Physiology. Dr. Zoltan Ungvari serves as Editor-in-Chief for GeroScience and as Consulting Editor for the American Journal of Physiology-Heart and Circulatory Physiology. The authors declare no other competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Baoqiang Li and Andriy Yabluchanskiy contributed equally to this study.
Contributor Information
Zoltan Ungvari, Email: Zoltan-Ungvari@ouhsc.edu.
Sava Sakadžić, Email: sava.sakadzic@mgh.harvard.edu.
References
- 1.Graus F, Walker RW, Allen JC. Brain metastases in children. J Pediatr. 1983;103:558–561. doi: 10.1016/S0022-3476(83)80583-6. [DOI] [PubMed] [Google Scholar]
- 2.Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14:48–54. doi: 10.1007/s11912-011-0203-y. [DOI] [PubMed] [Google Scholar]
- 3.Gaspar LE, et al. The role of whole brain radiation therapy in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:17–32. doi: 10.1007/s11060-009-0060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patil CG, et al. Whole brain radiation therapy (WBRT) alone versus WBRT and radiosurgery for the treatment of brain metastases. Cochrane Database Syst Rev. 2017;9:CD006121. doi: 10.1002/14651858.CD006121.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsao MN, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. 2018 doi: 10.1002/14651858.CD003869.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park K, et al. Radiotherapy for brain metastasis and long-term survival. Sci Rep. 2021;11:8046. doi: 10.1038/s41598-021-87357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YW, Cho HJ, Lee WH, Sonntag WE. Whole brain radiation-induced cognitive impairment: pathophysiological mechanisms and therapeutic targets. Biomol Ther. 2012;20:357–370. doi: 10.4062/biomolther.2012.20.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dieckmann K, Herrmann H. Are there still indications for whole brain irradiation in 2021? Memo - Mag Eur Med Oncol. 2021;14:204–207. [Google Scholar]
- 9.van Grinsven EE, Nagtegaal SHJ, Verhoeff JJC, van Zandvoort MJE. The impact of stereotactic or whole brain radiotherapy on neurocognitive functioning in adult patients with brain metastases: a systematic review and meta-analysis. Oncol Res Treat. 2021;44:622–636. doi: 10.1159/000518848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24:1295–1304. doi: 10.1200/JCO.2005.04.6185. [DOI] [PubMed] [Google Scholar]
- 11.Welzel G, et al. Acute neurocognitive impairment during cranial radiation therapy in patients with intracranial tumors. Strahlenther Onkol. 2008;184:647. doi: 10.1007/s00066-008-1830-6. [DOI] [PubMed] [Google Scholar]
- 12.Welzel G, et al. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int J Radiat Oncol Biol Phys. 2008;72:1311–1318. doi: 10.1016/j.ijrobp.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Brown PD, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyers CA, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol Off J Am Soc Clin Oncol. 2004;22:157–165. doi: 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- 15.Greene-Schloesser D, et al. Radiation-induced brain injury: a review. Front Oncol. 2012;2:73. doi: 10.3389/fonc.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yabluchanskiy A, et al. Pharmacological or genetic depletion of senescent astrocytes prevents whole brain irradiation-induced impairment of neurovascular coupling responses protecting cognitive function in mice. GeroScience. 2020;42:409–428. doi: 10.1007/s11357-020-00154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamproglou I, et al. Total body 4.5 Gy gamma irradiation-induced early delayed learning and memory dysfunction in the rat. Cell Mol Biol Noisy Gd Fr. 2001;47:453–457. [PubMed] [Google Scholar]
- 18.Shi L, et al. Spatial learning and memory deficits after whole-brain irradiation are associated with changes in NMDA receptor subunits in the hippocampus. Radiat Res. 2006;166:892–899. doi: 10.1667/RR0588.1. [DOI] [PubMed] [Google Scholar]
- 19.Soussain C, et al. CNS complications of radiotherapy and chemotherapy. The Lancet. 2009;374:1639–1651. doi: 10.1016/S0140-6736(09)61299-X. [DOI] [PubMed] [Google Scholar]
- 20.Warrington JP, Csiszar A, Mitschelen M, Lee YW, Sonntag WE. Whole brain radiation-induced impairments in learning and memory are time-sensitive and reversible by systemic hypoxia. PLoS ONE. 2012;7:e30444. doi: 10.1371/journal.pone.0030444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ungvari Z, et al. Cerebromicrovascular dysfunction predicts cognitive decline and gait abnormalities in a mouse model of whole brain irradiation-induced accelerated brain senescence. GeroScience. 2017;39:33–42. doi: 10.1007/s11357-017-9964-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robbins ME, Bourland JD, Cline JM, Wheeler KT, Deadwyler SA. A model for assessing cognitive impairment after fractionated whole-brain irradiation in nonhuman primates. Radiat Res. 2011;175:519–525. doi: 10.1667/RR2497.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanbury DB, et al. Pathology of fractionated whole-brain irradiation in rhesus monkeys ( Macaca mulatta ) Radiat Res. 2015;183:367–374. doi: 10.1667/RR13898.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown WR, Thore CR, Moody DM, Robbins ME, Wheeler KT. Vascular damage after fractionated whole-brain irradiation in rats. Radiat Res. 2005;164:662–668. doi: 10.1667/RR3453.1. [DOI] [PubMed] [Google Scholar]
- 25.Shi L, et al. Maintenance of white matter integrity in a rat model of radiation-induced cognitive impairment. J Neurol Sci. 2009;285:178–184. doi: 10.1016/j.jns.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Connor M, et al. Regional susceptibility to dose-dependent white matter damage after brain radiotherapy. Radiother Oncol. 2017;123:209–217. doi: 10.1016/j.radonc.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96:17–42. doi: 10.1016/j.neuron.2017.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashpole NM, et al. Systemic influences contribute to prolonged microvascular rarefaction after brain irradiation: a role for endothelial progenitor cells. Am J Physio - Heart Circ Physiol. 2014;307:H858–H868. doi: 10.1152/ajpheart.00308.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee WH, Cho HJ, Sonntag WE, Lee YW. Radiation attenuates physiological angiogenesis by differential expression of VEGF, Ang-1, tie-2 and Ang-2 in rat brain. Radiat Res. 2011;176:753–760. doi: 10.1667/RR2647.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warrington JP, et al. Whole brain radiation-induced vascular cognitive impairment: mechanisms and implications. J Vasc Res. 2013;50:445–457. doi: 10.1159/000354227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warrington JP, et al. Cerebral microvascular rarefaction induced by whole brain radiation is reversible by systemic hypoxia in mice. Am. J. Physiol.-Heart Circ. Physiol. 2011;300:H736–H744. doi: 10.1152/ajpheart.01024.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Correa DD, et al. Longitudinal cognitive assessment in patients with primary CNS lymphoma treated with induction chemotherapy followed by reduced-dose whole-brain radiotherapy or autologous stem cell transplantation. J Neurooncol. 2019;144:553–562. doi: 10.1007/s11060-019-03257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stokes TB, et al. White matter changes in breast cancer brain metastases patients who undergo radiosurgery alone compared to whole brain radiation therapy plus radiosurgery. J Neurooncol. 2015;121:583–590. doi: 10.1007/s11060-014-1670-4. [DOI] [PubMed] [Google Scholar]
- 34.Cheng H, Chen H, Lv Y, Chen Z, Li CR. Prospective memory impairment following whole brain radiotherapy in patients with metastatic brain cancer. Cancer Med. 2018;7:5315–5321. doi: 10.1002/cam4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bovi JA, et al. Pretreatment volume of MRI-determined white matter injury predicts neurocognitive decline after hippocampal avoidant whole-brain radiation therapy for brain metastases: secondary analysis of NRG Oncology Radiation Therapy Oncology Group 0933. Adv Radiat Oncol. 2019;4:579–586. doi: 10.1016/j.adro.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayinger M, et al. Leukoencephalopathy after prophylactic whole-brain irradiation with or without hippocampal sparing: a longitudinal magnetic resonance imaging analysis. Eur J Cancer. 2020;124:194–203. doi: 10.1016/j.ejca.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 37.Yoo DH, et al. MR imaging evaluation of intracerebral hemorrhages and T2 hyperintense white matter lesions appearing after radiation therapy in adult patients with primary brain tumors. PLoS ONE. 2015;10:e0136795. doi: 10.1371/journal.pone.0136795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andrews RN, et al. White matter is the predilection site of late-delayed radiation-induced brain injury in non-human primates. Radiat Res. 2019;191:217–231. doi: 10.1667/RR15263.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panagiotakos G, et al. Long-term impact of radiation on the stem cell and oligodendrocyte precursors in the brain. PLoS ONE. 2007;2:e588. doi: 10.1371/journal.pone.0000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li B, Ohtomo R, Thunemann M, Adams SR, Yang J, Fu B, Yaseen MA, et al. Two-photon microscopic imaging of capillary red blood cell flux in mouse brain reveals vulnerability of cerebral white matter to hypoperfusion. J Cereb Blood Flow Metab. 2020;40(12):501–12. 10.1177/0271678X19831016. [DOI] [PMC free article] [PubMed]
- 41.Ahn SJ, et al. Label-free assessment of hemodynamics in individual cortical brain vessels using third harmonic generation microscopy. Biomed Opt Express. 2020;11:2665–2678. doi: 10.1364/BOE.385848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esipova TV, et al. Oxyphor 2P: A high-performance probe for deep-tissue longitudinal oxygen imaging. Cell Metab. 2019;29:736–744.e7. doi: 10.1016/j.cmet.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li B, et al. More homogeneous capillary flow and oxygenation in deeper cortical layers correlate with increased oxygen extraction. ELife. 2019;8:e42299. doi: 10.7554/eLife.42299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demaria M, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldey GJ, et al. Removable cranial windows for long-term imaging in awake mice. Nat Protoc. 2014;9:2515–2538. doi: 10.1038/nprot.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Komiyama T, et al. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature. 2010;464:1182–1186. doi: 10.1038/nature08897. [DOI] [PubMed] [Google Scholar]
- 47.Sakadžić S, et al. Two-photon high-resolution measurement of partial pressure of oxygen in cerebral vasculature and tissue. Nat Methods. 2010;7:755–759. doi: 10.1038/nmeth.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yaseen MA, et al. Multimodal optical imaging system for in vivo investigation of cerebral oxygen delivery and energy metabolism. Biomed Opt Express. 2015;6:4994–5007. doi: 10.1364/BOE.6.004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Unekawa M, et al. RBC velocities in single capillaries of mouse and rat brains are the same, despite 10-fold difference in body size. Brain Res. 2010;1320:69–73. doi: 10.1016/j.brainres.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 50.Li B, Lee J, Boas DA, Lesage F. Contribution of low- and high-flux capillaries to slow hemodynamic fluctuations in the cerebral cortex of mice. J Cereb Blood Flow Metab. 2016;36(8):1351–6. 10.1177/0271678X16649195. [DOI] [PMC free article] [PubMed]
- 51.Lee J, Wu W, Lesage F, Boas DA. Multiple-capillary measurement of RBC speed, flux, and density with optical coherence tomography. J. Cereb. Blood Flow Metab. Off J Int Soc Cereb Blood Flow Metab. 2013;33:1707–1710. doi: 10.1038/jcbfm.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Srinivasan VJ, et al. Quantitative cerebral blood flow with optical coherence tomography. Opt Express. 2010;18:2477. doi: 10.1364/OE.18.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Finikova OS, et al. Oxygen microscopy by two-photon-excited phosphorescence. ChemPhysChem. 2008;9:1673–1679. doi: 10.1002/cphc.200800296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uchida K, Reilly MP, Asakura T. Molecular stability and function of mouse hemoglobins. Zoolog Sci. 1998;15:703–706. doi: 10.2108/zsj.15.703. [DOI] [Google Scholar]
- 55.Lecoq J, et al. Simultaneous two-photon imaging of oxygen and blood flow in deep cerebral vessels. Nat Med. 2011;17:893–898. doi: 10.1038/nm.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parpaleix A, Houssen YG, Charpak S. Imaging local neuronal activity by monitoring PO2 transients in capillaries. Nat Med. 2013;19:241–246. doi: 10.1038/nm.3059. [DOI] [PubMed] [Google Scholar]
- 57.Tahir W, Kura S, Zhu J, Cheng X, Damseh R, Tadesse F, et al. Anatomical modeling of brain vasculature in two-photon microscopy by generalizable deep learning. BME Frontiers. 2020. 10.34133/2020/8620932. [DOI] [PMC free article] [PubMed]
- 58.Damseh R, Delafontaine-Martel P, Pouliot P, Cheriet F, Lesage F. Laplacian flow dynamics on geometric graphs for anatomical modeling of cerebrovascular networks. IEEE Trans Med Imaging. 2021;40:381–394. doi: 10.1109/TMI.2020.3027500. [DOI] [PubMed] [Google Scholar]
- 59.Gui C, et al. A prospective evaluation of whole brain volume loss and neurocognitive decline following hippocampal-sparing prophylactic cranial irradiation for limited-stage small-cell lung cancer. J Neurooncol. 2019;144:351–358. doi: 10.1007/s11060-019-03235-7. [DOI] [PubMed] [Google Scholar]
- 60.Takeshita Y, et al. Early volume reduction of the hippocampus after whole-brain radiation therapy: an automated brain structure segmentation study. Jpn J Radiol. 2020;38:118–125. doi: 10.1007/s11604-019-00895-3. [DOI] [PubMed] [Google Scholar]
- 61.Shibamoto Y, et al. Incidence of brain atrophy and decline in Mini-Mental State Examination score after whole-brain radiotherapy in patients with brain metastases: a prospective Study. Int J Radiat Oncol. 2008;72:1168–1173. doi: 10.1016/j.ijrobp.2008.02.054. [DOI] [PubMed] [Google Scholar]
- 62.Lyons DG, Parpaleix A, Roche M, Charpak S. Mapping oxygen concentration in the awake mouse brain. ELife. 2016;5:e12024. doi: 10.7554/eLife.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moeini M, et al. Compromised microvascular oxygen delivery increases brain tissue vulnerability with age. Sci Rep. 2018;8:8219. doi: 10.1038/s41598-018-26543-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blinder P, et al. The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat Neurosci. 2013;16:889–897. doi: 10.1038/nn.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ballabh P, Braun A, Nedergaard M. Anatomic analysis of blood vessels in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatr Res. 2004;56:117–124. doi: 10.1203/01.PDR.0000130472.30874.FF. [DOI] [PubMed] [Google Scholar]
- 66.Cavaglia M, et al. Regional variation in brain capillary density and vascular response to ischemia. Brain Res. 2001;910:81–93. doi: 10.1016/S0006-8993(01)02637-3. [DOI] [PubMed] [Google Scholar]
- 67.Müller K, Courtois G, Ursini MV, Schwaninger M. New insight into the pathogenesis of cerebral small-vessel diseases. Stroke. 2017;48:520–527. doi: 10.1161/STROKEAHA.116.012888. [DOI] [PubMed] [Google Scholar]
- 68.Chojdak-Łukasiewicz J, Dziadkowiak E, Zimny A, Paradowski B. Cerebral small vessel disease: a review. Adv. Clin. Exp Med Off Organ Wroclaw Med Univ. 2021;30:349–356. doi: 10.17219/acem/131216. [DOI] [PubMed] [Google Scholar]
- 69.Pantoni L, Poggesi A, Inzitari D. The relation between white-matter lesions and cognition. Curr Opin Neurol. 2007;20:390–397. doi: 10.1097/WCO.0b013e328172d661. [DOI] [PubMed] [Google Scholar]
- 70.Horsfield MA, Jones DK. Applications of diffusion-weighted and diffusion tensor MRI to white matter diseases – a review. NMR Biomed. 2002;15:570–577. doi: 10.1002/nbm.787. [DOI] [PubMed] [Google Scholar]
- 71.Nakao S, Yamamoto T, Kimura S, Mino T, Iwamoto T. Brain white matter lesions and postoperative cognitive dysfunction: a review. J Anesth. 2019;33:336–340. doi: 10.1007/s00540-019-02613-9. [DOI] [PubMed] [Google Scholar]
- 72.Golub AS, Pittman RN. Erythrocyte-associated transients in PO2 revealed in capillaries of rat mesentery. Am J Physiol Heart Circ Physiol. 2005;288:H2735–2743. doi: 10.1152/ajpheart.00711.2004. [DOI] [PubMed] [Google Scholar]
- 73.Cabrales P, Intaglietta M. Time-dependant oxygen partial pressure in capillaries and tissue in the hamster window chamber model. Antioxid Redox Signal. 2007;9:845–853. doi: 10.1089/ars.2007.1584. [DOI] [PubMed] [Google Scholar]
- 74.Barker MC, Golub AS, Pittman RN. Erythrocyte-associated transients in capillary PO2: an isovolemic hemodilution study in the rat spinotrapezius muscle. Am J Physiol Heart Circ Physiol. 2007;292:H2540–2549. doi: 10.1152/ajpheart.00915.2006. [DOI] [PubMed] [Google Scholar]
- 75.Wälchli T, et al. Hierarchical imaging and computational analysis of three-dimensional vascular network architecture in the entire postnatal and adult mouse brain. Nat Protoc. 2021;16:4564–4610. doi: 10.1038/s41596-021-00587-1. [DOI] [PubMed] [Google Scholar]
- 76.Akiyama K, Tanaka R, Sato M, Takeda N. Cognitive dysfunction and histological findings in adult rats one year after whole brain irradiation. Neurol Med Chir (Tokyo) 2001;41:590–598. doi: 10.2176/nmc.41.590. [DOI] [PubMed] [Google Scholar]
- 77.Nakagaki H, Brunhart G, Kemper TL, Caveness WF. Monkey brain damage from radiation in the therapeutic range. J Neurosurg. 1976;44:3–11. doi: 10.3171/jns.1976.44.1.0003. [DOI] [PubMed] [Google Scholar]
- 78.Andrews RN, et al. Cerebrovascular remodeling and neuroinflammation is a late effect of radiation-induced brain injury in non-human primates. Radiat Res. 2017;187:599–611. doi: 10.1667/RR14616.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Constanzo J, et al. Diffusion MRI monitoring of specific structures in the irradiated rat brain. Magn Reson Med. 2018;80:1614–1625. doi: 10.1002/mrm.27112. [DOI] [PubMed] [Google Scholar]
- 80.Peiffer AM, Shi L, Olson J, Brunso-Bechtold JK. Differential effects of radiation and age on diffusion tensor imaging in rats. Brain Res. 2010;1351:23–31. doi: 10.1016/j.brainres.2010.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Correa DD, et al. Cognitive functions in survivors of primary central nervous system lymphoma. Neurology. 2004;62:548–555. doi: 10.1212/01.WNL.0000109673.75316.D8. [DOI] [PubMed] [Google Scholar]
- 82.DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789–796. doi: 10.1212/WNL.39.6.789. [DOI] [PubMed] [Google Scholar]
- 83.Duan F, et al. Whole-brain changes in white matter microstructure after radiotherapy for nasopharyngeal carcinoma: a diffusion tensor imaging study. Eur. Arch. Oto-Rhino-Laryngol. Off. J. Eur. Fed. Oto-Rhino-Laryngol. Soc. EUFOS Affil. Ger Soc Oto-Rhino-Laryngol. - Head Neck Surg. 2016;273:4453–4459. doi: 10.1007/s00405-016-4127-x. [DOI] [PubMed] [Google Scholar]
- 84.Frytak S, et al. Magnetic resonance imaging for neurotoxicity in long-term survivors of carcinoma. Mayo Clin Proc. 1985;60:803–812. doi: 10.1016/S0025-6196(12)64785-5. [DOI] [PubMed] [Google Scholar]
- 85.King TZ, Wang L, Mao H. Disruption of white matter integrity in adult survivors of childhood brain tumors: correlates with long-term intellectual outcomes. PLoS ONE. 2015;10:e0131744. doi: 10.1371/journal.pone.0131744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Conill C, et al. Incidence of radiation-induced leukoencephalopathy after whole brain radiotherapy in patients with brain metastases. Clin Transl Oncol. 2007;9:590–595. doi: 10.1007/s12094-007-0108-2. [DOI] [PubMed] [Google Scholar]
- 87.Bompaire F, et al. New insights in radiation-induced leukoencephalopathy: a prospective cross-sectional study. Support. Care Cancer Off. J. Multinatl Assoc Support Care Cancer. 2018;26:4217–4226. doi: 10.1007/s00520-018-4296-9. [DOI] [PubMed] [Google Scholar]
- 88.Rubinstein LJ, Herman MM, Long TF, Wilbur JR. Disseminated necrotizing leukoencephalopathy: a complication of treated central nervous system leukemia and lymphoma. Cancer. 1975;35:291–305. doi: 10.1002/1097-0142(197502)35:2<291::AID-CNCR2820350202>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 89.Lee JS, et al. Neurotoxicity in long-term survivors of small cell lung cancer. Int J Radiat Oncol Biol Phys. 1986;12:313–321. doi: 10.1016/0360-3016(86)90344-5. [DOI] [PubMed] [Google Scholar]
- 90.Lee YY, Nauert C, Glass JP. Treatment-related white matter changes in cancer patients. Cancer. 1986;57:1473–1482. doi: 10.1002/1097-0142(19860415)57:8<1473::AID-CNCR2820570807>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 91.Leng X, et al. Application of a machine learning method to whole brain white matter injury after radiotherapy for nasopharyngeal carcinoma. Cancer Imaging Off. Publ Int Cancer Imaging Soc. 2019;19:19. doi: 10.1186/s40644-019-0203-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Monaco EA, et al. Leukoencephalopathy after whole-brain radiation therapy plus radiosurgery versus radiosurgery alone for metastatic lung cancer. Cancer. 2013;119:226–232. doi: 10.1002/cncr.27504. [DOI] [PubMed] [Google Scholar]
- 93.Schuitema I, et al. Accelerated aging, decreased white matter integrity, and associated neuropsychological dysfunction 25 years after pediatric lymphoid malignancies. J Clin Oncol Off J Am Soc Clin Oncol. 2013;31:3378–3388. doi: 10.1200/JCO.2012.46.7050. [DOI] [PubMed] [Google Scholar]
- 94.Szerlip N, et al. Factors impacting volumetric white matter changes following whole brain radiation therapy. J Neurooncol. 2011;103:111–119. doi: 10.1007/s11060-010-0358-7. [DOI] [PubMed] [Google Scholar]
- 95.Vigliani MC, et al. Dementia following treatment of brain tumors with radiotherapy administered alone or in combination with nitrosourea-based chemotherapy: a clinical and pathological study. J Neurooncol. 1999;41:137–149. doi: 10.1023/A:1006183730847. [DOI] [PubMed] [Google Scholar]
- 96.Wang S, et al. Longitudinal diffusion tensor magnetic resonance imaging study of radiation-induced white matter damage in a rat model. Cancer Res. 2009;69:1190–1198. doi: 10.1158/0008-5472.CAN-08-2661. [DOI] [PubMed] [Google Scholar]
- 97.Moretti R, Caruso P. An iatrogenic model of brain small-vessel disease: post-radiation encephalopathy. Int J Mol Sci. 2020;21:6506. doi: 10.3390/ijms21186506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hattori H, Takeda M, Kudo T, Nishimura T, Hashimoto S. Cumulative white matter changes in the gerbil brain under chronic cerebral hypoperfusion. Acta Neuropathol (Berl) 1992;84:437–442. doi: 10.1007/BF00227672. [DOI] [PubMed] [Google Scholar]
- 99.Dalby RB, Eskildsen SF, Videbech P, Frandsen J, Mouridsen K, Sørensen L, et al. Oxygenation differs among white matter hyperintensities, intersected fiber tracts and unaffected white matter. Brain Commun. 1(1):fcz033. 10.1093/braincomms/fcz033. [DOI] [PMC free article] [PubMed]
- 100.Brown WR, Moody DM, Thore CR, Challa VR, Anstrom JA. Vascular dementia in leukoaraiosis may be a consequence of capillary loss not only in the lesions, but in normal-appearing white matter and cortex as well. J Neurol Sci. 2007;257:62–66. doi: 10.1016/j.jns.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Joutel A, et al. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest. 2010;120:433–445. doi: 10.1172/JCI39733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miura M, et al. High prevalence of small vessel disease long after cranial irradiation. J Clin Neurosci Off J Neurosurg Soc Australas. 2017;46:129–135. doi: 10.1016/j.jocn.2017.09.008. [DOI] [PubMed] [Google Scholar]
- 103.Lo EH, Frankel KA, Steinberg GK, DeLaPaz RL, Fabrikant JI. High-dose single-fraction brain irradiation: MRI, cerebral blood flow, electrophysiological, and histological studies. Int J Radiat Oncol Biol Phys. 1992;22:47–55. doi: 10.1016/0360-3016(92)90981-M. [DOI] [PubMed] [Google Scholar]
- 104.Remler MP, Marcussen WH, Tiller-Borsich J. The late effects of radiation on the blood brain barrier. Int J Radiat Oncol Biol Phys. 1986;12:1965–1969. doi: 10.1016/0360-3016(86)90133-1. [DOI] [PubMed] [Google Scholar]
- 105.Nakata H, et al. Early blood-brain barrier disruption after high-dose single-fraction irradiation in rats. Acta Neurochir (Wien) 1995;136:82–86. doi: 10.1007/BF01411440. [DOI] [PubMed] [Google Scholar]
- 106.Yoshida Y, et al. X-ray irradiation induces disruption of the blood-brain barrier with localized changes in claudin-5 and activation of microglia in the mouse brain. Neurochem Int. 2018;119:199–206. doi: 10.1016/j.neuint.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 107.Yuan H, Gaber MW, Boyd K, Wilson CM, Kiani MF, Merchant TE. Effects of fractionated radiation on the brain vasculature in a murine model: blood-brain barrier permeability, astrocyte proliferation, and ultrastructural changes. Int J Radiat Oncol Biol. Phys. 2006;66(3):860–6. 10.1016/j.ijrobp.2006.06.043. [DOI] [PubMed]
- 108.Balasubramanian P, et al. Obesity-induced cognitive impairment in older adults: a microvascular perspective. Am J Physiol Heart Circ Physiol. 2021;320:H740–H761. doi: 10.1152/ajpheart.00736.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ungvari Z, et al. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol. 2021;17:639–654. doi: 10.1038/s41581-021-00430-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Toth P, et al. Age-related autoregulatory dysfunction and cerebromicrovascular injury in mice with angiotensin II-induced hypertension. J. Cereb. Blood Flow Metab. Off J Int Soc Cereb Blood Flow Metab. 2013;33:1732–1742. doi: 10.1038/jcbfm.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tucsek Z, et al. Aging exacerbates obesity-induced cerebromicrovascular rarefaction, neurovascular uncoupling, and cognitive decline in mice. J Gerontol A Biol Sci Med Sci. 2014;69:1339–1352. doi: 10.1093/gerona/glu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fu Z, et al. Age-dependent responses of brain myelin integrity and behavioral performance to radiation in mice. Radiat Res. 2017;188:505–516. doi: 10.1667/RR14732.1. [DOI] [PubMed] [Google Scholar]
- 113.Li J, Xiao L, He D, Luo Y, Sun H. Mechanism of white matter injury and promising therapeutic strategies of MSCs after intracerebral hemorrhage. Front Aging Neurosci. 2021;13:632054. doi: 10.3389/fnagi.2021.632054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hung P-L, Huang C-C, Huang H-M, Tu D-G, Chang Y-C. Thyroxin treatment protects against white matter injury in the immature brain via brain-derived neurotrophic factor. Stroke. 2013;44:2275–2283. doi: 10.1161/STROKEAHA.113.001552. [DOI] [PubMed] [Google Scholar]
- 115.Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89–91. doi: 10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 116.Furuse M, Nonoguchi N, Kawabata S, Miyatake S-I, Kuroiwa T. Delayed brain radiation necrosis: pathological review and new molecular targets for treatment. Med Mol Morphol. 2015;48:183–190. doi: 10.1007/s00795-015-0123-2. [DOI] [PubMed] [Google Scholar]
- 117.Abayomi OK. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 1996;35:659–663. doi: 10.3109/02841869609083995. [DOI] [PubMed] [Google Scholar]
- 118.Ungvari Z, et al. Ionizing radiation promotes the acquisition of a senescence-associated secretory phenotype and impairs angiogenic capacity in cerebromicrovascular endothelial cells: role of increased DNA damage and decreased DNA repair capacity in microvascular radiosensitivity. J Gerontol Ser A. 2013;68:1443–1457. doi: 10.1093/gerona/glt057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Imaizumi N, Monnier Y, Hegi M, Mirimanoff R-O, Rüegg C. Radiotherapy suppresses angiogenesis in mice through TGF-betaRI/ALK5-dependent inhibition of endothelial cell sprouting. PLoS ONE. 2010;5:e11084. doi: 10.1371/journal.pone.0011084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Robbins MEC, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol. 2004;80:251–259. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- 121.Greene-Schloesser D, Moore E, Robbins ME. Molecular pathways: radiation-induced cognitive impairment. Clin. Cancer Res. Off J Am Assoc Cancer Res. 2013;19:2294–2300. doi: 10.1158/1078-0432.CCR-11-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fleckenstein K, et al. Temporal onset of hypoxia and oxidative stress after pulmonary irradiation. Int J Radiat Oncol. 2007;68:196–204. doi: 10.1016/j.ijrobp.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Todorović A, Kasapović J, Pejić S, Stojiljković V, Pajović SB. Differences in antioxidative response of rat hippocampus and cortex after exposure to clinical dose of gamma-rays. Ann N Y Acad Sci. 2005;1048:369–372. doi: 10.1196/annals.1342.041. [DOI] [PubMed] [Google Scholar]
- 124.Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol Zurich Switz. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Peuchen S, et al. Interrelationships between astrocyte function, oxidative stress and antioxidant status within the central nervous system. Prog Neurobiol. 1997;52:261–281. doi: 10.1016/S0301-0082(97)00010-5. [DOI] [PubMed] [Google Scholar]
- 126.Institoris A, et al. Whole brain irradiation in mice causes long-term impairment in astrocytic calcium signaling but preserves astrocyte-astrocyte coupling. GeroScience. 2021;43:197–212. doi: 10.1007/s11357-020-00289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Srinivasan VJ, Mandeville ET, Can A, Blasi F, Climov M, Daneshmand A, et al. Multiparametric, longitudinal optical coherence tomography imaging reveals acute injury and chronic recovery in experimental ischemic stroke. PLoS One. 2013;8(8):e71478. 10.1371/journal.pone.0071478. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.