Abstract
Sodium glucose cotransporter-2 inhibitors (SGLT2is) promote urinary glucose excretion and decrease plasma glucose levels independent of insulin. Canagliflozin (CANA) is an SGLT2i, which is widely prescribed, to reduce cardiovascular complications, and as a second-line therapy after metformin in the treatment of type 2 diabetes mellitus. Despite the robust metabolic benefits, reductions in bone mineral density (BMD) and cortical fractures were reported for CANA-treated subjects. In collaboration with the National Institute on Aging (NIA)–sponsored Interventions Testing Program (ITP), we tested skeletal integrity of UM-HET3 mice fed control (137 mice) or CANA-containing diet (180 ppm, 156 mice) from 7 to 22 months of age. Micro-computed tomography (micro-CT) revealed that CANA treatment caused significant thinning of the femur mid-diaphyseal cortex in both male and female mice, did not affect trabecular bone architecture in the distal femur or the lumbar vertebra-5 in male mice, but was associated with thinning of the trabeculae at the distal femur in CANA-treated female mice. In male mice, CANA treatment is associated with significant reductions in cortical bone volumetric BMD by micro-CT, and by quantitative backscattered scanning electron microscopy. Raman microspectroscopy, taken at the femur mid-diaphyseal posterior cortex, showed significant reductions in the mineral/matrix ratio and an increased carbonate/phosphate ratio in CANA-treated male mice. These data were supported by thermogravimetric assay (TGA) showing significantly decreased mineral and increased carbonate content in CANA-treated male mice. Finally, the sintered remains of TGA were subjected to X-ray diffraction and showed significantly higher fraction of whitlockite, a calcium orthophosphate mineral, which has higher resorbability than hydroxyapatite. Overall, long-term CANA treatment compromised bone morphology and mineral composition of bones, which likely contribute to increased fracture risk seen with this drug.
Keywords: Bone, Micro-CT, BMD, SGLT2i, Canagliflozin
Introduction
Sodium-glucose cotransporters (SGLTs) mediate glucose uptake across apical cell membranes against a concentration gradient, coupled to active transport of sodium. SGLT1 mediates primarily sodium-dependent glucose uptake in the small intestine, while SGLT2 is mainly localized to the apical membrane of the S1/S2 segments of the renal proximal tubules [1–3] and is responsible for more than 90% of glucose reabsorption in the kidneys. Canagliflozin (CANA) is a selective SGLT2 inhibitor (SGLT2i), which is ~160-fold more selective for renal SGLT2 (IC50 4.1 nM) than for its family member SGLT1 (IC50 664 nM), making the renal tubules the primary target organ for CANA.
Due to their glucosuric effects, SGLT2is are now widely used in type-2 diabetes mellitus (T2DM) to reduce blood glucose levels. When given to T2DM patients without chronic kidney disease, SGLT2i therapy was proven effective in decreasing body weight (BW) and blood pressure, and in controlling blood glucose levels, while preserving kidney function [4–7]. However, early clinical studies reported adverse effects on bone, specifically an increased risk of bone fractures, particularly those associated with CANA [6, 8–10]. The majority of patients who presented with cortical bone fractures experienced traumatic falls, which may have resulted from postural hypotension [6]. A smaller fraction of SGLT2i-treated patients presented with a small (1.2%), but significant site-specific (total hip) reduction in bone mineral density (BMD) that is expected to lead to an increase in fracture risk [11]. In the CANVAS study [12], 4.9% of the participants experienced a fracture event during follow-up, of which 49.4% were women. One possible cause for loss of BMD could be attributed to the weight loss following treatment. Indeed, weight loss was shown to correlate with increased collagen type 1 β-carboxy-telopeptide, a measure of bone resorption and could explain 3% of fractures [11]. It was these observations that led the FDA to issue a warning about an increased risk of fractures with SGLT2i treatment. However, it should be noted that the mechanisms underlying bone loss with weight loss therapies are not well delineated, and, in some studies, have been shown to be independent of the magnitude of weight loss.
Acute and chronic toxicity studies in several species, including Sprague Dawley (SD) rats, CD1 mice, dogs, and rabbits [13], identified two key target organs for CANA, kidney and bone. In mice and dogs, there were minimal renal findings, likely due to limited diuresis (kidney pathology was mostly affected in rat studies [13]). However, consistent effects were detected in bone. Four weeks exposure of rats to CANA significantly affected compact bone evidenced by decreases in bone turnover enzymes, lower BMD, and decreased bone strength [14]. The changes in compact bone correlated with decreased body weight (BW). A similar pattern was also observed in dogs. Male dogs that lost weight also had lower femur BMD [15]. Likewise, mechanical strength of L5 lumbar vertebral bone was decreased at all doses in male rats and at a high dose of 100 mg/kg body weight per day in female rats [16]. Notably however, SGLT2 expression was not detected in whole bone, nor in primary cells, nor in cell lines of the osteoblast (OB) or osteoclast (OC) lineages in mice [17] nor in humans [18]. Thus, the effects of SGLT2i on bone are probably non-cell autonomous.
The National Institute on Aging (NIA)–sponsored Interventions Testing Program (ITP) was established to identify therapeutic interventions that slow aging and increase lifespan in genetically diverse UM-HET3 mice. These mice were used in several studies to map genes controlling skeletal morphology [19–22]. A recent report by the ITP shows that CANA (given in food, mixed at a dose of 180 milligrams per kilogram diet (180 ppm)) extended the median survival of male mice by 14%, with no survival effect on female mice [23]. In addition to lowering fasting blood glucose levels and improved glucose tolerance in both male and female mice, CANA treatment was associated with reduced BW and reductions in body adiposity in female mice only.
Here, we report the skeletal phenotype of a cross-sectional cohort of ~280 UM-HET3 mice, treated with CANA from 7 to 22 months of age. Histopathological studies of this specific cohort revealed that CANA treatment diminished the incidence or severity of cardiomyopathy, glomerulonephropathy, arteriosclerosis, hepatic microvesicular cytoplasmic vacuolation (lipidosis), and adrenal cortical neoplasms in male mice only [24]. We dissected bones from these mice and tested 3 skeletal sites: cortical bone (femur mid-diaphysis), trabecular bone of the appendicular skeleton (femur distal metaphysis), and trabecular bone of the axial skeleton (L5 vertebra).
Results
Effects of long-term CANA treatment on bone morphology
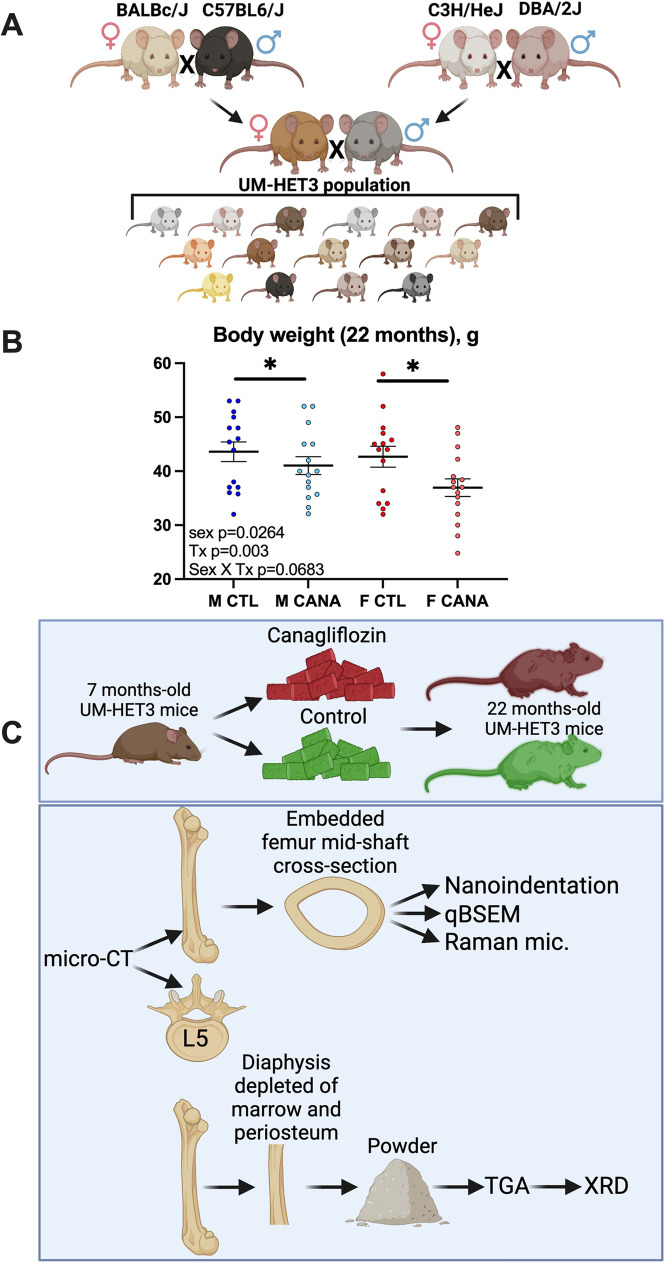
UM-HET3 are genetically diverse mice generated by a 4-way cross of BALBc/ByJ, C57BL6/J, C3H/HeJ, and DBA/2J (Fig. 1A). As was previously reported [23], CANA treatment from 7 to 22 months of age showed significant reductions in body weight (p=0.003), which were more prevalent in female mice but did not show significant interactions between sex and treatment (p=0.0683) (Fig. 1B).
Fig. 1.
Experimental design using the UM-HET3 mice. (A) UM-HET3 crossing strategy. The UM-HET3 mice were generated via a 4-way cross of C3/D2-F1 males and CBy/B6-F1 females resulting in F1 progeny in which each mouse shares 50% of its genetic load with other subjects in the cohort. (B) Body weights were taken at sacrifice from control and CANA-treated mice following 17 months of CANA treatment (from 7 to 22 months of age). M CTL, n = 15 M CANA, n = 15 F CTL, n = 15 F CANA, n = 16. Data tested based on 2 × 2 factorial design, described in methods. Data presented as mean ± SEM. Significance is labeled for treatment effect only (*p < 0.05). (C) Schematic illustration of the analyses performed with femur and vertebra. qBSEM-quantitative backscattered electron microscopy; TGA—thermogravimetric assay; XRD—X-ray diffraction; Raman mic—Raman microspectroscopy
To assess the effects of CANA on skeletal properties, we used a cohort of mice at 22 months of age. Carcasses of CANA-treated and control UM-HET3 mice were obtained from three ITP centers (University of Michigan UM, University of Texas UT, and The Jackson Laboratory JL). Bone specimens (femur and lumbar vertebra-5, L5) were dissected and subjected to several assays including micro-computed tomography (micro-CT), quantitative backscattered electron microscopy (qBSEM), Raman microspectroscopy, nanoindentation, thermogravimetric assay (TGA), and X-ray diffraction (XRD) (Fig. 1C).
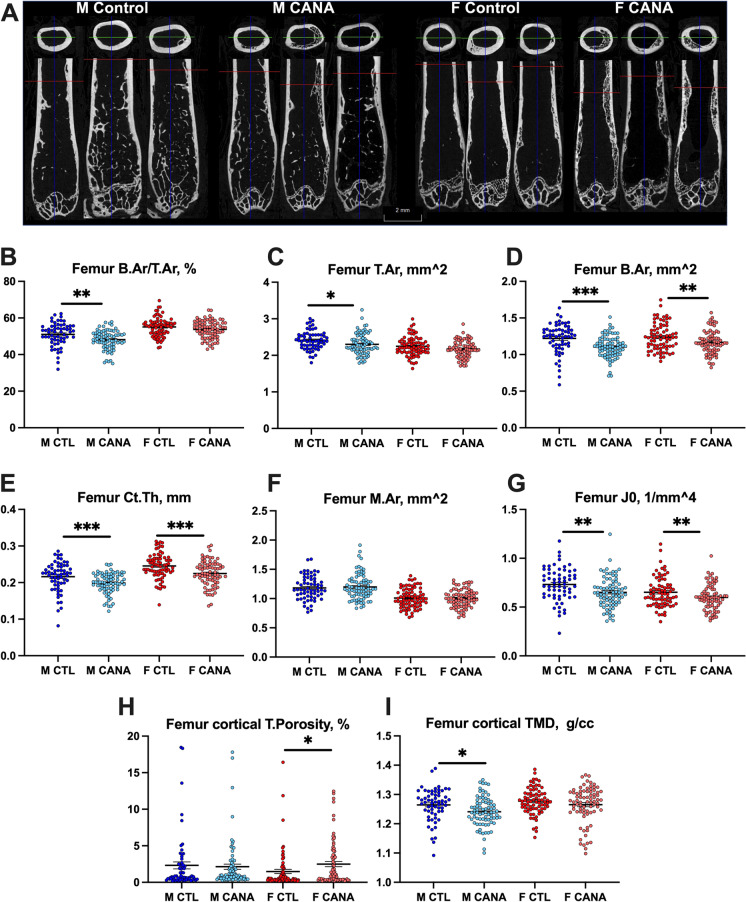
Cortical bone parameters were analyzed in the femur mid-diaphysis, and statistical analyses were performed on samples from each site separately and on the combined samples from all sites (Fig. 2, Table 1). We found that three parameters significantly differed by sex in all sites. These included relative cortical area (calculated as percent of bone area (B.Ar) per total cross-sectional area (T.Ar)), cortical bone thickness (Ct.Th), and marrow area (M.Ar). In all sites, we found that relative cortical area was higher in the female mice compared to male, regardless of treatment. Similarly, M.Ar was significantly larger in male mice as compared to female mice, at all sites, regardless of treatment. Interestingly however, Ct.Th was significantly wider in female compared to male mice, and CANA treatment significantly reduced Ct.Th in both male and female mice in all sites. No significant interaction between sex and treatment was detected for Ct.Th. These data indicate that male and female mice responded similarly to CANA treatment, resulting in thinning of the cortex. T.Ar was reduced in male mice only, while B.Ar was reduced in both sexes treated with CANA; however, no significant interactions were found between sex and treatment for both parameters. Polar moments of inertia (MMI) were greater in male mice relative to female mice, and reduced by CANA treatment in both sexes. Interestingly, we observed increased cortical porosity in femurs of aged female mice treated with CANA. Cortical porosity was analyzed in 3D for each region of interest and included pores that are larger than the typical vascular porosity found in cortical bone. The collective data for cortical bone parameters from all sites (with increased power) yielded significant differences across treatment, as well as significant differences across sex, but there was no differential effect between the two, i.e., treatment effects did not vary by sex (Fig. 2, Table 1). Finally, loss of body weight (BW) is often accompanied by loss of bone mineral density (BMD). Thus, we tested whether treatment, body weight, and sex are predictive of cortical BMD (also known as tissue mineral density, TMD). We found that only sex, but not BW, is a significant predictor of TMD (p=0.048). Furthermore, there was no interaction between treatment and BW at 22 months of age on TMD (p=0.135).
Fig. 2.
Cortical bone morphology in response to long-term CANA administration. Cortical bone parameters were taken at the femur mid-diaphysis using SkyScan 1172 micro-CT at 9.7-um resolution. (A) Representative 2D images of femur from control and CANA-treated mice. Note the cortical porosity/trabeculation at the femur mid-diaphyses of CANA-treated mice. (B) Relative bone area (B.Ar)/total cross-sectional area (T.Ar), (C) T.Ar, (D) B.Ar, (E) cortical bone thickness (Ct.Th), (F) bone marrow area (M.Ar), (G) J0-MMI-polar moment of inertia, (H) cortical bone porosity. (I) Cortical bone tissue mineral density (TMD). M CTL, n = 62; M CANA, n = 79; F CTL, n = 75; F CANA, n = 77. Data presented as mean ± SEM (*p < 0.05, **p < 0.01, ***p < 0.001). Data tested based on 2 × 2 factorial design, described in methods. Significance is labeled for treatment effect only
Table 1.
Cortical bone parameters were taken at the femur mid-diaphysis using SkyScan 1172 micro-CT at 9.7-um resolution. T.Ar total cross-sectional area, B.Ar bone area, Ct.Th cortical bone thickness, M.Ar marrow area, MMI polar moment of inertia, TMD cortical tissue mineral density. Data presented as mean ± SD of pooled samples from all centers, and tested based on 2 × 2 factorial design, described in methods
| Femur mid diaphysis | M CTL (n = 62) |
M CANA (n = 79) |
F CTL (n = 75) |
F CANA (n = 77) |
Adjusted p values | ||
|---|---|---|---|---|---|---|---|
| mean ± SD | mean ± SD | mean ± SD | mean ± SD | Tx | Sex | Tx*Sex | |
| T.Ar, mm^2 | 2.386 ± 0.330 | 2.303 ± 0.286 | 2.247 ± 0.258 | 2.172 ± 0.233 | 0.0182 | < 0.0001 | > 0.1 |
| B.Ar, mm^2 | 1.220 ± 0.200 | 1.105 ± 0.150 | 1.238 ± 0.182 | 1.167 ± 0.160 | < 0.0001 | > 0.1 | > 0.1 |
| Ct.Th, mm | 0.225 ± 0.083 | 0.199 ± 0.028 | 0.246 ± 0.034 | 0.225 ± 0.034 | < 0.0001 | < 0.0001 | > 0.1 |
| M.Ar, mm^2 | 1.166 ± 0.240 | 1.199 ± 0.220 | 1.009 ± 0.165 | 1.005 ± 0.152 | > 0.1 | < 0.0001 | > 0.1 |
| B.Ar/T.Ar, % | 51.538 ± 7.273 | 48.144 ± 5.126 | 55.118 ± 5.116 | 53.758 ± 4.799 | 0.0003 | < 0.0001 | > 0.1 |
| MMI(polar), mm^-4 | 0.732 ± 0.172 | 0.651 ± 0.154 | 0.652 ± 0.152 | 0.599 ± 0.130 | 0.001 | < 0.0001 | > 0.1 |
| TMD, g/cm^3 | 1.257 ± 0.082 | 1.241 ± 0.050 | 1.277 ± 0.044 | 1.265 ± 0.062 | 0.015 | < 0.0001 | > 0.1 |
| Total Porosity, % | 2.324 ± 3.778 | 2.137 ± 3.218 | 1.482 ± 2.573 | 2.484 ± 3.073 | 0.046 | > 0.1 | > 0.1 |
Bolded values significance accepted at p <0.05
Trabecular bone parameters were analyzed in the femur distal metaphysis, and statistical analyses were performed on samples of each site separately and on the combined samples from all sites (Fig. 3, Table 2). Note that at 22 months of age, the trabecular bone compartment at the femur distal metaphysis is almost deleted, so that only large magnitude differences could be detected. Overall, we did not detect treatment effects in that bone compartment. The only consistent change at all sites was trabecular separation (Tb.Sp), which was significantly increased in female as compared to male mice.
Fig. 3.
Trabecular bone morphology in response to long-term CANA administration. Trabecular bone was assessed in the femur distal metaphysis (A–E) and the L5 vertebra (F–J). Trabecular bone parameters included bone volume/total volume (BV/TV), bone mineral density (BMD), trabecular number (Tb.N), and trabecular thickness (Tb.Th). M CTL, n = 62; M CANA, n = 79; F CTL, n = 75; F CANA, n = 76. Data presented as mean ± SEM (*p < 0.05). Data tested based on 2 × 2 factorial design, described in methods. Significance is labeled for treatment effect only
Table 2.
Trabecular bone parameters were taken at the femur distal metaphysis using SkyScan 1172 micro-CT at 9.7-um resolution. BV/TV bone volume/total volume, Tb.Th trabecular thickness, Tb.Sp trabecular spacing, Tb.N trabecular number, BMD bone mineral density. Data presented as mean ± SD of pooled samples from all centers, and tested based on 2 × 2 factorial design, described in methods
| Femur distal metaphysis | M CTL (n = 62) |
M CANA (n = 79) |
F CTL (n = 75) |
F CANA (n = 76) |
Adjusted p values | ||
|---|---|---|---|---|---|---|---|
| mean ± SD | mean ± SD | mean ± SD | mean ± SD | Tx | Sex | Tx*Sex | |
| BV/TV, % | 5.811 ± 4.065 | 5.664 ± 2.918 | 5.982 ± 4.698 | 6.805 ± 6.816 | > 0.1 | > 0.1 | > 0.1 |
| Tb.Th, mm | 0.072 ± 0.009 | 0.069 ± 0.008 | 0.072 ± 0.013 | 0.066 ± 0.008 | < 0.0001 | 0.038 | > 0.1 |
| Tb.Sp, mm | 0.503 ± 0.150 | 0.480 ± 0.149 | 0.657 ± 0.173 | 0.649 ± 0.158 | > 0.1 | < 0.0001 | > 0.1 |
| Tb.N, 1/mm | 0.808 ± 0.571 | 0.826 ± 0.432 | 0.801 ± 0.582 | 0.988 ± 0.926 | > 0.1 | > 0.1 | > 0.1 |
| BMD, g/cm^3 | 0.091 ± 0.043 | 0.084 ± 0.038 | 0.084 ± 0.050 | 0.089 ± 0.063 | > 0.1 | > 0.1 | > 0.1 |
Bolded values significance accepted at p <0.05
To better understand the effects of CANA on trabecular bone, we analyzed the lumbar vertebra-5 (L5) of the axial skeleton (Fig. 3, Table 3). We found significant differences between sexes in all parameters, including bone volume per total volume (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and Tb.Sp. As expected, BV/TV and Tb.N were significantly higher in male as compared to female mice and Tb.Sp was significantly higher in female as compared to male mice, regardless of treatment.
Table 3.
Trabecular bone parameters of the L-5 vertebrae were taken using SkyScan 1172 micro-CT at 7.5-um resolution. BV/TV bone volume/total volume, Tb.Th trabecular thickness, Tb.Sp trabecular spacing, Tb.N trabecular number, BMD bone mineral density. Data presented as mean ± SD of pooled samples from all centers, and tested based on 2 × 2 factorial design, described in methods
| L5 vertebra | M CTL (n = 62) |
M CANA (n = 78) |
F CTL (n = 74) |
F CANA (n = 75) |
Adjusted p values | ||
|---|---|---|---|---|---|---|---|
| mean ± SD | mean ± SD | mean ± SD | mean ± SD | Tx | Sex | Tx*Sex | |
| BV/TV, % | 18.827 ± 6.090 | 14.999 ± 4.560 | 12.757 ± 3.128 | 15.025 ± 5.464 | > 0.1 | 0.038 | > 0.1 |
| Tb.Th, mm | 0.066 ± 0.007 | 0.063 ± 0.008 | 0.074 ± 0.006 | 0.071 ± 0.008 | > 0.1 | > 0.0001 | > 0.1 |
| Tb.Sp, mm | 0.287 ± 0.083 | 0.311 ± 0.088 | 0.450 ± 0.055 | 0.430 ± 0.066 | > 0.1 | > 0.0001 | > 0.1 |
| Tb.N, 1/mm | 2.852 ± 0.938 | 2.403 ± 0.758 | 1.729 ± 0.410 | 2.123 ± 0.723 | > 0.1 | > 0.0001 | > 0.1 |
| BMD, g/cm^3 | 0.433 ± 0.076 | 0.383 ± 0.061 | 0.360 ± 0.049 | 0.393 ± 0.070 | > 0.1 | > 0.1 | 0.088 |
Bolded values significance accepted at p <0.05
Spatial distribution of bone mineral in response to long-term CANA treatment using backscattered surface electron microscopy (qBSEM)
qBSEM was performed on cross-sectioned samples of the femur mid-diaphysis embedded in PMMA (Fig. 4A). Samples were carbon coated prior to scanning. Since we compared the degree of mineralization between groups within the same study, we directly compared grey values between the groups, instead of translating these values to mineral density [25], which requires many assumptions to convert grey values to mineral density [26]. Combined grayscale histograms were plotted for each group in order to compute average pixel distributions (Fig. 4B). We found small, but significantly increased cortical tissue grayscale values (indicative of mineral) in female vs male mice, regardless of treatment (Fig. 4C). Males treated with CANA showed decreased grayscale values as compared to controls, but this did not reach statistical significance.
Fig. 4.
Spatial distribution of cortical bone mineral following long-term CANA treatment. PMMA embedded femur cross-sections were used for qBSEM analyses. (A) Representative qBSEM images converted to indexed color and subject to an 8bin color lookup table (inset: cool colors less dense, warm colors to gray more dense) and (B) representative grayscale histograms from control and CANA-treated male and female mice. (C) Degree of cortical mineral density reported from the grayscale values. M CTL n = 8, M CANA n = 22, F CTL n = 21, F CANA n = 20. (D) PMMA sections that were used for qBSEM were polished and used for Raman microspectroscopy using 785-nm laser beam. (E) Mineral to matrix ratio (MMR) (v1PO4 [950–970 cm−1]/amide I [1660–1690 cm−1]), (F) Carbonate [1050–1070 cm−1] to phosphate ratio (CO32−/PO43−), and (G) mineral crystallinity based on the phosphate peak [950–970 cm−1] were calculated according to Morris et al. [66]. Data presented as mean ± SEM (*p < 0.05, **p < 0.01)
Spatial distribution of bone mineral in response to long-term CANA treatment using Raman microspectroscopy
The predominant mineral component of bone is carbonated hydroxyapatite. To gain more details on bone mineral composition, we used Raman microspectroscopy, which gives a spatial resolution (1 μm) of bone compositional information in phosphate (P) and carbonate (CO3) contents, mineral/matrix ratio (MMR), and mineral crystallinity (Fig. 4D). Spectra were taken at the mid posterior cortex (Fig. 4D). Raman studies of male bones showed that MMR, which is one of the strongest predictors of bone mechanical properties [27], was significantly lower (p=0.043) in CANA-treated mice (13.68±0.28) as compared to controls (14.7±0.44) (Fig. 4E). We found a significantly higher (p=0.0091) CO32−/PO43− ratio in CANA-treated male mice (0.0774±0.002) as compared to controls (0.0866±0.002) (Fig. 4F).
Thermogravimetric analysis (TGA)
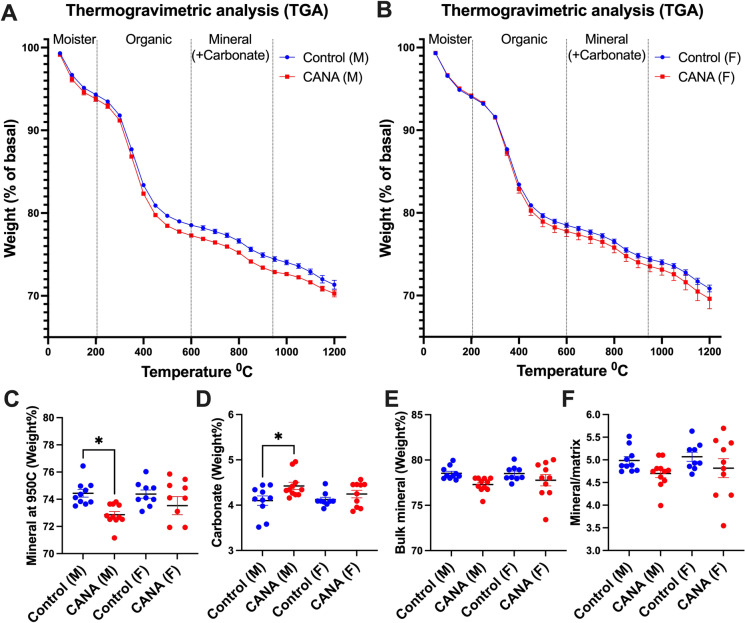
To complement the above structural techniques (Raman, qBSEM) used to study special bone tissue mineral composition, we used TGA, which quantifies bulk mineral and organic contents, including carbonated mineral and non-collagenous proteins (Fig. 5). The weight loss between ~20 and 200°C is due to moisture acquired during sample preparation. Weight loss between 200 and 600°C represents the organic content of bone and did not differ between control (15.61±0.33 wt%) and CANA-treated (15.87±0.41 wt%) male mice. Mineral content at 950°C was significantly (p=0.005) decreased in CANA-treated male mice (72.75±0.23 wt%) as compared to controls (74.74±0.55 wt%). Carbonate levels in the mineral crystal lattice vary between 2 and 7.4 wt%, depending largely on age [28, 29]. We found that carbonate content (calculated as weight loss between 600 and 950°C) significantly (p=0.021) increased in CANA-treated male mice (4.46±0.14 wt%) as compared to controls (3.89±0.16 wt%). Bulk mineral (calculated as wt% at 950°C summed with carbonate content) also showed significant (p=0.014) decrease in CANA-treated male mice (77.22±0.28 wt%) as compared to controls (78.63±0.42 wt%). Mineral to matrix ratio was decreased in CANA-treated male as compared to control mice, but this did not reach significance. TGA parameters did not differ significantly between control and CANA-treated female mice.
Fig. 5.
Cortical bone mineral content following long-term CANA treatment by TGA analyses. TGA profiles of femur shaft powder (10–15 mg) bones heated from ambient temperature (37 °C) to 1200 °C in a platinum crucible at a constant rate of 10 °C/min in controlled air atmosphere from male (A) and female (B) mice. Changes in mass were used to estimate (C) mineral content, (D) carbonate, (E) bulk mineral content including carbonate, and (F) mineral to matrix ratio. M CTL n = 10, M CANA n = 11, F CTL n = 9, F CANA n = 10. Data presented as mean ± SEM (*p < 0.05)
Powder X-ray diffraction (XRD)
Following TGA (heated to 1200°C), the sintered remains were subjected to XRD. Two main minerals were identified by XRD: hydroxyapatite (HA) and Whitlockite (WH). We found that the ratio between HA and WH was ~3:1 in femur diaphysis from control male mice, while HA/WH ratio was ~3:2 in femur diaphysis from CANA-treated male mice. HA/WH ratio in female mice was ~3:1, regardless of treatment (Fig. 6). Taken together, we noted significant changes in mineral composition with CANA that may contribute to structural changes and enhanced skeletal fragility.
Fig. 6.
Cortical bone mineral composition following long-term CANA treatment by XRD analyses. (A) XRD profiles of femur mid-diaphysis powder heated to 1200 °C from control and CANA-treated male mice. XRD scans were collected at 45 kV and 40 mA with a step size of 0.01° and 52 s/step and a 2-theta range from 20 to 80°. Spectra were analyzed to identify peaks corresponding to hydroxyapatite (HA) (traced using HA reference code: 01–074-0566) and calcium phosphate (traced using β-TCP (reference code: 01–070-2065). Pie graphs represent the ratio of HA:WH (Whitlockite). (B) Relative fraction of HA in the bone mineral. (C) Mineral crystal size along the c-axis was calculated from full width at half max (FWHM) of the 26° peak, which corresponds to the (0 0 2) c-axis and is the only peak that does not exhibit overlapping in the XRD spectra. Data presented as mean ± SEM. M CTL n = 3, M CANA n = 4, F CTL n = 5, F CANA n = 4
Effects of long-term CANA treatment on nano-mechanical properties of cortical bone
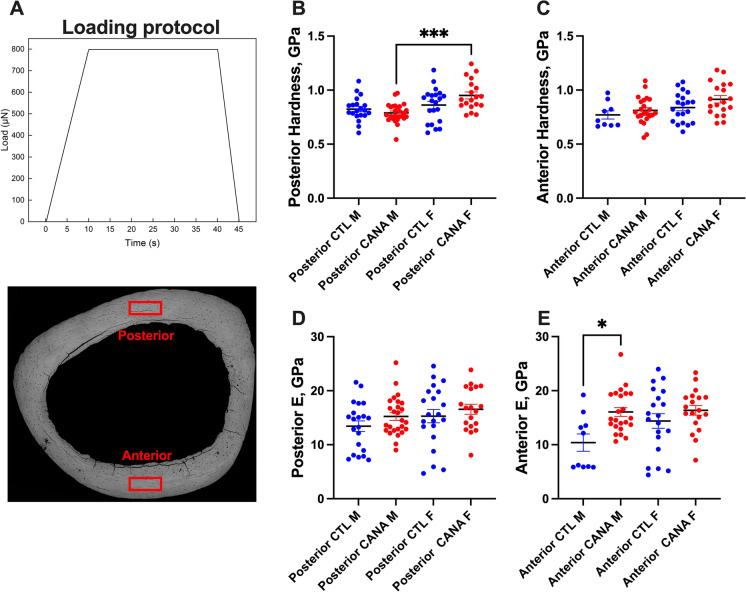
To gain data on bone tissue elastic properties, we applied nanoindentation, which allows single-point physical measurement of tissue elastic modulus (E) at the nanometer scale. This method has been used previously for studies of cortical bone of inbred mice [30, 31]. Nanoindentation was performed on cross-sectional samples of the femur mid-diaphysis as in [32]. We used repolished cross-sections analyzed by qBSEM and addressed the nano-mechanical properties at the posterior quadrant of the cortex. Eighteen indents were made in each specimen and viscoelastic analysis was used to evaluate elastic properties [32]. We found no differences in E between the anterior and posterior quadrants of the same animal. No significant differences in E (Fig. 7A) or tissue hardness (Fig. 7B) were detected with treatment in male or female mice.
Fig. 7.
Nano-mechanical properties of cortical bone following long-term CANA treatment. Nanoindentation was performed on mid-diaphyseal cross-sections of the PMMA-embedded femurs at the anterior and posterior quadrants using a Hysitron TI 950 system (Hysitron, Minneapolis, MN, USA). (A) A trapezoidal loading protocol was applied longitudinally to a maximum load of 8 mN with a rising time of 10 s and a holding of 30 s as in [32]. Eighteen indents were made in each specimen with a minimum spacing of 15 μm between indents. Viscoelastic analysis was used to evaluate elastic properties [32]. Bone tissue hardness measured at the (B) posterior (M CTL n = 20, M CANA n = 26, F CTL n = 21, F CANA n = 19) and (C) anterior quadrants (M CTL n = 9, M CANA n = 23, F CTL n = 20, F CANA, n = 19) of the cross-sections. Bone tissue elastic modulus (E) measured at the (D) posterior (M CTL n = 21, M CANA n = 26, F CTL n = 21, F CANA n = 19) or (E) anterior quadrants (M CTL n = 10, M CANA n = 23, F CTL n = 20, F CANA, n = 19) of the cross-sections. Data presented as mean ± SEM (*p < 0.05, **p < 0.01, ***p < 0.001)
Discussion
The effects of long-term CANA treatment on the skeleton of non-diabetic adult subjects are a matter of debate. Clinical use of canagliflozin (CANA), which exceeded 1.3 million prescriptions between the years 2013 and 2019, has markedly increased recently as a result of widespread acceptance of its use as a second-line therapy after metformin in the treatment of T2DM. The CREDENCE trial [33–35], in which CANA reduced the risk of hospitalization for heart failure or cardiovascular (CV) death by 31%, led to further increase in CANA use in both diabetic and non-diabetic patients. However, a recent meta-analysis including 78 randomized controlled trials with >85,000 patients indicated that the use of CANA, but not dapagliflozin or empagliflozin, increased fracture risk by 17%. Importantly, while short-term (<52 weeks) use of CANA decreased fracture risk, long-term (>52 weeks) use was associated with an increased risk for fractures [36]. Experiments described here using genetically diverse healthy adult mice describe, for the first time, the effects of long-term CANA treatment (17 months of treatment) on skeletal integrity. Our findings indicate that long-term CANA treatment affected mostly compact, cortical bone of the femur diaphysis, with little to no effects on the trabecular bone compartment. Skeletal changes included thinning of the cortical cortex in both male and female mice treated with CANA, changes that are expected to impair whole-bone mechanical properties, which largely depend on cortical morphology. Multiple analyses revealed that over time CANA treatment led to bone mineral loss specifically in males. The cause for this sexual dimorphism is unclear, but was also shown in studies reporting body composition [23] and cognitive function [37]. Toxicology studies have indicated that repeated administration of CANA in female dogs increased drug exposure by 2.5-fold [13], and was also reported for female mice and rats [38], suggesting drug accumulation in tissues that may be sex-dependent. However, more detailed pharmacokinetic studies are needed to test that possibility. Other possible explanations for the sexual dimorphic effects of CANA on bone metabolism may rise from negative side effects of the drug in the males or alterations in specific pathways that modulate bone turnover [39]. Finally, CANA effects on BMD in male mice may be indirect via enhanced weight loss (as compared to CANA-treated female mice). Weight loss significantly associates with increases in the serum bone turnover markers osteocalcin and CTX. In overweight and obese adults, weight loss is associated with decrease in total hip BMD but not spinal BMD [40]. In patients with T2D treated with CANA, approximately 40% of the reduction in total hip BMD was associated with weight loss [11]. Reductions in body weight are associated with modulations in adiponectin and leptin, which are produced by adipose tissues, and affect bone cells [41, 42]. However, a few studies suggested that decreased body weight regulates bone turnover independent of leptin [43]. The scientific query of how CANA treatment affects bone turnover, and whether the loss of BMD with treatment is sex specific, is yet to be answered.
The hormonal or cellular mechanisms responsible for mineral loss following CANA treatment were not addressed in this cross-sectional study. In a study of streptozotocin-induced hyperglycemia in male DBA/2J mice, CANA treatment for 10 weeks caused decreased in femur cortical thickness, increased cortical porosity, and impaired mechanical properties that were associated with increased parathyroid hormone (PTH) and bone resorption markers in diabetic, but not normoglycemic mice [44]. In a consequent study, the authors reported that CANA treatment of control mice imparted secondary effects on bone turnover, evidenced by decreased C-terminal telopeptides of type I collagen (a bone resorption marker), increased osteoid surface, and increased urine calcium-creatinine ratio [17]. Using the polygenic TallyHO mice (a model of early onset T2DM), CANA treatment normalized blood glucose but did not improve urine mineral wasting or bone deficits [45]. These reports indicate secondary effects of CANA treatment on bone tissue homeostasis. Importantly, SGLT2 expression was not detected in whole bone, nor in primary cells or cell lines of the osteoblast (OB) or osteoclast (OC) lineages in mice [17] or in humans [18], suggesting that the effects of CANA on bone are non-cell autonomous. Several possible mechanisms may underly the overall reduced BMD in response to CANA treatment, including reductions in body weight seen in rodents [16, 46, 47] even when fed HFD [48], and in patients with or without T2DM [49, 50]. The use of SGLT2i leads to adaptive changes in whole-body metabolism including glucose utilization, hormonal responses (insulin/glucagon ratio) [51], substrate selection (lipid vs carbohydrates) [52–55], and probably energy expenditure, all which have indirect effects on bone metabolism. Impaired calcium metabolism following CANA treatment can also contribute to decreased BMD. In rats, a single dose of CANA increased urine calcium excretion, which was attributed to increased gut calcium absorption (as determined by radio-labeled calcium) [16]. Interestingly, in humans, short-term CANA treatment (300 mg, for 5 days) led to a 16% increase in serum phosphorus (Pi) levels, a 20% increase in plasma FGF23 along with a 25% increase in PTH, and a 10% decrease in plasma 1,25OHD, all of which could affect bone integrity and increase fracture risk [56]. However, reports of 1–2 years of therapy [57] were unable to show association between Ca2+/Pi, PTH, 1,25OHD, and CANA treatment. Our study addressed the effects of long-term CANA treatment in non-diabetic UM-HET3 mice. It is possible that the skeletal response to CANA in diabetic mice will differ from that of non-diabetic UM-HET3 mice.
Changes in bone mineral content and composition in the current study were detected using multiple methods. Micro-CT analyses revealed that CANA treatment and sex had significant effects on cortical TMD (without significant interaction, Table 1) in males. These findings were in agreement with those obtained by qBSEM. We found that grayscale histograms were significantly higher in female as compared to male mice, and that CANA-treated males showed reduced cortical tissue grayscale values as compared to control males, but this did not reach significance. Accordingly, Raman microspectroscopy revealed that mineral to matrix ratio in the posterior cortex of CANA-treated mice was significantly reduced, while carbonate/phosphate ratio was increased. These ratios are often used to assess bone mineral quality and correlate positively with bone fracture risk in human and animal models [58–66]. Carbonate in the bone mineral modifies apatite crystallinity, and increases the solubility of the apatite crystal. Our Raman data were in agreement with the TGA data from the femur diaphysis. We found significantly reduced mineral with increased carbonate content in femurs of CANA-treated male mice. These data were further supported by XRD. HA comprises ~70–75% of the bone minerals, and is the most stable calcium-phosphate compound under physiological conditions, i.e., at temperature, pH, and composition of the body fluids [67]. WH is the second most abundant mineral in bone, comprising approximately 20–35% of the total mineral weight. WH is a calcium orthophosphate crystal, Ca9Mg(HPO4)(PO4)6, with an unusual form of calcium phosphate that has an intermediate resorbability compared to HA and β-tricalcium phosphate (TCP) [68]. We found increased proportions of WH in the mineral from CANA-treated male bones as compared to controls, which would be expected to contribute to decreased bone tissue quality and mechanical properties.
Finally, we studied the nano-mechanical properties of the posterior cortical bone tissue (of the femur mid-diaphysis) in male and female mice. No significant differences in tissue elastic modulus or tissue hardness were found with CANA treatment. It is generally accepted that bone density correlates with elastic modulus. However, this is not always true in pathologic bones. Decreased tissue elastic modulus was reported in inbred mice with brittle (oim−/− osteogenesis imperfecta model with increased tissue mineralization) or ductile bones (Phospho1−/− model with decreased tissue mineral content) [32], suggesting that in addition to the mineral composition and crystal size, collagen content, architecture, and cross-links affect tissue properties. Studies are ongoing to better understand the short- and long-term effects of CANA on mouse bone tissue properties.
Our study had a few limitations that were inherent due to the preservation of the bone specimens (fixed >48 h), available from the ITP centers following CANA treatment, which led us analyzing dry tissue that may affect the nano-mechanical and Raman properties of the bone tissue. Additionally, the skeletal specimens were of mice at one age, preventing us from studying longitudinal effects of CANA treatment over time. We were not able to measure bone resorption or formation markers in serum, due to limited sample availability. Finally, we did not compare CANA to other SGLT2is that may elicit a different effect on bone. However, we unraveled significant information on the long-term effects of CANA on skeletal integrity. We showed that CANA treatment mainly altered mainly compact bone morphology in both sexes causing thinning of the cortex of long bones, which is expected to compromise whole bone mechanical properties. CANA minimally affected the trabecular bone compartment, where significant changes were only seen in trabecular thickness. Interestingly, we found sexually dimorphic effects of CANA treatment on bone mineral content and composition. While male mice treated with CANA showed overall decreased mineral content by micro-CT, qBSEM, and TGA, mineral content or composition in female mice treated with CANA did not differ from that of control female mice. Further studies are needed to understand how CANA treatment affects osteogenesis during aging.
Methods
Animals
UM-HET3 are genetically heterogeneous mice, the F1 offspring of CByB6F1 female (JAX stock #100009) mated with C3D2F1 male (JAX stock #100004) mice. Each UM-HET3 mouse is genetically unique, but shares 50% of its genetic material with every other UM-HET3 mouse. UM-HET3 mice were weaned to same-sex cages containing either 3 male or 4 female mice, and assigned for control or CANA treatment using a random number table.
All animal experiments were approved by the IACUC of JAX, UM, and UT.
Diet
Mice were fed CANA-containing chow at 180 mg/kg chow, from 7 to 22 months of age. This dose is equivalent to 30 mg/kg mouse body weight for a 30-g mouse eating 5 g of food each day.
Data acquisition
When reached 22 months of age, mice were sacrificed and fixed in 10% buffered formalin. Researchers that dissected, scanned, and analyzed the bones were blinded to sample IDs. Sample size for each assay is reported in tables and figures.
Micro-computed tomography (micro-CT)
All bone specimens were scanned using the same instrument under the same conditions. Micro-CT was done in accordance with the American Society for Bone and Mineral Research (ASBMR) guidelines [69]. Bones were scanned using a high-resolution SkyScan micro-CT system (SkyScan 1172, Kontich, Belgium) with 10-MP digital detector, 10W of energy (70 kV and 142 mA), and a pixel size of 9.7microns, exposure 850 ms/frame rotation step 0.3 degrees with ×10 frame averaging, 0.5-mm aluminum filter (to increase the transmission) and scan rotation was 180 degrees. All samples were scanned in the air. Global thresholding was applied to all datasets prior to morphometric analysis. Image reconstruction was done with NRecon using GPU acceleration (version 1.7.3.0; Bruker micro-CT, Kontich, Belgium). Gaussian smoothing with a 2voxel radius, ring artifact, and beam hardening corrections were applied during reconstruction. Ring artifact reduction was set to 7 pixels. Beam hardening correction was set to 40%. The volume of interest (VOI) was selected from the reconstructed μCT image stacks. Femur cortical bone was analyzed in the mid-diaphysis. Trabecular bone measurements were taken at the femur distal metaphysis and the 5th lumbar vertebra (L5). Structural analysis was performed using the CTAn software (version 1.17.7.2+; Bruker micro-CT). 3D images were constructed using CT Vox software (version 3.3.0 r1403; Bruker micro-CT, Kontich, Belgium). The scan of hydroxyapatite phantoms was incorporated into the analysis. Calibration phantoms (CaHA of 0.25 and 0.75 g.cm-3, Buker) were scanned and reconstructed at the same settings used for bone samples. The BMD/TMD calibration against attenuation coefficient was completed in CTAn analyzer. Porosity analysis was done in 3D within the region of interest, and we activated the “Additional Values” in the task list using CTAn Analyser. Porosity analysis is contained in the final check box for the number of objects. We measured the total porosity (%) as the volume of all open plus closed pores as a percent of the total VOI.
Quantitative backscattered surface electron microscopy (qBSEM)
Fixed femur diaphyseal segments were dehydrated and embedded undecalcified in poly-methyl methacrylate (PMMA). Cross-sections (200 µm) were cut, adhered to plastic slides, and polished on a Buehler Metaserv 250 (Lake Bluff, IL) to a 1-µm surface finish. Sections were carbon coated, and imaged at high vacuum by backscattered electron imaging in the scanning electron microscope (BSE-SEM) with a Zeiss Gemini 300 field emission-scanning electron microscope (White Plains, NY) operated by Zeiss SmartSEM software (v. 6.09). Digital grey-level BSE-SEM images were collected at an 8.5-mm working distance, 10-kV accelerating voltage, and a ca. 300-pA beam current at 487 nm/pixel resolution. Zeiss Atlas 5 software (v. 5.3.3.9) was used to provide automated image acquisition of each bone block and tiling of BSE-SEM images for about 12 blocks in one preprogrammed imaging run. The electron beam was turned on at least 1 h prior to automated montaging, allowing the gun head to warm up and for the beam to stabilize. Using halogenated dimethacrylate standards provided by A. Boyde, Queen Mary, University of London, closely bracketing the densities of bone, beam conditions, and stability of the electron beam were established and monitored. To permit quantitative BSE-SEM comparisons between all bones within and between imaging runs, we employed the standards using a method similar to that of Goldman [70]. The mean of the histogram peak for each standard was determined by adjustments of brightness and contrast, such that a grey level value of 5 was assigned to the mean of the first iodo standard [71] and a value of 250 was assigned to that of the second iodo standard [72]. The 5/250 grey level peaks were established at the beginning of the imaging run, and once again checked at the end of the automated run. In all cases, the beam either did not drift from 5/250 conditions, or drift did not exceed ±1 grey level from one or both of the initial 5/250 values.
Raman microspectroscopy
Fixed femur diaphyseal segments were dehydrated and embedded undecalcified in poly-methyl methacrylate (PMMA). Cross-sections (200 µm) were cut, adhered to plastic slides, surface polished, and used for Raman microspectroscopy. Spectra were collected using Thermo-Fisher DXR2 confocal Raman microscope. We used laser (at 20mW) with 785-nm wavelength excitation at 50X/0.75 BD objective, 50-µm entrance slit provided a spectral resolution of 50–3300 cm−1 and a 0.96-µm diameter spot size, as in our previous studies [73]. Data was acquired (collection exposure time of 4 s, with 40 exposures) from the middle of the posterior cortex (starting 20-µm distance from the periosteum and ending 20-µm distance from the endosteum). Approximately 20 spectra from 4 spots were averaged per sample (after fluorescence background substruction). Analysis was performed using OMNIC spectroscopy software (Omnic for dispersive Raman version 9.6), in accordance with Morris et al. [66]. Our principal focus was on the following: (1) mineral to matrix (amide I) ratio (n1PO4 [950–970] peak/amide I [1660–1690] collagen peak), (2) carbonate to phosphate ratio, ([1050–1070] and the [950–970] peaks, respectively), and (3) crystallinity that was calculated as the inverse of the width of the phosphate symmetric stretch band at half the maximum intensity value (full width at half maximum, crystallinity=1/FWHM). Bands were fitted with Gaussian curve and integrated area ratio was used for calculations.
Nanoindentation
Nanoindentation was performed as in [32] on femur mid-diaphyseal sections embedded in PMMA, in dry conditions, using a nanoindenter (Hysitron TI 950, Minneapolis, MN) equipped with a Berkovich diamond 3-sided pyramid probe. A loading profile with peak load of 8mN at a rate of 0.8mN/s was applied, with a holding period of 30 s. Nine indents with a minimum spacing of 15µm between indents were made in each location including the posterior and anterior quadrants of the cortex (Fig. 7). Tissue E and hardness were calculated using the TriboScan software from slopes that yielded the reduced modulus (Er) and hardness (H). Data were analyzed using two-way analysis of variance and Tukey tests in SPSS (Version 26.0; IBM Corp, Armonk, NY).
Thermogravimetric analysis (TGA)
TGA was performed as in [32], using TGA Q600 Series system (TA instruments, New Castel, DE). Femur shafts were dried in 60°C oven overnight and powderized in 2-ml metal lysing tubes (MP Biomedicals LLC, Cat#116991006) using TissueLyser II Bead Mill (Qiagen, cat#44213). Femur shaft dried powder (10–15mg) was heated from ambient temperature (37°C) to 1200°C in a platinum crucible at a constant rate of 10°C/min in controlled air atmosphere. Changes in mass up to 200°C were considered as loss of water, changes between 200 and 600°C were considered as loss of organic matter, and changes between 600 and 950°C were considered as loss of carbonate. Mass left after heating to 1200°C were subsequently studied by X-ray diffraction.
X-ray diffraction of bone powder (XRD)
The sintered products of TGA 1200°C that represent mainly hydroxyapatite (HA) and tricalcium phosphate (TCP) were identified by XRD (X’pert powder Panalytical, Almelo, Netherlands). XRD scans were collected at 45 kV and 40mA with a step size of 0.01° and 52 s/step and a 2-theta range from 20 to 80°. Spectra were analyzed to identify peaks corresponding to hydroxyapatite (HA) (traced using HA; reference code: 01-074-0566) and calcium phosphate (traced using β-TCP; reference code: 01-070-2065).
Statistical analysis
Descriptive statistics for this 2×2 factorial design were computed overall as well as stratified by site and treatment. Study outcomes were first analyzed using nonparametric multivariate analysis of variance with permutation tests (10,000 permutations per outcome). Significant multivariate results were subsequently followed up with two-factor aligned rank transformation analysis of variance with factors for sex, treatment, and the sex*treatment interaction. The false discovery rate for each F-test was controlled for using the method of Benjamini and Hochberg [74]. Finally, p-values from post hoc tests for each significant factor were adjusted using the Bonferroni method. Separately, the relationship between cortical BMD and treatment, body weight, sex, and the treatment-body weight interaction were modeled using linear regression. Analysis was conducted in R v4.1.3.
Author contribution
Conceptualization: Investigators of the ITP program RAM, RS, DEH, WL. Funding acquisition: SY. Formal analyses: SY, CJR. Investigation: GY, ETPB, SBP, MD, BH, DM, LW, CC, TGB. Statistical analyses: RRR. Resources: ITP centers (UM, UT, JL). Writing original draft: SY. Writing review and editing: SY, CJR.
Funding
Financial support is received from the National Institutes of Health Grant R01AG056397 to SY, U54GM115516 to CJR, U01-AG022303 to RAM, UO1-AG022308 to DEH, and U01-AG013319 to RS; RS is supported by a Senior Research Career Scientist Award from the Department of Veterans Affairs Office of Research and Development, S10 OD010751-01A1 for micro-computed tomography, S10 OD026989 for Zeiss Gemini 300 FE-SEM.
Data availability
The datasets generated and analyzed during the current study are available upon request. Our studies do not include the use of custom code or mathematical algorithms. We have included citations for available data in the references section.
Declarations
Ethics approval
The project was reviewed and approved by the Institutional Animal Care and Use committees at the University of Michigan, University of Texas Health Science Center at San Antonio, and The Jackson Laboratory.
Conflict of interest
The authors declare no competing interests. All authors have discussed the results and approved the final version of the manuscript. SY is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All authors concur with the submission. The material submitted for publication has not been previously reported and is not under consideration for publication elsewhere.
Gozde Yildirim, Edmara T. P. Bergamo, and Sher Bahadur Poudel contributed equally to this work.
Contributor Information
Gozde Yildirim, Email: yildirimgina@gmail.com.
Edmara T. P. Bergamo, Email: edmaratatiely@gmail.com
Sher Bahadur Poudel, Email: sbp4@nyu.edu.
Ryan R. Ruff, Email: ryan.ruff@nyu.edu
Manisha Dixit, Email: dixitm01@nyu.edu.
Bin Hu, Email: bin.hu@nyu.edu.
Dindo Q. Mijares, Email: dqm1@nyu.edu
Lukasz Witek, Email: Lukasz.witek@nyu.edu.
Carolyn Chlebek, Email: carolyn.chlebek@mainehealth.org.
David E. Harrison, Email: david.harrison@jax.org
Randy Strong, Email: STRONG@uthscsa.edu.
Richard A. Miller, Email: millerr@umich.edu
Warren Ladiges, Email: wladiges@uw.edu.
Timothy G. Bromage, Email: tim.bromage@nyu.edu
Clifford J. Rosen, Email: cjrofen@gmail.com
Shoshana Yakar, Email: sy1007@nyu.edu.
References
- 1.Zhao FQ, et al. Cloning and expression of bovine sodium/glucose cotransporter SGLT2. J Dairy Sci. 2005;88(8):2738–2748. doi: 10.3168/jds.S0022-0302(05)72953-2. [DOI] [PubMed] [Google Scholar]
- 2.Vrhovac I, et al. Localizations of Na(+)-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch. 2015;467(9):1881–1898. doi: 10.1007/s00424-014-1619-7. [DOI] [PubMed] [Google Scholar]
- 3.Sabolic I, et al. Expression of Na+-D-glucose cotransporter SGLT2 in rodents is kidney-specific and exhibits sex and species differences. Am J Physiol Cell Physiol. 2012;302(8):C1174–C1188. doi: 10.1152/ajpcell.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perkovic V, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 5.Wiviott SD, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 6.Watts NB, et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2016;101(1):157–166. doi: 10.1210/jc.2015-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zinman B, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 8.Fralick M, et al. Fracture risk after initiation of use of canagliflozin: a cohort study. Ann Intern Med. 2019;170(3):155–163. doi: 10.7326/M18-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor SI, Blau JE, Rother KI. Possible adverse effects of SGLT2 inhibitors on bone. Lancet Diabetes Endocrinol. 2015;3(1):8–10. doi: 10.1016/S2213-8587(14)70227-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohan DE, et al. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85(4):962–971. doi: 10.1038/ki.2013.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bilezikian JP, et al. Evaluation of bone mineral density and bone biomarkers in patients with type 2 diabetes treated with canagliflozin. J Clin Endocrinol Metab. 2016;101(1):44–51. doi: 10.1210/jc.2015-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Z, et al. Canagliflozin and fracture risk in individuals with type 2 diabetes: results from the CANVAS Program. Diabetologia. 2019;62(10):1854–1867. doi: 10.1007/s00125-019-4955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mamidi RN, et al. Metabolism and excretion of canagliflozin in mice, rats, dogs, and humans. Drug Metab Dispos. 2014;42(5):903–916. doi: 10.1124/dmd.113.056440. [DOI] [PubMed] [Google Scholar]
- 14.Londzin P, et al. Unfavorable effects of sodium-glucose cotransporter 2 (SGLT2) inhibitors on the skeletal system of nondiabetic rats. Biomed Pharmacother. 2022;155:113679. doi: 10.1016/j.biopha.2022.113679. [DOI] [PubMed] [Google Scholar]
- 15.FDA, U.S., U.S. Food and Drug Administration, Center for Drug Evaluation and Research. Pharmacology/Toxicology Review(s) of Canagliflozin NDA 204042 for the treatment of type 2 diabetes submitted by Janssen Pharmaceuticals Inc. on 31 May 2014, Pharmacology/Toxicology NDA Review and Evaluation. 264 pgs. 2014.
- 16.De Jonghe S, et al. Carcinogenicity in rats of the SGLT2 inhibitor canagliflozin. Chem Biol Interact. 2014;224:1–12. doi: 10.1016/j.cbi.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 17.Thrailkill KM, et al. The impact of SGLT2 inhibitors, compared with insulin, on diabetic bone disease in a mouse model of type 1 diabetes. Bone. 2017;94:141–151. doi: 10.1016/j.bone.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab Pharmacokinet. 2005;20(6):452–477. doi: 10.2133/dmpk.20.452. [DOI] [PubMed] [Google Scholar]
- 19.Miller RA, et al. Preservation of femoral bone thickness in middle age predicts survival in genetically heterogeneous mice. Aging Cell. 2011;10(3):383–391. doi: 10.1111/j.1474-9726.2011.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reeves GM, et al. Quantitative trait loci modulate vertebral morphology and mechanical properties in a population of 18-month-old genetically heterogeneous mice. Bone. 2007;40(2):433–443. doi: 10.1016/j.bone.2006.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volkman SK, et al. Quantitative trait loci that modulate femoral mechanical properties in a genetically heterogeneous mouse population. J Bone Miner Res. 2004;19(9):1497–1505. doi: 10.1359/JBMR.040506. [DOI] [PubMed] [Google Scholar]
- 22.Volkman SK, et al. Quantitative trait loci for femoral size and shape in a genetically heterogeneous mouse population. J Bone Miner Res. 2003;18(8):1497–1505. doi: 10.1359/jbmr.2003.18.8.1497. [DOI] [PubMed] [Google Scholar]
- 23.Miller RA, et al. Canagliflozin extends life span in genetically heterogeneous male but not female mice. JCI Insight. 2020;5(21):e140019. 10.1172/jci.insight.140019. [DOI] [PMC free article] [PubMed]
- 24.Snyder JM, et al. Canagliflozin retards age-related lesions in heart, kidney, liver, and adrenal gland in genetically heterogenous male mice. Geroscience. 2023;45(1):385–397. doi: 10.1007/s11357-022-00641-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyde A, et al. Mineral density quantitation of the human cortical iliac crest by backscattered electron image analysis: variations with age, sex, and degree of osteoarthritis. Bone. 1995;16(6):619–627. doi: 10.1016/8756-3282(95)00119-X. [DOI] [PubMed] [Google Scholar]
- 26.Vanleene M, et al. Ultra-structural defects cause low bone matrix stiffness despite high mineralization in osteogenesis imperfecta mice. Bone. 2012;50(6):1317–1323. doi: 10.1016/j.bone.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris MD, Mandair GS. Raman assessment of bone quality. Clin Orthop Relat Res. 2011;469(8):2160–2169. doi: 10.1007/s11999-010-1692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao S, et al. The preparation and characteristics of a carbonated hydroxyapatite/collagen composite at room temperature. J Biomed Mater Res B Appl Biomater. 2005;74(2):817–821. doi: 10.1002/jbm.b.30315. [DOI] [PubMed] [Google Scholar]
- 29.Meneghini C, et al. Rietveld refinement on x-ray diffraction patterns of bioapatite in human fetal bones. Biophys J. 2003;84(3):2021–2029. doi: 10.1016/S0006-3495(03)75010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silva MJ, et al. Nanoindentation and whole-bone bending estimates of material properties in bones from the senescence accelerated mouse SAMP6. J Biomech. 2004;37(11):1639–1646. doi: 10.1016/j.jbiomech.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 31.Chen Q, et al. Congenital lack of COX-2 affects mechanical and geometric properties of bone in mice. Calcif Tissue Int. 2003;73(4):387–392. doi: 10.1007/s00223-002-0009-x. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Florez N, et al. An investigation of the mineral in ductile and brittle cortical mouse bone. J Bone Miner Res. 2015;30(5):786–795. doi: 10.1002/jbmr.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarraju A, et al. Effects of canagliflozin on cardiovascular, renal, and safety outcomes in participants with type 2 diabetes and chronic kidney disease according to history of heart failure: Results from the CREDENCE trial. Am Heart J. 2021;233:141–148. doi: 10.1016/j.ahj.2020.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Bakris G, et al. Effects of canagliflozin in patients with baseline eGFR <30 ml/min per 1.73 m(2): subgroup analysis of the randomized CREDENCE trial. Clin J Am Soc Nephrol. 2020;15(12):1705–1714. [DOI] [PMC free article] [PubMed]
- 35.Jardine MJ, et al. Renal, cardiovascular, and safety outcomes of canagliflozin by baseline kidney function: a secondary analysis of the CREDENCE randomized trial. J Am Soc Nephrol. 2020;31(5):1128–1139. doi: 10.1681/ASN.2019111168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lou Y, et al. Sodium-glucose cotransporter 2 inhibitors and fracture risk in patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Ther Adv Chronic Dis. 2020;11:2040622320961599. doi: 10.1177/2040622320961599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jayarathne HSM, et al. Neuroprotective effects of canagliflozin: lessons from aged genetically diverse UM-HET3 mice. Aging Cell. 2022;21(7):e13653. doi: 10.1111/acel.13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inc., J.P.U.S.F.a.D.A., Center for Drug Evaluation and Research. Pharmacology/Toxicology Review(s) of Canagliflozin NDA 204042 for the treatment of type 2 diabetes submitted by Janssen Pharmaceuticals Inc., Pharmacology/Toxicology NDA Review and Evaluation. 264 pgs. 2014.
- 39.Ye Y, et al. Effect of sodium-glucose co-transporter 2 inhibitors on bone metabolism and fracture risk. Front Pharmacol. 2018;9:1517. doi: 10.3389/fphar.2018.01517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zibellini J, et al. Does diet-induced weight loss lead to bone loss in overweight or obese adults? A systematic review and meta-analysis of clinical trials. J Bone Miner Res. 2015;30(12):2168–2178. doi: 10.1002/jbmr.2564. [DOI] [PubMed] [Google Scholar]
- 41.Williams GA, et al. In vitro and in vivo effects of adiponectin on bone. Endocrinology. 2009;150(8):3603–3610. doi: 10.1210/en.2008-1639. [DOI] [PubMed] [Google Scholar]
- 42.Tamura T, et al. Serum leptin and adiponectin are positively associated with bone mineral density at the distal radius in patients with type 2 diabetes mellitus. Metabolism. 2007;56(5):623–628. doi: 10.1016/j.metabol.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Jansson JO, et al. Body weight homeostat that regulates fat mass independently of leptin in rats and mice. Proc Natl Acad Sci U S A. 2018;115(2):427–432. doi: 10.1073/pnas.1715687114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thrailkill KM, et al. SGLT2 inhibitor therapy improves blood glucose but does not prevent diabetic bone disease in diabetic DBA/2J male mice. Bone. 2016;82:101–107. doi: 10.1016/j.bone.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thrailkill KM, et al. Canagliflozin, an SGLT2 inhibitor, corrects glycemic dysregulation in TallyHO model of T2D but only partially prevents bone deficits. Bone. 2020;141:115625. doi: 10.1016/j.bone.2020.115625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawarasaki S, et al. Comparative analysis of the preventive effects of canagliflozin, a sodium-glucose co-transporter-2 inhibitor, on body weight gain between oral gavage and dietary administration by focusing on fatty acid metabolism. Diabetes Metab Syndr Obes. 2020;13:4353–4359. doi: 10.2147/DMSO.S269916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang X, et al. The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-Pgc-1alpha signalling pathway. Adipocyte. 2020;9(1):484–494. doi: 10.1080/21623945.2020.1807850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naznin F, et al. Canagliflozin, a sodium glucose cotransporter 2 inhibitor, attenuates obesity-induced inflammation in the nodose ganglion, hypothalamus, and skeletal muscle of mice. Eur J Pharmacol. 2017;794:37–44. doi: 10.1016/j.ejphar.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 49.Hollander P, et al. Coadministration of canagliflozin and phentermine for weight management in overweight and obese individuals without diabetes: a randomized clinical trial. Diabetes Care. 2017;40(5):632–639. doi: 10.2337/dc16-2427. [DOI] [PubMed] [Google Scholar]
- 50.Cefalu WT, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013;382(9896):941–950. doi: 10.1016/S0140-6736(13)60683-2. [DOI] [PubMed] [Google Scholar]
- 51.Merovci A, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest. 2014;124(2):509–514. doi: 10.1172/JCI70704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu L, et al. SGLT2 Inhibition by empagliflozin promotes fat utilization and browning and attenuates inflammation and insulin resistance by polarizing M2 macrophages in diet-induced obese mice. EBioMedicine. 2017;20:137–149. doi: 10.1016/j.ebiom.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hawley SA, et al. The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular aMP levels. Diabetes. 2016;65(9):2784–2794. doi: 10.2337/db16-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferrannini E, et al. Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest. 2014;124(2):499–508. doi: 10.1172/JCI72227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrannini E, et al. Shift to fatty substrate utilization in response to sodium-glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65(5):1190–1195. doi: 10.2337/db15-1356. [DOI] [PubMed] [Google Scholar]
- 56.Blau JE, et al. Canagliflozin triggers the FGF23/1,25-dihydroxyvitamin D/PTH axis in healthy volunteers in a randomized crossover study. JCI Insight. 2018;3(8):e99123. 10.1172/jci.insight.99123. [DOI] [PMC free article] [PubMed]
- 57.Bays HE, et al. Canagliflozin: effects in overweight and obese subjects without diabetes mellitus. Obesity (Silver Spring) 2014;22(4):1042–1049. doi: 10.1002/oby.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Creecy A, et al. Changes in the fracture resistance of bone with the progression of type 2 diabetes in the ZDSD rat. Calcif Tissue Int. 2016;99(3):289–301. doi: 10.1007/s00223-016-0149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fu X, et al. Effects of ovariectomy on rat mandibular cortical bone: a study using Raman spectroscopy and multivariate analysis. Anal Chem. 2012;84(7):3318–3323. doi: 10.1021/ac300046x. [DOI] [PubMed] [Google Scholar]
- 60.Iwasaki Y, et al. Changes in chemical composition of cortical bone associated with bone fragility in rat model with chronic kidney disease. Bone. 2011;48(6):1260–1267. doi: 10.1016/j.bone.2011.03.672. [DOI] [PubMed] [Google Scholar]
- 61.Shen J, et al. A longitudinal Raman microspectroscopic study of osteoporosis induced by spinal cord injury. Osteoporos Int. 2010;21(1):81–87. doi: 10.1007/s00198-009-0949-3. [DOI] [PubMed] [Google Scholar]
- 62.Kohn DH, et al. Exercise alters mineral and matrix composition in the absence of adding new bone. Cells Tissues Organs. 2009;189(1–4):33–37. doi: 10.1159/000151452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCreadie BR, et al. Bone tissue compositional differences in women with and without osteoporotic fracture. Bone. 2006;39(6):1190–1195. doi: 10.1016/j.bone.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 64.Yerramshetty JS, Lind C, Akkus O. The compositional and physicochemical homogeneity of male femoral cortex increases after the sixth decade. Bone. 2006;39(6):1236–1243. doi: 10.1016/j.bone.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 65.Akkus O, Adar F, Schaffler MB. Age-related changes in physicochemical properties of mineral crystals are related to impaired mechanical function of cortical bone. Bone. 2004;34(3):443–453. doi: 10.1016/j.bone.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 66.Tarnowski CP, Ignelzi MA, Jr, Morris MD. Mineralization of developing mouse calvaria as revealed by Raman microspectroscopy. J Bone Miner Res. 2002;17(6):1118–1126. doi: 10.1359/jbmr.2002.17.6.1118. [DOI] [PubMed] [Google Scholar]
- 67.Tuncer M, et al. Capacitive behaviour of nanocrystalline octacalcium phosphate (OCP) (Ca(8)H(2)(PO(4))(6).5H(2)O) as an electrode material for supercapacitors: biosupercaps. Nanoscale. 2019;11(39):18375–18381. doi: 10.1039/C9NR07108C. [DOI] [PubMed] [Google Scholar]
- 68.Jang HL, et al. In vitro and in vivo evaluation of Whitlockite biocompatibility: comparative study with hydroxyapatite and beta-tricalcium phosphate. Adv Healthc Mater. 2016;5(1):128–136. doi: 10.1002/adhm.201400824. [DOI] [PubMed] [Google Scholar]
- 69.Bouxsein ML, et al. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25(7):1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 70.Goldman HM, et al. Intrapopulation variability in mineralization density at the human femoral mid-shaft. J Anat. 2003;203(2):243–255. doi: 10.1046/j.1469-7580.2003.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kingsmill VJ, Boyde A. Mineralisation density of human mandibular bone: quantitative backscattered electron image analysis. J Anat. 1998;192(Pt 2):245–256. doi: 10.1046/j.1469-7580.1998.19220245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Davy KWM. Novel aromatic dimethacrylate esters as dental resins. J Mater Sci - Mater Med. 1994;5:350–352. doi: 10.1007/BF00058961. [DOI] [Google Scholar]
- 73.Kaya S, et al. Lactation-induced changes in the volume of osteocyte lacunar-canalicular space alter mechanical properties in cortical bone tissue. J Bone Miner Res. 2017;32(4):688–697. doi: 10.1002/jbmr.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.YoavBenjamini YH. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc: Ser B (Methodol) 1995;57(1):289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are available upon request. Our studies do not include the use of custom code or mathematical algorithms. We have included citations for available data in the references section.