Abstract
The right ventrolateral prefrontal cortex (rVLPFC) is highly engaged in emotion regulation of social pain. However, there is still lack of both inhibition and excitement evidence to prove the causal relationship between this brain region and voluntary emotion regulation. This study used high‐frequency (10 Hz) and low‐frequency (1 Hz) repetitive transcranial magnetic stimulation (rTMS) to separately activate or inhibit the rVLPFC in two groups of participants. We recorded participants' emotion ratings as well as their social attitude and prosocial behaviors following emotion regulation. Also, we used eye tracker to record the changes of pupil diameter to measure emotional feelings objectively. A total of 108 healthy participants were randomly assigned to the activated, inhibitory or sham rTMS groups. They were required to accomplish three sequential tasks: the emotion regulation (cognitive reappraisal) task, the favorability rating task, and the donation task. Results show that the rVLPFC‐inhibitory group reported more negative emotions and showed larger pupil diameter while the rVLPFC‐activated group showed less negative emotions and reduced pupil diameter during emotion regulation (both compared with the sham rTMS group). In addition, the activated group gave more positive social evaluation to peers and donated more money to a public welfare activity than the rVLPFC‐inhibitory group, among which the change of social attitude was mediated by regulated emotion. Taken together, these findings reveal that the rVLPFC plays a causal role in voluntary emotion regulation of social pain and can be a potential brain target in treating deficits of emotion regulation in psychiatric disorders.
Keywords: emotion regulation, prosocial behavior, social exclusion, transcranial magnetic stimulation, ventrolateral prefrontal cortex
By using 10 Hz and 1 Hz rTMS to activate and inhibit the rVLPFC, we uncovered the causal role of this prefrontal region in voluntary emotion regulation. Meanwhile, rTMS‐induced emotion regulation effect changed participants' social attitudes and prosocial behaviors.
1. INTRODUCTION
Social pain is defined as painful feelings following negative social evaluation, interpersonal rejection, social exclusion, or loss of relatives and friends (Eisenberger, 2012, 2015). Social pain can be emotionally devastating, which might threaten fundamental needs such as meaningful existence and sense of belonging (Williams, 2007; Zadro et al., 2004), promote aggressive behaviors (Twenge et al., 2001), and even result in mental disorders such as anxiety (Fung & Alden, 2017) and depression (Wang et al., 2017). Emotion regulation is a powerful way to alleviate negative experiences and neural responses to social pain (Gross, 2002; He et al., 2018; He, Liu, et al., 2020; He, Zhao, et al., 2020; Zhao et al., 2021). Cognitive reappraisal is an often employed emotion regulation strategy that involves modifying emotional responses by reinterpreting situations (Ochsner et al., 2012). Cognitive reappraisal is an effective strategy for regulating emotions adaptively and is associated with enduring positive impacts on both physical and mental well‐being (McRae & Gross, 2020).
The ventrolateral prefrontal cortex, especially its right portion (rVLPFC), is considered as an important brain region for down‐regulating negative emotions when participants experienced social pain (Eisenberger et al., 2003; Masten et al., 2009; Onoda et al., 2010; Vijayakumar et al., 2017). For instance, Eisenberger et al. (2003) examined the neural activities during social exclusion induced by a virtual ball tossing game. They found that social pain evoked not only the anterior cingulate cortex but also the rVLPFC; also, the activation of the rVLPFC was negatively correlated with the subjective reported distress during the task (see also Masten et al., 2009). Thereafter, Riva et al. (2012); Riva, Romero Lauro, DeWall, et al. (2015) applied anodal transcranial direct current stimulation (tDCS) to activate the rVLPFC and found this manipulation decreased negative emotions as well as aggressive behaviors after the participants experienced social exclusion. Meanwhile, the same group of researchers used the cathode tDCS to inhibit the rVLPFC and reached the opposite result, that is, participants felt more negative in response to social exclusion (Riva, Romero Lauro, Vergallito, et al., 2015).
However, it is worth noting that these studies did not give participants specific instructions to regulate their emotion. Without a manipulation of voluntary emotion regulation, these studies thus mainly proved the role of rVLPFC during automatic and implicit regulation of social pain. Voluntary emotion regulation is an efficient and self‐controllable way to improve mood, which is very important in our daily life (Phillips et al., 2008). However, voluntary emotion regulation and automatic, implicit emotion regulation depend on nonoverlapping neural substrates (Braunstein et al., 2017). To fill this gap, our lab implemented a series of studies that used an explicit cognitive reappraisal task to explore the role of the rVLPFC on voluntary down‐regulation of social pain. These studies supposed to facilitate the rVLPFC by using the anodal tDCS (He et al., 2018; He, Liu, et al., 2020) or high‐frequency repetitive transcranial magnetic stimulation (rTMS) (He, Zhao, et al., 2020; Li et al., 2022; Zhao et al., 2021), which found that the participants in active tDCS/rTMS group performed better in reappraising social pain compared with the sham tDCS/rTMS group.
However, it seems that the excitatory effect of anodal tDCS and high frequency rTMS is lack of clear evidence because the exact mechanisms underlying these electrical and magnetic techniques have not been clearly identified (Chase et al., 2020; Klomjai et al., 2015). Sometimes an “active” parameter of these devices gives an “inhibitory” effect. For instance, Daskalakis et al. (2006) found that cortical silent period was significantly prolonged (i.e., indicating cortical inhibition) by using high stimulation frequency (10 or 20 Hz) of rTMS. Also, Jung et al. (2008) found that 5 s of 10 Hz rTMS decreased the activation of the primary motor cortex. More recently, Matsushita et al. (2021) applied anodal tDCS over the right auditory cortex and found the pitch discrimination learning was disturbed, which indicated an inhibitory effect of anodal tDCS. Similarly, the rTMS parameters that theoretically produce cortical inhibition do not always work well. For example, Li et al. (2016) found that both low‐ (1 Hz) and high‐frequency (10 Hz) rTMS sessions significantly improved cortical excitability in active rTMS groups compared with sham group, that is, low frequency rTMS also promoted the recovery of upper limb motor function in patients with cerebral infarction. Therefore, considering the uncertainty of tDCS/rTMS application that might give contradictory effects in various cognitive tasks and various brain regions, we cannot conclude that the rVLPFC was indeed activated in our previous studies. To obtain convincing evidence for the essential role of the rVLPFC in voluntary regulation of social pain, this study both excited and inhibited this brain region using rTMS in two groups of participants and examined the neural modulation effects of rTMS in both the groups.
Besides the main purpose, this study also explored the impact of down‐regulating social pain on changing social attitude and prosocial behaviors. People usually feel distressed or become hostile and aggressive when they receive social isolation or criticism (DeWall et al., 2009). This negative emotion affects social attitude (Manstead, 1991). For instance, angry‐induced participants had automatic prejudice against the outgroup (DeSteno et al., 2004), and happy‐induced participants judged a given character happier than sad‐induced and control participants (Innes‐Ker & Niedenthal, 2002). Emotion regulation especially cognitive reappraisal is helpful to change attitude (Cancino‐Montecinos et al., 2018; Jones et al., 2014). For instance, the participants who had undergone cognitive reappraisal training were more supportive of conciliatory policies than aggressive policies (Halperin et al., 2013). Also, people who better regulated their emotions were more empathetic and more likely to engage in prosocial behaviors (Damon et al., 2006; Vohs & Baumeister, 2016). Meanwhile, it is found that habitual use of cognitive reappraisal strategy can predict empathic concern and prosocial behaviors (Lebowitz & Dovidio, 2015).
Taken together, the current study has two purposes. The first is to verify the critical role of rVLPFC in down‐regulating social pain using high‐ (>5 Hz) and low‐frequency (≤1 Hz) rTMS to hypothetically activate and inhibit this brain region (Fitzgerald et al., 2006). Consistent with our previous studies (He et al., 2018; He, Liu, et al., 2020; He, Zhao, et al., 2020; Li et al., 2022; Zhao et al., 2021), we chose the subjective rating of emotional feelings as the main index to evaluate the effect of emotional regulation. Besides, the pupil diameter was used to objectively assess physiological arousal of participants during the emotion regulation task. It has been shown that pupil diameter is sensitive to emotional components of stimuli (Bradley et al., 2008) and it is more dilated after viewing negative emotion pictures (Kinner et al., 2017; Snowden et al., 2016). The stronger the emotional experience, the higher the arousal, and the larger the pupil diameter (De Witte et al., 2017). Specifically, greater emotion regulation success was associated with smaller pupil size (Bebko et al., 2011; Kinner et al., 2017; Urry et al., 2009). Another purpose of this study is to explore the impact of emotion regulation of social pain on subsequent social attitude and prosocial behaviors. Here the favorability rating task and the donation task were carried out to measure social attitude and prosocial behaviors. We hypothesized that the VLPFC‐activated group would rate peers more favorably and donate more money compared to the VLPFC‐inhibitory group.
2. METHOD
2.1. Participants
Three rTMS groups were included in this study: the rVLPFC‐activated group, the rVLPFC‐inhibitory group, and the vertex (sham) group. We performed a priori power analysis utilizing the G*Power 3.1.9 software (F tests, ANOVA: repeated measures and within‐between interaction). The a priori data for our analysis was the effect size ( = 0.083) reported in our previous rTMS study (Zhao et al., 2021). Our power analysis result indicated that a total of 48 participants would be required to achieve a statistical power of 95%. However, a sample size of 16 participants per group is too small in current neuroscience research. Therefore, we ultimately decided to include 36 participants in each rTMS group, which ensured a statistical power close to 100%. As a result, a total of 108 healthy, right‐handed college students were recruited from Shenzhen University. None of the participants had any previous exposure or experience to TMS before the experiment. They completed six questionnaires on the date of the experiment, including the Beck Depression Inventory Second Edition (BDI‐II; Beck et al., 1996), the Spielberger's State–Trait Anxiety Inventory (STAI‐T; Spielberger et al., 1983), the Rosenberg Self‐Esteem Scale (RSES; Rosenberg, 1965), the Rejection Sensitivity Questionnaire (RSQ; Downey & Feldman, 1996), the Toronto Alexithymia Scale (TAS‐20; Parker et al., 2003), and the Liebowitz Social Anxiety Scale (LSAS; Liebowitz, 1987). No significant differences were found in these measured characteristics across the three groups (Table 1). All participants signed informed consent prior to the experiment. The Ethics Committee of Shenzhen University approved the study.
TABLE 1.
Demographical characteristics of the three groups.
| Items | Inhibitory group (n = 36) | Sham group (n = 36) | Activated group (n = 36) | Statistics a | |
|---|---|---|---|---|---|
| F (2,105) | p | ||||
| Gender (male/female) | 18/18 | 18/18 | 18/18 | ||
| Age (year) | 20.75 ± 0.31 | 20.31 ± 0.37 | 20.22 ± 0.29 | 0.76 | 0.472 |
| BDI | 7.92 ± 1.24 | 8.25 ± 1.38 | 8.58 ± 1.31 | 0.07 | 0.937 |
| STAI‐T | 40.72 ± 1.71 | 43.44 ± 1.89 | 43.36 ± 1.76 | 0.75 | 0.476 |
| RSES | 29.14 ± 0.85 | 27.97 ± 0.68 | 27.97 ± 0.84 | 0.72 | 0.490 |
| RSQ | 10.88 ± 0.37 | 10.67 ± 0.42 | 10.48 ± 0.35 | 0.27 | 0.765 |
| TAS‐20 | 50.58 ± 1.82 | 54.00 ± 1.75 | 50.64 ± 1.85 | 1.18 | 0.312 |
| LSAS | 47.78 ± 4.14 | 51.06 ± 3.57 | 49.08 ± 3.55 | 0.19 | 0.825 |
Abbreviations: BDI, the Beck Depression Inventory Second Edition; STAI‐T, the trait form of Spielberger's State–Trait Anxiety Inventory; RSES, the Rosenberg Self‐Esteem Scale; RSQ, the Rejection Sensitivity Questionnaire; TAS‐20, the Toronto Alexithymia Scale; LSAS, the Liebowitz Social Anxiety Scale.
One‐way ANOVA across the three groups.
2.2. Materials and experimental procedure
The experimental materials were 60 social exclusion pictures selected from the Image database of social inclusion and exclusion in Asian young adults (Zheng et al., 2022), which was developed by our lab and has been used to successfully evoke social pain in our previous studies (He et al., 2018; He, Liu, et al., 2020; He, Zhao, et al., 2020; Zhao et al., 2021). During the experiment, all images were presented in the center of an LCD monitor (3.0 × 3.5° visual angle).
The study was a two (regulation type: passive viewing and cognitive reappraisal) by three (rTMS group: VLPFC‐inhibitory, VLPFC‐activated and sham) mixed design. The regulation type was the within‐subject factor and the rTMS group was the between‐subject factor. The experimental task was divided into two blocks, of which the first block included the passive viewing task and the second block included the cognitive reappraisal task. This setting was to avoid any carry‐over effect of the explicit instruction for cognitive reappraisal (see also He et al., 2018; He, Liu, et al., 2020; He, Zhao, et al., 2020; Zhao et al., 2021). Each block is followed by a favorability rating task and a financial donation task (Figure 1a). Participants underwent two 15‐min rTMS sessions in the whole experiment. The two sessions of rTMS pulses were administered separately before the passive viewing task and before the cognitive reappraisal task.
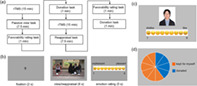
FIGURE 1.
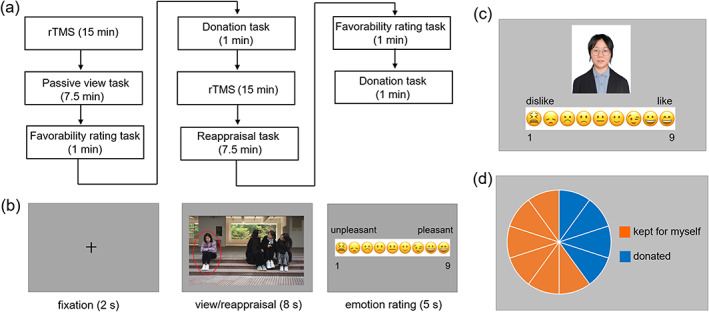
Illustration of Methods. (a) Experimental procedure. (b) One trial in the emotion regulation task. (c) The favorability rating task. The person in the photo is replaced by the first author of the paper to avoid portrait rights issues. (d) The donation task.
Emotion regulation task (Figure 1b). The main task was divided into two blocks. In the beginning of the passive viewing block, the instruction was as follows: “In this section, please imagine yourself as the person circled in the picture and experience how you would feel in that situation.” In the beginning of the cognitive reappraisal block, participants were instructed as follows: “In this section, still imagine that you are the person circled in the picture, and at the same time think about a better outcome or reinterpret the situation. For example, you could imagine the persons sitting away from you are discussing a topic that you are not interested in or you could easily make some change and join the group soon.” As shown in Figure 1b, a trial began with a 2‐s fixation followed by the picture presentation for 8 s. During this period, participants were required to watch passively or to regulate their emotion via cognitive reappraisal strategy. After that, they were asked to report their feelings on a continuous scale ranging from 1 to 9 (1 = maximal negativity and 9 = maximal positivity) by clicking the left button on the mouse.
Favorability rating task (Figure 1c). This task had two rounds. We used 12 identity photos of peers (6 males and 6 females) with neutral facial expressions. The background color (white), resolution and brightness of all photos were standardized. The face attractiveness of the photos was rated by another group of homogeneous participants (Li et al., 2022). The face attractiveness and gender of the photos used in the study were counterbalanced in the two rounds. In each round, participants rated their favorability toward six unfamiliar peers. They can use the left mouse button to click on a continuous scale ranging from 1 to 9 (“1” for extremely dislike, “9” for extremely like) to report their first‐impression favorability toward the presented peer within 5 s.
Financial donation task (Figure 1d). This task also had two rounds. Participants were instructed before the task that: “Now we give you a bonus of 10 RMB yuan (approximately $1.5). Recently we have launched a public welfare activity of economic donation. You can choose to donate some money to buy educational toys and give them to the children with audio‐visual or intellectual disabilities in Shenzhen Special Education School. There will be two rounds of donation activities, and your final bonus is the remaining amount of money you kept for yourself.” In each round, participants had a bonus of 10 RMB yuan and they could use mouse to click in the pie chart to indicate the amount of donation. After the experiment, the participants were asked whether they believed the donation was true. Finally, the payment of each participant was 60 RMB yuan (approximately $9.0) adding their bonus left in the two donation rounds.
2.3. Repetitive TMS (rTMS)
Offline rTMS was applied using a figure‐of‐eight coil connected to the magnetic stimulator (M‐100 Ultimate; Shenzhen Yingchi Technology Co., Ltd, Shenzhen, China). The stimulation target of the inhibitory and activated groups were the rVLPFC. The control site in the sham group was the vertex (Zhao et al., 2021), which has been proved to produce scalp sensation similar to the experimental groups and has relatively little influence over on‐going brain processes involved in most experimental tasks (Jung et al., 2016). Location of the coil was based on the International 10/20 electroencephalogram system. The rVLPFC is at the F8 and the vertex is at the Cz (Li et al., 2022; Zhao et al., 2021). The resting motor threshold (rMT) of each participant was measured in the motor cortex (the C3) and the threshold was defined as 50% of the pulses that reliably produced thumb twitch. In the VLPFC‐activated group, participants received 10 Hz rTMS at 90% of rMT (Ahn et al., 2013; Zhao et al., 2021). A total of 30 trains (i.e., 1170 pulses) are included in one 15‐min session, each lasting 3.9 s and separated by 26.1 s resting period (Zhao et al., 2021). In the VLPFC‐inhibitory group, participants received 1 Hz rTMS at 110% of rMT for 15 min (Fitzsimmons et al., 2020; Knecht et al., 2003), that is, 900 pulses in each session. In the sham group, half of the participants received 1 Hz rTMS at 110% rMT and the other half received 10 Hz rTMS at 90% rMT over the vertex. In the post‐interview, all participants stated that they were unaware of which group they belonged to.
2.4. Eye‐tracker recording and data analysis
The experimental room was illuminated by a LED ceiling lamp with constant brightness (25 lx). Pupil data were recorded during the emotion regulation task using an EyeLink 1000 Plus infrared eyetracker (SR Research Ltd, Ottawa, Canada). The sampling rate was 500 Hz. Participants sat with their head fixed on a chinrest at a distance of about 60 cm from the eyetracker. Calibration and validation procedures were conducted during rTMS stimulation. Participants were told to avoid any head movement during rTMS sessions and the emotion regulation task.
Pupillometry data were preprocessed using the Pupillometry Pipeliner (PUPI) in Matlab (Kinley & Levy, 2022). The data in the two blocks were concatenated separately. Blink samples were identified and removed using the pupillometry noise method (Hershman et al., 2019). Then, we applied the linear interpolation method to fill in any missing data points (15.33% ± 1.32%), and subsequently transformed the data into z‐scores (Hershman et al., 2019). This study defined the mean of the 200 ms interval preceding stimulus onset as the baseline (Leknes et al., 2013; Snowden et al., 2016). The time window for calculating mean pupil diameter was selected as 2–8 s after the picture onset, since previous studies have suggested that pupil dilation induced by emotion events usually occurs 2 s after the presentation of emotional images (Bradley et al., 2008; Henderson et al., 2014; Kinner et al., 2017; Partala & Surakka, 2003).
2.5. Statistics
Statistical analyses were performed using SPSS Statistics 20.0 (IBM, Somers, USA). Descriptive data are presented as mean ± standard error. Subjective ratings and pupil diameter were analyzed using repeated‐measures ANOVAs. Significance level was set at p < .05. Since there were no significant differences in dependent variables between vertex‐activated and vertex‐inhibitory participants (Fs<1), we combined the two subsets of the sham group as one group in all the statistical analyses. We performed two‐tailed Pearson's correlations between individual characteristics and emotion rating as well as pupil diameter. Multiple comparisons were corrected using the false discovery rate (FDR) method.
3. RESULTS
3.1. Emotion rating
The main effect of rTMS group was found to be significant (F (2,105) = 7.48, p = .001, = 0.125): the VLPFC‐activated group reported more positive feelings (4.85 ± 0.12) when compared with the sham rTMS group (4.38 ± 0.12, p = .018) and the VLPFC‐inhibitory group (4.22 ± 0.12, p = .001), whereas the emotion rating between the sham and the inhibitory group did not differ (p = 1.000). Also, there was a significant main effect of regulation type (F (1,105) = 670.77, p < .001, = 0.865): the emotion rating was more positive in the reappraisal block (6.01 ± 0.09) as compared to that in the passive viewing block (2.95 ± 0.09).
More importantly, a significant interaction between rTMS group and regulation type was observed (F (2,105) = 7.89, p = .001, = 0.131; Figure 2a). In the reappraisal block, there were significant differences in emotion rating among the three rTMS groups (F (2,105) = 13.54, p < .001, = 0.205): the activated group reported the most positive feelings (6.59 ± 0.16), followed by the sham group (6.03 ± 0.16), while the inhibitory group had the most negative emotion ratings (5.42 ± 0.16) (pairwise p values: activated vs. sham = .043; sham vs. inhibitory = .023; activated vs. inhibitory <.001). However, no significant difference was observed among the three groups in the passive viewing block (F (2,105) = 1.73, p = .183, = 0.032; activated = 3.12 ± 0.16, sham = 2.72 ± 0.16, and inhibitory = 3.02 ± 0.16).
FIGURE 2.
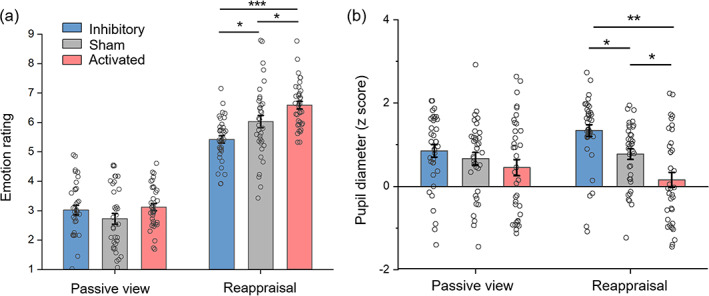
Statistical results of emotion rating and pupil diameter in the emotion regulation task. (a) Subjective emotion rating. (b) Mean pupil diameter during 2–8 s of the image presentation. Bars represent SE of the mean. The small circle represents individual data. *p < .05; **p < .01; and ***p < .001.
Exploratory analysis found that the subjective emotion rating was negatively correlated with the participants' depression, trait anxiety, and alexithymia during cognitive reappraising (Table 2).
TABLE 2.
Correlations between emotion rating and individual characteristics.
| Individual characteristics | Inhibitory (n = 36) | Sham (n = 36) | Activated (n = 36) | |||
|---|---|---|---|---|---|---|
| View | Reappraisal | View | Reappraisal | View | Reappraisal | |
| BDI |
r = −.282 p = .152 |
r = −.055 p = .750 |
r = −.494 p = .008 |
r = −.405 p = .037 |
r = −.238 p = .185 |
r = −.258 p = .172 |
| STAI‐T |
r = −.445 p = .011 |
r = −.267 p = .131 |
r = −.584 p < .001 |
r = −.396 p = .023 |
r = −.251 p = .140 |
r = −.458 p = .010 |
| RSES |
r = .308 p = .091 |
r = −.017 p = .923 |
r = .368 p = .072 |
r = .338 p = .088 |
r = .383 p = .084 |
r = .318 p = .093 |
| RSQ |
r = −.284 p = .248 |
r = .078 p = .746 |
r = −.525 p = .008 |
r = −.283 p = .188 |
r = .039 p = .820 |
r = .114 p = .676 |
| TAS‐20 |
r = −.519 p = .003 |
r = −.238 p = .184 |
r = −.529 p = .004 |
r = −.398 p = .026 |
r = −.288 p = .119 |
r = −.154 p = .371 |
| LSAS |
r = −.169 p = .433 |
r = .023 p = .895 |
r = −.440 p = .028 |
r = −.346 p = .101 |
r = −.254 p = .270 |
r = −.123 p = .544 |
Note: Significant correlations were thresholded using the false discovery rate (FDR) method. Bold values indicate p < 0.05 after FDR correction.
3.2. Pupil response
Figure 3 illustrates evoked pupil responses to social exclusion pictures in the three rTMS groups. The rTMS resulted in a main effect (F (2,105) = 6.64, p = .002, = 0.112): the pupil diameter was larger in the inhibitory group (1.10 ± 0.15) than that in the activated group (0.30 ± 0.15, p = .001). There was no significant difference between the sham (0.72 ± 0.15) and the activated (p = .178) or between the sham and the inhibitory group (p = .256). Meanwhile, the main effect of regulation type was significant (F (1,105) = 4.92, p = .029, = 0.045): the pupil diameter was larger in the cognitive reappraisal task (0.76 ± 0.09) than that in the passive viewing task (0.65 ± 0.10, p = .029).
FIGURE 3.
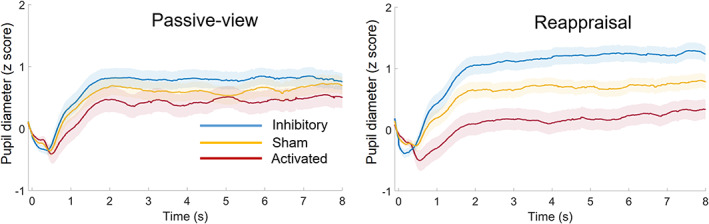
Pupillary responses in the passive viewing block and the reappraisal block.
A significant interaction between rTMS group and regulation type was observed (F (2,105) = 24.05, p < .001, = 0.314; Figure 2b). The pupil diameter significantly differed across the three groups in the cognitive reappraisal task (F (2,105) = 15.17, p < .001, = 0.224): it was the largest in the inhibitory group (1.34 ± 0.15), median in the sham group (0.77 ± 0.15), and was the smallest in the activated group (0.16 ± 0.15) (pairwise p values: activated vs. sham = .015; sham vs. inhibitory = .029; activated vs. inhibitory <.001). However, there was no significant difference among the three rTMS groups in the passive viewing task (F (2,105) = 1.48, p = .232, = 0.027; activated = 0.45 ± 0.17, sham = 0.66 ± 0.17, and inhibitory = 0.85 ± 0.17).
We also analyzed the Pearson correlations between the pupil diameter and the emotion ratings (Table 3). Results showed that there is a negative correlation between subjective and objective measurements in all the conditions, which indicated the consistency of the two dependent variables.
TABLE 3.
Correlations between pupil diameter and emotion rating.
| Emotion rating | Inhibitory (n = 36) | Sham (n = 36) | Activated (n = 36) | Total (n = 108) | ||||
|---|---|---|---|---|---|---|---|---|
| View | Reappraisal | View | Reappraisal | View | Reappraisal | View | Reappraisal | |
| View |
r = −.369 p = .027 |
r = −.450 p = .016 |
r = −.443 p = .011 |
r = −.450 p = .012 |
r = −.425 p = .013 |
r = −.421 p = .013 |
r = −.393 p < .001 |
r = −.386 p < .001 |
| Reappraisal |
r = −.340 p = .043 |
r = −.391 p = .024 |
r = −.369 p = .031 |
r = −.467 p = .011 |
r = −.427 p = .014 |
r = −.434 p = .016 |
r = −.394 p < .001 |
r = −.534 p < .001 |
Note: Significant correlations were thresholded using the false discovery rate (FDR) method.
3.3. Favorability rating
The main effect of regulation type was significant (F (1,105) = 13.67, p < .001, = 0.115). Favorability ratings were higher in the reappraisal block (5.87 ± 0.08) than that in the passive viewing block (5.61 ± 0.08, p < .001). The main effect of rTMS group was not significant (F (2,105) = 0.12, p = .888, = 0.002).
There was a significant interaction between rTMS group and regulation type (F (2,105) = 5.66, p = .005, = 0.097). The activated group gave more positive evaluation after cognitive reappraisal block (5.98 ± 0.14) than after passive viewing block (5.48 ± 0.14) (F (1,105) = 17.18, p < .001, = 0.141). The same result pattern was found in the sham group (F (1,105) = 7.57, p = .007, = 0.067; reappraisal = 5.95 ± 0.14, viewing = 5.62 ± 0.14). However, there was no significant difference in the inhibitory group between the two task conditions (F (1,105) = 0.24, p = .625, = 0.002; viewing = 5.73 ± 0.14, reappraisal = 5.67 ± 0.14). In order to highlight the group difference, we calculated the reappraisal effect by subtracting the favorability rating in the passive viewing block from that in the reappraisal block. The reappraisal effect showed significant differences among the three groups (F (2,105) = 5.66, p = .005): the VLPFC‐activated group (0.50 ± 0.13) showed a stronger reappraisal promotion effect than the VLPFC‐inhibitory group (−0.06 ± 0.12, p = .004; Figure 4a).
FIGURE 4.
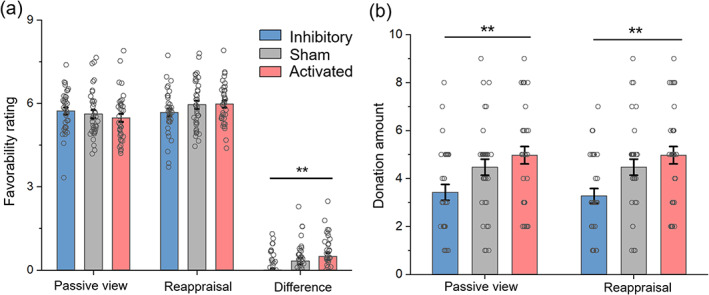
Statistical results of social behaviors following the emotion regulation task. (a) Favorability rating. (b) Amount of donated money. Bars represent SE of the mean. The small circle represents individual data. **p < .01.
In order to further explore the relationship between rTMS effect, emotion experience and social attitude, we performed a mediation analysis using the PROCESS Macro for SPSS (Model 4; Hayes, 2013), with 5000 bootstrapping samples (Preacher & Hayes, 2008). We took rTMS group as independent variable, emotion indexes (i.e., emotion rating and pupil diameter in the reappraisal block) as mediators, and favorability rating as dependent variable. The rTMS group was transformed to dummy code using the sham group as the comparison group. We examined mediation paths based on a 95% bias‐corrected bootstrap confidence interval (CI) and considered that the indirect effect was significant when zero was not included in the CI. First, we tested the mediating role of the emotion rating. Results showed that the indirect effect of both inhibitory group (B = −0.61 × 0.41 = −0.25, SE = 0.12, 95% CI = [−0.50, −0.05]) and activated group (B = 0.56 × 0.41 = 0.23, SE = 0.11, 95% CI = [0.03, 0.46]) through emotion rating were significant (Figure 5a). Similarly, the indirect effect of both inhibitory group (B = 0.56 × −0.3 = −0.17, SE = 0.08, 95% CI = [−0.35, −0.04]) and activated group (B = −0.62 × −0.3 = 0.18, SE = 0.09, 95% CI = [0.04, 0.41]) through pupil diameter were also significant (Figure 5b). These findings showed that emotion experience in the reappraisal condition exhibited a mediating effect between rTMS effect and favorability rating.
FIGURE 5.
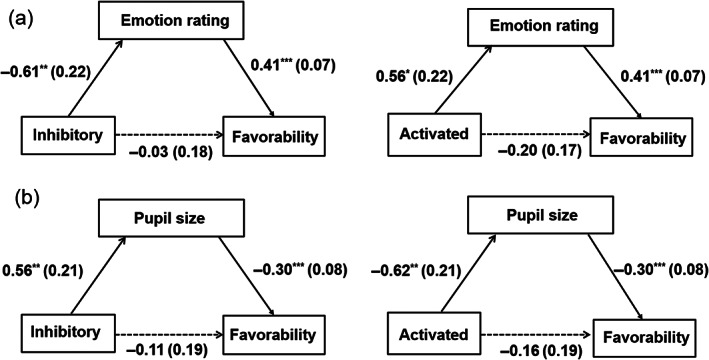
Mediating effect of emotion experience on rTMS and favorability in the reappraisal condition using the sham group as the comparison group. The mediating models were examined using (a) emotion rating, or (b) pupil diameter as a mediator. Unstandardized coefficients are shown as mean (SE). Statistically significant pathways are represented using solid lines. *p < .05; **p < .01; ***p < .001.
3.4. Amount of donated money
The 102 out of the 108 participants believed that the donation task was true. Therefore, the analyses of the amount of donated money were based on these 102 datasets. The main effect of rTMS group was significant (F (2,99) = 5.473, p = .006, = 0.100; Figure 4b): the activated group (4.84 ± 0.33) donated more money than the inhibitory group (3.35 ± 0.34, p = .006). No significant difference was found between the sham (4.47 ± 0.33, p = 1.000) and the activated group or between the sham and the inhibitory group (p = .058).
4. DISCUSSION
The current research employed low‐ and high‐frequency rTMS to examine the causal role of the rVLPFC in voluntary emotion regulation of social pain. Results showed that using low‐frequency rTMS to inhibit the rVLPFC increased the negative emotional experience of social exclusion (reflected by more negative emotional ratings and larger pupil diameters), while using high‐frequency rTMS to activate this brain region reduced social pain (reflected by more positive emotional ratings and smaller pupil diameters). We also found that the VLPFC‐activated group gave more positive social evaluation to peers and donated more money than the VLPFC‐inhibitory group, among which the change of social attitude is mediated by regulated emotion.
The main finding was while the low frequency rTMS at the rVLPFC obstructed the emotional regulation of social pain and resulted in more negative feelings, high‐frequency rTMS promoted the emotion regulation process. This bidirectional (i.e., inhibited and activated the rVLPFC) result strengthens our previous findings supporting the key role of the rVLPFC in voluntarily reducing negative emotions (He et al., 2018; He, Liu, et al., 2020; He, Zhao, et al., 2020; Li et al., 2022; Zhao et al., 2021). Also, we found that the result of pupil diameter was consistent with the subjective emotion rating, reflected by the significant correlation between these two measures across all conditions. Previous studies have demonstrated that emotional arousal is accompanied by increased pupil diameters (Bradley et al., 2008; Kinner et al., 2017; Partala & Surakka, 2003). In the cognitive reappraisal block, the pupil diameter of the VLPFC‐inhibitory group was significantly larger than that of the sham group, while the pupil diameter of the VLPFC‐activated group was significantly smaller than that of the sham group. This between‐group finding shows that the neural activation of the rVLPFC directly influenced the emotional arousal experienced by participants during explicit emotion regulation. However, someone may note that the pupil diameter during cognitive reappraisal was larger than that during the passive viewing condition. We argue that this effect was not due to the effect of emotion regulation between the two conditions. Instead, it may be because cognitive reappraisal required significantly more cognitive resources than the passive viewing condition; and the increased cognitive load led to amplified pupil diameter (Johnstone et al., 2007; Kinner et al., 2017; Urry et al., 2006; Urry et al., 2009; van Reekum et al., 2007). Altogether, this study provides congruent evidence for the causal role of the rVLPFC in emotion regulation.
The above findings support the excitatory effect of high‐frequency and the inhibitory effect of low‐frequency rTMS when targeting on the rVLPFC in emotion regulation. However, some rTMS studies using the same frequency (10 Hz or 1 Hz) did not produce the same excitatory or inhibitory effects on certain brain regions, as mentioned in the introduction (Daskalakis et al., 2006; Gilio et al., 2003; Jung et al., 2008; Li et al., 2016; Schambra et al., 2003). In our opinion, the inconsistency might be due to different stimulus parameters (e.g., length and intensity of magnetic train: Fitzgerald et al., 2006) and initial cortical excitability/cognitive states (Bergmann et al., 2021; Silvanto et al., 2008). For the second reason, some studies have discovered that the same rTMS parameters sometimes produce very different neural (e.g., Blankenburg et al., 2008) and behavioral (e.g., Borgomaneri et al., 2020) outcomes in the same participants when they had different initial cortical excitability or cognitive states. In view of the numerous stimulus parameters and brain states that might influence rTMS effects, it is necessary to both excite and inhibit the brain region of interest to verify neural modulation outcomes, thus improving the reproducibility and reliability of rTMS studies (Klomjai et al., 2015). This gap has been filled in this study for the rVLPFC during emotion regulation.
An interesting finding was that subjective emotion rating of the sham group was negatively correlated with individual's depression, trait anxiety, social anxiety, rejection sensitivity, and alexithymia during passive viewing, whereas these correlations were largely attenuated in the two active rTMS groups. It is reasonable that emotional experience is affected by individual characteristics such as depression (Rive et al., 2013), trait anxiety (Campbell‐Sills et al., 2011), social anxiety (Jazaieri et al., 2015), rejection sensitivity (Silvers et al., 2012), and alexithymia (van der Velde et al., 2015) in the absence of rTMS intervention. In this study, rTMS was used to activate or inhibit rVLPFC, which is equivalent to enhancing the effect of emotion regulation (implicit emotion regulation for the passive viewing condition). When the effect of emotion regulation is amplified, the effect of individual difference on emotion rating might be relatively reduced.
Another finding was that the rTMS‐facilitated reappraisal effect promoted the participants to give more positive evaluations to unfamiliar peers, in which the experienced emotional feeling played a mediating role. This result suggests that the application of rTMS changed people's social attitude by influencing their emotion through cognitive reappraisal. Consistent with our finding, some studies have also observed the role of emotion in attitude change. Cancino‐Montecinos et al. (2018) found that reduced negative emotion introduced by cognitive reappraisal correlated with positive attitude change in a compliance inducing paradigm. Another intriguing finding is that the rVLPFC‐activated group showed more prosocial behaviors, i.e., they donated more money than rVLPFC‐inhibitory group in the public donation task, irrespective of the reappraisal or passive viewing conditions. The influence of rTMS on donation was observed as a main effect among groups, indicating that the rTMS‐modulated effect of rVLPFC on public donation affected general social decisions among all the participants. Making prosocial decision is a complex process requiring self‐control to solve the conflict between own benefit and the interests of others (Bellucci et al., 2020; Fehr & Camerer, 2007; Telzer et al., 2011). In accordance with this notion, the VLPFC is widely implicated in cognitive flexibility and inhibitory control, which might help to inhibit individuals' impulsive, selfish response and facilitate to consider the needs of others (Cohen & Lieberman, 2010; Samson et al., 2005; Vogeley et al., 2001). In line with the inhibitory control role of the VLPFC, the rTMS‐activated rVLFPC might promote participants to override their own interests and make other‐oriented decisions, i.e., donated more money to public welfare projects. The reason why we chose social attitude and prosocial behavior as two separate tests here was that the changes in attitude and behavior are not necessarily consistent (Anker et al., 2010). For example, some studies have shown that while prosocial behavior diminished due to depletion of self‐control, the level of trust in others was not changed (Osgood & Muraven, 2015).
It is worth noting that this study still has some limitations. Specifically, we used the International 10/20 electroencephalogram system to locate the TMS coil, which could potentially result in inaccurate coil location. We suggest future studies utilize a neuronavigational system to identify personalized stimulation target, thereby enhancing the accuracy of coil localization (Cash et al., 2021; Douw et al., 2020; Feng et al., 2022).
In conclusion, the current study provided bidirectional (inhibition and excitement) evidence for the pivotal role of the rVLPFC in voluntarily regulating emotions. We found that, activating and inhibiting this brain region not only facilitated and restrained, respectively, the effect of cognitive reappraisal, resulting in more positive or negative emotional feelings and social attitudes towards unfamiliar peers, but also influenced prosocial behaviors such as public donation. These findings deepen our understanding of neural mechanisms underlying voluntary emotion regulation, and highlight the rVLPFC as a potential brain target in treating deficits of emotion regulation in psychiatric disorders. Nevertheless, it should be noted that the VLPFC is one node of the whole emotion regulation network. Uncovering how the VLPFC works with other prefrontal regions (including, e.g., the dorsolateral and ventromedial prefrontal cortices) during emotion regulation should be the next focus of future work.
AUTHOR CONTRIBUTIONS
Dandan Zhang and Wenwen Yu designed the research. Yiwei Li, Xueying Cao, and Licheng Mo performed the experiment. Wenwen Yu and Yuming Chen analyzed the data. Dandan Zhang, Wenwen Yu, and Yiwei Li wrote the paper.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This study was funded by the National Natural Science Foundation of China (32271102; 31970980; 31920103009), the Shenzhen‐Hong Kong Institute of Brain Science (2022SHIBS0003), and the Major Project of National Social Science Foundation (20&ZD153).
Yu, W. , Li, Y. , Cao, X. , Mo, L. , Chen, Y. , & Zhang, D. (2023). The role of ventrolateral prefrontal cortex on voluntary emotion regulation of social pain. Human Brain Mapping, 44(13), 4710–4721. 10.1002/hbm.26411
DATA AVAILABILITY STATEMENT
The data and code of this study would be available upon reasonable request and with approval of the Institute of Brain and Psychological Sciences, Sichuan Normal University. More information on making this request can be obtained from the corresponding author, D. Zhang (zhangdd05@gmail.com).
REFERENCES
- Ahn, H. M. , Kim, S. E. , & Kim, S. H. (2013). The effects of high‐frequency rTMS over the left dorsolateral prefrontal cortex on reward responsiveness. Brain Stimulation, 6(3), 310–314. [DOI] [PubMed] [Google Scholar]
- Anker, A. E. , Feeley, T. H. , & Kim, H. (2010). Examining the attitude‐behavior relationship in prosocial donation domains. Journal of Applied Social Psychology, 40(6), 1293–1324. [Google Scholar]
- Bebko, G. M. , Franconeri, S. L. , Ochsner, K. N. , & Chiao, J. Y. (2011). Look before you regulate: Differential perceptual strategies underlying expressive suppression and cognitive reappraisal. Emotion, 11(4), 732–742. [DOI] [PubMed] [Google Scholar]
- Beck, A. , Steer, R. , & Brown, G. (1996). Beck depression inventory–Second edition (BDI‐II). The Psychological Corporation. [Google Scholar]
- Bellucci, G. , Camilleri, J. A. , Eickhoff, S. B. , & Krueger, F. (2020). Neural signatures of prosocial behaviors. Neuroscience & Biobehavioral Reviews, 118, 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann, T. O. , Varatheeswaran, R. , Hanlon, C. A. , Madsen, K. H. , Thielscher, A. , & Siebner, H. R. (2021). Concurrent TMS‐fMRI for causal network perturbation and proof of target engagement. NeuroImage, 237, 118093. [DOI] [PubMed] [Google Scholar]
- Blankenburg, F. , Ruff, C. C. , Bestmann, S. , Bjoertomt, O. , Eshel, N. , Josephs, O. , Weiskopf, N. , & Driver, J. (2008). Interhemispheric effect of parietal TMS on somatosensory response confirmed directly with concurrent TMS‐fMRI. Journal of Neuroscience, 28(49), 13202–13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgomaneri, S. , Battaglia, S. , Garofalo, S. , Tortora, F. , Avenanti, A. , & di Pellegrino, G. (2020). State‐dependent TMS over prefrontal cortex disrupts fear‐memory reconsolidation and prevents the return of fear. Current Biology, 30(18), 3672–3679. [DOI] [PubMed] [Google Scholar]
- Bradley, M. M. , Miccoli, L. , Escrig, M. A. , & Lang, P. J. (2008). The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology, 45(4), 602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein, L. M. , Gross, J. J. , & Ochsner, K. N. (2017). Explicit and implicit emotion regulation: A multi‐level framework. Social Cognitive and Affective Neuroscience, 12(10), 1545–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell‐Sills, L. , Simmons, A. N. , Lovero, K. L. , Rochlin, A. A. , Paulus, M. P. , & Stein, M. B. (2011). Functioning of neural systems supporting emotion regulation in anxiety‐prone individuals. NeuroImage, 54(1), 689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancino‐Montecinos, S. , Bjorklund, F. , & Lindholm, T. (2018). Dissonance reduction as emotion regulation: Attitude change is related to positive emotions in the induced compliance paradigm. PLoS One, 13(12), e0209012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash, R. F. H. , Cocchi, L. , Lv, J. , Wu, Y. , Fitzgerald, P. B. , & Zalesky, A. (2021). Personalized connectivity‐guided DLPFC‐TMS for depression: Advancing computational feasibility, precision and reproducibility. Human Brain Mapping, 42(13), 4155–4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase, H. W. , Boudewyn, M. A. , Carter, C. S. , & Phillips, M. L. (2020). Transcranial direct current stimulation: A roadmap for research, from mechanism of action to clinical implementation. Molecular Psychiatry, 25(2), 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. R. , & Lieberman, M. D. (2010). The common neural basis of exerting self control in multiple domains. In Trope Y., Hassin R., & Ochsner K. N. (Eds.), Self control in society, mind, and brain. Oxford University Press. [Google Scholar]
- Damon, W. , Lerner, R. M. , & Eisenberg, N. (2006). Handbook of child psychology, social, emotional, and personality development. John Wiley & Sons. [Google Scholar]
- Daskalakis, Z. J. , Moller, B. , Christensen, B. K. , Fitzgerald, P. B. , Gunraj, C. , & Chen, R. (2006). The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Experimental Brain Research, 174(3), 403–412. [DOI] [PubMed] [Google Scholar]
- De Witte, N. A. , Sutterlin, S. , Braet, C. , & Mueller, S. C. (2017). Psychophysiological correlates of emotion regulation training in adolescent anxiety: Evidence from the novel PIER task. Journal of Affective Disorders, 214, 89–96. [DOI] [PubMed] [Google Scholar]
- DeSteno, D. , Dasgupta, N. , Bartlett, M. Y. , & Cajdric, A. (2004). Prejudice from thin air: The effect of emotion on automatic intergroup attitudes. Psychological Science, 15(5), 319–324. [DOI] [PubMed] [Google Scholar]
- DeWall, C. N. , Twenge, J. M. , Gitter, S. A. , & Baumeister, R. F. (2009). It's the thought that counts: The role of hostile cognition in shaping aggressive responses to social exclusion. Journal of Personality and Social Psychology, 96(1), 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douw, L. , Quaak, M. , Fitzsimmons, S. M. D. D. , de Wit, S. J. , van der Werf, Y. D. , van den Heuvel, O. A. , & Vriend, C. (2020). Static and dynamic network properties of the repetitive transcranial magnetic stimulation target predict changes in emotion regulation in obsessive‐compulsive disorder. Brain Stimulation, 13(2), 318–326. [DOI] [PubMed] [Google Scholar]
- Downey, G. , & Feldman, S. I. (1996). Implications of rejection sensitivity for intimate relationships. Journal of Personality and Social Psychology, 70(6), 1327–1343. [DOI] [PubMed] [Google Scholar]
- Eisenberger, N. I. (2012). The pain of social disconnection: Examining the shared neural underpinnings of physical and social pain. Nature Reviews: Neuroscience, 13(6), 421–434. [DOI] [PubMed] [Google Scholar]
- Eisenberger, N. I. (2015). Social pain and the brain: Controversies, questions, and where to go from here. Annual Review of Psychology, 66, 601–629. [DOI] [PubMed] [Google Scholar]
- Eisenberger, N. I. , Lieberman, M. D. , & Williams, K. D. (2003). Does rejection hurt? An fMRI study of social exclusion. Science, 302(5643), 290–292. [DOI] [PubMed] [Google Scholar]
- Fehr, E. , & Camerer, C. F. (2007). Social neuroeconomics: The neural circuitry of social preferences. Trends in Cognitive Sciences, 11(10), 419–427. [DOI] [PubMed] [Google Scholar]
- Feng, Z. J. , Deng, X. P. , Zhao, N. , Jin, J. , Yue, J. , Hu, Y. S. , … Wang, J. (2022). Resting‐state fMRI functional connectivity strength predicts local activity change in the dorsal cingulate cortex: A multi‐target focused rTMS study. Cerebral Cortex, 32(13), 2773–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald, P. B. , Fountain, S. , & Daskalakis, Z. J. (2006). A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clinical Neurophysiology, 117(12), 2584–2596. [DOI] [PubMed] [Google Scholar]
- Fitzsimmons, S. , Douw, L. , van den Heuvel, O. A. , van der Werf, Y. D. , & Vriend, C. (2020). Resting‐state and task‐based centrality of dorsolateral prefrontal cortex predict resilience to 1 Hz repetitive transcranial magnetic stimulation. Human Brain Mapping, 41(11), 3161–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung, K. , & Alden, L. E. (2017). Once hurt, twice shy: Social pain contributes to social anxiety. Emotion, 17(2), 231–239. [DOI] [PubMed] [Google Scholar]
- Gilio, F. , Rizzo, V. , Siebner, H. R. , & Rothwell, J. C. (2003). Effects on the right motor hand‐area excitability produced by low‐frequency rTMS over human contralateral homologous cortex. Journal of Physiology, 551(Pt 2), 563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, J. J. (2002). Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology, 39(3), 281–291. [DOI] [PubMed] [Google Scholar]
- Halperin, E. , Porat, R. , Tamir, M. , & Gross, J. J. (2013). Can emotion regulation change political attitudes in intractable conflicts? From the laboratory to the field. Psychological Science, 24(1), 106–111. [DOI] [PubMed] [Google Scholar]
- Hayes, A. F. (2013). Mediation, moderation, and conditional process analysis. Guilford. [DOI] [PubMed] [Google Scholar]
- He, Z. , Lin, Y. , Xia, L. , Liu, Z. , Zhang, D. , & Elliott, R. (2018). Critical role of the right VLPFC in emotional regulation of social exclusion: A tDCS study. Social Cognitive and Affective Neuroscience, 13(4), 357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Z. , Liu, Z. , Zhao, J. , Elliott, R. , & Zhang, D. (2020). Improving emotion regulation of social exclusion in depression‐prone individuals: A tDCS study targeting right VLPFC. Psychological Medicine, 50(16), 2768–2779. [DOI] [PubMed] [Google Scholar]
- He, Z. , Zhao, J. , Shen, J. , Muhlert, N. , Elliott, R. , & Zhang, D. (2020). The right VLPFC and downregulation of social pain: A TMS study. Human Brain Mapping, 41(5), 1362–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, R. R. , Bradley, M. M. , & Lang, P. J. (2014). Modulation of the initial light reflex during affective picture viewing. Psychophysiology, 51(9), 815–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershman, R. , Henik, A. , & Cohen, N. (2019). CHAP: Open‐source software for processing and analyzing pupillometry data. Behavior Research Methods, 51(3), 1059–1074. [DOI] [PubMed] [Google Scholar]
- Innes‐Ker, A. , & Niedenthal, P. M. (2002). Emotion concepts and emotional states in social judgment and categorization. Journal of Personality and Social Psychology, 83(4), 804–816. [PubMed] [Google Scholar]
- Jazaieri, H. , Morrison, A. S. , Goldin, P. R. , & Gross, J. J. (2015). The role of emotion and emotion regulation in social anxiety disorder. Current Psychiatry Reports, 17(1), 531. [DOI] [PubMed] [Google Scholar]
- Johnstone, T. , van Reekum, C. M. , Urry, H. L. , Kalin, N. H. , & Davidson, R. J. (2007). Failure to regulate: Counterproductive recruitment of top‐down prefrontal‐subcortical circuitry in major depression. Journal of Neuroscience, 27(33), 8877–8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, C. R. , Kirkland, T. , & Cunningham, W. A. (2014). Attitudes, evaluation, and emotion regulation. In Gross J. J. (Ed.), Handbook of emotion regulation (pp. 251–266). The Guilford Press. [Google Scholar]
- Jung, J. , Bungert, A. , Bowtell, R. , & Jackson, S. R. (2016). Vertex stimulation as a control site for transcranial magnetic stimulation: A concurrent TMS/fMRI study. Brain Stimulation, 9(1), 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, S. H. , Shin, J. E. , Jeong, Y. S. , & Shin, H. I. (2008). Changes in motor cortical excitability induced by high‐frequency repetitive transcranial magnetic stimulation of different stimulation durations. Clinical Neurophysiology, 119(1), 71–79. [DOI] [PubMed] [Google Scholar]
- Kinley, I. , & Levy, Y. (2022). PuPl: An open‐source tool for processing pupillometry data. Behavior Research Methods, 54(4), 2046–2069. [DOI] [PubMed] [Google Scholar]
- Kinner, V. L. , Kuchinke, L. , Dierolf, A. M. , Merz, C. J. , Otto, T. , & Wolf, O. T. (2017). What our eyes tell us about feelings: Tracking pupillary responses during emotion regulation processes. Psychophysiology, 54(4), 508–518. [DOI] [PubMed] [Google Scholar]
- Klomjai, W. , Katz, R. , & Lackmy‐Vallee, A. (2015). Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Annals of Physical and Rehabilitation Medicine, 58(4), 208–213. [DOI] [PubMed] [Google Scholar]
- Knecht, S. , Ellger, T. , Breitenstein, C. , Ringelstein, E. B. , & Henningsen, H. (2003). Changing cortical excitability with low‐frequency transcranial magnetic stimulation can induce sustained disruption of tactile perception. Biological Psychiatry, 53(2), 175–179. [DOI] [PubMed] [Google Scholar]
- Lebowitz, M. S. , & Dovidio, J. F. (2015). Implications of emotion regulation strategies for empathic concern, social attitudes, and helping behavior. Emotion, 15(2), 187–194. [DOI] [PubMed] [Google Scholar]
- Leknes, S. , Wessberg, J. , Ellingsen, D. M. , Chelnokova, O. , Olausson, H. , & Laeng, B. (2013). Oxytocin enhances pupil dilation and sensitivity to 'hidden' emotional expressions. Social Cognitive and Affective Neuroscience, 8(7), 741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Meng, X. M. , Li, R. Y. , Zhang, R. , Zhang, Z. , & Du, Y. F. (2016). Effects of different frequencies of repetitive transcranial magnetic stimulation on the recovery of upper limb motor dysfunction in patients with subacute cerebral infarction. Neural Regeneration Research, 11(10), 1584–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S. , Xie, H. , Zheng, Z. , Chen, W. , Xu, F. , Hu, X. , & Zhang, D. (2022). The causal role of the bilateral ventrolateral prefrontal cortices on emotion regulation of social feedback. Human Brain Mapping, 43(9), 2898–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz, M. (1987). Social Phobia. Modern Problems in Pharmacopsychiatry, 22, 141–173. [DOI] [PubMed] [Google Scholar]
- Manstead, A. S. R. (1991). Emotion in social life. Cognition & Emotion, 5(5–6), 353–362. [Google Scholar]
- Masten, C. L. , Eisenberger, N. I. , Borofsky, L. A. , Pfeifer, J. H. , McNealy, K. , Mazziotta, J. C. , & Dapretto, M. (2009). Neural correlates of social exclusion during adolescence: Understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience, 4(2), 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita, R. , Puschmann, S. , Baillet, S. , & Zatorre, R. J. (2021). Inhibitory effect of tDCS on auditory evoked response: Simultaneous MEG‐tDCS reveals causal role of right auditory cortex in pitch learning. NeuroImage, 233, 117915. [DOI] [PubMed] [Google Scholar]
- McRae, K. , & Gross, J. J. (2020). Emotion regulation. Emotion, 20(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Ochsner, K. N. , Silvers, J. A. , & Buhle, J. T. (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the new York Academy of Sciences, 1251, E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda, K. , Okamoto, Y. , Nakashima, K. , Nittono, H. , Yoshimura, S. , Yamawaki, S. , Yamaguchi, S. , & Ura, M. (2010). Does low self‐esteem enhance social pain? The relationship between trait self‐esteem and anterior cingulate cortex activation induced by ostracism. Social Cognitive and Affective Neuroscience, 5(4), 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osgood, J. M. , & Muraven, M. (2015). Self‐control depletion does not diminish attitudes about being prosocial but does diminish prosocial behaviors. Basic and Applied Social Psychology, 37(1), 68–80. [Google Scholar]
- Parker, J. D. , Taylor, G. J. , & Bagby, R. M. (2003). The 20‐item Toronto alexithymia scale: III. Reliability and factorial validity in a community population. Journal of Psychosomatic Research, 55(3), 269–275. [DOI] [PubMed] [Google Scholar]
- Partala, T. , & Surakka, V. (2003). Pupil size variation as an indication of affective processing. International Journal of Human‐Computer Studies, 59(1–2), 185–198. [Google Scholar]
- Phillips, M. L. , Ladouceur, C. D. , & Drevets, W. C. (2008). A neural model of voluntary and automatic emotion regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13(9), 833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher, K. J. , & Hayes, A. F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40(3), 879–891. [DOI] [PubMed] [Google Scholar]
- Riva, P. , Romero Lauro, L. J. , Dewall, C. N. , & Bushman, B. J. (2012). Buffer the pain away: Stimulating the right ventrolateral prefrontal cortex reduces pain following social exclusion. Psychological Science, 23(12), 1473–1475. [DOI] [PubMed] [Google Scholar]
- Riva, P. , Romero Lauro, L. J. , DeWall, C. N. , Chester, D. S. , & Bushman, B. J. (2015). Reducing aggressive responses to social exclusion using transcranial direct current stimulation. Social Cognitive and Affective Neuroscience, 10(3), 352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva, P. , Romero Lauro, L. J. , Vergallito, A. , DeWall, C. N. , & Bushman, B. J. (2015). Electrified emotions: Modulatory effects of transcranial direct stimulation on negative emotional reactions to social exclusion. Social Neuroscience, 10(1), 46–54. [DOI] [PubMed] [Google Scholar]
- Rive, M. M. , van Rooijen, G. , Veltman, D. J. , Phillips, M. L. , Schene, A. H. , & Ruhe, H. G. (2013). Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neuroscience & Biobehavioral Reviews, 37(10 Pt 2), 2529–2553. [DOI] [PubMed] [Google Scholar]
- Rosenberg, M. (1965). Society and adolescent child. Princeton University Press. [Google Scholar]
- Samson, D. , Apperly, I. A. , Kathirgamanathan, U. , & Humphreys, G. W. (2005). Seeing it my way: A case of a selective deficit in inhibiting self‐perspective. Brain, 128(Pt 5), 1102–1111. [DOI] [PubMed] [Google Scholar]
- Schambra, H. M. , Sawaki, L. , & Cohen, L. G. (2003). Modulation of excitability of human motor cortex (M1) by 1 Hz transcranial magnetic stimulation of the contralateral M1. Clinical Neurophysiology, 114(1), 130–133. [DOI] [PubMed] [Google Scholar]
- Silvanto, J. , Cattaneo, Z. , Battelli, L. , & Pascual‐Leone, A. (2008). Baseline cortical excitability determines whether TMS disrupts or facilitates behavior. Journal of Neurophysiology, 99(5), 2725–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers, J. A. , McRae, K. , Gabrieli, J. D. , Gross, J. J. , Remy, K. A. , & Ochsner, K. N. (2012). Age‐related differences in emotional reactivity, regulation, and rejection sensitivity in adolescence. Emotion, 12(6), 1235–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden, R. J. , O'Farrell, K. R. , Burley, D. , Erichsen, J. T. , Newton, N. V. , & Gray, N. S. (2016). The pupil's response to affective pictures: Role of image duration, habituation, and viewing mode. Psychophysiology, 53(8), 1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger, C. D. , Gorsuch, R. L. , Lushene, R. , Vagg, P. R. , & Jacobs, G. A. (1983). Manual for the state‐trait anxiety inventory. Consulting Psychologists Press. [Google Scholar]
- Telzer, E. H. , Masten, C. L. , Berkman, E. T. , Lieberman, M. D. , & Fuligni, A. J. (2011). Neural regions associated with self control and mentalizing are recruited during prosocial behaviors towards the family. NeuroImage, 58(1), 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twenge, J. M. , Baumeister, R. F. , Tice, D. M. , & Stucke, T. S. (2001). If you can't join them, beat them: Effects of social exclusion on aggressive behavior. Journal of Personality and Social Psychology, 81(6), 1058–1069. [DOI] [PubMed] [Google Scholar]
- Urry, H. L. , van Reekum, C. M. , Johnstone, T. , & Davidson, R. J. (2009). Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. NeuroImage, 47(3), 852–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry, H. L. , van Reekum, C. M. , Johnstone, T. , Kalin, N. H. , Thurow, M. E. , Schaefer, H. S. , Jackson, C. A. , Frye, C. J. , Greischar, L. L. , Alexander, A. L. , & Davidson, R. J. (2006). Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience, 26(16), 4415–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velde, J. , Gromann, P. M. , Swart, M. , Wiersma, D. , de Haan, L. , Bruggeman, R. , Krabbendam, L. , & Aleman, A. (2015). Alexithymia influences brain activation during emotion perception but not regulation. Social Cognitive and Affective Neuroscience, 10(2), 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reekum, C. M. , Johnstone, T. , Urry, H. L. , Thurow, M. E. , Schaefer, H. S. , Alexander, A. L. , & Davidson, R. J. (2007). Gaze fixations predict brain activation during the voluntary regulation of picture‐induced negative affect. NeuroImage, 36(3), 1041–1055. [DOI] [PubMed] [Google Scholar]
- Vijayakumar, N. , Cheng, T. W. , & Pfeifer, J. H. (2017). Neural correlates of social exclusion across ages: A coordinate‐based meta‐analysis of functional MRI studies. NeuroImage, 153, 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeley, K. , Bussfeld, P. , Newen, A. , Herrmann, S. , Happe, F. , Falkai, P. , Maier, W. , Shah, N. J. , Fink, G. R. , & Zilles, K. (2001). Mind reading: Neural mechanisms of theory of mind and self‐perspective. NeuroImage, 14(1 Pt 1), 170–181. [DOI] [PubMed] [Google Scholar]
- Vohs, K. D. , & Baumeister, R. F. (2016). Handbook of self‐regulation: Research, theory, and applications. Guilford Publications. [Google Scholar]
- Wang, H. , Braun, C. , & Enck, P. (2017). How the brain reacts to social stress (exclusion)—A scoping review. Neuroscience & Biobehavioral Reviews, 80, 80–88. [DOI] [PubMed] [Google Scholar]
- Williams, K. D. (2007). Ostracism. Annual Review of Psychology, 58, 425–452. [DOI] [PubMed] [Google Scholar]
- Zadro, L. , Williams, K. D. , & Richardson, R. (2004). How low can you go? Ostracism by a computer is sufficient to lower self‐reported levels of belonging, control, self‐esteem, and meaningful existence. Journal of Experimental Social Psychology, 40(4), 560–567. [Google Scholar]
- Zhao, J. , Mo, L. , Bi, R. , He, Z. , Chen, Y. , Xu, F. , Xie, H. , & Zhang, D. (2021). The VLPFC versus the DLPFC in downregulating social pain using reappraisal and distraction strategies. Journal of Neuroscience, 41(6), 1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Z. , Li, S. , Mo, L. , Chen, W. , & Zhang, D. (2022). ISIEA: An image database of social inclusion and exclusion in young Asian adults. Behavior Research Methods, 54(5), 2409–2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and code of this study would be available upon reasonable request and with approval of the Institute of Brain and Psychological Sciences, Sichuan Normal University. More information on making this request can be obtained from the corresponding author, D. Zhang (zhangdd05@gmail.com).


