Abstract
Many mucosal pathogens invade the host by initially infecting the organized mucosa-associated lymphoid tissue (o-MALT) such as Peyer’s patches or nasal cavity-associated lymphoid tissue (NALT) before spreading systemically. There is no clear demonstration that serum antibodies can prevent infections in o-MALT. We have tested this possibility by using the mouse mammary tumor virus (MMTV) as a model system. In peripheral lymph nodes or in Peyer’s patches or NALT, MMTV initially infects B lymphocytes, which as a consequence express a superantigen (SAg) activity. The SAg molecule induces the local activation of a subset of T cells within 6 days after MMTV infection. We report that similar levels of anti-SAg antibody (immunoglobulin G) in serum were potent inhibitors of the SAg-induced T-cell response both in peripheral lymph nodes and in Peyer’s patches or NALT. This result clearly demonstrates that systemic antibodies can gain access to Peyer’s patches or NALT.
The development of vaccines against infectious pathogens such as pneumococci, meningococci, rotavirus, herpesvirus, human papillomaviruses, and human immunodeficiency virus (HIV) is a priority in the next decade. It is well established that effective antigen presentation is a key factor in successful immunization, and many efforts are devoted to selecting the best adjuvant, the best carrier, or the appropriate live attenuated pathogens to deliver the vaccines (5). In addition to being adapted to the pathogen (antibody and/or cytotoxic responses), the immune response must also be well distributed spatially, i.e., the immune effectors need to gain access to the site of infection in order to control infections. This question is particularly relevant in the development of mucosal vaccines. It is thought that the immune effectors protecting mucosal surfaces are secretory immunoglobulin A (sIgA) and mucosal cytotoxic T lymphocytes (CTL) (14). Therefore, a vaccine that induces the production of pathogen-specific sIgA in mucosal secretions and mucosal CTL is expected to block infection. However, it is known that sIgA is not secreted uniformly over the mucosal surfaces. Indeed, the epithelial cells covering the organized mucosa-associated lymphoid tissue (o-MALT), due to a lack of poly-Ig receptor expression (19), do not secrete sIgA. Hence, o-MALT will not be protected from pathogen invasion by a sIgA antibody response and thus provides gateways for many mucosal pathogens (18). The identification of immune effectors that clear pathogens from o-MALT is crucial to the design of immunization protocols aimed at blocking early stages of infection with mucosal pathogens. In this study we examined whether serum IgG antibodies can block a critical event initiated in o-MALT which results in the dissemination of retrovirus. For this purpose, we used as a model the mouse mammary tumor virus (MMTV), a type B retrovirus transmitted from the mother to the offspring through milk (6), which crosses the intestinal barrier of the neonate by an unknown process. MMTV initially infects Peyer’s patch B lymphocytes (13), which produce a superantigen (SAg) that triggers a T-cell response (9, 10). Later the virus spreads systemically to all lymphoid organs and to the mammary glands. Recently, we reported that adult mice are susceptible to mucosal MMTV infection via the nasal route, which results in a SAg response in the o-MALT of the nasal cavity (referred to as the nasal cavity-associated lymphoid tissue [NALT]) (23). Adult mice can also be infected systemically: MMTV is injected in the hind footpad, infects B lymphocytes, and triggers a SAg response in the draining popliteal lymph node (9, 10). The SAg-reactive T-cell response, which is restricted to the site of entry of MMTV (Peyer’s patches [PP], NALT, or the popliteal lymph node), is critical for the viral infection (9, 10, 23). By systemic injection of IgG antibodies directed against the SAg molecule into mice mucosally or systemically infected by MMTV, we observed that equivalent antibody levels were potent inhibitors of the SAg response in PP, NALT and the popliteal lymph node.
Serum IgG reaches peripheral lymph nodes and blocks the MMTV-driven SAg response.
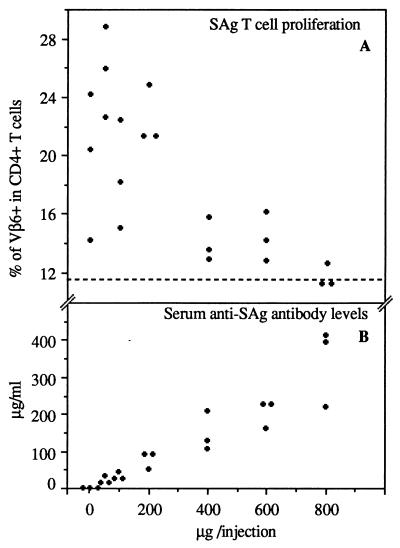
We have previously produced a monoclonal antibody specific to the COOH-terminal end of the SAg molecule encoded by the SW strain of MMTV (1). First, we tested whether the injection of this antibody could inhibit the SAg-induced T-cell response in a peripheral lymph node. BALB/c mice were injected intraperitoneally with anti-SAg antibodies (1) purified on protein G-Sepharose (Pharmacia Biotech Europe GmbH, Dübendorf, Switzerland) and subcutaneously in the hind footpad with MMTV-infected milk. BALB/c mice were originally purchased from Harlan Olac (London, United Kingdom). Infected milk was aspirated from lactating MMTV(SW)-infected mice which were obtained from IFFA Credo (l’Arlabesques, France) and bred in our animal facility. The milk was diluted to 1:1,000 in phosphate-buffered saline (PBS) and 20 μl was injected into the hind footpad. Within 6 days, the lymphoid population of the draining popliteal lymph node was recovered and labelled with a mixture of anti-CD4 (phycoerythrin-conjugated anti-L3T4; Caltag, San Francisco, Calif.) and affinity-purified, fluorescein isothiocyanate-conjugated anti-Vβ6 (44-22-1) (20), anti-Vβ14 (14.2) (16), or anti-Vβ2 (17). All samples were analyzed by using a FACScan and the Lysys II program (Becton Dickinson and Co.). Dead cells were excluded by a combination of forward and side scatter. In mice infected with MMTV, the percentage of SAg-reactive CD4+ Vβ6+ T cells increased from 12 to as much as 30% (Fig. 1). The percentage of non-SAg-reactive CD4+ Vβ2+ T cells (around 7.0%) was not affected by MMTV(SW) infection (data not shown). When MMTV-infected mice were injected with anti-SAg antibodies, the SAg-reactive T-cell response was inhibited in a dose-dependent manner (Fig. 1). When mice were injected with 800 μg of anti-SAg antibody on days 0, 2, and 4 after infection, the T-cell response was fully inhibited. Mice injected with 600 or 400 μg showed partial inhibition of the SAg T-cell response (Fig. 1). We determined the serum anti-SAg antibody levels at sacrifice in the different groups of mice (Fig. 1) and found that the serum antibody level needed to fully inhibit the SAg T-cell response in the popliteal lymph node is approximately 300 μg/ml. The antibody levels were determined as follows. Nunc (Roskilde, Denmark) immunoplates I were coated with the COOH-terminal MMTV(SW) SAg peptide KILYNMKYTHGGRVGFDPF (0.2 μM) and incubated overnight at 4°C. After washings and the saturation of nonspecific sites, serial dilutions of serum were added and incubated for 2 h at 37°C. The bound anti-SAg antibodies were detected by the addition of biotin-labelled anti-mouse IgG (RPN 1177; Amersham, Little Chalfont, United Kingdom) for 1 h at 37°C; this was followed by the addition of alkaline phosphatase coupled to avidin (A-2527; Sigma, FLUKA Division, Buchs, Switzerland) and para-nitrophenyl phosphate (Art. 6850; Merck). The optical density was read at 405 nm. The antibody concentrations were established by comparison of the tested serum with purified anti-SAg antibodies of known concentrations. Our results indicate that serum antibody levels of >300 μg/ml are required to block a systemic SAg response.
FIG. 1.
Systemic anti-SAg antibodies can inhibit the SAg response triggered by MMTV infection in the popliteal lymph node. (A) Percentages of CD4+ Vβ6+ T cells in popliteal lymph nodes of MMTV-infected mice injected with anti-SAg antibodies. Groups of mice were injected at days 0, 2, and 4 after infection with 800, 600, 400, 200, 100, 50, or 0 μg of antibodies. The dotted horizontal line represents the upper limit of percentages of CD4+ Vβ6+ T cells in the popliteal lymph nodes of noninfected mice. (B) Anti-SAg antibody levels determined in sera of mice at sacrifice.
Serum IgG reaches the PP and blocks the MMTV-driven SAg response.
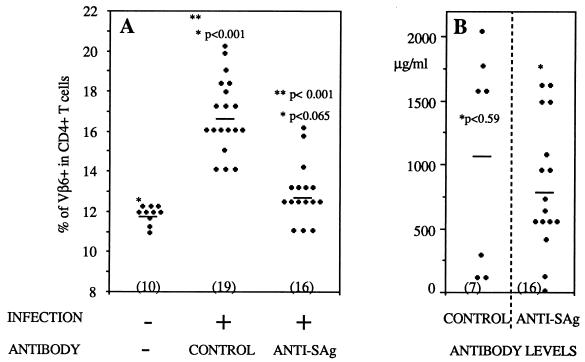
Many environmental pathogens, eluding the immune exclusion mechanism mediated by sIgA, initiate their infectious cycles in o-MALT (15, 18). There is a need to identify the immune effectors capable of clearing such pathogens from o-MALT. Here we tested the ability of a systemic antibody to reach the PP. Intestinal MMTV infection is initially restricted to the PP during the neonatal period (13), and within 6 days it triggers the response of SAg-reactive T cells locally. To test whether systemic antibodies gain access to the PP, we systemically injected MMTV-infected neonates with anti-SAg antibodies and monitored the percentage of SAg-reactive T cells by flow cytometry. Three-day-old neonates were injected subcutaneously in the back with 0.5 × 106 hybridoma cells (26) or received injections of purified antibodies (450 μg/injection) at days 10, 12, 13, 14, 15, 16, and 17. Neonates were infected by foster nursing on MMTV-infected lactating females between the ages of 8 and 12 days and were sacrificed at day 18, and PP lymphocytes were recovered and analyzed by flow cytometry, as previously described (13). Four independent experiments were performed. In each experiment, we compared the results obtained for injected and noninjected neonates foster-nursed by the same infected mother to those for unmanipulated neonates from the same litter left with the uninfected mother. Figure 2 shows the results for mice either implanted with hybridoma cells or injected with purified antibodies, since no difference was found between these two antibody delivery protocols. In noninfected 18-day-old mice, PP CD4+ Vβ6+ T cells represent 11 ± 1% of the total CD4+ T-cell population (Fig. 2A). In contrast, in age-matched MMTV(SW)-infected pups, the proportion of PP CD4+ Vβ6+ T cells increased up to 17% (Fig. 2A). The percentage of non-SAg-reactive CD4+ Vβ2+ T cells (7.2%) was not affected by MMTV(SW) infection (data not shown). When anti-SAg antibodies were injected into neonates challenged with MMTV(SW), the SAg-reactive T-cell response was inhibited in 11 of 16 mice (Fig. 2A). At the time of sacrifice, the serum anti-SAg antibody levels varied from 4 to 1,610 μg/ml with hybridoma cells and from 405 to 1,600 μg/ml with purified antibodies (Fig. 2B). In neonates injected with isotype-matched control antibodies (Helicobacter pylori urease-specific IgG2b), the SAg-induced T-cell response was not inhibited (7 of 7 mice) (Fig. 2A). As in the experimental group, the serum antibody levels in control mice ranged from 106 to 2,036 μg/ml (Fig. 2B). For serum control antibody level determinations, we performed the same procedure as described above for the anti-SAg antibody levels, except that the Nunc immunoplates I were coated with recombinant Helicobacter pylori urease (kindly provided by Oravax, Boston, Mass.). In addition, we tested the ability of the sera of neonates injected with the anti-SAg antibody to block in vitro the activation of Vβ6+ T hybridoma (RG17) (measured by interleukin 2 secretion) in response to the SAg molecules expressed by LBB cells (1). In contrast to the sera of neonates injected with control antibodies, which were not able to inhibit Vβ6+ T hybridoma activation, the sera of neonates injected with the anti-SAg antibody were potent inhibitors (data not shown). This result confirms that the inhibition of the SAg-induced T-cell response is mediated by seric anti-SAg antibodies. To further characterize the specific inhibition of the SAg-induced T-cell response by the anti-SAg antibody, mice were challenged with the C4 strain of MMTV (22). The SW and C4 SAgs differ in their COOH-terminal sequences; this polymorphism is distinguishable by the anti-SAg antibody and is responsible for the specificity of the T-cell response, i.e., C4 and SW strains induce the responses of CD4+ Vβ2+ and CD4+ Vβ6+ T cells, respectively (11, 22). The anti-SAg antibody that we used was unable to prevent the response of CD4+ Vβ2+ T cells in the PP following challenge with the C4 strain of MMTV (Fig. 3A). Taken together, our results indicate that serum anti-SAg antibodies can block the SAg-induced T-cell response in the PP.
FIG. 2.
Systemic anti-SAg antibodies can inhibit the SAg response triggered by MMTV infection in the PP of neonates. (A) Percentages of CD4+ Vβ6+ T cells in PP of MMTV-infected or uninfected neonates injected with anti-SAg or anti-SAg hybridoma cells or control antibodies. The number of mice in each group is given in parentheses. ∗, statistical comparison between uninfected and infected pups; ∗∗, statistical comparison between infected neonates injected with control or anti-SAg antibodies. The Mann-Whitney Wilcoxon test was used for statistical analysis; differences were taken as statistical significant for P values of <0.05. (B) Control or anti-SAg antibody levels determined in sera of neonates at sacrifice. ∗, statistical comparison between control and anti-SAg antibody levels. Horizontal lines in both panels represent means.
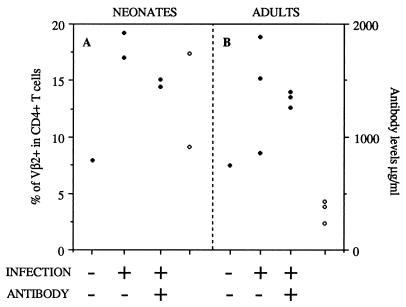
FIG. 3.
Systemic anti-SAg antibodies cannot inhibit the SAg response triggered by the C4 strain of MMTV. (A) Percentages of CD4+ Vβ2+ T cells (solid symbols) in the PP of MMTV(C4)-infected or uninfected neonates injected with anti-SAg antibodies. Two neonates were infected and injected with hybridoma cells secreting anti-SAg antibodies. Two neonates were infected but not injected with anti-SAg antibodies. One neonate was left unmanipulated. Serum antibody levels (in micrograms per milliliter; right y axis) were determined at sacrifice (open symbols). (B) Percentages of CD4+ Vβ2+ T cells (solid symbols) in NALT of MMTV(C4)-infected or uninfected adult mice injected with anti-SAg antibodies. Four mice were infected and injected with hybridoma cells secreting anti-SAg antibodies. Three mice were infected and not injected with antibodies. Open symbols, serum anti-SAg antibody levels determined at sacrifice.
Serum IgG reaches the NALT and blocks the MMTV-driven SAg response.
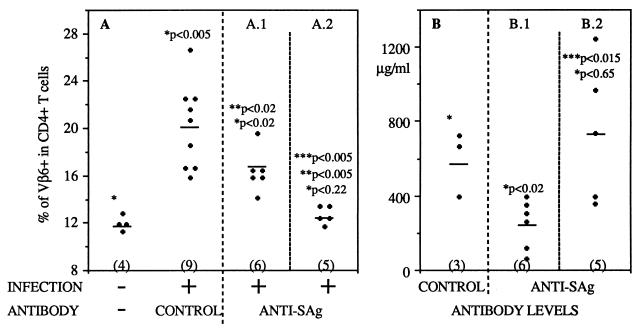
Here we showed that in newborn mice, serum antibodies are able to traverse PP capillaries, where they inhibit the SAg activity of MMTV. Whether the capillaries of the PP are equally permeable to antibodies in adult mice cannot be answered by using the MMTV model because the acidic conditions and digestive-enzyme secretions of the adult gastrointestinal tract are likely to inactivate the virus and prevent PP infection (24). However, we could extend our study to another o-MALT located in the adult mouse nasal cavity: the NALT. The NALT has been suggested to be a portal of entry for many mucosal pathogens (12), and notably for MMTV when the virus is given by the nasal route. Furthermore, the human homologue of the NALT is the Waldeyer rings (7), which have been recently implicated in HIV transmission during oral sexual intercourse (3, 21). Hence, in order to develop an efficient HIV vaccine, it is important to know whether systemic antibodies can gain access to the mucosal lymphoid organs associated with the mucosa of the upper respiratory airways. By using the newly developed adult model of MMTV mucosal infection (23), we examined whether serum anti-SAg IgG reached the NALT and inhibited the SAg response caused by nasal MMTV infection. Six days after nasal instillation of MMTV, a typical SAg-reactive T-cell response was detected in NALT (Fig. 4A). For nasal MMTV infection, adult mice (8 to 10 weeks old) were anesthetized and 20 μl of infected milk diluted to 1:2 in PBS was inserted into the nostrils. The method used for NALT isolation was described previously (23). Seven adult mice were injected subcutaneously with hybridoma cells secreting the anti-SAg antibody and were challenged 10 days later. Six days after infection, the mice were sacrificed and the NALT cells were analyzed by flow cytometry. Four mice were not protected, and a significant SAg response was measured (Fig. 4A). In three mice the T-cell response was blocked (Fig. 4A). We correlated protection with serum antibody concentration and found that nonprotected mice had low levels of antibodies (30 to 250 μg/ml), while protected mice had titers higher than 350 μg/ml at the time of sacrifice (Fig. 4B). All the mice infected with MMTV but not injected with antibodies developed SAg responses (Fig. 4A). In a parallel experiment, purified anti-SAg antibodies were injected prior to MMTV challenge. Four mice were injected with anti-SAg antibodies and were infected via the nasal route with MMTV. Half the mice developed SAg responses, with serum anti-SAg antibody levels around 295 to 340 μg/ml (Fig. 4), while the two which were protected had higher antibody titers (380 and 350 μg/ml) (Fig. 4). Figure 4 shows the results for mice either implanted with hybridoma cells or injected with purified antibodies, since no difference was found between these two antibody delivery protocols. The inhibition of the SAg-induced T-cell response was specific, since serum control antibody levels as high as 600 μg/ml were unable to provide inhibition (Fig. 4). Furthermore, 425 μg of anti-SAg antibody/ml did not block the SAg response triggered by MMTV(C4) (Fig. 3B). Our data demonstrate that serum anti-SAg antibodies can inhibit the SAg-induced T-cell response in NALT tissue.
FIG. 4.
Systemic anti-SAg antibodies can inhibit the SAg response triggered by MMTV infection in NALT of adult mice. (A) Percentages of CD4+ Vβ6+ T cells in NALT of MMTV-infected or uninfected adult mice injected with anti-SAg or control antibodies. The number of mice used in each group is given in parentheses. (B) Control or anti-SAg antibody levels determined in the sera of mice at sacrifice. Panels A.1 and B.1 correspond to one group of mice, and panels A.2 and B.2 correspond to another. ∗, statistical comparison between uninfected and infected pups; ∗∗, statistical comparison between infected neonates injected with control or anti-SAg antibodies; ∗∗∗, statistical comparison between A.1 and A.2 results or between B.1 and B.2 results. Horizontal lines represent means.
In contrast to the findings of a previous report (2), we demonstrate that systemic antibodies can reach o-MALT and peripheral lymph nodes with the same efficiency, since similar levels of anti-SAg antibody (IgG) in serum were potent inhibitors of the SAg-induced T-cell response both in peripheral lymph nodes and in the PP or NALT. Until now, it was assumed that an efficient vaccine directed against a mucosal pathogen must elicit a strong sIgA response directed against the exposed surface structures of the pathogen. Although a sIgA response causing immune exclusion should be protective in the intestinal, nasal, or respiratory secretions, there is evidence to the contrary for the o-MALT. Indeed, sIgA has been shown to attach and translocate into the o-MALT via M cells. Pathogens nonspecifically or specifically associated with sIgA could follow the same transcytotic pathway and infect o-MALT by this mechanism (25). A vaccine inducing a systemic antibody response would therefore compensate for the ineffectiveness (19) or the harmful consequences (25) of a sIgA response. Furthermore, an appropriate systemic antibody response might also be effective at overcoming an inefficient T cytotoxic response, which could be due to the upregulation of Fas ligand by virus-infected cells (such as HIV [4]), creating an immunoprivileged site for the virus (8).
Acknowledgments
We thank Sally Hopkins for the critical reading of the manuscript and Hiltrud Stubbe, Lene Gamborg, and Corinne Tallichet-Blanc for excellent technical help.
This work was supported by grants to J.-P.K. from the Swiss National Science Foundation (31-37612.93), the Swiss AIDS program (3139-37155.93), and the Swiss Research against Cancer Foundation (AKT 622) and by a grant to H.A.-O. from the Swiss National Science Foundation (31-42468.94).
REFERENCES
- 1.Acha Orbea H, Scarpellino L, Shakhov A N, Held W, MacDonald H R. Inhibition of mouse mammary tumor virus-induced T cell responses in vivo by antibodies to open reading frame protein. J Exp Med. 1992;176:1769–1772. doi: 10.1084/jem.176.6.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan C H, Trier J S. Structure and permeability differ in subepithelial villus and Peyer’s patch follicle capillaries. Gastroenterology. 1991;100:1172–1179. [PubMed] [Google Scholar]
- 3.Baba T W, Trichel A M, Liska V, Martin L N, Murphey-Corb M, Ruprecht R M. Infection and AIDS in adult macaques after nontraumatic oral exposure to cell-free SIV. Science. 1996;272:1486–1489. doi: 10.1126/science.272.5267.1486. [DOI] [PubMed] [Google Scholar]
- 4.Badley A D, McElhinny J A, Leibson P J, Lynch D H, Alderson M R, Paya C V. Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J Virol. 1996;70:199–206. doi: 10.1128/jvi.70.1.199-206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beverley P C L. Vaccine immunity. Immunol Today. 1997;18:413–415. doi: 10.1016/s0167-5699(97)01120-1. [DOI] [PubMed] [Google Scholar]
- 6.Bittner J J. The milk influence of breast tumors in mice. Science. 1942;95:462–463. doi: 10.1126/science.95.2470.462. [DOI] [PubMed] [Google Scholar]
- 7.Frieke K C, Koornstra P J, Hameleers D M H, Biewenga J, Spit B J, Duijvestijn A M, van Breda Vriesman P J C, Sminia T. The role of nasopharyngeal lymphoid tissue. Immunol Today. 1992;13:219–224. doi: 10.1016/0167-5699(92)90158-4. [DOI] [PubMed] [Google Scholar]
- 8.Griffith T S, Ferguson T A. The role of FasL-induced apoptosis in immune privilege. Immunol Today. 1997;18:240–244. doi: 10.1016/s0167-5699(97)81663-5. [DOI] [PubMed] [Google Scholar]
- 9.Held W, Waanders G, Shakhov A N, Scarpellino L, Acha-Orbea H, MacDonald H R. Superantigen-induced immune stimulation amplifies mouse mammary tumor virus infection and allows virus transmission. Cell. 1993;74:529–540. doi: 10.1016/0092-8674(93)80054-i. [DOI] [PubMed] [Google Scholar]
- 10.Held W, Shakhov A N, Izui S, Waanders G, Scarpellino L, MacDonald H R, Acha-Orbea H. Superantigen reactive CD4+ T cells are required to stimulate B cells after infection with mouse mammary tumor virus. J Exp Med. 1993;177:359–366. doi: 10.1084/jem.177.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Held W, Shakhov A N, Waanders G, Scarpellino L, Luethy R, Kraehenbuhl J P, MacDonald H R, Acha-Orbea H. An exogenous mouse mammary tumor virus with properties of mls-1a (mtv-7) J Exp Med. 1992;175:1623–1633. doi: 10.1084/jem.175.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hopkins S, Fisher G, Kraehenbuhl J P, Velin D. Nasal associated lymphoid tissue—a site for vaccination and pathogen entry. STP Pharm Sci. 1998;8:47–51. [Google Scholar]
- 13.Karapetian O, Shakhov A N, Kraehenbuhl J P, Acha-Orbea H. Retroviral infection of neonatal Peyer’s patch lymphocytes: the mouse mammary tumor virus model. J Exp Med. 1994;180:1511–1516. doi: 10.1084/jem.180.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kraehenbuhl J P, Neutra M R. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol Rev. 1992;72:853–879. doi: 10.1152/physrev.1992.72.4.853. [DOI] [PubMed] [Google Scholar]
- 15.Kraehenbuhl J P, Neutra M R. Transepithelial transport and mucosal defense: secretion of IgA. Trends Cell Biol. 1992;2:170–174. doi: 10.1016/0962-8924(92)90036-m. [DOI] [PubMed] [Google Scholar]
- 16.Liao N S, Maltzman J, Raulet D H. Positive selection determines T cell receptor Vβ14 gene usage by CD8+ T cells. J Exp Med. 1989;170:135–141. doi: 10.1084/jem.170.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Necker A, Rebai N, Matthes M, Jouvin-Marche E, Cazenave P A, Swarnworawong P, Palmer E, MacDonald H R, Malissen B. Monoclonal antibodies raised against engineered soluble mouse T cell receptors and specific for V alpha 8-, V beta 2- or V beta 10-bearing T cells. Eur J Immunol. 1991;21:3035–3040. doi: 10.1002/eji.1830211220. [DOI] [PubMed] [Google Scholar]
- 18.Neutra M R, Frey A, Kraehenbuhl J P. Epithelial M cells: gateways for mucosal infection and immunization. Cell. 1996;86:345–348. doi: 10.1016/s0092-8674(00)80106-3. [DOI] [PubMed] [Google Scholar]
- 19.Pappo J, Owen R L. Absence of secretory component expression by epithelial cells overlying rabbit gut-associated lymphoid tissue. Gastroenterology. 1988;95:1173–1177. doi: 10.1016/0016-5085(88)90347-2. [DOI] [PubMed] [Google Scholar]
- 20.Payne J, Huber B T, Cannon N A, Schneider R, Schilham M W, Acha-Orbea H, MacDonald H R, Hengartner H. Two monoclonal rat antibodies with specificity for the beta-chain variable region Vβ6 of the murine T-cell receptor. Proc Natl Acad Sci USA. 1988;85:7695–7698. doi: 10.1073/pnas.85.20.7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schacker T, Collier A C, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med. 1996;125:257–261. doi: 10.7326/0003-4819-125-4-199608150-00001. [DOI] [PubMed] [Google Scholar]
- 22.Shakhov A N, Wang H, Acha-Orbea H, Pauley R J, Wei W Z. A new infectious mammary tumor virus in the milk of mice implanted with C4 hyperplastic alveolar nodules. Eur J Immunol. 1993;23:2765–2769. doi: 10.1002/eji.1830231107. [DOI] [PubMed] [Google Scholar]
- 23.Velin D, Fotopoulos G, Lüthi F, Kraehenbuhl J P. The nasal associated lymphoid tissue of adult mice acts as an entry site for the mouse mammary tumor virus. J Exp Med. 1997;185:1871–1876. doi: 10.1084/jem.185.10.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velin D, Acha-Orbea H, Kraehenbuhl J-P. The neonatal Fc receptor is not required for mucosal infection by mouse mammary tumor virus. J Virol. 1996;70:7250–7254. doi: 10.1128/jvi.70.10.7250-7254.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weltzin R A, Lucia Jandris P, Michetti P, Fields B N, Kraehenbuhl J P, Neutra M R. Binding and transepithelial transport of immunoglobulins by intestinal M cells: demonstration using monoclonal IgA antibodies against enteric viral proteins. J Cell Biol. 1989;108:1673–1685. doi: 10.1083/jcb.108.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winner L S I, Mack J, Weltzin R, Mekalanos J J, Kraehenbuhl J-P, Neutra M R. New model for analysis of mucosal immunity: intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect Immun. 1991;59:977–982. doi: 10.1128/iai.59.3.977-982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]