Abstract
目的
初步构建基于软骨钙化层损伤重建的有机—无机复合组织工程支架,探究掺镁硅灰石含量与支架抗压性能之间的关系。
方法
利用质量分数分别为1%、3%、5%的高生物活性钙镁硅酸盐超细颗粒复合Ⅰ型胶原—透明质酸钠进行三维打印,经海藻酸钠—氯化钙气雾交联成型,电镜下观察表面孔隙、孔径、无机相分布,万能材料试验机测试抗压性能,并计算支架孔隙率。
结果
支架表面平均孔径(212.3±34.2)μm,平均孔隙率(48.3±5.9)%,不同质量分数的高生物活性钙镁硅酸盐超细颗粒复合Ⅰ型胶原—透明质酸钠支架压缩模量差异无统计学意义( P>0.05),平均压缩模量(7.2±1.2)MPa,介于软骨和软骨下骨之间。
结论
利用三维打印技术成功构建出多孔钙化层仿生重建支架,可为今后研制多层次复合支架治疗骨—软骨损伤奠定基础。
Abstract
Objective
To fabricate organic-inorganic composite tissue engineering scaffolds for reconstructing calcified cartilage layer based on three-dimensional (3D) printing technique.
Methods
The scaffolds were developed by 3D-printing technique with highly bioactive calcium-magnesium silicate ultrafine particles of 1%, 3% and 5% of mass fraction, in which the organic phases were composed of type I collagen and sodium hyaluronate. The 3D-printed scaffolds were then crosslinked and solidified by alginate and CaCl 2 aerosol. The pore size and distribution of inorganic phase were observed with scanning electron microscope (SEM); the mechanical properties were tested with universal material testing machine, and the porosity of scaffolds was also measured.
Results
Pore size was approximately (212.3±34.2) μm with a porosity of (48.3±5.9)%, the compressive modulus of the scaffolds was (7.2±1.2) MPa, which was irrelevant to the percentage changes of calcium-magnesium silicate, the compressive modulus was between that of cartilage and subchondral bone.
Conclusion
The porous scaffolds for calcified cartilage layer have been successfully fabricated, which would be used for multi-layered composite scaffolds in osteochondral injury.
Keywords: Cartilage diseases/therapy; Silicon; Calcium compounds; Hyaluronic acid; Collagen type I; Computer-aided design; Imaging, three-dimensional; Sodiwm alginate; Scaffolds
骨—软骨联合损伤是临床常见疾病,其修复治疗一直是临床难题之一。目前的传统治疗方法诸如清创、骨髓刺激、骨软骨移植和软骨细胞移植等治疗方法可能因血管长入而产生纤维软骨,导致远期修复效果不理想 [ 1- 3] 。组织工程是一种使用可降解的仿生支架复合协调处理的种子细胞和有生物活性的信号分子来修复损伤的方法,可避免上述弊端,因而具有应用潜力 [ 4] 。其中支架为细胞增殖生长和迁移分化提供了适宜的空间与环境,是组织工程中最重要的部分。本文在骨—软骨损伤修复中利用三维打印技术制造掺镁硅灰石钙化层重建支架,通过体视镜和电镜观察、力学性能检测、孔隙率计算等方法,分析了钙化层重建支架的特性,以期为今后含钙化层骨—软骨多层次复合支架研制提供实验基础。
海藻酸钠、氯化钙、四水硝酸钙、六水硝酸镁、九水偏硅酸钠、浓氨水、无水乙醇均购自国药集团化学试剂有限公司;透明质酸钠购自上海圻明生物科技有限公司;Ⅰ型胶原购自美国Sigma-Aldrich公司;聚乙烯醇购自上海阿拉丁生化科技股份有限公司;1 mL针筒购自江西洪达医疗器械集团有限公司;超声雾化加湿器购自中国SKEEN公司;TL-LL-100W恒流泵购自中国无锡天利流体公司;SU8010场发射电镜购自日本日立公司;SZX7连续变倍体视镜购自日本奥林巴斯公司;WD-T万能材料试验机购自上海卓技公司。
取九水偏硅酸钠129.1 g溶于1 L去离子水;取四水硝酸钙96.5 g、六水硝酸镁11.6 g溶于1 L去离子水,滴加浓氨水调节其酸碱度为10.0后滴入九水偏硅酸钠溶液中并搅拌12 h后抽滤,用去离子水洗涤4次,再用无水乙醇洗涤2次,90 ℃干燥24 h,180 ℃干燥8 h,880 ℃干燥150 min后得到掺镁硅灰石粉末。按质量分数1%、3%和5%的比例加到10 g 2%聚乙烯醇溶液内,均匀搅拌2 h后加入0.20 g Ⅰ型胶原粉末静置溶解,再加入0.03 g透明质酸钠和0.60 g海藻酸钠粉末后搅拌为均匀溶液后放入37 ℃培养箱溶胀1 h后置于4 ℃冰箱保存待用。使用1 mL针筒作为墨盒,选用400 μm直径喷头,设定线宽400 μm、层高320 μm、孔隙宽度470 μm,将墨盒内不同比例的掺镁硅灰石粉末溶液在电机驱动下使其水平移动并挤出溶液,逐层打印后使线条堆积形成三维状,利用海藻酸钠与10%氯化钙气雾化学交联成型原理,打印后向支架滴加5滴10%氯化钙以提高交联度,再滴加3~5滴无水乙醇冲洗。
支架上滴加5滴无水乙醇后置于37 ℃生化培养箱中烘干后,使用体视镜在适宜倍率下观察支架上表面、侧面和纵切面色泽和支架线条和孔隙形态、大小。
支架上滴加5滴无水乙醇后置37 ℃生化培养箱中烘干后,使用扫描电子显微镜在不同倍率下观察支架表面的线条形态、粗细、孔径形态、大小和支架表面掺镁硅灰石无机相分布。
使用万能材料试验机测试钙化层支架抗压性能,样品干燥后体积约4 mm×4 mm×3 mm,测试温度为室温,测试速度1 mm/min( n=6)。
采用相对密度法计算支架孔隙率 P,公式:
| () |
其中 M、 V分别为单个支架测得的质量、体积, ρ为凝胶真密度( n=3)。
采用SPSS 20.0软件进行统计学分析。各组数据经方差齐性检验呈正态分布,以均数±标准差( x ± s)表示,采用单因素方差分析比较组间差异,以 P<0.05为差异有统计学意义。
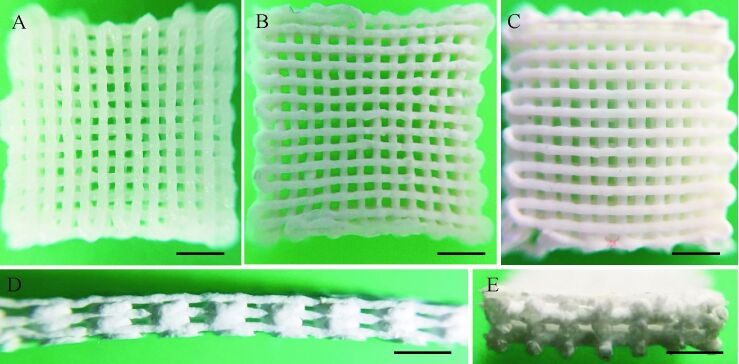
体视镜下观察可见各组钙化层支架均线条粗细基本均匀、排列有序,大部分孔隙均匀,而边缘处孔隙略小。由于含有掺镁硅灰石无机粉末,支架呈莹白色,见 图 1A、 1B、 1C,其中1%组支架较3%组、5%组更透明。观察支架侧面和纵切面,可见制备的支架孔隙连通度为100%,支架侧面、纵切面孔隙呈长方形,孔径高度[(101.9±4.9) μm]小于孔径宽度(均大于200 μm),见 图 1D、 1E。
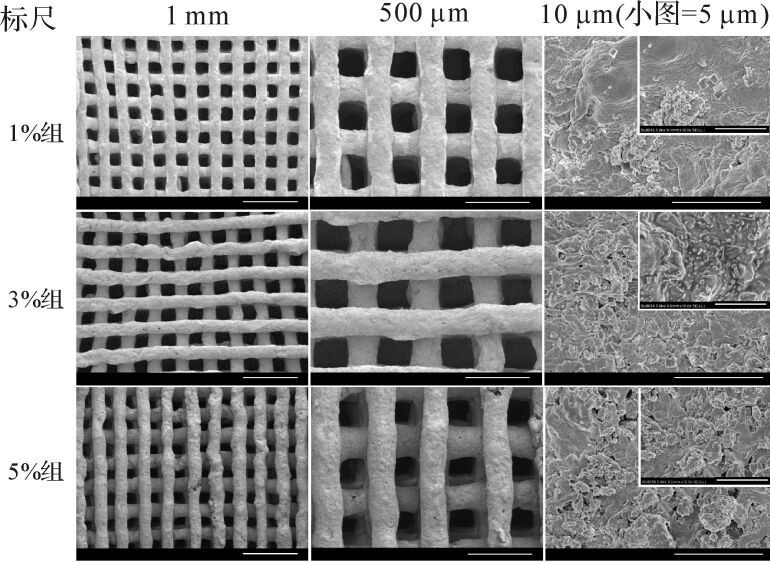
扫描电镜观察钙化层支架表面,低倍电镜下观察结果与体视镜下类似,其中5%组支架边缘附近孔隙明显较中心部分孔隙小,且越靠近边缘孔隙越小,但是其中心部分孔隙与1%组、3%组支架相似。高倍电镜下可见各组掺镁硅灰石超细颗粒呈不规则形状,在支架中分布均匀,掺镁硅灰石掺杂比例越高其镜下密度越高,见 图 2。每组支架在标尺=1 mm的电镜图中随机选取10个表面孔隙并计算平均孔径大小,各组孔径分别为(215.1±25.1)μm、(226.8±24.5)μm、(195.1±44.3)μm,支架孔径各组之间差异无统计学意义( P=0.105),平均孔径为(212.3±34.2) μm。以上结果提示,本研究制备的钙化层支架可为细胞黏附增殖提供空间,并为营养物质、代谢产物以及气体交换提供通道。
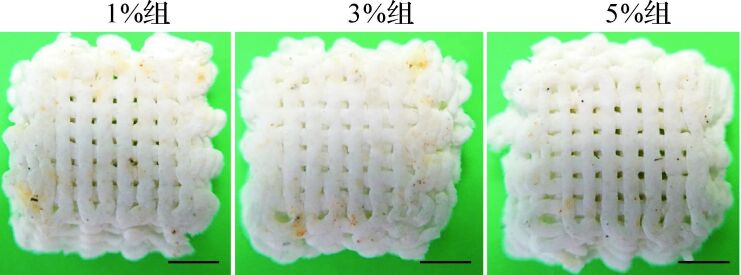
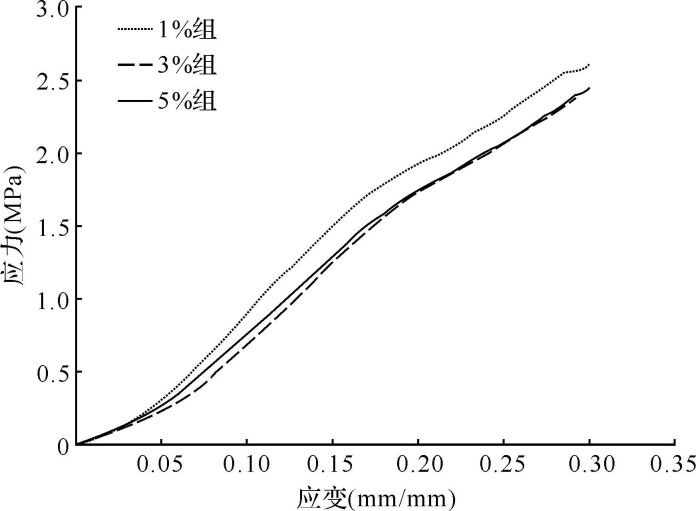
由于钙化支架主要承受压力和亚拉伸力,本研究测试钙化层支架的抗压性能。测试中,钙化层支架可被持续压缩形成紧密堆积片状物,随着压缩程度增大,支架形变越大,直至孔隙消失,线条之间融合,支架内结构未发生断裂,见 图 3A、 3B、 3C。选择应力—应变比值曲线中初始阶段弹性形变部分(≤10%)计算应力—应变比值( 图 4),即支架的压缩模量。各组与不掺镁硅灰石粉末支架的压缩模量[分别为(7.5±1.2)MPa、(7.9±0.6)MPa、(6.3±1.5)MPa、(7.4±1.1)MPa]差异无统计学意义( P=0.591),平均压缩模量为(7.2±1.2) MPa。以上结果提示,制备的支架对钙化层抗压性能有部分仿生,可在一定程度保证支架在修复过程中始终为细胞提供机械力保护。
通过三维软件设计,打印相关参数相同,因此理论上三组支架孔隙率相同。三组支架孔隙率分别为(48.8±6.0)%、(50.8±7.0)%、(45.4±6.0)%,孔隙率各组之间差异无统计学意义( P=0.603),平均孔隙率为(48.3±5.9)%。
三维打印是目前常用的组织工程支架制备方法之一,具有不需模具或机械加工的特点,非常适合个性化制造,相比其他制造方法节省大量时间和成本。三维打印的材料来源广泛,如天然和合成聚合物、生物陶瓷和复合材料。这些材料打印的支架具有良好的生物相容性,可控的支架孔隙大小、形状有利于种植细胞的迁移、增殖与分化。Shor等 [ 5] 使用精密挤压沉积法制造出的聚己内酯(PCL)支架拥有组织工程所必需的机械性能、结构整体性、可控孔径与良好孔通性。体内实验结果显示支架的压缩模量和抗压强度与密质骨匹配有利于成骨细胞黏附增殖 [ 5] 。在骨组织工程中,磷酸钙(如β-磷酸三钙、羟基磷灰石、磷酸八钙)是一类成骨能力极佳的材料,早已广泛使用。Khanarian等 [ 6] 将琼脂糖与羟基磷灰石复合,发现3%微米级羟基磷灰石最适合钙化软骨生成。其他学者均发现镁硅酸钙材料相比于β-磷酸三钙可更好地促进骨髓间充质干细胞向成骨细胞分化,即成骨作用更强 [ 7- 8] ;而透明质酸与Ⅰ型胶原为细胞外基质的成分,均具有良好的生物相容性和低抗原性,可以介导细胞间传导,利于细胞黏附、增殖和分化 [ 9- 10] 。透明质酸具有良好的可降解性和生物相容性,有保护软骨、润滑关节作用 [ 11- 12] 。因此本研究采用掺镁硅灰石复合Ⅰ型胶原—透明质酸钠三维打印重建钙化层支架。
软骨钙化层是连接关节中软骨—骨的结构,其能使软骨—骨实现功能性地整合并在承受生理负荷过程中将剪切力(应力从关节软骨向干骺端传导过程中形成)转化为压力和张力后传导至软骨下骨;同时,钙化层也可限制透明软骨与软骨下骨之间物质交换,在软骨再生时阻止血管由软骨下骨长入,保证新生软骨处于缺氧环境,避免纤维软骨的形成。值得注意的是,关节软骨损伤常常合并软骨下骨损伤 [ 13] 。骨和软骨合并损伤后,软骨钙化层及软骨下骨微环境均遭受破坏 [ 14] 。传统的软骨组织工程研究多侧重单纯的软骨修复,忽略了软骨下骨及钙化层的损伤修复,缺少对软骨与软骨下骨之间钙化层的整合和隔离作用的认识。2007年Allan等 [ 15] 构建的具有钙化层界面的骨—软骨复合支架比单纯骨—软骨双相支架具有更好的分散应力和抗剪切力作用。2012年Da等 [ 16] 在双相支架中加入了致密层以仿生钙化层,发现致密层明显提高了双相支架强度与体内的骨软骨组织再生。随着对界面组织研究的深入,越来越多的学者认同韧带—骨、肌腱—骨和软骨—骨这三种不同软/硬组织连接界面的重要性 [ 17] ,骨软骨组织工程也因此在未来能更多地应用于临床 [ 18] 。本研究的钙化层重建支架孔径适中,钙化层结构和抗压性能具有一定程度的仿生性。
支架的孔径对成骨有重要作用,有研究指出骨组织工程支架孔径需要在200~400 μm之间 [ 19- 21] ,软骨组织工程支架的孔径在100~300 μm之间 [ 22] ,本研究的支架孔径为(212.3±34.2) μm,较为合适,因为较小的孔径可营造缺氧的环境,诱导可骨化的软骨形成 [ 23] ;且较小的孔径也是对钙化层屏障作用的仿生。电镜下观察可见含5%掺镁硅灰石支架边缘附近孔隙明显较中心部分孔隙小,且越靠近边缘孔隙越小;但是中心部分孔隙与含1%、3%掺镁硅灰石支架并无差异,其原因是干燥时支架内部收缩度不完全均匀,支架两端略微翘起,导致观察角度与支架表面不垂直。组织工程支架的孔隙率对于体内外骨形成有重要影响,适当的孔径和连通的孔隙提供细胞、血管的再生所需要的空间,还可提供营养物质、代谢产物以及气体交换的通道,利于细胞附着、迁移和增殖。本研究制备的支架孔隙率高,孔间连通度为100%,在理论上可为细胞生长提供空间,还可为营养物质、代谢产物以及气体交换提供通道。体视镜下可观察到支架孔径的高度小于宽度,但这并不阻碍细胞长入水凝胶支架。造成这一现象的原因可能是:①交联前线条处于凝胶状态,由于自身重力导致线条高度降低;②上方支架的重力对下方线条产生压迫导致线条部分融合;③后续干燥失水导致线条高度降低。一般来说,有机—无机复合可增强材料的抗压性能 [ 24] ,但也不乏强度不变甚至降低的例子 [ 25- 26] ,这可能与干燥方法有关。本研究钙化层支架未经过高温烧结或真空干燥,含水量较多,故支架表现出水凝胶的压缩性质。
支架的压缩模量和抗压强度与密质骨匹配,体内实验提示其有利于成骨细胞黏附增殖 [ 5] 。Cui等 [ 27] 以聚乙二醇二甲基丙烯酸酯(PEGDMA)和软骨细胞为生物墨水,同时利用光聚合精确固定细胞位置,得到了有足够机械强度的支架,其压缩模量与天然软骨匹配。本研究支架压缩模量介于软骨和软骨下骨之间 [ 28- 29] ,这是对钙化层抗压性能的部分仿生,在一定程度上保证了支架在修复过程中始终为细胞提供机械力保护。
本研究利用三维打印技术首次成功构建出基于钙化层重建的多孔生物支架,制备的支架对软骨钙化层有一定仿生,这些研究有望为今后软骨层、软骨下骨层支架复合及其体内外实验研究奠定基础。
Funding Statement
国家自然科学基金(81472063);浙江省自然科学基金(LZ14E020001)
References
- 1.FRISBIE D D, TROTTER G W, POWERS B E, et al. Arthroscopic subchondral bone plate microfracture technique augments healing of large chondral defects in the radial carpal bone and medial femoral condyle of horses. Vet Surg. 1999;28(4):242–255. doi: 10.1053/jvet.1999.0242. [FRISBIE D D, TROTTER G W, POWERS B E, et al. Arthroscopic subchondral bone plate microfracture technique augments healing of large chondral defects in the radial carpal bone and medial femoral condyle of horses[J]. Vet Surg, 1999, 28(4):242-255.] [DOI] [PubMed] [Google Scholar]
- 2.BEDI A, FEELEY B T, WILLIAMS R J 3RD. Management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92(4):994–1009. doi: 10.2106/JBJS.I.00895. [BEDI A, FEELEY B T, WILLIAMS R J 3RD. Management of articular cartilage defects of the knee[J]. J Bone Joint Surg Am, 2010, 92(4):994-1009.] [DOI] [PubMed] [Google Scholar]
- 3.HUNZIKER E B. Articular cartilage repair: basic science and clinical progress. Osteoarthritis Cartilage. 2002;10(6):432–463. doi: 10.1053/joca.2002.0801. [HUNZIKER E B. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects[J]. Osteoarthritis Cartilage, 2002, 10(6):432-463.] [DOI] [PubMed] [Google Scholar]
- 4.YE K, DI BELLA C, MYERS D E, et al. The osteochondral dilemma: review of current management and future trends. ANZ J Surg. 2014;84(4):211–217. doi: 10.1111/ans.2014.84.issue-4. [YE K, DI BELLA C, MYERS D E, et al. The osteochondral dilemma: review of current management and future trends[J]. ANZ J Surg, 2014, 84(4):211-217.] [DOI] [PubMed] [Google Scholar]
- 5.SHOR L, GVÜERI S, CHANG R, et al. Precision extruding deposition(PED) fabrication of polycaprolactone(PCL) scaffolds for bone tissue engineering. Biofabrication. 2009;1(1):015003. doi: 10.1088/1758-5082/1/1/015003. [SHOR L, GVÜERI S, CHANG R, et al. Precision extruding deposition(PED) fabrication of polycaprolactone(PCL) scaffolds for bone tissue engineering[J]. Biofabrication, 2009, 1(1):015003.] [DOI] [PubMed] [Google Scholar]
- 6.KHANARIAN N T, HANEY N M, BURGA R A, et al. A functional agarose-hydroxyapatite scaffold for osteochondral interface regeneration. Biomaterials. 2012;33(21):5247–5258. doi: 10.1016/j.biomaterials.2012.03.076. [KHANARIAN N T, HANEY N M, BURGA R A, et al. A functional agarose-hydroxyapatite scaffold for osteochondral interface regeneration[J]. Biomaterials, 2012, 33(21):5247-5258.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SUN H, WU C, DAI K, et al. Proliferation and osteoblastic differentiation of human bone marrow-derived stromal cells on akermanite-bioactive ceramics. Biomaterials. 2006;27(33):5651–5657. doi: 10.1016/j.biomaterials.2006.07.027. [SUN H, WU C, DAI K, et al. Proliferation and osteoblastic differentiation of human bone marrow-derived stromal cells on akermanite-bioactive ceramics[J]. Biomaterials, 2006, 27(33):5651-5657.] [DOI] [PubMed] [Google Scholar]
- 8.HUANG Y, JIN X, ZHANG X, et al. In vitro and in vivo evaluation of akermanite bioceramics for bone regeneration . Biomaterials. 2009;30(28):5041–5048. doi: 10.1016/j.biomaterials.2009.05.077. [HUANG Y, JIN X, ZHANG X, et al. In vitro and in vivo evaluation of akermanite bioceramics for bone regeneration[J]. Biomaterials, 2009, 30(28):5041-5048. ] [DOI] [PubMed] [Google Scholar]
- 9.TAN H, CHU C R, PAYNE K A, et al. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30(13):2499–2506. doi: 10.1016/j.biomaterials.2008.12.080. [TAN H, CHU C R, PAYNE K A, et al. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering[J]. Biomaterials, 2009, 30(13):2499-2506.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.KAWASAKI K, OCHI M, UCHIO Y, et al. Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels. J Cell Physiol. 1999;179(2):142–148. doi: 10.1002/(ISSN)1097-4652. [KAWASAKI K, OCHI M, UCHIO Y, et al. Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels[J]. J Cell Physiol, 1999, 179(2):142-148.] [DOI] [PubMed] [Google Scholar]
- 11.CIANFLOCCO A J. Viscosupplementation in patients with osteoarthritis of the knee. Postgrad Med. 2013;125(1):97–105. doi: 10.3810/pgm.2013.01.2618. [CIANFLOCCO A J. Viscosupplementation in patients with osteoarthritis of the knee[J]. Postgrad Med, 2013, 125(1):97-105.] [DOI] [PubMed] [Google Scholar]
- 12.RESPONTE D J, NATOLI R M, ATHANASIOU K A. Identification of potential biophysical and molecular signalling mechanisms underlying hyaluronic acid enhancement of cartilage formation. J R Soc Interface. 2012;9(77):3564–3573. doi: 10.1098/rsif.2012.0399. [RESPONTE D J, NATOLI R M, ATHANASIOU K A. Identification of potential biophysical and molecular signalling mechanisms underlying hyaluronic acid enhancement of cartilage formation[J]. J R Soc Interface, 2012, 9(77):3564-3573.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.RUDERT M. Histological evaluation of osteochondral defects: consideration of animal models with emphasis on the rabbit, experimental setup, follow-up and applied methods. Cells Tissues Organs. 2002;171(4):229–240. doi: 10.1159/000063125. [RUDERT M. Histological evaluation of osteochondral defects: consideration of animal models with emphasis on the rabbit, experimental setup, follow-up and applied methods[J]. Cells Tissues Organs, 2002, 171(4):229-240.] [DOI] [PubMed] [Google Scholar]
- 14.HUNZIKER E B, DRIESANG I M, SAAGER C. Structural barrier principle for growth factor-based articular cartilage repair. Clin Orthop Relat Res. 2001;(391 Suppl):S182–S189. doi: 10.1097/00003086-200110001-00018. [HUNZIKER E B, DRIESANG I M, SAAGER C. Structural barrier principle for growth factor-based articular cartilage repair[J]. Clin Orthop Relat Res, 2001(391 Suppl):S182-S189.] [DOI] [PubMed] [Google Scholar]
- 15.ALLAN K. S, PILLIAR R M, WANG J, et al. Formation of biphasic constructs containing cartilage with a calcified zone interface. Tissue Eng. 2007;13(1):167–177. doi: 10.1089/ten.2006.0081. [ALLAN K. S, PILLIAR R M, WANG J, et al. Formation of biphasic constructs containing cartilage with a calcified zone interface[J]. Tissue Eng, 2007, 13(1):167-177.] [DOI] [PubMed] [Google Scholar]
- 16.DA H, JIA S J, MENG G L, et al. The impact of compact layer in biphasic scaffold on osteochondral tissue engineering. PLoS One. 2013;8(1):e54838. doi: 10.1371/journal.pone.0054838. [DA H, JIA S J, MENG G L, et al. The impact of compact layer in biphasic scaffold on osteochondral tissue engineering[J]. PLoS One, 2013, 8(1):e54838.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SEIDI A, RAMALINGAM M, ELLOUMI-HANNACHI I, et al. Gradient biomaterials for soft-to-hard interface tissue engineering. Acta Biomater. 2011;7(4):1441–1451. doi: 10.1016/j.actbio.2011.01.011. [SEIDI A, RAMALINGAM M, ELLOUMI-HANNACHI I, et al. Gradient biomaterials for soft-to-hard interface tissue engineering[J]. Acta Biomater, 2011, 7(4):1441-1451.] [DOI] [PubMed] [Google Scholar]
- 18.GETGOOD A, BROOKS R, FORTIER L, et al. Articular cartilage tissue engineering: today's research, tomorrow's practice? J Bone Joint Surg Br. 2009;91(5):565–576. doi: 10.1302/0301-620X.91B5.21832. [GETGOOD A, BROOKS R, FORTIER L, et al. Articular cartilage tissue engineering: today's research, tomorrow's practice?[J]. J Bone Joint Surg Br, 2009, 91(5):565-576.] [DOI] [PubMed] [Google Scholar]
- 19.BOYAN B D, HUMMERT T W, DEAN D D, et al. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials. 1996;17(2):137–146. doi: 10.1016/0142-9612(96)85758-9. [BOYAN B D, HUMMERT T W, DEAN D D, et al. Role of material surfaces in regulating bone and cartilage cell response[J]. Biomaterials, 1996, 17(2):137-146.] [DOI] [PubMed] [Google Scholar]
- 20.LEONG K F, CHEAH C M, CHUA C K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials. 2003;24(13):2363–2378. doi: 10.1016/S0142-9612(03)00030-9. [LEONG K F, CHEAH C M, CHUA C K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs[J]. Biomaterials, 2003, 24(13):2363-2378.] [DOI] [PubMed] [Google Scholar]
- 21.ROBINSON B P, HOLLINGER J O, SZACHOWICZ E H, et al. Calvarial bone repair with porous D, L-polylactide. Otolaryngol Head Neck Surg. 1995;112(6):707–713. doi: 10.1016/S0194-5998(95)70180-X. [ROBINSON B P, HOLLINGER J O, SZACHOWICZ E H, et al. Calvarial bone repair with porous D, L-polylactide[J]. Otolaryngol Head Neck Surg, 1995, 112(6):707-713.] [DOI] [PubMed] [Google Scholar]
- 22.EL-AYOUBI R, DEGRANDPRE C, DIRADDO R, et al. Design and dynamic culture of 3D-scaffolds for cartilage tissue engineering. J Biomater Appl. 2011;25(5):429–444. doi: 10.1177/0885328209355332. [EL-AYOUBI R, DEGRANDPRE C, DIRADDO R, et al. Design and dynamic culture of 3D-scaffolds for cartilage tissue engineering[J]. J Biomater Appl, 2011, 25(5):429-444.] [DOI] [PubMed] [Google Scholar]
- 23.KARAGEORGIOU V, KAPLAN D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [KARAGEORGIOU V, KAPLAN D. Porosity of 3D biomaterial scaffolds and osteogenesis[J]. Biomaterials, 2005, 26(27):5474-5491.] [DOI] [PubMed] [Google Scholar]
- 24.ZHANG Y, ZHANG M. Microstructural and mechanical characterization of chitosan scaffolds reinforced by calcium phosphates. J Non-Cryst Solids. 2001;282(2-3):159–164. doi: 10.1016/S0022-3093(01)00345-3. [ZHANG Y, ZHANG M. Microstructural and mechanical characterization of chitosan scaffolds reinforced by calcium phosphates[J]. J Non-Cryst Solids, 2001, 282(2-3):159-164.] [DOI] [Google Scholar]
- 25.MISRA S K, NAZHAT S N, VALAPPIL S P, et al. Fabrication and characterization of biodegradable poly(3-hydroxybutyrate) composite containing bioglass. Biomacromolecules. 2007;8(7):2112–2119. doi: 10.1021/bm0701954. [MISRA S K, NAZHAT S N, VALAPPIL S P, et al. Fabrication and characterization of biodegradable poly(3-hydroxybutyrate) composite containing bioglass[J]. Biomacromolecules, 2007, 8(7):2112-2119.] [DOI] [PubMed] [Google Scholar]
- 26.LEVINGSTONE T J, MATSIKO A, DICKSON G R, et al. A biomimetic multi-layered collagen-based scaffold for osteochondral repair. Acta Biomaterialia. 2014;10(5):1996–2004. doi: 10.1016/j.actbio.2014.01.005. [LEVINGSTONE T J, MATSIKO A, DICKSON G R, et al. A biomimetic multi-layered collagen-based scaffold for osteochondral repair[J]. Acta Biomaterialia, 2014, 10(5):1996-2004.] [DOI] [PubMed] [Google Scholar]
- 27.CUI X, BREITENKAMP K, FINN M G, et al. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A. 2012;18(11-12):1304–1312. doi: 10.1089/ten.tea.2011.0543. [CUI X, BREITENKAMP K, FINN M G, et al. Direct human cartilage repair using three-dimensional bioprinting technology[J]. Tissue Eng Part A, 2012, 18(11-12):1304-1312.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.AMINI A R, LAURENCIN C T, NUKAVARAPU S P. Bone tissue engineering: recent advances and challenges. Crit Rev Biomed Eng. 2012;40(5):363–408. doi: 10.1615/CritRevBiomedEng.v40.i5. [AMINI A R, LAURENCIN C T, NUKAVARAPU S P. Bone tissue engineering: recent advances and challenges[J]. Crit Rev Biomed Eng, 2012, 40(5):363-408.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SHAO X, GOH J C, HUTMACHER D W, et al. Repair of large articular osteochondral defects using hybrid scaffolds and 0 bone marrow-derived mesenchymal stem cells in a rabbit model. Tissue Eng. 2006;12(6):1539–1551. doi: 10.1089/ten.2006.12.1539. [SHAO X, GOH J C, HUTMACHER D W, et al. Repair of large articular osteochondral defects using hybrid scaffolds and 0 bone marrow-derived mesenchymal stem cells in a rabbit model[J]. Tissue Eng, 2006, 12(6):1539-1551.] [DOI] [PubMed] [Google Scholar]