Summary
Background
The abdominal obesity trends and prevalence are important contributing factors to significant rise of many noncommunicable diseases in Vietnam but have not been well-documented in the literature. This study aimed to describe the prevalence and trends of obesity and abdominal obesity in Vietnam from 2009 to 2015 and evaluate how different definitions of obesity and abdominal obesity are associated with metabolic-related conditions.
Methods
We conducted a secondary analysis based on the Vietnam STEPS (STEPwise approach to Surveillance) cross-sectional Survey 2009 and 2015. Obesity and abdominal obesity were defined using the body mass index (BMI), waist circumference (WC), and waist-hip ratio (WHR) cut-offs from the World Health Organization (WHO) and International Diabetes Federation (IDF).
Findings
Depending on the specific cut-offs, from 2009 to 2015, obesity prevalence increased from 0.8%–10% to 1.7%–16.4% in women and from 0.8%–10.3% to 1.7%–15% in men; abdominal obesity prevalence increased from 3%–31.3% to 8%–41.7% in women and from 0.3%–19.3% to 0.4%–25% in men. Abdominal obesity using WC-IDF and WHR-WHO definitions had noticeably higher sensitivity and lower specificity for metabolic-related conditions compared to the other four criteria. All anthropometric measurements were statistically correlated with biomarkers/blood pressure in 2009 and 2015 except for fasting glucose. Only WC-IDF and WHR-WHO definitions showed consistent association with all reported metabolic-related conditions regardless of sex and survey years.
Interpretation
The prevalence of obesity and abdominal obesity in Vietnam is increasing rapidly, especially abdominal obesity in women regardless of the criteria used. More studies are needed to investigate how using different diagnostic criteria for obesity and abdominal obesity could better identify metabolic-related conditions.
Funding
Authors received no funding for this study.
Keywords: Prevalence, Obesity, Abdominal obesity, Vietnam, National survey
Research in context.
Evidence before this study
The increase in obesity prevalence has been recognized as one contributing factor to the significant rise of many non-communicable diseases (NCDs) in Vietnam. While BMI is a good proxy measure of total body fat in the general population, many studies have shown that abdominal adiposity, measured by waist circumference (WC) or waist-hip ratio (WHR), could be a better indicator of health risk compared to total body adiposity, as measured by BMI. Despite its importance, the prevalence of and trends in abdominal obesity in Vietnam are not well-documented in the literature. Furthermore, different diagnosis criteria can yield greatly different prevalence estimates, which may provide disparate implications to policymakers.
Added value of this study
Our findings suggest that depending on the specific cut-offs, from 2009 to 2015, obesity prevalence increased from 0.8%–10% to 1.7%–16.4% in women and from 0.8%–10.3% to 1.7%–15% in men; abdominal obesity prevalence increased from 3%–31.3% to 8%–41.7% in women and from 0.3%–19.3% to 0.4%–25% in men. Abdominal obesity using WC-IDF and WHR-WHO definitions had noticeably higher sensitivity and lower specificity in screening for metabolic-related conditions compared to the other criteria. Only WC-IDF and WHR-WHO definitions showed consistent association with all reported metabolic-related conditions regardless of sex and survey years.
Implications of all the available evidence
While the prevalence of obesity in Vietnam using BMI-based definitions similarly increased in women and men over the years, the prevalence of abdominal obesity in Vietnam is much higher and increased more rapidly in women compared to men. The agreements between BMI-based definitions and WC/WHR definitions were low, especially with BMI cut-off based on WHO standard definitions. The findings reinforce the importance of monitoring abdominal obesity based on WC-IDF or WHR-WHO criteria as a risk factor for metabolic-related conditions in clinical settings and support further studies to explore the contributors to the rapid increase of abdominal obesity in Vietnam.
Introduction
As a transitional economy, the pattern of diseases in Vietnam has shifted rapidly in the last 20 years.1, 2, 3, 4 Among the changes, the increase in obesity prevalence has been recognized as one contributing factor to the significant rise of many non-communicable diseases (NCDs).4,5 Several studies from 2009 to 2015 showed that the prevalence of overweight or obesity in Vietnam ranged from 15.6% to nearly 30% using the WHO general cut-off values for body mass index (BMI).5, 6, 7 Using the WHO 2004 standard BMI cut-off for Asian populations, the prevalence ranged from 26.1% to 33.7%.5,8 Unfortunately, the burden of obesity in Vietnam will probably continue to increase in the next decade due to the ongoing changes in diet patterns, favoring Westernization, and increased sedentary lifestyle.4,5 Therefore, the Vietnamese government created the “National Strategy for the prevention and control of non-communicable diseases 2015–2025” and aimed to keep the prevalence of overweight and obesity (BMI ≥25 kg/m2) under 10% in children and 15% in adults.9
Most studies that served as evidence for the National Strategy were based on either the WHO general cut-off (BMI ≥25 for overweight and BMI ≥30 for obesity) or the WHO 2004 standard cut-off for the Asian population (BMI ≥ 23 for overweight and BMI ≥ 27.5 for obesity pertaining to the health risks that trigger public health action).5,6,8,9However, the most recent guideline for obesity diagnosis and treatment of the Ministry of Health (MoH) in Vietnam used WHO 2000 standard cut-off for Asian population (BMI ≥23 for overweight and BMI ≥ 25 for obesity).10 Use of varied criteria of BMI for defining obesity makes it difficult to tracking of obesity trends.
While BMI is a rather good proxy measure of total body fat in the general population, many studies have shown that abdominal adiposity, measured by Waist circumference (WC) or Waist-hip ratio (WHR), could be a better indicator of health risk compared to total body adiposity, as measured by BMI.11, 12, 13, 14, 15, 16 Some studies have shown that individuals with normal BMI but high WC or WHR could still have an elevated risk for various common obesity-related conditions such as cardiometabolic diseases, cancer, and premature mortality.11,12,16 The reasons for this phenomenon could be that the increase in abdominal or visceral adiposity leads to insulin resistance, impaired insulin secretion, and inflammations, which are precursors for many NCDs.17 As abdominal obesity is more pathogenic than general obesity, it is useful to monitor WC or WHR to estimate risk for metabolic diseases rather than BMI alone.
Despite its importance, the national prevalence of and trends in abdominal obesity in Vietnam are not well-documented in the literature. Some studies reported abdominal obesity prevalence in Vietnam, as a component of metabolic syndrome, ranging between 8.7% and 30.2% from 2004 to 2019.18, 19, 20, 21, 22, 23 However, these studies were conducted in different provinces and used different sampling schemes, which cannot provide national estimates to inform key decision makers in planning national NCD prevention strategies. Meanwhile, WHO has provided publicly available datasets collected from nationally representative surveys that contains important information related to these health conditions in Vietnam.24 Therefore, we conducted a secondary analysis of the WHO STEPwise approach to chronic disease risk factor surveillance (STEPS) surveys in Vietnam to describe the obesity and abdominal obesity trends and prevalence in Vietnam from 2009 to 2015 and evaluate how different definitions of obesity and abdominal obesity are associated with metabolic-related conditions. To our knowledge, this study is the first study that provides an overview picture of national prevalence of obesity and abdominal obesity in Vietnam adults from different aspects based on reliable national surveys.
Methods
Study design and study population
We performed a secondary analysis on data from two nationally representative household-based cross-sectional surveys of the WHO and MoH in Vietnam (among adults 25–64 years in 2009 and on adults 18–69 years in 2015)—the details of the sampling method were described elsewhere.7,25, 26, 27 In brief, the STEPS survey utilized the standard procedure from the WHO STEPwise approach to monitoring NCDs and their risk factors.28
For both the 2009 and 2015 surveys, information was collected in three STEPS. STEPS 1 was an interviewer-administered survey on demographic and behavioral risk factors; STEPS 2 was physical measurements (height, weight, blood pressure, waist circumference, hip circumference); STEPS 3 was blood and urine sample collection to measure blood glucose, blood cholesterol, HDL, creatinine, and sodium concentration in urine.7,25,27
In 2009, 22,940 individuals (aged 25–64) were invited to join the survey, the overall response rate was 64% (14,706 participants); whereas, in 2015, 3856 individuals (aged 18–64) were invited, the response rate was 97.4% for STEPS 1 (3758 participants) and 79.8% for both STEPS 2 and STEPS 3, collected at the same time (3080 participants).7,26,27 The sample size calculations and methods for the 2009 and 2015 survey were reported elsewhere, and the sample calculation of STEPS 2009 is not well-documented compared to STEPS 2015.7,29
In this study, we used public STEPS data requested from WHO's NCD Microdata Repository24 and further obtained information on residence, ethnicity, asset, hip circumference from colleagues from MoH in Vietnam, which were merged by participant de-identified id to the public dataset for our analysis with their permission.
Exclusion criteria
Our study excluded any participants who were pregnant at enrollment (90 cases). Implausible values of weight (<25 kg or >170 kg), height (<100 cm or >220 cm) and other biological and anthropometric measurements were changed to missing (Supplemental File S1). Biological implausible values limits were internally defined among the research team based on this study sample.30
Obesity and abdominal obesity assessment
All physical measurements in the STEPS survey were conducted by provincial preventive medicine centers using standardized tools recommended by the WHO in Vietnam, including standard electronic scales, stadiometers, and constant tension tape.7,31 Weight (in kilograms) and height (in centimeters) were measured in bare feet with light clothing in a standing posture. Waist circumference (in centimeters) was measured horizontally in the midpoint between the lowest inferior point of the last rib and the iliac crest while standing. Hip circumference (in centimeter) was measured horizontally at the largest posterior protuberance of the buttocks while standing.31 Waist-hip ratio was calculated by dividing the measurement of waist circumference by the measurement of hip circumference. In Table 1, we present the definitions of obesity and abdominal obesity for both sexes in the scope of this study. Abdominal obesity was defined using the “World Health Organization cut-off points and risk of metabolic complications” or the IDF–“International Diabetes Federation cut-off points for different ethnic groups”.32 Obesity was defined using the WHO general BMI cut-off or WHO BMI cut-off in 2000 or 2004 for the Asian population.32, 33, 34 We added the WHO BMI cut-off in 2000 for the Asian population into our analysis because the most recent guideline for obesity diagnosis and treatment of the Ministry of Health (MoH) in Vietnam in 2022 recommended this cut-off.10 For WHO 2004 cut-off points, we based on the proposed trigger point for public health action to define obesity as ≥27.5 kg/m2 which is considered associated with higher higher-risk for chronic diseases and death.33
Table 1.
Obesity and abdominal obesity definitions.
| Indicator | Cut-off points for female | Cut-off points for male |
|---|---|---|
| Obesity using BMI–WHO general definition | BMI ≥30 kg/m2 for obesity | |
| Obesity using BMI–WHO 2004 definition in Asian population | BMI ≥27.5 kg/m2 for obesity | |
| Obesity using BMI–WHO 2000 definition in Asian population | BMI ≥25 kg/m2 for obesity | |
| Abdominal obesity using WC–WHO definition | WC > 88 cm | WC > 102 cm |
| Abdominal obesity using WC–IDF definition (for South Asians, Chinese, and Japanese) | WC > 80 cm | WC > 90 cm |
| Abdominal obesity using WHR–WHO definition | WHR ≥ 0.85 | WHR ≥ 0.90 |
Abbreviation: BMI–Body mass index (kg/m2); WC–Waist circumference (cm); WHR–Waist-to-hip ratio.
Metabolic-related condition assessment
The measurement of blood glucose, blood cholesterol, HDL was conducted at the community health station by three trained health staff in the early morning to ensure that the subject was fasting according to WHO STEPS protocol, and the detailed information on these measurements was reported elsewhere.7,29
Blood pressure was taken three times at the midpoint of the right arm after at least 5 min of rest.35 Hypertension was defined as having the mean systolic blood pressure ≥130 mmHg or mean diastolic blood pressure ≥80 mmHg according to the recommendation of the current American Heart Association.36
The biochemical measures, including fasting blood glucose, fasting total cholesterol and high-density cholesterol (HDL) were collected by finger capillary blood tests.7,31 Diabetes type 2 was defined as having fasting blood glucose ≥7 mmol/l (i.e., 126 mg/dl) according to the recommendation of the American Diabetes Association37 or having used any medication for diabetes prescribed by a doctor or other health worker in the past two weeks. High total cholesterol was defined as having the level of total cholesterol over 6.2 mmol/l (i.e., 240 mg/dl) and low HDL was defined as <1 mmol/l (i.e., 40 mg/dl) in men and <1.3 mmol/l in women (i.e., 50 mg/dl).38
Information regarding sampling and covariate assessments was further presented in Supplemental File S1.
Data analysis
We used Stata 16.1 survey command (svy) to adjust for sampling weight, cluster sampling, and calculated the prevalence of obesity and abdominal obesity. The survey weight variables for each STEP survey provided in the obtained data were calculated based on the inverse of the probability of selection, adjustment for the non-response, and population structure of Vietnam in the survey year.
To assess the correlation between different adiposity measurements and biomarkers, we calculated the weighted Spearman's correlation coefficients for complex survey data by transforming these continuous variables into rank-order measures (using the rank command in Stata) and using the CORR_SVY pack to calculate correlation matrix based on these rank-order measure.39 For individuals currently taking medication for hypertension, diabetes, or hyperlipidemia, we assigned diagnostic threshold values to mean systolic and diastolic blood pressure, fasting blood glucose, and total cholesterol if their current values were below the diagnostic thresholds (Supplemental File S1). Spearman's correlation coefficients range from −1 to +1 and represent the direction and strength of monotonic relationship between the rank-ordered values of two variables.40 Due to the differences in scale and distributions of our continuous measurements, Spearman's correlation coefficients produce more stable estimates compared to Pearson's correlation coefficient. The sensitivity, specificity, the weighted positive likelihood ratio (LR+), which equals to [sensitivity/(1-specificity)], and the negative likelihood ratio (LR−), which equals to [(1-sensitivity)/specificity],41 of each obesity criterion for diagnosing metabolic conditions were also calculated using weighted data. When compared to the gold standard, the further LR + goes beyond 1, the more posttest odds of the disease or outcome would increase.42 On the other hand, the more LR-is smaller than 1, the more posttest odds of the disease or outcome would decrease.42
To examine the association of each obesity criteria with type 2 diabetes, high total cholesterol, hypertension, and low HDL, we calculated prevalence ratios (PRs) using univariable modified Poisson regression models with robust error variances stratified by sex and survey year.43,44
We used the statistical significance at the alpha level of 0.05 and the Bonferroni correction to adjust for multiple testing in supplement Figures S1 and S2.45
Our study is a secondary analysis on publicly available and deidentified datasets, which does not require an IRB approval in Vietnam. The original STEPS surveys were approved by the Ethics Committee of Vietnam Ministry of Health and the Tasmanian Health and Medical Human Research Ethics Committee in 200946 and Hanoi School of Public Health in 2015.7 All participants provided verbal and/or written informed consent and could decline or withdraw from the study at any time.
Role of the funding source
Authors received no funding for this study.
Results
In Table 2, we present the weighted characteristics of the surveyed participants in 2009 and 2015. The 2015 study population was more educated than the 2009 study population. Noticeably, there were some striking differences in lifestyle factors between two surveyed populations. Participants in 2015 were more likely to drink alcohol in the past 12 months, meet WHO recommendations fruit/vegetable consumption, and be slightly more physically active than participants in 2009. However, the 2015 group also had more individuals with more than 6 h spent in sedentary activities per day than 2009 group. Other physical measurements, such as height, weight, BMI, and WC were higher in the 2015 group than in 2009. The prevalence of smoking also seemed to be higher in 2009 than 2015, but the amount of missing data in 2009 for this variable (63.2%) makes this estimate unreliable.
Table 2.
Characteristics of the surveyed participants in 2009 and 2015.
| 2009 |
2015 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female |
Male |
Total |
Missings in the unweighted sample |
Female |
Male |
Total |
Missings in the unweighted sample |
|||||||
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Missing/N (%) | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | Missing/N (%) | |
| Demographic | ||||||||||||||
| Age group (%) | 0/14,519 (0.00) | 0/3720 (0.00) | ||||||||||||
| 18–24 | N/A | N/A | N/A | 18.08 | 15.76–20.65 | 21.11 | 18.20–24.34 | 19.66 | 17.63–21.86 | |||||
| 25–29 | 14.72 | 12.25–17.59 | 14.83 | 12.01–18.18 | 14.78 | 12.26–17.70 | 16.72 | 14.37–19.37 | 15.64 | 12.90–18.83 | 16.16 | 14.33–18.16 | ||
| 30–39 | 31.71 | 30.10–33.37 | 33.88 | 31.67–36.17 | 32.77 | 31.24–34.33 | 20.36 | 18.34–22.55 | 21.80 | 19.77–23.98 | 21.12 | 19.63–22.68 | ||
| 40–49 | 28.56 | 26.74–30.44 | 28.33 | 26.67–30.05 | 28.45 | 26.94–30.01 | 21.01 | 19.13–23.03 | 21.61 | 19.18–24.27 | 21.33 | 19.79–22.95 | ||
| 50–64 | 25.01 | 22.70–27.47 | 22.96 | 20.70–25.39 | 24.01 | 21.91–26.25 | 20.09 | 18.19–22.14 | 16.10 | 14.33–18.04 | 18.00 | 16.61–19.48 | ||
| 65–69 | N/A | N/A | N/A | 3.74 | 2.97–4.69 | 3.74 | 2.99–4.67 | 3.74 | 3.15–4.42 | |||||
| Residential area (%) | 14,519/14,519 (100.00) | 0/3720 (0.00) | ||||||||||||
| Urban | N/A | N/A | N/A | 41.45 | 39.03–43.91 | 38.78 | 36.10–41.52 | 40.05 | 38.39–41.74 | |||||
| Rural | N/A | N/A | N/A | 58.55 | 56.09–60.97 | 61.22 | 58.48–63.90 | 59.95 | 58.26–61.61 | |||||
| Years of education (mean) | 7.45 | 6.33–8.57 | 8.49 | 7.60–9.37 | 7.95 | 6.98–8.93 | 547/14,519 (3.77) | 8.36 | 8.02–8.71 | 9.03 | 8.67–9.38 | 8.70 | 8.41–8.98 | 679/3720 (18.25) |
| Education levels (%) | 31/14,519 (0.21) | 3/3720 (0.08) | ||||||||||||
| Primary and below | 51.68 | 35.25–67.75 | 42.50 | 31.99–53.72 | 47.21 | 33.77–61.07 | 38.88 | 35.64–42.23 | 31.96 | 28.48–35.65 | 35.26 | 32.41–38.22 | ||
| Secondary school | 25.70 | 17.46–36.13 | 29.69 | 24.39–35.60 | 27.64 | 20.82–35.69 | 24.55 | 22.04–27.24 | 26.75 | 23.85–29.85 | 25.70 | 23.54–27.98 | ||
| High school | 11.82 | 7.45–18.27 | 14.49 | 11.40–18.25 | 13.12 | 9.36–18.10 | 17.43 | 15.24–19.86 | 22.83 | 20.06–25.86 | 20.25 | 18.46–22.17 | ||
| College and above | 10.80 | 6.40–17.64 | 13.33 | 7.95–21.48 | 12.03 | 7.20–19.42 | 19.14 | 16.84–21.66 | 18.46 | 15.89–21.35 | 18.78 | 16.80–20.94 | ||
| Employment status (%) | 1090/14,519 (7.51) | 2/3720 (0.05) | ||||||||||||
| Currently employed | 77.60 | 64.00–87.10 | 92.32 | 88.45–94.97 | 84.76 | 76.52–90.47 | 79.36 | 76.52–81.94 | 91.75 | 89.75–93.39 | 85.84 | 84.11–87.41 | ||
| Not currently employed | 22.40 | 12.90–36.00 | 7.68 | 5.03–11.55 | 15.24 | 9.53–23.48 | 20.64 | 18.06–23.48 | 8.25 | 6.61–10.25 | 14.16 | 12.59–15.89 | ||
| Lifestyle factors | ||||||||||||||
| Smoking status (%) | 9173/14,519 (63.18) | 10/3720 (0.27) | ||||||||||||
| Never smoker | 11.84 | 4.30–28.63 | 3.09 | 1.67–5.63 | 3.48 | 1.92–6.23 | 97.82 | 96.54–98.63 | 33.86 | 30.68–37.20 | 64.35 | 62.11–66.54 | ||
| Former smoker | 17.60 | 13.18–23.10 | 21.17 | 16.99–26.06 | 21.01 | 16.84–25.89 | 0.69 | 0.40–1.18 | 16.31 | 14.35–18.48 | 8.86 | 7.84–10.01 | ||
| Current smoker | 70.56 | 60.09–79.24 | 75.74 | 71.10–79.85 | 75.51 | 70.98–79.54 | 1.49 | 0.84–2.62 | 49.83 | 46.59–53.06 | 26.78 | 24.69–28.98 | ||
| Drinking status in the past 12 months (%) | 64/14,519 (0.44) | 11/3720 (0.30) | ||||||||||||
| Abstainer in past 12 months | 88.68 | 84.90–91.60 | 18.38 | 15.26–21.97 | 54.58 | 51.27–57.85 | 57.09 | 54.13–60.01 | 8.41 | 6.83–10.31 | 31.70 | 29.76–33.70 | ||
| Less than once a month | 6.39 | 4.69–8.63 | 11.85 | 7.62–17.98 | 9.04 | 6.85–11.84 | 36.09 | 33.40–38.87 | 22.08 | 19.32–25.11 | 28.78 | 26.87–30.78 | ||
| More than once a month but not daily | 4.48 | 2.94–6.77 | 56.58 | 50.27–62.69 | 29.75 | 26.21–33.55 | 6.48 | 5.06–8.27 | 58.71 | 55.51–61.85 | 33.73 | 31.53–36.00 | ||
| Daily | 0.46 | 0.20–1.04 | 13.19 | 8.25–20.43 | 6.63 | 4.12–10.50 | 0.33 | 0.15–0.75 | 10.80 | 9.11–12.76 | 5.79 | 4.87–6.88 | ||
| Eat more than 5 daily servings of fruits or vegetables per day (%) | 79/14,519 (0.54) | 25/3720 (0.67) | ||||||||||||
| No | 71.23 | 61.28–79.47 | 70.82 | 62.61–77.87 | 71.03 | 62.58–78.24 | 29.75 | 26.78–32.91 | 36.32 | 33.22–39.53 | 33.18 | 30.94–35.49 | ||
| Yes | 28.77 | 20.53–38.72 | 29.18 | 22.13–37.39 | 28.97 | 21.76–37.42 | 70.25 | 67.09–73.22 | 63.68 | 60.47–66.78 | 66.82 | 64.51–69.06 | ||
| Met the WHO recommendation on physical activity (%) | 2218/14,519 (15.28) | 695/3720 (18.68) | ||||||||||||
| No | 23.63 | 12.99–39.06 | 20.72 | 10.70–36.30 | 22.22 | 11.93–37.59 | 36.97 | 33.89–40.16 | 20.77 | 18.39–23.37 | 28.55 | 26.40–30.80 | ||
| Yes | 76.37 | 60.94–87.01 | 79.28 | 63.70–89.30 | 77.78 | 62.41–88.07 | 63.03 | 59.84–66.11 | 79.23 | 76.63–81.61 | 71.45 | 69.20–73.60 | ||
| Levels of total physical activity (%) | 2218/14,519 (15.28) | 695/3720 (18.68) | ||||||||||||
| Low | 26.15 | 15.28–41.01 | 23.82 | 13.06–39.43 | 25.02 | 14.27–40.09 | 39.65 | 36.46–42.92 | 26.43 | 23.79–29.25 | 32.78 | 30.50–35.14 | ||
| Moderate | 22.31 | 15.07–31.72 | 16.19 | 11.31–22.64 | 19.34 | 13.29–27.27 | 24.03 | 21.61–26.62 | 15.61 | 13.63–17.82 | 19.65 | 18.07–21.34 | ||
| High | 51.54 | 31.70–70.91 | 59.99 | 41.50–76.02 | 55.64 | 36.39–73.33 | 36.33 | 33.21–39.57 | 57.96 | 54.81–61.05 | 47.57 | 45.00–50.15 | ||
| Total time (hours) spent in sedentary activities per day (mean) | 3.79 | 2.73–4.85 | 3.74 | 2.70–4.79 | 3.77 | 2.72–4.81 | 240/14,519 (1.65) | 4.28 | 4.05–4.50 | 4.00 | 3.78–4.22 | 4.13 | 3.95–4.31 | 27/3720 (0.73) |
| More than 6 h spent in sedentary activities per day (%) | 240/14,519 (1.65) | 27/3720 (0.73) | ||||||||||||
| No | 81.06 | 65.62–90.56 | 82.63 | 66.08–92.07 | 81.83 | 66.08–91.23 | 74.56 | 71.47–77.42 | 78.35 | 75.50–80.96 | 76.54 | 74.19–78.74 | ||
| Yes | 18.94 | 9.44–34.38 | 17.37 | 7.93–33.92 | 18.17 | 8.77–33.92 | 25.44 | 22.58–28.53 | 21.65 | 19.04–24.50 | 23.46 | 21.26–25.81 | ||
| Body measurement | ||||||||||||||
| Height in cm(mean) | 152.18 | 151.57–152.79 | 162.25 | 161.68–162.82 | 157.08 | 156.52–157.64 | 8/14,519 (0.06) | 152.59 | 152.15–153.02 | 162.43 | 161.97–162.89 | 157.55 | 157.16–157.94 | 682/3720 (18.33) |
| Weight in kg (mean) | 49.21 | 47.55–50.88 | 56.02 | 54.18–57.87 | 52.53 | 50.85–54.21 | 6/14,519 (0.04) | 51.15 | 50.57–51.73 | 58.14 | 57.43–58.85 | 54.67 | 54.16–55.19 | 682/3720 (18.33) |
| BMI in kg/m2(mean) | 21.23 | 20.61–21.85 | 21.24 | 20.69–21.80 | 21.23 | 20.67–21.80 | 8/14,519 (0.06) | 21.96 | 21.74–22.19 | 22.02 | 21.77–22.27 | 21.99 | 21.81–22.17 | 682/3720 (18.33) |
| Waist circumference in cm (mean) | 71.11 | 68.44–73.79 | 74.05 | 71.48–76.63 | 72.54 | 69.94–75.14 | 8/14,519 (0.06) | 75.49 | 74.76–76.21 | 77.79 | 77.01–78.58 | 76.65 | 76.05–77.25 | 685/3720 (18.41) |
| Hip circumference in cm (mean) | 86.17 | 84.41–87.94 | 87.17 | 85.28–89.07 | 86.66 | 84.85–88.47 | 11/14,519 (0.08) | 89.99 | 89.46–90.52 | 90.72 | 90.15–91.28 | 90.36 | 89.92–90.79 | 685/3720 (18.41) |
| Waist-to-hip ratio (mean) | 0.82 | 0.81–0.84 | 0.85 | 0.83–0.86 | 0.84 | 0.82–0.85 | 13/14,519 (0.09) | 0.84 | 0.83–0.84 | 0.86 | 0.85–0.86 | 0.85 | 0.84–0.85 | 685/3720 (18.41) |
In Table S1, we presented the weighted prevalence of obesity and abdominal obesity by age groups in 2009. Using BMI as the measure reference, all WHO definitions provided similar results for obesity between males and females. However, there was a large discrepancy among males and females regarding abdominal obesity in 2009. Using WC-WHO definition, the prevalence for men was only 0.3% (95% CI: 0.1%–1.1%) while it was 3.0% (95% CI: 1.5%–5.7%) for women. The difference also was present when using WC with IDF definition, as the prevalence for men and women was 4.5% (95% CI: 2.1%–9.1%) and 14.4% (95% CI: 8.9%–22.3%), respectively. We also observed different patterns of obesity across age groups for men and women. In both sexes, obesity and abdominal obesity prevalence tended to increase with age groups except for obesity using BMI-WHO general definition.
The weighted prevalence of obesity and abdominal obesity in the 2015 survey across age groups is presented in Table S2. In terms of obesity prevalence, there were small differences between men and women when using BMI-WHO general definition and two WHO definitions for the Asian population. However, we continued to observe a large discrepancy in abdominal obesity between the two sexes. The prevalence of abdominal obesity ranged from 8% to 41.7% in women depends on the specific cut-off and definitions, while this prevalence ranged from 0.4% to 25% in men. We also observed the same pattern of increasing obesity and abdominal obesity prevalence across age groups in both men and women, but more pronounced in women. In order to directly compare results with data in 2009, we also restricted the analysis of data in 2015 to a subpopulation aged 25–64. We found that prevalence of obesity and abdominal obesity in both sexes in this subpopulation were consistent with the corresponding prevalence in the whole population.
The sex differences in obesity and abdominal obesity prevalence between the two survey years are summarized in Fig. 1. There was an increasing trend in both obesity and abdominal obesity over time in Vietnam, which was greater in women than in men. Using obesity definitions with BMI–WHO references, we noticed only a slight absolute increase in prevalence of obesity in both men and women from 2009 to 2015. However, for abdominal obesity, the differences between men and women over time were large regardless of the definitions.
Fig. 1.
Prevalence of obesity and abdominal obesity in 2009 and 2015.
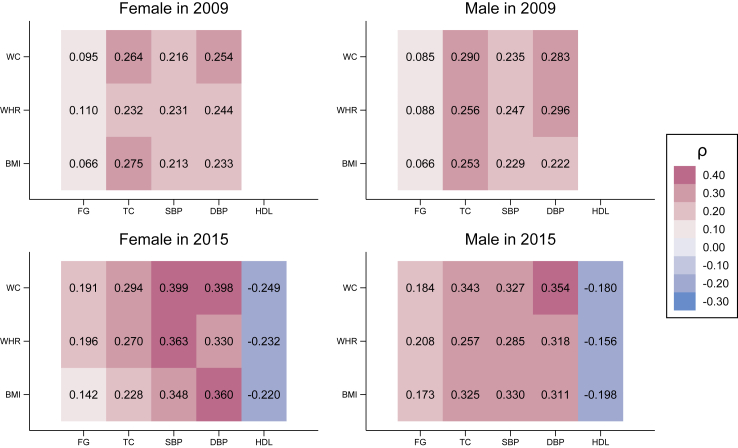
In Fig. 2, we present the Spearman correlation coefficients between different anthropometric measurements and biomarkers/blood pressure in 2009 and 2015. All anthropometric measurements were statistically correlated with biomarkers/blood pressure in 2009 and 2015 except for fasting glucose. The correlations were positive for most reported biomarkers and negative for HDL cholesterol. Regarding the strength of the correlations, we could see that the correlations appeared to be weak or modest, and these coefficients were stronger in 2015 compared to 2009. In 2009, weak correlations were observed between total cholesterol and WC/BMI in women and men, and between diastolic blood pressure and WC/WHR in men. On the other hand, in 2015, noticeable correlations were observed between systolic blood pressure and WC/WHR in women, diastolic blood pressure and WC/BMI in women and men. We further present the scatter plots and median-spline relationship between anthropometric measurements and biomarkers/blood pressure in Figures S1 and S2.
Fig. 2.
Spearman's correlation coefficients between anthropometric measurements and biomarkers/blood pressure. WC: waist circumference, WHR: waist-to-hip ratio, BMI: body mass index, FG: fasting glucose, TC: total cholesterol, SBP: systolic blood pressure, DBP: diastolic blood pressure, HDL: high-density lipoprotein cholesterol.
We estimated how obesity and abdominal obesity could be used to screen for some metabolic-related conditions in Table 3. In both years, obesity based on BMI-WHO general definition consistently had the highest specificity (98.4%–99.5%) and lowest sensitivity (1.3%–10.1%) for screening for diabetes type 2, high total cholesterol, hypertension, and low HDL cholesterol (in 2015 only). Consistently, only obesity based on BMI-WHO general definition yielded LR+ > 4 for classifying diabetes type 2 (in both 2009 and 2015) and hypertension (in 2015). Obesity using BMI definitions and abdominal obesity using WC-WHO definition had quite similar values of sensitivity and specificity in both survey years. Abdominal obesity using WC-IDF definition and WHR-WHO definition had noticeably higher sensitivity and lower specificity compared to the other three criteria. Interestingly, while the specificity of all obesity criteria was rather similar over time, the sensitivity increased from 2009 to 2015. LR− was close to 1 with obesity using BMI definitions and abdominal obesity using WC-WHO definition and were lowest with abdominal obesity using WHR-WHO definition in all diseases. In addition, the areas under the ROC curve of those anthropometries for chronic diseases screening ranged from 0.58 to 0.76, which were showed in Figure S3. Noticeably, WHR generally yielded higher AUC values for diabetes in both sexes compared to other measurements. When compared to BMI, WC and WHR consistently showed better AUC values except for high total cholesterol and low HDL cholesterol in males in 2015. The Kappa statistics comparing multiple obesity criteria in 2009 and 2015 are also presented in Table S3.
Table 3.
Sensitivity, specificity, and likelihood ratio of obesity and abdominal obesity in screening for metabolic-related conditions.
 |
BMI–WHO general: Obesity using BMI–WHO general definition.
BMI–WHO 2004 Asian: Obesity using BMI–WHO definition in 2004 for Asian population.
BMI–WHO 2000 Asian: Obesity using BMI–WHO definition in 2000 for Asian population.
WC–WHO: Abdominal obesity using WC–WHO definition.
WC–IDF: Abdominal obesity using WC–IDF definition.
WHR–WHO: Abdominal obesity using WHR–WHO definition.
CI: 95% confidence interval.
Table 4 presents the crude associations between obesity and metabolic-related conditions in Poisson regression models.
Table 4.
Univariate associations between obesity and metabolic-related conditions in Poisson regression models.
| 2009 |
2015 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence of metabolic related conditions among obese individuals (PPV) | Prevalence of obesity among individuals with metabolic related conditions (Sensitivity) | PR | 95% CI | p-value | Prevalence of metabolic related conditions among obese individuals (PPV) | Prevalence of obesity among individuals with metabolic related conditions (Sensitivity) | PR | 95% CI | p-value | |
| Female | ||||||||||
| Diabetes | ||||||||||
| BMI–WHO general | 5.12% | 2.38% | 3.036 | 0.649–14.203 | 0.13562 | 13.05% | 7.84% | 4.664 | 1.585–13.724 | 0.00530 |
| BMI–WHO 2004 Asian | 3.17% | 5.24% | 1.899 | 0.586–6.152 | 0.24361 | 6.90% | 15.07% | 2.547 | 1.152–5.630 | 0.02103 |
| BMI–WHO 2000 Asian | 3.25% | 18.37% | 2.099 | 1.394–3.158 | 0.00307 | 5.13% | 28.16% | 2.002 | 1.131–3.544 | 0.01739 |
| WC–WHO | 5.88% | 9.98% | 3.701 | 2.181–6.283 | 0.00045 | 8.80% | 23.57% | 3.551 | 1.870–6.744 | 0.00012 |
| WC–IDF | 4.69% | 38.54% | 3.827 | 2.806–5.219 | 0.00001 | 5.16% | 50.08% | 2.466 | 1.443–4.213 | 0.00103 |
| WHR–WHO | 3.81% | 68.99% | 4.949 | 3.413–7.176 | 0.00001 | 5.55% | 77.62% | 4.843 | 2.467–9.509 | 0.00001 |
| High total cholesterol | ||||||||||
| BMI–WHO general | 11.30% | 1.96% | 2.406 | 1.190–4.863 | 0.02065 | 19.86% | 3.14% | 1.775 | 0.814–3.870 | 0.14837 |
| BMI–WHO 2004 Asian | 11.98% | 7.20% | 2.637 | 1.371–5.073 | 0.00911 | 12.97% | 7.45% | 1.155 | 0.647–2.063 | 0.62518 |
| BMI–WHO 2000 Asian | 11.17% | 23.19% | 2.759 | 2.096–3.631 | 0.00003 | 16.68% | 24.10% | 1.620 | 1.158–2.266 | 0.00497 |
| WC–WHO | 10.55% | 6.55% | 2.305 | 1.379–3.851 | 0.00562 | 19.40% | 13.67% | 1.823 | 1.196–2.777 | 0.00536 |
| WC–IDF | 10.37% | 30.81% | 2.708 | 2.011–3.645 | 0.00006 | 19.15% | 48.84% | 2.344 | 1.703–3.228 | 0.00016 |
| WHR–WHO | 8.01% | 52.39% | 2.439 | 1.977–3.009 | 0.00001 | 17.27% | 63.52% | 2.430 | 1.708–3.457 | 0.00049 |
| Hypertension | ||||||||||
| BMI–WHO general | 62.74% | 1.64% | 2.126 | 1.696–2.665 | 0.00006 | 75.92% | 3.63% | 2.205 | 1.668–2.915 | <0.00001 |
| BMI–WHO 2004 Asian | 63.60% | 6.21% | 2.212 | 1.872–2.613 | <0.00001 | 63.39% | 11.13% | 1.905 | 1.544–2.352 | <0.00001 |
| BMI–WHO 2000 Asian | 54.97% | 18.39% | 2.037 | 1.666–2.490 | 0.00004 | 57.81% | 26.97% | 1.884 | 1.597–2.223 | <0.00001 |
| WC–WHO | 63.81% | 6.39% | 2.222 | 1.908–2.588 | <0.00001 | 65.16% | 14.75% | 2.001 | 1.687–2.373 | <0.00001 |
| WC–IDF | 51.18% | 24.73% | 1.956 | 1.659–2.307 | 0.00001 | 57.20% | 46.35% | 2.169 | 1.855–2.536 | <0.00001 |
| WHR–WHO | 42.82% | 45.04% | 1.956 | 1.659–2.307 | 0.00001 | 49.94% | 59.17% | 2.030 | 1.717–2.399 | <0.00001 |
| Low HDL cholesterol | ||||||||||
| BMI–WHO general | 92.61% | 2.21% | 1.236 | 1.132–1.349 | <0.00001 | |||||
| BMI–WHO 2004 Asian | 81.93% | 7.10% | 1.095 | 0.979–1.225 | 0.11029 | |||||
| BMI–WHO 2000 Asian | 80.90% | 17.61% | 1.091 | 1.009–1.180 | 0.02956 | |||||
| WC–WHO | 84.83% | 9.01% | 1.140 | 1.047–1.240 | 0.00251 | |||||
| WC–IDF | 84.63% | 32.54% | 1.185 | 1.109–1.266 | <0.00001 | |||||
| WHR–WHO | 82.10% | 45.53% | 1.167 | 1.084–1.256 | 0.00005 | |||||
| Male | ||||||||||
| Diabetes | ||||||||||
| BMI–WHO general | 6.88% | 3.93% | 5.466 | 0.754–39.641 | 0.08344 | 17.11% | 12.64% | 7.490 | 1.399–40.093 | 0.01882 |
| BMI–WHO 2004 Asian | 3.44% | 7.64% | 2.781 | 1.210–6.389 | 0.02200 | 10.12% | 21.44% | 4.750 | 1.164–19.379 | 0.02997 |
| BMI–WHO 2000 Asian | 2.68% | 20.99% | 2.347 | 1.448–3.804 | 0.00356 | 7.49% | 41.61% | 4.291 | 1.717–10.722 | 0.00192 |
| WC–WHO | 14.49% | 3.34% | 11.494 | 1.672–79.023 | 0.01927 | 0.00% | 0.00% | N/A | N/A | N/A |
| WC–IDF | 5.68% | 18.94% | 5.154 | 3.290–8.073 | 0.00003 | 11.32% | 39.07% | 6.600 | 2.555–17.047 | 0.00011 |
| WHR–WHO | 4.01% | 59.10% | 6.083 | 4.198–8.814 | <0.00001 | 5.81% | 55.94% | 3.869 | 1.646–9.096 | 0.00201 |
| High total cholesterol | ||||||||||
| BMI–WHO general | 11.13% | 2.31% | 3.044 | 1.175–7.891 | 0.02725 | 13.43% | 2.92% | 1.879 | 0.561–6.295 | 0.30557 |
| BMI–WHO 2004 Asian | 11.82% | 9.51% | 3.410 | 1.630–7.133 | 0.00499 | 20.07% | 14.34% | 3.066 | 1.707–5.507 | 0.00020 |
| BMI–WHO 2000 Asian | 9.77% | 27.76% | 3.258 | 1.917–5.537 | 0.00089 | 12.04% | 23.29% | 1.863 | 1.118–3.104 | 0.01715 |
| WC–WHO | 16.73% | 1.37% | 4.553 | 2.446–8.478 | 0.00050 | 32.31% | 2.02% | 4.529 | 1.098–18.679 | 0.03672 |
| WC–IDF | 12.37% | 14.95% | 3.739 | 1.822–7.673 | 0.00288 | 18.69% | 22.21% | 3.030 | 1.811–5.072 | 0.00003 |
| WHR–WHO | 8.69% | 45.60% | 3.467 | 2.366–5.080 | 0.00007 | 11.46% | 38.69% | 1.948 | 1.203–3.156 | 0.00690 |
| Hypertension | ||||||||||
| BMI–WHO general | 58.02% | 1.04% | 1.280 | 0.954–1.717 | 0.08887 | 87.11% | 3.07% | 1.797 | 1.494–2.161 | <0.00001 |
| BMI–WHO 2004 Asian | 70.67% | 4.65% | 1.582 | 1.361–1.839 | 0.00011 | 84.26% | 9.37% | 1.788 | 1.573–2.033 | <0.00001 |
| BMI–WHO 2000 Asian | 63.96% | 14.52% | 1.477 | 1.273–1.714 | 0.00031 | 71.70% | 21.91% | 1.587 | 1.383–1.822 | <0.00001 |
| WC–WHO | 69.14% | 0.50% | 1.525 | 1.117–2.082 | 0.01407 | 93.74% | 0.82% | 1.912 | 1.639–2.232 | <0.00001 |
| WC–IDF | 68.85% | 6.73% | 1.553 | 1.348–1.790 | 0.00010 | 79.58% | 14.85% | 1.725 | 1.468–2.026 | <0.00001 |
| WHR–WHO | 63.19% | 26.84% | 1.535 | 1.361–1.732 | 0.00004 | 66.17% | 33.60% | 1.519 | 1.330–1.735 | <0.00001 |
| Low HDL cholesterol | ||||||||||
| BMI–WHO general | 80.45% | 1.94% | 1.236 | 0.985–1.552 | 0.06697 | |||||
| BMI–WHO 2004 Asian | 87.01% | 6.83% | 1.357 | 1.215–1.515 | <0.00001 | |||||
| BMI–WHO 2000 Asian | 79.25% | 16.95% | 1.257 | 1.143–1.382 | <0.00001 | |||||
| WC–WHO | 75.50% | 0.52% | 1.157 | 0.656–2.038 | 0.61358 | |||||
| WC–IDF | 78.99% | 10.35% | 1.234 | 1.091–1.395 | 0.00089 | |||||
| WHR–WHO | 73.04% | 27.31% | 1.163 | 1.054–1.283 | 0.00278 | |||||
Bold values indicated statistical significance at alpha = 0.05 level. P-values were not adjusted for multiple testing.
PPV: Positive predictive value.
BMI–WHO general: Obesity using BMI–WHO general definition.
BMI–WHO 2004 Asian: Obesity using BMI–WHO definition in 2004 for Asian population.
BMI–WHO 2000 Asian: Obesity using BMI–WHO definition in 2000 for Asian population.
WC–WHO: Abdominal obesity using WC–WHO definition.
WC–IDF: Abdominal obesity using WC–IDF definition.
WHR–WHO: Abdominal obesity using WHR–WHO definition.
PR: prevalence ratio.
For diabetes type 2 in 2009, abdominal obesity using either WC (both WHO and IDF definition) or WHR appeared to be a better predictor than other criteria; but in 2015, BMI-WHO defined obesity was shown to be the better predictor among male and second best in female coming only after WHR. For high total cholesterol, all abdominal obesity criteria showed an association with the outcome among female in both 2009 and 2015. In 2015, only obesity using BMI-WHO general or BMI-WHO 2004 for Asian population was not associated with this outcome. Regarding hypertension, all criteria demonstrated to be similarly associated with this condition except for BMI-WHO general criterion among male in 2009. For low HDL cholesterol, all criteria also demonstrated to be similarly associated with the outcome except for BMI-WHO 2004 for Asian population among female and BMI-WHO, WC-WHO criteria among males. Overall, only abdominal obesity based on WC-IDF or WHR-WHO definition showed consistent association with all outcomes regardless of sex and survey years. When further adjusted for sex, age, education level, weekly physical activity, daily sitting hours, alcohol drinking in the past 12 months, fruit or vegetable consumption, the association between abdominal obesity using WC-IDF or WHR-WHO definition with metabolic-related conditions remained consistently significant, which is shown in Table S4. When combining the two survey datasets without taking survey weight into account in Table S5, we also found that, generally, abdominal obesity appeared to be a better predictor with higher significant prevalence ratios for most metabolic-related conditions in both sexes compared to obesity using BMI-WHO definitions.
Discussion
Our study showed that depending on the specific cut-offs, from 2009 to 2015, obesity prevalence increased from 0.8%–10% to 1.7%–16.4% in women and from 0.8%–10.3% to 1.7%–15% in men; abdominal obesity prevalence increased from 3%–31.3% to 8%–41.7% in women and from 0.3%–19.3% to 0.4%–25% in men. Regardless of the specific cut-off values used, we found that the prevalence of obesity and abdominal obesity increased in Vietnam between 2009 and 2015, in both men and women. A handful of studies in Vietnam have reported on prevalence of abdominal obesity in different regions over the last two decades18, 19, 20, 21, 22, 23,47,48 which was mostly similar to our findings that showed higher prevalence of abdominal obesity in women compared to men.18, 19, 20, 21,23 In 2004, Tran et al. conducted a survey in Ho Chi Minh City, the largest city in Vietnam, using a random sample and reported that the prevalence of abdominal obesity was 8.1% for women and 12.0% for men.47 However, Tran et al. used the same WC cut-off for both sexes (WC ≥ 86 cm), explaining why the prevalence was higher among men than women in this study. Furthermore, this survey was also conducted in 2004, which is 5 and 11 years prior to our data, which can partly explain the reported lower prevalence. Other Pubmed-indexed studies that employed WC-IDF definition with Asian cutoffs to define abdominal obesity found the prevalence of abdominal obesity was 8.7%–30.2% over the past two decades depending on study site.18, 19, 20, 21, 22, 23
In 2019, Tran et al. reported that the prevalence of abdominal obesity in urban Ho Chi Minh City was 49.4% using WHR-WHO definition.48 However, this study suffered from two significant limitations: the study did not employ random sampling, and more than half of the participants were over 45 years old. We also observed a high prevalence of abdominal obesity with WHR-WHO definition in this age group (39.4%–64.2%). Moreover, this study was conducted in an urban area, which could probably have a high prevalence of obesity and other chronic diseases compared to rural areas.
In other countries in Asia, the burden of abdominal obesity is also rising.49 Several studies in China reported that abdominal obesity prevalence in 2011 was 43.2% using the WC-IDF cut-off.50 The International Day for the Evaluation of Abdominal Obesity in Asian (IDEA) regions study in 2007 showed that South Asians' prevalence was 78% for women and 58% for men using WC-WHO definition.51 Our results were somewhat similar to China and lower compared to other South Asian countries. The similarities in time trends in Vietnamese and Chinese economic development, diets, and lifestyles in recent years could partly explain the comparable prevalence between the two countries.
Our study also showed that the prevalence of abdominal obesity was much higher in women than men and that this prevalence tended to increase with age. One biological hypothesis explaining these differences is changing fat distribution and adipocyte differentiation due to sex hormones and older age.52 However, we could not rule out the role of behaviors (e.g., smoking or sedentary behaviors) and socioeconomic (e.g., access to healthy food) in this disparity.52 The cut points used to diagnose abdominal obesity are arbitrary in both men and women so it is difficult to make direct comparisons. We also showed that the correlation between anthropometric measurements and biomarkers/blood pressure among Vietnamese population were modest, which is very similar to other recent studies reported correlations on Chinese,53 Brazillian,54 Turkey,55 and US population.56
Our results on the positive association between obesity and abdominal obesity with metabolic diseases were also consistent with previous reports.11, 12, 13,57, 58, 59, 60 That et al. found that obesity (BMI ≥25) and WC-based abdominal obesity were independently associated with diabetes risk in the population of Danang city, Vietnam in 2020 (aOR:1.36 (95% CI: 1.16,1.58) and aOR: 1.53 (95% CI: 1.31, 1.80), respectively).59 Hien et al. reported increased risk for hypertension in participants with overweight/obese (BMI ≥ 23) or WHR-based abdominal obesity (aOR: 1.82 (1.35–2.45) and aOR: 1.40 (1.03–1.91), respectively).60 Other prospective studies with very large sample sizes showed the stronger associations between abdominal obesity and major adverse cardiac events11 and mortality13 compared to general obesity. In our study, abdominal obesity based on WC-IDF and WHR-WHO criteria also showed the highest sensitivity when used as screening proxy for major chronic conditions. As WC-IDF and WHR-WHO criteria seem to be strong risk factors for diabetes type 2, hypertension, high cholesterol, and low HDL cholesterol, it would be useful to refer to this abdominal obesity criteria to predict better the risk of these health conditions compared to BMI-based criteria.61 Clinical implications for such differences between abdominal obesity and obesity for screening diseases in Vietnam are not entirely clear at the moment; however, BMI measures total body fat (and lean mass) and WC/WHR measures abdominal fat.62 Such discrepancies between abdominal obesity and obesity prevalence could be better understood by examining the distribution of participants' fat and muscle mass. Further studies with better measurements, such as dual-energy X-ray absorptiometry, MRI, or CT scan, are needed to investigate this issue.
Our study's strength was a nationally representative sample that allowed us to calculate the obesity and abdominal prevalence among the target population. The survey in 2015 achieved a reasonably good response rate (around 80%), which enhanced the internal validity of our study. Moreover, all questionnaires and physical measurements were standardized by WHO in Vietnam and collected by trained data collectors. The measurement errors of BMI, WC, and WHR compared to other specialized tools in measuring body fat mass and muscle mass were relatively low.56
However, our study had several limitations. First, the non-response issue (36% in the 2009 sample and 20% in the 2015 sample) probably causes non-response bias in the prevalence estimates. Secondly, the information about health behavior such as smoking and diet patterns was limited, so we cannot reduce confounding caused by these factors in our models. Moreover, the datasets used for this analysis were collected 14 and 8 years ago, which may not reflect the current prevalence and associations between anthropometric measures and metabolic-related conditions well. Finally, the cross-sectional study design's nature, which only allowed us to explore associations between anthropometric measurements and metabolic-related conditions, is prone to reverse causation. We believe that a prospective cohort would be needed to explore the causal relationship between obesity and metabolic-related diseases and provide stronger and more informative evidence to the policymakers regarding the obesity pandemic in Vietnam.
Conclusions
In summary, the prevalence of obesity in Vietnam using BMI-based definitions similarly increased in women and men over years, but the prevalence of abdominal obesity in Vietnam is much higher and increased more rapidly in women compared to men. The agreements between BMI-based definitions and WC/WHR definitions were low, especially with BMI cut-off based on WHO standard definitions. More studies are needed to explore the contributors to abdominal obesity in Vietnam.
Contributors
Tung Pham∗: Conceptualization, Formal analysis, Methodology, Project administration, Software, Visualization, Writing—original draft, Writing—review & editing.
Linh Bui∗: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Supervision, Writing—original draft, Writing—review & editing.
∗Tung Pham and Linh Bui contributed equally as co-first authors.
Edward Giovannucci: Conceptualization, Formal analysis, Verifying the reported data, Methodology, Writing—original draft, Writing—review & editing.
Minh Hoang: Data curation, Resources, Investigation, Writing—review & editing.
Bao Tran: Data curation, Resources, Investigation, Writing—review & editing.
Jorge Chavarro: Conceptualization, Methodology, Writing—original draft, Writing—review & editing.
Walter Willett: Conceptualization, Formal analysis, Verifying the reported data, Methodology, Writing—original draft, Writing—review & editing.
Data sharing statement
This article uses data from the Vietnam 2009 and 2015 STEPS survey, implemented by the MoH with ts The datasets can be assessed free-of-charge by contacting the NCD Surveillance Team (email: ncdmonitoring@who.int) as mentioned in the NCD Microdata Repository's website (https://extranet.who.int/ncdsmicrodata/).
Declaration of interests
We declare no conflict of interests.
Acknowledgements
We would like to thank WHO and the MoH in Vietnam for providing such valuable resources for the public use. The findings and views reported in this article are those of the authors and should not be attributed to WHO or MoH. In no event shall the World Health Organization be liable for damages arising from its use.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100859.
Appendix A. Supplementary data
References
- 1.Vos T., Lim S.S., Abbafati C., et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Institute for Health Metrics and Evaluation (IHME) Institute for Health Metrics and Evaluation; Vietnam: 2015. https://www.healthdata.org/vietnam [Google Scholar]
- 3.Nhung N.T.T., Long T.K., Linh B.N., Vos T., Huong N.T., Anh N.D. Estimation of Vietnam national burden of disease 2008. Asia Pac J Public Health. 2014;26:527–535. doi: 10.1177/1010539513510556. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen T.T., Hoang M.V. Non-communicable diseases, food and nutrition in Vietnam from 1975 to 2015: the burden and national response. Asia Pac J Clin Nutr. 2018;27 doi: 10.6133/apjcn.032017.13. [DOI] [PubMed] [Google Scholar]
- 5.Vietnam Ministry of Health . Vietnam Ministry of Health; Hanoi, Vietnam: 2015. Vietnam Joint annual health review 2014. Strengthening prevention and control of non-communicable disease. [Google Scholar]
- 6.Ly K.A., Ton T.G., Ngo Q.V., Vo T.T., Fitzpatrick A.L. Double burden: a cross-sectional survey assessing factors associated with underweight and overweight status in Danang, Vietnam. BMC Public Health. 2013;13:35. doi: 10.1186/1471-2458-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.General Department of Preventive Medicine . Vietnam Ministry of Health; Hanoi: 2016. National survey on the risk factors of non-communicable diseases (STEPS) Viet Nam 2015.https://extranet.who.int/ncdsmicrodata/index.php/catalog/590/download/4309 [Google Scholar]
- 8.Walls H.L., Peeters A., Son P.T., et al. Prevalence of underweight, overweight and obesity in urban Hanoi, Vietnam. Asia Pac J Clin Nutr. 2009;18:234–239. [PubMed] [Google Scholar]
- 9.MoH . Hanoi; Vietnam: 2015. National Strategy for the prevention and control of noncommunicable diseases 2015-2025. [Google Scholar]
- 10.Về việc ban hành tài liệu chuyên môn “Hướng dẫn chẩn đoán và điều trị bệnh béo phì”. 2022. https://emohbackup.moh.gov.vn/publish/home?documentId=8975 [Google Scholar]
- 11.Choi D., Choi S., Son J.S., Oh S.W., Park S.M. Impact of discrepancies in general and abdominal obesity on major adverse cardiac events. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.013471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janssen I., Katzmarzyk P.T., Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79:379–384. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z., Klimentidis Y.C., Bea J.W., et al. Body mass index, waist circumference, and mortality in a large multiethnic postmenopausal cohort-results from the women's health initiative. J Am Geriatr Soc. 2017;65:1907–1915. doi: 10.1111/jgs.14790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamer M., O'Donovan G., Stensel D., Stamatakis E. Normal-weight central obesity and risk for mortality. Ann Intern Med. 2017;166:917. doi: 10.7326/L17-0022. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C., Rexrode K.M., van Dam R.M., Li T.Y., Hu F.B. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008;117:1658–1667. doi: 10.1161/CIRCULATIONAHA.107.739714. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y., Liu B., Snetselaar L.G., et al. Association of normal-weight central obesity with all-cause and cause-specific mortality among postmenopausal women. JAMA Netw Open. 2019;2 doi: 10.1001/jamanetworkopen.2019.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tchernof A., Després J.-P. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 18.Trinh O.T.H., Nguyen N.D., Phongsavon P., Dibley M.J., Bauman A.E. Metabolic risk profiles and associated risk factors among Vietnamese adults in Ho Chi Minh City. Metab Syndr Relat Disord. 2010;8:69–78. doi: 10.1089/met.2009.0018. [DOI] [PubMed] [Google Scholar]
- 19.Binh T.Q., Phuong P.T., Nhung B.T., Tung D.D. Metabolic syndrome among a middle-aged population in the Red River Delta region of Vietnam. BMC Endocr Disord. 2014;14:77. doi: 10.1186/1472-6823-14-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran H.V., Truong M.T., Nguyen T. Prevalence of metabolic syndrome in adults in Khanh Hoa, Viet Nam. J Genet Cardiol. 2004;1:95–100. [Google Scholar]
- 21.Pham D.T., Nguyen H.T., Tran A.T.V., Tran T.K., Phan D.H., Ninh N.T. Prevalence of metabolic syndrome in rural areas of Vietnam: a selected-randomized study. Arch Pharm Pract. 2019;10 [Google Scholar]
- 22.Son L.N.T.D., Kunii D., Hung N.T.K., Sakai T., Yamamoto S. The metabolic syndrome: prevalence and risk factors in the urban population of Ho Chi Minh City. Diabetes Res Clin Pract. 2005;67:243–250. doi: 10.1016/j.diabres.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 23.Dang A.K., Le H.T., Nguyen G.T., et al. Prevalence of metabolic syndrome and its related factors among Vietnamese people: a systematic review and meta-analysis. Diabetes Metabol Syndr: Clin Res Rev. 2022;16 doi: 10.1016/j.dsx.2022.102477. [DOI] [PubMed] [Google Scholar]
- 24.WHO NCD Microdata repository–central data catalog. https://extranet.who.int/ncdsmicrodata/index.php/catalog#_r=&collection=&country=196&dtype=&from=1999&page=1&ps=&sid=&sk=&sort_by=nation&sort_order=&to=2019&topic=&view=s&vk=
- 25.Tran Q.B., Hoang V.M., Vu H.L., et al. Risk factors for non-communicable diseases among adults in Vietnam: findings from the Vietnam STEPS survey 2015. J Glob Health Sci. 2020;2 doi: 10.35500/jghs.2020.2.e7. [DOI] [Google Scholar]
- 26.Vietnam Ministry of Health . Vietnam Ministry of Health; Hanoi, Vietnam: 2010. Fact sheet: Vietnam STEPwise approach to surveillance (STEPS) 2009–2010. [Google Scholar]
- 27.Bui T.V., Blizzard C.L., Luong K.N., et al. National survey of risk factors for non-communicable disease in Vietnam: prevalence estimates and an assessment of their validity. BMC Public Health. 2016;16 doi: 10.1186/s12889-016-3160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asia WHORO for S-E . 2018. WHO STEPS survey: step towards a healthier world: monitoring noncommunicable diseases and their risk factors.https://apps.who.int/iris/handle/10665/275391 [Google Scholar]
- 29.Bui T.V., Blizzard L., Luong K.N., et al. Declining prevalence of tobacco smoking in Vietnam. Nicotine Tob Res. 2015;17:831–838. doi: 10.1093/ntr/ntu202. [DOI] [PubMed] [Google Scholar]
- 30.Lawman H.G., Ogden C.L., Hassink S., Mallya G., Vander Veur S., Foster G.D. Comparing methods for identifying biologically implausible values in height, weight, and body mass index among youth. Am J Epidemiol. 2015;182:359–365. doi: 10.1093/aje/kwv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pham L.H., Au T.B., Blizzard L., et al. Prevalence of risk factors for non-communicable diseases in the Mekong Delta, Vietnam: results from a STEPS survey. BMC Public Health. 2009;9:291. doi: 10.1186/1471-2458-9-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization . World Health Organization; Geneva: 2011. Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva, 8-11 December 2008. [Google Scholar]
- 33.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 34.IASO . 2000. The Asia-Pacific Perspective: redefining obesity and its treatment.https://apps.who.int/iris/bitstream/handle/10665/206936/0957708211_eng.pdf [Google Scholar]
- 35.World Health Organization . WHO; Geneva: 2005. WHO steps surveillance manual: the WHO stepwise approach to chronic disease risk factor surveillance. [Google Scholar]
- 36.Understanding blood pressure readings. www.heart.orghttps://www.heart.org/en/health-topics/high-blood-pressure/understanding-blood-pressure-readings
- 37.Diagnosis | ADA. https://www.diabetes.org/a1c/diagnosis
- 38.National Cholesterol Education Program . 2012. Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) executive summary.https://www.nhlbi.nih.gov/files/docs/guidelines/atp3xsum.pdf [Google Scholar]
- 39.Winter N. 2001. CORR_SVY: Stata module to compute correlation tables for survey data.https://ideas.repec.org/c/boc/bocode/s422701.html [Google Scholar]
- 40.Schober P., Boer C., Schwarte L.A. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126:1763–1768. doi: 10.1213/ANE.0000000000002864. [DOI] [PubMed] [Google Scholar]
- 41.McGee S. Simplifying likelihood ratios. J Gen Intern Med. 2002;17:647–650. doi: 10.1046/j.1525-1497.2002.10750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.American Academy of Family Physician Likelihood ratios, predictive values, and post-test probabilities. https://www.aafp.org/dam/AAFP/documents/journals/afp/Likelihood_Ratios.pdf
- 43.Chen W., Qian L., Shi J., Franklin M. Comparing performance between log-binomial and robust Poisson regression models for estimating risk ratios under model misspecification. BMC Med Res Methodol. 2018;18 doi: 10.1186/s12874-018-0519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 45.Streiner D.L. Best (but oft-forgotten) practices: the multiple problems of multiplicity-whether and how to correct for many statistical tests. Am J Clin Nutr. 2015;102:721–728. doi: 10.3945/ajcn.115.113548. [DOI] [PubMed] [Google Scholar]
- 46.Tran N.T.T., Blizzard C.L., Luong K.N., et al. The importance of waist circumference and body mass index in cross-sectional relationships with risk of cardiovascular disease in Vietnam. PLoS One. 2018;13 doi: 10.1371/journal.pone.0198202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cuong T.Q., Dibley M.J., Bowe S., Hanh T.T.M., Loan T.T.H. Obesity in adults: an emerging problem in urban areas of Ho Chi Minh City, Vietnam. Eur J Clin Nutr. 2007;61:673–681. doi: 10.1038/sj.ejcn.1602563. [DOI] [PubMed] [Google Scholar]
- 48.Quoc Cuong T., Van Bao L., Anh Tuan N., et al. Associated factors of hypertension in women and men in Vietnam: a cross-sectional study. Int J Environ Res Publ Health. 2019;16 doi: 10.3390/ijerph16234714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramachandran A., Chamukuttan S., Shetty S.A., Arun N., Susairaj P. Obesity in Asia—is it different from rest of the world. Diabetes Metabol Res Rev. 2012;28:47–51. doi: 10.1002/dmrr.2353. [DOI] [PubMed] [Google Scholar]
- 50.Chen Y., Peng Q., Yang Y., Zheng S., Wang Y., Lu W. The prevalence and increasing trends of overweight, general obesity, and abdominal obesity among Chinese adults: a repeated cross-sectional study. BMC Public Health. 2019;19:1293. doi: 10.1186/s12889-019-7633-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balkau B., Deanfield J.E., Després J.-P., et al. International Day for the Evaluation of Abdominal Obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168,000 primary care patients in 63 countries. Circulation. 2007;116:1942–1951. doi: 10.1161/CIRCULATIONAHA.106.676379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olinto M.T.A., Theodoro H., Canuto R. 2017. Epidemiology of abdominal obesity. Adiposity–epidemiology and treatment modalities. [DOI] [Google Scholar]
- 53.Zhu Q., Wang X.-B., Yao Y., et al. Association between anthropometric measures and cardiovascular disease (CVD) risk factors in Hainan centenarians: investigation based on the Centenarian's health study. BMC Cardiovasc Disord. 2018;18:73. doi: 10.1186/s12872-018-0810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cassani R.S.L., Nobre F., Pazin-Filho A., Schmidt A. Relationship between blood pressure and anthropometry in a cohort of Brazilian men: a cross-sectional study. Am J Hypertens. 2009;22:980–984. doi: 10.1038/ajh.2009.104. [DOI] [PubMed] [Google Scholar]
- 55.Erdal İ., Yalçin S.S., Aksan A., Gençal D., Kanbur N. How useful are anthropometric measurements as predictive markers for elevated blood pressure in adolescents in different gender? J Pediatr Endocrinol Metab. 2020;33:1203–1211. doi: 10.1515/jpem-2020-0175. [DOI] [PubMed] [Google Scholar]
- 56.Lee D.H., Keum N., Hu F.B., et al. Development and validation of anthropometric prediction equations for lean body mass, fat mass and percent fat in adults using the National Health and Nutrition Examination Survey (NHANES) 1999-2006. Br J Nutr. 2017;118:858–866. doi: 10.1017/S0007114517002665. [DOI] [PubMed] [Google Scholar]
- 57.Andersen C.J., Murphy K.E., Fernandez M.L. Impact of obesity and metabolic syndrome on immunity. Adv Nutr. 2016;7:66–75. doi: 10.3945/an.115.010207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Després J.-P., Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 59.Ton T.T., Tran A.T.N., Do I.T., et al. Trends in prediabetes and diabetes prevalence and associated risk factors in Vietnamese adults. Epidemiol Health. 2020;42 doi: 10.4178/epih.e2020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hien H.A., Tam N.M., Tam V., Derese A., Devroey D. Prevalence, awareness, treatment, and control of hypertension and its risk factors in (central) Vietnam. Int J Hypertens. 2018;2018 doi: 10.1155/2018/6326984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vazquez G., Duval S., Jacobs D.R., Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev. 2007;29:115–128. doi: 10.1093/epirev/mxm008. [DOI] [PubMed] [Google Scholar]
- 62.Hu F.B. Oxford University Press; Oxford; New York: 2008. Obesity epidemiology. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.