Abstract
Dynamin 2 (DNM2) is a ubiquitously expressed GTPase regulating membrane trafficking and cytoskeleton dynamics. Heterozygous dominant mutations in DNM2 cause centronuclear myopathy (CNM), associated with muscle weakness and atrophy and histopathological hallmarks as fiber hypotrophy and organelles mis-position. Different severities range from the severe neonatal onset form to the moderate form with childhood onset and to the mild adult onset form. No therapy is approved for CNM. Here we aimed to validate and rescue a mouse model for the moderate form of DNM2-CNM harboring the common DNM2 R369W missense mutation. Dnm2R369W/+ mice presented with increased DNM2 protein level in muscle and moderate CNM-like phenotypes with force deficit, muscle and fiber hypotrophy, impaired mTOR signaling, and progressive mitochondria and nuclei mis-position with age. Molecular analyses revealed a fiber type switch toward oxidative metabolism correlating with decreased force and alteration of mitophagy markers paralleling mitochondria structural defects. Normalization of DNM2 levels through intramuscular injection of AAV-shDnm2 targeting Dnm2 mRNA significantly improved histopathology and muscle and myofiber hypotrophy. These results showed that the Dnm2R369W/+ mouse is a faithful model for the moderate form of DNM2-CNM and revealed that DNM2 normalization after a short 4-week treatment is sufficient to improve the CNM phenotypes.
Keywords: MT: Oligonucleotides: Therapies and Applications, congenital myopathy, myotubular myopathy, centronuclear myopathy, dynamin, gene therapy, adeno-associated virus, RNA interference, mitophagy, mouse model
Graphical abstract

Heterozygous dominant mutations in Dynamin 2 gene cause centronuclear myopathy. Here we created and characterized the Dnm2R369W/+ mouse as a faithful disease model with a moderate severity phenotype. Intramuscular injection of AAV-shDnm2 normalized DNM2 levels, rescued the defective muscle trophism and histopathology, and improved muscle force.
Introduction
Dynamin 2 (DNM2) is a large mechanochemical GTPase that is implicated in several cellular processes including endocytosis, apoptosis, cytokinesis, phagocytosis, and cell migration,1,2,3 being engaged in cell membrane trafficking and cytoskeleton remodeling.4 DNM2 is composed of several protein domains: the N-terminal GTPase domain, the middle domain (MID) and GTPase effector domain forming a stalk responsible for protein oligomerization, a pleckstrin homology domain (PH) binding membrane, and the C-terminal proline-arginine-rich domain (PRD) binding to effector proteins with an Src homology (SH3) domain (Figure 1A).5,6,7,8,9 DNM2 is ubiquitously expressed and has different isoforms, either containing exon 10a/b in mutually exclusive splicing or including the alternative spliced exons 12b and 13b.10,11 Exon12b inclusion is specific to muscles and increases during development, while human and mouse adult muscles express this muscle-specific and the ubiquitous DNM2 isoforms equally.11
Figure 1.
Creation and validation of Dnm2R369W/+ mice
(A) Dnm2 exonic representation and the respective DNM2 protein domains: GTPase, middle, pleckstrin homology, GTPase effector, and proline rich. The exons 10a, 10b, 12b, and 13b can be alternatively spliced. DNM2 R369W mutation was knocked in by CRISPR-Cas9 in the exon 8. The shDnm2 that was used in this study to downregulate DNM2 targets exon 4. (B) Chromatopherograms of WT and Dnm2R369W/+ mice, which present a GAG>GAA synonym mutation for genotyping purpose and the Arg>Trp R369W CNM mutation (CGC to TGG). (C) Example of genotyping with amplicons of different molecular weights: the WT allele as a 427-bp band and the Dnm2R369W mutated alleles as two bands of 243 bp and a 184 bp, generated by the cleavage of the Glu GAA site by the restriction enzyme XmnI. (D) Proportion of genotypes in the mouse offspring at 8 weeks of life (n = 30 mice). Chi-square test, p = 0.75. (E) Body weight (n = 10 up to 8 weeks, n = 5 from 12 to 24 weeks). (F) Dnm2 mRNA expression of pan (ubiquitous + muscle-specific), muscle-specific (exon 12B), and ubiquitous and Dnm2 isoforms (n = 5). (G) Absolute quantification of mutated and WT forms of Dnm2 by ddPCR. Two-way ANOVA for allelic source of variation: p = 0.0002. (H) DNM2 protein expression in Dnm2R369W/+ mice (n = 10, ∼110, and 120 kDa) or (I) patients harboring the CNM DNM2 R369W mutation (n = 3). (J) Immunofluorescence of longitudinal and (K) transversal muscle sections for DNM2 localization with the Z line marker α-actinin and the satellite cell marker PAX7 (n = 3). Samples from mice at 8 weeks old. Mean ± SEM, unpaired t test, ∗∗p < 0.01: CNM DNM2R369W/+ differs from healthy controls, ∗∗∗∗p < 0.0001: Dnm2R369W/+ differs from WT. Bars represent 20 μm.
Heterozygous mutations in DNM2 gene are one of the main causes of centronuclear myopathy (CNM), a subclass of congenital myopathies with slowly progressive muscle weakness affecting children to adults of both sexes and diverge ethnic origin (ADCNM, MIM: 160150).12 These mutations act in a dominant inheritance pattern and are believed to be gain of function. Indeed, in vitro experiments with mutated DNM2 revealed increased stability of oligomers and GTPase activity, independently of lipid binding,13,14,15 and overexpression of wild-type (WT) DNM2 triggers a CNM-like phenotype in mice.16,17 Patients carrying DNM2 mutations present a broad variability in the symptoms severity and age of onset, from a severe neonatal form to a moderate (intermediate) severity and to a mild form with adult onset.12,18,19,20,21,22,23,24 In early onset severe cases, the symptoms include generalized muscle weakness, hypotonia, facial weakness, ophthalmoplegia, ptosis, and breathing difficulties.20 Moderate cases have delayed motor development milestones during childhood, difficulties for walking, running, or climbing stairs, while mild cases display a diffuse and slowly progressive myopathy from 20 to 40 years. As histopathological hallmarks, muscle biopsies show a predominance of oxidative fibers, fiber size heterogeneity, and central accumulation of oxidative activity and nuclei.25
The CNM pathomechanism is only partially understood. Analysis of zebrafish models and mice knockin for a severe (S619L) or mild (R465W) mutation reported defects in neuromuscular junctions, organelle positioning, calcium handling, and autophagy.26,27,28,29,30 No therapies are currently available to treat CNM patients, but therapeutic proof of concepts have been successful in pre-clinical models. Acetylcholine esterase inhibitors and DNM2 downregulation were proposed for DNM2-CNM. Acetylcholine esterase inhibitors target the alteration of neuromuscular junctions.31,32 DNM2 downregulation, with antisense oligonucleotides or shRNA, rescued the locomotor, histological, and force defects of Dnm2S619L/+ and Dnm2R465W/+ mice, models for the severe and mild DNM2-CNM respectively.30,33 Specific reduction of the Dnm2 mutated allele with siRNA also improved the phenotypes of the Dnm2R465W/+ mice.34
A main bottleneck in this field is whether DNM2 downregulation would be therapeutic on different severity forms and mutations in DNM2.35 Here, we first created a mammalian model for the moderate DNM2-CNM form and tested DNM2 downregulation with in vivo expression of a specific shRNA against Dnm2. We selected the R369W DNM2 mutation, as among the most common mutation linked to moderate cases with age of onset ranging from childhood to adulthood and with variable symptoms that may include muscle weakness (either proximal, distal, or diffuse), facial weakness, ophthalmologic involvement, hyperlordosis, walking difficulties, and impairment of tendon reflexes at different intensities.20 We found the Dnm2R369W/+ mouse is a faithful model for the moderate CNM form and presents increased DNM2 level and alteration of metabolic and mitochondria functions in addition to a CNM histopathology and muscle involvement. DNM2 normalization upon a single injection of adeno-associated virus (AAV)-shDnm2 was sufficient to improve these alterations.
Results
Creation and initial characterization of the Dnm2R369W/+ mouse for moderate DNM2-CNM
The DNM2 R369W mutation in the middle domain was introduced in mice using CRISPR-Cas9. Three point mutations were created in the exon 8 of the Dnm2 gene: the CGC>TGG mutations resulting in the substitution of an arginine by a tryptophan and a flanking synonymous mutation GAG>GAA (Glu) for genotyping purposes (Figures 1A and 1B). The presence of these heterozygous mutations was confirmed by genotyping and Sanger sequencing (Figures 1B and 1C).
Dnm2R369W/+ males and females were successfully generated and fertile. They were bred with WT mice, producing offspring following Mendelian proportion (Figure 1D). Dnm2R369W/+ mice were studied at different ages from 3 to 24 weeks to assess the clinical, histological, and force phenotypes. No postnatal deaths were observed up to 24 weeks of life. At all ages studied, Dnm2R369W/+ mice presented no clear physical signs associated with myopathy such as kyphosis, breathing or walking difficulties, nor body weight loss (Figure 1E), albeit unilateral ptosis was observed in 2% of them. We observed three males of each genotype up to their natural death, and their lifespan was around 1 year and 8 months, regardless of the genotype.
We next assessed if the mutation in Dnm2 gene impacts the corresponding mRNA and protein expression. No alteration was observed in gene expression of pan-Dnm2, muscle-specific, nor ubiquitous isoforms in the TA (tibialis anterior) muscles of Dnm2R369W/+ mice at 8 weeks of age (Figure 1F), and the expression of the mutated and WT forms of Dnm2 mRNA each accounted for about 50%, as verified by reverse transcription droplet digital PCR (RT-ddPCR, Figure 1G). However, DNM2 protein level was significantly increased by 50% in the Dnm2R369W/+ mouse model (Figure 1H), mimicking the upregulation found in the muscles of CNM patients carrying the DNM2 R369W mutation versus healthy controls (Figure 1I).
Immunofluorescence in longitudinal muscle sections indicated that DNM2 was mainly colocalized with α-actinin, a Z line marker (Figure 1J). DNM2 was also present in PAX7-negative cells adjacent to muscle fibers and at the periphery of the fiber in transverse sections (Figure 1K). All these results indicate that the R369W mutation has no strong effect on protein localization but causes DNM2 increased levels in muscles of the Dnm2R369W/+ mouse.
Muscle weakness in Dnm2R369W/+ mice
We next submitted Dnm2R369W/+ mice to force grip and four limbs hanging tests from 3 to 24 weeks of age and verified the contractile properties of TA muscles at 8 weeks of age. Dnm2R369W/+ mice had the same performance in the grip and hanging tests as the control WT mice; however, they may have compensated a lack of distal force by relying on the forelimbs to keep holding the grid (Figures 2A–2C and Video S1). Thus, the contractile properties were measured based on the force response by isolated TA muscles after sciatic nerve stimulation to simulate the maximum force production (stimulations at ascendant frequencies) or the susceptibility to fatigue (repeated 40-Hz stimulations). Dnm2R369W/+ performed as WT mice in the simulation of susceptibility to fatigue (Figure 2D). However, the experiment of force production revealed decreased submaximal force (≥50 Hz) and decreased maximum absolute and specific force (at 100 Hz), supporting that Dnm2R369W/+ mice present force deficit (Figures 2E and 2F).
Figure 2.
Muscle strength tests and deficits in the contractile properties
(A) Four limbs grip test and (B) hanging test (n = 10 up to 8 weeks, n = 5 from 12 to 24 weeks). (C) Representative rotatory movements of the tail and forelimbs during the hanging test at 6 weeks of age (see Video S1). (D) TA muscle resistance to fatigue after sciatic nerve stimulations at 40 Hz. (E) Submaximal and maximum absolute force outputs and (F) maximum absolute force and (G) maximum specific force (n = 5 at 8 weeks old). The maximum force was calculated at 100 Hz. The specific force is expressed as the ratio of the force produced (mN) by the cross-sectional area of the muscle analyzed (CSA = muscle weight (mg)/muscle length (mm) x muscle density 1.06 mg/mm3). Mean ± SEM, Mann-Whitney or unpaired t test, ∗p < 0.05 and ∗∗p < 0.01: Dnm2R369W/+ differs from WT.
Representative video of the characteristic rotatory movements of the tail and the forelimbs of Dnm2R369W/+ mice during the hanging test at 6 weeks of age.
Dnm2R369W/+ muscle recapitulates CNM histological hallmarks
Alteration in fiber diameter is among the histological hallmarks of CNM, also including centralized nuclei and abnormal oxidative staining.25 At 8 weeks of age, the TA muscles from Dnm2R369W/+ mice were hypotrophic compared to WT, as indicated by the reduction of 10% in the ratio of TA muscle weight normalized to body weight (Figure 3A). Transverse sections of soleus muscles stained with hematoxylin and eosin (H&E) did not present obvious alterations (Figure S1), while TA muscles had fibers with smaller diameter (<40 μm), a decreased number of large fibers (>40 μm), and nuclei correctly placed at the periphery of the fibers (Figures 3B–3D). The succinate dehydrogenase (SDH) staining presented central and necklace-like accumulation of oxidative activity in 9.6% and 2.17% of the fibers, respectively, while these defects were never noted in WT (Figure 3E). In the soleus muscles, central staining was found in 3.23%, while absent in WT (Figure S1). Together, the impaired histology, the deficits in muscle force, and DNM2 overexpression validate the Dnm2R369W/+ mice as a faithful CNM mouse model.
Figure 3.
Muscle hypotrophy and histological hallmarks of centronuclear myopathy
(A) Ratio of hindlimbs muscle weight per body weight at 8 and 24 weeks of age (8w and 24w). GA, gastrocnemius; TA, tibialis anterior; SOL, soleus; EDL, extensor digitorum longus. Analyses of muscle sections at 8 weeks old, with 2 mm2 analyzed per muscle. The TA muscles sections stained with hematoxylin and eosin were used for determining (B) the general histological aspect (n = 5), (C) the percentage of fibers presenting internalized nuclei (n = 3), and (D) the fiber diameter (n = 4). (E) SDH oxidative staining showed abnormal central accumulation (c), necklace fibers (n), or spokes of wheels aspect (s) (n = 3). Mean ± SEM, Mann-Whitney or unpaired t test, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001: Dnm2R369W/+ differs from WT at the same age; α p < 0.05, αα p < 0.01, and ααα p < 0.001: Dnm2R369W/+ at 8 weeks differs from Dnm2R369W/+ at 24 weeks; n p < 0.05: Dnm2R369W/+ necklace fibers at 8 weeks differs from Dnm2R369W/+ 24 weeks. Bars represent 50 μm.
Dnm2R369W/+ mice were also analyzed at 24 weeks old to assess a potential progression of the muscle disease. The gastrocnemius (GA) and TA muscles from mutated mice presented a reduction in the mass when comparing 24 and 8 weeks of life. In the case of the TA muscles, the ratio of muscle per body weight decreased by 22% (Figure 3A), and the number of internalized nuclei was five times higher compared to controls (Figures 3B and 3C). Although the SDH abnormal staining remained at the same levels, the profile of accumulation changed from predominantly centralized (9.6%) at 8 weeks to a half-half mix of centralized SDH staining and necklace-like staining (7.7% and 7.2% respectively) at 24 weeks (Figure 3E). Nearly 1% of the fibers evolved to a radial disposition of SDH staining (aspect of "spoke of wheels"), a typical pattern in CNM patients. These results suggest that the TA muscle phenotype of Dnm2R369W/+ mice progressed from 8 to 24 weeks old.
Muscle fiber disorganization underlies the muscle weakness
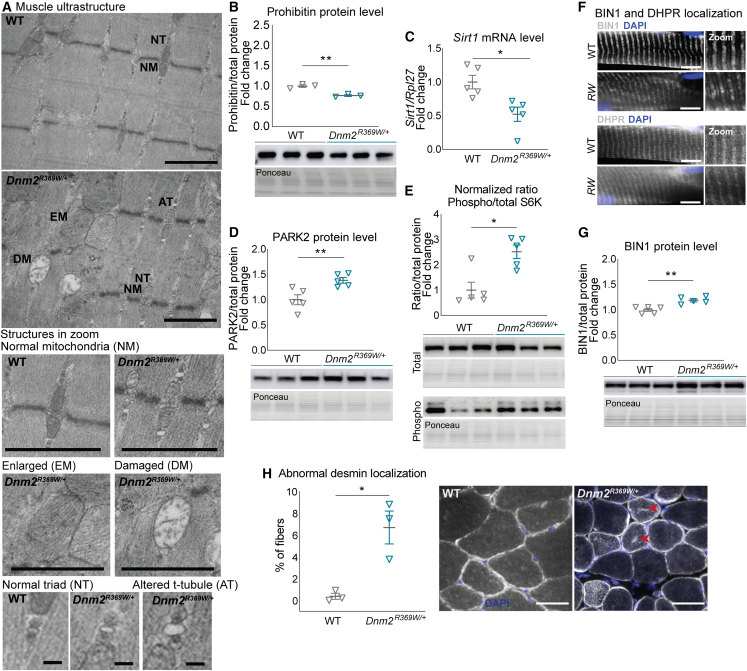
To assess the cause of the muscle weakness, we investigated further the fiber ultrastructural organization. In accordance with the result of abnormal SDH oxidative staining, structural mitochondrial defects were found in electron microscopy (n = 2, Figure 4A). Dnm2R369W/+ muscles presented with a subgroup of mitochondria with normal shape and size but also enlarged mitochondria sometimes with disrupted cristae. In corroboration, we found that prohibitin, a marker of inner mitochondrial membrane and a mitophagy receptor,36 was downregulated in Dnm2R369W/+ mice (Figure 4B). No mitochondrial fragmentation nor autophagic vacuoles were observed in electron microscopy.
Figure 4.
Muscle structure disorganization in Dnm2R369W/+ mice
(A) Different populations of mitochondria and triads found in electron microscopy: normal mitochondria (NM), enlarged mitochondria (EM), damaged mitochondria (DM, with abnormal cristae), normal triad (NT) and altered T-tubule (AT) (n = 2). Bars in the larger panel and mitochondria represent 1 μm; bars in the triads represent 0.1 μm. (B) Prohibitin protein levels (∼30 kDa, n = 3), (C) mRNA level of Sirt1 and protein levels of (D) PARK2 (∼55 kDa, n = 4), and (E) phosphorylated and total S6K (60 kDa, n = 5). (F) BIN1 and DHPR localization in longitudinal muscle sections (n = 3, bar represents 10 μm) and (G) BIN1 protein expression (n = 5, ∼60 kDa). (H) Desmin mislocalization (arrows) in transverse muscle sections (n = 3). Mice at 8 weeks old. Bar represents 50 μm. Mean ± SEM. Unpaired t test, ∗p < 0.05 and ∗∗p < 0.01: Dnm2R369W/+ differs from WT.
One of the main mitophagy pathways is mediated by PTEN-induced putative kinase 1 (PINK1)/Parkin 2 (PARK2)37,38,39 and regulated by Sirt1.40 We found that the mRNA levels of Sirt1 were half of the values found for the WTs (Figure 4C) and, although Pink1 and Park2 mRNA expression did not differ between genotypes (values normalized to the WTs = 1: Pink1 0.97 SEM ± 0.07 p = 0.11, Park2 1.10 ± 0.03 SEM p = 0.23, n = 5 each genotype), the E3 ubiquitin ligase PARK2 was found increased (Figure 4D). The mTOR pathway is related with mitochondrial homeostasis and implicated in mitochondrial function41 and autophagy suppression.42 We investigated the activity of the mTOR pathway through the phosphorylation level of S6K, a major downstream target. The phosphorylation of S6K was indicated by the 2.5-fold higher ratio of phosphorylated per total form in Dnm2R369W/+ (Figure 4E). Altogether, these results suggest that mitophagy is impaired in the TA muscles of Dnm2R369W/+ mice.
The electron microscopy also revealed a combination of triads with normal aspect and others with shape defects in the Dnm2R369W/+ muscles, as enlargement of the T-tubule (Figure 4A). Triads are essential membrane structures for excitation-contraction coupling and are composed of two terminal cisternae from the sarcoplasmic reticulum and a central T-tubule.43 To evaluate further the T-tubules, immunofluorescence experiments were performed for dihydropyridine receptor (DHPR), a voltage-gated calcium channel found at the T-tubule,44,45 and for amphiphysin 2 (BIN1), a protein implicated in T-tubules formation.46,47 While DHPR and BIN1 localization was not obviously altered (Figure 4F), correlating with the normal localization of triad seen by electron microscopy, BIN1 protein was found 20% overexpressed in TA muscles of Dnm2R369W/+ mice at 8 weeks, confirming alteration of T-tubule maintenance as a pathomechanism (Figure 4G).
Desmin is a protein found at the Z line that is pivotal for maintaining the muscle structure and function48 and the correct mitochondrial and nuclei positioning.49 DNM2-CNM patients accumulate desmin in the center of the fibers.25 Accordingly, 6.7% of the Dnm2R369W/+ fibers were positive for internal desmin accumulation at 8 weeks of age versus 0.38% in WT controls (n = 3, p = 0.015) (Figure 4H). These results indicate that Dnm2R369W/+ mice present disorganization of the muscle fiber structure and accumulation of muscle-specific proteins, similarly to DNM2-CNM patients.
Dnm2R369W/+ muscles present alterations in muscle fiber metabolism
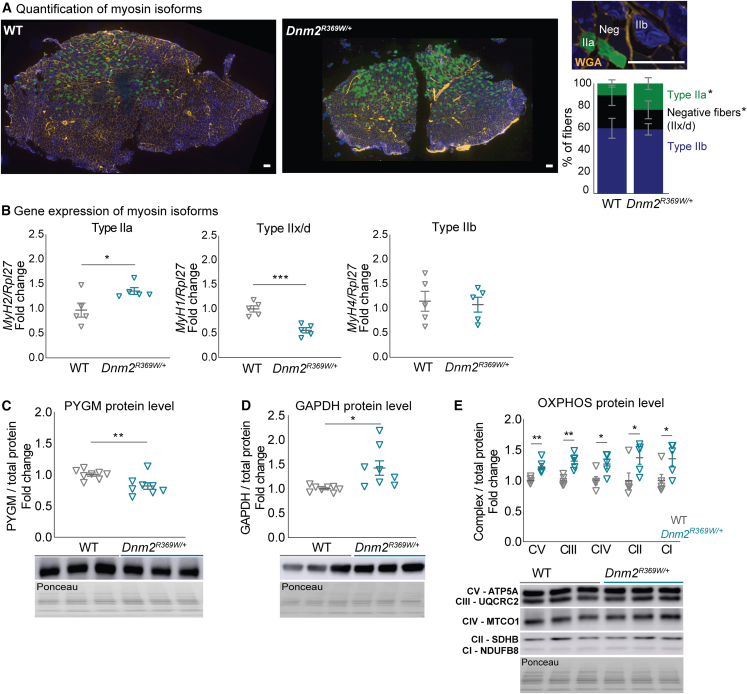
Alteration in fiber type specification toward an increase in slow-twitch fibers with high oxidative capacity (i.e., type I) is a hallmark of CNM and more generally congenital myopathies.25 However, a previous study conducted in Dnm2R465W/+ mice showed WT fiber type composition may be linked to the mild-form muscle phenotypes of this model.50 Here, we estimated the myofibers profile through mRNA expression of myosin isoforms and immunofluorescent co-labeling of myosins type I (MYH7), IIa (MYH2), and IIb (MYH4) in the TA muscles of Dnm2R369W/+ mice at 8 weeks of age. We focused on the TA muscles as they were the most affected of the muscles tested (Figure 5A) and are fast-twitch muscles with no slow type I fibers and very low intermediate type IIa fibers,51,52 where a slower specification would be easy to highlight. Fast fibers positive for type IIb myosin accounted for around 60% in both Dnm2R369W/+ and WT mice (Figure 5A). However, a fiber switch occurred toward intermediate type IIa fibers with 25.5% in Dnm2R369W/+ versus 10.5% in WT muscles. In addition, fibers with negative labeling most probably representing type IIx/d fibers decreased to 15.5% in Dnm2R369W/+ compared to 29.6% in WT muscles. No type I fibers were found for any genotypes. The mRNA expression of genes coding for the myosin isoforms IIa, IIx/d, and IIb (MyH7, MyH2, MyH1, and MyH4) corroborated the immunofluorescence results with overexpression of type IIa (1.36-fold) and downregulation of IIx/d (0.57-fold) corresponding myosin transcripts (Figure 5B). This fiber type profile indicates a significant switch to slower fibers at 8 weeks and a similar tendency at 24 weeks (p = 0.057) (Figure S2). This fiber type switch could explain the force decrease and suggests a trend from a glycolytic toward a more oxidative metabolism in Dnm2R369W/+ muscles.
Figure 5.
Alterations in muscle fiber metabolism in Dnm2R369W/+ mice
(A) TA muscle fibers positive for myosin isoforms IIa (MYH2) and IIb (MYH4) per muscle section (total area of 2 mm2). Negative or light labeling were considered mixed fiber type or type IIx/d. No fiber type I fiber (MYH7) was found. (B) qRT-PCR of genes coding for myosins type IIa, IIx/d, and IIb (MyH2, MyH1, and MyH4, respectively; n = 4) and (C) PYGM (n = 8, ∼100 kDa), (D) GAPDH (n = 8, ∼37 kDa), and (E) OXPHOS (n = 5, ∼15, 25, 35, 40, and 55 kDa) protein levels. Mice at 8 weeks old. Bar represents 100 μm. Mean ± SEM, Mann-Whitney test or unpaired t test: ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001: Dnm2R369W/+ differs from WT.
To confirm the metabolic switch, we investigated the expression of the muscle isoform of glycogen phosphorylase (PYGM), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and the mitochondrial oxidative phosphorylation system (OXPHOS). PYGM is implicated in energy generation for muscle contraction through glycogenolysis, and GAPDH participates in the sixth step of glycolysis.53,54 The OXPHOS (mitochondrial complexes I to V) regulates the electron transport chain in the inner mitochondrial membrane for mitochondrial oxidation.55 The expression of these three markers on western blot was altered in the TA muscles of Dnm2R369W/+ mice at 8 weeks of age when compared to WT (Figures 5C–5E). PYGM was downregulated (18.2%), GAPDH was upregulated (42.5%), and the quantification of OXPHOS indicated increased expression of all five mitochondrial complexes. These data corroborate with the fiber type switch and support a metabolic alteration toward oxidation in Dnm2R369W/+ mice.
Normalization of DNM2 levels by shDnm2 injection improved the Dnm2R369W/+ phenotypes
As we found the CNM-like phenotypes of Dnm2R369W/+ mice, i.e., muscle hypotrophy with decreased muscle force and altered structural organization of myofibers, correlates with increased level of DNM2 protein, we sought to reduce DNM2 level to improve the disease. shDnm2 targeting the exon 4 of Dnm2 mRNA was expressed from adeno-associated virus (AAV9) (Figure 1A). One of the TA muscles of Dnm2R369W/+ mice and WT littermates received a single intramuscular injection of AAV-shDnm2 at 4 weeks old, before reaching mature adulthood.56 The contralateral TA muscles of the same mice were injected with control AAV-shScramble, which does not hybridize with endogenous Dnm2 mRNA. The muscles were analyzed at 8 weeks.
The injection of shDnm2 caused downregulation of pan-Dnm2 by 25% and 27% in the TA muscles of WT and Dnm2R369W/+ mice, respectively, and the reduction in DNM2 protein expression by 14% in WT and 38% in Dnm2R369W/+ mice, in comparison to contralateral muscles injected with scramble (Figures 6A and 6B). Importantly, the reduction caused the normalization of Dnm2 expression in the TA muscles of Dnm2R369W/+ mice compared to the level of WT scramble. Dnm2R369W/+ mice injected with shDnm2 displayed normalization of muscle hypotrophy, as indicated by the ratio of muscle to body weight (Figure 6C). The reason for this normalization was the increase in myofiber diameter noted from the (H&E) staining. In particular, there was a significant improvement in the number of large fibers (>40μm minFeret) reaching WT level (Figures 6D and 6E). The aspect of SDH oxidative staining was also improved in Dnm2R369W/+ mice with the reduction of the number of fibers with abnormal central accumulation of oxidative staining from 14% in scramble to 10.3% in shDnm2 (Figures 6D and 6F). The injection of shDnm2 did not have a noticeable impact in WT muscle (Figure S3).
Figure 6.
Intramuscular injections of AAV-shDnm2 normalized DNM2 expression, rescued the histopathology, and improved muscle force and ultrastructure
Injections of TA muscles at 4 weeks old, muscles analyzed at 8 weeks old. (A) Dnm2 mRNA and (B) DNM2 protein expression (n = 5–6). (C) TA muscle weight (n = 6). (D) Transversal muscle sections stained with hematoxylin and eosin and SDH (c: central accumulation) (n = 4). (E) Sections stained with H&E were used to calculate fiber diameter (n = 4). (F) Quantification of SDH staining (n = 3). (G) Electron microscopy of TA muscles from Dnm2R369W/+ mice injected with AAV-shDnm2 presenting a mix of enlarged mitochondria (EM) and normal triads (NT) (n = 2). (H) Maximum absolute force and (I) specific force at 100 Hz (n = 6). Mean ± SEM. DNM2 protein level: Wilcoxon matched pairs test, ∗p < 0.05: Dnm2R369W/+ shDnm2 differs from Dnm2R369W/+ scramble; Mann-Whitney test, α p < 0.05: Dnm2R369W/+ scramble differs from WT scramble. Central staining ∗p < 0.05: Dnm2R369W/+ shDnm2 vs. Dnm2R369W/+ scramble; αααα p < 0.0001: Dnm2R369W/+ shDnm2 vs. WT shDnm2 or Dnm2R369W/+ scramble vs. WT scramble. Others: unpaired t test, α p < 0.05, αα p < 0.01, and ααα p < 0.001: Dnm2R369W/+ differs from WT injected with the same treatment; paired t test, ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗∗p < 0.0001: scramble vs. shDnm2 within the same genotype.
Normalization of DNM2 levels improved myofiber organization
We observed through electron microscopy that DNM2 normalization by AAV-shDnm2 injection in TA muscles of Dnm2R369W/+ mice led to some improvements in myofiber organization (Figure 6G). In particular, mitochondria with disrupted cristae were no longer present, albeit there were still some enlarged mitochondria. Concerning the ultrastructure of the triads, they were in normal shape without T-tubules alterations in muscles of Dnm2R369W/+ mice after shDnm2 injection (Figure 6G). Thus, the improvement of the functional and histological CNM phenotypes of the Dnm2R369W/+ mice correlated with some improvement in myofiber intracellular organization.
Correlating with the amelioration of the histology and the aspect of the mitochondria and triads in electron microscopy, the absolute force and the specific force of the isolated TA injected with shDnm2 increased by 26% and 16% respectively, compared to the contralateral scramble TA muscles (Figures 6H and 6I). Thus, DNM2 reduction through shDnm2 intramuscular expression improves muscle hypotrophy and force and the CNM-like histopathology of Dnm2R369W/+ mice. Noteworthy, DNM2 normalization to WT levels is sufficient to achieve this rescue.
Discussion
This study aimed to create, validate, and rescue a mouse model for the moderate form of DNM2-CNM harboring the common Dnm2 R369W missense mutation and to assess to which extent Dnm2 targeting is sufficient for a potential therapy. Dnm2R369W/+ mice presented with increased DNM2 protein level as in patients and moderate CNM-like phenotypes in the TA muscles at 8 weeks, including force deficit, muscle and fiber hypotrophy, and alterations in the myofibers organization including mitochondria with disrupted cristae and some triad structural defects. Normalization of DNM2 levels by AAV-shDnm2 injection targeting Dnm2 mRNA improved the different phenotypes. These results reveal that the Dnm2R369W/+ mouse is a faithful model for the moderate form of DNM2-CNM and suggest that DNM2 normalization would be enough to improve the phenotypes in young patients carrying the common DNM2 R369W mutation.
The Dnm2R369W/+ mouse is a faithful model for the moderate form of DNM2-CNM
DNM2 R369W patients may manifest a variable number of CNM symptoms at different intensities, such as muscle weakness (proximal, distal, or diffuse) and muscle hypotrophy, with onset ranging from early childhood to early adulthood.20,23,24 Fiber diameter heterogeneity, mis-localized organelles like nuclei and mitochondria, and altered oxidative stain are histological hallmarks of DNM2 R369W patients.12,25 Dnm2R369W/+ mice reproduce the muscle hypotrophy and decreased muscle force. These defects are obvious in the TA muscle, a muscle especially affected in other mouse models for different genetic forms of CNM.28,57 DNM2-CNM is described as slowly progressive in patients.12 Comparative analysis of the Dnm2R369W/+ phenotypes at 8 and 24 weeks showed worsening of muscle hypotrophy in the TA and GA muscles, and the histology was progressively impacted concerning the number of internalized nuclei and alterations in SDH oxidative stains. A typical and specific oxidative pattern found in DNM2-CNM patients and named “spokes of a wheel” (radial arrangements of sarcoplasmic strands) could also be observed at 24 weeks.
Apart from this new Dnm2R369W/+ mouse, two other Dnm2-CNM mice were characterized and represent models for the mild and severe forms of the disease, respectively Dnm2R465W/+ and Dnm2S619L/+. Dnm2R465W/+ mice present progressive CNM phenotypes including muscle histology disorganization (mainly central accumulation of oxidative stains), muscle and fiber hypotrophy, and compromised muscle contraction.28,33 TA muscles were affected by hypotrophy at 2 months of age, but the overall histological aspect in H&E staining remained close to normal up to 8 months of age. DNM2 levels were normal at 8 weeks, although contractile properties were impaired at that age. Dnm2S619L/+ mice present decreased body weight since the very early days postnatal, important impairment in motor function, muscle fiber hypotrophy, abnormal oxidative staining, and enlarged and round-shaped mitochondria with disrupted cristae from 3 weeks of age.30 Obviously, the phenotype onset and severity of the Dnm2R369W/+ mouse are intermediate compared to the Dnm2R465W/+ and Dnm2S619L/+ mice, indicating the Dnm2R369W/+ mouse is a faithful model for the moderate DNM2-CNM form (Figure 7B). Thus, the Dnm2R369W/+ mouse is a valuable tool to further decipher the phenotype-genotype correlation, to better understand CNM pathomechanism and disease progression, and to assess if a potential therapy will be applicable to different DNM2 mutations and CNM severity.
Figure 7.
Improvement in CNM-like muscle phenotypes of Dnm2R369W/+ mice after DNM2 normalization with shDnm2
(A) Summary of the mean results obtained in WT and Dnm2R369W/+ mice at 8 weeks old submitted to a single injection of AAV-shDnm2 in the TA muscle and scramble in the contralateral TA at 4 weeks old. 100% represents the normalized values for WT muscles injected with scramble. (B) Mean results obtained for the characterization of Dnm2R369W/+ mice compared to previously published Dnm2-mutated CNM mouse models at 8 weeks old: the severe Dnm2S619L/+30 and the mild Dnm2R465W/+.28 The data are normalized to the WT values originally reported for each model. ∗No hanging was test described in the original publication of Dnm2R465W/+ mice, but it was assumed as equal to the WT. Some of these parameters were not analyzed in (A) as the treatment was intramuscular (for body weight and hanging test) or as centralization of nuclei appeared later than the age of analysis.
Pathological mechanism linked to DNM2-CNM
In patients with DNM2-CNM, the main histopathological hallmarks are organelle mis-positioning and a switch of fiber type toward oxidative metabolism (type I fiber predominance).58 Dnm2R369W/+ presented alterations in the expression of myosin isoforms, supporting a switch toward a more oxidative metabolism. Indeed, oxidative or glycolytic fibers correlate with force production: slow oxidative type I fibers are known to produce less force than fast glycolytic type II fibers.59,60 This difference may be based on the energy production, myosin isoforms, and sensitivity of the contractile apparatus to calcium. Thus, the myosin isoform and metabolic switch discovered in the Dnm2R369W/+ mouse is a plausible explanation for the force decrease. We cannot exclude additional explanation as alteration of excitation-contraction coupling that was reported in the Dnm2R465W/+ mouse.27 The upregulation of the muscle-specific isoform of BIN1 that regulates T-tubule biogenesis and maintenance can potentially represent a compensatory mechanism to such ECC defects. This hypothesis is in accordance with the demonstration that overexpression of BIN1 through transgenesis or AAV transduction improves Dnm2R465W/+ phenotypes.61
Organelles mis-positioning was evidenced in the Dnm2R369W/+ mouse, and we showed it is progressive with age concerning mitochondria and nuclei. In addition to accumulating toward the central area of the fiber, mitochondria can show enlargement and disrupted cristae. We discovered alteration of upstream and downstream mitophagy markers, strongly supporting that mitochondria defects may be partly caused by defective mitochondrial quality control. It is yet unknown if altered mitophagy is a common finding to several forms of DNM2-CNM as it remains to be tested in the models for the other forms. Of note, we also found internal accumulation of desmin that is found in the Dnm2R465W/+ mouse and in the MTM1-CNM form,26,62,63,64 suggesting general alteration of autophagy is a common pathological mechanism.
Normalization of DNM2 level is sufficient for phenotypic amelioration
DNM2 is overexpressed in patients and mouse models harboring other CNM mutations,30,57,65 and we confirmed that DNM2 levels were increased in the Dnm2R369W/+ mice and DNM2 R369W patients. Local DNM2 reduction was achieved by intramuscular expression of shDnm2 targeting Dnm2 mRNA through AAV transduction (Figure 7A). A rather short treatment period (4 weeks) was sufficient to achieve both DNM2 normalization to WT level and improvement of the main phenotypes including the muscle hypotrophy, myofiber hypotrophy, and mitochondria mis-position. Similar rescue results were reported for mouse models of the other forms of DNM2-CNM.30,33 Here we demonstrate that DNM2 normalization is enough to achieve a therapeutic benefit. These data also highlight that a similar treatment could work for different mutations and severity of CNM linked to DNM2.
Concerning the choice of AAV-shRNA instead of siRNA for reducing DNM2 level through RNA interference, the AAV-shRNA approach that was chosen as a single injection was enough to downregulate DNM2 and improve the phenotypes. siRNA has a transient nature that usually requires repeated injections. Moreover, specific and efficient delivery to the tissue of interest is still a major bottleneck. For AAV-shRNA, efficient muscle transduction and long-term expression after several years in human were reported.66 However, a high dose of AAV induced liver toxicity in specific disease populations like X-linked CNM.67 The recent and ongoing development of novel AAV serotype de-targeting the liver and with increased muscle transduction efficiency (thus requiring a lower dose) could help reducing the AAV-shRNA bottlenecks.68
Materials and methods
Mice
Dnm2R369W/+ C57BL/6N knockin mice (MGI nomenclature Dnm2em1Ics) were created by PHENOMIN-Institut Clinique de la Souris (www.phenomin.fr/en-us, Illkirch-Graffenstaden, France) using CRISPR-Cas9 to insert a mutation in the Dnm2 gene (RefSeq: NM_001039520.2): the CNM-related R369W mutations 1293C>T and 1295C>G leading to the substitution Arg – CGC per Trp – TGG, plus a synonym mutation 1292G>A (GAG>GAA) creating an XmnI restriction site for genotyping purposes. C57BL/6N fertilized oocytes were microinjected with a mix of sgRNA (TGAGCGCTTTCCCTTTGAAC, 12 ng/μL), spCas9 mRNA (25 g/μL), and single-stranded oligodeoxynucleotides (ssODN, AGATCAAGTAGACACACTAGAGTTGTCTGGTGGAGCCCGCATCAATCGTATCTTTCATGAATGGTTTCCCTTTGAACTGGTAAAGGTAGGTGTTCAGCCTGGAGTTAAGTCAGACACTCTCATGCTTGGTCTTTG, 10 ng/μL). The microinjected eggs were re-implanted in CD1 foster females and the germ line transmission was achieved in pups carrying the XmnI restriction site and the R369W mutation. The mice were genotyped with the primers 5′-CATGCCTCCTTCCAATACACAAAT-3′ and 5′-GAGATCTGGGAAGATGGGGACATGT-3′, and the PCR products were digested with XmnI restriction enzyme (20 u/μL) for 30 min at 37°C, followed by electrophoresis and Sanger sequencing.
The mice were housed in the Institut Clinique de la Souris animal facility in 12-h/12-h light/dark cycles, under controlled conditions of temperature and humidity, food and water ad libitum. The mice were euthanized by CO2 inhalation followed by cervical dislocation. All experiments were conducted in accordance with French and European legislations (ethics approval 25185–2020042209385997).
Muscle strength assessment
The hanging test was performed in triplicate by placing the mice horizontally on a grid in suspension, where they were expected to hold against the gravity force for up to 60 s. For the grip test, the mice were weighted, and their force was measured in triplicate using a grip meter (Bioseb Grip Test V3.22, Pinellas Park, FL, USA). The mice were allowed to grab the grip grid and were then gently pulled backward in the horizontal plane. The force obtained was normalized to the body weight.
AAV-shDnm2
AAV2/9-shDnm2 under the control of a U6 promoter was modified from the sequences created and validated in Buono et al. and Tasfaout et al.33,56 for the removal of the plasmid region responsible for the GFP expression. The AAV-shDnm2 targeting Dnm2 exon 4 (shDnm2, AAGGACATGATCCTGCAGTTCAT, Figure 1A) was injected in the TA muscle. This sequence has 100% homology to the human genome sequence RefSeq: NM_001005360.3.69 The control AAV-shScramble has no homology to mouse genome (scramble, GGGCTATCCCAACGCTATTAGT) and was injected in the contralateral muscle of 4-week-old-mice at 1.2 × 1010 vg in a final volume of 20 μL. At 8 weeks old, mice were euthanized, and muscles were processed.
Muscle contractile properties
The mice were anesthetized by sequential intraperitoneal injections of Domitor (1 mg/kg)/fentanyl (0.14 mg/kg), diazepam (4 mg/kg), and fentanyl alone (0.14 mg/kg). In sequence, the sciatic nerve was exposed trough a small opening in the gluteus, and the ipsilateral TA muscle was detached and connected to a force transducer Complete1300A mouse Test System (Aurora Scientific, Aurora, Canada). The TA muscle contraction was assessed after sciatic nerve tetanic stimulations at 1, 10, 20, 30, 50, 75, 100 and 150 Hz. The specific force values were obtained by normalizing the absolute force indexes by the cross-sectional area of the muscle analyzed (CSA, TA muscle weight [mg]/TA muscle length [mm] x skeletal muscle density [1.06 mg/mm3]).70,71 The fatigue simulation was realized after 3 min of rest by repeated stimulations of TA muscles at 40 Hz (from 1 to 80 pulses).
Tissue processing and histology
The hindlimb muscles were dissected, weighed, snap frozen in liquid nitrogen, cooled in isopentane, and stored at −80°C until processing. Transverse sections of TA muscles (8 μm width) were cut for H&E and SDH stainings and scanned in a NanoZoomer 2HT (Hamamatsu, Iwata, Japan) and processed using ImageJ software (NIH, Bethesda, MD, USA).
Electron microscopy
The TA muscles were fixed in 2.5% glutaraldehyde and 2.5% paraformaldehyde diluted in 0.1 M cacodylate buffer (pH 7.4), washed in cacodylate buffer, postfixed in 1% osmium tetroxide, and dehydrated in ascendant graded alcohol and propylene oxide. The samples were oriented and embedded in Epon 812, sectioned at 70 nm in Ultracut UCT (Leica Biosystems, Wetzlar, Germany) and contrasted with uranyl acetate and lead citrate. In sequence, they were examined at 70 kv with a Morgagni 268D electron microscope (FEI Electron Optics, Eindhoven, Netherlands), and the images were captured using the Mega View III camera (Olympus Soft Imaging Solutions, Tokyo, Japan).
Protein extraction and western blotting
TA muscles from the mice and muscle biopsies from patients (healthy controls: 1 and 2 paraspinal, 3 latissimus dorsi; CNM DNM2 R369W: 1 deltoid, 2 and 3 vastus lateralis) were lysed in RIPA buffer supplemented with protease inhibitor cocktail EDTA-free (Roche 11873580001, Basel, Switzerland). A total of 10 μg of proteins was applied to SDS-PAGE gels, and the proteins were transferred to nitrocellulose membranes using Trans-blot semi-dry transfer system (Bio-Rad, Hercules, CA, USA). Protein load control was obtained as quantification of Ponceau S staining in the membranes photographed in an Amersham Imager 600 (GE Healthcare Life Sciences, Boston, MA). The membranes were blocked in 5% milk and incubated with anti-BIN1 (1:20,000, homemade 2405), anti-DNM2 (1:50,000, homemade 2865), anti-GAPDH (1:10,000, Millipore MAB374, Burlington, MA, USA), anti-OXPHOS (1:500, ABCAM ab110413, Cambridge, United Kingdom), anti-PARK2 (1:3,000, Invitrogen, 702785, Waltham, MA, USA), anti-prohibitin (1:1000, ABCAM, ab28172, Cambridge, United Kingdom), anti-PYGM (1:1000, ABCAM ab63158, Cambridge, United Kingdom), anti-total S6K (1:1000, Cell Signaling, 2708, Danvers, MA, USA) or anti-phosphorylated S6K (1:1000, Cell Signaling, 9208, Danvers, MA, USA) and for 1 h with 1:10,000 secondary antibodies conjugated with horseradish. The membranes were exposed to ECL and scanned in an Amersham Imager 600. Blots and total protein (Ponceau S) densities were quantified in ImageJ.
RNA extraction, qRT-PCR, and RT-ddPCR
RNA was extracted from TA muscles with Tri Reagent (Molecular Research Center TR118, Cincinnati, OH, USA) according to the manufacturer’s indications, and the cDNA was transcribed using SuperScriptIV Reverse Transcriptase (Thermo Fisher Scientific 18090050, Waltham, MA, USA) and ribonuclease inhibitors (40 U/μL, Promega N2511, Madison, WI, USA). The qRT-PCRs were performed in a LightCycler 480 (Roche, Basel, Switzerland) in triplicate reactions set up with SYBR Green (Qiagen 204145, Hilden, Germany) and Rpl2772 as the housekeeping gene (primers72,73,74,75 in Table S1).
The RT-ddPCR experiments were performed as previously described.76 In brief, RNA was extracted from the TA muscles of mice at 8 weeks old and transcribed into the cDNA. The RT-ddPCR used FAM or HEX reporter dyes and ZEN/Iowa Black FQ double-quenched nuclease probes (IDT, Coralville, IA, USA) either targeting the 5′ region of WT or mutated form of Dnm2. The reactions were conducted in a QX200 Droplet Digital PCR System (Bio-Rad, Hercules, CA, USA), and the Hprt gene was used for housekeeping (Table S2).
Immunofluorescence
For immunofluorescence, TA muscles from mice were used. Longitudinal sections of TA muscles were fixed in PFA 4% overnight, cryopreserved in sucrose 30% and sectioned in cryostat. Transverse sections were fixed in PFA 4% prior to the immunofluorescence reactions. The samples were permeabilized with Triton 0.2% and blocked in 5% BSA. The slides were incubated with 1:100 of anti-α actinin (Sigma-Aldrich A7811, St. Louis, MO, USA), anti-BIN1 IgG2 (Sigma-Aldrich B9428, St. Louis, MO, USA), anti-desmin (ABCAM AB15200, Cambridge, United Kingdom), anti-DHPR IgG1 (ABCAM ab2862, Cambridge, United Kingdom), anti-DNM2 (homemade 2680) or anti-PAX7 (Developmental Studies Hybridoma Bank PAX7, Iowa, IA, USA), and 1:250 of the suitable fluorescent secondary antibody plus 1:1,000 DAPI for 1 h 30 min at room temperature.
The fiber type immunofluorescence was performed in non-fixed and non-permeabilized transverse muscle sections. The samples were blocked in 3% BSA and incubated with a mix of the primary antibodies 1:50 anti-myosin type I, MYH7 (Developmental Studies Hybridoma Bank BA-D5, IgG2b, Iowa, IA, USA), 1:50 anti-myosin type IIa, MYH2 (Developmental Studies Hybridoma Bank SC-71, IgG1, Iowa, IA, USA), and 1:50 anti-myosin type IIb, MYH4 (Developmental Studies Hybridoma Bank BF-F3, IgM, Iowa, IA, USA). Next, the samples were incubated with a mix of the secondary antibodies 1:100 anti-IgG2b Cy3 dye (Jackson ImmunoResearch 115-165-207 West Grove, PA, USA), 1:100 anti-IgG1 Alexa Fluor 488 dye (Jackson ImmunoResearch 115-545-205 West Grove, PA, USA), 1:100 anti-IgM DyLight 405 dye (Jackson ImmunoResearch 115-475-075 West Grove, PA, USA), plus 1:200 wheat germ agglutinin Alexa Fluor 647 dye (Invitrogen W32466, Waltham, MA, USA).
Statistics
Statistical analyses were performed in the GraphPad Prism 8 software (GraphPad, San Diego, CA, USA). After the determination of samples distribution profile by Shapiro-Wilk test, the appropriate statistic test was performed as indicated in the figure legends. Significant variations were accepted when p < 0.05.
Study approval
All experiments were conducted in accordance with French and European legislations and approved by the institutional ethics committee (project number 25185–2020042209385997 and CE-2022-3).
Acknowledgments
The authors would like to thank the scientific platforms at the Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC) and Institut Clinique de la Souris (ICS), mainly Nadia Messaddeq for the electron microscopy preparation and images, Pascale Koebel for producing the plasmids used in this study, Loic Lindner and Pauline Cayrou for the RT-ddPCR reactions, the PHENOMIN-ICS for the establishment of the Dnm2 mouse mutant line, and Dr Karlin Boujman, Dr Nicol C. Voermans (Donders Institute for Brain, Cognition and Behavior, Radboud University Medical Center, Netherlands), Dr Stephane Vasseur (Myobank-AFM, Institut de Myologie, France), and Dr Yvan De Feraudy (Médecine translationnelle et neurogénétique, IGBMC, France) for the human samples. The graphical abstract was created with BioRender.com. This work is part of the Interdisciplinary Thematic Institute IMCBio, as part of the ITI 2021–2028 program of the University of Strasbourg, CNRS and Inserm, and was supported by IdEx Unistra (ANR-10-IDEX-0002) and by SFRI-STRAT’US project (ANR 20-SFRI-0012) and EUR IMCBio (ANR-17-EURE-0023) under the framework of the French Investments for the Future Program. This work was also supported by a donation from Roland Sackers.
Author contributions
J.C.N. and J.L. conceived the project and analyzed the data. J.C.N. and F.M.F. performed experiments. J.C.N. and J.L. wrote the manuscript. J.B. and J.L. provided funding. J.L. supervised the work.
Declaration of interests
J.L. is co-founder of Dynacure.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtn.2023.07.003.
Supplemental information
Data and code availability
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplemental material.
References
- 1.Gold E.S., Underhill D.M., Morrissette N.S., Guo J., McNiven M.A., Aderem A. Dynamin 2 is required for phagocytosis in macrophages. J. Exp. Med. 1999;190:1849–1856. doi: 10.1084/jem.190.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kruchten A.E., McNiven M.A. Dynamin as a mover and pincher during cell migration and invasion. J. Cell Sci. 2006;119:1683–1690. doi: 10.1242/jcs.02963. [DOI] [PubMed] [Google Scholar]
- 3.Praefcke G.J.K., McMahon H.T. The dynamin superfamily: universal membrane tubulation and fission molecules? Nat. Rev. Mol. Cell Biol. 2004;5:133–147. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson S.M., De Camilli P. Dynamin, a membrane-remodelling GTPase. Nat. Rev. Mol. Cell Biol. 2012;13:75–88. doi: 10.1038/nrm3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chappie J.S., Acharya S., Liu Y.W., Leonard M., Pucadyil T.J., Schmid S.L. An intramolecular signaling element that modulates dynamin function in vitro and in vivo. Mol. Biol. Cell. 2009;20:3561–3571. doi: 10.1091/mbc.E09-04-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faelber K., Posor Y., Gao S., Held M., Roske Y., Schulze D., Haucke V., Noé F., Daumke O. Crystal structure of nucleotide-free dynamin. Nature. 2011;477:556–560. doi: 10.1038/nature10369. [DOI] [PubMed] [Google Scholar]
- 7.Jimah J.R., Hinshaw J.E. Structural Insights into the Mechanism of Dynamin Superfamily Proteins. Trends Cell Biol. 2019;29:257–273. doi: 10.1016/j.tcb.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein D.E., Lee A., Frank D.W., Marks M.S., Lemmon M.A. The pleckstrin homology domains of dynamin isoforms require oligomerization for high affinity phosphoinositide binding. J. Biol. Chem. 1998;273:27725–27733. doi: 10.1074/jbc.273.42.27725. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan S., Dharmarajan V., Reed D.K., Griffin P.R., Schmid S.L. Identification and function of conformational dynamics in the multidomain GTPase dynamin. EMBO J. 2016;35:443–457. doi: 10.15252/embj.201593477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNiven M.A., Cao H., Pitts K.R., Yoon Y. The dynamin family of mechanoenzymes: pinching in new places. Trends Biochem. Sci. 2000;25:115–120. doi: 10.1016/s0968-0004(99)01538-8. [DOI] [PubMed] [Google Scholar]
- 11.Cowling B.S., Prokic I., Tasfaout H., Rabai A., Humbert F., Rinaldi B., Nicot A.S., Kretz C., Friant S., Roux A., Laporte J. Amphiphysin (BIN1) negatively regulates dynamin 2 for normal muscle maturation. J. Clin. Invest. 2017;127:4477–4487. doi: 10.1172/JCI90542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bitoun M., Maugenre S., Jeannet P.Y., Lacène E., Ferrer X., Laforêt P., Martin J.J., Laporte J., Lochmüller H., Beggs A.H., et al. Mutations in dynamin 2 cause dominant centronuclear myopathy. Nat. Genet. 2005;37:1207–1209. doi: 10.1038/ng1657. [DOI] [PubMed] [Google Scholar]
- 13.Kenniston J.A., Lemmon M.A. Dynamin GTPase regulation is altered by PH domain mutations found in centronuclear myopathy patients. EMBO J. 2010;29:3054–3067. doi: 10.1038/emboj.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chin Y.H., Lee A., Kan H.W., Laiman J., Chuang M.C., Hsieh S.T., Liu Y.W. Dynamin-2 mutations associated with centronuclear myopathy are hypermorphic and lead to T-tubule fragmentation. Hum. Mol. Genet. 2015;24:5542–5554. doi: 10.1093/hmg/ddv285. [DOI] [PubMed] [Google Scholar]
- 15.Wang L., Barylko B., Byers C., Ross J.A., Jameson D.M., Albanesi J.P. Dynamin 2 mutants linked to centronuclear myopathies form abnormally stable polymers. J. Biol. Chem. 2010;285:22753–22757. doi: 10.1074/jbc.C110.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowling B.S., Toussaint A., Amoasii L., Koebel P., Ferry A., Davignon L., Nishino I., Mandel J.L., Laporte J. Increased expression of wild-type or a centronuclear myopathy mutant of dynamin 2 in skeletal muscle of adult mice leads to structural defects and muscle weakness. Am. J. Pathol. 2011;178:2224–2235. doi: 10.1016/j.ajpath.2011.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu N., Bezprozvannaya S., Shelton J.M., Frisard M.I., Hulver M.W., McMillan R.P., Wu Y., Voelker K.A., Grange R.W., Richardson J.A., et al. Mice lacking microRNA 133a develop dynamin 2-dependent centronuclear myopathy. J. Clin. Invest. 2011;121:3258–3268. doi: 10.1172/JCI46267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bitoun M., Bevilacqua J.A., Eymard B., Prudhon B., Fardeau M., Guicheney P., Romero N.B. A new centronuclear myopathy phenotype due to a novel dynamin 2 mutation. Neurology. 2009;72:93–95. doi: 10.1212/01.wnl.0000338624.25852.12. [DOI] [PubMed] [Google Scholar]
- 19.Bitoun M., Bevilacqua J.A., Prudhon B., Maugenre S., Taratuto A.L., Monges S., Lubieniecki F., Cances C., Uro-Coste E., Mayer M., et al. Dynamin 2 mutations cause sporadic centronuclear myopathy with neonatal onset. Ann. Neurol. 2007;62:666–670. doi: 10.1002/ana.21235. [DOI] [PubMed] [Google Scholar]
- 20.Böhm J., Biancalana V., Dechene E.T., Bitoun M., Pierson C.R., Schaefer E., Karasoy H., Dempsey M.A., Klein F., Dondaine N., et al. Mutation spectrum in the large GTPase dynamin 2, and genotype-phenotype correlation in autosomal dominant centronuclear myopathy. Hum. Mutat. 2012;33:949–959. doi: 10.1002/humu.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biancalana V., Romero N.B., Thuestad I.J., Ignatius J., Kataja J., Gardberg M., Héron D., Malfatti E., Oldfors A., Laporte J. Some DNM2 mutations cause extremely severe congenital myopathy and phenocopy myotubular myopathy. Acta Neuropathol. Commun. 2018;6:93. doi: 10.1186/s40478-018-0593-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abath Neto O., Martins C.d.A., Carvalho M., Chadi G., Seitz K.W., Oliveira A.S.B., Reed U.C., Laporte J., Zanoteli E. DNM2 mutations in a cohort of sporadic patients with centronuclear myopathy. Genet. Mol. Biol. 2015;38:147–151. doi: 10.1590/S1415-4757382220140238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fattori F., Maggi L., Bruno C., Cassandrini D., Codemo V., Catteruccia M., Tasca G., Berardinelli A., Magri F., Pane M., et al. Centronuclear myopathies: genotype-phenotype correlation and frequency of defined genetic forms in an Italian cohort. J. Neurol. 2015;262:1728–1740. doi: 10.1007/s00415-015-7757-9. [DOI] [PubMed] [Google Scholar]
- 24.Mori-Yoshimura M., Okuma A., Oya Y., Fujimura-Kiyono C., Nakajima H., Matsuura K., Takemura A., Malicdan M.C.V., Hayashi Y.K., Nonaka I., et al. Clinicopathological features of centronuclear myopathy in Japanese populations harboring mutations in dynamin 2. Clin. Neurol. Neurosurg. 2012;114:678–683. doi: 10.1016/j.clineuro.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 25.Romero N.B. Centronuclear myopathies: a widening concept. Neuromuscul. Disord. 2010;20:223–228. doi: 10.1016/j.nmd.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Puri C., Manni M.M., Vicinanza M., Hilcenko C., Zhu Y., Runwal G., Stamatakou E., Menzies F.M., Mamchaoui K., Bitoun M., Rubinsztein D.C. A DNM2 Centronuclear Myopathy Mutation Reveals a Link between Recycling Endosome Scission and Autophagy. Dev. Cell. 2020;53:154–168.e6. doi: 10.1016/j.devcel.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 27.Kutchukian C., Szentesi P., Allard B., Trochet D., Beuvin M., Berthier C., Tourneur Y., Guicheney P., Csernoch L., Bitoun M., Jacquemond V. Impaired excitation-contraction coupling in muscle fibres from the dynamin2(R465W) mouse model of centronuclear myopathy. J. Physiol. 2017;595:7369–7382. doi: 10.1113/JP274990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durieux A.C., Vignaud A., Prudhon B., Viou M.T., Beuvin M., Vassilopoulos S., Fraysse B., Ferry A., Lainé J., Romero N.B., et al. A centronuclear myopathy-dynamin 2 mutation impairs skeletal muscle structure and function in mice. Hum. Mol. Genet. 2010;19:4820–4836. doi: 10.1093/hmg/ddq413. [DOI] [PubMed] [Google Scholar]
- 29.Zhao M., Smith L., Volpatti J., Fabian L., Dowling J.J. Insights into wild type dynamin 2 and the consequences of DNM2 mutations from transgenic zebrafish. Hum. Mol. Genet. 2019;28:4186–4196. doi: 10.1093/hmg/ddz260. [DOI] [PubMed] [Google Scholar]
- 30.Massana Muñoz X., Kretz C., Silva-Rojas R., Ochala J., Menuet A., Romero N.B., Cowling B.S., Laporte J. Physiological impact and disease reversion for the severe form of centronuclear myopathy linked to dynamin. JCI Insight. 2020;5 doi: 10.1172/jci.insight.137899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dowling J.J., Joubert R., Low S.E., Durban A.N., Messaddeq N., Li X., Dulin-Smith A.N., Snyder A.D., Marshall M.L., Marshall J.T., et al. Myotubular myopathy and the neuromuscular junction: a novel therapeutic approach from mouse models. Dis. Model. Mech. 2012;5:852–859. doi: 10.1242/dmm.009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robb S.A., Sewry C.A., Dowling J.J., Feng L., Cullup T., Lillis S., Abbs S., Lees M.M., Laporte J., Manzur A.Y., et al. Impaired neuromuscular transmission and response to acetylcholinesterase inhibitors in centronuclear myopathies. Neuromuscul. Disord. 2011;21:379–386. doi: 10.1016/j.nmd.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Buono S., Ross J.A., Tasfaout H., Levy Y., Kretz C., Tayefeh L., Matson J., Guo S., Kessler P., et al. Reducing dynamin 2 (DNM2) rescues DNM2-related dominant centronuclear myopathy. Proc. Natl. Acad. Sci. USA. 2018;115:11066–11071. doi: 10.1073/pnas.1808170115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trochet D., Prudhon B., Beuvin M., Peccate C., Lorain S., Julien L., Benkhelifa-Ziyyat S., Rabai A., Mamchaoui K., Ferry A., et al. Allele-specific silencing therapy for Dynamin 2-related dominant centronuclear myopathy. EMBO Mol. Med. 2018;10:239–253. doi: 10.15252/emmm.201707988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dudhal S., Mekzine L., Prudhon B., Soocheta K., Cadot B., Mamchaoui K., Trochet D., Bitoun M. Development of versatile allele-specific siRNAs able to silence all the dominant dynamin 2 mutations. Mol. Ther. Nucleic Acids. 2022;29:733–748. doi: 10.1016/j.omtn.2022.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nijtmans L.G., de Jong L., Artal Sanz M., Coates P.J., Berden J.A., Back J.W., Muijsers A.O., van der Spek H., Grivell L.A. Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins. EMBO J. 2000;19:2444–2451. doi: 10.1093/emboj/19.11.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harper J.W., Ordureau A., Heo J.M. Building and decoding ubiquitin chains for mitophagy. Nat. Rev. Mol. Cell Biol. 2018;19:93–108. doi: 10.1038/nrm.2017.129. [DOI] [PubMed] [Google Scholar]
- 38.Pickles S., Vigié P., Youle R.J. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. 2018;28:R170–R185. doi: 10.1016/j.cub.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuda N., Sato S., Shiba K., Okatsu K., Saisho K., Gautier C.A., Sou Y.S., Saiki S., Kawajiri S., Sato F., et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J. Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wan W., Hua F., Fang P., Li C., Deng F., Chen S., Ying J., Wang X. Regulation of Mitophagy by Sirtuin Family Proteins: A Vital Role in Aging and Age-Related Diseases. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.845330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tran Q., Jung J.H., Park J., Lee H., Hong Y., Cho H., Kim M., Park S., Kwon S.H., Kim S.H., et al. S6 kinase 1 plays a key role in mitochondrial morphology and cellular energy flow. Cell. Signal. 2018;48:13–24. doi: 10.1016/j.cellsig.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Saxton R.A., Sabatini D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;169:361–371. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 43.Shishmarev D. Excitation-contraction coupling in skeletal muscle: recent progress and unanswered questions. Biophys. Rev. 2020;12:143–153. doi: 10.1007/s12551-020-00610-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curtis B.M., Catterall W.A. Purification of the calcium antagonist receptor of the voltage-sensitive calcium channel from skeletal muscle transverse tubules. Biochemistry. 1984;23:2113–2118. doi: 10.1021/bi00305a001. [DOI] [PubMed] [Google Scholar]
- 45.Fosset M., Jaimovich E., Delpont E., Lazdunski M. [3H]nitrendipine receptors in skeletal muscle. J. Biol. Chem. 1983;258:6086–6092. [PubMed] [Google Scholar]
- 46.Lee E., Marcucci M., Daniell L., Pypaert M., Weisz O.A., Ochoa G.C., Farsad K., Wenk M.R., De Camilli P. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science. 2002;297:1193–1196. doi: 10.1126/science.1071362. [DOI] [PubMed] [Google Scholar]
- 47.Razzaq A., Robinson I.M., McMahon H.T., Skepper J.N., Su Y., Zelhof A.C., Jackson A.P., Gay N.J., O'Kane C.J. Amphiphysin is necessary for organization of the excitation-contraction coupling machinery of muscles, but not for synaptic vesicle endocytosis in Drosophila. Genes Dev. 2001;15:2967–2979. doi: 10.1101/gad.207801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agnetti G., Herrmann H., Cohen S. New roles for desmin in the maintenance of muscle homeostasis. FEBS J. 2022;289:2755–2770. doi: 10.1111/febs.15864. [DOI] [PubMed] [Google Scholar]
- 49.Roman W., Martins J.P., Carvalho F.A., Voituriez R., Abella J.V.G., Santos N.C., Cadot B., Way M., Gomes E.R. Myofibril contraction and crosslinking drive nuclear movement to the periphery of skeletal muscle. Nat. Cell Biol. 2017;19:1189–1201. doi: 10.1038/ncb3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fongy A., Falcone S., Lainé J., Prudhon B., Martins-Bach A., Bitoun M. Nuclear defects in skeletal muscle from a Dynamin 2-linked centronuclear myopathy mouse model. Sci. Rep. 2019;9:1580. doi: 10.1038/s41598-018-38184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Augusto V., Padovani C.R., Campos G.E.R. Skeletal muscle fiber types in C57BL6J mice. Journal of Morphological Sciences. 2004;21:89–94. [Google Scholar]
- 52.Kammoun M., Cassar-Malek I., Meunier B., Picard B. A simplified immunohistochemical classification of skeletal muscle fibres in mouse. Eur. J. Histochem. 2014;58:2254. doi: 10.4081/ejh.2014.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Llavero F., Arrazola Sastre A., Luque Montoro M., Gálvez P., Lacerda H.M., Parada L.A., Zugaza J.L. McArdle Disease: New Insights into Its Underlying Molecular Mechanisms. Int. J. Mol. Sci. 2019;20:5919. doi: 10.3390/ijms20235919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Song S., Finkel T. GAPDH and the search for alternative energy. Nat. Cell Biol. 2007;9:869–870. doi: 10.1038/ncb0807-869. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez-Vizarra E., Zeviani M. Mitochondrial disorders of the OXPHOS system. FEBS Lett. 2021;595:1062–1106. doi: 10.1002/1873-3468.13995. [DOI] [PubMed] [Google Scholar]
- 56.Tasfaout H., Lionello V.M., Kretz C., Koebel P., Messaddeq N., Bitz D., Laporte J., Cowling B.S. Single Intramuscular Injection of AAV-shRNA Reduces DNM2 and Prevents Myotubular Myopathy in Mice. Mol. Ther. 2018;26:1082–1092. doi: 10.1016/j.ymthe.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cowling B.S., Chevremont T., Prokic I., Kretz C., Ferry A., Coirault C., Koutsopoulos O., Laugel V., Romero N.B., Laporte J. Reducing dynamin 2 expression rescues X-linked centronuclear myopathy. J. Clin. Invest. 2014;124:1350–1363. doi: 10.1172/JCI71206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fischer D., Herasse M., Bitoun M., Barragán-Campos H.M., Chiras J., Laforêt P., Fardeau M., Eymard B., Guicheney P., Romero N.B. Characterization of the muscle involvement in dynamin 2-related centronuclear myopathy. Brain. 2006;129:1463–1469. doi: 10.1093/brain/awl071. [DOI] [PubMed] [Google Scholar]
- 59.Schiaffino S., Reggiani C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011;91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 60.Frontera W.R., Ochala J. Skeletal muscle: a brief review of structure and function. Calcif. Tissue Int. 2015;96:183–195. doi: 10.1007/s00223-014-9915-y. [DOI] [PubMed] [Google Scholar]
- 61.Lionello V.M., Kretz C., Edelweiss E., Crucifix C., Gómez-Oca R., Messaddeq N., Buono S., Koebel P., Massana Muñoz X., Diedhiou N., et al. BIN1 modulation in vivo rescues dynamin-related myopathy. Proc. Natl. Acad. Sci. USA. 2022;119 doi: 10.1073/pnas.2109576119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Durieux A.C., Vassilopoulos S., Lainé J., Fraysse B., Briñas L., Prudhon B., Castells J., Freyssenet D., Bonne G., Guicheney P., Bitoun M. A centronuclear myopathy--dynamin 2 mutation impairs autophagy in mice. Traffic. 2012;13:869–879. doi: 10.1111/j.1600-0854.2012.01348.x. [DOI] [PubMed] [Google Scholar]
- 63.Al-Qusairi L., Prokic I., Amoasii L., Kretz C., Messaddeq N., Mandel J.L., Laporte J. Lack of myotubularin (MTM1) leads to muscle hypotrophy through unbalanced regulation of the autophagy and ubiquitin-proteasome pathways. Faseb. J. 2013;27:3384–3394. doi: 10.1096/fj.12-220947. [DOI] [PubMed] [Google Scholar]
- 64.Hnia K., Tronchère H., Tomczak K.K., Amoasii L., Schultz P., Beggs A.H., Payrastre B., Mandel J.L., Laporte J. Myotubularin controls desmin intermediate filament architecture and mitochondrial dynamics in human and mouse skeletal muscle. J. Clin. Invest. 2011;121:70–85. doi: 10.1172/JCI44021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toussaint A., Cowling B.S., Hnia K., Mohr M., Oldfors A., Schwab Y., Yis U., Maisonobe T., Stojkovic T., Wallgren-Pettersson C., et al. Defects in amphiphysin 2 (BIN1) and triads in several forms of centronuclear myopathies. Acta Neuropathol. 2011;121:253–266. doi: 10.1007/s00401-010-0754-2. [DOI] [PubMed] [Google Scholar]
- 66.Mendell J.R., Al-Zaidy S.A., Rodino-Klapac L.R., Goodspeed K., Gray S.J., Kay C.N., Boye S.L., Boye S.E., George L.A., Salabarria S., et al. Current Clinical Applications of In Vivo Gene Therapy with AAVs. Mol. Ther. 2021;29:464–488. doi: 10.1016/j.ymthe.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kishimoto T.K., Samulski R.J. Addressing high dose AAV toxicity - 'one and done' or 'slower and lower. Expet Opin. Biol. Ther. 2022;22:1067–1071. doi: 10.1080/14712598.2022.2060737. [DOI] [PubMed] [Google Scholar]
- 68.Tabebordbar M., Lagerborg K.A., Stanton A., King E.M., Ye S., Tellez L., Krunnfusz A., Tavakoli S., Widrick J.J., Messemer K.A., et al. Directed evolution of a family of AAV capsid variants enabling potent muscle-directed gene delivery across species. Cell. 2021;184:4919–4938.e22. doi: 10.1016/j.cell.2021.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.National Center for Biotechnology Information; 1988. Nucleotide. https://www.ncbi.nlm.nih.gov/nucleotide/ [Google Scholar]
- 70.Brooks S.V., Faulkner J.A. Contractile properties of skeletal muscles from young, adult and aged mice. J. Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Distefano G., Ferrari R.J., Weiss C., Deasy B.M., Boninger M.L., Fitzgerald G.K., Huard J., Ambrosio F. Neuromuscular electrical stimulation as a method to maximize the beneficial effects of muscle stem cells transplanted into dystrophic skeletal muscle. PLoS One. 2013;8 doi: 10.1371/journal.pone.0054922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thomas K.C., Zheng X.F., Garces Suarez F., Raftery J.M., Quinlan K.G.R., Yang N., North K.N., Houweling P.J. Evidence based selection of commonly used RT-qPCR reference genes for the analysis of mouse skeletal muscle. PLoS One. 2014;9 doi: 10.1371/journal.pone.0088653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Honda M., Hidaka K., Fukada S.I., Sugawa R., Shirai M., Ikawa M., Morisaki T. Vestigial-like 2 contributes to normal muscle fiber type distribution in mice. Sci. Rep. 2017;7:7168. doi: 10.1038/s41598-017-07149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gómez-Oca R., Edelweiss E., Djeddi S., Gerbier M., Massana-Muñoz X., Oulad-Abdelghani M., Crucifix C., Spiegelhalter C., Messaddeq N., Poussin-Courmontagne P., et al. Differential impact of ubiquitous and muscle dynamin 2 isoforms in muscle physiology and centronuclear myopathy. Nat. Commun. 2022;13:6849. doi: 10.1038/s41467-022-34490-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luo H., Zhou M., Ji K., Zhuang J., Dang W., Fu S., Sun T., Zhang X. Expression of Sirtuins in the Retinal Neurons of Mice, Rats, and Humans. Front. Aging Neurosci. 2017;9:366. doi: 10.3389/fnagi.2017.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lindner L., Cayrou P., Jacquot S., Birling M.C., Herault Y., Pavlovic G. Reliable and robust droplet digital PCR (ddPCR) and RT-ddPCR protocols for mouse studies. Methods. 2021;191:95–106. doi: 10.1016/j.ymeth.2020.07.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative video of the characteristic rotatory movements of the tail and the forelimbs of Dnm2R369W/+ mice during the hanging test at 6 weeks of age.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its supplemental material.