Abstract.
Membrane trafficking of post-synaptic cargo is a key determinant of synaptic transmission and synaptic plasticity. We describe here the latest developments in visualizing individual exocytosis and endocytosis events in neurons using pH-sensitive tags. We show how these tools help decipher the spatial and temporal regulation of membrane trafficking steps during synaptic plasticity.
Keywords: endocytosis, exocytosis, live cell fluorescence imaging, membrane trafficking, synaptic plasticity
1. Introduction
Membrane trafficking in dendrites, in particular exocytosis, endocytosis, and recycling of post-synaptic receptors, is a key determinant of synaptic transmission and synaptic plasticity.1 Indeed, blocking exocytosis mediated by VAMP1-3 with post-synaptic dialysis of tetanus toxin, through a patch-clamp recording pipette, bocks long-term potentiation (LTP)2–4 while blocking dynamin mediated endocytosis with a peptide interfering with its function blocks long-term depression (LTD).5 In addition, exocytosis of recycling endosomes (REs) increases following LTP induction6 and endocytosis of post-synaptic receptors increases following LTD induction.7 Based on these data, a model has been built describing endocytosis, sorting and recycling of post-synaptic receptors within dendrites [Fig. 1(a)]. However, its spatial organization and dynamics, as well as many molecular players involved are still unknown. Therefore, live cell imaging of individual exocytic or endocytic events is crucial to determine if and how membrane trafficking is modulated following the induction of synaptic plasticity, and whether these processes contribute to its spatial selectivity.11 Here we will review the methods developed to image individual exocytosis and endocytosis events, how it helped decipher the cellular and molecular mechanisms, and the challenges ahead to deal with their limitations.
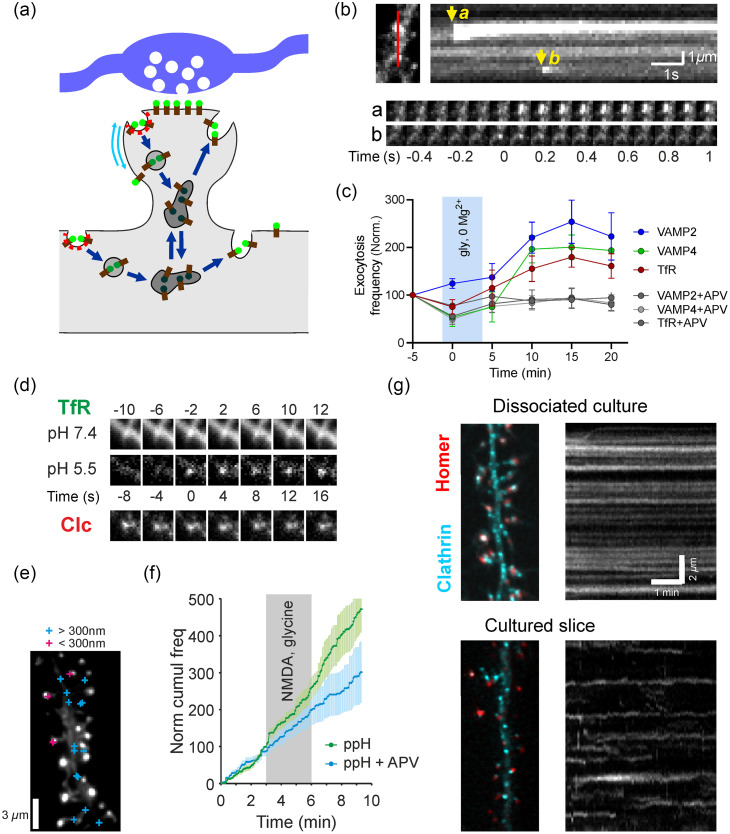
Fig. 1.
Visualization of post-synaptic membrane trafficking in neurons. (a) Scheme of the steps of membrane trafficking visualized with SEP-labeled cargo (brown sticks), visible at neutral pH (green lollipops) but not at the acidic pH of REs (dark gray). A presynaptic terminal is depicted in blue, and the PSD facing this axon in the post-synaptic spine has concentrated receptors. The internalization of receptors occurs near the PSD (red stippled line, figuring clathrin) or further away in the dendritic shaft. Blue arrows depict the progression of trafficking along the endosomal pathway to end in exocytosis near the PSD or further away. (b) Kymograph showing the detection of two exocytosis events in a portion of dendrite of a neuron transfected with TfR-SEP. For the event , the vesicle is visible for several seconds, due to kiss-and-run exocytosis, while for event , it is transient due to receptor diffusion. Reprinted from Ref. 8. (c) The frequency of exocytosis events, visualized by VAMP2-SEP, VAMP4-SEP, or TfR-SEP, increases after induction of LTP by the perfusion of a solution containing glycine () and no , which activates synaptic NMDA receptors. Reprinted from Ref. 9. (d) Images of a portion of dendrite taken at alternating pH of 7.4 and 5.5 enable the detection of an endocytic vesicle containing TfR-SEP at time 0. It appears at a CCS labeled with clathrin. (e) Location of endocytic events (blue and magenta crosses) relative to PSDs labeled with Homer1c-tdTomato (image of the fluorescent label). (f) Cumulative frequency of endocytic events labeled with SEP-GluA2. It increases during induction of LTD with NMDA. (d)–(f) Reprinted from Ref. 10. (g) Images of a cultured neuron (top) and a CA1 pyramidal neuron in a cultured hippocampal slice (bottom) transfected with Homer1c-tdTomato and clathrin-GFP. In both cases, CCS are visible throughout the dendritic shaft and in most spines. Right, kymographs of clathrin-GFP show that they are very stable in cultured neurons but transient in the slice.
2. Imaging of Individual Exocytosis Events
The method of choice to image exocytosis events has been to rely on the fact that intraluminal pH of secretory vesicles, recycling endosomes (REs), or synaptic vesicles is acidic (pH to 6). Therefore, the fast change in pH (from 5.5 to extracellular pH 7.4) occurring at the time of exocytosis can be detected by a pH-sensitive fluorophore conveniently positioned at the intraluminal/extracellular side of the transmembrane cargo of interest, such as post-synaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs), or the transferrin receptor (TfR), a ubiquitous RE marker. The pH-sensitive protein with the close to ideal properties to sense this transition is the GFP mutant super-ecliptic pHluorin (SEP), isolated more than 20 years ago.12 The SEP is virtually non-fluorescent at pH 5.5, making the SEP-labeled cargo in REs or secretory vesicles invisible, such that single exocytosis events can be visualized as bright punctae throughout somatodendritic compartments of the neuron, including dendritic spines. Modeling of exocytosis and diffusion in the plasma membrane with experimentally derived parameters shows that AMPAR exocytosis must occur in the vicinity of synapses for rapid control of AMPAR number at synapses.13 After exocytosis, fluorescence decays with various kinetics, revealing different modes of exocytosis [Fig. 1(b)].8,14,15 Photobleaching of parts, or even the whole cell, nearly erases the fluorescence of fluorescent of cargo residing in the plasma membrane while preserving the non-fluorescent cargo in acidic compartments, allowing a better isolation of exocytic events and quantification of whole-cell exocytosis rates.9,16 In addition, the red pH-sensitive fluorescent proteins, pHuji and pHmScarlet17,18 or SNAPtag ligands labeled with the pH-sensitive red fluorophore Virginia Orange,19 are available to permit multicolor imaging of various cargo proteins. Recent developments in research on post-synaptic exocytosis and synaptic plasticity include the effect of local induction of LTP at single spines on exocytosis,20–22 the role of L-type calcium channels in controlling exocytosis after LTP induction,22 and the identification of several classes of REs containing either VAMP2 or VAMP4 having distinct roles in LTP [Fig. 1(c)].9
3. Imaging of Individual Endocytosis Events: Clathrin Dynamics
Unlike exocytosis, the formation of endocytic vesicles is not accompanied by sudden changes in pH, rendering imaging of endocytosis at high speed with single-vesicle resolution difficult.23 In the case of clathrin-mediated endocytosis (CME), one solution is to label clathrin or associated proteins and image clathrin-coated structures (CCSs). The CCSs are located all over dendrites, but a significant proportion is located in the vicinity of post-synaptic densities (PSD), away, such that 75% to 85% of PSDs have a nearby CCS.10,24–26 The proportion of PSDs bearing a CCS is decreased to when the three isoforms of Shank1-3, PSD proteins that interact with endocytic and actin binding proteins, are downregulated by a miRNA27 or when the immediate early gene Homer1a is overexpressed, which displaces Shank proteins from PSDs.25 Peri-PSD CCSs play a specific role in post-synaptic receptor endocytosis,25 even if some AMPARs may internalize through clathrin independent endocytosis.28–30 How does imaging of CCSs reveal the dynamics of CME? The CCSs are transient structures appearing and disappearing in living cells: this would reflect the clustering of cargo, invagination to form a vesicle, scission, clathrin uncoating, and movement away from the plasma membrane. Therefore, CCS lifetime, around 1-2 min in most cell types, can be used as a proxy for endocytic activity with single CCS resolution.31 However, super-resolution microscopy and correlative light electron microscopy have revealed the existence of complex CCSs that produce more than one endocytic vesicle.32,33 Therefore, vesicles can form without CCS disappearance or even measurable changes observed with classical wide field microscopy, so-called non-terminal endocytic events (Refs. 34 and 35; see also following paragraph). In dendrites of mature neurons in culture, CCSs are almost all stable in a period of at least 10 min, despite the fact that they internalize CME cargo at a much higher rate.10,24–26 Therefore, even if some changes in the size or number of CCS have been observed after induction of LTP or LTD,26 observing CCSs in living neurons cannot reveal the precise moment of vesicle formation, hence the rate of endocytosis.
4. Imaging of Individual Endocytosis Events: Direct Detection of Vesicle Formation
By definition, endocytosis depicts the process of vesicle formation, i.e., the isolation of a membrane cargo from the extracellular space. Testing this connection could thus provide a direct assay for vesicle formation, overcoming the limitation of observing CCS dynamics. This connection can be tested with a cargo protein labeled with pH-sensitive tag such as SEP and with repeated pulsed pH changes (ppH) from 7.4 to 5.5. Cargo on the plasma membrane becomes invisible at extracellular pH 5.5 while cargo in non-acidic vesicles (e.g., endocytic vesicles not yet acidified) will remain visible. The moment of vesicle formation is thus detected when a pH resistant vesicle appears in an image taken at extracellular pH 5.5, with a temporal precision matching the ability to exchange the two solutions rapidly, typically less than 2 s for whole cells but faster for small neurites [Fig. 1(d)].8 A good cargo protein to use is, like for RE exocytosis, TfR-SEP. In addition to its location in RE (which are acidic and thus invisible with the SEP tag), it is also located on the plasma membrane and constitutively internalized through CME. Multiple control experiments show that CME is not affected during the ppH protocol.34,35 In neurons, despite the activation of acid-sensing ion channels by the low pH solution, blocking these channels does not affect the rate of CME, and quenching SEP fluorescence with cell-impermeable Trypan purple instead of acidic pH enables the detection of vesicles, albeit for only a few minutes due to progressive accumulation of the dye on the plasma membrane.10 Multiple endocytic events indeed appear at individual CCSs with a median inter-event interval of 168 s.10 Moreover, endocytic vesicles containing the AMPAR subunit SEP-GluA2 detected with the ppH protocol form preferentially near PSDs while those containing the non-synaptic TfR-SEP do not [Fig. 1(e)].10 Moreover, application of N-methyl-D-aspartic acid (NMDA), which leads to internalization of AMPARs and LTD, provokes a transient increase in the frequency of SEP-GluA1 and SEP-GluA2 endocytic events [Fig. 1(f)].10,29,36,37 The characterization of the dynamics of post-synaptic endocytosis is thus well under way. Nevertheless, several outstanding questions remain. How is endocytosis regulated after LTD at individual spines? What is the fate of endocytic vesicles? Are specific populations targeted to degradation or recycled? Does it occur locally? To address these questions at the single event level, new protocols and tools are required with new types of markers, such as protocols, inducing LTD in individual spines38 or fluorophores with inverse pH sensitivity.39
5. Current Limitations and Perspectives
Endogenous AMPARs are for the most part heteromers of GluA subunits associated with several accessory proteins,40 so overexpression of SEP-GluA subunits likely biases the labeling towards homomeric receptors with specific trafficking routes.30 Genome editing of GluA genes to tag endogenous AMPARs, either in single neurons41,42 or in transgenic mice with bi-allelic gene editing43 should reveal the unbiased trafficking of post-synaptic receptors. Another important step towards understanding membrane trafficking in a physiological context is the ability to image tagged proteins of interest in more intact systems. Although most live imaging studies have been performed in cultured neurons grown on glass coverslips, imaging of exocytosis in cultured slices has been proven possible by two photon microscopy.20 Imaging endocytosis in slices remains more challenging, as the ppH-based methods may lack the required time resolution due to the convoluted extracellular space. Nevertheless, our preliminary data show that CCSs are more dynamic in slices than in cultured neurons on glass [Fig. 1(g)], paving the way for an estimation of local endocytic activity and its modulation during synaptic plasticity based on imaging of CCSs.
Finally, to better assess the membrane trafficking of receptors in a physiological context in situ, we can anticipate the development of brighter pH-sensitive fluorescent proteins (it is remarkable that SEP was not optimized since its discovery in 2000, despite the constant improvement of GFP based fluorescent proteins44) and fast, sensitive imaging techniques, such as lattice light-sheet microscopy with adaptive optics.45
Acknowledgments
Research in the Perrais lab is supported by the Agence Nationale de la Recherche (Grant Nos. ANR-19-CE16-0003, ANR-20-CE92-0053, and ANR-21-CE44-0019). S.S. is supported by a Sir Henry Wellcome Postdoctoral Fellowship (218650/Z/19/Z).
Biographies
David Perrais uses electrophysiology and live cell fluorescence imaging to study exocytosis, endocytosis and synaptic transmission, first as a post-doctoral fellow with Wolf Almers at the Vollum Institute (Portland, Oregon, United States), then with Christophe Mulle at the University of Bordeaux. He obtained a CNRS researcher position in 2005 and became a research director in 2015, moving to the team of Daniel Choquet in 2010 at the Interdisciplinary Institute of Neuroscience (IINS) in Bordeaux. Since 2020, he leads the team “Membrane Trafficking at Synapses” at IINS.
Silvia Sposini studied, as a PhD student at Imperial College, under the supervision of Aylin Hanyaloglu, the trafficking of various classes of G protein coupled receptors, uncovering a new type of endosome for receptor recycling. In 2018, she became a post-doctoral fellow in the team of David Perrais and received in 2020 a Sir Henry Wellcome post-doctoral fellowship to pursue work on GPCR trafficking in neurons.
Julie Angibaud obtained an engineer position at the University of Bordeaux after a PhD on the role of the immune response in neuronal development. Between 2010 and 2019, she worked in the team of Valentin Nagerl at IINS, working on the application of STED microscopy for imaging neurons in brain slices. In 2020, she joined the team of David Perrais to develop the imaging of endocytic zones in brain slices.
Disclosure
No conflicts of interest, financial or otherwise, are declared by the authors.
Contributor Information
David Perrais, Email: david.perrais@u-bordeaux.fr.
Silvia Sposini, Email: silvia.sposini@u-bordeaux.fr.
Julie Angibaud, Email: julie.angibaud@u-bordeaux.fr.
References
- 1.Huganir R. L., Nicoll R. A., “AMPARs and synaptic plasticity: the last 25 years,” Neuron 80(3), 704–717 (2013). 10.1016/j.neuron.2013.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lledo P. M., et al. , “Postsynaptic membrane fusion and long-term potentiation,” Science 279(5349), 399–403 (1998). 10.1126/science.279.5349.399 [DOI] [PubMed] [Google Scholar]
- 3.Lu W., et al. , “Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons,” Neuron 29(1), 243–254 (2001). 10.1016/S0896-6273(01)00194-5 [DOI] [PubMed] [Google Scholar]
- 4.Penn A. C., et al. , “Hippocampal LTP and contextual learning require surface diffusion of AMPA receptors,” Nature 549(7672), 384–388 (2017). 10.1038/nature23658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lüscher C., et al. , “Role of AMPA receptor cycling in synaptic transmission and plasticity,” Neuron 24(3), 649–658 (1999). 10.1016/S0896-6273(00)81119-8 [DOI] [PubMed] [Google Scholar]
- 6.Park M., et al. , “Recycling endosomes supply AMPA receptors for LTP,” Science 305(5692), 1972–1975 (2004). 10.1126/science.1102026 [DOI] [PubMed] [Google Scholar]
- 7.Beattie E. C., et al. , “Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD,” Nat. Neurosci. 3(12), 1291–1300 (2000). 10.1038/81823 [DOI] [PubMed] [Google Scholar]
- 8.Jullié D., Choquet D., Perrais D., “Recycling endosomes undergo rapid closure of a fusion pore on exocytosis in neuronal dendrites,” J. Neurosci. 34(33), 11106–11118 (2014). 10.1523/JNEUROSCI.0799-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakr M., et al. , “The vSNAREs VAMP2 and VAMP4 control recycling and intracellular sorting of post-synaptic receptors in neuronal dendrites,” Cell Rep. 36(10), 109678 (2021). 10.1016/j.celrep.2021.109678 [DOI] [PubMed] [Google Scholar]
- 10.Rosendale M., et al. , “Spatial and temporal regulation of receptor endocytosis in neuronal dendrites revealed by imaging of single vesicle formation,” Cell Rep. 18(8), 1840–1847 (2017). 10.1016/j.celrep.2017.01.081 [DOI] [PubMed] [Google Scholar]
- 11.Hiester B. G., et al. , “Mechanisms and role of dendritic membrane trafficking for long-term potentiation,” Front. Cell. Neurosci. 12, 391 (2018). 10.3389/fncel.2018.00391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sankaranarayanan S., et al. , “The use of pHluorins for optical measurements of presynaptic activity,” Biophys. J. 79(4), 2199–2208 (2000). 10.1016/S0006-3495(00)76468-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czondor K., et al. , “Unified quantitative model of AMPA receptor trafficking at synapses,” Proc. Natl. Acad. Sci. 109(9), 3522–3527 (2012). 10.1073/pnas.1109818109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy M. J., et al. , “Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines,” Cell 141(3), 524–535 (2010). 10.1016/j.cell.2010.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roman-Vendrell C., et al. , “Imaging of kiss-and-run exocytosis of surface receptors in neuronal cultures,” Front. Cell. Neurosci. 8, 363 (2014). 10.3389/fncel.2014.00363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu D., et al. , “Postsynaptic synaptotagmins mediate AMPA receptor exocytosis during LTP,” Nature 544(7650), 316–321 (2017). 10.1038/nature21720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen Y., et al. , “pHuji, a pH-sensitive red fluorescent protein for imaging of exo- and endocytosis,” J. Cell Biol. 207(3), 419–432 (2014). 10.1083/jcb.201404107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu A., et al. , “pHmScarlet is a pH-sensitive red fluorescent protein to monitor exocytosis docking and fusion steps,” Nat. Commun. 12(1), 1413 (2021). 10.1038/s41467-021-21666-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martineau M., et al. , “Semisynthetic fluorescent pH sensors for imaging exocytosis and endocytosis,” Nat. Commun. 8(1), 1412 (2017). 10.1038/s41467-017-01752-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patterson M. A., Szatmari E. M., Yasuda R., “AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation,” Proc. Natl. Acad. Sci. U. S. A. 107(36), 15951–15956 (2010). 10.1073/pnas.0913875107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harward S. C., et al. , “Autocrine BDNF–TrkB signalling within a single dendritic spine,” Nature 538(7623), 99–103 (2016). 10.1038/nature19766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiester B. G., et al. , “L-type voltage-gated Ca2+ channels regulate synaptic activity-triggered recycling endosome fusion in neuronal dendrites,” Cell Rep. 21(8), 2134–2146 (2017). 10.1016/j.celrep.2017.10.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosendale M., Perrais D., “Imaging in focus: imaging the dynamics of endocytosis,” Int. J. Biochem. Cell Biol. 93(Supplement C), 41–45 (2017). 10.1016/j.biocel.2017.10.010 [DOI] [PubMed] [Google Scholar]
- 24.Blanpied T. A., Scott D. B., Ehlers M. D., “Dynamics and regulation of clathrin coats at specialized endocytic zones of dendrites and spines,” Neuron 36(3), 435–449 (2002). 10.1016/S0896-6273(02)00979-0 [DOI] [PubMed] [Google Scholar]
- 25.Lu J., et al. , “Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to homer,” Neuron 55(6), 874–889 (2007). 10.1016/j.neuron.2007.06.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catsburg L. A., et al. , “Dynamics and nanoscale organization of the postsynaptic endocytic zone at excitatory synapses,” eLife 11, e74387 (2022). 10.7554/eLife.74387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scheefhals N., et al. , “Shank proteins couple the endocytic zone to the postsynaptic density to control trafficking and signaling of metabotropic glutamate receptor 5,” Cell Rep. 29(2), 258–269.e8 (2019). 10.1016/j.celrep.2019.08.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glebov O. O., et al. , “Clathrin-independent trafficking of AMPA receptors,” J. Neurosci. 35(12), 4830–4836 (2015). 10.1523/JNEUROSCI.3571-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fujii S., Tanaka H., Hirano T., “Detection and characterization of individual endocytosis of AMPA-type glutamate receptor around postsynaptic membrane,” Genes Cells 22(6), 583–590 (2017). 10.1111/gtc.12493 [DOI] [PubMed] [Google Scholar]
- 30.Azarnia Tehran D., et al. , “Selective endocytosis of Ca2+-permeable AMPARs by the Alzheimer’s disease risk factor CALM bidirectionally controls synaptic plasticity,” Science Adv. 8(21), eabl5032 (2022). 10.1126/sciadv.abl5032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaksonen M., Roux A., “Mechanisms of clathrin-mediated endocytosis,” Nat. Rev. Mol. Cell Biol. 19(5), 313–326 (2018). 10.1038/nrm.2017.132 [DOI] [PubMed] [Google Scholar]
- 32.Li D., et al. , “Extended-resolution structured illumination imaging of endocytic and cytoskeletal dynamics,” Science 349(6251), aab3500 (2015). 10.1126/science.aab3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sochacki K. A., et al. , “The structure and spontaneous curvature of clathrin lattices at the plasma membrane,” Dev. Cell 56(8), 1131–1146.e3 (2021). 10.1016/j.devcel.2021.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taylor M. J., Perrais D., Merrifield C. J., “A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis,” PLoS Biol. 9(3), e1000604 (2011). 10.1371/journal.pbio.1000604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merrifield C. J., Perrais D., Zenisek D., “Coupling between clathrin-coated-pit invagination, cortactin recruitment, and membrane scission observed in live cells,” Cell 121(4), 593–606 (2005). 10.1016/j.cell.2005.03.015 [DOI] [PubMed] [Google Scholar]
- 36.Fujii S., Tanaka H., Hirano T., “Suppression of AMPA receptor exocytosis contributes to hippocampal LTD,” J. Neurosci. 38(24), 5523–5537 (2018). 10.1523/JNEUROSCI.3210-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Compans B., et al. , “NMDAR-dependent long-term depression is associated with increased short term plasticity through autophagy mediated loss of PSD-95,” Nat. Commun. 12(1), 2849 (2021). 10.1038/s41467-021-23133-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh W. C., Hill T. C., Zito K., “Synapse-specific and size-dependent mechanisms of spine structural plasticity accompanying synaptic weakening,” Proc. Natl. Acad. Sci. U.S.A. 110(4), E305–E312 (2013). 10.1073/pnas.1214705110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayashi A., et al. , “High affinity receptor labeling based on basic leucine zipper domain peptides conjugated with pH-sensitive fluorescent dye: visualization of AMPA-type glutamate receptor endocytosis in living neurons,” Neuropharmacology 100(Supplement C), 66–75 (2016). 10.1016/j.neuropharm.2015.07.026 [DOI] [PubMed] [Google Scholar]
- 40.Yu J., et al. , “Hippocampal AMPA receptor assemblies and mechanism of allosteric inhibition,” Nature 594, 448–453 (2021). 10.1038/s41586-021-03540-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Willems J., et al. , “ORANGE: a CRISPR/Cas9-based genome editing toolbox for epitope tagging of endogenous proteins in neurons,” PLOS Biol. 18(4), e3000665 (2020). 10.1371/journal.pbio.3000665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang H., et al. , “An optimized CRISPR/Cas9 approach for precise genome editing in neurons,” eLife 10, e65202 (2021). 10.7554/eLife.65202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Graves A. R., et al. , “Visualizing synaptic plasticity in vivo by large-scale imaging of endogenous AMPA receptors,” eLife 10, e66809 (2021). 10.7554/eLife.66809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Campbell B. C., et al. , “mGreenLantern: a bright monomeric fluorescent protein with rapid expression and cell filling properties for neuronal imaging,” Proc. Natl. Acad. Sci. U.S.A. 117(48), 30710–30721 (2020). 10.1073/pnas.2000942117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu T.-L., et al. , “Observing the cell in its native state: imaging subcellular dynamics in multicellular organisms,” Science 360(6386), eaaq1392 (2018). 10.1126/science.aaq1392 [DOI] [PMC free article] [PubMed] [Google Scholar]