Abstract
Background/objective
Osteoarthritis (OA) is a whole joint disorder with no disease modifying treatment currently available. The Epidermal Growth Factor Receptor (EGFR) signaling pathway plays an important role in cartilage/bone development and its ligand transforming growth factor-α (TGFα) is upregulated in OA. In contrast, Mitogen-inducible gene 6 (Mig6) is a negative regulator of EGFR, and cartilage-specific Mig-6 deletion results in anabolic effects on cartilage and formation of chondro-osseus nodules (CON). We aimed to attenuate EGFR signaling by inhibiting TGFα production in cartilage-specific Mig6 deficient mice, to test whether this would prevent the formation of CONs.
Methods
We generated double knockout mice by crossing cartilage-specific Mig-6fl/flCol2a1-Cre+/− and whole-body Tgfa± mice to generate experimental and control wild-type mice. Knee and elbow sections were used to examine articular cartilage thickness, cell density, and osteoclast presence. Additionally, immunohistochemistry was completed to analyze phospho-EGFR and SOX9.
Results
Mig-6 deficient mice display cartilage thickening and CONs at 12 weeks in both the elbow and knee joints, which is independent of TGFα ligand presence. Similarly, articular cartilage cell density is increased in Mig6-cKO/Tgfa-KO and Mig6-cKOmice, but not Tgfa-KO mice, and displays increased SOX9 and phospho-EGFR staining.
Conclusion
The articular cartilage displays increased thickness/cell density and CON formation independent of the presence of TGFα, suggesting the anabolic phenotype in the Mig6-deficient mice is independent of TGFα/EGFR binding. The anabolic phenotype may be due to an alternative EGFR ligand activation, or other non-EGFR specific mechanism. More research is required to elucidate the exact pathway responsible for the anabolic effects.
Keywords: Epidermal growth factor receptor, Mitogen inducible gene 6, Transgenic mice, Chondrocyte, Animal model, Transforming growth factor-alpha
1. Introduction
Osteoarthritis (OA) is the most common form of arthritis, defined as a whole joint disorder characterized by cartilage degeneration, subchondral bone remodeling, osteophyte formation, and synovitis, with complex tissue cross-talk [1,2]. The multifactorial nature of OA has proven to be a challenge in the effort to identify therapeutic targets and there is currently no disease modifying OA drug (DMOAD) to stop, slow, or reverse OA progression, and so treatment focuses on education, exercise, pain management, and surgery [3].
The Epidermal Growth Factor Receptor (EGFR) signaling pathway plays an important role in cartilage/bone development and homeostasis as it leads to the downstream activation of many important pathways such as MAPK/ERK, PI3K/AKT, and JAK/STAT, respectively causing cell proliferation/gene expression changes, survival/protein synthesis, and survival/proliferation [4]. The importance of EGFR cannot be overstated, as almost all cell types express EGFR family members and the knockout of any one of them is lethal in mice [5]. Interestingly, EGFR signaling has been reported to both attenuate and aggravate OA, demonstrating very context-dependent activity with factors such as disease stage, age, sex, and ligand-receptor combinations affecting outcomes [6].
Transforming growth factor-alpha (TGFα), an EGFR ligand, is increased in the synovial membrane and synovial fluid of OA patients [7]. Indeed, TGFα expression is increased 4-fold in rat chondrocytes following surgical induction of OA [8]. Our group previously demonstrated catabolic effects of TGFα in ex vivo rat osteochondral explants, including degradation of ECM proteins, loss of chondrocyte phenotype, chondrocyte clustering, and increased levels of the OA marker MMP-13 [9,10]. Indeed, pharmacological inhibition of EGFR slows OA progression in a rat model of OA [11], and mice deficient for TGFα are protected from post-traumatic OA, at least at young age [12]. On the other hand, genetic loss or pharmacological inhibition of EGFR disrupts cartilage homeostasis and accelerates OA progression [13,14]. Furthermore, intra-articular delivery of TGFα polymeric micellar nanoparticles attenuated surgery-induced OA in a mouse model [15].
EGFR signaling is regulated by mitogen inducible gene 6 (Mig6), a scaffold protein that down-regulates EGFR signaling [16]. It is also referred to as Gene 33, ErbB receptor feedback inhibitor 1 (ERRFI1), or receptor-associated late transducer (RALT), although it will be referred to as Mig6 in this paper for simplicity. Mig6 plays an important role in joint homeostasis; in fact, whole-body Mig-6 deficient mice develop joint deformities and most die within 6 months due to temporomandibular joint ankylosis [17]. Using the Col2a1 Cre system to specifically delete Mig-6 from mouse chondrocytes provides improved survival compared to global knockout, and the mutant mice exhibit increased cartilage thickness at 12-weeks. These mice also display severe osteophyte-like chondro-osseus nodules (CON) in the knee and spine, severely impairing ambulation [18,19]. At 12 weeks of age, the CONs appear as a diffusely radiopaque material on micro-CT that are less intense than bone, but by 36 weeks they are of similar intensity as bone. Detailed micro-CT images are available via Pest et al. [18].
In the present study, we aim to attenuate EGFR signaling by inhibiting TGFα production in cartilage-specific Mig-6 deficient mice. We hypothesized that eliminating one major EGFR ligand would reduce the effect of Mig6 deficiency, possibly preventing the formation of CONs in mice with an anabolic cartilage phenotype.
2. Materials and methods
2.1. Animals and surgery
Mice deficient in TGFα were developed using embryonic stem cell gene targeting via phosphoglycerate kinase (PGK)-neo expression cassette by Mann and colleagues [12,20]. Cartilage-specific Mig6 deficient mice were established by breeding Mig-6fl/fl mice [21] with Col2a1Cre mice [22]. We generated double knockout mice by crossing cartilage-specific Mig-6fl/flCol2a1-Cre+/− and whole-body Tgfa −/− mice to generate experimental (Mig-6fl/flCol2a1-Cre+/−;Tgfa−/−, Mig-6fl/flCol2a1-Cre+/−;Tgfa+/+, Mig-6fl/flCol2a1-Cre−/−;Tgfa−/−) and control wild-type mice (Mig-6fl/flCol2a1-Cre−/−;Tgfa+/+). Henceforth, double-knockout mice are referenced as Mig6-cKO/Tgfa-KO, Mig6 deficient mice as Mig6-cKO and TGFα deficient mice as Tgfa−ΚΟ. Male mice from each group were selected for further experimentation, using age-match litters to achieve N = 5. Mice were bred in-house and maintained in a temperature- and humidity-controlled room with water and standard chow freely available. All mice were genotyped using extracted DNA from ear biopsies at 21 days of age. Mice were euthanized by CO2 asphyxiation at 12-weeks of age. All animal experiments were in accordance with the Canadian Council on Animal Care guidelines and were approved by the Animal Use Subcommittee at Western University (2019–035).
2.2. Histological assessment and immunohistochemistry
Knee and elbow joints were dissected and fixed in 4% paraformaldehyde at 4 °C overnight. Joint decalcification with 5% ethylenediaminetetraacetic acid (EDTA) in phosphate buffered saline (PBS) at 7.0 pH was carried out for 11 days at room temperature. Following processing and paraffin embedding, joints were sectioned at 5 μm in the sagittal or coronal planes. Samples from the left knee and elbow were stained in 0.04% Toluidine Blue in 0.2 M acetate buffer, pH 4.0 to assess glycosaminoglycan content and general histology.
Right elbow and knee samples were dewaxed using xylene and rehydrated using a graded series of ethanol washes, then incubated in 3% hydrogen peroxide in methanol for 15 min to remove endogenous peroxidase activity. 0.1% Triton X-100 was used for antigen retrieval and then blocked with 5% serum in PBS. Sections were then stained overnight at 4 °C using primary antibody for SOX9 (R&D Systems, AF3075) or phosphor-EGFR (phosphoTyr-1173; Cell Signaling Technology), and incubated with secondary antibody conjugated to horseradish peroxidase (Santa Cruz Biotechnology). Sections were exposed to DAB + chromogen (Dako Canada) and counterstained with 0.5% methyl green in 0.1 M sodium acetate buffer 4.2 pH. Tartrate-resistant acid phosphatase (TRAP; Sigma Canada) staining was also performed to assess osteoclast presence. TRAP staining was done in accordance to manufacturers instructions. Images for all stained slides were taken on a Leica DFC295 digital camera affixed to a Leica DM1000 microscope on the Leica Application Suite software (v3.8.0).
2.3. Articular cartilage measurements
Coronal knee and elbow sections were blinded and randomized for analysis. The articular cartilage thickness was measured at three evenly spaced points and averaged. Measurements were done on a minimum of three sections per joint per animal, with an average of 50 μm between sections using ImageJ (v.1.52) software.
Coronal knee sections were blinded and randomized for analysis of the uncalcified cartilage (superficial tangential zone to the tidemark), calcified cartilage (tidemark to the subchondral bone), and subchondral plate thickness. Measurements were done on three sections per joint per animal and averaged. Measurements were taken across the medial/lateral tibial plateau and medial/lateral femoral condyle. OsteoMetric OsteoMeasure software was used as previously described [23].
2.4. Cell density and immunohistochemistry quantification
Cell density was calculated within central regions (200 μm wide x 70 μm tall) on the articular cartilage of the humerus and tibia. Lacunae with nuclear staining were counted as cells using the ImageJ (v.1.52) software on a minimum of three sections per animal. Sections were blinded and randomized for analysis. A similar protocol was followed to quantify positive cells (brown nuclear staining) for phospho-EGFR and SOX9 in knee articular cartilage samples.
2.5. Statistical analysis
Statistical analysis was performed in GraphPad Prism v.9.5.0. Normality was assessed via the D'Agostino & Pearson test. A one-way analysis of variance (ANOVA) with Tukey's multiple comparison test was run for articular cartilage thickness and cell density. Data is presented as mean with 95% CI. Data was considered statistically significant when p < 0.05.
3. Results
3.1. Anabolic articular cartilage phenotype in Mig6 deficient mice
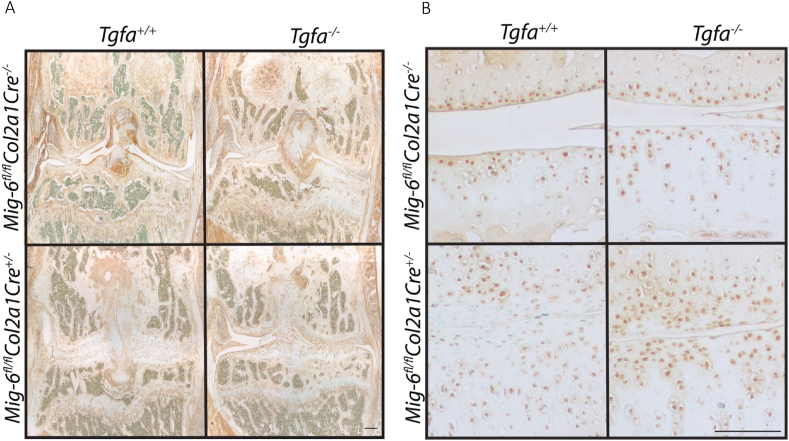
All genotypes were confirmed through PCR, and phospho-EGFR immunohistochemistry was performed to demonstrate EGFR activity [18]. As expected, phospho-EGFR staining appears to be increased in Mig6-cKO mice compared to wild-type mice (Fig. 1). The number of positive cells is significantly increased in the Mig6-cKO/Tgfa-KO group compared to wild type (mean difference = −82.73, 95% CI [−113.1, −52.33]), and trends towards an increase in the Mig6-KO group (mean difference = −22.70, 95% CI [−51.35, 5.95]). Tgfa−ΚΟ mice trend towards having fewer positive cells compared to the wild-type and Mig6-KO mice, and significantly less positive cells than the Mig6-cKO/Tgfa-KO mice (mean difference = −83.13, 95% CI [−113.5, −52.73]) (Fig. 1).
Fig. 1.
Phospho-EGFR activity is upregulated in Mig6 deficient mice. Brown staining indicates positive phospho-EGFR staining, which appears more intense and present in more chondrocytes in Mig6 deficient mice, with opposite effects in Tgfa-KO mice. The Mig6-cKO/Tgfa-KO mice seem to have a similar amount of staining to the Mig6-cKO mice. Images represent coronal knee sections, counterstained in methyl green at (A) 4x and (B) 20x. Scale bars = 100 μm. N = 5.
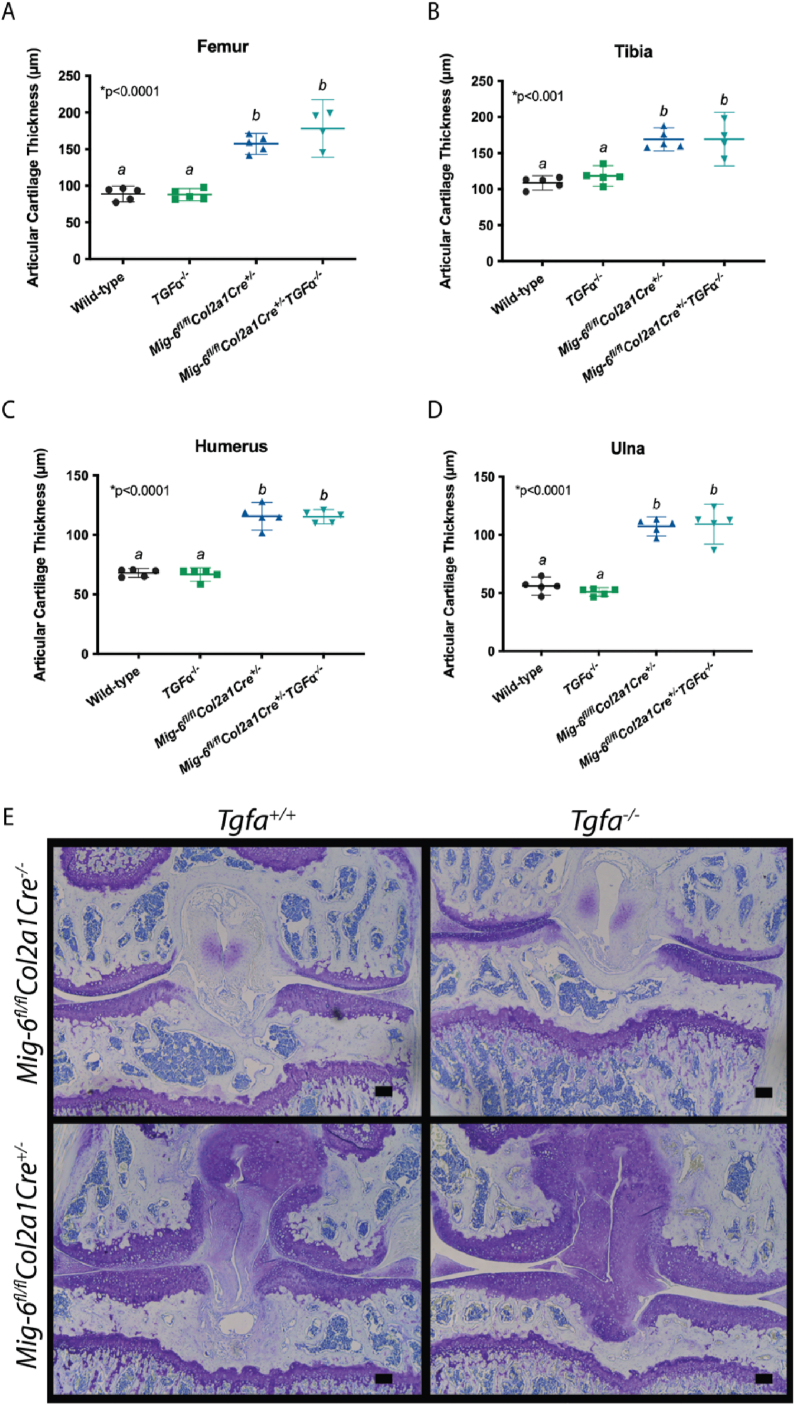
The thickness of articular cartilage was significantly increased in the femur (Fig. 2 A, E), tibia (Fig. 2 B, E), humerus (Fig. 2C), and ulna (Fig. 2 D) of Mig6-cKO mice, regardless of the presence of TGFα. There was no evidence of changes in articular cartilage thickness in Tgfa−ΚΟ mice compared to wild-type mice in knee or elbow joints. Interestingly, there was no difference in articular cartilage thickness between the Mig6-cKO/Tgfa-KO and Mig6-cKO (femur mean difference = −20.99 μm, 95% CI [−47.65, 5.67]).
Fig. 2.
Mig6 deficient mice display articular cartilage thickening at 12 weeks, regardless of TGFα. Average articular cartilage thickness was calculated using three measurements at different locations on the articular cartilage using ImageJ software on coronal sections. Average articular cartilage thickness was significantly increased in Mig6 deficient mice, regardless of TGFα presence in the (A, E) femur, (B, E) tibia, (C) humerus, and (D) ulna. Graphed data represents a non-parametric one-way ANOVA with Tukey's multiple comparison test, where the individual mean and 95% CI is presented, p values are presented on each graph. (E) Representative images of toluidine blue stained knee sections. Scale bars = 100 μm. N = 5.
In the Mig6-cKO and Mig6-cKO/Tgfa-KO mice, the articular cartilage had a significantly higher proportion of uncalcified cartilage (Fig. 3 C, F) and a lower proportion of calcified cartilage (Fig. 3 D, F). The uncalcified cartilage in these groups was significantly thicker (Fig. 3 A) compared to the wild-type and Tgfa-KO mice, although the calcified cartilage thickness was not (Fig. 3 B). Additionally, the subchondral plate was thinner in the Mig6-cKO and Mig6-cKO/Tgfa-KO mice compared to the other two groups (Fig. 3 E). The data presented represents the medial femoral condyle, although similar trends were seen in the medial tibial plateau, lateral femoral condyle, and lateral tibial plateau (data available upon request).
Fig. 3.
Mig6 deficient mice have significantly more uncalcified cartilage. Average uncalcified and calcified cartilage thickness was calculated on Osteomeasure across three sections, with the medial femoral condyle results presented. (A) The uncalcified cartilage thickness was significantly greater in the Mig6-cKO and Mig6-cKO/Tgfa-KO compared to wild-type and Tgfa-KO, and (C) made up a greater percentage of total cartilage. (B) The thickness of the calcified cartilage was not significantly different between groups, but when looking at the percentage of total calcified cartilage in (D), the Mig6-cKO and Mig6-cKO/Tgfa-KO mice are significantly decreased compared to the wild type and Tgfa-KO mice. The breakdown of uncalcified vs. calcified cartilage can be seen in (F). Subchondral plate thickness was measured (E), with the Mig6-cKO and Mig6-cKO/Tgfa-KO groups demonstrating significantly less thickness compared to the wild-type and Tgfa-KO groups. Graphed data represents a one-way ANOVA with Tukey's multiple comparison test, where the individual mean and 95% CI is presented, p values are presented on each graph. N = 5.
3.2. Cell density is not affected by TGFα deficiency
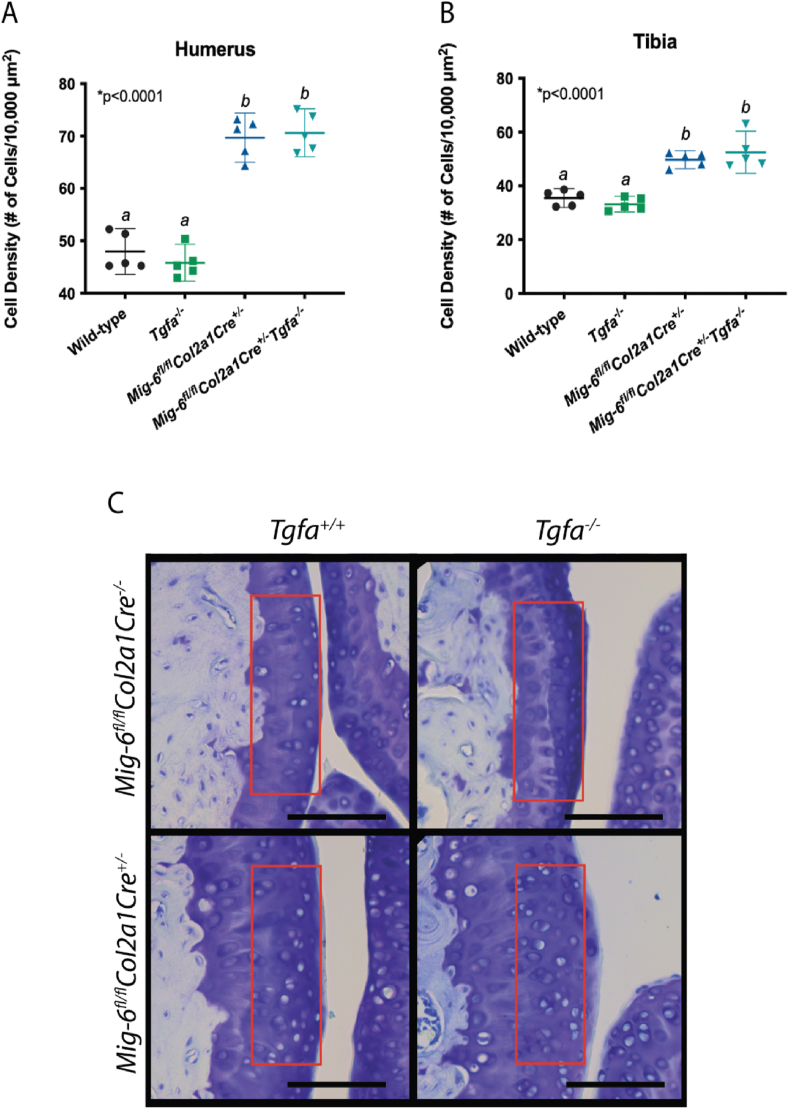
Mice with cartilage-specific deletion of Mig6 (with and without TGFα presence) were found to have a 1.5-fold increase in cell density in the cartilage of the humerus, compared to wild-type (Mig6-cKO mean difference = −21.75, 95% CI [−28.03, −15.47]; Mig6-cKO/Tgfa-KO mean difference = −22.69, 95% CI [−28.97, −16.41]) and Tgfa−ΚΟ mice (Mig6-cKO mean difference = −23.89, 95% CI [−30.17, −17.61]; Mig6-cKO/Tgfa-KO mean difference = −24.83, 95% CI [−31.11, −18.55]; Fig. 4 A, C). Similar to articular cartilage thickness, there was no difference between the Mig6-cKO/Tgfa-KO and Mig6-cKO mice (mean difference = −0.941, 95% CI [−7.221, 5.339]). The Mig6-cKO and Mig6-cKO/Tgfa-KO mice had a similar increase in cell density in the tibia, compared to wild-type and Tgfa-KO mice (Fig. 4 B). Immunohistochemistry staining for SOX9 was positive in all groups, although it trends towards more positive cells in the Mig6-cKO mice compared to the Tgfa−ΚΟ and wild type mice. The Mig6-cKO/Tgfa-KO mice had significantly more positive cells than the wild-type (mean difference = −56.20, 95% CI [−106.1, −6.32]) and Tgfa-KO mice (mean difference = −59.1, 95% CI [−109.0 to −9.22]) (Fig. 5).
Fig. 4.
Cell density is not affected by TGFα deficiency. Cell density was calculated within the articular cartilage of toluidine blue stained elbow and knee joints. Lacunae with nuclear staining was counted within a 70 μm × 200 μm region, indicated by the red box (C), representative images of the sagittal elbow is presented. Scale bars = 100 μm. Cell density is significantly increased in Mig6 deficient mice, regardless of TGFα presence in the (A) humerus and (B) tibia. Graphed data represents a non-parametric one-way ANOVA with Tukey's multiple comparison test, where the individual mean and 95% CI is presented, p values are presented on each graph. N = 5.
Fig. 5.
SOX9 staining is increased in Mig6 deficient groups. Brown staining indicates positive SRY-box transcription factor 9 (SOX9) nuclear staining, which is present in all groups. The Mig6-cKO/Tgfa-KO mice and Mig6-cKO mice appear to have more positively stained chondrocytes than the Tgfa-KO and wild-type mice. All sections are of the coronal knee, counterstained with methyl green at (A) 4x and (B) 20x. Scale bars = 100 μm. N = 5.
3.3. CONs develop in Mig6 deficient mice independent of TGFα ligand presence
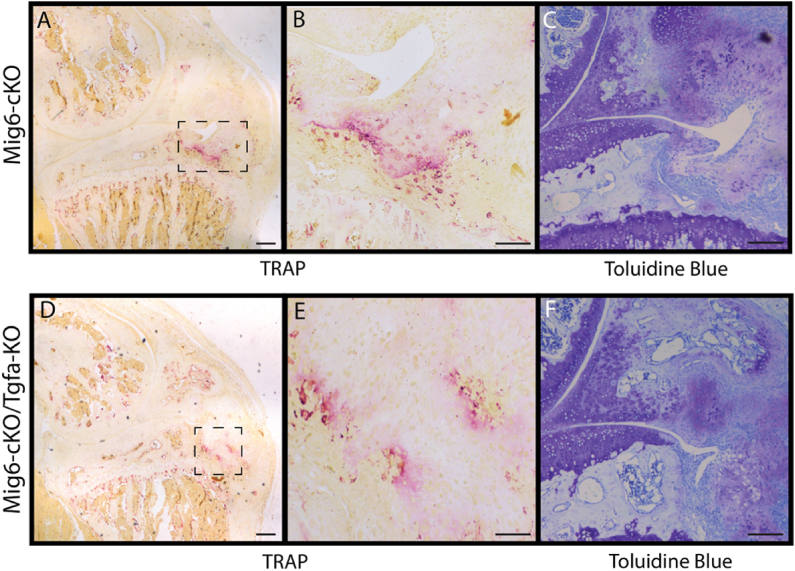
Chondro-osseus nodules (CONs) around the articulating knee surface were present in Mig6-cKO/Tgfa-KO (3 of n = 5) and Mig6-cKO mice (2 of n = 5). CON development does not appear in wild-type or Tgfa−ΚΟ mice (Fig. 6). TRAP staining reveals an increase in osteoclasts within CONs, which appears similar between the two Mig6-deficient groups (Fig. 7).
Fig. 6.
CONs develop in Mig6 deficient mice independent of TGFα ligand presence. Toluidine blue stained sagittal knee sections of 12-week-old mice demonstrate CON development in Mig6 deficient mice, with or without TGFα. CON development does not appear in wild type or Tgfa-KO mice. Images are representative of N = 2 for the Mig6-cKO/Tgfa-KO mice and N = 3 for the remaining groups. All sections are of the sagittal knee at 10x magnification. Scale bar = 1000 μm.
Fig. 7.
TRAP staining is increased within CONs. TRAP staining reveals an increase in osteoclasts within CONs, which appears similar between Mig6-cKO and Mig6-cKO/Tgfa-KO mice. Images (A, D) represent TRAP staining of sagittal knee sections at 4x magnification and (B, E) 20x magnification. (C, F) There is also an increase in purple proteoglycan staining in sagittal knee sections within CONs. Images (B) and (E) are magnified from the dotted box within images (A) and (D), respectively. (A, D) Scale bars = 200 μm and (B, C, E, F) scale bars = 100 μm.
4. Discussion
Regulation of the EGFR pathway via Mig6 signaling results in an anabolic cartilage phenotype at 12-weeks. In the present study, we aimed to assess the relationship TGFα plays in this phenotype, e.g. to examine whether absence of one major EGFR ligand would affect the anabolic effects of Mig6-deficiency. We noted that upon Mig6 deletion, the articular cartilage displays increased thickness, cell density, and CON formation independent of TGFα, suggesting the anabolic phenotype in the Mig6 mice is independent of TGFα/EGFR binding. These data do not support our initial hypothesis.
Our results demonstrate an increase in articular cartilage thickness in both the knee and elbow joints of 12-week old Mig6-cKO/Tgfa-KO and Mig6-cKO mice, similar to previous studies. Pest et al. [18] reported a ∼1.5-fold increase in knee articular cartilage thickness in Mig6-cKO mice, and we have similarly noted a ∼1.6-fold increase. This cartilage appears to be composed of a higher percentage of uncalcified cartilage and proportionally less calcified cartilage. Additionally, the subchondral plate appears to be thinner in Mig6-deficient mice. Pest et al. [18] noted that this anabolic cartilage phenotype is transient, as the cartilage thickness is not significantly different from the wild-type mice at 36-weeks. Perhaps this is due to advancement of the subchondral plate and tidemark, as we noted here that the 12-week old mice have significantly more uncalcified cartilage and a thinner subchondral plate, although further analysis of the 36-week old mice is required. The transient nature of the anabolic articular cartilage phenotype, as well as the ability of this cartilage to adapt to mechanical stress requires further analysis to understand the potential importance of this process in OA.
Our data on cellular density, SOX9, and EGFR staining is similar to those described in Pest et al. [18]. We also noted lesions at the anterior and posterior cruciate ligament entheses that appear to be associated with the CONs. A more detailed analysis of these ligament changes can be seen in Pest et al. [18]. In contrast, we noted CON development in 2–3 animals with Mig6 deficiency (with or without TGFα presence) at 12-weeks of age, whereas Pest et al. [18] noted all Mig6 deficient mice developed CONs in at least 1 knee joint. This may be due to a sex difference, as our work was solely performed in male mice. In contrast, Pest et al. [18] noted this occurrence in female mice. Unfortunately, the N = ≤ 3 prevents us from performing further analysis on those mice that did develop CONs.
The transcription factor SOX9 is a key marker of chondrocyte development and phenotype maintenance [24]. In our study, SOX9 antibody staining trends towards more positive cells in the Mig6-deficient mice, which was expected due to the increase in articular cartilage thickness and cell density. Interestingly, the stain appears more intense in the Mig6-cKO/Tgfa-KO mice compared to the Mig6-cKO, and the Mig6-cKO/Tgfa-KO group had significantly more positive cells than the wild-type mice, whereas this was only a trend in the Mig6-cKO mice. This finding is consistent with previous work demonstrating a reduction of SOX9 mRNA and protein expression following in vitro TGFα treatment [9]. There was no significant difference between the percentage of positive cells in the Mig6-cKO and Mig6-cKO/Tgfa-KO groups, although we aim to more accurately quantify SOX9 (and phospho-EGFR) protein expression in these groups in future studies to validate the results.
We did not note a decrease in CON formation or cartilage thickening in the Mig6-cKO/Tgfa-KO mice compared to Mig6-cKO mice, which we had expected. This may be due to a number of factors. Firstly, EGFR has multiple binding ligands, so perhaps a ligand other than TGFα is responsible or compensates [4]. For example, heparin binding EGF-growth factor (HB-EGF) is an EGFR ligand that may be protective of surgically-induced OA in mice when it is overexpressed [25]. The relationship between EGFR and OA is very context dependent [6], and so exploring alternative ligand activation is a worthwhile future endeavor to understanding its role.
Other non-EGFR specific mechanisms may also be responsible for the lack of effects of TGFα deficiency in our study. Mig6 is a negative regulatory of the c-Met receptor, which mediates cell proliferation and migration via hepatocyte growth factor (HGF) [26,27]. Previous studies have demonstrated HGF and c-Met expression by chondrocytes in vivo, both in healthy and OA tissue [28]. HGF and c-Met are produced by the calcified and deep zone chondrocytes. The calcified cartilage and deep zone chondrocytes have a larger portion of c-Met receptors, compared to the mid and superficial zone chondrocytes [28]. Additionally, there is research pointing to a change in HGF/c-Met activity in synovial joints during OA [29]. It is therefore possible that our Mig6-cKO mice de-regulate c-Met signaling, which may contribute to the anabolic phenotype.
Overall, it appears that the anabolic phenotype found in Mig6-cKO mice is independent of TGFα/EGFR binding. There are a number of other ligand interactions our group will examine in the future to understand the mechanisms behind this anabolic phenotype.
Author contributions
Conceptualization and design, B.T, M.A.P, and F.B; analysis and interpretation of data, E.H, B.T, M.A.P, and F.B; article drafting, E.H; article revision, B.T, M.A.P, L.Q, and F.B; final article approval, E.H, B.T, M.A.P, L.Q, and F.B; provision of study materials, L.Q and F.B; statistics, E.H and B.T; obtaining of funding, F.B; technical/logistical support, M.A.P and F.B; collection and assembly of data, E.H, B.T, and M.A.P.
Role of the funding source
F.B. is the Canada Research Chair in Musculoskeletal Research. Work in the Beier laboratory is supported by operating grants from the Canadian Institute of Health Research (Grant #332438).
E.H. is funded by the Arthritis Society Canada PhD Salary Award 22–0000000154 (and matched NIH funding), the Ontario Graduate Scholarship, and a Transdisciplinary Training Award from the Bone and Joint Institute at Western University, Canada.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
The authors would like to thank all members of the Beier lab for ongoing support.
References
- 1.Osteoarthritis cartilage, standardization of osteoarthritis definitions. 2015. https://oarsi.org/research/standardization-osteoarthritis-definitions [Google Scholar]
- 2.Badley E.M., Wilfong J.M., Zahid S., Perruccio A. Arthritis community research and evaluation unit. The status of arthritis in Canada: national report. Arthritis Society. 2019:1–34. [Google Scholar]
- 3.Bannuru R.R., Osani M.C., Vaysbrot E.E., Arden N.K., Bennel K., Bierma-Zeinstra S.M.A., et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Citri A., Yarden Y. EGF-ERBB signalling: towards the systems level. Nat. Rev. Mol. Cell Biol. 2006;7:505–516. doi: 10.1038/nrm1962. [DOI] [PubMed] [Google Scholar]
- 5.Olayioye M.A., Neve R.M., Lane H.A., Hynes N.E. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19(13):3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin L., Beier F. EGFR signaling: friend or foe for cartilage? JBMR Plus. 2019;3(2) doi: 10.1002/jbm4.10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallbeck A.L., Walz T.M., Briheim K., Wasteson A. TGF- and ErbB2 production in synovial joint tissue: increased expression in arthritic joints. Scand. J. Rheumatol. 2005;34(3):204–211. doi: 10.1080/03009740510017715. [DOI] [PubMed] [Google Scholar]
- 8.Appleton C.T.G., Pitelka V., Henry J., Beier F. Global analyses of gene expression in early experimental osteoarthritis. Arthritis Rheum. 2007;56(6):1854–1868. doi: 10.1002/art.22711. [DOI] [PubMed] [Google Scholar]
- 9.Appleton C.T.G., Usmani S.E., Bernier S.M., Aigner T., Beier F. Transforming growth factor alpha suppression of articular chondrocyte phenotype and Sox9 expression in a rat model of osteoarthritis. Arthritis Rheum. 2007;56(11):3693–3705. doi: 10.1002/art.22968. [DOI] [PubMed] [Google Scholar]
- 10.Appleton C.T.G., Usmani S.E., Mort J.S., Beier F. Rho/ROCK and MEK/ERK activation by transforming growth factor-alpha induces articular cartilage degradation. Lab. Invest. 2010;90(1):20–30. doi: 10.1038/labinvest.2009.111. [DOI] [PubMed] [Google Scholar]
- 11.Appleton C.T.G., Usmani S.E., Pest M.A., Pitelka V., Mort J.S., Beier F. Reduction in disease progression by inhibition of transforming growth factor-alpha-CCL2 signaling in experimental posttraumatic osteoarthritis. Arthritis Rheum. 2015;67(10):2691–2701. doi: 10.1002/art.39255. [DOI] [PubMed] [Google Scholar]
- 12.Usmani S.E., Ulici V., Pest M.A., Hill T.L., Welch I.D., Beier F. Context-specific protection of TGFα null mice from osteoarthritis. Sci. Rep. 2016;6 doi: 10.1038/srep30434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X., Zhu J., Liu F., Li Y., Chandra A., Beier F., et al. Reduced EGFR signaling enhances cartilage destruction in a mouse osteoarthritis model. Bone Res. 2014;2(14015) doi: 10.1038/boneres.2014.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei Y., Ma X., Sun H., Gui T., Yao L., Han B., et al. EGFR signaling is required for maintaining adult cartilage homeostasis and attenuating osteoarthritis progression. J. Bone Miner. Res. 2022;37(5):1012–1023. doi: 10.1002/jbmr.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei Y., Luo L., Gui T., Yu F., Yan L., Yao L., et al. Targeting cartilage EGFR pathway for osteoarthritis treatment. Sci. Transl. Med. 2021;13(576) doi: 10.1126/scitranslmed.abb3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferby A., Reschke M., Kudlacek O., Knyazev P., Pante G., Amann K., et al. Mig6 is a negative regulator of EGF receptor–mediated skin morphogenesis and tumor formation. Nat. Med. 2006;12(5):568–573. doi: 10.1038/nm1401. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y., Su Y., Lanning N., Swiatek P.J., Bronson R.T., Sigler R., et al. Targeted disruption of Mig-6 in the mouse genome leads to early onset degenerative joint disease. Proc. Natl. Acad. Sci. U.S.A. 2005;102(33):11740–11745. doi: 10.1073/pnas.0505171102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pest M.A., Russell B.A., Zhang Y., Jeong J., Beier F. Disturbed cartilage and joint homeostasis resulting from a loss of mitogen-inducible gene 6 in a mouse model of joint dysfunction. Arthritis Rheumatol. 2014;66(10):2816–2827. doi: 10.1002/art.38758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staal B., Williams B.O., Beier F., Woude G.F.V., Zhang Y. Cartilage-specific deletion of Mig-6 results in osteoarthritis-like disorder with excessive articular chondrocyte proliferation. Proc. Natl. Acad. Sci. U.S.A. 2014;111(7):2590–2595. doi: 10.1073/pnas.1400744111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mann G.B., Fowler K.J., Gabriel A., Nice E.C., Williams R.L., Dunn A.R. Mice with a null mutation of the TGF alpha gene have abnormal skin architecture, wavy hair, and curly whiskers and often develop corneal inflammation. Cell. 1993;73(2):249–261. doi: 10.1016/0092-8674(93)90227-h. [DOI] [PubMed] [Google Scholar]
- 21.Jin N., Gilbert J.L., Broaddus R.R., Demayo F.J., Jeong J. Generation of a Mig-6 conditional null allele. Genesis. 2007;45(11):716–721. doi: 10.1002/dvg.20348. [DOI] [PubMed] [Google Scholar]
- 22.Terpstra L., Prud'homme J., Arabian A., Takeda S., Karsenty G., Dedhar S., et al. Reduced chondrocyte proliferation and chondrodysplasia in mice lacking the integrin-linked kinase in chondrocytes. J. Cell Biol. 2003;162(1):139–148. doi: 10.1083/jcb.200302066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moon P.M., Shao Z.Y., Wambiekele G., Appleton C.T.G., Laird D.W., Penuela S., et al. Global deletion of pannexin 3 resulting in accelerated development of aging-induced osteoarthritis in mice. Arthritis Rheumatol. 2021;73(7):1178–1188. doi: 10.1002/art.41651. [DOI] [PubMed] [Google Scholar]
- 24.Ng L.J., Wheatley S., Muscat G.E., Conway-Campbell J., Bowles J., Wright E., et al. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev. Biol. 1997;183(1):108–121. doi: 10.1006/dbio.1996.8487. [DOI] [PubMed] [Google Scholar]
- 25.Wei Y., Luo L., Gui T., Yu F., Yan L., Yao L., et al. Targeting cartilage EGFR pathway for osteoarthritis treatment. Sci. Transl. Med. 2021;13(576) doi: 10.1126/scitranslmed.abb3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pante G., Thompson J., Lamballe F., Iwata T., Ferby I., Barr F.A., et al. Mitogen-inducible gene 6 is an endogenous inhibitor of HGF/Met-induced cell migration and neurite growth. J. Cell Biol. 2005;171(2):337–348. doi: 10.1083/jcb.200502013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trusolino L L., Comoglio P.M. Scatter-factor and semaphoring receptors: cell signalling for invasive growth. Nat. Rev. Cancer. 2002;2(4):289–300. doi: 10.1038/nrc779. [DOI] [PubMed] [Google Scholar]
- 28.Pfander D., Cramer T., Weseloh G., Pullig O., Schuppan D., Bauer M., et al. Hepatocyte growth factor in human osteoarthritic cartilage. Osteoarthritis Cartilage. 1999;7(6):548–559. doi: 10.1053/joca.1999.0259. [DOI] [PubMed] [Google Scholar]
- 29.Tonomura H., Nagae M., Takatori R., Ishibashi H., Itsuji T., Takahashi K. The potential role of hepatocyte growth factor in degenerative disorders of the synovial joint and spine. Int. J. Mol. Sci. 2020;21(22):8717. doi: 10.3390/ijms21228717. [DOI] [PMC free article] [PubMed] [Google Scholar]