Highlights
-
•
Schizophrenia patients with moderate severity negative symptoms were recruited.
-
•
Mu opioid receptor (MOR) availability was measured using [11C]-Carfentanil PET.
-
•
Patients had lower MOR availability in the striatum compared with controls.
-
•
Patients had striatal hypoactivation when performing a monetary incentive delay task.
-
•
Brain activity during reward anticipation was not related to MOR availability in either group.
Keywords: Mu opioid receptor, Reward, Functional MRI, PET
Abstract
Background
Reward processing deficits are a core feature of schizophrenia and are thought to underlie negative symptoms. Pre-clinical evidence suggests that opioid neurotransmission is linked to reward processing. However, the contribution of Mu Opioid Receptor (MOR) signalling to the reward processing abnormalities in schizophrenia is unknown. Here, we examined the association between MOR availability and the neural processes underlying reward anticipation in patients with schizophrenia using multimodal neuroimaging.
Method
37 subjects (18 with Schizophrenia with moderate severity negative symptoms and 19 age and sex-matched healthy controls) underwent a functional MRI scan while performing the Monetary Incentive Delay (MID) task to measure the neural response to reward anticipation. Participants also had a [11C]-carfentanil PET scan to measure MOR availability.
Results
Reward anticipation was associated with increased neural activation in a widespread network of brain regions including the striatum. Patients with schizophrenia had both significantly lower MOR availability in the striatum as well as striatal hypoactivation during reward anticipation. However, there was no association between MOR availability and striatal neural activity during reward anticipation in either patient or controls (Pearson's Correlation, controls df = 17, r = 0.321, p = 0.18, patients df = 16, r = 0.295, p = 0.24). There was no association between anticipation-related neural activation and negative symptoms (r = –0.120, p = 0.14) or anhedonia severity (social r = –0.365, p = 0.14 physical r = –0.120, p = 0.63).
Conclusions
Our data suggest reduced MOR availability in schizophrenia might not underlie striatal hypoactivation during reward anticipation in patients with established illness. Therefore, other mechanisms, such as dopamine dysfunction, warrant further investigation as treatment targets for this aspect of the disorder.
1. Introduction
Negative symptoms, including amotivation and anhedonia, are a major cause of functional impairment in schizophrenia (Galderisi et al., 2018). Existing pharmacotherapies fail to target this symptom domain, highlighting the need to better understand their underlying neurobiology (Kaar et al., 2020, Lobo et al., 2022). Reward processing deficits are extensively reported in schizophrenia, with impaired learning from reward shown in patients with high levels of negative symptoms (Deserno et al., 2017, Gold et al., 2012, Radua et al., 2015, Strauss et al., 2014).
Functional magnetic resonance imaging (fMRI) studies have localised the circuitry involved in anticipatory and consummatory reward, with a recent meta-analysis of 45 studies showing reward anticipation is associated with activation in the ventral striatum, middle cingulate cortex/supplementary motor area and insula (Jauhar et al., 2021). The largest meta-analysis to date in patients with schizophrenia (patients n = 917), found that patients have significant hypoactivation of the ventral striatum during reward anticipation when compared to control subjects (Radua et al., 2015). More recent work meta-analysing monetary incentive delay task fMRI found that during reward anticipation schizophrenia patients had hypoactivation in the striatum, anterior cingulate cortex, median cingulate cortex, amygdala, precentral gyrus and superior temporal gyrus (Zeng et al., 2022). Of these regions, specifically greater degree of striatal hypoactivation was associated with greater negative symptom severity (Zeng et al., 2022).
Another meta-analysis of fMRI studies that looked at consummatory reward tasks reported that during these tasks schizophrenia patients had significant hypoactivation in the cingulate cortex, orbitofrontal cortex, and basal ganglia (Zhang et al., 2016). More recent work has also shown reduced neural activation of the striatum during reward anticipation to be associated with measures of apathy in patients with schizophrenia (Kluge et al., 2018), together raising the question of the biological mechanisms underlying aberrant reward processing in schizophrenia.
Historically, the opioid system has been repeatedly discussed in the context of schizophrenia pathophysiology (Bloom et al., 1976, Clark and Abi-Dargham, 2019, Comfort, 1977, Jacquet and Marks, 1976, Quednow et al., 2008, Schmauss and Emrich, 1985, Terenius et al., 1976). More recently, there has been growing evidence for a role of opioid-signalling specifically in reward processing. Preclinical studies have reported that dopamine-depleted rats can still acquire a morphine-conditioned place preference (Hnasko et al., 2005). Mu-opioid receptor (MOR) knockout mice show reduced motivation to eat (Papaleo et al., 2007), reduced anticipation of food reward (Kas et al., 2004, Selleck and Baldo, 2017), and reduced maternal attachment (Moles et al., 2004). MOR knockout mice also have reduced reward responses to morphine (Contet et al., 2004, Hall et al., 2001, Norman and D’Souza, 2017), as well as to cocaine and alcohol (Becker et al., 2002). Consistent with preclinical studies, human studies have reported that food reward processing in humans is mediated by MOR (Loseth et al., 2014, Nummenmaa et al., 2018, Rabiner et al., 2011). Moreover, the administration of the opioid receptor antagonist, nalmefene, reduces BOLD response in the mesolimbic system during monetary reward anticipation (Quelch et al., 2017).
Furthermore, we have shown that patients with schizophrenia have lower MOR availability in the striatum, as well as the hedonic brain network consisting of the insula, amygdala, anterior cingulate cortex, and orbitofrontal cortex (Ashok et al., 2019). The increasing evidence for the role of the MOR in regulating reward processing raises the question if reduced MOR might underlie reward deficits in schizophrenia.
To test this, we selected patients with schizophrenia with negative symptoms. We hypothesized that we would identify striatal hypoactivation during reward anticipation in these patients relative to controls, in line with the fMRI findings that have been published previously (Leroy et al., 2020, Radua et al., 2015, Zeng et al., 2022), and set out to test whether reward anticipation-related activation in the striatum would be associated with MOR availability, which we have shown to be lower in patients in this region previously. We additionally tested whether activation of the striatum was associated with measures of anhedonia, consummatory and anticipatory pleasure and conducted exploratory analyses testing for BOLD-MOR relationships in extra-striatal hedonic regions (amygdala, insula, anterior cingulate and orbitofrontal cortices), which had previously been shown to have reduced MOR density in patients with schizophrenia and have been implicated in fMRI studies of reward anticipation in schizophrenia.
2. Methods
The study was approved by the London - Camberwell St Giles Research Ethics Committee and the Administration of Radioactive Substances Advisory Committee (ARSAC, UK). After receiving a description of the study all participants provided written informed consent to participate.
We recruited 20 patients with schizophrenia from secondary mental health services. PET data were not available in one subject as they dropped out of the study, another subject was excluded from the final analyses due to high motion during the fMRI scan and the final sample included 18 patients and 19 controls, PET data for these participants was included in Ashok et al., 2019. All patients met the DSM-5 criteria for schizophrenia. The inclusion criteria were a minimum score ≥ 4 on at least one item on the Positive and Negative Symptom Scale (PANSS) negative symptom sub-scale (Kay et al., 1987) or two or more negative symptoms with a score ≥ 3 on the PANSS negative symptom sub-scale to ensure current negative symptoms. All patients were on a stable dose of an antipsychotic for at least four weeks before the scan (supplementary table 1). Nineteen healthy volunteers were recruited from the same local catchment area through public advertisement. Inclusion criteria comprised no psychiatric morbidity as assessed by the Structured Clinical Interview for DSM 5 (SCID) and no family history of psychosis. Exclusion criteria for all subjects were: history or current substance use disorder (other than tobacco) as assessed by clinical interview, history of head injury or neurological abnormality, present or recent (1 month) use of opiates, antidepressants or other psychoactive medications including antiepileptics, significant physical comorbidity (minor self-limiting illnesses were permitted) as assessed by history and physical examination, and contraindications to PET or MRI scanning.
Subjects underwent a screening assessment which included medical and psychiatric history as well as the history of alcohol, tobacco, and other substance use and a physical examination. In addition, the physical and social anhedonia rating scale, and temporal experience of pleasure scale (TEPS) were administered to assess anhedonia in both patients and controls (Gard et al., 2006). The Calgary depression scale was used to assess depressive symptoms (Addington et al., 1990).
2.1. Magnetic resonance imaging acquisition
Data were collected using a 3T Siemens Verio system running the syngo MR B17 software with a Siemens 32 channel receive-only phased-array head coil. At the start of each scanning session a high-resolution T1-weighted volume was acquired for the purpose of fMRI and PET coregistration using a Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence and parameters from the Alzheimer’s Disease Research Network (ADNI-GO; 160 slices × 240 × 256, TR = 2300 ms, TE = 2.98 ms, flip angle = 9°, 1 mm isotropic voxels, bandwidth = 240 Hz/pixel, parallel imaging (PI) factor = 2; Jack et al., 2008). Subsequent functional images were acquired using a multiband sequence based on the multiband EPI WIP v012b provided by the University of Minnesota (Auerbach et al., 2013; Cauley et al., 2014; Setsompop et al., 2012; Xu et al., 2013). This sequence featured 42 interleaved slices, with a TR of 1200 ms, TE of 30 ms, 3 mm isotropic voxels in a 64x64 matrix, flip angle of 62°, and bandwidth of 1906 Hz/pixel. To achieve the desired resolution and repetition time, parallel imaging using Generalized Autocalibrating Partially Parallel Acquisition (GRAPPA) with an acceleration factor of 2 was conducted, and the multiband acceleration factor was also set to 2. The first six volumes of each functional run were discarded to allow for T1 saturation effects. These were not included in any number of volumes reported here.
2.2. Monetary incentive delay task
This task was designed to probe the neural correlates of reward anticipation (Knutson et al., 2000). The task contained two trial types (win trials and neutral trials) and participants could win money depending upon how quickly they reacted to a target stimulus. Each trial began with the presentation of the cue stimulus, which was either an orange (win trial) or blue (neutral trial) square, shown at the centre of the screen for 0.5 s. Following the cue, there was an anticipation period which varied randomly between 2, 3, and 4 s. The target stimulus (a white square) was then presented for a variable duration (see below), and participants were instructed to respond as fast as possible to this stimulus, by pressing a button using an MR-compatible button box. Following the target, feedback was presented.
For the win trials, participants won £1 if they responded during the target stimulus presentation. In this case, the participant received the feedback message “Hit! You won £1”, in green text for 1000 ms. On neutral trials, participants neither won nor lost money. If the current trial was a neutral trial, and the subject responded successfully to the target, the feedback message was simply “Hit!” in green text for 1000 ms. For both win and neutral trials, if the subject failed to respond during stimulus presentation the word “Miss” appeared at the centre of the screen in red text for 1000 ms. The additional message “Current Total = £XX” was always displayed below the feedback message, and showed the subject’s current total winnings during the task. All participants watched a demo video of the task and fully understood the rules and cue outcomes before completing the task in the scanner.
The duration of the target stimulus was dynamic in order to account for individual differences in response time and ensure a similar level of task difficulty for each subject. If the subject had missed a response, 16.66 ms (one screen refresh, on a standard 60 Hz monitor) was added to the duration of the target stimulus on the next trial. If the subject responded successfully, 16.66 ms was subtracted from the target stimulus’ duration on the next trial. The default duration of the target stimulus at the beginning of the task was 300 ms, and the duration increased up to a maximum of 400 ms, or down to a minimum of 200 ms. Time was also added or subtracted to the feedback stimulus proportionately in order to ensure that the total duration of the target plus the feedback was always exactly 1300 ms. Following the feedback, an inter-trial interval consisting of a fixation point was presented, which varied randomly within the range 2.2 to 10.2 s in one-second increments, on an approximately Poisson distribution (Hagberg et al., 2001). The total task duration was 12 min (plus a 10-second buffer period in the end) or 608 scanning volumes. In total there were 24 win trials and 48 neutral trials, the 2:1 ratio of win:neutral trials ensured win trials were perceived as more rewarding by participants and more money (£1) could be paid out per trial (Skumlien et al., 2023, Skumlien et al., 2022). All money won during the task was paid out to the participants at the end of the study.
2.3. Functional magnetic resonance imaging analysis
Image processing was performed using FSL version 6.0 (FMRIB’s Software Library; Oxford Centre for Functional Resonance Imaging of the Brain [FMRIB], https://www.fmrib.ox.ac.uk/fsl/). BET was used for brain extraction of the anatomical data and FSLanat was used for additional anatomical data pre-processing. Motion correction was performed with FMRIB Linear Image Registration Tool (MCFLIRT), with spatial smoothing using a Gaussian kernel of full width at half maximum (FWHM) 6 mm. High motion for a subject was defined as a mean relative root-mean square displacement that exceeds 0.5 mm. For subjects with high motion, plots of their mean and relative displacement were visually inspected and if high motion affected over 30 consecutive volumes, they were excluded from the analyses. Temporal high-pass filtering was applied with a 100 s cut-off threshold. A two-step co-registration to the subject’s individual anatomical image and an anatomical template image in standard stereotactic space (MNI152) was performed, with no temporal filtering applied.
First-level analyses were carried out in FSL’s FEAT module, data were combined using mixed effects (FLAME-1) models and analysed using the general linear model and FILM (FMRIB’s Improved Linear Model) pre-whitening. The blood-level oxygen dependent response was modelled with blocks of reward anticipation and neutral anticipation conditions as explanatory variables, with standard head motion regressors (3 translations and 3 rotations). Task regressors were convoluted with a standard Gamma function (SD = 3 s, Mean lag = 6 s), with added temporal derivative and temporal filtering to match the pre-processing steps applied to the data. Contrasts were computed to model effects of reward anticipation > neutral anticipation.
Second (group) level analyses were conducted using a whole-brain cluster-corrected significance threshold (cluster defining threshold of Z = 2.3, whole-brain family-wise error corrected p < 0.05). Our primary fMRI hypothesis concerned a group difference in the reward anticipation > neutral contrast, which we tested using a two sample t-tests, implemented in FSL’s FLAME-1 model.
We extracted single subject reward > neutral anticipation contrast estimates from bilateral striatum using an a priori striatal region of interest (ROI), which was defined using a mask derived from the CIC Neuroanatomical atlas (Tziortzi et al., 2011), to spatially match ROIs extracted from PET analyses. Data was extracted by back-projecting the ROI mask in individual subject space using FSL’s Featquery.
2.4. PET acquisition
[11C]-carfentanil, a selective MOR agonist, was synthesized following methods described previously by Ashok et al., 2019. Following a transmission CT scan, a maximum of 300 MBq of [11C]-carfentanil was administered. PET emission data were collected for 90 min in 26 frames (8 × 15 s, 3 × 60 s, 5 × 120 s, 5 × 300 s and 5 × 600 s, to a total of 5400 s). PET scans were acquired on a Siemens HiRez 6 PET/computed tomography scanner (Siemens Healthcare, Erlangen, Germany). PET and fMRI data were acquired within the same week for most participants, maximum time between scans was within a month of each other.
2.5. PET image analysis
PET data presented in this study has been published previously in Ashok et al. (2019), and the present study uses an identical analysis pipeline for PET analysis. Image pre-processing and PET modelling were carried out using MIAKAT™ software (https://www.miakat.org). Dynamic PET data were corrected for attenuation and scatter correction, and for motion by frame-by-frame realignment to frame 16. Each scan was rigid-body coregistered to the structural MRI. Regions of interest (ROIs) were defined using the same neuroanatomical atlas as described above (Tziortzi et al., 2011), applied to the PET image by non-linear deformation parameters derived using unified segmentation of the structural MRI using statistical parametric mapping (SPM12) functions implemented in MIAKAT. The template and atlas fits were confirmed visually for each participant. [11C]-carfentanil binding potential (BPND) values were quantified using the simplified reference tissue model (SRTM) with occipital lobe grey matter as the reference (Colasanti et al., 2012, Lammertsma and Hume, 1996). This approach shows good agreement on comparison with the arterial input function derived volume of distribution (Frost et al., 1985, Hirvonen et al., 2009), and the occipital cortex has negligible MOR availability (Hiller and Fan, 1996, Mick et al., 2016, Mick et al., 2014, Rabiner et al., 2011, Turton et al., 2018).
2.6. Statistical analysis
Statistical analyses of fMRI data included group-level analyses to confirm the effect of task in each group, followed by group-level analyses comparing patients and controls (both stages described above) and region of interest (ROI) analyses. Final statistical analyses were performed with SPSS (version 27) and included a primary analysis that examined group differences in parameter estimates during reward anticipation in the striatum using a two sample t-test, as well as correlations between PET and fMRI measures using Pearson’s correlation. A statistical threshold of p < 0.05 was applied to the primary analyses. Correlation coefficients were compared by transforming the r-coefficients for each group to z-scores using Fisher’s r to z transform, followed by calculating the Zobserved value using the following formula: Zobserved = (z1 – z2) / (square root of [(1/N1 −3) + (1/N2 −3)]) (Hinkle et al., 1988). A p-value of p < 0.05 corresponding to the Zobserved was used to identify r-coefficients that we’re significantly different between group. Additional analyses included Pearson’s correlations to assess relationships between measures of anhedonia (physical and social anhedonia rating scale and temporal experience of pleasure scales) and striatal activation during reward anticipation. Further exploratory analyses included examining group differences during reward anticipation in preselected ROIs spanning the hedonic network (amygdala, insula, anterior cingulate and orbitofrontal cortex) and correlations between PET and fMRI measures using Pearson’s correlation in these regions as well as in subdivisions of the striatum (nucleus accumbens, caudate, putamen, pallidum). Exploratory analyses were not corrected for multiple comparisons. All data are presented as mean ± SEM.
3. Results
For this study, one patient was excluded from the original sample published by Ashok et al. (2019) due to excessive motion during the fMRI scan. Demographic details for all participants are given in Table 1, with medication details provided in supplementary table 1. There was no significant difference between groups in age, sex, radioactive dose, and injected mass per body weight (µgm/kg) received. As expected, there was a significant group difference in anhedonia, with higher anhedonia ratings in patients (social anhedonia t(35) = 2.764, p = 0.009, physical anhedonia t(35) = 2.190, p = 0.035, two-sample t-test) and in BMI (Table 1).
Table 1.
Demographic details of subjects. BMI – body mass index, PANSS – Positive and Negative Symptom Scale, SANS – Scale for Assessment of Negative Symptoms.
|
Schizophrenia patients (n = 18) (mean ± SEM) |
Controls (n = 19) | p-value | |
|---|---|---|---|
| Age (years) | 35.6 ± 2.1 | 37.84 ± 2.6 | 0.51 |
| Gender | 18/0 | 17/2 | 0.17 |
| Injected radioactivity (MBq) | 203.0 ± 8 | 196.9 ± 10 | 0.65 |
| Injected mass per body weight (µgm/kg) | 0.023 ± 0.001 | 0.024 ± 0.001 | 0.61 |
| BMI (kg/m2) | 30.0 ± 0.80 | 25.2 ± 0.93 | 0.001* |
| Mean age at onset (years) | 22.89 ± 1.2 | n/a | n/a |
| Mean duration of illness (years) | 11.7 ± 2.3 | n/a | n/a |
| PANSS Positive Negative General Total |
14.5 ± 0.5 21.4 ± 1.1 26.7 ± 0.8 62.6 ± 2.0 |
n/a | n/a |
| SANS-25 | 55.7 ± 5 | n/a | n/a |
| Revised social anhedonia scale | 17.4 ± 2.0 | 10.2 ± 1.8 | 0.009* |
| Revised physical anhedonia scale | 23.0 ± 2.9 | 13.7 ± 3 | 0.035* |
| Temporal experience pleasure scale Anticipatory pleasure scale Consummatory pleasure scale |
37.1 ± 2.4 28.0 ± 2.4 |
42.5 ± 2 34.8 ± 1.7 |
0.36 0.028* |
| Calgary depression scale total score | 8.5 ± 1.7 | n/a | n/a |
| Reaction time (s) Neutral trial Reward anticipation trial |
0.22 ± 0.01 0.21 ± 0.01 |
0.24 ± 0.01 0.23 ± 0.01 |
0.15 0.11 |
| Accuracy in reward anticipation (fraction of total trial) |
0.57 ± 0.05 |
0.6 ± 0.13 |
0.62 |
3.1. Behavioural measures
There was no significant difference between groups in the reaction time in reward trials (Table 1). The reaction time difference between reward and neutral trials, which is a putative measure of motivational salience, also did not differ between group [patients vs controls (mean in s ± SEM): -0.0042 ± 0.004 vs −0.005 ± 0.004 respectively, t(35) = –1.508, p = 0.9, two sample t-test], there was no significant change in reaction time between neutral and reward trials in either controls (t(16) = –1.161, p = 0.26, paired t-test), patients (t(15) = –0.551, p = 0.59, paired t-test), or in the full sample (t(32) = –1.259, p = 0.22, paired t-test). There was no correlation between clinical measures and motivational salience in either group (p > 0.05, Pearson’s correlation coefficient). As shown in Table 1, patients with schizophrenia and control subjects had comparable scores on the anticipatory subscale of the TEPS (t(35) = –0.911, p = 0.36, two sample t-test) and patients had significantly lower scores on the consummatory subscale of the TEPS (t(35) = –2.289, p = 0.028, two sample t-test) than control subjects. Patients has significantly higher levels of anhedonia (Table 1), which included higher scores on revised social anhedonia scale (t(35) = 2.764, p = 0.009 and revised physical anhedonia scale (t(35) = 2.190, p = 0.035, two-sample t-test), than control subjects.
3.2. fMRI measures
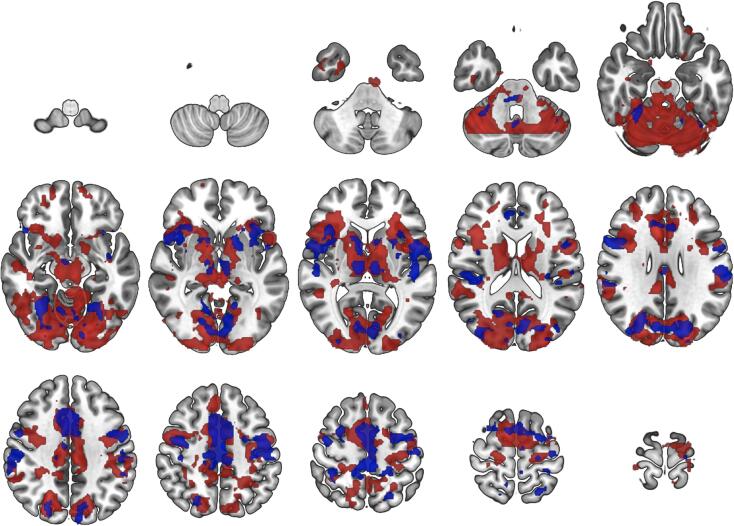
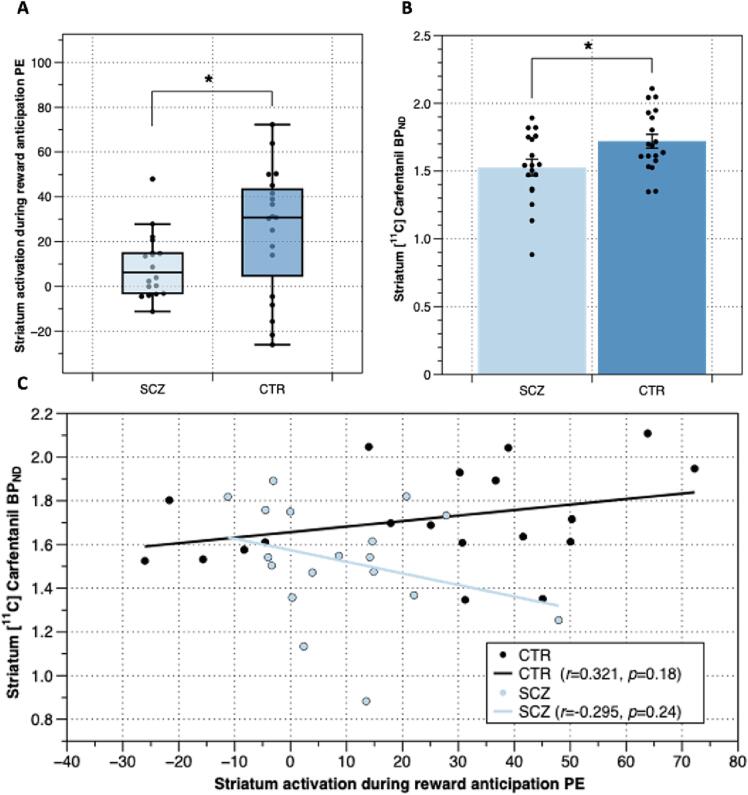
In both patients and controls, whole-brain analyses showed an effect of task in the reward > neutral anticipation condition across the frontal pole, superior, middle, inferior frontal gyrus, cingulate gyrus, striatum, temporal gyrus, insular cortex, amygdala, thalamus and occipital cortex in both patients and controls, as seen in Fig. 1 and supplementary Fig. 2. In patients with schizophrenia there was significant hypoactivation at the whole-brain level spanning parietal, occipital and cerebellar regions as well as the thalamus, caudate, putamen and amygdala relative to controls, as seen in supplementary Fig. 2. Patients did not show increased activation for reward > neutral anticipation in any brain region compared to healthy controls at the whole-brain level. Striatal activation during reward anticipation was significantly lower in patients with schizophrenia in the ROI analyses (t(35) = –2.084, p = 0.044, uncorrected, independent t-test), as seen in Fig. 2a. Exploratory ROI analyses showed that compared to controls patients had significantly lower activation during reward anticipation in the amygdala (t(35) = –2.518, p = 0.017, uncorrected, independent t-test, supplementary figure 3) and orbitofrontal cortex (t(35) = –2.193, p = 0.035, uncorrected, independent t-test, supplementary figure 4) and comparable activation in the insular and anterior cingulate cortices (supplementary figures 5 and 6).
Fig. 1.
Group-level effects of reward anticipation during the monetary incentive delay task in control subjects (red) and patients with schizophrenia (blue). Images show statistical Z-maps (Gaussianised T/F) of brain regions showing significantl activation during reward anticipation (reward anticipation > neutral anticipation) in patients with schizophrenia (n = 18, shown in blue) and control subjects (n = 19, shown in red). Images thresholded at Z > 3.0 and a whole-brain corrected cluster significance threshold of p = 0.05, for Z > 2.3 see supplementary Fig. 2 Slices shown are z = -64––58 −52––46 −40––34; −28––20 −14––8 −2 4; 10 18 24 30 36 42; 48 54 62 68 74 80.
Fig. 2.
Striatal reward anticipation related activation and mu opioid receptor (MOR) density in the striatum in patients with schizophrenia and respective controls: A) Lower Mean parameter estimate values during reward > neutral anticipation in the striatum in patients with schizophrenia (n = 18) compared to respective controls (n = 19) (p = 0.044, paired t-test). B) Mean MOR density measures as [11C]Cafentanil BPND in the striatum in patients with schizophrenia and respective controls (p = 0.021, independent t-test). C) Plot relating [11C]Cafentanil BPND and parameter estimate values for the reward anticipation condition in the striatum patients with schizophrenia, shown in blue and respective control subjects shown in black. Trend lines indicate lack of significant association between MOR availability and activation in the striatum (Pearson's Correlation, controls r = 0.321, p > 0.05, patients r = 0.295, p > 0.05). * indicates p < 0.05.
3.3. Correlation between PET and fMRI measures
We again report that our patient sample also had significantly lower MOR availability in the striatum relative to controls (Ashok et al., 2019), shown in Fig. 2b (t(35) = –2.422, p = 0.021, independent t-test). In the striatum, there was no association between MOR availability and the parameter estimate values during reward > neutral anticipation in either patients or controls, as shown in Fig. 2c (Pearson's Correlation, controls df = 17, r = 0.321, p = 18, patients df = 16, r = –0.295, p = 0.24). Correlation coefficients in patients were significantly lower than in controls (Zobserved = 1.778, p = 0.038). There were no associations between MOR availability and the parameter estimate values during reward > neutral anticipation in any of the striatal subdivisions (nucleus accumbens, caudate, putamen, pallidum) in either group (supplementary table 4). There was no association between imaging measures (MOR availability/ fMRI parameter estimates) and the reaction-time-based measure of motivational salience in either group (Pearson’s coefficient, p > 0.05, Table 2). Exploratory analyses conducted on the amygdala, orbitofrontal, insular and anterior cingulate cortices all showed comparable MOR availability between patients and controls (amygdala t(35) = –1.557, orbitofrontal cortex t(35) = –1.956, insular cortex t(35) = –1.385, anterior cingulate cortex t(35) = –1.469, all p > 0.05, independent t-tests) and no association between MOR availability and parameter estimate values during reward > neutral anticipation in either patients or controls in any of the regions analysed (supplementary figures 3–6), with comparable correlation coefficients the two groups (All Zobserved < 1.5, p > 0.05).
Table 2.
Associations between striatal activation during reward anticipation, striatal Mu Opioid Receptor (MOR) availability and measures of hedonic responses/symptoms. BPND – binding potential, PANSS – positive and negative symptoms scale, SANS – scale for the assessment of negative symptoms, TEPS – temporal experience of pleasure scale.
| Measure |
Striatal Reward > neutral anticipation parameter estimate |
Striatal MOR BPND |
||||||
|---|---|---|---|---|---|---|---|---|
| SCZ |
CTR |
SCZ |
CTR |
|||||
| r | p | r | p | r | p | r | p | |
| PANSS | −0.196 | 0.43 | n/a | n/a | −0.014 | 0.96 | n/a | n/a |
| PANSS-positive | −0.172 | 0.50 | n/a | n/a | 0.081 | 0.75 | n/a | n/a |
| PANSS-negative | −0.120 | 0.63 | n/a | n/a | −0.065 | 0.80 | n/a | n/a |
| PANSS-general | −0.216 | 0.39 | n/a | n/a | 0.008 | 0.97 | n/a | n/a |
| SANS-25 | 0.106 | 0.32 | n/a | n/a | −0.166 | 0.51 | n/a | n/a |
| Revised social anhedonia | −0.365 | 0.14 | 0.299 | 0.21 | 0.334 | 0.16 | 0.379 | 0.11 |
| Revised physical anhedonia | −0.231 | 0.36 | 0.281 | 0.24 | 0.197 | 0.43 | 0.281 | 0.24 |
| TEPS anticipatory pleasure scale | 0.383 | 0.12 | −0.270 | 0.26 | 0.109 | 0.67 | −0.451 | 0.05 |
| TEPS consummatory pleasure scale | 0.188 | 0.46 | −0.244 | 0.31 | 0.246 | 0.33 | −0.244 | 0.31 |
| Reaction time (neutral) | −0.254 | 0.34 | −0.088 | 0.74 | −0.215 | 0.42 | −0.039 | 0.88 |
| Reaction time (anticipation) | −0.139 | 0.61 | −0.286 | 0.27 | −0.198 | 0.46 | −0.284 | 0.27 |
| Accuracy in reward anticipation | −0.08 | 0.77 | 0.309 | 0.23 | −0.102 | 0.71 | −0.088 | 0.74 |
3.4. Correlation between behavioural measures and neuroimaging measures
In our patient group, we found no associations between striatal MOR availability and negative symptoms as measured by the PANSS and SANS, and there were no associations between striatal activation during reward anticipation and negative symptoms (see Table 2). We found no relationships between striatal MOR availability and the anticipatory or consummatory temporal experience of pleasure scale in either patients or controls (Table 2).
4. Discussion
Our study shows that patients with schizophrenia with moderate negative symptoms that have significantly lower MOR availability in the striatum also have striatal hypoactivation during reward anticipation. We further show there is no association between MOR availability and striatal neural activity during reward anticipation in both patients and in healthy controls. While correlation coefficients for this relationship differed between groups, our results suggest mu-opioid receptor levels are unlikely to be a major determinant of neural activity during reward processing, which extends prior knowledge on the role of striatal opioid signalling during reward in both schizophrenia and the healthy brain.
In the sample as a whole, reward anticipation was associated with neural activation in superior frontal gyrus, middle frontal gyrus, paracentral lobule, inferior temporal gyrus, left occipital gyrus, parahippocampal gyrus, insula, nucleus accumbens, caudate, putamen, thalamus, amygdala, and anterior cingulate cortex. The effect of task in our study is consistent with findings of a meta-analyses of data from subjects performing the monetary incentive delay task, which identified similar spatial patterns of activity related to reward anticipation (Jauhar et al., 2021, Oldham et al., 2018, Wilson et al., 2018). We found hypoactivation of the striatum during reward anticipation in schizophrenia patients compared to controls, consistent with previous findings of lower striatal response in schizophrenia during reward anticipation (Zeng et al., 2022). However, in our sample striatal hypoactivation was not associated with severity of negative symptoms, contrary to findings of a recent meta-analysis of the MID tasks in schizophrenia (Zeng et al., 2022). A possible explanation for this is that our sample may lack the power to detect this association.
Our findings in control subjects suggest that interindividual differences in MOR availability do not underlie interindividual differences in striatal activation during monetary reward anticipation. However, previous multimodal neuroimaging studies suggest that the opioid system plays a key role in regulating reward function. This contrasts with findings of an inverse relationship between MOR availability in ventral striatum, amygdala and hypothalamus and activation of these regions during food reward processing (Nummenmaa et al., 2018). The difference between our finding with a monetary reward task and this finding with a food stimulus may suggest that monetary and food stimuli have differential neural regulation. Additionally, a lack of association between MOR availability in the striatum and reward activation, does not exclude the possibility that opioid release may play a role in modulating striatal reward function. For example, work by Saanijoki et al. (2018) showed that exercise-induced changes in MOR binding negatively correlated with the reward anticipation signal measured using fMRI for palatable food stimuli in orbitofrontal and cingulate cortices, insula, ventral striatum, amygdala, and thalamus (Saanijoki et al., 2018). Future studies examining the association of opioid release in schizophrenia and reward anticipation neural activity would extend our findings and understanding of the functional consequences of altered opioid signalling in schizophrenia.
A consideration in our study is that patients were taking antipsychotic medication, largely second-generation drugs. Evidence suggests that second generation antipsychotics may normalise reward-related striatal neural responses in patients with schizophrenia (Juckel et al., 2006, Nielsen et al., 2018). For example, Juckel et al. reported a reduction in ventral striatal activation during reward anticipation in patients treated with first-generation antipsychotic but not in patients treated with second-generation antipsychotics (Juckel et al., 2006). Another study showed switching patients from first generation to second-generation antipsychotics (Schlagenhauf et al., 2008) and treatment of medication naïve first episode patients with second-generation antipsychotics normalized activation deficits in the striatum (Nielsen et al., 2012). Other cross-sectional, studies have also reported no difference in striatal activation during activation of monetary reward in patients treated with second-generation antipsychotics (Mucci et al., 2015, Walter et al., 2009) and a recent meta-analysis of MID fMRI studies found that striatal hypoactivation was positivity associated with the percentage of second-generation antipsychotic users (Zeng et al., 2022). Whilst we cannot exclude the possibility that treatment had reduced the altered neural response to reward in the patients, we nevertheless found significantly lower activation in patients, suggesting that reward processing deficits persisted in our sample.
Given the role of MOR in reward processing, a remaining question is whether MOR deficits may underlie dysfunction in consummatory reward, which is also reported in patients with schizophrenia. In our study, we found no association between reduced MOR availability and lower scores on the consummatory pleasure subscale of the Temporal Experience Pleasure Scale (TEPS). However, given that the fMRI task we used was not designed to probe the neural basis of consummatory reward, the relationship between MOR and consummatory reward dysfunction in schizophrenia remains a question for future research.
Overall, our findings suggest that reward anticipation is not driven by MOR density in control subjects and that lower MOR availability in schizophrenia does not underlie striatal hypoactivation during reward anticipation in patients. One alternative biological mechanism that could drive striatal hypoactivity during reward anticipation in schizophrenia may be dysfunctional dopaminergic signalling (Schott et al., 2008).
5. Conclusions
To conclude, there is hypoactivation of the striatum in schizophrenia patients during reward anticipation, but this isn’t associated with lower striatal MOR availability in this patient group or in healthy controls, suggesting other neurochemical mechanisms underlie altered reward processing in schizophrenia.
6. Funding/support
This study was funded by grants MC-A656-5QD30 from the Medical Research Council-UK, 666 from the Maudsley Charity 094849/Z/10/Z from the Brain and Behavior Research Foundation, and Wellcome Trust to Dr Howes and King’s College London scholarship to Dr Ashok.
7. Role of the funder/sponsor
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
CRediT authorship contribution statement
Ekaterina Shatalina: Methodology, Investigation, Formal analysis, Data curation, Writing – original draft, Writing – review & editing, Visualization. Abhishekh H. Ashok: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Writing – original draft, Writing – review & editing, Project administration. Matthew B. Wall: Methodology, Formal analysis, Investigation, Writing – review & editing, Supervision. Matthew M. Nour: Methodology, Writing – review & editing. Jim Myers: Methodology, Data curation, Formal analysis, Writing – review & editing. Tiago Reis Marques: Supervision, Funding acquisition, Writing – review & editing. Eugenii A. Rabiner: Supervision, Methodology, Writing – review & editing. Oliver D. Howes: Conceptualization, Supervision, Funding acquisition, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Conflict of Interest Disclosure: Dr Ashok conducts research funded by the National Institute of Health Research (Reference number: NIHR ACF-2019-14-004). Prof Howes conducts research funded by the Medical Research Council (UK), the National Institute of Health Research (UK) and the Maudsley Charity. Prof Howes has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organized by Astra-Zeneca, BMS, Eli Lilly, Janssen, Lundbeck, Lyden-Delta, Servier, and Roche. Neither Prof Howes or his family have been employed by or have holdings/a financial stake in any biomedical company. Dr Wall’s and Dr Rabiner's primary employer is Invicro , a private company which performs contract research work for the pharmaceutical and biotechnology industries.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2023.103481.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Addington D., Addington J., Schissel B. A depression rating scale for schizophrenics. Schizophr. Res. 1990;3(4):247–251. doi: 10.1016/0920-9964(90)90005-r. [DOI] [PubMed] [Google Scholar]
- Ashok A.H., Myers J., Reis Marques T., Rabiner E.A., Howes O.D. Reduced mu opioid receptor availability in schizophrenia revealed with [11C]-carfentanil positron emission tomographic Imaging. Nat. Commun. 2019;10:1–9. doi: 10.1038/s41467-019-12366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A., Grecksch G., Kraus J., Loh H.H., Schroeder H., Hollt V. Rewarding effects of ethanol and cocaine in mu opioid receptor-deficient mice. Naunyn. Schmiedebergs Arch. Pharmacol. 2002;365:296–302. doi: 10.1007/s00210-002-0533-2. [DOI] [PubMed] [Google Scholar]
- Bloom F., Segal D., Ling N., Guillemin R. Endorphins: profound behavioral effects in rats suggest new etiological factors in mental illness. Science. 1976;194(4265):630–632. doi: 10.1126/science.185694. [DOI] [PubMed] [Google Scholar]
- Clark S.D., Abi-Dargham A. The role of dynorphin and the kappa opioid receptor in the symptomatology of schizophrenia: a review of the evidence. Biol. Psychiatry. 2019;86(7):502–511. doi: 10.1016/j.biopsych.2019.05.012. [DOI] [PubMed] [Google Scholar]
- Colasanti A., Searle G.E., Long C.J., Hill S.P., Reiley R.R., Quelch D., Erritzoe D., Tziortzi A.C., Reed L.J., Lingford-Hughes A.R., Waldman A.D., Schruers K.R.J., Matthews P.M., Gunn R.N., Nutt D.J., Rabiner E.A. Endogenous opioid release in the human brain reward system induced by acute amphetamine administration. Biol. Psychiatry. 2012;72(5):371–377. doi: 10.1016/j.biopsych.2012.01.027. [DOI] [PubMed] [Google Scholar]
- Comfort A. Morphine as an antipsychotic? Clin. Toxicol. 1977;11(4):383–386. doi: 10.3109/15563657708988200. [DOI] [PubMed] [Google Scholar]
- Contet C., Kieffer B.L., Befort K. Mu opioid receptor: a gateway to drug addiction. Curr. Opin. Neurobiol. 2004;14(3):370–378. doi: 10.1016/j.conb.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Deserno L., Heinz A., Schlagenhauf F. Computational approaches to schizophrenia: a perspective on negative symptoms. Schizophr. Res. 2017;186:46–54. doi: 10.1016/j.schres.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Frost J.J., Wagner H.N., Dannals R.F., Ravert H.T., Links J.M., Wilson A.A., Burns H.D., Wong D.F., McPherson R.W., Rosenbaum A.E., Kuhar M.J., Snyder S.H. Imaging opiate receptors in the human brain by positron tomography. J. Comput. Assist. Tomogr. 1985;9(2):231–236. doi: 10.1097/00004728-198503000-00001. [DOI] [PubMed] [Google Scholar]
- Galderisi S., Mucci A., Buchanan R.W., Arango C. Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry. 2018;5(8):664–677. doi: 10.1016/S2215-0366(18)30050-6. [DOI] [PubMed] [Google Scholar]
- Gard D.E., Gard M.G., Kring A.M., John O.P. Anticipatory and consummatory components of the experience of pleasure: a scale development study. J. Res. Pers. 2006;40(6):1086–1102. [Google Scholar]
- Gold J.M., Waltz J.A., Matveeva T.M., Kasanova Z., Strauss G.P., Herbener E.S., Collins A.G., Frank M.J. Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Arch. Gen. Psychiatry. 2012;69:129–138. doi: 10.1001/archgenpsychiatry.2011.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg G.E., Zito G., Patria F., Sanes J.N. Improved detection of event-related functional MRI signals using probability functions. Neuroimage. 2001;14(5):1193–1205. doi: 10.1006/nimg.2001.0880. [DOI] [PubMed] [Google Scholar]
- Hall F.S., Sora I., Uhl G.R. Ethanol consumption and reward are decreased in mu-opiate receptor knockout mice. Psychopharmacology (Berl) 2001;154:43–49. doi: 10.1007/s002130000622. [DOI] [PubMed] [Google Scholar]
- Hiller J.M., Fan L.-Q. Laminar distribution of the multiple opioid receptors in the human cerebral cortex. Neurochem. Res. 1996;21(11):1333–1345. doi: 10.1007/BF02532374. [DOI] [PubMed] [Google Scholar]
- Hinkle D.E., Wiersma W., Jurs S.G. Houghton Mifflin; 1988. Solutions Manual: Applied Statistics for the Behavioral Sciences. [Google Scholar]
- Hirvonen J., Aalto S., Hagelberg N., Maksimow A., Ingman K., Oikonen V., Virkkala J., Nagren K., Scheinin H. Measurement of central mu-opioid receptor binding in vivo with PET and [11C]carfentanil: a test-retest study in healthy subjects. Eur. J. Nucl. Med. Mol. Imaging. 2009;36:275–286. doi: 10.1007/s00259-008-0935-6. [DOI] [PubMed] [Google Scholar]
- Hnasko T.S., Sotak B.N., Palmiter R.D. Morphine reward in dopamine-deficient mice. Nature. 2005;438(7069):854–857. doi: 10.1038/nature04172. [DOI] [PubMed] [Google Scholar]
- Jacquet Y.F., Marks N. The C-fragment of β-lipotropin: an endogenous neuroleptic or antipsychotogen? Science. 1976;194(4265):632–635. doi: 10.1126/science.185695. [DOI] [PubMed] [Google Scholar]
- Jauhar S., Fortea L., Solanes A., Albajes-Eizagirre A., McKenna P.J., Radua J., Jung W.H. Brain activations associated with anticipation and delivery of monetary reward: A systematic review and meta-analysis of fMRI studies. PLoS One. 2021;16(8):e0255292. doi: 10.1371/journal.pone.0255292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G., Schlagenhauf F., Koslowski M., Filonov D., Wüstenberg T., Villringer A., Knutson B., Kienast T., Gallinat J., Wrase J., Heinz A. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology (Berl) 2006;187(2):222–228. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- Kaar S.J., Natesan S., McCutcheon R., Howes O.D. Antipsychotics: mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology. 2020;172:107704. doi: 10.1016/j.neuropharm.2019.107704. [DOI] [PubMed] [Google Scholar]
- Kas M.J.H., van den Bos R., Baars A.M., Lubbers M., Lesscher H.M.B., Hillebrand J.J.G., Schuller A.G., Pintar J.E., Spruijt B.M. Mu-opioid receptor knockout mice show diminished food-anticipatory activity. Eur. J. Neurosci. 2004;20(6):1624–1632. doi: 10.1111/j.1460-9568.2004.03581.x. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kluge A., Kirschner M., Hager O.M., Bischof M., Habermeyer B., Seifritz E., Walther S., Kaiser S. Combining actigraphy, ecological momentary assessment and neuroimaging to study apathy in patients with schizophrenia. Schizophr. Res. 2018;195:176–182. doi: 10.1016/j.schres.2017.09.034. [DOI] [PubMed] [Google Scholar]
- Knutson B., Westdorp A., Kaiser E., Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12(1):20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Lammertsma A.A., Hume S.P. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4(3):153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Leroy A., Amad A., D'Hondt F., Pins D., Jaafari N., Thomas P., Jardri R. Reward anticipation in schizophrenia: a coordinate-based meta-analysis. Schizophr. Res. 2020;218:2–6. doi: 10.1016/j.schres.2019.12.041. [DOI] [PubMed] [Google Scholar]
- Lobo M.C., Whitehurst T.S., Kaar S.J., Howes O.D. New and emerging treatments for schizophrenia: a narrative review of their pharmacology, efficacy and side effect profile relative to established antipsychotics. Neurosci. Biobehav. Rev. 2022;132:324–361. doi: 10.1016/j.neubiorev.2021.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loseth G.E., Ellingsen D.M., Leknes S. State-dependent mu-opioid modulation of social motivation. Front. Behav. Neurosci. 2014;8:430. doi: 10.3389/fnbeh.2014.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick I., Myers J., Stokes P.R.A., Erritzoe D., Colasanti A., Bowden-Jones H., Clark L., Gunn R.N., Rabiner E.A., Searle G.E., Waldman A.D., Parkin M.C., Brailsford A.D., Nutt D.J., Lingford-Hughes A.R. Amphetamine induced endogenous opioid release in the human brain detected with [(1)(1)C]carfentanil PET: replication in an independent cohort. Int. J. Neuropsychopharmacol. 2014;17(12):2069–2074. doi: 10.1017/S1461145714000704. [DOI] [PubMed] [Google Scholar]
- Mick I., Myers J., Ramos A.C., Stokes P.R.A., Erritzoe D., Colasanti A., Gunn R.N., Rabiner E.A., Searle G.E., Waldman A.D., Parkin M.C., Brailsford A.D., Galduróz J.C.F., Bowden-Jones H., Clark L., Nutt D.J., Lingford-Hughes A.R. Blunted endogenous opioid release following an oral amphetamine challenge in pathological gamblers. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2016;41(7):1742–1750. doi: 10.1038/npp.2015.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A., Kieffer B.L., D'Amato F.R. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304:1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- Mucci A., Dima D., Soricelli A., Volpe U., Bucci P., Frangou S., Prinster A., Salvatore M., Galderisi S., Maj M. Is avolition in schizophrenia associated with a deficit of dorsal caudate activity? A functional magnetic resonance imaging study during reward anticipation and feedback. Psychol. Med. 2015;45(8):1765–1778. doi: 10.1017/S0033291714002943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M.O., Rostrup E., Wulff S., Bak N., Broberg B.V., Lublin H., Kapur S., Glenthoj B. Improvement of brain reward abnormalities by antipsychotic monotherapy in schizophrenia. Arch. Gen. Psychiatry. 2012;69:1195–1204. doi: 10.1001/archgenpsychiatry.2012.847. [DOI] [PubMed] [Google Scholar]
- Nielsen M.Ø., Rostrup E., Broberg B.V., Wulff S., Glenthøj B. Negative symptoms and reward disturbances in schizophrenia before and after antipsychotic monotherapy. Clin. EEG Neurosci. 2018;49(1):36–45. doi: 10.1177/1550059417744120. [DOI] [PubMed] [Google Scholar]
- Norman H., D’Souza M.S. Endogenous opioid system: a promising target for future smoking cessation medications. Psychopharmacology (Berl) 2017;234(9-10):1371–1394. doi: 10.1007/s00213-017-4582-0. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L., Saanijoki T., Tuominen L., Hirvonen J., Tuulari J.J., Nuutila P., Kalliokoski K. mu-opioid receptor system mediates reward processing in humans. Nat. Commun. 2018;9:1500. doi: 10.1038/s41467-018-03848-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldham S., Murawski C., Fornito A., Youssef G., Yücel M., Lorenzetti V. The anticipation and outcome phases of reward and loss processing: a neuroimaging meta-analysis of the monetary incentive delay task. Hum Brain Mapp. 2018;39(8):3398–3418. doi: 10.1002/hbm.24184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F., Kieffer B.L., Tabarin A., Contarino A. Decreased motivation to eat in mu-opioid receptor-deficient mice. Eur. J. Neurosci. 2007;25:3398–3405. doi: 10.1111/j.1460-9568.2007.05595.x. [DOI] [PubMed] [Google Scholar]
- Quednow B.B., Csomor P.A., Chmiel J., Beck T., Vollenweider F.X. Sensorimotor gating and attentional set-shifting are improved by the μ-opioid receptor agonist morphine in healthy human volunteers. The International Journal of Neuropsychopharmacology. 2008;11:655–669. doi: 10.1017/S1461145707008322. [DOI] [PubMed] [Google Scholar]
- Quelch D.R., Mick I., McGonigle J., Ramos A.C., Flechais R.S.A., Bolstridge M., Rabiner E., Wall M.B., Newbould R.D., Steiniger-Brach B., van den Berg F., Boyce M., Østergaard Nilausen D., Breuning Sluth L., Meulien D., von der Goltz C., Nutt D., Lingford-Hughes A. Nalmefene reduces reward anticipation in alcohol dependence: an experimental functional magnetic resonance imaging study. Biol. Psychiatry. 2017;81(11):941–948. doi: 10.1016/j.biopsych.2016.12.029. [DOI] [PubMed] [Google Scholar]
- Rabiner E.A., Beaver J., Makwana A., Searle G., Long C., Nathan P.J., Newbould R.D., Howard J., Miller S.R., Bush M.A., Hill S., Reiley R., Passchier J., Gunn R.N., Matthews P.M., Bullmore E.T. Pharmacological differentiation of opioid receptor antagonists by molecular and functional imaging of target occupancy and food reward-related brain activation in humans. Mol. Psychiatry. 2011;16(8):826–835. doi: 10.1038/mp.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radua J., Schmidt A., Borgwardt S., Heinz A., Schlagenhauf F., McGuire P., Fusar-Poli P. Ventral striatal activation during reward processing in psychosis: a neurofunctional meta-analysis. JAMA Psychiat. 2015;72:1243–1251. doi: 10.1001/jamapsychiatry.2015.2196. [DOI] [PubMed] [Google Scholar]
- Saanijoki T., Nummenmaa L., Tuulari J.J., Tuominen L., Arponen E., Kalliokoski K.K., Hirvonen J. Aerobic exercise modulates anticipatory reward processing via the μ-opioid receptor system. Hum. Brain Mapp. 2018;39(10):3972–3983. doi: 10.1002/hbm.24224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlagenhauf F., Juckel G., Koslowski M., Kahnt T., Knutson B., Dembler T., Kienast T., Gallinat J., Wrase J., Heinz A. Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine. Psychopharmacology (Berl) 2008;196(4):673–684. doi: 10.1007/s00213-007-1016-4. [DOI] [PubMed] [Google Scholar]
- Schmauss C., Emrich H.M. Dopamine and the action of opiates: a reevaluation of the dopamine hypothesis of schizophrenia with special consideration of the role of endogenous opioids in the pathogenesis of schizophrenia. Biol. Psychiatry. 1985;20(11):1211–1231. doi: 10.1016/0006-3223(85)90179-9. [DOI] [PubMed] [Google Scholar]
- Schott B.H., Minuzzi L., Krebs R.M., Elmenhorst D., Lang M., Winz O.H., Seidenbecher C.I., Coenen H.H., Heinze H.-J., Zilles K., Düzel E., Bauer A. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J. Neurosci. 2008;28(52):14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selleck R.A., Baldo B.A. Feeding-modulatory effects of mu-opioids in the medial prefrontal cortex: a review of recent findings and comparison to opioid actions in the nucleus accumbens. Psychopharmacology (Berl) 2017;234(9-10):1439–1449. doi: 10.1007/s00213-016-4522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skumlien M., Mokrysz C., Freeman T.P., Wall M.B., Bloomfield M., Lees R., Borissova A., Petrilli K., Carson J., Coughlan T., Ofori S., Langley C., Sahakian B.J., Curran H.V., Lawn W. Neural responses to reward anticipation and feedback in adult and adolescent cannabis users and controls. Neuropsychopharmacology. 2022;47(11):1976–1983. doi: 10.1038/s41386-022-01316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skumlien M., Freeman T.P., Hall D., Mokrysz C., Wall M.B., Ofori S., Petrilli K., Trinci K., Borissova A., Fernandez-Vinson N., Langley C., Sahakian B.J., Curran H.V., Lawn W. The effects of acute cannabis with and without cannabidiol on neural reward anticipation in adults and adolescents. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2023;8(2):219–229. doi: 10.1016/j.bpsc.2022.10.004. [DOI] [PubMed] [Google Scholar]
- Strauss G.P., Waltz J.A., Gold J.M. A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull. 2014;40(Suppl 2):S107–S116. doi: 10.1093/schbul/sbt197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenius L., Wahlström A., Lindström L., Widerlöv E. Increased CSF levels of endorphines in chronic psychosis. Neurosci. Lett. 1976;3(3):157–162. doi: 10.1016/0304-3940(76)90086-0. [DOI] [PubMed] [Google Scholar]
- Turton S., Myers J.FM., Mick I., Colasanti A., Venkataraman A., Durant C., Waldman A., Brailsford A., Parkin M.C., Dawe G., Rabiner E.A., Gunn R.N., Lightman S.L., Nutt D.J., Lingford-Hughes A. Blunted endogenous opioid release following an oral dexamphetamine challenge in abstinent alcohol-dependent individuals. Mol. Psychiatry. 2020;25(8):1749–1758. doi: 10.1038/s41380-018-0107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziortzi A.C., Searle G.E., Tzimopoulou S., Salinas C., Beaver J.D., Jenkinson M., Laruelle M., Rabiner E.A., Gunn R.N. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. Neuroimage. 2011;54(1):264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Walter H., Kammerer H., Frasch K., Spitzer M., Abler B. Altered reward functions in patients on atypical antipsychotic medication in line with the revised dopamine hypothesis of schizophrenia. Psychopharmacology (Berl) 2009;206(1):121–132. doi: 10.1007/s00213-009-1586-4. [DOI] [PubMed] [Google Scholar]
- Wilson R.P., Colizzi M., Bossong M.G., Allen P., Kempton M., Bhattacharyya S. The neural substrate of reward anticipation in health: a meta-analysis of fMRI findings in the monetary incentive delay task. Neuropsychol. Rev. 2018;28(4):496–506. doi: 10.1007/s11065-018-9385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J., Yan J., Cao H., Su Y., Song Y., Luo Y., Yang X. Neural substrates of reward anticipation and outcome in schizophrenia: a meta-analysis of fMRI findings in the monetary incentive delay task. Transl. Psychiatry. 2022;12:1–14. doi: 10.1038/s41398-022-02201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Lin P., Shi H., Öngür D., Auerbach R.P., Wang X., Yao S., Wang X. Mapping anhedonia-specific dysfunction in a transdiagnostic approach: an ALE meta-analysis. Brain Imaging Behav. 2016;10(3):920–939. doi: 10.1007/s11682-015-9457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.