Highlights
-
•
Organoids are excellent preclinical models that retain genome stability and the tumor microenvironment.
-
•
GC organoids are of great value in basic life science research and clinical research such as organ development and drug susceptibility screening.
-
•
Cisplatin resistance worsens the prognosis of gastric cancer patients.
-
•
ATR inhibition can sensitize cisplatin in vivo and in vitro by inhibiting DDR.
Keywords: ATR inhibitor, Cisplatin, Gastric cancer, Chemoresistance, Organoid
Abstract
Background
Chemoresistance is a common event after cancer chemotherapy, including gastric cancer (GC). Cisplatin has been reported to induce the DNA damage response (DDR), thus leading to chemoresistance. VE-821, a specific inhibitor of ATR, has been proven to suppress a variety of solid malignancies effectively. Our study aimed to explore the effect of VE-821 on enhancing the chemical sensitivity to cisplatin and clarify the potential molecular mechanisms.
Methods
Cell viability and apoptosis of MKN-45 and AGS were measured by CCK8 and flow cytometry assay respectively. Western blotting was used to detect the expression of target proteins. TCGA database was used to analyze the correlation between the ATR expression with the prognosis of GC patients. The viability of GC organoids was detected by Cell Titer Glo (CTG) through luminescence.
Results
Cisplatin inhibited the proliferation and induced apoptosis of GC cells with a relatively high IC50 value, and increased the phosphorylation levels of ATR-CHK1 and H2AX. VE-821 achieved the same effects but by downregulating the phosphorylation levels of the ATR-CHK1 pathway. Besides, higher ATR expression in GC tissues was positively correlated with higher pathological stage in GC patients. Interestingly, ATR inhibition reversed cisplatin-induced STAT3 activation and enhanced H2AX levels. Moreover, VE-821 significantly sensitized GC cells to cisplatin, and these two drugs had synergistic effects in GC cell lines, organoids, and in vivo.
Conclusion
Our results suggested VE-821 sensitized GC cells to cisplatin via reversing DDR activation. And VE-821 treatment may be a promising therapeutic strategy for GC patients with cisplatin resistance.
Introduction
Gastric cancer (GC) is one of the most malignant tumors in the world. Its morbidity rate ranks fifth in the world, and its death rate ranks fourth in the world. The incidence is increasing every year, especially among young adults (aged<50 years) [1]. Major risk factors for gastric cancer include Helicobacter pylori infection, age, high salt intake, low vegetable and high fat diet [1,2]. According to the Laurén classification, GC is pathologically divided into intestinal, diffuse, and indeterminate types [3]. Currently, early GC can be treated with endoscopic resection, non-early operable GC is treated with surgery, and advanced GC is usually treated with first-line platinum (cisplatin or oxaliplatin) and fluoropyrimidine (5-fluorouracil or cephalosporin), or perioperative chemotherapy followed by double sequential chemotherapy or surgery. Although different treatment methods can be used for advanced GC, the median survival time of patients is only about 1 year, and the prognosis is poor. One of the major factors is chemotherapy resistance, which is a common event after cancer chemotherapy [2,3]. Therefore, new methods to sensitize GC cells to chemotherapy are urgently needed.
During the process of cell proliferation and differentiation, the DNA inside the cell is constantly damaged in various ways. Intracellular DNA can be affected by a variety of endogenous events, such as transcriptional replication disturbances or reactive oxygen species (ROS), and exogenous factors, such as chemotherapy, ultraviolet radiation, and ionizing radiation [4]. To maintain the integrity of the genome, cells have evolved a DNA damage response (DDR) that detects and repairs DNA damage and interacts with checkpoints in the cell cycle to reduce cell proliferation [5]. Three core kinases in the phosphatidylinositol 3-kinase-related kinase family act as DNA damage responders (DDRs) during the cell cycle, including ATM (ataxia telangiectasia mutated), ATR (ataxia-telangiectasia, and Rad3-related), and DNA-PKcs (DNA-dependent protein kinase catalytic subunit) [5,6]. Due to the mechanism of action of DDR, radiotherapy and most chemotherapy drugs can damage DNA and only partially suppress tumors.
Platinum-based drugs, especially cisplatin, are used to treat most solid malignancies, including GC, where DNA damage is one of the key anticancer modes of action [7]. Cisplatin binds to DNA, forms intrastrand and interstrand crosslinks, disrupts the DNA double helix structure, and prevents DNA replication and transcription. Cisplatin-DNA crosslinking and adduct formation induces DNA damage and promotes apoptosis [7,8]. Activation of the DDR signaling pathway following cisplatin-induced DNA damage is a key factor leading to cisplatin resistance [7,9]. Once DNA is damaged, the ATR-CHK1 and ATM-CHK2 signaling pathways halt cell cycle progression at the G2/M and G1/S checkpoints, respectively, to allow sufficient time for DNA repair. Activation of the ATR-CHK1 signaling pathway initiates cell cycle arrest, repairs DNA and stabilizes replication forks [10]. Inhibition of ATR accelerates the development of DNA damaged cells in G2 phase to M phase, disrupting DNA repair; DNA damaged cells cannot divide to the next generation, but enter mitotic cell disaster [11].
VE-821 is one of the ATR inhibitors, which can effectively inhibit the signal transduction of ATR-CHK1 pathway with high selectivity and specificity. Thus, VE-821 inhibits the activation of S-phase and G2/M-phase checkpoints, leading to the progression of cells into mitosis and ultimately resulting in cell death. Research has shown that certain chemotherapeutic drugs, which are resistant due to DNA damage repair caused by ATR-CHK1, can be made more effective in killing tumors by combining them with VE-821. This has been observed in various types of tumors, including ovarian cancer, pancreatic cancer, lung cancer, breast cancer and esophageal squamous cell carcinoma [12], [13], [14], [15], [16]. But there are few reports that VE-821 increases the chemosensitivity of gastric cancer.
STAT3 (Signal Transducer and Activator of Transcription 3) is a transcription factor that plays an important role in various biological processes such as inflammation, metabolism and tumorigenesis [17], [18], [19]. Cumulated evidences support that STAT3 is essential for tumor cells proliferation and apoptosis, even more, high expression of STAT3 usually links to poor prognosis of patients [20,21]. Constitutively activation of STAT3 by phosphorylation can regulate various STAT3 target genes such as Bcl-2, c-Myc, cyclin D1 and other proteins affecting apoptosis and the cell cycle [22,23], meanwhile inactivate tumor-suppressor gene p53 [23]. In addition, a previous study by our laboratory indicated that STAT3 may influence the drug resistance of gastric cancer cells to cisplatin [24]. This study provided evidence that constitutively activated STAT3 in GC cells contributed to multidrug resistance by conferring apoptosis resistance to chemotherapy. Effective blockade of the STAT3 pathway both with drugs and small-interfering RNA (si-RNA) remarkably sensitized gastric cancer cells to chemotherapeutic agents. Similar phenomenon was reported in laryngeal cancer stem cells by down-regulating the IL-6/STAT3 pathway [20,25].
It has been reported that STAT3 is closely related to DDR [26]. One study evidenced that STAT3 interrupts ATR-CHK1 signaling to allow oncovirus-mediated cell proliferation [27]. While another study observed that ATR depletion suppressed downstream CHK1 and STAT3 phosphorylation in response to DNA damage [28]. Therefore, here we examined Stat3 activation (p-STAT3) to determine whether VE-821-induced DDR inhibition contributes to Stat3-associated susceptibility to cisplatin resistance.
Due to the lack of research models characterizing the occurrence and development of GC, the research on the pathogenesis and therapeutic drugs of GC is limited. Organoids are a source of stem cells that are cultured in three dimensions in vitro while retaining various properties of living tissue, such as self-organization and self-renewal. It is an excellent preclinical model for basic life science research and applied clinical research such as organ development studies, pathogen discovery, disease modeling, and drug screening [29], [30], [31]. Morphologically, GC organoids are monolithic spherical structures containing gastric acid and cellular secretions [32], [33], [34], that rapidly proliferate, renew, and differentiate. Luminescent pH is lowered by histamine stimulation, which is reversed by the proton pump inhibitor omeprazole [35]. The establishment of GC organoids retains the characteristics of genome stability and tumor heterogeneity, and is of great value in the pathogenesis of GC, tumor microenvironment, functional detection of gastric cancer-related genes, drug sensitivity screening, and individualized treatment of GC [32,36]. It has been reported that human GC organoids can be obtained from surgical resection specimens and tissue biopsies [37], [38], [39]. Therefore, in our study, we established GC organoids to test the efficacy of cisplatin and/or VE-821.
Here, we found that high ATR expression was associated with higher pathological stage. The IC50 values of VE-821 combined with cisplatin in GC cell lines and organoid models were significantly lower than those of single drug or drug loading, consistent with the results of in vivo experiments. Our experiments also showed that the ATR inhibitor VE-821 attenuated GC resistance to cisplatin by reversing DDR activation, which may be related to the inhibition of constitutive activation of STAT3.
Methods and materials
Cell culture and treatment
Human GC cells (AGS and MKN-45) were purchased from the Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, China. All cell lines were authenticated by Short-Tandem Repeats (STRs) by the supplier and tested for mycoplasma contamination using commercial mycoplasma PCR (Mycoplasma PCR Detection Kit, Beyotime, China) every 2–3 months. The cells were passaged for less than 6 months before use and maintained in RPMI-1640 medium (Invitrogen, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Biological Industries, Cromwell, CT, USA) and 10 mg/mL penicillin-streptomycin (Invitrogen) (100 U/ml) at 37 °C with a 5% CO2 atmosphere. The cells were used for experiments after they reached 70–80% confluence.
Reagents and antibodies
The cells were treated with cisplatin and/or VE-821. All drugs were purchased from Selleck Chemicals. Cisplatin was dissolved in saline and VE-821 in dimethylsulfoxide (DMSO). Antibodies to various antigens were as follows: phospho-CHK1 (Ser 345) (Proteintech Cat# 28803–1-AP, RRID:AB_2918206, 1:1000), CHK 1 (Proteintech Cat# 25887–1-AP, RRID:AB_2880283, 1:2000), phospho-ATR (Thr 1989) (Cell Signaling Technology Cat# 58014, RRID:AB_2722679, 1:1000), ATR (Proteintech Cat# 19787–1-AP, RRID:AB_10639516, 1:1000), phospho-H2AX (Ser139) (Abcam Cat# ab81299, RRID:AB_1640564, 1:3000), phospho-STAT3 (Cell Signaling Technology Cat# 9145, RRID:AB_2491009, 1:2000), STAT3 (Proteintech Cat# 10253–2-AP, RRID:AB_2302876, 1:2000), β-actin (Sigma-Aldrich Cat# A5316, RRID:AB_476743, 1:5000), Anti-rabbit IgG (Cell Signaling Technology Cat# 7074, RRID:AB_2099233, 1:3000), Anti-mouse IgG (Cell Signaling Technology Cat# 7076, RRID:AB_330924, 1:3000), and Goat Anti-Rabbit IgG H&L(Alexa Fluor ® 647) (Abcam Cat# ab150083, RRID:AB_2714032, 1:500).
Cell viability assay
A total of 3000 cells suspended in a volume of 100μl complete medium per well were cultured in a 96-wells plate. We used a CCK-8 kit solution (Dojindo, Minato-ku, Tokyo, Japan) to measure the cell viability of AGS and MKN-45 cells with different concentrations of VE-821 and/or cisplatin. A volume of 10μl CCK-8 reagent was added to each well and incubated at 37 °C for 1.5 h. Absorbance was recorded at 450 nm. We performed this assay at 24 h, 48 h, and 72 h respectively after treating with VE-821 and/or cisplatin. All experiments were repeated at least three times.
Apoptosis assay
Flow cytometry was used to detect the apoptosis rate with the Annexin V-fluorescein Isothiocyanate (FITC) /Propidium Iodide (PI) (556, 547, BD Biosciences, USA) staining according to the manufacturer's protocol. For flow analysis, about (5–10) × 105 GC cells were seeded in 6-well culture plates and adhered for 24 h at 37 °C with a 5% CO2 atmosphere. Following the addition of the drugs, the GC cells were incubated for 72 h at 37 °C.
The supernatant and adherent GC cells per well were collected and centrifuged with 2000 rpm for 5 min for the following steps. The precipitations were washed twice with PBS and resuspended with 100μl 1 × binding buffer (556, 547, BD Biosciences, USA), then stained with Annexin V-FITC/PI for 15 min and detected with Flow Cytometer (BD Accuri™ C6 Plus).
Western blotting (WB)
The GC cells were collected and washed three times with PBS. Total protein from the GC cells was lysed using RIPA lysis buffer (150 mM NaCl, 50 mM Tris–HCl at pH 7.4, 1 mM EDTA, 0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate, and 1% NP-40) mixed with protease and phosphatase inhibitor cocktail (Roche Diagnostics GmbH, Mannheim, Germany) and PMSF (Biosharp, Hefei, China) for 15–20 min on shaker 4 °C according to the instructions provided with the kit. The proteins were quantified via the bicinchoninic acid (BCA) method and resolved by SDS–PAGE, then transferred to polyvinylidene difluoride membranes (PVDF membranes). Primary antibodies blotted with PVDF membranes were those in the part of Reagents and antibodies. The protein expression was visualized using the 5200 Multi Chemiluminescent Imaging System (Tanon, China).
Immunofluorescence (IF)
Twenty-five thousand cells were seeded into a 24-well plate within round coverslips and then adhere overnight. After incubating with different reagents for about 48 h, cells were fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.5% Triton-X-100 for 20 min, and then blocked with 5% bovine serum albumin (BSA) at room temperature for 30 min. Antibody against γ-H2AX was incubated with the cells on slices at 4 °C overnight. After incubating with a secondary antibody for 1 h and counterstaining with DAPI, the images were visualized by confocal microscopy.
The cancer genome atlas (TCGA) database analysis
RNA expression in terms of RNA-seq and the clinical feature were downloaded from TCGA database. In total 169 patients were classified to “Low expression” and “High expression” by a cutoff point which was the median of ATR expression level, and analyzed with Kaplan-Meier plotter. Differences were considered statistically significant when p < 0.05.
Human organoid culture
Human GC tissues were obtained from patients who underwent surgery at Nanjing Drum Tower Hospital, the affiliated hospital of Nanjing University Medical School. Patients enroled with none of radiotherapy, chemotherapy, or other related anti-tumor therapies, and signed informed consent before surgery.
The experimental method of isolating and cultured human GC organoids referred to previous studies with minor modification [39,40] (Supplementary Methods). And organoids were passaged twice a week with a split ratio of 1:2/1:3.
Treatment with chemotherapeutics was performed 72 h after seeding. Selected organoids were treated with cisplatin and/or VE-821. The study was complied with the principles of Declaration of Helsinki and approved by the institutional review board of the Ethics Committee of Nanjing Drum Tower Hospital affiliated to Nanjing University Medical School (approval number: 2020–183).
Cell-titer GLO 3D cell viability assay
After two weeks of treatment with cisplatin and/or VE-821, viability was analyzed with Cell-titer GLO 3D (Promega, Germany) cell viability reagent which was optimized for organoid models according to the manufacturer's instruction, and luminescence was measured on an enzyme-labeled instrument.
Xenograft model
The GC xenograft model used the athymic BALB/c nude mice (4–5 weeks old) which were purchased from Beijing Vital River Laboratory Animal Technology. And the assays were approved by the Institutional Animal Care and Use Committee of Nanjing Drum Tower Hospital (20201105). After about 3 days of adaptation, each of the 20 nude mice was subcutaneously injected into the right hind leg with 2 × 106 MKN45 cells suspended in 100μl PBS and 100μl Matrigel. Tumors were measured using a caliper and the volumes were calculated as follows: Volume=(L × W2)/2. When tumors reached approximately 100mm [3], the mice were randomized to following 4 groups (n = 5 for each group): (i) control group receiving saline i.p.+CMC-Na p.o.; (ii) saline i.p.+VE-821 3 mg/kg p.o.; (iii) cisplatin 5 mg/kg i.p.+CMC-Na p.o.; (iv)cisplatin 5 mg/kg i.p.+VE-821 3 mg/kg p.o. VE-821 and cisplatin were commonly administered every three days. All mice were killed when they met the humane endpoint criteria. Part of the tumors was fixed with 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin-eosin (HE). The remaining tissues were frozen in liquid nitrogen and then stored at −80 °C for subsequent analyses.
Immunohistochemistry (IHC)
Paraffin-embedded tissue sections were deparaffinized, rehydrated, subjected to antigen retrieval, and blocked, then incubated at 4 °C overnight with primary antibodies, followed by secondary antibodies at room temperature for 1–2 h. Ki-67 (Proteintech), and phospho-H2AX (Ser139) (Abcam) antibodies were used.
Statistical analysis
Each experiment has been repeated a minimum of three times. All results were expressed as the mean ± SEM and performed using GraphPad Prism 9 software (GraphPad Software, La Jolla, CA, USA) and SPSS software (IBM SPSS-statistics, China). A two-sided Student t-test was performed when appropriate. The combined index (CI) was calculated by CompuSyn joint index calculation software. WB experiments and immunofluorescence experiments were quantitatively analyzed using Image J software. Differences were considered statistically significant at p < 0.05(* p < 0.05, **p < 0.01, *** p < 0.001, **** p < 0.0001).
Results
Cisplatin inhibits the proliferation of GC cells and induces DNA damage
Platin-based chemotherapy is one of the first-line drugs for advanced GC. The mechanism behind the negative effect on GC cells resulting from cisplatin is DNA damage reportedly. To confirm the above point, first of all, we selected two GC cell lines, AGS and MKN-45, for in vitro experiments. After treatment of the two cells with cisplatin for 48 h and 72 h, it was suggested that cisplatin could inhibit cell proliferation in a concentration-dependent manner via the CCK-8 experiment (Fig. 1A). The IC50 values of AGS and MKN-45 treated with cisplatin for 72 h were 39.3 μM and 35.9 μM respectively. In addition, in a long-term clonogenic assay, cisplatin significantly inhibited the formation of AGS and MKN-45 cell colonies (Fig. 1D and 1E). Meanwhile, the results of flow cytometry indicated that cisplatin can slightly induce apoptosis of GC cells after treating for 72 h (Fig. 1B and 1C). To explore the possible role of the DDR signaling pathway in the mechanism of cisplatin resistance, the expression levels of ATR, p-ATR, CHK1, p-CHK1, and γ-H2AX proteins in the two cells were observed by the way of western blot following with different concentrations of cisplatin. Compared with the control group, cisplatin facilitated the phosphorylation of ATR and CHK1 (Fig. 1F and Supplementary Fig. 1B, C, F, G). Moreover, as was shown in Fig. 1G and 1H, stronger fluorescence intensity of γ-H2AX was observed in the cisplatin group than in the control group. Studies have shown that STAT3 is necessary to effect chemoresistance [24] and repair damaged DNA [41]. We, thus, tested the level of STAT3 and p-STAT3 through WB and quantitative analysis of images. We found that STAT3 was activated in both cell lines after cisplatin treatment, that is, p-STAT3 but not STAT3 levels increased (Fig. 1F and Supplementary Fig. 1A, E). The results are consistent with our previous finding that STAT3 activation affects cisplatin resistance in gastric cancer cells [24]. To sum up, cisplatin induces the formation of intra- and inter-DNA strand cross-linkages, alters the DNA double helical structure, and causes DNA damage. Furthermore, it activates a DDR signaling pathway mediated by ATR-CHK1 in GC cells, which may contribute to cisplatin chemoresistance and associate with STAT3 activation.
Fig. 1.
Cisplatin inhibits the proliferation of GC cells and induces DNA damage. (A) The efforts for the proliferation of AGS and MKN-45 cells after treatment with cisplatin for 48 h and 72 h were detected by CCK-8. (B, C) The apoptosis of AGS and MKN-45 cells treated with 5 μM cisplatin for 72 h was detected and quantified by Flow cytometry. (D, E) Colony-formation assay of AGS and MKN-45 cells treated with Cisplatin (2 μM, 5 μM, and 10 μM). (F) The protein expression of ATR, CHK1, STAT3 and p-ATR, p-CHK1, p-STAT3, γ-H2AX in AGS and MKN-45 cells after treatment with cisplatin for 48 h. (G, H) The expression and quantification of signal intensities of γ-H2AX in AGS and MKN-45 cells after adding cisplatin were determined by IF. The data are presented as mean ± SEM of three independent experiments. (Bar=25 μm. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
VE-821 inhibits GC cells proliferation and alters the DDR
After treating AGS and MKN-45 cells with VE-821 for 48 h and 72 h, the CCK-8 experiment resulted that VE-821 could significantly inhibit cell proliferation in a concentration-dependent manner (Fig. 2A). The IC50 values of VE-821 in AGS and MKN-45 cells were calculated to be 13.7 μM and 11.3 μM respectively at the time of 72 h, meanwhile, as shown in Fig. 2B and 2C, VE-821 promotes apoptosis of GC cells. Beside, VE-821 also decreased the colony-formation capacities of the GC cells dose-dependently (Fig. 2D and 2E). When it comes to the effort of VE-821 to DNA damage and DDR, we discovered downregulation of the ratios of p-ATR to ATR, and p-CHK1 to CHK1, and upregulation of γ-H2AX in cells exposed to VE-821 in a dose-dependent manner (Fig. 2F and Supplementary Fig. 2B, C, F, G). Then we stained PE to visualize the intensity of γ-H2AX. And we figured out that in both two cells, the levels of γ-H2AX were significantly upregulated in the VE-821-treated group (Fig. 2G and 2H). Furthermore, ATR inhibition prevented STAT3 activation (Fig. 2F and Supplementary Fig. 2A, E), which is consistent with another previous study [28]. In conclusion, our experiments demonstrated that VE-821 inhibits proliferation and induces apoptosis of GC cells by attenuating DDR, inducing DNA damage and increasing DNA replication pressure.
Fig. 2.
VE-821 inhibits GC cells proliferation and alters the DDR. (A)Proliferation ability of AGS and MKN-45 cells treated with VE-821 for 48 h and 72 h was detected by CCK-8. (B, C) Apoptosis of AGS and MKN-45 cells treated with VE-821 at 2 μM for 72 h was detected by flow cytometry. (D, E) Colony-formation assay of AGS and MKN-45 cells treated with VE-821 (1 μM, 2 μM, and 5 μM). (F) Expression of ATR, CHK1, STAT3 and phosphorylated ATR, CHK1, STAT3, γ-H2AX in AGS and MKN-45 cells treated with different concentrations of VE-821 for 48 h were detected by WB. (G, H) The expression of signal intensities of γ-H2AX in AGS and MKN-45 cells after treating with VE-821 was determined by IF. The data are presented as mean ± SEM of three independent experiments. (Bar=25 μm. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Relationship between ATR expression and clinicopathological features
The RNA expression data of GC tissues and the corresponding clinical information of patients were downloaded from the TCGA database. The inclusion criteria refer to complete RNA expression data and clinical parameter information including survival time, age, gender, tumor size, lymph node metastasis, TNM stage, and tumor stage (Table 1). Table 1 suggested that ATR expression is highly correlated with the clinical stage of patients, which indicated that patients with higher ATR expression have a higher pathological stage (Table 1).
Table 1.
The expression and clinicopathologic significance of ATR in gastric cancer.
| Characteristic | ATR expression |
p Value | ||
|---|---|---|---|---|
| Low(n = 112) | High(n = 57) | |||
| Gender | 0.048* | |||
| Female | 43 | 31 | ||
| Male | 69 | 26 | ||
| Age(year) | 0.083 | |||
| >66 | 53 | 35 | ||
| ≤66 | 59 | 22 | ||
| Tumor size(cm) | 0.159 | |||
| >1 | 52 | 20 | ||
| ≤1 | 60 | 37 | ||
| Tumor differentiation | 0.511 | |||
| High & Middle | 37 | 16 | ||
| Low | 75 | 41 | ||
| Tumor invasion | 0.59 | |||
| T1–2 | 38 | 17 | ||
| T3–4 | 74 | 40 | ||
| TNM stage | 0.003⁎⁎ | |||
| I∼II | 64 | 19 | ||
| III∼IV | 48 | 38 | ||
| Lymph node metastasis | 0.091 | |||
| No | 42 | 14 | ||
| Yes | 70 | 43 | ||
p < 0.05.
p < 0.01.
Inhibition of ATR enhances the chemosensitivity of GC cells to cisplatin
Cisplatin chemoresistance remains the major obstacle to achieving optimal prognosis in GC patients, and it may be related to ATR-mediated DNA damage and DDR. ATR is located upstream of CHK1, which can regulate the cell cycle and initiate DDR by activating CHK1 during replication stress. Therefore, we chose ATR inhibitor VE-821 to further explore the effect of the ATR/CHK1 pathway on cisplatin sensitivity. The inhibitory effects of cisplatin combined with VE-821 on the proliferation of AGS and MKN-45 cells were investigated by CCK8 assay. To reduce the cytotoxicity of VE-821, the concentrations were determined to be 1 μM, 2 μM, and 5 μM. GC cells were treated with a fixed concentration of VE-821 and different concentrations of cisplatin for 72 h. The result showed that there was a dose-dependent relationship between cell survival and cisplatin concentration, and the outcome of the two drugs combined was better than that of a single drug (Fig. 3A). To determine the synergistic effect, CompuSyn joint index calculation software was used to calculate the cell inhibition rate data obtained from the CCK8 experiment which was shown in Fig. 3B. The combined index (CI) of VE-821 and cisplatin were mostly between 0 and 0.5, which indicated that these two drugs had a high synergistic effect. Similar synergistic effect was verified in colony-formation assays (Fig. 3E and 3F). According to flow cytometry, the percentage of apoptotic cells in the combined treatment group increased compared with those treated with any one drug alone (Fig. 3C and 3D). Furthermore, WB was used to detect the trend of downstream signaling pathways to explore the potential mechanism of cisplatin sensitivity via ATR inhibition. In the combination groups with cisplatin and VE-821, we found further inhibition of phosphorylation of STAT3 and significant upregulation of γ-H2AX (Fig. 3G and Supplementary Fig. 3). Even more, the expression levels of p-ATR, and p-CHK1 were lower compared with those in the cisplatin group, which means DDR, which is induced via cisplatin-based DNA damage, was effectively reversed with ATR inhibition (Fig. 3G and Supplementary Fig. 3). Meanwhile, we incubated GC cells with the fluorescent secondary antibody to detect levels of γ-H2AX, as same as the WB result, the stronger intensity was unfolded in the combination group compared with any drug alone (Fig. 3H and 3I). Taken together, these results indicated that VE-821, an ATR inhibitor, could enhance the sensitivity of GC cell lines to cisplatin via reducing DDR pathway, and this process may also relate to the attenuation of STAT3 constitutive activation.
Fig. 3.
Inhibition of ATR enhances the chemosensitivity of GC cells to cisplatin. (A) Proliferation ability of AGS and MKN-45 cells treated with fixed VE-821 concentration and different cisplatin concentrations for 72 h was detected by CCK-8. When combined with different concentrations (0 μM, 1 μM, 2 μM, 5 μM) of VE-821, the IC50 of cisplatin in AGS was respectively 20.7 μM, 4.53 μM, 1.60 μM, 0.33 μM, and the IC50 in MKN45 was respectively 18.9 μM, 3.60 μM, 2.66 μM, 0.72 μM. (B) The CI values were calculated with CompuSyn joint index calculation software. (C, D) Apoptosis of AGS and MKN-45 cells treated with VE-821 at 2 μM and cisplatin at 5 μM for 72 h was detected by flow cytometry. (E, F) Colony-formation assay of AGS and MKN-45 cells treated with combination of VE-821 and cisplatin. (G) The protein expressions of ATR, CHK1, STAT3 and p-ATR, p-CHK1, p-STAT3, γ-H2AX in AGS and MKN-45 cells treated with VE-821 and cisplatin for 48 h were detected by WB. (H, I) The IF was used to detect the expression of signal intensities of γ-H2AX in AGS and MKN-45 cells in four groups. The data are presented as mean ± SEM of three independent experiments. (Bar=25 μm. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
VE-821 enhances the sensitivity of GC organoids to cisplatin
According to Lauren's classification, GC organoid models can be divided into three types: intestinal type, diffuse type, and mixed type [3]. To verify that ATR inhibitor VE-821 synergistically enhances the chemosensitivity of GC cells to cisplatin, the drugs were added to three types of GC organoids. The typical visual field was selected and photographed every 3-to 4 days to show the cell shrinkage and apoptosis after drug treatment, and the effect of the combined group was better than that of a single drug (Fig. 4A, 4B, and 4C). After that, the cell luminescence index was detected by Cell Titer Glo 3D experiment, as shown in Fig. 4D, 4E, and 4F, and it was found that the combined effect of two drugs in a mixed gastric cancer organoid model was significantly better than that of a single drug, and the curative effect was better than that of diffuse type and intestinal type, which was also verified in the calculation of IC50 (Fig. 4 G).
Fig. 4.
VE-821 enhances the sensitivity of GC organoids to cisplatin. (A, B, C) Morphological changes of organoid cells treated with cisplatin and VE-821 alone or in combination in GC organoid model classified as mixed by Lauren, and detection of cell activity after dosing treatment. (D, E, F) Carrying out Cell Titer Glo 3D detection after drug treatment in intestinal GC organoids and reading luminescence index through microplate reader. (G) IC50 value of the drugs in different Lauren classifications of GC organoid models. The data are presented as mean ± SEM of three independent experiments.
The ATR inhibitor, VE-821, effectively sensitive GC to cisplatin in vivo
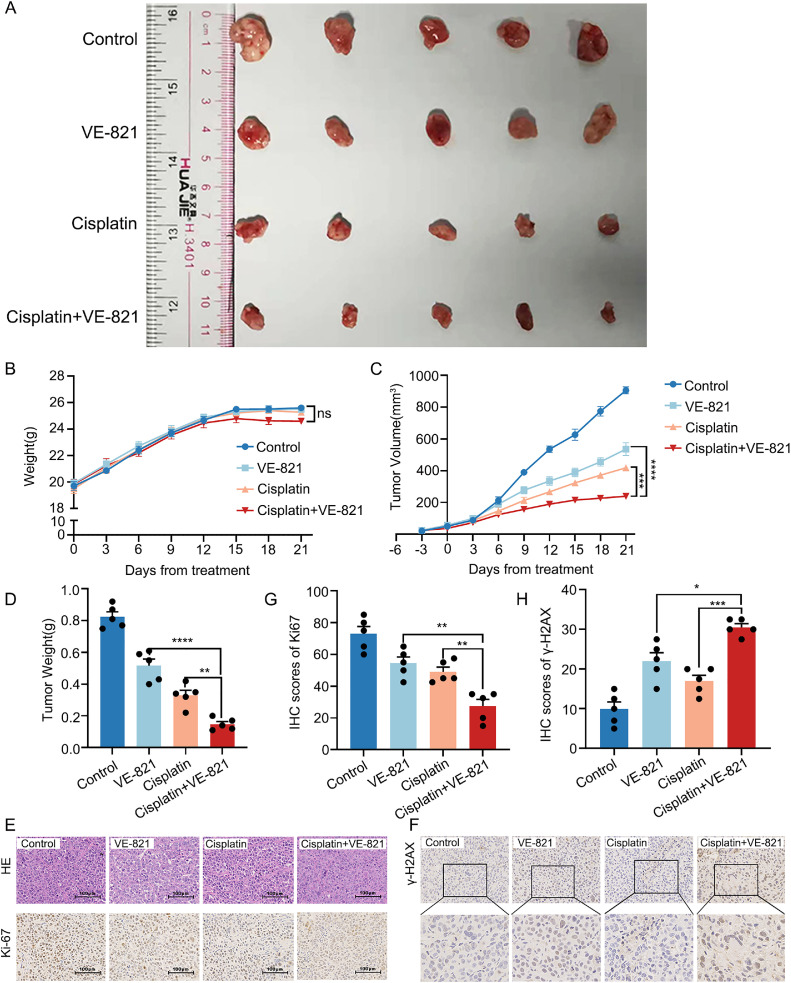
We first validated whether inhibition of ATR affects the sensitivity of GC to cisplatin in vivo. We hypothesized that VE-821 may sensitize GC to cisplatin via up-regulation of γ-H2AX. Remarkably reductions in tumor volumes and weights in the group treated with VE-821 and cisplatin were observed, compared with those in the VE-821 or cisplatin alone, or in the vehicle-treated group (p < 0.0001) (Fig. 5A, 5C and 5D). Little differences in mice weights between the four groups verified the safety of VE-821 and cisplatin (Fig. 5B). To further evaluate the toxicity of the therapy, we collected eyeball blood samples after 3 weeks of administration for blood testing. As depicted in Supplementary Fig. 4, the results indicated no significant differences in the blood routine, liver function, and renal function among the four groups after the 3 weeks of administration period. In addition, we observed fewer levels of Ki-67 in the group with the combination of these two drugs (Fig. 5E and 5G). To determine whether the sensitizing effect of VE-821 was associated with DNA damage, γ-H2AX was measured in xenograft tumors using IHC. The result showed higher expression of γ-H2AX in the group treated with VE-821 and cisplatin than those in other groups (Fig. 5F and 5H). These results above showed that inhibition of ATR could enhance the sensitivity of GC to cisplatin via increasing DNA damage in vivo, which consisted with the experiments in vitro and organoid.
Fig. 5.
In vivo, VE-821 effectively enhances the sensitivity of GC to cisplatin. (A) Twenty mice were divided into 4 groups treated with VE-821, cisplatin, and the two drugs together. Tumors at 3 weeks after the initial treatment were harvested. All resected tumors were photographed after killing. (B) The body weights of each mouse were recorded every 3 days. (C) Changes in tumor volume during drugs treatment. Tumor volume was measured every 3 days. (D) The weights of resected tumors from each mouse were recorded. (E) IHC staining shows the weaker expression of Ki-67 in group VE-821+cisplatin. (F) The expression of γ-H2AX in group VE-821+cisplatin is stronger. (G, H) The IHC scores were separately completed by two professional pathologists. The data are presented as mean ± SEM of three independent experiments. (Bar=100 μm, 25 μm. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
Discussion
In our study, enhanced levels of p-CHK1 and p-ATR was observed in GC cells after cisplatin exposure. This finding provides important information for the application of ATR inhibitors in the treatment of gastric cancer. Vertex Pharmaceuticals discovered the ATR inhibitor berzosertib (M6620) and simultaneously performed high-throughput testing for potent ATR inhibition [42] . The drug, developed on the basis of VE-821, is the first ATR inhibitor to enter clinical research. Among patients with small cell lung cancer (SCLC), the majority (68.0%) of patients treated with berzosertib plus topotecan experienced tumor regression, according to official data from a phase II clinical trial. Late disease progression was also observed in platinum-resistant patients (30.0%) [43].
In the TCGA database, the expression of ATR was closely related to the clinical stage of patients. It shows that the expression of ATR can be used as a biological index to evaluate the prognosis of gastric cancer. Therefore, we speculate that ATR inhibition combined with cisplatin is an effective way to treat gastric cancer. In vitro experiments showed that GC cells were sensitized to cisplatin after exposure to ATR inhibitors, which was supported by experimental data on CCK8.
Preliminary studies in our laboratory have reported that the activation of STAT3 plays a key role in the induction of multidrug resistance in gastric cancer, and the inhibition of STAT3 may sensitize gastric cancer cells to cisplatin [24]. In addition, STAT3 is considered to be one of the independent risk factors in patients with advanced gastric cancer [21], and has been reported to be closely related to DDR [26]. In this study, through quantitative analysis of WB images, we found that cisplatin-induced high level of p-STAT3 can be further suppressed by VE-821. Meanwhile, VE-821 can promote the susceptibility to cisplatin resistance in GC cells. Thus, we suggested that STAT3 activation may play a key role in VE-821 mediated effects.
Furthermore, we demonstrated that the combination of cisplatin and VE-821 is effective on organoid cells. We randomly selected three representative organoid cells of diffuse type, intestinal type and mixed type, and found that the apoptosis of intestinal organoid cells was the most significant after the combination of the two drugs. Literature shows that intestinal GC patients are the most sensitive to chemotherapy. Combining the above points, organoid drug sensitivity test found that intestinal organoid cells have the best chemosensitivity to cisplatin. The telephone call back found that the patient's condition was developing rapidly and died, which further highlighted the importance of GC organ cell culture medium drug screening for individualized precision treatment.
In conclusion, our study indicated that the activation of DDR signaling plays an important role in the mechanism of cisplatin resistance in GC cells, and the combination of ATR inhibitor VE-821 and cisplatin may be an effective treatment for GC because VE-821 can increase the sensitivity of GC cells to cisplatin. The results of this study may contribute to the selection of ATR inhibitor combination regimens and provide a solid theoretical basis for clinical trials of gastric cancer. The underlying mechanisms behind cisplatin sensitivity remain to be further explored.
Ethics approval and consent to participate
The study was approved by the institutional review board of the Ethics Committee of Nanjing Drum Tower Hospital affiliated to Nanjing University Medical School (approval number: 2020–183). All patients have signed the inform consents before surgery. All the animal experiments complied with the Institutional Animal Care and Use Committee of Nanjing Drum Tower Hospital (20201105).
Availability of data and materials
Not applicable.
CRediT authorship contribution statement
Haochen Su: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Yue Yuan: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Visualization. Jiatong Tang: Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Visualization. Yixuan Zhang: Conceptualization, Investigation, Writing – review & editing, Supervision. Hao Wu: Investigation. Yin Zhang: Investigation, Validation. Jiawei Liang: Investigation, Validation. Lei Wang: Resources. Xiaoping Zou: Resources. Shuling Huang: Resources, Funding acquisition. Shu Zhang: Conceptualization, Methodology, Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition. Ying Lv: Conceptualization, Methodology, Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81802396), Natural Science Foundation of Jiangsu Province (BK20191113 & BK20180117), General Project of Nanjing Medical Science and Technology Development Project (YKK17077), Nanjing Science and Technology Development Plan Project (201715023), Nanjing Medical Science and Technology Development Key Project (ZKX18022), Nanjing Science and Technology project (201911038), and Special fund of Nanjing Health and Technology development (JQX18002).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2023.101743.
Contributor Information
Shu Zhang, Email: zhangsgastro@nju.edu.cn.
Ying Lv, Email: lvying@njglyy.com.
Appendix. Supplementary materials
References
- 1.Sung H., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 Cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Smyth E.C., Nilsson M., Grabsch H.I., van Grieken N.C., Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 3.Biagioni A., et al. Update on gastric cancer treatments and gene therapies. Cancer Metastasis Rev. 2019;38:537–548. doi: 10.1007/s10555-019-09803-7. [DOI] [PubMed] [Google Scholar]
- 4.Saldivar J.C., Cortez D., Cimprich K.A. The essential kinase ATR: ensuring faithful duplication of a challenging genome. Nat. Rev. Mol. Cell Biol. 2017;18:622–636. doi: 10.1038/nrm.2017.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciccia A., Elledge S.J. The DNA damage response: making it safe to play with knives. Mol. Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blackford A.N., Jackson S.P. ATM, ATR, and DNA-PK: the trinity at the heart of the DNA damage response. Mol. Cell. 2017;66:801–817. doi: 10.1016/j.molcel.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Galluzzi L., et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–1883. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh S. Cisplatin: the first metal based anticancer drug. Bioorg. Chem. 2019;88 doi: 10.1016/j.bioorg.2019.102925. [DOI] [PubMed] [Google Scholar]
- 9.Kryczka J., Kryczka J., Czarnecka-Chrebelska K.H., Brzezianska-Lasota E. Molecular mechanisms of chemoresistance induced by cisplatin in NSCLC cancer therapy. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22168885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weber A.M., Ryan A.J. ATM and ATR as therapeutic targets in cancer. Pharmacol. Ther. 2015;149:124–138. doi: 10.1016/j.pharmthera.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Smith J., Tho L.M., Xu N., Gillespie D.A. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv. Cancer. Res. 2010;108:73–112. doi: 10.1016/B978-0-12-380888-2.00003-0. [DOI] [PubMed] [Google Scholar]
- 12.Huntoon C.J., et al. ATR inhibition broadly sensitizes ovarian cancer cells to chemotherapy independent of BRCA status. Cancer Res. 2013;73:3683–3691. doi: 10.1158/0008-5472.CAN-13-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prevo R., et al. The novel ATR inhibitor VE-821 increases sensitivity of pancreatic cancer cells to radiation and chemotherapy. Cancer Biol. Ther. 2012;13:1072–1081. doi: 10.4161/cbt.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanjiv K., et al. Cancer-Specific Synthetic Lethality between ATR and CHK1 Kinase Activities. Cell Rep. 2016;14:298–309. doi: 10.1016/j.celrep.2015.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomes L.R., et al. ATR mediates cisplatin resistance in 3D-cultured breast cancer cells via translesion DNA synthesis modulation. Cell Death. Dis. 2019;10:459. doi: 10.1038/s41419-019-1689-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi Q., et al. The identification of the ATR inhibitor VE-822 as a therapeutic strategy for enhancing cisplatin chemosensitivity in esophageal squamous cell carcinoma. Cancer Lett. 2018;432:56–68. doi: 10.1016/j.canlet.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Nie Y., et al. STAT3 inhibition of gluconeogenesis is downregulated by SirT1. Nat. Cell Biol. 2009;11:492–500. doi: 10.1038/ncb1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S., et al. SIRT1 inhibits gastric cancer proliferation and metastasis via STAT3/MMP-13 signaling. J. Cell. Physiol. 2019;234:15395–15406. doi: 10.1002/jcp.28186. [DOI] [PubMed] [Google Scholar]
- 19.Gao Q., et al. Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4661–4666. doi: 10.1073/pnas.0303992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun C.Y., Nie J., Huang J.P., Zheng G.J., Feng B. Targeting STAT3 inhibition to reverse cisplatin resistance. Biomed. Pharmacother. 2019;117 doi: 10.1016/j.biopha.2019.109135. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S., et al. Co-ordinated overexpression of SIRT1 and STAT3 is associated with poor survival outcome in gastric cancer patients. Oncotarget. 2017;8:18848–18860. doi: 10.18632/oncotarget.14473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fletcher S., Turkson J., Gunning P.T. Molecular approaches towards the inhibition of the signal transducer and activator of transcription 3 (Stat3) protein. ChemMedChem. 2008;3:1159–1168. doi: 10.1002/cmdc.200800123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niu G., et al. Role of Stat3 in regulating p53 expression and function. Mol. Cell. Biol. 2005;25:7432–7440. doi: 10.1128/MCB.25.17.7432-7440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang S., et al. Inhibition of activated Stat3 reverses drug resistance to chemotherapeutic agents in gastric cancer cells. Cancer Lett. 2012;315:198–205. doi: 10.1016/j.canlet.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 25.Fu Q., et al. Ribonucleic acid interference knockdown of IL-6 enhances the efficacy of cisplatin in laryngeal cancer stem cells by down-regulating the IL-6/STAT3/HIF1 pathway. Cancer Cell Int. 2017;17:79. doi: 10.1186/s12935-017-0448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barry S.P., et al. STAT3 modulates the DNA damage response pathway. Int. J. Exp. Pathol. 2010;91:506–514. doi: 10.1111/j.1365-2613.2010.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao X.H., et al. STAT3 regulated ATR via microRNA-383 to control DNA damage to affect apoptosis in A431 cells. Cell. Signal. 2015;27:2285–2295. doi: 10.1016/j.cellsig.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh R.C., et al. ATR-mediated CD47 and PD-L1 up-regulation restricts radiotherapy-induced immune priming and abscopal responses in colorectal cancer. Sci. Immunol. 2022;7:eabl9330. doi: 10.1126/sciimmunol.abl9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lancaster M.A., Knoblich J.A. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 2014;345 doi: 10.1126/science.1247125. [DOI] [PubMed] [Google Scholar]
- 30.Fatehullah A., Tan S.H., Barker N. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 2016;18:246–254. doi: 10.1038/ncb3312. [DOI] [PubMed] [Google Scholar]
- 31.Drost J., Clevers H. Organoids in cancer research. Nat. Rev. Cancer. 2018;18:407–418. doi: 10.1038/s41568-018-0007-6. [DOI] [PubMed] [Google Scholar]
- 32.Lau H.C.H., Kranenburg O., Xiao H., Yu J. Organoid models of gastrointestinal cancers in basic and translational research. Nat. Rev. Gastroenterol. Hepatol. 2020;17:203–222. doi: 10.1038/s41575-019-0255-2. [DOI] [PubMed] [Google Scholar]
- 33.McCracken K.W., et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature. 2014;516:400–404. doi: 10.1038/nature13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pompaiah M., Bartfeld S. Gastric organoids: an emerging model system to study helicobacter pylori pathogenesis. Curr. Top. Microbiol. Immunol. 2017;400:149–168. doi: 10.1007/978-3-319-50520-6_7. [DOI] [PubMed] [Google Scholar]
- 35.Schumacher M.A., et al. The use of murine-derived fundic organoids in studies of gastric physiology. J. Physiol. 2015;593:1809–1827. doi: 10.1113/jphysiol.2014.283028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seidlitz T., et al. Human gastric cancer modelling using organoids. Gut. 2019;68:207–217. doi: 10.1136/gutjnl-2017-314549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao M., et al. Development of patient-derived gastric cancer organoids from endoscopic biopsies and surgical tissues. Ann. Surg. Oncol. 2018;25:2767–2775. doi: 10.1245/s10434-018-6662-8. [DOI] [PubMed] [Google Scholar]
- 38.Yan H.H.N., et al. A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell. 2018;23:882–897. doi: 10.1016/j.stem.2018.09.016. e811. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y., et al. Hesperadin suppresses pancreatic cancer through ATF4/GADD45A axis at nanomolar concentrations. Oncogene. 2022;41:3394–3408. doi: 10.1038/s41388-022-02328-4. [DOI] [PubMed] [Google Scholar]
- 40.Boj S.F., et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun C.Y., Nie J., Huang J.P., Zheng G.J., Feng B. Targeting STAT3 inhibition to reverse cisplatin resistance. Biomed. Pharmacother. 2019;117 doi: 10.1016/j.biopha.2019.109135. [DOI] [PubMed] [Google Scholar]
- 42.Charrier J.D., et al. Discovery of potent and selective inhibitors of ataxia telangiectasia mutated and Rad3 related (ATR) protein kinase as potential anticancer agents. J. Med. Chem. 2011;54:2320–2330. doi: 10.1021/jm101488z. [DOI] [PubMed] [Google Scholar]
- 43.Thomas A., et al. Therapeutic targeting of ATR yields durable regressions in small cell lung cancers with high replication stress. Cancer Cell. 2021;39 doi: 10.1016/j.ccell.2021.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.